Abstract
Background: Common bile duct (CBD) treatments are often associated with complications, limiting long-term efficacy. To overcome these issues, polymeric grafts have been suggested as promising alternatives, since they are highly customizable, biocompatible, and may reduce side effects frequency. Methods: A systematic review was conducted, interrogating MEDLINE and Cochrane Library. Next, an in vivo study involved 20 pigs, which underwent a former controlled biliary injury. To repair the defect, a α,β-Poly(N-2-hydroxyethyl)-DL-Aspartamide (PHEA)–Polylactic-acid (PLA)–Polycaprolactone (PCL) scaffold was implanted. The animals were sacrificed at one and three months for gross and histological examinations, to assess tissue integration and healing outcomes. Results: The systematic review highlighted that such scaffolds have shown promising results in CBD regeneration, both in single and joined applications. These findings were confirmed by the in vivo study, where the use of such scaffolds—particularly, the planar ones—led to safe and complete bile duct regeneration. Histological analysis revealed lymphomonocytic infiltrates and neovascularization, while microscopic examination showed progressive scaffold degradation accompanied by biliary tissue regeneration. Conclusions: Experimental results are consistent with the literature, confirming the potential of such polymeric scaffolds in aiding complete CBD regeneration and being reabsorbed shortly after. Still, further studies are needed to fully validate their translational application. PROSPERO ID: CRD420251115056.
1. Introduction
Laparoscopic cholecystectomy represents the gold standard treatment of cholelithiasis, and it is commonly preferred to the open approach because of its more favorable outcomes. However, many studies showed that this procedure is associated with an increased risk of common bile duct (CBD) injuries, with an incidence of 0.5–0.8% [1,2,3].
In CBD injury, treatment requires a multidisciplinary approach depending on the size of the lesion. Surgical options may vary from the placement of subhepatic drainage to the performance of biliodigestive anastomosis, which can restore intestinal continuity [4]. However, these interventions are associated with both short- and long-term complications [5,6,7]. Indeed, these are burdened by high morbidity and mortality rates (6.5% and 4.2%, respectively), thus reducing the patients’ Quality of Life (QoL) [8,9].
In this context, the rapidly advancing field of Tissue Engineering and Regenerative Medicine (TERM) presents potential solutions. Such an innovative field of medical sciences is constantly testing and developing new natural or synthetic 3D biocompatible and resorbable scaffolds that may support and promote CBD regeneration; indeed, these can be fine-tuned to properly fit a certain microenvironment [10] and its tissue characteristics [11]. Doing so, such scaffolds may provide support for adhesion and implantation of various cellular populations, promoting the growth of new tissues with the same morpho-functional features as the native ones [12].
Moreover, these scaffolds are designed to provide always just the right grade of mechanical support; in the early phases of regeneration, they maintain their mechanical integrity to provide strong support to the growing tissues against the forces acting in vivo; eventually, they are completely degraded, allowing for tissue remodeling [10].
Still, previous studies underlined that natural and autologous grafts, such as those derived from animal intestines, are plagued by early degradation and immune rejection, hampering long-lasting performance [13,14]. Therefore, new polymeric scaffolds are in development. Among these, the copolymeric scaffold made by α,β-Poly(N-2-hydroxyethyl)-DL-Aspartamide (PHEA), Polylactic acid (PLA) and Polycaprolactone (PCL)—namely, PHEA-PLA-PCL, is emerging as a valuable alternative, designed to ensure biocompatibility and long-term stability. Indeed, several studies previously carried out by the authors proved that PHEA-PLA-PCL has a high mechanical resistance and good biocompatibility. Such assessments regarded bile duct regeneration in the rabbit model and arteriovenous fistula in the pig model [15,16,17].
To properly evaluate PHEA-PLA-PCL scaffold characteristics in the context of CBD reconstruction, a systematic review of the literature will be conducted, thus gathering and analyzing existing evidence on its behalf and, therefore, providing a comprehensive overview of the subject. Moreover, this will be compared to the experimental results obtained in a proper in vivo study on pigs; here, the ability to withstand the lytic action of bile and to result in the complete CBD regeneration will be assessed. The goal will be to identify best practices, potential issues, and optimization criteria to allow the application of such scaffolds in human biliary diseases.
2. Results
2.1. Systematic Review
Table 1 depicts the results of the systematic review, along with the Quality Assessment (QA) of each inserted article. To note, QA results were low, as the systematic review only included D5 articles, i.e., reviews and preclinical studies. Nonetheless, the reported information can still be useful for making initial assessments about the experimental efficacy of such devices.

Table 1.
Table of Results.
2.1.1. Analysis of In Vitro Studies
Several in vitro studies assessed the effectiveness of polymeric scaffolds for CBD tissue engineering. Xu [21] investigated a PLGA–BaSO4 scaffold, noting that its structural integrity remained stable during the first two weeks of bile exposure, followed by a predictable degradation pattern via hydrolysis, without toxic byproducts. This was key in suggesting its potential for safe in vivo translation. Zhou [23] evaluated electrospun PLGA scaffolds seeded with CBD-derived endothelial cells from swine BMCs, showing high cellular viability, uniform attachment, and biliary epithelial marker expression, without evidence of cytotoxicity. The scaffold maintained its fibrous architecture for up to two weeks, making it suitable for bile duct support. Kim [25] applied electrospray deposition to create sorafenib-loaded PCL films on metallic stents, aimed at delivering a localized chemotherapeutic effect against cholangiocarcinoma. In vitro tests showed a sustained drug release for up to 30 days, significantly reducing cancer cell proliferation and angiogenesis, and suggesting its utility for malignant biliary obstruction. Cai [26] developed PLA/PCL drug-eluting stents embedded with EDTA and SC to promote CBD stone dissolution. In ex vivo perfusion models mimicking bile flow, 50% EDTA/SC stents achieved up to 26.2% stone mass loss over ~13 weeks, balancing degradation and therapeutic efficacy. Notably, higher concentrations accelerated degradation but eventually reduced overall effectiveness, underlining the importance of polymer tuning. Huang [28] expanded upon such a scaffold by comparing three fabrication techniques (i.e., dip coating, coaxial electrospinning, and their combination), revealing that the combined method ensured the most controlled and prolonged drug release, resulting in superior stone dissolution in vitro. Buisson [32] employed a multilayered 3D-printed PCL scaffold with distinct fibrous and microporous regions, seeded with hCdH-Chols. The inner fibrous layer promoted differentiation and epithelialization within 14 days, while the outer microporous layer provided mechanical robustness. Finally, Hallett [33] validated the functionality of a novel human biliary epithelial cell (hBEC) line derived from cadaveric liver tissue, demonstrating high proliferative and clonogenic capacities. When seeded on electrospun PCL scaffolds, hBECs retained their cholangiocyte phenotype, further confirming the biocompatibility and regenerative suitability of these polymeric constructs.
2.1.2. Analysis of In Vivo Studies
A substantial body of research supports the safety and functionality of scaffold-based CBD substitutes in animal models, confirming the biocompatibility, mechanical resilience, and regenerative potential of such functionalized devices. Miyazawa [18] reported that PCL–PLA/PGA scaffolds, whether seeded with BMCs or not, supported full epithelialization and structural regeneration in pigs after six months, with no adverse events (AEs) or histological discrepancies between groups. Laukkarinen [19] introduced a radiopaque, biodegradable PLA–BaSO4 stent in a porcine model, significantly reducing bile leakage and eliminating the need for secondary endoscopic removal. The stents degraded fully by six months, and histological analysis revealed only mild inflammation, demonstrating both functional and safety advantages over non-biodegradable PE controls. Xu [21] found that radiopaque PLGA scaffolds implanted in dogs maintained bile duct patency, induced minimal inflammation, and allowed normalization of hepatic biomarkers by week five, confirming their degradability and biocompatibility in a large animal model. Aikawa [22] showed that even unseeded PCL–PLA/PGA patches could regenerate ductal tissue in pigs, with cuboidal columnar epithelium formation and no residual fibrosis or scarring after four months. Kim [25] validated the in vivo anti-tumor efficacy of sorafenib-loaded PCL stents in murine xenograft models, with marked tumor inhibition and apoptosis, without systemic toxicity or structural failure upon stent deployment. Buscemi [16] reported that PHEA-PLA/PCL scaffolds resisted bile corrosion while facilitating epithelial and glandular cell regeneration in rabbits, indicating strong regenerative performance even in smaller animal models. Huang [28] confirmed just the biocompatibility of EDTA/SC-eluting stents in vivo, not being able to test stone mass loss due to a high mortality shown in a preliminary test, when scaffolds were placed with biliary stones. Girard [29] designed a radiopaque, resorbable biliary stent composed of PLA/PEG with TIB–PCL. In vivo and in vitro studies obtained similar results, with a quasi-linear degradation of the PLA/PEG component, only late structural fragmentation, a fibrous encapsulation by 6 months, and preservation of mechanical properties for 4 weeks, thus ensuring duct patency during healing. Cadaveric testing by four surgeons yielded a 75% implantation success rate, confirmed by CT imaging. Buisson [32] demonstrated significantly higher survival and functional recovery in rabbits implanted with hCdH-Chol-seeded PCL scaffolds compared to unseeded controls, which all died within five days. Histological analyses confirmed integration and absence of stricture formation. Kim [34] divided 14 pigs into a 3D-printed PCL/BaSO4 stent group and a sham group; while radiological patency was confirmed, complications included three early deaths (one in the test and two in the control group), stent migration, fracture, and moderate inflammation. Finally, Valderrama-Treviño [37] demonstrated long-term scaffold integration over an 18-month period using PLGA–PCL–Gel CBD scaffold in pigs, with full epithelial regeneration and sustained bile duct functionality, supporting the potential of prolonged regenerative applications.
2.1.3. Analysis of Review Articles
The included reviews offered comprehensive overviews of scaffold technologies, biomaterials, and tissue engineering approaches for bile duct regeneration and disease modeling. Aikawa [20] and Justin [27] underscored the regenerative capacity of bioabsorbable polymer scaffolds, particularly PCL–PLA/PGA composites, in promoting early tissue remodeling and epithelial recovery, while also discussing the importance of novel surgical techniques and cell-based therapies. Kasuya [24] compared collagen and PLGA-based matrices for bile duct formation in vitro, showing that although collagen supported functionality, it lacked integration with other hepatic structures—an issue resolved by PLGA membranes, which demonstrated improved modularity and compatibility for 3D co-culture systems. Brevini [30] reviewed the potential of synthetic materials like PLA in constructing scaffold-based bile ducts, highlighting their adaptability and mechanical control, yet pointing out the limited number of preclinical validations available to date. Wang [31] provided a detailed evaluation of TEBDs, emphasizing the necessity for scaffolds that balance biocompatibility with mechanical strength and tunable degradation rates. The review eventually advocated hybrid scaffolds, i.e., the ones that combine natural and synthetic elements, as promising tools to mimic physiological bile duct architecture. Miyazawa [35] proposed a classification of bile duct substitutes based on their source materials and underscored critical design factors such as wound healing dynamics, biliary fluid properties, and tissue integration at the anastomotic site—all essential for ensuring scaffold functionality and long-term efficacy. Finally, De Siervi [36] focused on the utility of synthetic 3D scaffolds in modeling primary liver cancer, arguing that their mechanical reproducibility and controlled degradation outperform natural matrices in experimental standardization and therapeutic testing, further confirming the translational potential of these bioengineered platforms.
2.2. In Vivo Study
All the animals completed the planned follow-up without spontaneous deaths or the need for early euthanasia due to clinical deterioration. No fatal AEs occurred during the observation period.
As yet described in the method section, the 20 animals were divided as reported in the following Table 2.

Table 2.
Description of study groups.
2.2.1. Obtained Results
Hereafter, the results obtained in the in vivo study, divided into groups:
- Group A: Gallbladder wall planar scaffold
All pigs survived the procedure, and no complications occurred. Animals were sacrificed after three months, and a cholecystectomy was performed. The structure of the scaffold was macroscopically indistinguishable from the gallbladder.
Histological examination showed the complete reconstitution of the gallbladder mucosa with a simple columnar epithelium and a widespread monocyte infiltration. Neoangiogenesis was evidenced, as well as an intense inflammatory response. The scaffold sample was examined by immunofluorescence using anti-CD31 monoclonal antibodies. Staining revealed the presence of numerous vessels around the fibrous tissue. Serial imaging along the z axis allowed us to determine the correct organization of the vessels and confirmed the good response in terms of neoangiogenesis (Figure 1A).
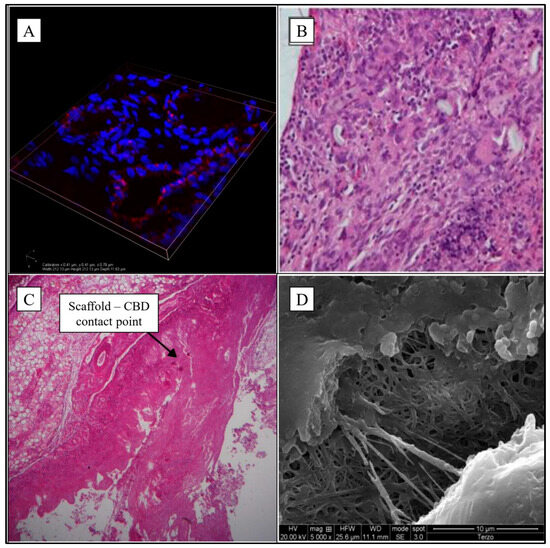
Figure 1.
Microphotographs of implanted scaffolds. (A) 3D organization of endothelial cells with confocal microscope (Nikon a1—width 212.13 µm, height 212.13 µm, depth 11.63 µm—cellular nucleus in blue, antibodies anti-CD31 in red); (B) Gallbladder-jejunal scaffold histological examination (EE20×) shows intense chronic inflammatory infiltration in the portion of the scaffold; (C) CBD tubular scaffold histological examination shows the contact point of proximal anastomosis (EE5×); (D) the scaffold patch of CBD is partially degraded at electron microscopy 5000×. Sources: (A,C,D) are reprinted from Ref. [38]; (B) = owned by the co-authors.
- Group B: Gallbladder-jejunal tubular scaffold
The postoperative course was regular, and sacrifice of animals was scheduled at three months.
An intense phlogistic reaction was found in the new bile duct made up by the scaffold, which was only partially reabsorbed. There was evidence of a foreign body granulomatous reaction, confirmed by the histological examination that showed an intense chronic inflammatory infiltration in the portion of the scaffold (lymphocytes, macrophages and giant cells) (Figure 1B).
- Group C: CBD tubular scaffold
The postoperative course was normal. Laparotomy was performed after three months, and the scaffold and its anastomoses were in place without leaking. The microscopical exam of the scaffold revealed signs of integration between the scaffold and CBD (Figure 1C).
- Group D: CBD patch
Given that the CBD lesion to be repaired in this group was planar and only 2 cm in size, it was decided to sacrifice the animals just one month after surgery, in order to evaluate the early effects of the implant. Although there was macroscopic evidence of the complete integrity of the CBD without any scaffold adhesion left, the patch was only partially degraded. SEM examination further confirmed this, showing an almost intact fibrillar structure after only one month (Figure 1D).
2.2.2. Semiquantitative Histopathological Analysis
Semiquantitative histopathological analysis confirmed the microscopic observations, allowing for an objective comparison of the regenerative process obtained in the different experimental groups. Table 3 shows a semiquantitative comparison of the results.

Table 3.
Semiquantitative histopathological results. Here, the number of “+” symbols represents the intensity of response obtained. These are shown with a color code, with green representing an optimal response, yellow an intermediate response, and red a suboptimal response.
As could be seen, groups A (planar on the gallbladder wall) and D (CBD patch) showed the best results in terms of complete epithelial regeneration and intense neovascularization, accompanied by just mild or moderate inflammation, compatible with a physiological response to the biomaterial. These data suggest that in limited parietal defects, the regenerative process is faster and more favorable, probably due to the presence of vital tissue margins that facilitate cell colonization and vascularization.
In contrast, in groups B and C, where the defect was reconstructed with complete tubular scaffolds, a more intense inflammatory reaction was observed, associated with partial epithelial regeneration and less advanced scaffold degradation. This could be attributed to the greater structural complexity of tubular regeneration, which requires more extensive neoangiogenesis and centripetal cell infiltration, conditions that are more difficult to achieve in a three-dimensional implant without residual epithelial support.
Focusing on scaffold degradation, the results show that planar scaffolds degrade faster than tubular ones, thus providing structural support to the regenerating tissue in the early stages of the healing process and beginning to degrade from the third month onwards. On the contrary, after three months, tubular scaffolds are still only partially degraded, remaining in the tissues for a longer period.
These results seem to suggest that the use of PHEA-PLA+PCL is more effective in partial bile duct defects, while in complete reconstructions, prolonged follow-up or modification of the material formulation may be necessary to optimize scaffold degradation and tissue response.
3. Discussion and Conclusions
TERM technologies rely on biomimetic and biocompatible materials to create artificial substitutes, aimed at restoring and maintaining the normal function of ill and damaged tissues or organs without any AEs. Particularly, the use of synthetic polymers has several advantages, primarily relative to their fine-tuning capabilities. By, for instance, the formation of copolymers, it is possible to modulate their chemical, physical and mechanical properties. Even their porosity may be regulated to resemble one of the natural tissues. Finally, the presence of free chemical groups can be used to incorporate other substances (e.g., heparin, antibiotics and growth factors) by direct binding [39].
Previous studies proved the ineffectiveness of natural grafts in CBD application, due to frequent AEs and lower tuning capabilities. Indeed, CBD reconstruction needs to be pursued carefully to avoid medium and long-term serious complications, such as biliary fistulas and stenosis with obstructive jaundice [40]. Therefore, polymeric scaffolds—although not yet utilized in clinical practice but just assessed in preclinical studies—may be the ones of choice in this context. This is exactly the hypothesis this paper aimed to confirm, through a systematic review and an in vivo study.
The systematic review confirmed the growing interest in synthetic polymers—particularly PCL, PLA, PGA and their copolymers—for the engineering of bile duct substitutes. In vitro results highlighted the favorable mechanical behavior, degradation profiles, and cytocompatibility of such materials, especially when combined with functional molecules or layered architectures that promote cellular viability, tissue-specific differentiation and the treatment of the underlying pathology. In vivo studies consistently demonstrated good biocompatibility and successful regeneration of biliary structures across several animal models, showing restored bile flow, normalized liver function markers, and histological reconstitution of ductal epithelia. However, clinical translation is still unachieved, as no clinical studies were retrieved. Consequently, the overall quality of evidence is low.
Notably, some studies did report relevant complications, such as post-operative mortality, inflammation, stent migration, and mechanical failure. Yet these AEs were not consistently linked to a specific polymer or strategy. In fact, some techniques that yielded poor outcomes in certain studies—e.g., drug-eluting stents or unseeded scaffolds—proved effective in others. This heterogeneity in protocols, models, and evaluation endpoints hinders the possibility of establishing which combination of material, structure, and cell seeding approach is most promising. To this end, comparative studies under controlled and standardized conditions are urgently needed, particularly in large animals and in long-term follow-up settings, to define clear efficacy and safety profiles for each strategy. To support the design of such future studies, researchers may benefit from the methodological recommendations outlined in the included reviews; for instance, the reviews by Justin [27] and Miyazawa [35] offer structured frameworks for scaffold development with therapeutic intent, while those by Wang [31] and De Siervi [36] are more suitable for disease modeling applications, particularly in the context of cancer research and drug testing.
The results of the preclinical study show that the PHEA-PLA/PCL scaffold can stimulate the regeneration of portions of pig biliary tissue. Moreover, microscopic analysis at three months showed a progressive degradation of the scaffold and a concomitant regeneration of the biliary tissue, as shown by the presence of new biliary epithelium in the implantation site under optic microscopy. Therefore, the material proved to be resistant to the lytic action of bile, as well as conducive to tissue regeneration. This might initially suggest possible clinical use of such a polymer conduit to achieve CBD regeneration in human patients. However, long-term exposure to bile acids in humans, where biliary pH and microbial flora may differ significantly, remains untested. Dedicated durability studies will be needed to confirm the scaffold’s chemical and mechanical stability in human settings.
In detail, in all animals, there was no obliteration of the new ducts, and anastomoses between the tubular scaffold and the CBD were successful from a technical point of view. Nonetheless, microscopic examination of the surgical specimens did not show complete integration between the native tissue and the scaffold, probably due to the short follow-up. Therefore, three months does not seem to be enough for the complete degradation of the scaffold and its replacement with a new duct. Still, no sign of leakage or stenosis was noted, and there was partial integration in some regions, indicating the initial stimulation of the regenerative process.
The functional properties of the scaffold, its structural resistance to bile, and its ability to induce tissue regeneration are further supported by the results from the CBD patch model. In this group, the limited size of the defect likely favored a more rapid and complete regeneration, with full-depth tissue reconstitution and scaffold resorption already achieved after one month. This suggests that the scaffold is more effective in the treatment of partial defects, where native tissue can support colonization and healing.
This interpretation is corroborated by the semiquantitative histopathological analysis, which allowed direct comparison of the regenerative processes among the four experimental groups. The planar scaffold (group A) and the patch model (group D) yielded the best results, with intense neovascularization, complete epithelial regeneration, and only mild to moderate inflammation—responses that are consistent with physiological healing. Conversely, the groups treated with complete tubular scaffolds (groups B and C) exhibited more intense inflammatory infiltrates, delayed scaffold degradation, and incomplete epithelial repair. This outcome likely reflects the greater complexity of tubular regeneration, which lacks residual epithelial support and depends on more demanding centripetal infiltration and vascularization processes. In this context, the scaffold degradation may trigger an exaggerated inflammatory response that could potentially slow the healing process. Future studies shall evaluate this phenomenon in greater detail to avoid chronic inflammation or fibrosis.
Such modulation could be achieved by refining scaffold features such as wall thickness, fiber alignment, porosity, and polymer composition, to facilitate better vascularization and centripetal colonization of the lumen. Moreover, improvements in surgical technique, such as minimizing manipulation, ensuring tension-free anastomosis, and standardizing suture type, may also influence outcomes and graft integration.
Although the preliminary results give reason to be cautiously optimistic, further technical adjustments are needed to assess the ability of the polymeric conduit to guide the complex regeneration process of tubular structures such as the CBD. On the other hand, the planar and patch versions of the same material have shown encouraging performance in regenerating damaged biliary tissue. Still, independently of its geometry, the material showed resistance to the lytic action of bile, and its 3D structure allowed performing complex anastomosis, with calibers as small as 4 mm. Indeed, from a surgical standpoint, the material was easy to handle, resisted suture, and showed favorable elasticity and porosity. Moreover, the scaffold demonstrated excellent biocompatibility, with biodegradation mediated by a moderate inflammatory response, which in turn appeared to support tissue regeneration through vessel ingrowth and immune cell recruitment. Particularly, no AEs related to residual scaffold fragments were observed during follow-up, although this was limited to a maximum of three months; longer studies are required to rule out late complications such as foreign body granulomas or persistent inflammation.
Still, it is particularly relevant that such results were achieved in the biliary environment, where regenerative stimuli are intrinsically poor, given the absence of circulating progenitor cells or reparative elements. Therefore, the scaffold’s ability to promote neovascularization and epithelialization without anatomical or functional complications suggests its suitability for further development.
This study has several limitations: most included studies were of low quality, often lacking standardized endpoints or controls, which precluded meta-analysis. The pig model, while anatomically relevant, differs from humans both at the cytological and physiological levels, and only a small sample of healthy pigs was tested for a limited follow-up, thus, limiting the strength of the conclusions. Finally, no molecular markers of regeneration were evaluated, nor was immune cell infiltration assessed beyond standard histology.
Nonetheless, this work provided a comprehensive literature overview and an original observational study that may contribute to advancing the current knowledge in the field. As such, it may stimulate further investigations into the cellular and molecular mechanisms underlying bile duct regeneration. A deeper understanding of these dynamics could enable the identification of reliable biomarkers, allowing for more precise monitoring and modulation of the regenerative process.
In conclusion, this study demonstrated that PHEA-PLA+PCL scaffolds—and, more broadly, synthetic polymer-based materials—exhibit favorable properties for supporting biliary tissue repair. Given their biocompatibility, mechanical resilience, and degradation profile, as well as the scalability of their manufacturing processes, these biomaterials may represent promising candidates for future clinical applications in hepatobiliary surgery. Nonetheless, further research is mandatory before clinical implementation, with particular attention to long-term safety, immunological and molecular response, and effectiveness in diseased biliary environments. Particularly, such research should aim at directly comparing the various polymers and scaffold architectures tested to date, under standardized conditions and with long-term follow-up, to identify the most suitable formulations and functionalizations (e.g., with cellular components or growth factors) for clinical translation.
4. Materials and Methods
4.1. Systematic Review
4.1.1. PRISMA 2020 Flow Diagram and Checklist
Figure 2 presents the PRISMA 2020 Flow Diagram, while the PRISMA 2020 Checklist is reported in Table S1.
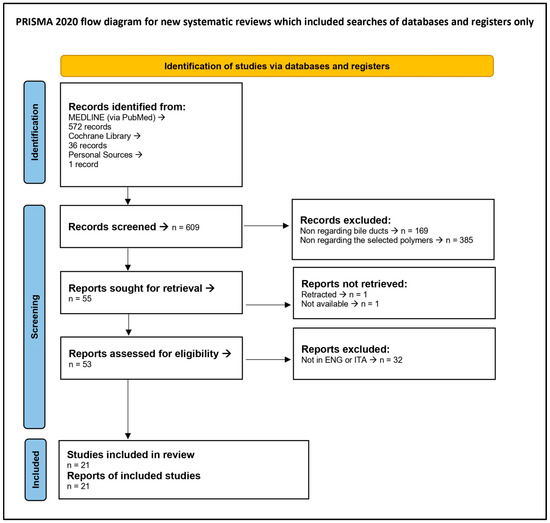
Figure 2.
PRISMA 2020 Flow Diagram.
4.1.2. Research Strings
All research was conducted on 18 March 2025, using both MEDLINE (via PubMed) and Cochrane Library.
- PubMed String → ((“Bile Ducts”[Mesh] OR “Biliary Tract”[Mesh] OR “Common Bile Duct” OR “CBD” OR “CBD injuries” OR “CBD repair” OR “Biliary injury repair” OR “Bile duct regeneration” OR “Biliary reconstruction”) AND (“Polymeric scaffold” OR “Bioabsorbable polymer” OR “Biodegradable scaffold” OR “Polycaprolactone” OR “PCL” OR “Polyhydroxyethyl aspartamide” OR “PHEA” OR “Polylactic Acid” OR “PLA” OR “Polylactide” OR “Tissue Engineering” OR “Regenerative Medicine”)).Used filters → Published in the last 20 years.Number of Results → 572.
- Cochrane Library String → (See Table 4).
 Table 4. Cochrane Library Search String.Used filters → Published in the last 20 years.Number of Results → 36.
Table 4. Cochrane Library Search String.Used filters → Published in the last 20 years.Number of Results → 36.
4.1.3. Screening
Once the 608 results were obtained, they were manually and independently screened by two authors (AG and GB) and their work was further evaluated by a third author (AILM). The screening was conducted according to the following inclusion criteria:
- Only papers in ENG or ITA.
- Only regarding bile ducts.
- Only regarding at least one of the following polymers:PLA, PCL, PHEA.
Doing so, 20 articles were included, along with one additional relevant paper [22], identified through personal knowledge and expertise.
4.1.4. Data Extraction
Data extraction was manually conducted by one author (AG) and his work was further evaluated by two other authors (GB and AILM). It covered the following parameters:
- First Author
- Publication Year
- PMID or DOI
- Type of Study
- Technical Details
- Results
4.1.5. QA and Data Synthesis
QA of included articles was manually and independently conducted by two authors (AG and GB) and further evaluated by a third author (AILM). Particularly, since both reviews and in vivo studies are included, only the modified presentation of the Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence scale [41] is adopted for any article. In detail, it rates the type of study by assessing the grade of recommendation (with A representing the maximum grade and D the minimum) and level of evidence (with 1 representing the maximum level and 5 the minimum). In this way, for each type of study, an ascending alphanumeric value will be produced, inversely proportional to the quality of the article in question. Therefore, the highest quality will be “Grade A—Level 1” (shown as “A1”) studies, while the lowest will be “Grade D—Level 5” (i.e., “D5”) studies.
Then, to further evaluate in vivo controlled studies, the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) risk of bias tool [42] is adopted, giving 0 point for “no”, 1 point for “unclear” and 2 points for “yes. By applying this tool, a scoring between 0 and 20 (reported as “value/20 points”) will be obtained, which is directly proportional to the quality of the work: the higher the quality, the higher the score obtained.
Finally, data synthesis was performed via ChatGPT (vers. 4 & 4o) by AGo. The obtained drafts were checked and re-elaborated by the same author. The final synthesis was checked by GB and AILM, then reported in Table 1.
4.2. In Vivo Study
4.2.1. Scaffolds Preparation
Polymeric scaffolds were prepared in the Biocompatible Polymers Laboratory of the Biological, Chemical and Pharmaceutical Sciences and Technologies Department (STEBICEF) of the University of Palermo. The starting polymer was PHEA, a biocompatible synthetic polymer already used as a drug carrier [43]. This polymer was combined with PCL and PLA to obtain the final scaffold by electrospinning [44]. As previously described [16,17], a 50:50 w/w mixture of PHEA-PLA and PCL was subjected to electrospinning at a voltage of 6–15 kV, a speed of 0.8–1 mL/min, and an extrusion distance between the needle and collector of 15–20 cm. The resulting nanofibers (500–1000 nm) were assembled to form tubular structures with a wall thickness of approximately 0.6 mm. At the end of the manufacturing process and before use, the scaffolds were washed repeatedly, vacuum dried, and sterilized. This procedure has already been shown, both in vitro and in mouse models, to produce biocompatible, structurally stable scaffolds capable of stimulating the growth of epithelial and subepithelial layers of the bile ducts. For this reason, this study aimed to test this protocol on a porcine model, in order to have a study model as similar as possible to humans, with a view to subsequent translation to actual clinical studies.
4.2.2. ARRIVE Checklist, Ethics Statements and Project Rationale
In Table S2, the Animal Research: Reporting of In Vivo Experiments (ARRIVE) Checklist is reported, fulfilled as needed. Furthermore, all animal experiments followed the ARRIVE Guidelines.
Study design was submitted to the Animal Welfare Body (O.P.B.A.) of the “A. Mirri” Institute for approval (according to Italian Legislative Decree No. 26 of 4 March 2014 transposing Directive 2010/63/EU on the protection of animals used for scientific purposes).
The “3R Principles” were used as a rationale for developing this study:
- Replacement → The pig model was selected for its high anatomical and physiological homology with humans, making it the most appropriate for testing bile duct regeneration using synthetic scaffolds. There are no validated in vitro or ex vivo alternative methods capable of comprehensively simulating the required tissue, immune, and biomechanical responses.
- Reduction → Although this is an exploratory study, the use of the minimum number of animals necessary to obtain meaningful data was ensured, in line with what is reported in the literature for similar models. The research team’s previous experience and a critical review of the literature allowed the experimental design to be optimized, reducing the need for unnecessary replications.
- Refinement → All surgical procedures were performed under general anesthesia, with subsequent postoperative pain management by the veterinarian, who applied an updated analgesic protocol and humane endpoint criteria. This minimized animal suffering and stress, ensuring compliance with the latest ethical standards.
This approach ensured maximum ethical and methodological compliance in the use of laboratory animals, promoting the reliability and reproducibility of the experimental results.
4.2.3. Study Subjects and Rationale for Surgery and Follow-Up
Study subjects were 20 healthy Mini-Pigs, all females of about 20 kg of weight and 4 months of age, not previously treated. No randomization, blinding, control for confounders, nor a priori sample size calculation were performed. Models were divided into four groups of five subjects each to test different reconstruction techniques. Therefore, they underwent a surgical bile duct injury, which was immediately repaired with interposition of a tubular or planar PHEA-PLA+PLC scaffold. Both 2 cm2 planar scaffolds and 4 mm × 40 mm tubular scaffolds are used. In both scaffolds, the wall thickness was 0.6 mm. All scaffolds obtained were washed several times with bidistilled water, then dried under vacuum and sterilized.
All surgical procedures were carried out on animals under adequate general anesthesia [45]. After surgery, all animals received antibiotic treatment for 3 days and were housed at the “A. Mirri” Experimental Zooprophylactic Institute of Sicily, where the personnel monitored each animal’s stay, taking care of its feeding and ensuring optimal recovery from surgery. The sacrifice of the animals was scheduled for one or three months, depending on the surgical procedure, except in case of worsening of clinical conditions.
Histological examination of the grafted sections was performed to assess the host’s response and the degree of tissue regeneration. In Group A, immunohistochemical examination was performed to search for specific endothelial markers by using Ig anti-CD31.
4.2.4. Details of Experimental Groups
Experimental groups were as follows:
- Group A: Gallbladder wall planar scaffoldThe fundus of the gallbladder was clamped, then an incision of about 2 cm2 was made. PHEA-PLA+PCL planar scaffold was sutured to replace the portion of the gallbladder wall by means of interrupted 5-0 absorbing monofilament stitches (Figure 3A).
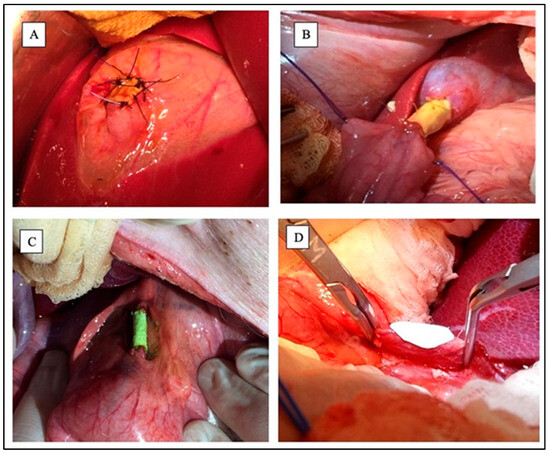 Figure 3. Experimental groups during surgeries. (A) Gallbladder wall planar scaffold; (B) Gallbladder-jejunal scaffold; (C) Common Bile Duct tubular scaffold; (D) Common Bile Duct patch during suturing. Sources: (A–C) are reprinted from Ref. [38]; (D) = owned by the co-authors.
Figure 3. Experimental groups during surgeries. (A) Gallbladder wall planar scaffold; (B) Gallbladder-jejunal scaffold; (C) Common Bile Duct tubular scaffold; (D) Common Bile Duct patch during suturing. Sources: (A–C) are reprinted from Ref. [38]; (D) = owned by the co-authors. - Group B: Gallbladder-jejunal tubular scaffoldThe gallbladder was isolated and a jejunal loop was mobilized. Both the gallbladder-scaffold and the scaffold-jejunal anastomoses were obtained by continuous suture with absorbing monofilament (Figure 3B).
- Group C: CBD tubular scaffoldA 3 cm length tract of the distal part of the CBD was replaced with a tubular scaffold. Both proximal and distal anastomoses were obtained by continuous suture using absorbing monofilament (Figure 3C).
- Group D: CBD patchA wedge choledochotomy was performed. The scaffold was then placed and sutured with absorbing monofilament stitches to replace the CBD wall (Figure 3D).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jfb16080297/s1, Table S1: PRISMA 2020 Checklist; Table S2: ARRIVE Checklist.
Author Contributions
Conceptualization, S.B., A.G. and A.I.L.M.; Data curation, S.B., G.B., A.G. and G.L.M.; Formal analysis, S.B., G.B., A.G., M.L., F.S.P., L.C., G.L.M. and R.P.; Funding acquisition, S.B. and A.I.L.M.; Investigation, S.B., G.B., A.G., G.L.M. and R.P.; Methodology, S.B., A.G., M.L., F.S.P., L.C., R.P. and A.I.L.M.; Project administration, S.B., M.L., G.C. and A.I.L.M.; Resources, M.L., F.S.P. and G.C.; Software, A.G. and L.C.; Supervision, G.C. and A.I.L.M.; Validation, S.B., G.B., A.G. and A.I.L.M.; Visualization, S.B., L.C. and R.P.; Writing—original draft, S.B., G.B. and A.G.; Writing—review and editing, S.B., G.B. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
During the course of preclinical studies, SB was supported by the Italian Ministry of University and Research (MIUR) (Linea di attività:
AIM1892002—3, CUP B78D18002280006).
Institutional Review Board Statement
The research is compliant with the ARRIVE Guidelines. According to Italian Legislative Decree No. 26 of 4 March 2014 transposing Directive 2010/63/EU on the protection of animals used for scientific purposes, study design was first submitted to the Animal Welfare Body (O.P.B.A.) of the “A. Mirri” Institute to approve the initial experimentation on 14 animals (OPBA report no. 1 of 10 September 2014); then, the trial was further extended to another 6 animals and such step was further authorized by the Italian Ministry of Health (authorization no. 259/2021-PR).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
A.G. and G.B. contributed to this paper under the “Experimental Oncology and Surgery” PhD program of the University of Palermo (IT). As specified in the “Method” section, during the preparation of this work, the authors used ChatGPT (vers. 4 & 4o) to initially resume the extracted data. The authors carefully reviewed and revised all content created with this tool, and they take full responsibility for the final manuscript. PROSPERO ID = CRD420251115056.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flum, D.R.; Cheadle, A.; Prela, C.; Dellinger, E.P.; Chan, L. Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA 2003, 290, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, G.; Giuliante, F.; Giovannini, I.; Ardito, F.; D’Acapito, F.; Vellone, M.; Murazio, M.; Capelli, G. Bile duct injury during laparoscopic cholecystectomy: Results of an Italian national survey on 56 591 cholecystectomies. Arch. Surg. 2005, 140, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, J.; Gullichsen, R.; Laine, S.; Salminen, P.; Grönroos, J.M. Bile duct injuries during laparoscopic cholecystectomy: Primary and long-term results from a single institution. Surg. Endosc. 2007, 21, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Eikermann, M.; Siegel, R.; Broeders, I.; Dziri, C.; Fingerhut, A.; Gutt, C.; Jaschinski, T.; Nassar, A.; Paganini, A.M.; Pieper, D.; et al. Prevention and treatment of bile duct injuries during laparoscopic cholecystectomy: The clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg. Endosc. 2012, 26, 3003–3039. [Google Scholar] [CrossRef] [PubMed]
- Törnqvist, B.; Zheng, Z.; Ye, W.; Waage, A.; Nilsson, M. Long-term effects of iatrogenic bile duct injury during cholecystectomy. Clin. Gastroenterol. Hepatol. 2009, 7, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Melton, G.B.; Lillemoe, K.D.; Cameron, J.L.; Sauter, P.A.; Coleman, J.; Yeo, C.J. Major bile duct injuries associated with laparoscopic cholecystectomy: Effect of surgical repair on quality of life. Ann. Surg. 2002, 235, 888–895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Judah, J.R.; Draganov, P.V. Endoscopic therapy of benign biliary strictures. World J. Gastroenterol. 2007, 13, 3531–3539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Głuszek, S.; Kot, M.; Bałchanowski, N.; Matykiewicz, J.; Kuchinka, J.; Kozieł, D.; Wawrzycka, I. Iatrogenic bile duct injuries—Clinical problems. Pol. Przegl Chir. 2014, 86, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Booij, K.A.; de Reuver, P.R.; Yap, K.; van Dieren, S.; van Delden, O.M.; Rauws, E.A.; Gouma, D.J. Morbidity and mortality after minor bile duct injury following laparoscopic cholecystectomy. Endoscopy 2015, 47, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Reagan, M.R.; Kaplan, D.L. Electrospun silk biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 988–1006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, S.W.; Zhang, Y.; Xia, Y. Three-dimensional scaffolds for tissue engineering: The importance of uniformity in pore size and structure. Langmuir 2010, 26, 19001–19006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Day, R.M.; Boccaccini, A.R.; Maquet, V.; Shurey, S.; Forbes, A.; Gabe, S.M.; Jérôme, R. In vivo characterisation of a novel bioresorbable poly(lactide-co-glycolide) tubular foam scaffold for tissue engineering applications. J. Mater. Sci. Mater. Med. 2004, 15, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Böttger, T.; Mann, B.; Pickel, B.; Weber, W.; Sorger, K.; Junginger, T. Animal experiment studies of pedicled small intestine transplantation as partial extrahepatic bile duct replacement. Langenbecks Arch. Chir. 1991, 376, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Bakir, B.; Kösem, M.; Kisli, E.; Gençcelep, M. Biliary tract reconstruction with autologous rectus sheath graft—An experimental study. Hepatogastroenterology 2005, 52, 1019–1022. [Google Scholar] [PubMed]
- Lo Monte, A.I.; Licciardi, M.; Bellavia, M.; Damiano, G.; Palumbo, V.D.; Palumbo, F.S.; Abruzzo, A.; Fiorica, C.; Pitarresi, G.; Cacciabaudo, F.; et al. Biocompatibility and biodegradability of electrospun PHEA-PLA scaffolds: Our preliminary experience in a murine animal model. Dig. J. Nanomater. Biostruct. 2012, 7, 841–851. [Google Scholar]
- Buscemi, S.; Damiano, G.; Fazzotta, S.; Maffongelli, A.; Palumbo, V.D.; Ficarella, S.; Fiorica, C.; Cassata, G.; Licciardi, M.; Palumbo, F.S.; et al. Electrospun Polyhydroxyethyl-Aspartamide-Polylactic Acid Scaffold for Biliary Duct Repair: A Preliminary In Vivo Evaluation. Transplant. Proc. 2017, 49, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Palumbo, V.D.; Maffongelli, A.; Fazzotta, S.; Palumbo, F.S.; Licciardi, M.; Fiorica, C.; Puleio, R.; Cassata, G.; Fiorello, L.; et al. Electrospun PHEA-PLA/PCL Scaffold for Vascular Regeneration: A Preliminary in Vivo Evaluation. Transplant. Proc. 2017, 49, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Torii, T.; Toshimitsu, Y.; Okada, K.; Koyama, I.; Ikada, Y. A tissue-engineered artificial bile duct grown to resemble the native bile duct. Am. J. Transplant. 2005, 5, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Laukkarinen, J.; Nordback, I.; Mikkonen, J.; Kärkkäinen, P.; Sand, J. A novel biodegradable biliary stent in the endoscopic treatment of cystic-duct leakage after cholecystectomy. Gastrointest. Endosc. 2007, 65, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, M.; Miyazawa, M.; Okada, K.; Toshimitsu, Y.; Torii, T.; Otani, Y.; Koyama, I.; Ikada, Y. Regeneration of extrahepatic bile duct--possibility to clinical application by recognition of the regenerative process. J. Smooth Muscle Res. 2007, 43, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, T.; Liu, S.; Zhang, K.; Shen, Z.; Li, Y.; Jing, X. Feasibility of biodegradable PLGA common bile duct stents: An in vitro and in vivo study. J. Mater. Sci. Mater. Med. 2009, 20, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, M.; Miyazawa, M.; Okamoto, K.; Toshimitsu, Y.; Torii, T.; Okada, K.; Akimoto, N.; Ohtani, Y.; Koyama, I.; Yoshito, I. A novel treatment for bile duct injury with a tissue-engineered bioabsorbable polymer patch. Surgery 2010, 147, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Y.; Yin, X.; Xu, Y.; Cao, Y.; Xu, Q. The compatibility of swine BMDC-derived bile duct endothelial cells with a nanostructured electrospun PLGA material. Int. J. Artif. Organs 2013, 36, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, J.; Tanishita, K. Microporous membrane-based liver tissue engineering for the reconstruction of three-dimensional functional liver tissues in vitro. Biomatter 2012, 2, 290–295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.H.; Jeong, Y.I.; Chung, C.W.; Kim, C.H.; Kwak, T.W.; Lee, H.M.; Kang, D.H. Preclinical evaluation of sorafenib-eluting stent for suppression of human cholangiocarcinoma cells. Int. J. Nanomed. 2013, 8, 1697–1711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, X.B.; Zhang, W.X.; Wan, X.J.; Yang, Q.; Qi, X.S.; Wang, X.P.; Lu, L.G. The effect of a novel drug-eluting plastic stent on biliary stone dissolution in an ex vivo bile perfusion model. Gastrointest. Endosc. 2014, 79, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Justin, A.W.; Saeb-Parsy, K.; Markaki, A.E.; Vallier, L.; Sampaziotis, F. Advances in the generation of bioengineered bile ducts. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cai, X.B.; Guo, L.L.; Qi, X.S.; Gao, Q.; Wan, X.J. Drug-eluting fully covered self-expanding metal stent for dissolution of bile duct stones. World J. Gastroenterol. 2019, 25, 3370–3379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Girard, E.; Chagnon, G.; Broisat, A.; Dejean, S.; Soubies, A.; Gil, H.; Sharkawi, T.; Boucher, F.; Roth, G.S.; Trilling, B.; et al. From in vitro evaluation to human postmortem pre-validation of a radiopaque and resorbable internal biliary stent for liver transplantation applications. Acta Biomater. 2020, 106, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Brevini, T.; Tysoe, O.C.; Sampaziotis, F. Tissue engineering of the biliary tract and modelling of cholestatic disorders. J. Hepatol. 2020, 73, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Faria, J.; Penning, L.C.; Masereeuw, R.; Spee, B. Tissue-Engineered Bile Ducts for Disease Modeling and Therapy. Tissue Eng. Part C Methods 2021, 27, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Buisson, E.M.; Park, S.H.; Kim, M.; Kang, K.; Yoon, S.; Lee, J.E.; Kim, Y.W.; Lee, N.K.; Jeong, M.A.; Kang, B.K.; et al. Transplantation of patient-specific bile duct bioengineered with chemically reprogrammed and microtopographically differentiated cells. Bioeng. Transl. Med. 2022, 7, e10252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hallett, J.M.; Ferreira-Gonzalez, S.; Man, T.Y.; Kilpatrick, A.M.; Esser, H.; Thirlwell, K.; Macmillan, M.T.; Rodrigo-Torres, D.; Dwyer, B.J.; Gadd, V.L.; et al. Human biliary epithelial cells from discarded donor livers rescue bile duct structure and function in a mouse model of biliary disease. Cell Stem Cell 2022, 29, 355–371.e310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.H.; Ha, D.H.; Han, E.S.; Choi, Y.; Koh, J.; Joo, I.; Cho, D.W.; Han, J.K. Feasibility and safety of a novel 3D-printed biodegradable biliary stent in an in vivo porcine model: A preliminary study. Sci. Rep. 2022, 12, 15875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyazawa, M.; Aikawa, M.; Takashima, J.; Kobayashi, H.; Ohnishi, S.; Ikada, Y. Pitfalls and promises of bile duct alternatives: A narrative review. World J. Gastroenterol. 2022, 28, 5707–5722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Siervi, S.; Turato, C. Liver Organoids as an In Vitro Model to Study Primary Liver Cancer. Int. J. Mol. Sci. 2023, 24, 4529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valderrama-Treviño, A.I.; Castell-Rodríguez, A.E.; Hernández-Muñoz, R.; Vázquez-Torres, N.A.; Macari-Jorge, A.; Barrera-Mera, B.; Maciel-Cerda, A.; Vera-Graziano, R.; Nuño-Lámbarri, N.; Montalvo-Javé, E.E. Development of a biodegradable prosthesis through tissue engineering, for the organ-replacement or substitution of the extrahepatic bile duct. Ann. Hepatol. 2024, 29, 101530. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S. A Tissue-Engineered Artificial Bile Duct Grown to Resemble the Native Bile Duct in a Porcine Model; University of Palermo, IRIS Portal: Palermo, Italy, 2016. [Google Scholar]
- Jun, S.; Hong, Y.; Imamura, H.; Ha, B.Y.; Bechhoefer, J.; Chen, P. Self-assembly of the ionic peptide EAK16: The effect of charge distributions on self-assembly. Biophys. J. 2004, 87, 1249–1259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haseltine, W.A. Regenerative Medicine 2003: An Overview. e-biomed J. Regen. Med. 2004, 4, 15–18. [Google Scholar] [CrossRef]
- Daramola, O.O.; Rhee, J.S. Rating evidence in medical literature. Virtual Mentor. 2011, 13, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, T.; Li, S.L.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Synthesis of poly(alpha,beta-[N-(2-hydroxyethyl)-L-aspartamide])-folate for drug delivery. J. Biomater. Sci. Polym. Ed. 2010, 21, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, X.; Xia, J.; Chen, P.; Zhou, X.; Huang, J.; Yu, J.; Gu, P. Electrospun chitosan-graft-poly (ε-caprolactone)/poly (ε-caprolactone) nanofibrous scaffolds for retinal tissue engineering. Int. J. Nanomed. 2011, 6, 453–461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cicero, L.; Fazzotta, S.; Palumbo, V.D.; Cassata, G.; Lo Monte, A.I. Anesthesia protocols in laboratory animals used for scientific purposes. Acta Biomed. 2018, 89, 337–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).