Enhancing Caries Preventive Effects of Nanomaterials with Phototherapy: A Scoping Review
Abstract
1. Introduction
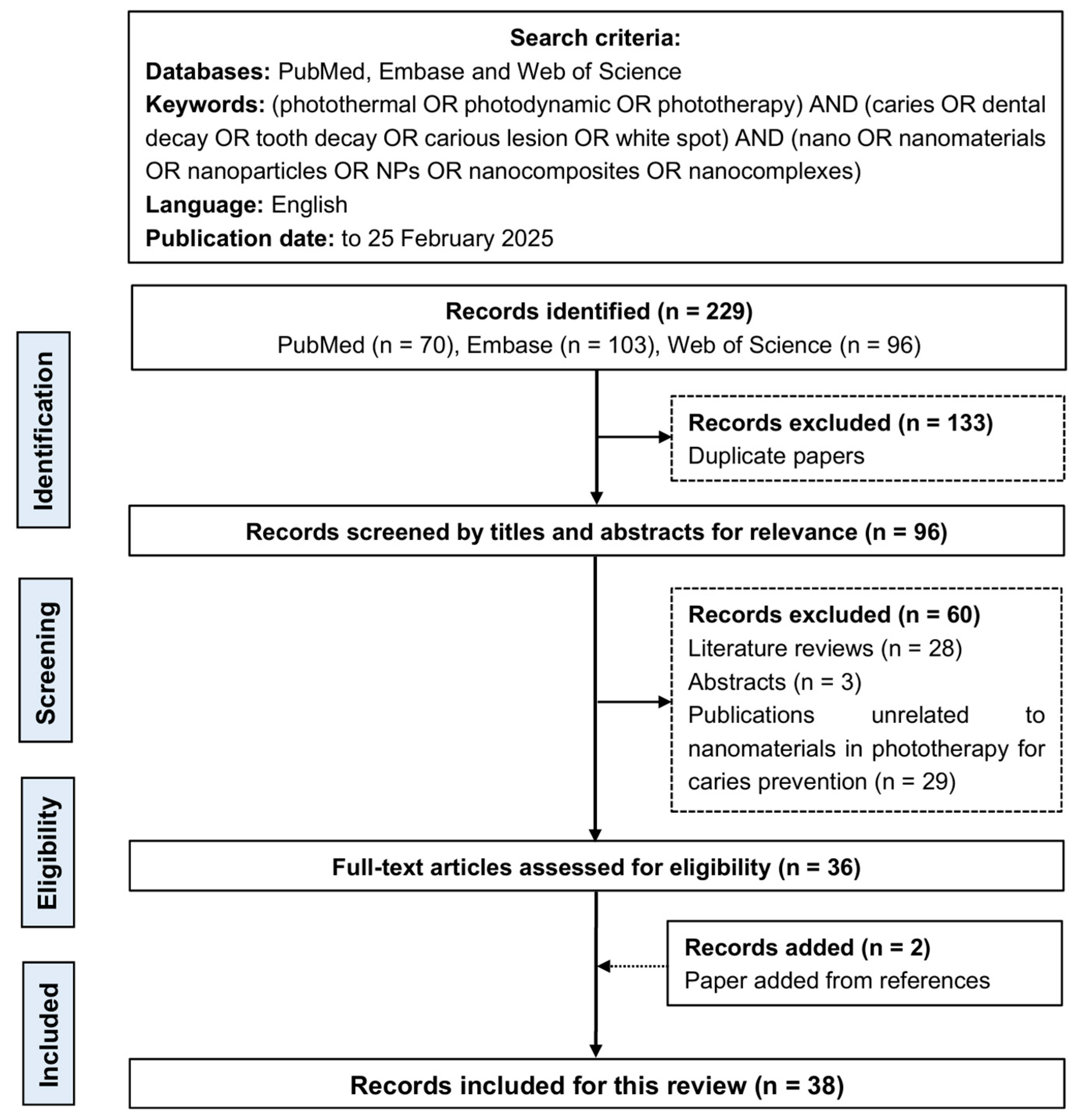
2. Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Assessment of the Risk of Bias
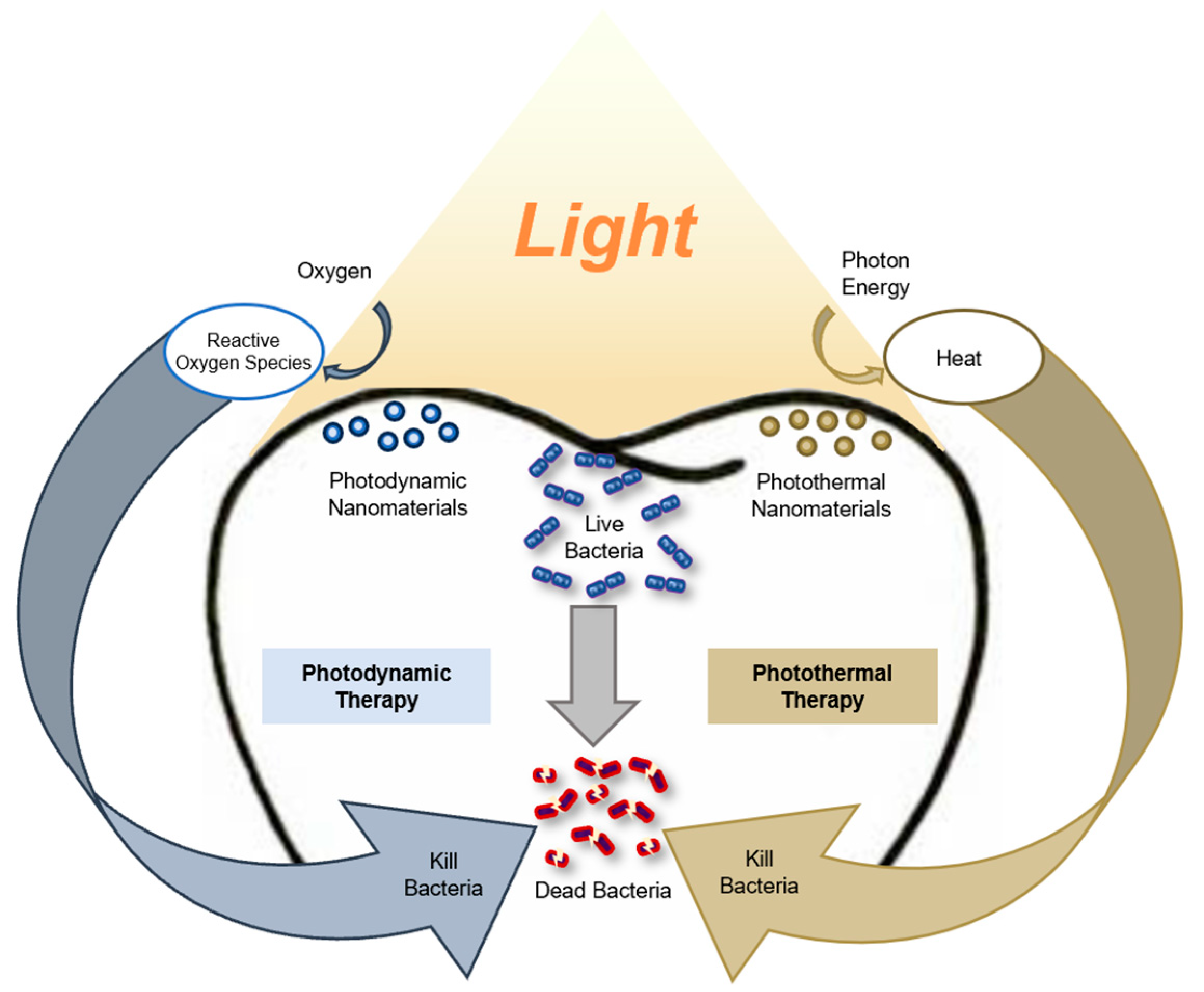
3. Results
3.1. Nanomaterials in Photodynamic Therapy
3.1.1. Natural Photodynamic Nanomaterials
3.1.2. Synthetic Photodynamic Nanomaterials
3.2. Nanomaterials in Photothermal Therapy
3.2.1. Metal-Based Photothermal Nanomaterials
3.2.2. Carbon-Based Photothermal Nanomaterials
3.2.3. Phosphorus-Based Nanomaterials
3.3. Nanomaterials in Combined Photothermal and Photodynamic Therapy
3.3.1. Metal-Based Dual-Modal Nanomaterials
3.3.2. Carbon-Based Dual-Modal Nanomaterials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Xu, V.W.; Yin, I.X.; Niu, J.Y.; Yu, O.Y.; Nizami, M.Z.I.; Chu, C.H. Developing a novel antibacterial copper tetraamine fluoride. J. Dent. 2024, 150, 105320. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Niu, J.Y.; Yu, O.Y.; Chu, C.H. Copper Materials for Caries Management: A Scoping Review. J. Funct. Biomater. 2023, 15, 10. [Google Scholar] [CrossRef]
- Ahmadian, E.; Shahi, S.; Yazdani, J.; Maleki Dizaj, S.; Sharifi, S. Local treatment of the dental caries using nanomaterials. Biomed. Pharmacother. 2018, 108, 443–447. [Google Scholar] [CrossRef]
- Schwendicke, F.; Paris, S.; Tu, Y.K. Effects of using different criteria for caries removal: A systematic review and network meta-analysis. J. Dent. 2015, 43, 1–15. [Google Scholar] [CrossRef]
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride Revolution and Dental Caries: Evolution of Policies for Global Use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of Antibiotics/Antimicrobial Agents on Dental Caries. Biomed. Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef] [PubMed]
- Kalka, K.; Merk, H.; Mukhtar, H. Photodynamic therapy in dermatology. J. Am. Acad. Dermatol. 2000, 42, 389–413. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Sun, J.; Song, L.; Fan, Y.; Tian, L.; Luan, S.; Niu, S.; Ren, L.; Ming, W.; Zhao, J. Synergistic Photodynamic and Photothermal Antibacterial Nanocomposite Membrane Triggered by Single NIR Light Source. ACS Appl. Mater. Interfaces 2019, 11, 26581–26589. [Google Scholar] [CrossRef]
- Warrier, A.; Mazumder, N.; Prabhu, S.; Satyamoorthy, K.; Murali, T.S. Photodynamic therapy to control microbial biofilms. Photodiagn. Photodyn. Ther. 2021, 33, 102090. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, Y.; Qi, H. Photothermal conversion and transfer in photothermal therapy: From macroscale to nanoscale. Adv. Colloid. Interface Sci. 2022, 308, 102753. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Chi, M.; Sun, X.; Xie, X.; Weir, M.D.; Oates, T.W.; Zhou, Y.; Wang, L.; Bai, Y.; Xu, H.H. Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int. J. Nanomed. 2019, 14, 6937–6956. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- da Mota, A.C.; Leal, C.R.; Olivan, S.; Leal Gonçalves, M.L.; de Oliveira, V.A.; Pinto, M.M.; Bussadori, S.K. Case Report of Photodynamic Therapy in the Treatment of Dental Caries on Primary Teeth. J. Lasers Med. Sci. 2016, 7, 131–133. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, J.; Qi, Y.; Yu, W.; Yang, S.; Zhang, J.; Zhao, J. Curcumin: A review of experimental studies and mechanisms related to periodontitis treatment. J. Periodontal Res. 2021, 56, 837–847. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Lung, C.Y.K.; Yu, O.Y.; Chu, C.H. Caries Management with Non-Metallic Nanomaterials: A Systematic Review. Int. J. Nanomed. 2022, 17, 5809–5824. [Google Scholar] [CrossRef]
- Raza, F.; Zafar, H.; Zhang, S.; Kamal, Z.; Su, J.; Yuan, W.E.; Mingfeng, Q. Recent Advances in Cell Membrane-Derived Biomimetic Nanotechnology for Cancer Immunotherapy. Adv. Heal. Mater. 2021, 10, e2002081. [Google Scholar] [CrossRef]
- Awad, M.; Thomas, N.; Barnes, T.J.; Prestidge, C.A. Nanomaterials enabling clinical translation of antimicrobial photodynamic therapy. J. Control Release 2022, 346, 300–316. [Google Scholar] [CrossRef]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Dilenko, H.; Bartoň Tománková, K.; Válková, L.; Hošíková, B.; Kolaříková, M.; Malina, L.; Bajgar, R.; Kolářová, H. Graphene-Based Photodynamic Therapy and Overcoming Cancer Resistance Mechanisms: A Comprehensive Review. Int. J. Nanomed. 2024, 19, 5637–5680. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Silvestre, A.L.P.; Di Filippo, L.D.; Besegato, J.F.; de Annunzio, S.R.; Almeida Furquim de Camargo, B.; de Melo, P.B.G.; Rastelli, A.N.S.; Fontana, C.R.; Chorilli, M. Current applications of drug delivery nanosystems associated with antimicrobial photodynamic therapy for oral infections. Int. J. Pharm. 2021, 592, 120078. [Google Scholar] [CrossRef]
- Wang, R.; Jia, C.; Zheng, N.; Liu, S.; Qi, Z.; Wang, R.; Zhang, L.; Niu, Y.; Pan, S. Effects of photodynamic therapy on Streptococcus mutans and enamel remineralization of multifunctional TiO(2)-HAP composite nanomaterials. Photodiagn. Photodyn. Ther. 2023, 42, 103141. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Salehi Vaziri, A.; Takzaree, N.; Ghorbanzadeh, R. Physico-mechanical and antimicrobial properties of an orthodontic adhesive containing cationic curcumin doped zinc oxide nanoparticles subjected to photodynamic therapy. Photodiagn. Photodyn. Ther. 2019, 25, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Hajfathalian, M.; de Vries, C.R.; Hsu, J.C.; Amirshaghaghi, A.; Dong, Y.C.; Ren, Z.; Liu, Y.; Huang, Y.; Li, Y.; Knight, S.A.; et al. Theranostic gold-in-gold cage nanoparticles enable photothermal ablation and photoacoustic imaging in biofilm-associated infection models. J. Clin. Investig. 2023, 133, e168485. [Google Scholar] [CrossRef]
- Ran, Y.; Shi, J.; Ding, Y.; Li, L.; Lu, D.; Zeng, Y.; Qiu, D.; Yu, J.; Cai, X.; Pan, Y. Black Phosphorus Nanosheets-Loaded Mussel-Inspired Hydrogel with Wet Adhesion, Photothermal Antimicrobial, and In Situ Remineralization Capabilities for Caries Prevention. Adv. Sci. 2024, 11, e2409155. [Google Scholar] [CrossRef]
- Ghanemi, M.; Salehi-Vaziri, A.; Pourhajibagher, M.; Bahador, A. Physico-mechanical and antimicrobial properties of an elastomeric ligature coated with reduced nanographene oxide-nano curcumin subjected to dual-modal photodynamic and photothermal inactivation against Streptococcus mutans biofilms. Photodiagn. Photodyn. Ther. 2023, 44, 103866. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O′Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J. Prosthet. Dent. 2024, 131, 1038–1042. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 2019, 366, l4898. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Khan, N.A.; Khan, A.A.; Bhutto, K.; Askary, S.H.; Askary, G.; Abrar, E.; Mahmood, S.J.; Qureshi, A. Titanium oxide and chitosan nanoparticles loaded in methylene blue activated by photodynamic therapy on caries affected dentin disinfection, bond strength, and smear layer removal efficacy. Photodiagn. Photodyn. Ther. 2024, 50, 104343. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, S.; Al Gburi, A.Q.K.; Ranjbar Omrani, L.; Chiniforush, N.; Moradi, Z. Evaluation of riboflavin, nanocurcumin, and hydrogen peroxide under light conditions: Reduction of mature dental biofilms and enamel mineral loss. Photodiagn. Photodyn. Ther. 2024, 50, 104379. [Google Scholar] [CrossRef]
- Hemmati, Y.B.; Bahrami, R.; Pourhajibagher, M. Assessing the physico-mechanical, anti-bacterial, and anti-demineralization properties of orthodontic resin composite containing different concentrations of photoactivated zinc oxide nanoparticles on Streptococcus mutans biofilm around ceramic and metal orthodontic brackets: An ex vivo study. Int. Orthod. 2024, 22, 100901. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Keshavarz Valian, N.; Bahador, A. Theranostic nanoplatforms of emodin-chitosan with blue laser light on enhancing the anti-biofilm activity of photodynamic therapy against Streptococcus mutans biofilms on the enamel surface. BMC Microbiol. 2022, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, R.; Hosseinpour Nader, A.; Salehi-Vaziri, A. The effects of bimodal action of photodynamic and photothermal therapy on antimicrobial and shear bond strength properties of orthodontic composite containing nano-graphene oxide. Photodiagn. Photodyn. Ther. 2021, 36, 102589. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Xia, Y.; Lan, Y.; Mokeem, L.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Magnetic-Responsive Photosensitizer Nanoplatform for Optimized Inactivation of Dental Caries-Related Biofilms: Technology Development and Proof of Principle. ACS Nano 2021, 15, 19888–19904. [Google Scholar] [CrossRef]
- Comeau, P.; Burgess, J.; Malekafzali, N.; Leite, M.L.; Lee, A.; Manso, A. Exploring the Physicochemical, Mechanical, and Photocatalytic Antibacterial Properties of a Methacrylate-Based Dental Material Loaded with ZnO Nanoparticles. Materials 2022, 15, 5075. [Google Scholar] [CrossRef]
- Binhasan, M.; Alsunbul, H.; Aljanakh, M.; Abduljabbar, T.; Vohra, F. Dentin disinfection and adhesive bond strength using modified photoactivated carbon nanoparticles. Photodiagn. Photodyn. Ther. 2023, 42, 103313. [Google Scholar] [CrossRef]
- Xu, X.Y.; Fan, M.L.; Yu, Z.H.; Zhao, Y.; Zhang, H.B.; Wang, J.; Wu, M.Z.; Sun, F.; Xu, X.N.; Ding, C.M.; et al. A removable photothermal antibacterial “warm paste” target for cariogenic bacteria. Chem. Eng. J. 2022, 429, 132491. [Google Scholar] [CrossRef]
- Guo, W.; Li, Y.; Wang, S.; Wang, Y.; Li, C.; Jin, Y.; Li, Y.; Chen, X.; Miao, W. Photodynamic nano hydroxyapatite with biofilm penetration capability for dental plaque eradication and prevention of demineralization. Colloids Surf. B Biointerfaces 2023, 225, 113242. [Google Scholar] [CrossRef]
- Lu, X.; Qu, Y.; Zhu, T.; Qu, X.; Zhang, Z.; Yu, Y.; Hao, Y. Applications of photothermally mediated nanohybrids for white spot lesions in orthodontics. Colloids Surf. B Biointerfaces 2023, 225, 113274. [Google Scholar] [CrossRef]
- Panda, S.; Rout, L.; Mohanty, N.; Satpathy, A.; Sankar Satapathy, B.; Rath, S.; Gopinath, D. Exploring the photosensitizing potential of Nanoliposome Loaded Improved Toluidine Blue O (NLITBO) Against Streptococcus mutans: An in-vitro feasibility study. PLoS ONE 2024, 19, e0312521. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Alaeddini, M.; Etemad-Moghadam, S.; Rahimi Esboei, B.; Bahrami, R.; Miri Mousavi, R.S.; Bahador, A. Quorum quenching of Streptococcus mutans via the nano-quercetin-based antimicrobial photodynamic therapy as a potential target for cariogenic biofilm. BMC Microbiol. 2022, 22, 125. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Pourhajibagher, M.; Chiniforush, N.; Bahador, A. Propolis nanoparticle enhances the potency of antimicrobial photodynamic therapy against Streptococcus mutans in a synergistic manner. Sci. Rep. 2020, 10, 15560. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.Y.; Zhu, G.Y.; Yu, C.H.; Chen, G.Y.; Zhang, C.L.; Zeng, X.; Chen, Q.M.; Peng, Q. Functionalized graphene oxide nanosheets with unique three-in-one properties for efficient and tunable antibacterial applications. Nano Res. 2021, 14, 185–190. [Google Scholar] [CrossRef]
- Silvestre, A.L.P.; Dos Santos, A.M.; de Oliveira, A.B.; Ferrisse, T.M.; Brighenti, F.L.; Meneguin, A.B.; Chorilli, M. Evaluation of photodynamic therapy on nanoparticles and films loaded-nanoparticles based on chitosan/alginate for curcumin delivery in oral biofilms. Int. J. Biol. Macromol. 2023, 240, 124489. [Google Scholar] [CrossRef]
- Hosseinpour-Nader, A.; Karimi, N.; Ghafari, H.A. Ex-vivo effects of propolis quantum dots-nisin-nanoquercetin-mediated photodynamic therapy on Streptococcus mutans biofilms and white spot lesions. Photodiagn. Photodyn. Ther. 2023, 41, 103255. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, Q.; Li, F.; Guo, J.; Huo, Y.; Xu, C.; Xiong, M.; Cheng, Z.; Liu, M.; Lin, J. Oxygen-carrying acid-responsive Cu/ZIF-8 for photodynamic antibacterial therapy against cariogenic Streptococcus mutans infection. Dalton Trans. 2023, 52, 16189–16196. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z.; Hu, Y.; Hu, L.; Zi, Y.; Wang, M.; Feng, X.; Huang, W. Bismuth Quantum Dot (Bi QD)/Polydimethylsiloxane (PDMS) Nanocomposites with Self-Cleaning and Antibacterial Activity for Dental Applications. Nanomaterials 2022, 12, 3911. [Google Scholar] [CrossRef]
- Ahrari, F.; Nazifi, M.; Mazhari, F.; Ghazvini, K.; Menbari, S.; Fekrazad, R.; Babaei, K.; Banihashemrad, A. Photoinactivation Effects of Curcumin, Nano-curcumin, and Erythrosine on Planktonic and Biofilm Cultures of Streptococcus mutans. J. Lasers Med. Sci. 2024, 15, e7. [Google Scholar] [CrossRef] [PubMed]
- Darvish, S.; Budala, D.G.; Goriuc, A. Antibacterial Properties of an Experimental Dental Resin Loaded with Gold Nanoshells for Photothermal Therapy Applications. J. Funct. Biomater. 2024, 15, 100. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Ranjbar Omrani, L.; Noroozian, M.; Ghorbanzadeh, Z.; Bahador, A. In vitro antibacterial activity and durability of a nano-curcumin-containing pulp capping agent combined with antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 33, 102150. [Google Scholar] [CrossRef] [PubMed]
- Ahrari, F.; Mazhari, F.; Ghazvini, K.; Fekrazad, R.; Menbari, S.; Nazifi, M. Antimicrobial photodynamic therapy against Lactobacillus casei using curcumin, nano-curcumin, or erythrosine and a dental LED curing device. Lasers Med. Sci. 2023, 38, 260. [Google Scholar] [CrossRef]
- Cai, Y.; Strømme, M.; Melhus, A.; Engqvist, H.; Welch, K. Photocatalytic inactivation of biofilms on bioactive dental adhesives. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 62–67. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z.; Pu, J.; Hu, L.; Zi, Y.; Wang, M.; Feng, X.; Huang, W. 2D MXene Ti(3)C(2)T (x) nanosheets in the development of a mechanically enhanced and efficient antibacterial dental resin composite. Front. Chem. 2022, 10, 1090905. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Haddadi-Asl, V.; Ghafari, H.A.; Ghorbanzadeh, R.; Mazlum, Y.; Bahador, A. Shear bond strength, adhesive remnant index, and anti-biofilm effects of a photoexcited modified orthodontic adhesive containing curcumin doped poly lactic-co-glycolic acid nanoparticles: An ex-vivo biofilm model of S. mutans on the enamel slab bonded brackets. Photodiagn. Photodyn. Ther. 2020, 30, 101674. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.T.; Wang, Y.; Wang, H.Y.; Hong, S.; Li, W.; Guo, D.S.; Zhang, X. A Supramolecular Nanoformulation with Adaptive Photothermal/Photodynamic Transformation for Preventing Dental Caries. ACS Nano 2024, 18, 27340–27357. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhang, Y.F.; Cheng, Y.J.; Wang, Y.X.; Chen, Z.Y.; Sun, H.N.; Wei, X.S.; Ma, Z.; Li, J.; Bai, Y.Y.; et al. NIR-activated nanosystems with self-modulated bacteria targeting for enhanced biofilm eradication and caries prevention. Bioact. Mater. 2022, 13, 269–285. [Google Scholar] [CrossRef]
- Shi, J.; Qi, X.; Ran, Y.; Zhou, Q.; Ding, Y.; Li, L.; Zeng, Y.; Qiu, D.; Cai, Z.; Cai, X.; et al. Saliva-acquired pellicle inspired multifunctional gargle with wet adhesion, photodynamic antimicrobial, and In situ remineralization properties for dental caries prevention. Bioact. Mater. 2025, 47, 212–228. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Xu, Y.; Liu, H.; Zhang, J.; Wang, Y.; Sun, Y.; Zhao, M.; Liao, L.; Wang, X. Fast Cross-Linked Hydrogel as a Green Light-Activated Photocatalyst for Localized Biofilm Disruption and Brush-Free Tooth Whitening. ACS Appl. Mater. Interfaces 2022, 14, 28427–28438. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Zhang, H.; Li, F.; Hu, J.; Qian, J.; Wang, Y.; Zhang, J.; Wu, Z. Dental Caries Management with Antibacterial Silver-Doped Prussian Blue Hydrogel by the Combined Effects of Photothermal Response and Ion Discharge. ACS Appl. Mater. Interfaces 2024, 16, 28172–28183. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, X.; Ji, Y.; Chen, R.; Zhou, S.; Yao, H.; Zhang, Z.; Ye, M.; Xu, Z.; Du, M. Bioresponsive nanotherapy for preventing dental caries by inhibiting multispecies cariogenic biofilms. Bioact. Mater. 2022, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Nader, A.; Karimi, N.; Ghafari, H.A.; Ghorbanzadeh, R. Effect of nanomicelle curcumin-based photodynamic therapy on the dynamics of white spot lesions and virulence of Streptococcus mutans in patients undergoing fixed orthodontic treatment: A randomized double-blind clinical trial. Photodiagn. Photodyn. Ther. 2022, 40, 103183. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Kolarikova, M.; Hosikova, B.; Dilenko, H.; Barton-Tomankova, K.; Valkova, L.; Bajgar, R.; Malina, L.; Kolarova, H. Photodynamic therapy: Innovative approaches for antibacterial and anticancer treatments. Med. Res. Rev. 2023, 43, 717–774. [Google Scholar] [CrossRef]
- Badran, Z.; Rahman, B.; De Bonfils, P.; Nun, P.; Coeffard, V.; Verron, E. Antibacterial nanophotosensitizers in photodynamic therapy: An update. Drug Discov. Today 2023, 28, 103493. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Garcia, I.M.; Ibrahim, M.S.; Rolim, J.; Gomes, E.A.B.; Martinho, F.C.; Collares, F.M.; Xu, H.; Melo, M.A.S. Prospects on Nano-Based Platforms for Antimicrobial Photodynamic Therapy Against Oral Biofilms. Photobiomodul Photomed. Laser Surg. 2020, 38, 481–496. [Google Scholar] [CrossRef]
- Yan, R.; Liu, J.; Dong, Z.; Peng, Q. Nanomaterials-mediated photodynamic therapy and its applications in treating oral diseases. Biomater. Adv. 2023, 144, 213218. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Baghdan, E.; Duse, L.; Schüer, J.J.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Development of inhalable curcumin loaded Nano-in-Microparticles for bronchoscopic photodynamic therapy. Eur. J. Pharm. Sci. 2019, 132, 63–71. [Google Scholar] [CrossRef]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-mediated Photodynamic Therapy for the treatment of oral infections-A review. Photodiagn. Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef]
- Etemadi, A.; Hamidain, M.; Parker, S.; Chiniforush, N. Blue Light Photodynamic Therapy With Curcumin and Riboflavin in the Management of Periodontitis: A Systematic Review. J. Lasers Med. Sci. 2021, 12, e15. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, F.; Fazlyab, M.; Chiniforush, N.; Akhavan, H. Comparative effects of SWEEPS technique and antimicrobial photodynamic therapy by using curcumin and nano-curcumin on Enterococcus faecalis biofilm in root canal treatment. Photodiagn. Photodyn. Ther. 2022, 40, 103130. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef]
- Yaghobee, S.; Pourhajibagher, M.; Bahrami, R.; Isaabadi, M. Nano-emodin mediated photodynamic therapy for wound healing of donor site after free gingival graft: A parallel clinical trial. Photodiagn. Photodyn. Ther. 2024, 45, 103958. [Google Scholar] [CrossRef] [PubMed]
- Monem Moharrer, S.; Pourhajibagher, M.; Azizi, A.; Alaee, A. Anti-virulence effect of photoactivated nano-quercetin by diode laser on Aggregatibacter actinomycetemcomitans. AMB Express 2025, 15, 55. [Google Scholar] [CrossRef]
- Najm, M.; Pourhajibagher, M.; Badirzadeh, A.; Razmjou, E.; Alipour, M.; Khoshmirsafa, M.; Bahador, A.; Hadighi, R. Photodynamic Therapy Using Toluidine Blue O (TBO) Dye as a Photosensitizer against Leishmania major. Iran J. Public Health 2021, 50, 2111–2120. [Google Scholar] [CrossRef]
- Rout, B.; Liu, C.H.; Wu, W.C. Increased anti-biofilm efficacy of toluidine blue on Staphylococcus species after nano-encapsulation. Photodiagn. Photodyn. Ther. 2018, 21, 190–200. [Google Scholar] [CrossRef]
- Lim, D.J. Methylene Blue-Based Nano and Microparticles: Fabrication and Applications in Photodynamic Therapy. Polym. 2021, 13, 3955. [Google Scholar] [CrossRef]
- Zada, L.; Anwar, S.; Imtiaz, S.; Saleem, M.; Shah, A.A. In vitro study: Methylene blue-based antibacterial photodynamic inactivation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2024, 108, 169. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.F.; Li, S.; Liang, L.; Huang, Q.; Yuwen, L.; Yang, W.; Wang, R.; Wang, L.H. Highly Biocompatible Chlorin e6-Loaded Chitosan Nanoparticles for Improved Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9980–9987. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, H.; Jiang, W.; Song, A.; Li, Z.; Luan, Y. Rational Design of IR820- and Ce6-Based Versatile Micelle for Single NIR Laser-Induced Imaging and Dual-Modal Phototherapy. Small 2018, 14, e1802994. [Google Scholar] [CrossRef]
- Ancona, A.; Dumontel, B.; Garino, N.; Demarco, B.; Chatzitheodoridou, D.; Fazzini, W.; Engelke, H.; Cauda, V. Lipid-Coated Zinc Oxide Nanoparticles as Innovative ROS-Generators for Photodynamic Therapy in Cancer Cells. Nanomaterials 2018, 8, 143. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Liu, X.; Chen, H.; Huang, Y.; Li, A.; Pu, Y.; Guo, L. Study on Mechanical Properties, Optical Properties, Cytotoxicity of TiO(2)-HAP Nanoparticles-Modified PMMA and Photodynamically Assisted Antibacterial Activity Against Candida Albicans in Vitro. Int. J. Nanomed. 2025, 20, 2695–2709. [Google Scholar] [CrossRef]
- Lucky, S.S.; Idris, N.M.; Huang, K.; Kim, J.; Li, Z.; Thong, P.S.; Xu, R.; Soo, K.C.; Zhang, Y. In vivo Biocompatibility, Biodistribution and Therapeutic Efficiency of Titania Coated Upconversion Nanoparticles for Photodynamic Therapy of Solid Oral Cancers. Theranostics 2016, 6, 1844–1865. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Liu, J.; Lin, Y.; Jiao, J.; Chen, B.; Wang, W.; Wu, S.; Li, C. Photothermal therapy with regulated Nrf2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mater. 2022, 9, 428–445. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Rahman, A.A.; Braim, F.S.; Mehrdel, B. Gold nanoparticles-based photothermal therapy for breast cancer. Photodiagn. Photodyn. Ther. 2023, 42, 103312. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ding, H.; Fu, S.; Li, D.; Yu, B. Bismuth-coated 80S15C bioactive glass scaffolds for photothermal antitumor therapy and bone regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1098923. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Wu, S.; Li, A.; Zhao, X.; Zhang, C.; Yu, B.; Zhao, N.; Xu, F.J. Silica-Coated Gold-Silver Nanocages as Photothermal Antibacterial Agents for Combined Anti-Infective Therapy. ACS Appl. Mater. Interfaces 2019, 11, 17177–17183. [Google Scholar] [CrossRef]
- Nirmal, G.R.; Lin, Z.C.; Chiu, T.S.; Alalaiwe, A.; Liao, C.C.; Fang, J.Y. Chemo-photothermal therapy of chitosan/gold nanorod clusters for antibacterial treatment against the infection of planktonic and biofilm MRSA. Int. J. Biol. Macromol. 2024, 268, 131673. [Google Scholar] [CrossRef]
- Dong, L.; Huang, W.; Chu, H.; Li, Y.; Wang, Y.; Zhao, S.; Li, G.; Zhang, H.; Li, D. Passively Q-switched near-infrared lasers with bismuthene quantum dots as the saturable absorber. Opt. Laser Technol. 2020, 128, 106219. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Laurent, S.; Port, M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics 2021, 13, 1793. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Li, X.; Hu, Y.; Zhang, X.; Lu, N.; Fang, Q.; Shao, J.; Li, S.; Xiu, W.; Song, Y.; et al. Recent advances in Prussian blue-based photothermal therapy in cancer treatment. Biomater. Sci. 2023, 11, 4411–4429. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Li, Y.; Zhang, C.; Fang, G. Recent Advances in Photothermal Therapy at Near-Infrared-II Based on 2D MXenes. Small 2024, 20, e2305645. [Google Scholar] [CrossRef] [PubMed]
- de Melo-Diogo, D.; Lima-Sousa, R.; Alves, C.G.; Correia, I.J. Graphene family nanomaterials for application in cancer combination photothermal therapy. Biomater. Sci. 2019, 7, 3534–3551. [Google Scholar] [CrossRef]
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, X.; Wang, D.; Zhang, H.; Xin, Q.; Wu, M.; Xu, X.; Sun, F.; Xing, Z.; Wang, L. Chloroplast-inspired scaffold for infected bone defect therapy: Towards stable photothermal properties and self-defensive functionality. Adv. Sci. 2022, 9, 2204535. [Google Scholar] [CrossRef]
- Shao, J.; Ruan, C.; Xie, H.; Chu, P.K.; Yu, X.F. Photochemical activity of black phosphorus for near-infrared light controlled in situ biomineralization. Adv. Sci. 2020, 7, 2000439. [Google Scholar] [CrossRef]
- Wen, F.; Li, P.; Meng, H.; Yan, H.; Huang, X.; Cui, H.; Su, W. Nitrogen-doped carbon dots/curcumin nanocomposite for combined Photodynamic/photothermal dual-mode antibacterial therapy. Photodiagn. Photodyn. Ther. 2022, 39, 103033. [Google Scholar] [CrossRef]
- An, Q.; Su, S.; Hu, W.; Wang, Y.; Liang, T.; Li, X.; Li, C. Dual-wavelength responsive CuS@COF nanosheets for high-performance photothermal/photodynamic combination treatments. Nanoscale 2023, 15, 19815–19819. [Google Scholar] [CrossRef]
- Luo, T.; Nash, G.T.; Xu, Z.; Jiang, X.; Liu, J.; Lin, W. Nanoscale Metal-Organic Framework Confines Zinc-Phthalocyanine Photosensitizers for Enhanced Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143, 13519–13524. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, X.; Shi, J.; Jiang, Z.; Zhang, C.Y. Graphene-based nanomaterials for cancer therapy and anti-infections. Bioact. Mater. 2022, 14, 335–349. [Google Scholar] [CrossRef]
- Sarcan, E.T.; Silindir-Gunay, M.; Ozer, A.Y. Theranostic polymeric nanoparticles for NIR imaging and photodynamic therapy. Int. J. Pharm. 2018, 551, 329–338. [Google Scholar] [CrossRef] [PubMed]
- AlRyalat, S.A.S.; Malkawi, L.W.; Momani, S.M. Comparing Bibliometric Analysis Using PubMed, Scopus, and Web of Science Databases. JOVE-J. Vis. Exp. 2019, 152, e58494. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, web of science, and Google scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Lazarinis, F.; Vilares, J.; Tait, J.; Efthimiadis, E.N. Current research issues and trends in non-English Web searching. Inf. Retr. 2009, 12, 230–250. [Google Scholar] [CrossRef]
- Amano, T.; Berdejo-Espinola, V.; Christie, A.P.; Willott, K.; Akasaka, M.; Báldi, A.; Berthinussen, A.; Bertolino, S.; Bladon, A.J.; Chen, M.; et al. Tapping into non-English-language science for the conservation of global biodiversity. PLoS Biol. 2021, 19, e3001296. [Google Scholar] [CrossRef]
- Sreenivasalu, P.K.P.; Dora, C.P.; Swami, R.; Jasthi, V.C.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, M.K. Nanomaterials in Dentistry: Current Applications and Future Scope. Nanomaterials 2022, 12, 1676. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef] [PubMed]
| Study Type | Assessment Tool [Ref.] | Criteria | Scoring System | Risk of Bias Classification |
|---|---|---|---|---|
| In vitro studies | Quality Assessment Tool for In Vitro Studies (QUIN Tool) [34] | (1) Clearly stated aims/objectives; (2) sample size calculation; (3) sampling technique; (4) details of comparison groups; (5) detailed methodology; (6) operator details; (7) randomisation; (8) measurement of outcomes; (9) outcome assessor details; (10) blinding; (11) statistical analysis; (12) presentation of results | Low risk (2 points); some concerns (1 point); high risk (0 points) | >70%: low risk; 50–70%: some concerns; <50%: high risk |
| Animal studies | Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE’s) Risk of Bias Tool [35] | (1) Allocation sequence; (2) baseline similarity; (3) allocation concealment; (4) random housing of animals; (5) caregiver/investigator blinding; (6) random outcome assessment; (7) outcome assessor blinding; (8) incomplete data addressed; (9) free of selective reporting; (10) free of other biases | ||
| Human studies | Cochrane Risk of Bias Tool for Randomised Trials Version 2 (RoB 2) [36] | (1) Randomization process bias; (2) intervention deviation bias; (3) missing outcome data; (4) outcome measurement bias; (5) reporting selection bias |
| First Author, Year [Ref.] | Items of Assessment | Score (%) | Risk of Bias | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clearly Stated Aims/Objectives | Sample Size Calculation | Sampling Technique | Details of Comparison Group | Detailed Methodology | Operator Details | Randomisation | Measurement of Outcome | Outcome Assessor Details | Blinding | Statistical Analysis | Presentation of Results | |||
| Alanazi, 2024 [37] |  |  |  |  |  |  |  |  |  |  |  |  | 83 | Low |
| Afrasiabi, 2024 [38] |  |  |  |  |  |  |  |  |  |  |  |  | 67 | Some concerns |
| Hemmati, 2024 [39] |  |  |  |  |  |  |  |  |  |  |  |  | 63 | Some concerns |
| Pourhajibagher, 2022 [40] |  |  |  |  |  |  |  |  |  |  |  |  | 63 | Some concerns |
| Ghorbanzadeh, [41] |  |  |  |  |  |  |  |  |  |  |  |  | 58 | Some concerns |
| Balhaddad, 2021 [42] |  |  |  |  |  |  |  |  |  |  |  |  | 58 | Some concerns |
| Ghanemi, 2023 [32] |  |  |  |  |  |  |  |  |  |  |  |  | 58 | Some concerns |
| Comeau, 2022 [43] |  |  |  |  |  |  |  |  |  |  |  |  | 58 | Some concerns |
| Binhasan, 2023 [44] |  |  |  |  |  |  |  |  |  |  |  |  | 58 | Some concerns |
| Xu, 2022 [45] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Guo, 2023 [46] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Lu, 2023 [47] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Panda, 2024 [48] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Wang, 2023 [28] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Pourhajibagher, 2022 [49] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Afrasiabi, 2020 [50] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Lu, 2021 [51] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Silvestre, 2023 [52] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Hosseinpour-Nader, 2023 [53] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Wang, 2023 [54] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Hu, 2022 [55] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Ahrari, 2024 [56] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Pourhajibagher, 2019 [29] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Darvish, 2024 [57] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Pourhajibagher, 2021 [58] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Ahrari, 2023 [59] |  |  |  |  |  |  |  |  |  |  |  |  | 54 | Some concerns |
| Cai, 2014 [60] |  |  |  |  |  |  |  |  |  |  |  |  | 50 | Some concerns |
| Hu, 2022 [61] |  |  |  |  |  |  |  |  |  |  |  |  | 50 | Some concerns |
| Ahmadi, 2020 [62] |  |  |  |  |  |  |  |  |  |  |  |  | 50 | Some concerns |
 ), some concerns (1 point, marked as
), some concerns (1 point, marked as  ), or high risk (0 points, marked as
), or high risk (0 points, marked as  ). Low risk of bias: >70% score; some concerns: between 50% and 70% scores.
). Low risk of bias: >70% score; some concerns: between 50% and 70% scores.| First Author, Year [Ref.] | Items of Assessment | Score (%) | Risk of Bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allocation Sequence | Baseline Similarity | Allocation Concealment | Random Housing of Animals | Caregiver/Investigator Blinding | Random Outcome Assessment | Outcome Assessor Blinding | Incomplete Data Addressed | Free of Selective Reporting | Free of Other Bias | |||
| Zhang, 2024 [63] |  |  |  |  |  |  |  |  |  |  | 80 | Low |
| Yu, 2022 [64] |  |  |  |  |  |  |  |  |  |  | 80 | Low |
| Shi, 2025 [65] |  |  |  |  |  |  |  |  |  |  | 75 | Low |
| Li, 2022 [66] |  |  |  |  |  |  |  |  |  |  | 75 | Low |
| Hajfathalian, 2023 [30] |  |  |  |  |  |  |  |  |  |  | 75 | Low |
| Li, 2024 [67] |  |  |  |  |  |  |  |  |  |  | 75 | Low |
| Ran, 2024 [31] |  |  |  |  |  |  |  |  |  |  | 75 | Low |
| Liu, 2022 [68] |  |  |  |  |  |  |  |  |  |  | 70 | Low |
 ) or some concerns (1 point, marked as
) or some concerns (1 point, marked as  ). Low risk of bias: >70% score.
). Low risk of bias: >70% score.| First Author, Year [Ref.] | Items of Assessment | Score (%) | Risk of Bias | ||||
|---|---|---|---|---|---|---|---|
| Randomization Process Bias | Intervention Deviation Bias | Missing Outcome Data | Outcome Measurement Bias | Reporting Selection Bias | |||
| Hosseinpour-Nader, 2022 [69] |  |  |  |  |  | 90 | Low |
 ) or some concerns (1 point, marked as
) or some concerns (1 point, marked as  ). Low risk of bias: >70% score.
). Low risk of bias: >70% score.| Nanomaterial [Ref.] | Microbes | Design (s) | Light Type, Wavelength | Initial Power Density | Exposure Time | Potential Use |
|---|---|---|---|---|---|---|
| Natural Photodynamic Nanomaterials | ||||||
| Curcumin nanomicelle [59] | L. casei | In vitro | Broad spectrum, 450 nm | 1200 mW/cm2 | 2 min | - |
| Curcumin nanomicelle [69] | S. mutans | Human | Single wavelength, 450 nm | 80 mW/cm2 | 3 min | Oral capsule |
| Curcumin nanoparticles [52] | S. mutans | In vitro | Single wavelength, 460 nm | 21 mW/cm2 | 12 min | Buccal film |
| Curcumin nanoparticles [56] | S. mutans | In vitro | Broad spectrum, 450 nm | 1200 mW/cm2 | 2 min | Topical agent |
| Curcumin nanomicelle [38] | S. mutans, L. acidophilus | In vitro | Single wavelength, 450 nm | 1000 mW/cm2 | 1 min | Topical agent |
| Curcumin nanoparticles [29] | S. mutans, S. sobrinus, L. acidophilus | In vitro | Broad spectrum, 435 nm | 1400 mW/cm2 | 5 min | Adhesive |
| Curcumin nanoparticles [62] | S. mutans | In vitro | Broad spectrum, 405 nm | 150 mW/cm2 | - | Adhesive |
| Curcumin nanoparticles [58] | S. mutans | In vitro | Broad spectrum, 435 nm | 1400 mW/cm2 | 5 min | Pulp capping agent |
| Emodin nanoparticles [40] | S. mutans | In vitro | Single wavelength, 405 nm | 150 mW/cm2 | 5 min | Topical agent |
| Quercetin nanoparticles [49] | S. mutans | In vitro | Broad spectrum, 405 nm | 150 mW/cm2 | 1 min | - |
| Quercetin nanoparticles [53] | S. mutans | In vitro | Broad spectrum, 450 nm | 150 mW/cm2 | 5 min | Topical agent |
| Synthetic Photodynamic Nanomaterials | ||||||
| Toluidine blue ortho nanoparticles [50] | S. mutans | In vitro | Single wavelength, 635 nm | 220 mW/cm2 | - | - |
| Toluidine blue ortho nanoparticles [48] | S. mutans | In vitro | Single wavelength, 650 nm | 100 mW/cm2 | 2 min | - |
| Toluidine blue ortho nanoparticles [42] | Saliva microbes | In vitro | Broad spectrum, 668 nm | 600 mW/cm2 | 5 min | Topical agent |
| Methylene blue nanoparticles [37] | S. mutans | In vitro | Single wavelength, 638 nm | 1500 mW/cm2 | 30 s | Adhesive |
| Methylene blue nanoparticles [44] | L. acidophilus | In vitro | Single wavelength, 660 nm | 40 mW/cm2 | 1 min | Topical agent |
| Chlorin e6 nanoparticles [68] | S. mutans, S. sobrinus, S. sanguinis | In vitro, animal | Single wavelength, 660 nm | 500 mW/cm2 | 5 min | Topical agent |
| Chlorin e6 nanoparticles [54] | S. mutans | In vitro | Single wavelength, 650 nm | 120 mW/cm2 | - | - |
| Chlorin e6 nanoparticles [46] | S. mutans | In vitro | Single wavelength, 660 nm | 100 mW/cm2 | 30 min | Topical agent |
| Chlorin e6 nanoparticles [65] | S. mutans, S. sanguis, C. albicans | In vitro, animal | Single wavelength, 665 nm | 214 mW/cm2 | 5 min | Gargle |
| Zinc oxide nanoparticles [39] | S. mutans | In vitro | Broad spectrum, 450 nm | 1400 mW/cm2 | 1 min | Adhesive |
| Zinc oxide nanoparticles [43] | S. mutans | In vitro | Broad spectrum, 450 nm | 23 mW/cm2 | 1 min | Restorative filler |
| Titanium dioxide nanoparticles [28] | S. mutans | In vitro | Broad spectrum, 450 nm | 150 mW/cm2 | 5 min | Topical agent |
| Titanium dioxide nanoparticles [60] | S. mutans | In vitro | Broad spectrum, 371 nm | 12 mW/cm2 | 25 h | Adhesive |
| Bismuth oxychloride nanoparticles [66] | S. aureus, S. mutans, E. coli | In vitro, animal | -, Green light | - | 5 min | Topical agent |
| Nanomaterial [Ref.] | Microbes | Design (s) | Light Type, Wavelength | Initial Power Density | Exposure Time | Potential Use |
|---|---|---|---|---|---|---|
| Metal-Based Photothermal Nanomaterials | ||||||
| Gold nanoparticles [57] | S. mutans | In vitro | Single wavelength, 660 nm | 100 mW/cm2 | 30 s | Restorative filler |
| Gold nanoparticles [30] | S. mutans, S. aureus | In vitro, animal | Single wavelength, 808 nm | 500 mW/cm2 | 5 min | Topical agent |
| Bismuth nanoparticles [55] | S. mutans | In vitro | Single wavelength, - | 12 mW/cm2 | 24 h | Restorative filler |
| Iron (III) ferrocyanide nanoparticles [67] | S. mutans, S. sobrinus, S. sanguinis | In vitro, animal | Single wavelength, 808 nm | 400 mW/cm2 | 3 min | Topical agent |
| Titanium aluminium carbide nanoparticles [61] | S. mutans | In vitro | Broad spectrum, Natural light | - | 5 min | Restorative filler |
| Carbon-Based Photothermal Nanomaterials | ||||||
| Graphene oxide nanosheets [51] | S. mutans | In vitro | Single wavelength, 808 nm | 880 mW/cm2 | 5 min | - |
| Polydopamine nanoparticles [45] | S. mutans | In vitro | Single wavelength, 808 nm | 750 mW/cm2 | 10 min | - |
| Polydopamine nanoparticles [47] | S. mutans | In vitro | Single wavelength, 808 nm | 1500 mW/cm2 | 10 min | Topical agent |
| Phosphorus-Based Photothermal Nanomaterials | ||||||
| Black phosphorus nanosheets [31] | S. mutans, S. sanguinis | In vitro, animal | Single wavelength, 808 nm | 1000 mW/cm2 | 5 min | Topical agent |
| Nanomaterial [Ref.] | Microbes | Design (s) | Light Type, Wavelength | Initial Power Density | Exposure Time | Potential Use |
|---|---|---|---|---|---|---|
| Metal-Based Dual-Modal Nanomaterials | ||||||
| Zinc phthalocyanine tetrasulfonate nanoparticles [63] | S. mutans | In vitro, animal | Single wavelength, 660 nm | 1000 mW/cm2 | 5 min | Topical agent |
| Carbon-Based Dual-Modal Nanomaterials | ||||||
| Graphene oxide nanoparticles [41] | S. mutans | In vitro | Single wavelength, 980 nm | 500 mW/cm2 | 5 min | Adhesive |
| Reduced graphene oxide nanosheets [32] | S. mutans | In vitro | Single wavelength, 980 nm | 1635 mW/cm2 | 2 min | Coating |
| Poly(2-(5,5-dimethyl-1,3-dioxan-2-yloxy)ethyl acrylate) [64] | S. mutans | In vitro, animal | Single wavelength, 808 nm | 1500 mW/cm2 | 5 min | Topical agent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, V.W.; Yin, I.X.; Niu, J.Y.; Chu, C.-H. Enhancing Caries Preventive Effects of Nanomaterials with Phototherapy: A Scoping Review. J. Funct. Biomater. 2025, 16, 308. https://doi.org/10.3390/jfb16090308
Xu VW, Yin IX, Niu JY, Chu C-H. Enhancing Caries Preventive Effects of Nanomaterials with Phototherapy: A Scoping Review. Journal of Functional Biomaterials. 2025; 16(9):308. https://doi.org/10.3390/jfb16090308
Chicago/Turabian StyleXu, Veena Wenqing, Iris Xiaoxue Yin, John Yun Niu, and Chun-Hung Chu. 2025. "Enhancing Caries Preventive Effects of Nanomaterials with Phototherapy: A Scoping Review" Journal of Functional Biomaterials 16, no. 9: 308. https://doi.org/10.3390/jfb16090308
APA StyleXu, V. W., Yin, I. X., Niu, J. Y., & Chu, C.-H. (2025). Enhancing Caries Preventive Effects of Nanomaterials with Phototherapy: A Scoping Review. Journal of Functional Biomaterials, 16(9), 308. https://doi.org/10.3390/jfb16090308








