Enhanced Osteogenic Response to an Osteochondral Scaffold Modified with BMP-2 or Strontium-Enriched Amorphous Calcium Phosphate in a Co-Culture In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Scaffolds
2.2. Cell Cultures
2.3. Co-Culture Model
2.4. Transmission Electron Microscopy (TEM) Analysis
2.5. Alamar Blue Assay
2.6. Gene Expression Analysis
2.7. Osteoclast Differentiation
2.8. Statistical Analysis
3. Results
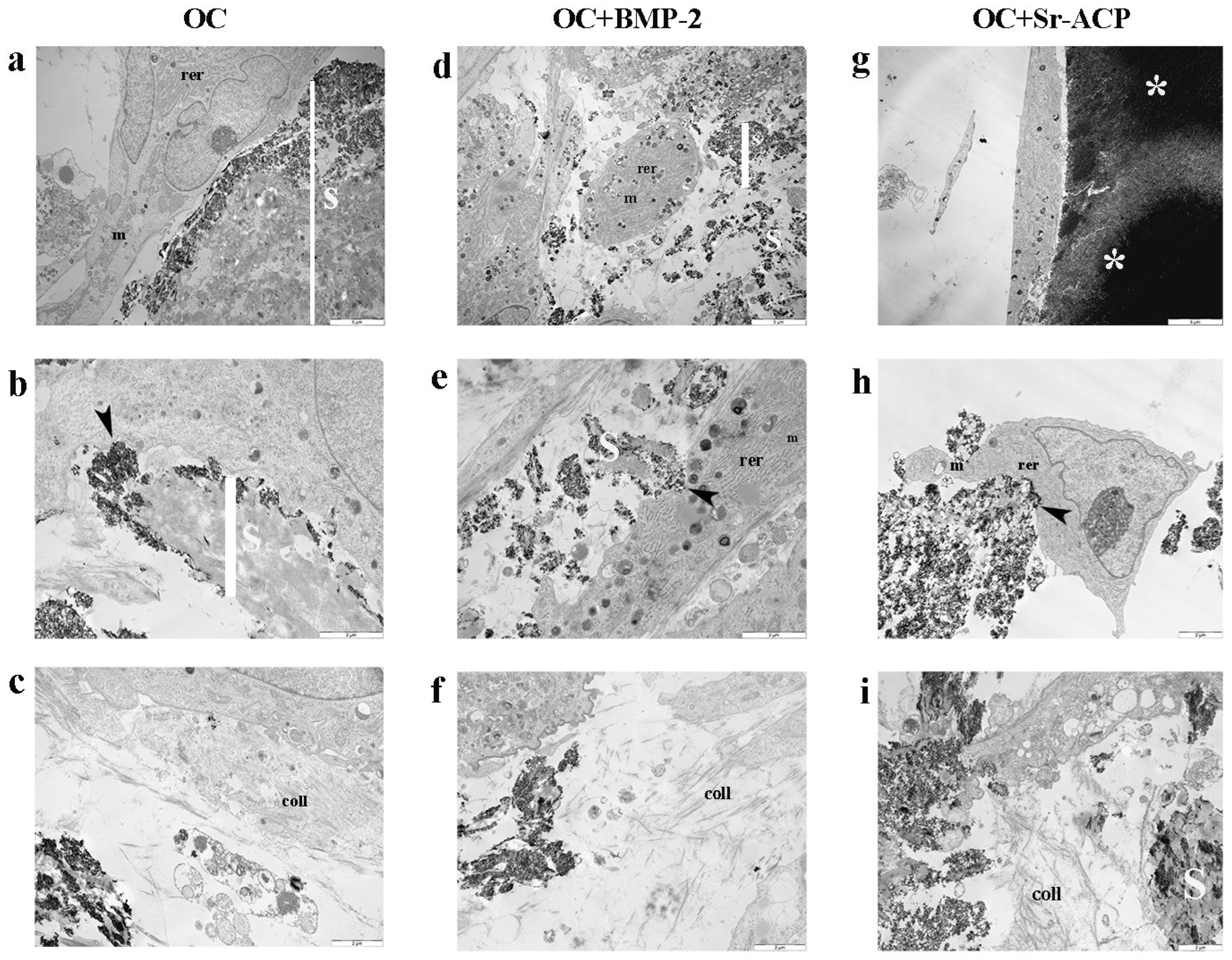
3.1. Effects of the Modified Scaffolds on NHOst Morphology and Matrix Formation
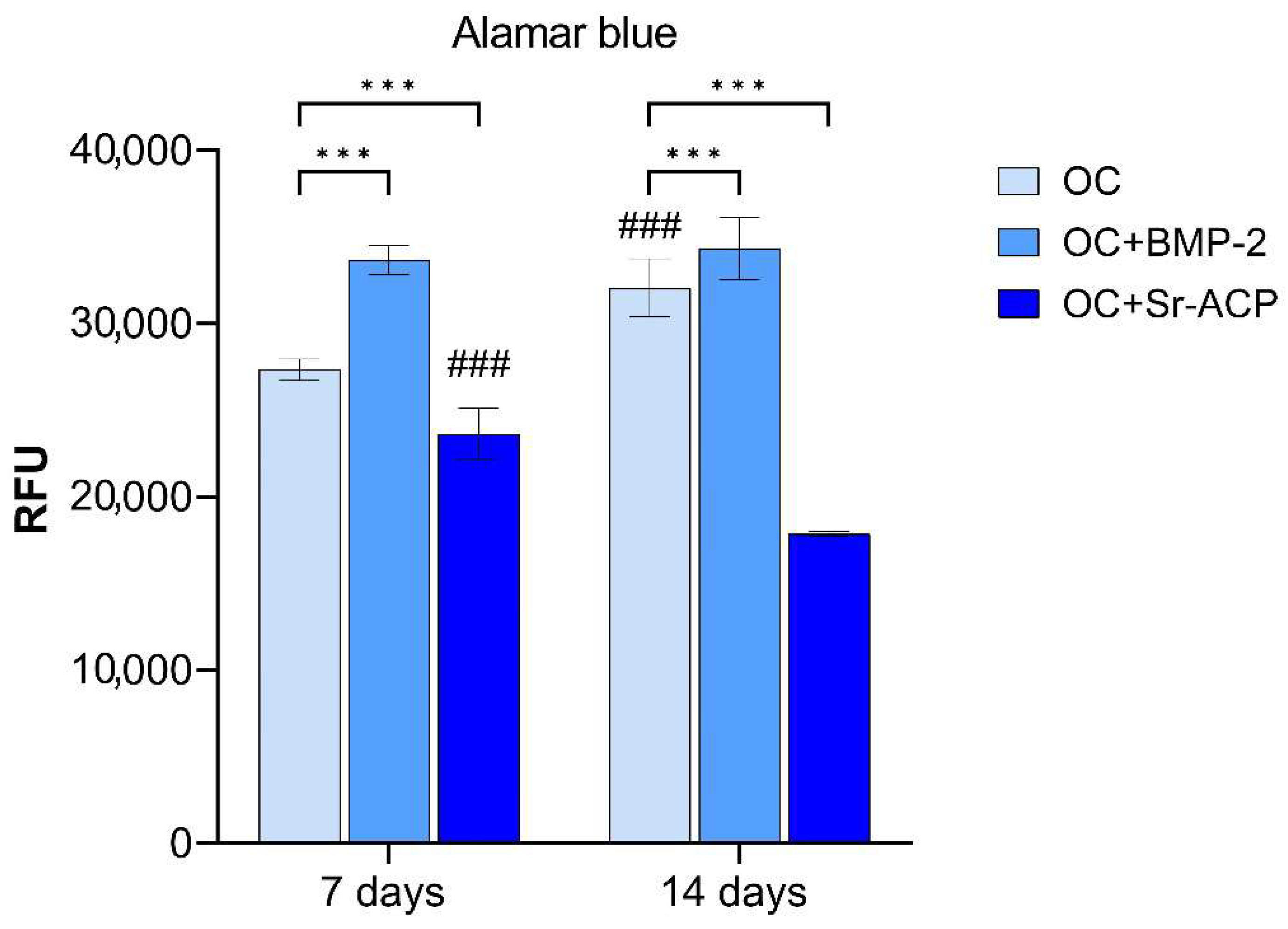
3.2. Alamar Blue Assay
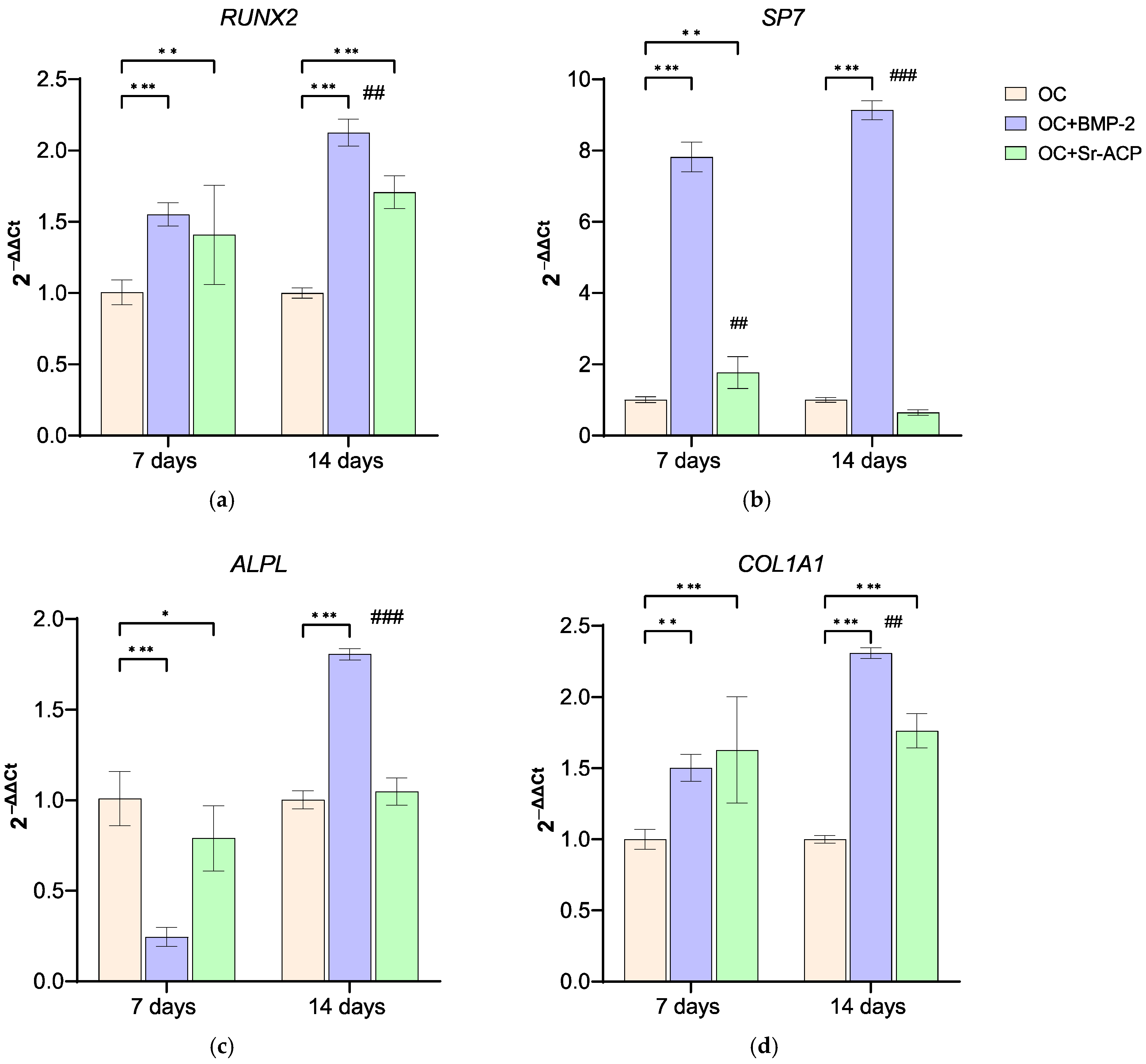
3.3. Effects of the Modified Scaffolds on Gene Expression
3.3.1. Effects of the Modified Scaffolds on NHOsts
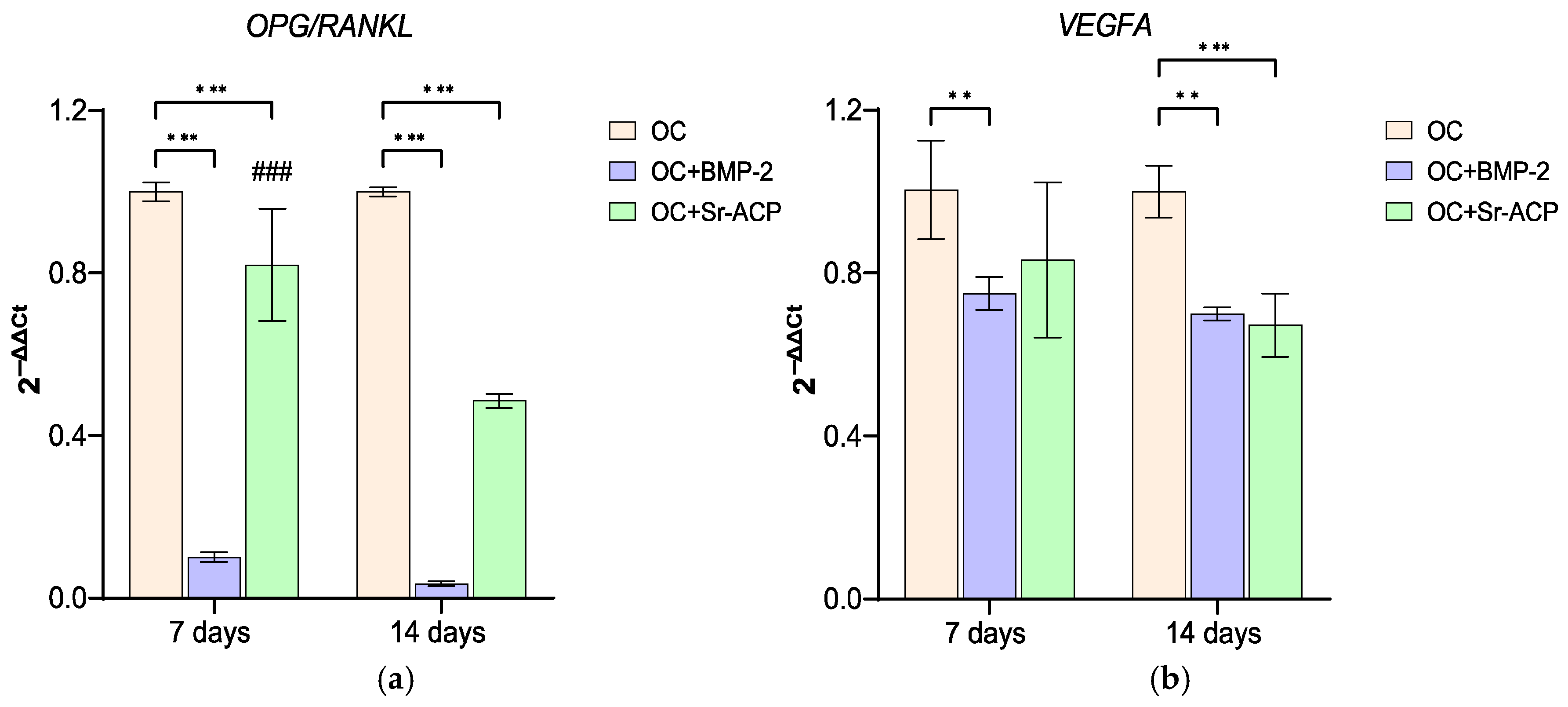
3.3.2. Effects of the Modified Scaffolds on NHOst Interaction with Osteoclasts and Endothelial Cells
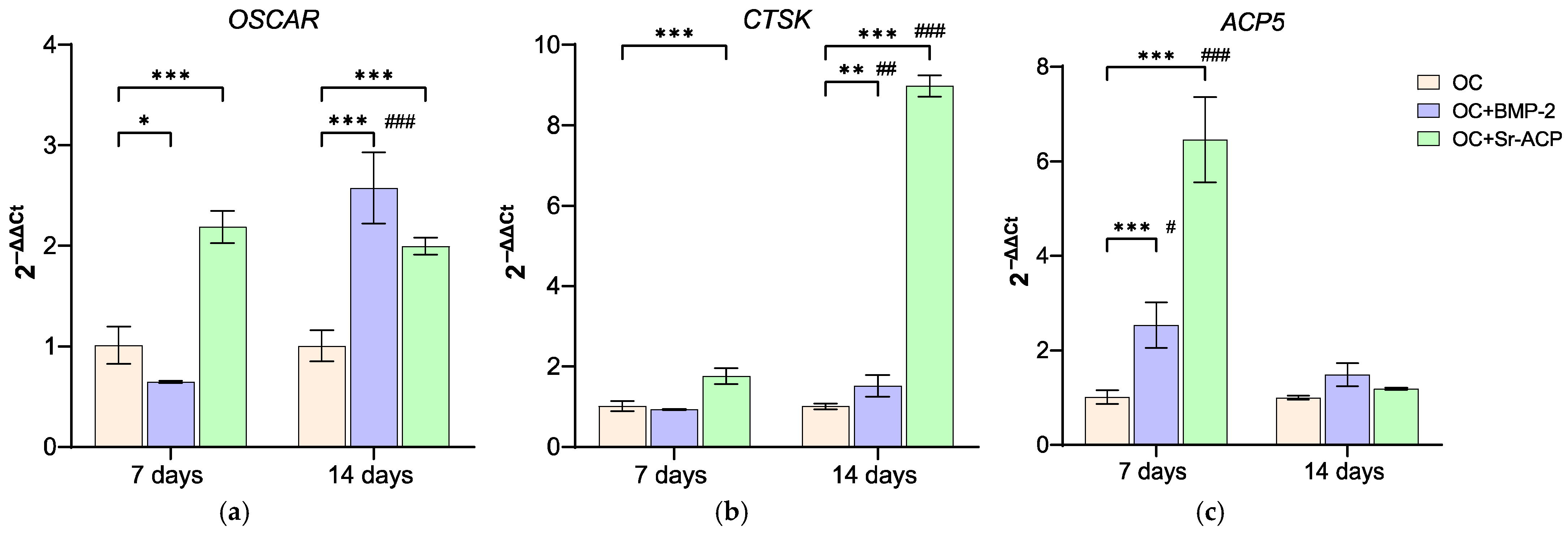
3.3.3. Effects of the Modified Scaffolds on Osteoclasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 1–18. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef]
- Solheim, E.; Krokeide, A.M.; Melteig, P.; Larsen, A.; Strand, T.; Brittberg, M. Symptoms and function in patients with articular cartilage lesions in 1000 knee arthroscopies. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1610–1616. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Kasaeian, A.; Roemer, F.W.; Ghotbi, E.; Ibad, H.A.; He, J.; Wan, M.; Zbijewski, W.B.; Guermazi, A.; Demehri, S. Subchondral bone in knee osteoarthritis: Bystander or treatment target? Skelet. Radiol. 2023, 52, 2069–2083. [Google Scholar] [CrossRef]
- Radin, E.L. Subchondral bone changes and cartilage damage. Equine. Vet. J. 1999, 31, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Z.; Zheng, H.A.; Jiang, Y.; Tu, Y.H.; Jiang, P.H.; Yang, A.L. Mechanical and biologic link between cartilage and subchondral bone in osteoarthritis. Arthritis Care Res. 2012, 64, 960–967. [Google Scholar] [CrossRef]
- Molfetta, L.; Casabella, A.; Rosini, S.; Saviola, G.; Palermo, A. Role of the Osteochondral Unit in the Pathogenesis of Osteoarthritis: Focus on the Potential Use of Clodronate. Curr. Rheumatol. Rev. 2022, 18, 2–11. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Perdisa, F.; Venieri, G.; Marcacci, M. Clinical results of multilayered biomaterials for osteochondral regeneration. J. Exp. Orthop. 2014, 1, 10. [Google Scholar] [CrossRef]
- Sessa, A.; Romandini, I.; Andriolo, L.; Di Martino, A.; Busacca, M.; Zaffagnini, S.; Filardo, G. Treatment of Juvenile Knee Osteochondritis Dissecans with a Cell-Free Biomimetic Osteochondral Scaffold: Clinical and MRI Results at Mid-Term Follow-up. Cartilage 2021, 13, 1137S–1147S. [Google Scholar] [CrossRef]
- Serre, C.M.; Papillard, M.; Chavassieux, P.; Voegel, J.C.; Boivin, G. Influence of magnesium substitution on a collagen-apatite biomaterial on the production of a calcifying matrix by human osteoblasts. J. Biomed. Mater. Res. 1998, 42, 626–633. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Busacca, M.; Di Martino, A.; Marcacci, M. Novel nano-composite multilayered biomaterial for osteochondral regeneration: A pilot clinical trial. Am. J. Sports Med. 2011, 39, 1180–1190. [Google Scholar] [CrossRef]
- Di Martino, A.; Perdisa, F.; Filardo, G.; Busacca, M.; Kon, E.; Marcacci, M.; Zaffagnini, S. Cell-Free Biomimetic Osteochondral Scaffold for the Treatment of Knee Lesions: Clinical and Imaging Results at 10-Year Follow-up. Am. J. Sports Med. 2021, 49, 2645–2650. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Di Martino, A.; Busacca, M.; Moio, A.; Perdisa, F.; Marcacci, M. Clinical results and MRI evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am. J. Sports Med. 2014, 42, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fahmy-Garcia, S.; Wesdorp, M.A.; Kops, N.; Forte, L.; De Luca, C.; Misciagna, M.M.; Dolcini, L.; Filardo, G.; Labberté, M.; et al. Effectiveness of BMP-2 and PDGF-BB Adsorption onto a Collagen/Collagen-Magnesium-Hydroxyapatite Scaffold in Weight-Bearing and Non-Weight-Bearing Osteochondral Defect Bone Repair: In Vitro, Ex Vivo and In Vivo Evaluation. J. Funct. Biomater. 2023, 14, 111. [Google Scholar] [CrossRef]
- Xu, J.; Vecstaudza, J.; Wesdorp, M.A.; Labberté, M.; Kops, N.; Salerno, M.; Kok, J.; Simon, M.; Harmand, M.F.; Vancíková, K.; et al. Incorporating strontium enriched amorphous calcium phosphate granules in collagen/collagen-magnesium-hydroxyapatite osteochondral scaffolds improves subchondral bone repair. Mater. Today Bio 2024, 25, 100959. [Google Scholar] [CrossRef]
- Kong, D.; Shi, Y.; Gao, Y.; Fu, M.; Kong, S.; Lin, G. Preparation of BMP-2 loaded MPEG-PCL microspheres and evaluation of their bone repair properties. Biomed. Pharmacother. 2020, 130, 110516. [Google Scholar] [CrossRef]
- Varkey, M.; Gittens, S.A.; Uludag, H. Growth factor delivery for bone tissue repair: An update. Expert Opin. Drug Deliv. 2004, 1, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Gelinsky, M. Strontium modified calcium phosphate cements-approaches towards targeted stimulation of bone turnover. J. Mater. Chem. B 2015, 3, 4626–4640. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Bomans, P.H.H.; George, A.; de With, G.; Sommerdijk, N.A.J.M. The role of the amorphous phase on the biomimetic mineralization of collagen. Faraday Discuss 2012, 159, 357–370. [Google Scholar] [CrossRef]
- Pagani, S.; Salerno, M.; Filardo, G.; Locs, J.; van Osch, G.J.V.M.; Vecstaudza, J.; Dolcini, L.; Borsari, V.; Fini, M.; Giavaresi, G.; et al. Human Osteoblasts’ Response to Biomaterials for Subchondral Bone Regeneration in Standard and Aggressive Environments. Int. J. Mol. Sci. 2023, 24, 14764. [Google Scholar] [CrossRef] [PubMed]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co–culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef]
- Pagani, S.; Torricelli, P.; Veronesi, F.; Salamanna, F.; Cepollaro, S.; Fini, M. An advanced tri-culture model to evaluate the dynamic interplay among osteoblasts, osteoclasts, and endothelial cells. J. Cell Physiol. 2018, 233, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Clark, C.C.; Brighton, C.T. Microvessel endothelial cells and pericytes increase proliferation and repress osteoblast phenotypic markers in rat calvarial bone cell cultures. J. Orthop. Res. 1995, 13, 553–561. [Google Scholar] [CrossRef]
- Huang, H.; Ma, L.; Kyrkanides, S. Effects of vascular endothelial growth factor on osteoblasts and osteoclasts. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 366–373. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, C.; Guan, D.; Ma, F. BMP2 and VEGF165 transfection to bone marrow stromal stem cells regulate osteogenic potential in vitro. Medicine 2018, 97, e9787. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Li, C.; Yu, Y. Role of bone morphogenetic protein-2 in osteogenic differentiation of mesenchymal stem cells. Mol. Med. Rep. 2015, 12, 4230–4237. [Google Scholar] [CrossRef]
- Lee, B.H.; Hong, M.H.; Kim, M.C.; Kwon, J.S.; Ko, Y.M.; Choi, H.J.; Lee, Y.K. Bone cement with a modified polyphosphate network structure stimulates hard tissue regeneration. J. Biomater. Appl. 2016, 31, 344–356. [Google Scholar] [CrossRef]
- Moon, H.J.; Kim, K.N.; Kim, K.M.; Choi, S.H.; Kim, C.K.; Kim, K.D.; LeGeros, R.Z.; Lee, Y.K. Bone formation in calvarial defects of Sprague-Dawley rats by transplantation of calcium phosphate glass. J. Biomed. Mater. Res. A 2005, 74, 497–502. [Google Scholar] [CrossRef]
- Syed-Picard, F.N.; Jayaraman, T.; Lam, R.S.K.; Beniash, E.; Sfeir, C. Osteoinductivity of calcium phosphate mediated by connexin 43. Biomaterials 2013, 34, 3763–3774. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E. Functionalized biomimetic calcium phosphates for bone tissue repair. J. Appl. Biomater. Funct. Mater. 2017, 15, e313–e325. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Boanini, E.; Gazzano, M.; Giardino, R.; Bigi, A. Interaction of Sr-doped hydroxyapatite nanocrystals with osteoclast and osteoblast-like cells. J. Biomed. Mater. Res. A 2009, 89, 594–600. [Google Scholar] [CrossRef]
- Yan, M.D.; Ou, Y.J.; Lin, Y.J.; Liu, R.M.; Fang, Y.; Wu, W.L.; Zhou, L.; Yao, X.; Chen, J. Does the incorporation of strontium into calcium phosphate improve bone repair? A meta-analysis. BMC Oral Health 2022, 22, 62. [Google Scholar] [CrossRef]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone Remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kumbar, S.G.; Nukavarapu, S.P. Biomaterial-directed cell behavior for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100260. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Vimalraj, S.; Arumugam, B.; Miranda, P.J.; Selvamurugan, N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015, 78, 202–208. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Yun, H.M.; Kim, B.; Jeong, Y.H.; Hong, J.T.; Park, K.R. Suffruticosol A elevates osteoblast differentiation targeting BMP2-Smad/1/5/8-RUNX2 in pre-osteoblasts. Biofactors 2023, 49, 127–139. [Google Scholar] [CrossRef]
- Gomathi, K.; Akshaya, N.; Srinaath, N.; Moorthi, A.; Selvamurugan, N. Regulation of Runx2 by post-translational modifications in osteoblast differentiation. Life Sci. 2020, 245, 117389. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Boccardi, V.; Taghizadeh, M.; Jafarnejad, S. Probiotics and bone disorders: The role of RANKL/RANK/OPG pathway. Aging Clin. Exp. Res. 2020, 32, 363–371. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast–osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nedeva, I.R.; Vitale, M.; Elson, A.; Hoyland, J.A.; Bella, J. Role of OSCAR Signaling in Osteoclastogenesis and Bone Disease. Front. Cell Dev. Biol. 2021, 9, 641162. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tian, L.; Pu, X.; Zeng, Q.; Xiao, Y.; Chen, X.; Zhang, X. Enhanced ectopic bone formation by strontium-substituted calcium phosphate ceramics through regulation of osteoclastogenesis and osteoblastogenesis. Biomater. Sci. 2022, 10, 5925–5937. [Google Scholar] [CrossRef]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Detsch, R.; Boccaccini, A.R. The role of osteoclasts in bone tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.G.; Grosso, A.; Briquez, P.S.; Born, G.M.E.; Lunger, A.; Schrenk, F.; Todorov, A.; Sacchi, V.; Hubbell, J.A.; Schaefer, D.J.; et al. Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater. 2022, 149, 111–125. [Google Scholar] [CrossRef] [PubMed]


| GENE | Primer Forward | Primer Reverse | Amplicon Length | Annealing Temperature |
|---|---|---|---|---|
| ACP5 | 5′-GAAGCGCAGATAGCCGTT-3′ | 5′-GGTCACTGCCTACCTGTG-3′ | 148 bp | 60 °C |
| ALPL | QuantiTect Primer Assay (Qiagen) Hs_ALPL_1_SG | 110 bp | 55 °C | |
| BGLAP | QuantiTect Primer Assay (Qiagen) Hs_BGLAP_1_SG | 90 bp | 55 °C | |
| COL1A1 | QuantiTect Primer Assay (Qiagen) Hs_COL1A1_1_SG | 118 bp | 55 °C | |
| CTSK | QuantiTect Primer Assay (Qiagen) Hs_CTSK-1_SG | 105 bp | 55 °C | |
| GAPDH | QuantiTect Primer Assay (Qiagen) Hs_GAPDH_1_SG | 95 bp | 55 °C | |
| OPG | QuantiTect Primer Assay (Qiagen) Hs_TNFRSF11B_1_SG | 107 bp | 55 °C | |
| OSCAR | QuantiTect Primer Assay (Qiagen) Hs_OSCAR_1_SG | 137 bp | 55 °C | |
| RANKL | QuantiTect Primer Assay (Qiagen) Hs_TNFSF11_1_SG | 91 bp | 55 °C | |
| RUNX2 | QuantiTect Primer Assay (Qiagen) Hs_RUNX2_1_SG | 101 bp | 55 °C | |
| SP7 | QuantiTect Primer Assay (Qiagen) Hs_SP7_1_SG | 120 bp | 55 °C | |
| SPARC | QuantiTect Primer Assay (Qiagen) Hs_SPARC_1_SG | 60 bp | 55 °C | |
| SPP1 | QuantiTect Primer Assay (Qiagen) Hs_SPP1_1_SG | 115 bp | 55 °C | |
| VEGFA | QuantiTect Primer Assay (Qiagen) Hs_VEGFA_6_SG | 99 bp | 55 °C | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagani, S.; Salerno, M.; Locs, J.; Vecstaudza, J.; Dolcini, L.; Fini, M.; Giavaresi, G.; Filardo, G.; Columbaro, M. Enhanced Osteogenic Response to an Osteochondral Scaffold Modified with BMP-2 or Strontium-Enriched Amorphous Calcium Phosphate in a Co-Culture In Vitro Model. J. Funct. Biomater. 2025, 16, 302. https://doi.org/10.3390/jfb16080302
Pagani S, Salerno M, Locs J, Vecstaudza J, Dolcini L, Fini M, Giavaresi G, Filardo G, Columbaro M. Enhanced Osteogenic Response to an Osteochondral Scaffold Modified with BMP-2 or Strontium-Enriched Amorphous Calcium Phosphate in a Co-Culture In Vitro Model. Journal of Functional Biomaterials. 2025; 16(8):302. https://doi.org/10.3390/jfb16080302
Chicago/Turabian StylePagani, Stefania, Manuela Salerno, Janis Locs, Jana Vecstaudza, Laura Dolcini, Milena Fini, Gianluca Giavaresi, Giuseppe Filardo, and Marta Columbaro. 2025. "Enhanced Osteogenic Response to an Osteochondral Scaffold Modified with BMP-2 or Strontium-Enriched Amorphous Calcium Phosphate in a Co-Culture In Vitro Model" Journal of Functional Biomaterials 16, no. 8: 302. https://doi.org/10.3390/jfb16080302
APA StylePagani, S., Salerno, M., Locs, J., Vecstaudza, J., Dolcini, L., Fini, M., Giavaresi, G., Filardo, G., & Columbaro, M. (2025). Enhanced Osteogenic Response to an Osteochondral Scaffold Modified with BMP-2 or Strontium-Enriched Amorphous Calcium Phosphate in a Co-Culture In Vitro Model. Journal of Functional Biomaterials, 16(8), 302. https://doi.org/10.3390/jfb16080302








