Influence of Drilling Protocol on Primary Implant Stability Depending on Different Bone Qualities and Implant Macro-Designs, Lengths, and Diameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Implants Characteristics
2.2. Implant Site Preparation
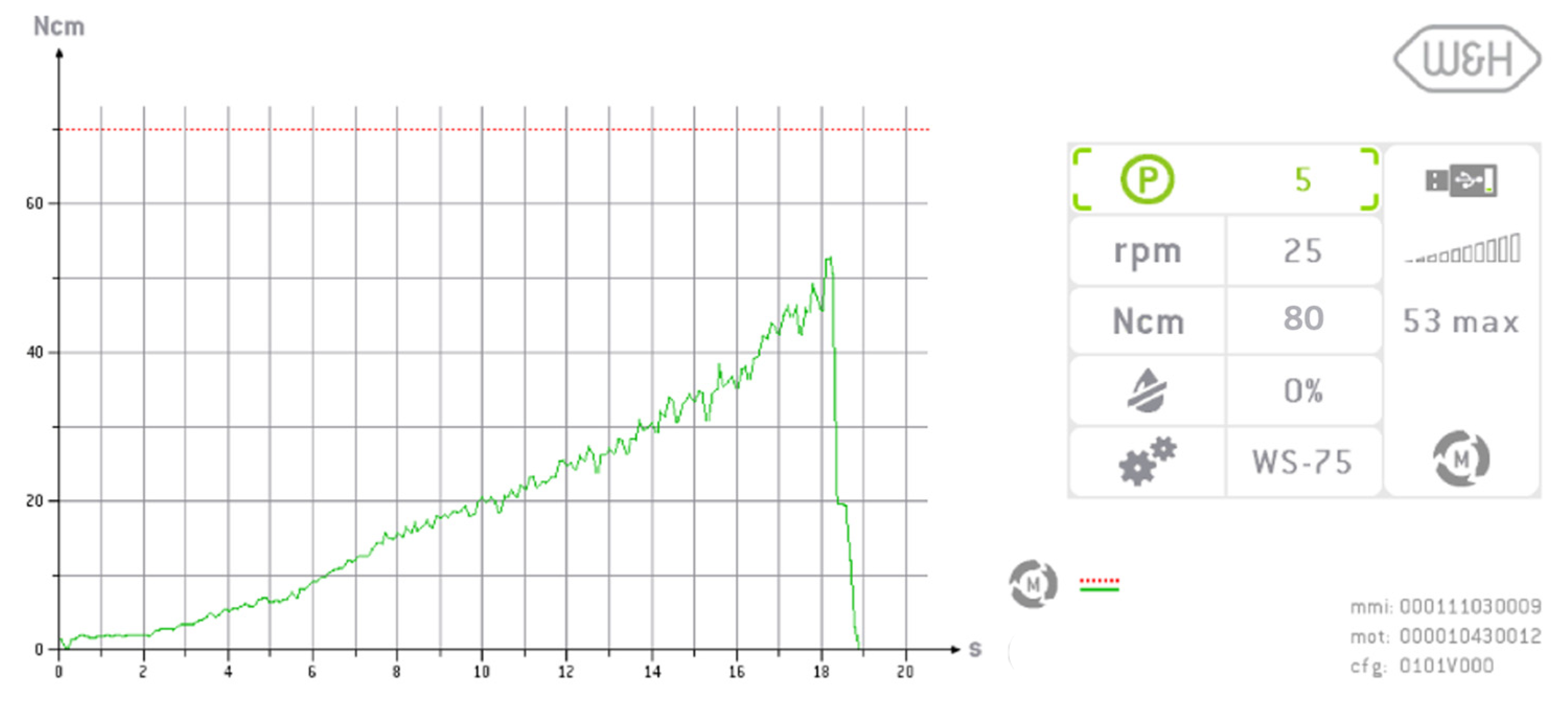
2.3. Implant Insertion and Measurement of Implant Stability
2.4. Statistical Analysis
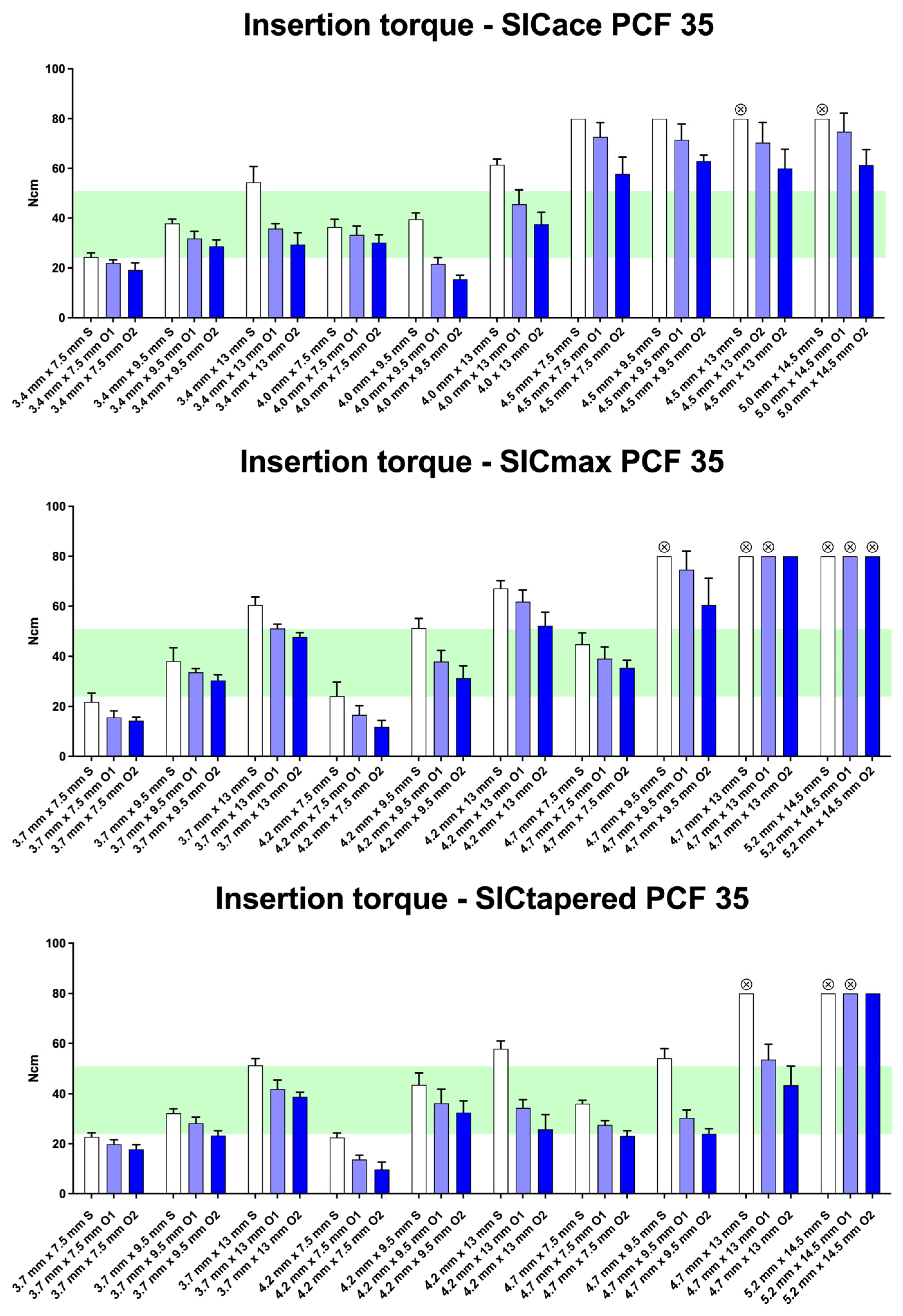
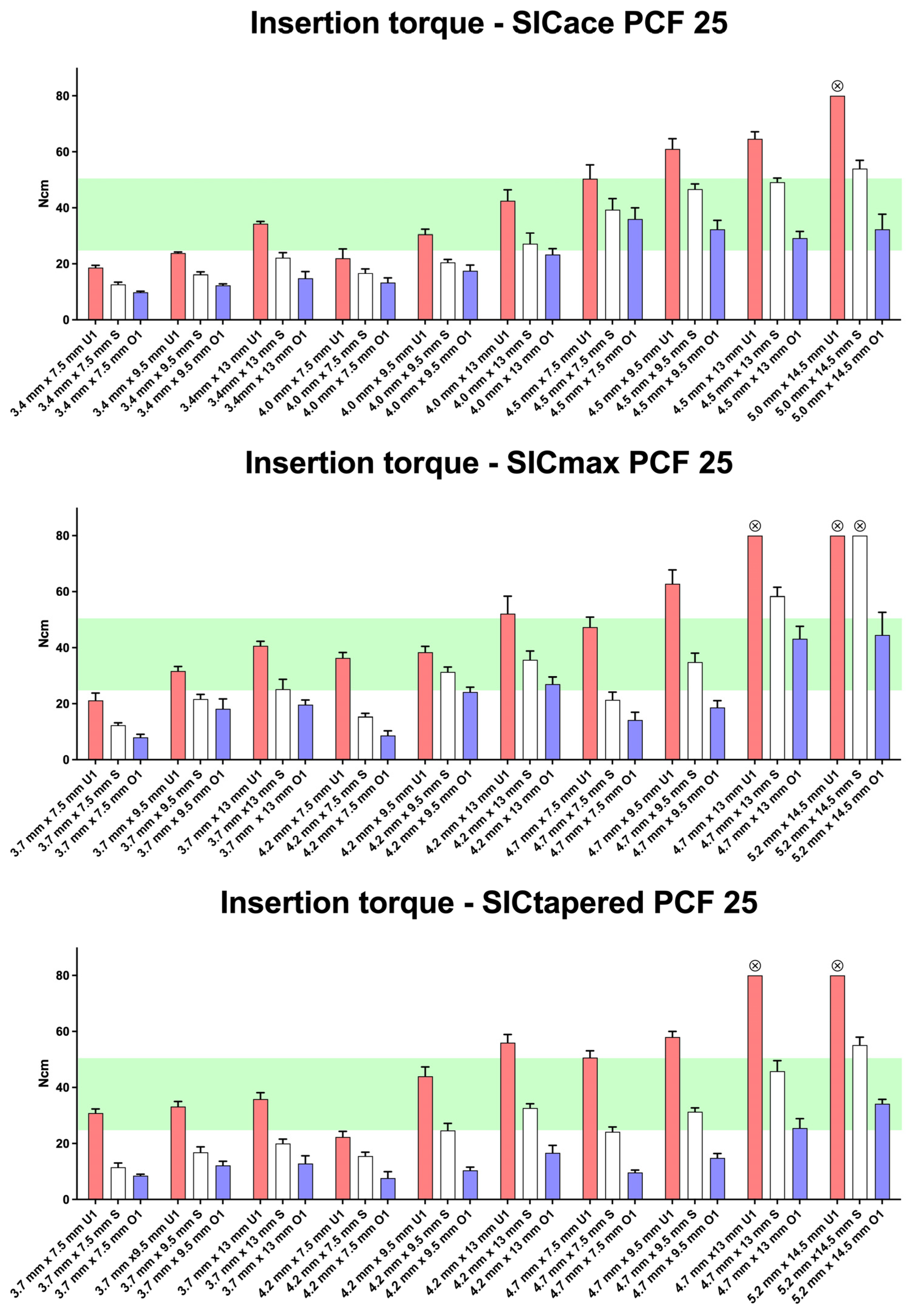
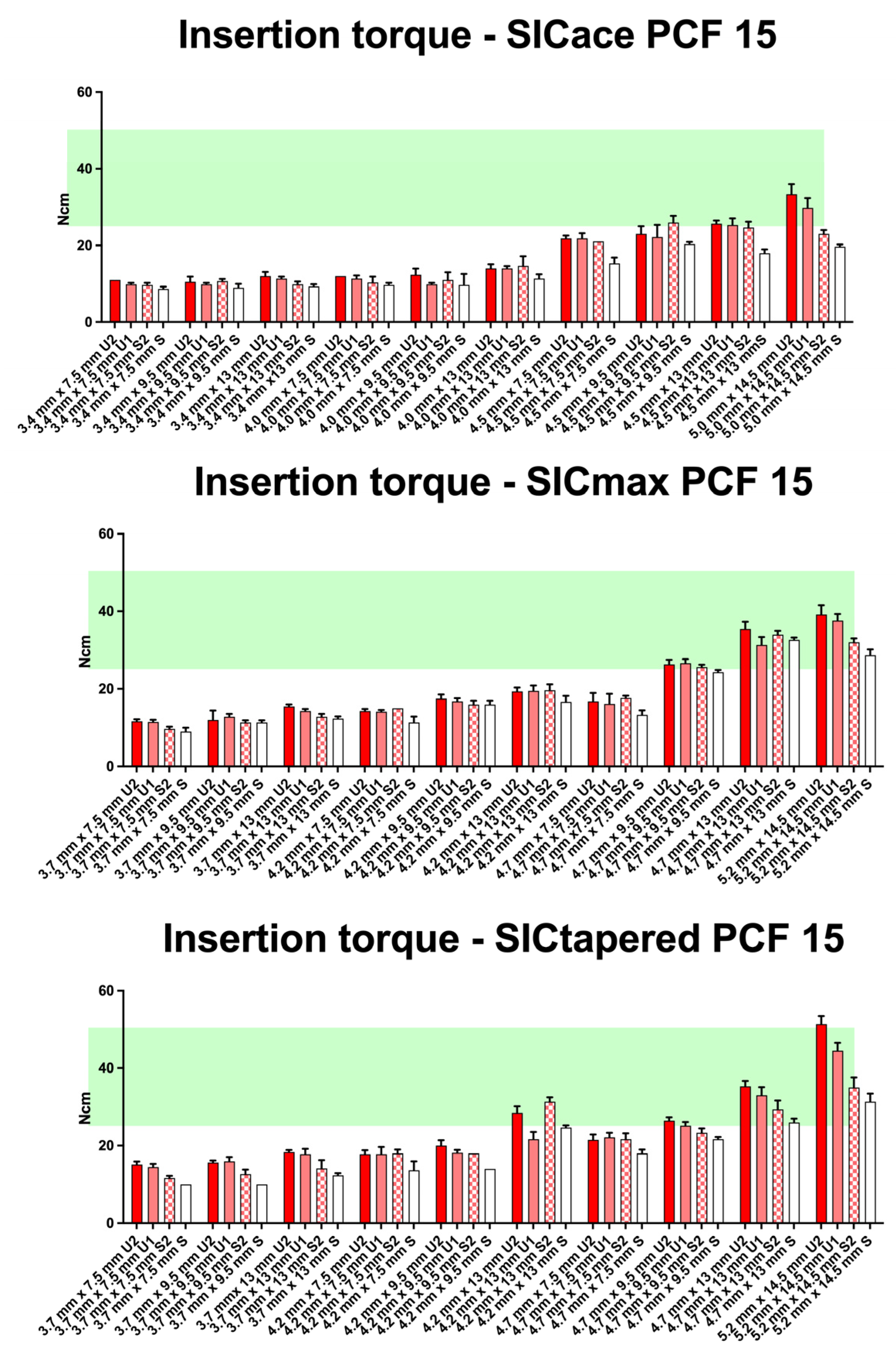
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atieh, M.A.; Alsabeeha, N.; Duncan, W.J. Stability of tapered and parallel-walled dental implants: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Climent, M.; Lemos, B.F.; Herrero-Climent, F.; Falcao, C.; Oliveira, H.; Herrera, M.; Gil, F.J.; Rios-Carrasco, B.; Rios-Santos, J.V. Influence of Implant Design and Under-Preparation of the Implant Site on Implant Primary Stability. An In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 4436. [Google Scholar] [CrossRef] [PubMed]
- Waechter, J.; Leite, F.R.; Nascimento, G.G.; Carmo Filho, L.C.; Faot, F. The split crest technique and dental implants: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Mohlhenrich, S.C.; Kniha, K.; Heussen, N.; Holzle, F.; Modabber, A. Effects on primary stability of three different techniques for implant site preparation in synthetic bone models of different densities. Br. J. Oral Maxillofac. Surg. 2016, 54, 980–986. [Google Scholar] [CrossRef]
- Mohlhenrich, S.C.; Heussen, N.; Elvers, D.; Steiner, T.; Holzle, F.; Modabber, A. Compensating for poor primary implant stability in different bone densities by varying implant geometry: A laboratory study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1514–1520. [Google Scholar] [CrossRef]
- Waechter, J.; Madruga, M.M.; Carmo Filho, L.C.D.; Leite, F.R.M.; Schinestsck, A.R.; Faot, F. Comparison between tapered and cylindrical implants in the posterior regions of the mandible: A prospective, randomized, split-mouth clinical trial focusing on implant stability changes during early healing. Clin. Implant. Dent. Relat. Res. 2017, 19, 733–741. [Google Scholar] [CrossRef]
- Sierra-Rebolledo, A.; Allais-Leon, M.; Maurette-O’Brien, P.; Gay-Escoda, C. Primary Apical Stability of Tapered Implants Through Reduction of Final Drilling Dimensions in Different Bone Density Models: A Biomechanical Study. Implant. Dent. 2016, 25, 775–782. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Branemark, P.I.; Lindhe, J.; Eriksson, B.; Sbordone, L. Marginal tissue reactions at osseointegrated titanium fixtures (I). A 3-year longitudinal prospective study. Int. J. Oral Maxillofac. Surg. 1986, 15, 39–52. [Google Scholar] [CrossRef]
- Gomez-Polo, M.; Ortega, R.; Gomez-Polo, C.; Martin, C.; Celemin, A.; Del Rio, J. Does Length, Diameter, or Bone Quality Affect Primary and Secondary Stability in Self-Tapping Dental Implants? J. Oral Maxillofac. Surg. 2016, 74, 1344–1353. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Albertini, M.; Rios-Santos, J.V.; Lazaro-Calvo, P.; Fernandez-Palacin, A.; Bullon, P. Resonance frequency analysis-reliability in third generation instruments: Osstell mentor(R). Med. Oral Patol. Oral Cir. Bucal 2012, 17, e801–e806. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Santos-Garcia, R.; Jaramillo-Santos, R.; Romero-Ruiz, M.M.; Fernandez-Palacin, A.; Lazaro-Calvo, P.; Bullon, P.; Rios-Santos, J.V. Assessment of Osstell ISQ’s reliability for implant stability measurement: A cross-sectional clinical study. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e877–e882. [Google Scholar] [CrossRef] [PubMed]
- Lages, F.S.; Douglas-de Oliveira, D.W.; Costa, F.O. Relationship between implant stability measurements obtained by insertion torque and resonance frequency analysis: A systematic review. Clin. Implant. Dent. Relat. Res. 2018, 20, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N.; Alleyne, D.; Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implant. Res. 1996, 7, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Ravida, A.; Wang, H.L.; Helms, J.A.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implant. 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Davies, J.E. Mechanisms of endosseous integration. Int. J. Prosthodont. 1998, 11, 391–401. [Google Scholar]
- Lekholm, U.; Zarb, G. Patient selection and preparation. In Tissue Integrated Prosthesis: Osseointegration in Clinical Dentistry; Albrektsson, T., Branemark, P.-I., Zarb, G., Eds.; Quintessence Publishing: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- He, J.; Zhao, B.; Deng, C.; Shang, D.; Zhang, C. Assessment of implant cumulative survival rates in sites with different bone density and related prognostic factors: An 8-year retrospective study of 2,684 implants. Int. J. Oral Maxillofac. Implant. 2015, 30, 360–371. [Google Scholar] [CrossRef]
- Licata, A. Bone density vs bone quality: What’s a clinician to do? Cleve Clin. J. Med. 2009, 76, 331–336. [Google Scholar] [CrossRef]
- Campos, F.E.; Gomes, J.B.; Marin, C.; Teixeira, H.S.; Suzuki, M.; Witek, L.; Zanetta-Barbosa, D.; Coelho, P.G. Effect of drilling dimension on implant placement torque and early osseointegration stages: An experimental study in dogs. J. Oral Maxillofac. Surg. 2012, 70, e43–e50. [Google Scholar] [CrossRef]
- Coelho, P.G.; Marin, C.; Teixeira, H.S.; Campos, F.E.; Gomes, J.B.; Guastaldi, F.; Anchieta, R.B.; Silveira, L.; Bonfante, E.A. Biomechanical evaluation of undersized drilling on implant biomechanical stability at early implantation times. J. Oral Maxillofac. Surg. 2013, 71, e69–e75. [Google Scholar] [CrossRef]
- Stocchero, M.; Toia, M.; Cecchinato, D.; Becktor, J.P.; Coelho, P.G.; Jimbo, R. Biomechanical, Biologic, and Clinical Outcomes of Undersized Implant Surgical Preparation: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2016, 31, 1247–1263. [Google Scholar] [CrossRef]
- Stoilov, M.; Shafaghi, R.; Stark, H.; Marder, M.; Kraus, D.; Enkling, N. Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols-An In Vitro Analysis. J. Funct. Biomater. 2023, 14, 469. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A.; Iezzi, G. Development of a new implant primary stability parameter: Insertion torque revisited. Clin. Implant. Dent. Relat. Res. 2013, 15, 637–644. [Google Scholar] [CrossRef]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontology 2000 2008, 47, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Gonzaga, L.; Amorim, K.; Wittneben, J.G.; Martig, L.; Morton, D.; Martin, W.; Gallucci, G.O.; Wismeijer, D. Selection criteria for immediate implant placement and immediate loading for single tooth replacement in the maxillary esthetic zone: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2023, 34 (Suppl. S26), 304–348. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A.; Meijer, G.J.; Cuijpers, V.; Frank Walboomers, X. Combined effect of undersized surgical technique and axial compression on the primary implant stability and host bone architecture. Saudi Dent. J. 2021, 33, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Oskouei, A.B.; Golkar, M.; Badkoobeh, A.; Jahri, M.; Sadeghi, H.M.M.; Mohammadikhah, M.; Abbasi, K.; Tabrizi, R.; Alam, M. Investigating the effect of insertion torque on marginal bone loss around dental implants. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101523. [Google Scholar] [CrossRef]
- ASTM F1839; Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments. ASTM: West Conshohocken, PA, USA, 1997. [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef]
- Tsolaki, I.N.; Tonsekar, P.P.; Najafi, B.; Drew, H.J.; Sullivan, A.J.; Petrov, S.D. Comparison of Osteotome and Conventional Drilling Techniques for Primary Implant Stability: An In Vitro Study. J. Oral Implantol. 2016, 42, 321–325. [Google Scholar] [CrossRef]
- Comuzzi, L.; Iezzi, G.; Piattelli, A.; Tumedei, M. An In Vitro Evaluation, on Polyurethane Foam Sheets, of the Insertion Torque (IT) Values, Pull-Out Torque Values, and Resonance Frequency Analysis (RFA) of NanoShort Dental Implants. Polymers 2019, 11, 1020. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Romasco, T.; Petrini, M.; Afrashtehfar, K.I.; Inchingolo, F.; Piattelli, A.; Di Pietro, N. Insertion Torque, Removal Torque, and Resonance Frequency Analysis Values of Ultrashort, Short, and Standard Dental Implants: An In Vitro Study on Polyurethane Foam Sheets. J. Funct. Biomater. 2022, 14, 10. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Di Pietro, N.; Romasco, T.; Heydari Sheikh Hossein, H.; Montesani, L.; Inchingolo, F.; Piattelli, A.; Covani, U. A Comparison of Conical and Cylindrical Implants Inserted in an In Vitro Post-Extraction Model Using Low-Density Polyurethane Foam Blocks. Materials 2023, 16, 5064. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.I.; Mir-Mari, J.; Hammerle, C.H. Loading protocols for single-implant crowns: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2014, 29, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Heschl, A.; Payer, M.; Platzer, S.; Wegscheider, W.; Pertl, C.; Lorenzoni, M. Immediate rehabilitation of the edentulous mandible with screw type implants: Results after up to 10 years of clinical function. Clin. Oral Implant. Res. 2012, 23, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, J.M.; Oliveira, Z.F.; Mansini, R.; Cabral, A.M. Correlation between placement torque and survival of single-tooth implants. Int. J. Oral Maxillofac. Implant. 2005, 20, 769–776. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Coulthard, P.; Worthington, H.V. Different loading strategies of dental implants: A Cochrane systematic review of randomised controlled clinical trials. Eur. J. Oral Implantol. 2008, 1, 259–276. [Google Scholar]
- Galli, F.; Capelli, M.; Zuffetti, F.; Testori, T.; Esposito, M. Immediate non-occlusal vs. early loading of dental implants in partially edentulous patients: A multicentre randomized clinical trial. Peri-implant bone and soft-tissue levels. Clin. Oral Implant. Res. 2008, 19, 546–552. [Google Scholar] [CrossRef]
- Barone, A.; Alfonsi, F.; Derchi, G.; Tonelli, P.; Toti, P.; Marchionni, S.; Covani, U. The Effect of Insertion Torque on the Clinical Outcome of Single Implants: A Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2016, 18, 588–600. [Google Scholar] [CrossRef]
- Marconcini, S.; Giammarinaro, E.; Toti, P.; Alfonsi, F.; Covani, U.; Barone, A. Longitudinal analysis on the effect of insertion torque on delayed single implants: A 3-year randomized clinical study. Clin. Implant. Dent. Relat. Res. 2018, 20, 322–332. [Google Scholar] [CrossRef]
- Ramesh, R.; Sasi, A.; Mohamed, S.C.; Joseph, S.P. “Compression Necrosis”—A Cause of Concern for Early Implant Failure? Case Report and Review of Literature. Clin. Cosmet. Investig. Dent. 2024, 16, 43–52. [Google Scholar] [CrossRef]
- Darriba, I.; Seidel, A.; Moreno, F.; Botelho, J.; Machado, V.; Mendes, J.J.; Leira, Y.; Blanco, J. Influence of low insertion torque values on survival rate of immediately loaded dental implants: A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 158–169. [Google Scholar] [CrossRef]
- Griggs, J.A. Dental Implants. Dent. Clin. N. Am. 2017, 61, 857–871. [Google Scholar] [CrossRef]
- Heimes, D.; Becker, P.; Pabst, A.; Smeets, R.; Kraus, A.; Hartmann, A.; Sagheb, K.; Kammerer, P.W. How does dental implant macrogeometry affect primary implant stability? A narrative review. Int. J. Implant. Dent. 2023, 9, 20. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G. Primary Stability Evaluation of a Novel Tapered Implant Inserted in Low-Density Bone Sites: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2023, 38, 334–337. [Google Scholar] [CrossRef]
- Abi-Aad, H.L.; Daher, F.I.; Baba, N.Z.; Cordioli, G.; Majzoub, Z.A.K. Insertion Torque of Variable-Thread Tapered Implants in the Posterior Maxilla: A Clinical Study. J. Prosthodont. 2019, 28, e788–e794. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yun, P.Y.; Yi, Y.J.; Kim, Y.K.; Lee, H.J.; Jo, D.W. Low Bone Density Predictability of CBCT and Its Relation to Primary Stability of Tapered Implant Design: A Pilot Study. J. Oral Implantol. 2023, 49, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Bagnasco, F.; Calimodio, I.; Di Tullio, N.; Delucchi, F.; Baldi, D.; Pera, F. Influence of Implant Thread Morphology on Primary Stability: A Prospective Clinical Study. Biomed. Res. Int. 2020, 2020, 6974050. [Google Scholar] [CrossRef] [PubMed]
- Summers, R. A new concept in maxillary implant surgery: The osteotome technique. Compendium 1994, 15, 152–162. [Google Scholar]
- Slete, F.B.; Olin, P.; Prasad, H. Histomorphometric Comparison of 3 Osteotomy Techniques. Implant. Dent. 2018, 27, 424–428. [Google Scholar] [CrossRef]
- Huwais, S.; Meyer, E.G. A Novel Osseous Densification Approach in Implant Osteotomy Preparation to Increase Biomechanical Primary Stability, Bone Mineral Density, and Bone-to-Implant Contact. Int. J. Oral Maxillofac. Implant. 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Althobaiti, A.K.; Ashour, A.W.; Halteet, F.A.; Alghamdi, S.I.; AboShetaih, M.M.; Al-Hayazi, A.M.; Saaduddin, A.M. A Comparative Assessment of Primary Implant Stability Using Osseodensification vs. Conventional Drilling Methods: A Systematic Review. Cureus 2023, 15, e46841. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.; Monje, F. The reliability of cone-beam computed tomography to assess bone density at dental implant recipient sites: A histomorphometric analysis by micro-CT. Clin. Oral Implants Res. 2013, 24, 871–879. [Google Scholar] [CrossRef]
- Parsa, A.; Ibrahim, N.; Hassan, B.; van der Stelt, P.; Wismeijer, D. Bone quality evaluation at dental implant site using multislice CT, micro-CT, and cone beam CT. Clin. Oral Implant. Res. 2015, 26, e1–e7. [Google Scholar] [CrossRef]
- Toia, M.; Stocchero, M.; Cecchinato, F.; Corra, E.; Jimbo, R.; Cecchinato, D. Clinical Considerations of Adapted Drilling Protocol by Bone Quality Perception. Int. J. Oral Maxillofac. Implant. 2017, 32, 1288–1295. [Google Scholar] [CrossRef]
- Gholami, H.; Mericske-Stern, R.; Joehren, P.; Enkling, N. Bone Resorption around Self-Tapping Implants in Bone Density Type I-II: 3-Years Results of a Prospective Clinical Study. Int. J. Dent. Oral Health 2020, 6. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Bone Quality and Quantity and Dental Implant Failure: A Systematic Review and Meta-analysis. Int. J. Prosthodont. 2017, 30, 219–237. [Google Scholar] [CrossRef]
- Pauwels, R.; Jacobs, R.; Singer, S.R.; Mupparapu, M. CBCT-based bone quality assessment: Are Hounsfield units applicable? Dentomaxillofac. Radiol. 2015, 44, 20140238. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Rahimi, H.; Motamedian, S.R.; Pirayesh, Z.; Haiat, A.; Zahedrozegar, S.; Mahmoudinia, E.; Rohban, M.H.; Krois, J.; Lee, J.H.; Schwendicke, F. Deep learning in periodontology and oral implantology: A scoping review. J. Periodontal Res. 2022, 57, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Alqutaibi, A.Y.; Algabri, R.S.; Elawady, D.; Ibrahim, W.I. Advancements in artificial intelligence algorithms for dental implant identification: A systematic review with meta-analysis. J. Prosthet. Dent. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Li, H.; Shimada, T.; Kita, S.; Iida, M.; Lee, C.; Nakano, T.; Yamaguchi, S.; Imazato, S. Development of artificial intelligence model for supporting implant drilling protocol decision making. J. Prosthodont. Res. 2023, 67, 360–365. [Google Scholar] [CrossRef]
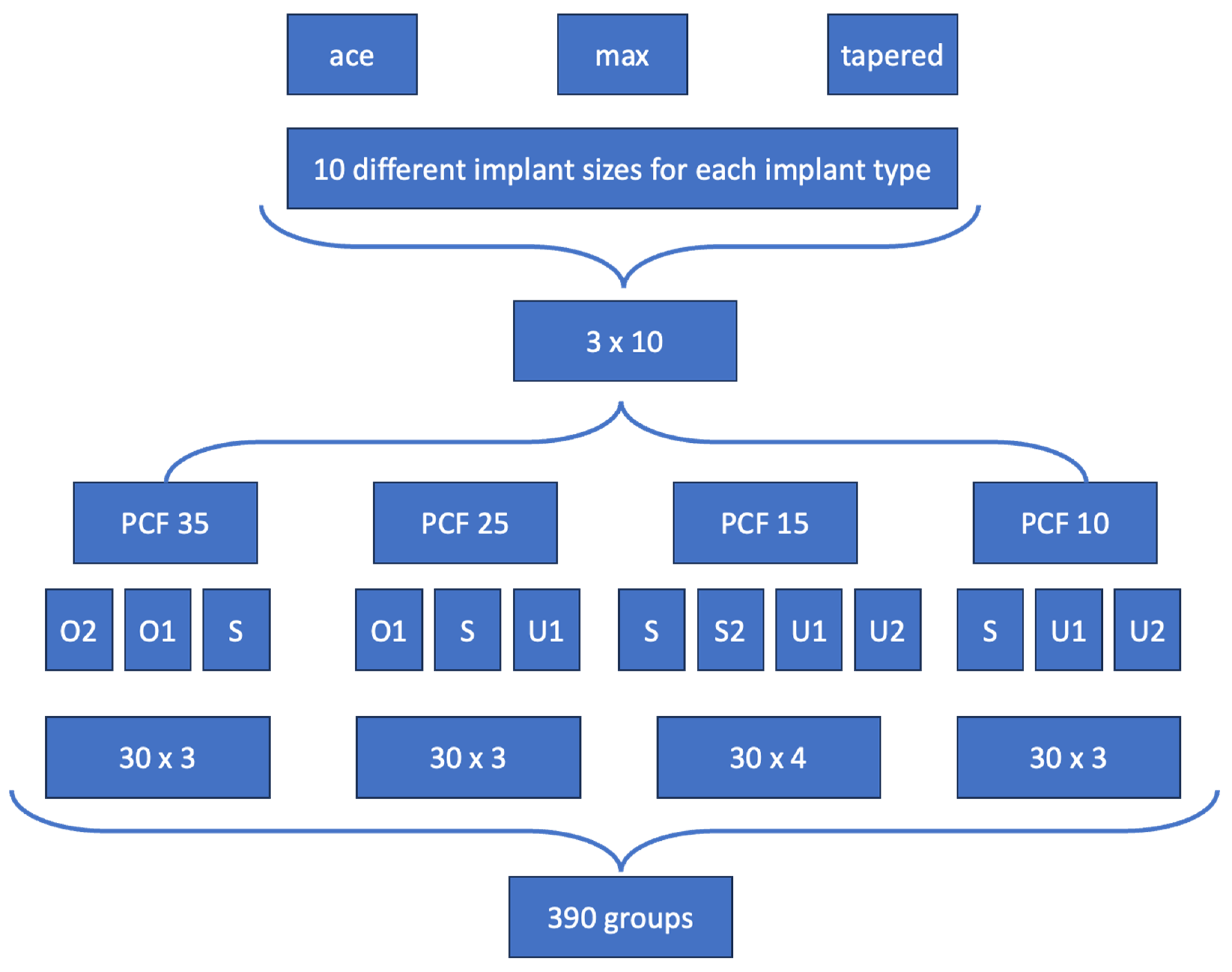

| Implant Type | Implant Ø in mm | Implant Length in mm |
|---|---|---|
| SICace® | 3.4 | 7.5 |
| SICmax® | 3.7 | 9.5 |
| SICtapered® | 3.7 | 13 |
| SICace® | 4.0 | 7.5 |
| SICmax® | 4.2 | 9.5 |
| SICtapered® | 4.2 | 13 |
| SICace® | 4.5 | 7.5 |
| SICmax® | 4.7 | 9.5 |
| SICtapered® | 4.7 | 13 |
| SICace® | 5.0 | 14.5 |
| SICmax® | 5.2 | |
| SICtapered® | 5.2 |
| 35 PCF (D1) | 25 PCF (D2) | 15 PCF (D3) | 10 PCF (D4) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O2 | O1 | S | O1 | S | U1 | S | S2 | U1 | U2 | S | U1 | U2 |
| Drilling Sequence | Last Drill | Drilling length | Crestal Drill | Tap Drill | ||||||||
| S (Standard) | Standard | 100% | ✓ | x | ||||||||
| S2 (Standard 2) | Standard | 100% | x | x | ||||||||
| O1 (Oversized Site 1) | Next bigger drill | 100% | ✓ | x | ||||||||
| O2 (Oversized Site 2) | Next bigger drill | 100% | ✓ | ✓ | ||||||||
| U1 (Undersized Site 1) | Standard | 70% | x | x | ||||||||
| U2 (Undersized Site 2) | Previous drill | 100% | x | x | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoilov, M.; Shafaghi, R.; Stoilov, L.; Stark, H.; Marder, M.; Enkling, N.; Kraus, D. Influence of Drilling Protocol on Primary Implant Stability Depending on Different Bone Qualities and Implant Macro-Designs, Lengths, and Diameters. J. Funct. Biomater. 2025, 16, 296. https://doi.org/10.3390/jfb16080296
Stoilov M, Shafaghi R, Stoilov L, Stark H, Marder M, Enkling N, Kraus D. Influence of Drilling Protocol on Primary Implant Stability Depending on Different Bone Qualities and Implant Macro-Designs, Lengths, and Diameters. Journal of Functional Biomaterials. 2025; 16(8):296. https://doi.org/10.3390/jfb16080296
Chicago/Turabian StyleStoilov, Milan, Ramin Shafaghi, Lea Stoilov, Helmut Stark, Michael Marder, Norbert Enkling, and Dominik Kraus. 2025. "Influence of Drilling Protocol on Primary Implant Stability Depending on Different Bone Qualities and Implant Macro-Designs, Lengths, and Diameters" Journal of Functional Biomaterials 16, no. 8: 296. https://doi.org/10.3390/jfb16080296
APA StyleStoilov, M., Shafaghi, R., Stoilov, L., Stark, H., Marder, M., Enkling, N., & Kraus, D. (2025). Influence of Drilling Protocol on Primary Implant Stability Depending on Different Bone Qualities and Implant Macro-Designs, Lengths, and Diameters. Journal of Functional Biomaterials, 16(8), 296. https://doi.org/10.3390/jfb16080296








