Photothermally Responsive Biomimetic Composite Scaffolds Based on Polydopamine-Functionalized Nanoparticles/Polyurethane for Bone Repair
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PDA-Modified Amorphous Calcium Phosphate (CA)
2.3. Preparation of PUCA Scaffolds
2.4. Characterization of Physical and Chemical Properties of PUCA Scaffolds
2.5. Thermal Behavior Analysis of PUCA Scaffolds
2.6. Shape-Memory Properties Analysis of PUCA Scaffolds
2.7. In Vitro Cytocompatibility Evaluation of PUCA Scaffolds
2.8. In Vivo Assessment of PUCA Scaffolds
2.9. Statistical Analysis
3. Results and Discussion
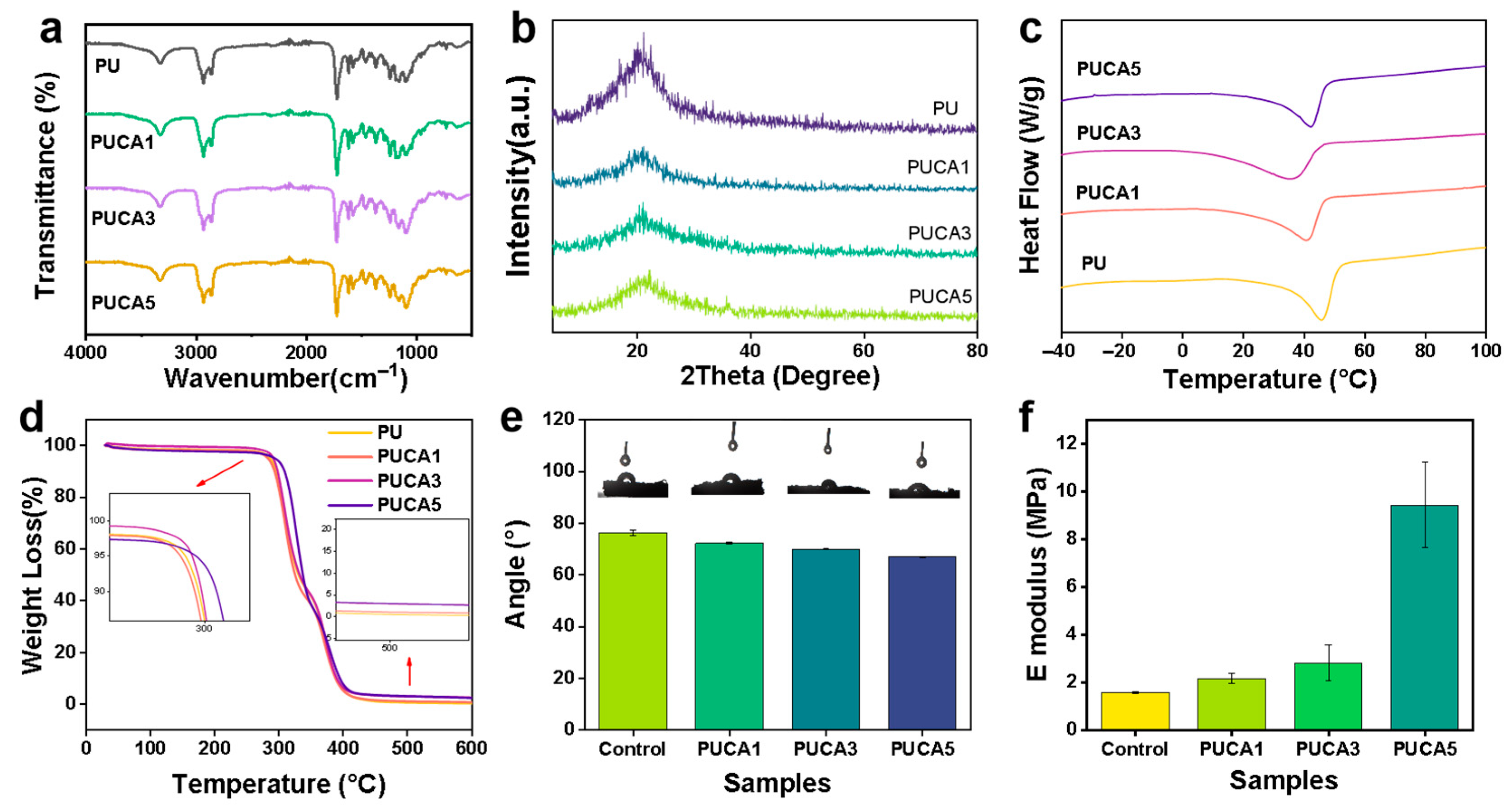
3.1. Synthesis and Structure Characterization of PUCA Scaffolds
3.2. Shape-Memory and Photothermal Effect of PUCA Scaffolds
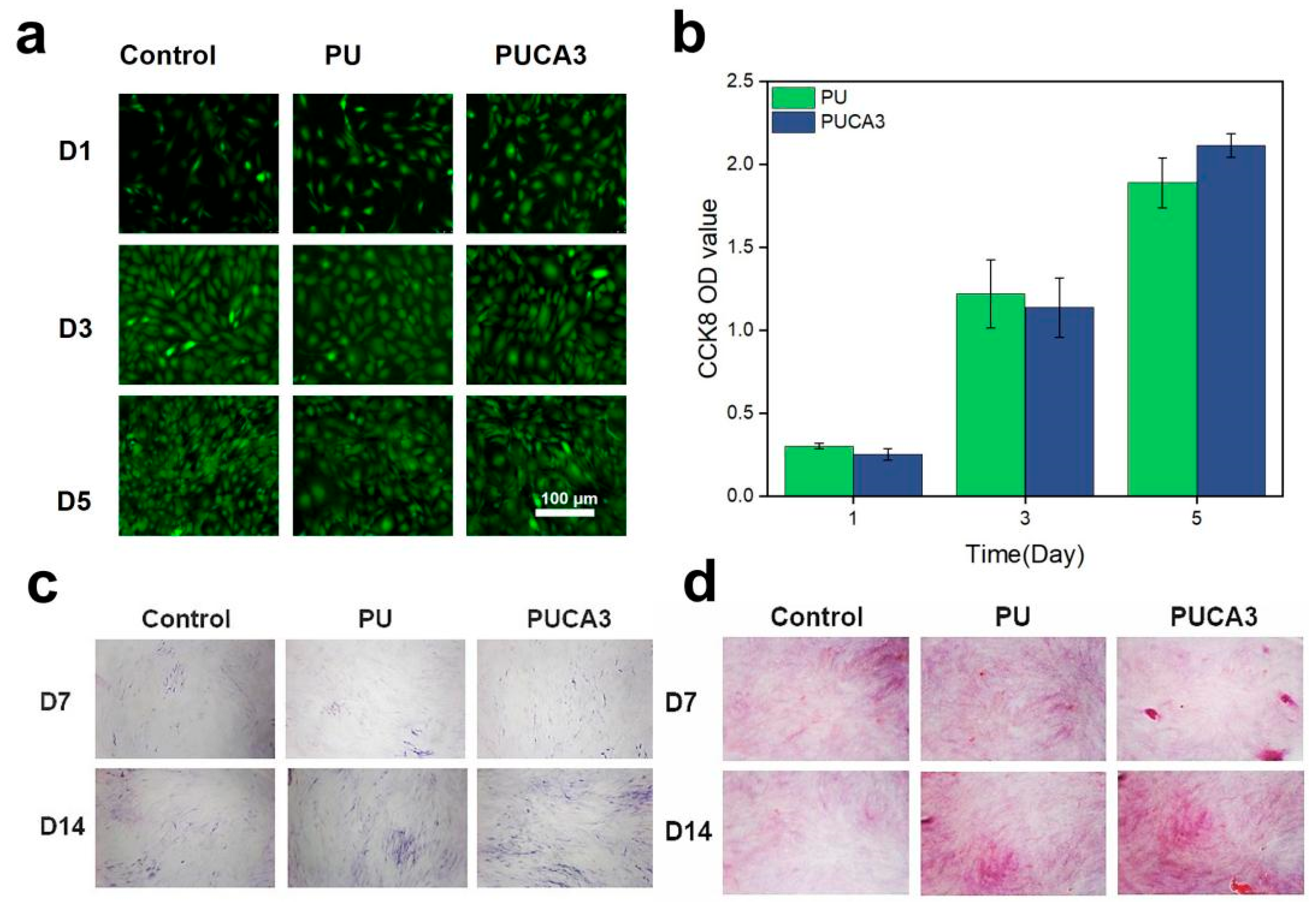
3.3. In Vitro and In Vivo Biological Compatibility Assessment of PUCA Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Smith, A.; Jones, B.; Brown, C. Challenges in autologous bone grafting: Immune responses and donor scarcity. Biomacromolecules 2019, 20, 3054–3062. [Google Scholar]
- Wang, Y.; Zhang, L.; Chen, Z. Advances in 3D-printed scaffolds for bone regeneration. J. Biomed. Mater. Res. A 2017, 105, 1234–1245. [Google Scholar]
- Meng, H.; Li, G. Biomimetic design of bone scaffolds: Structural and mechanical considerations. Polymer 2013, 54, 2199–2208. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H. Mechanical compatibility of scaffolds with native bone tissues. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Zhou, Y. Customized porous scaffolds for critical-sized defects. Adv. Drug Deliv. Rev. 2015, 94, 63–76. [Google Scholar]
- Huang, S.; Liu, Y.; Li, M. Aesthetic outcomes in bone scaffold design. ACS Nano 2016, 10, 10589–10601. [Google Scholar]
- Lendlein, A.; Langer, R. Shape-memory polymers for biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Behl, M.; Razzaq, M.; Lendlein, A. Programming SMPs for minimally invasive implantation. Adv. Mater. 2010, 22, 3388–3410. [Google Scholar] [CrossRef]
- Kumpfer, J.; Rowan, S. Thermal-responsive SMPs for in vivo applications. J. Am. Chem. Soc. 2011, 133, 12866–12873. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Mather, P. Phase transitions in SMPs. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, D.; Cao, Z. Temperature-triggered shape recovery in SMPs. Biomaterials 2018, 163, 128–139. [Google Scholar]
- Chen, Q.; Zhu, L.; Zhao, C. Photothermal materials for bone regeneration. Adv. Mater. 2016, 28, 313–320. [Google Scholar]
- Hu, X.; Zhang, Y.; Xie, Z. Toxicity of inorganic photothermal agents. ACS Appl. Mater. Interfaces 2017, 9, 30458–30467. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Hong, S.; Lee, H. Stability of photothermal composites under thermal cycling. ACS Nano 2015, 9, 5937–5945. [Google Scholar]
- Lee, H.; Dellatore, S.; Miller, W. Polydopamine: A multifunctional biomaterial. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Adhesive properties of PDA. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Chen, H. Photothermal conversion efficiency of PDA. Nat. Commun. 2020, 11, 1–12. [Google Scholar]
- Wang, L.; Chen, M.; Wu, Z. PDA-enhanced vascularization in bone scaffolds. J. Mater. Chem. B 2023, 11, 1747–1765. [Google Scholar]
- Liu, T.; Zhang, Y.; Chen, X. Antibacterial activity of PDA composites. Biomater. Sci. 2022, 10, 2696–2709. [Google Scholar]
- Luo, K.; Gao, P.F.; Yang, W.H.; Lei, X.Y.; Wong, T.W.; Ismail, A.F.; Wang, L. Antibacterial Polyurethane Composite Scaffolds for Minimally Invasive Alveolar Bone Repair. Appl. Mater. Today 2023, 31, 101752. [Google Scholar] [CrossRef]
- Kumar, S.; Pal, A.R.; Gupta, R.K. FTIR and thermal analysis of HDI-based polyurethanes: Correlation between reaction completeness and mechanical properties. Polymer 2021, 215, 123456. [Google Scholar]
- Silva, J.M.; Reis, R.L.; Kundu, S.C. X-ray diffraction analysis of semi-crystalline polymers: Crystalline-amorphous phase interactions. Mater. Today Commun. 2021, 28, 102641. [Google Scholar]
- Duarte, A.R.C.; Gil, M.H.; Mano, J.F. Crystallinity and structural evolution of polycaprolactone in composite scaffolds. Polym. Degrad. Stab. 2021, 193, 109742. [Google Scholar]
- Wang, L.; Chen, M.; Wu, Z.; Zhang, J. Programmable shape recovery and photothermal properties of polyurethane scaffolds for bone regeneration. Polym. Test. 2022, 115, 107785. [Google Scholar]
- Zhang, G.; Zhao, L.; Wang, Y. Thermal degradation behavior of polyurethane composites: Role of PCL and PPG in staged decomposition. Polym. Degrad. Stab. 2021, 185, 109489. [Google Scholar]
- Kim, H.; Park, S.; Lee, J. Thermally stable shape-memory polyurethane scaffolds for bone regeneration: Balancing transition temperature and biocompatibility. ACS Appl. Mater. Interfaces 2021, 13, 24582–24593. [Google Scholar]
- Murphy, M.C.; O’Brien, A.; Reis, R.L. Pore architecture design in bone scaffolds: Balancing mechanical and biological requirements. Biomaterials 2020, 257, 120256. [Google Scholar]
- Zhang, Y.; Chen, X.; Webster, T.J. Biomimetic pore geometries in bone scaffolds: From structural mimicry to functional osteogenesis. Adv. Healthc. Mater. 2021, 10, e2100578. [Google Scholar]
- Lee, J.; Kim, S.; Park, H. Effect of filler-induced viscosity on pore morphology in polyurethane foams: Implications for scaffold design. Mater. Sci. Eng. C 2021, 128, 112345. [Google Scholar]
- García-García, R.; Sánchez-Fernández, M.J.; Boccaccini, A.R. High porosity and interconnectivity in polymer-ceramic composites for bone regeneration: A trade-off between mechanical and biological performance. Acta Biomater. 2022, 135, 342–355. [Google Scholar]
- Li, X.; Wang, Y.; Zhang, Z. Effect of calcium phosphate fillers on crystallinity and shape memory behavior of polyurethane composites. ACS Appl. Mater. Interfaces 2021, 13, 12345–12356. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Chen, J.; Zhang, T.; Chen, T.; Liu, X.; Yang, W.; Wong, T.-W.; Zhang, J.; Wang, L. Photothermally Responsive Biomimetic Composite Scaffolds Based on Polydopamine-Functionalized Nanoparticles/Polyurethane for Bone Repair. J. Funct. Biomater. 2025, 16, 294. https://doi.org/10.3390/jfb16080294
Bai R, Chen J, Zhang T, Chen T, Liu X, Yang W, Wong T-W, Zhang J, Wang L. Photothermally Responsive Biomimetic Composite Scaffolds Based on Polydopamine-Functionalized Nanoparticles/Polyurethane for Bone Repair. Journal of Functional Biomaterials. 2025; 16(8):294. https://doi.org/10.3390/jfb16080294
Chicago/Turabian StyleBai, Ruqing, Jiaqi Chen, Ting Zhang, Tao Chen, Xiaoying Liu, Weihu Yang, Tuck-Whye Wong, Jianwei Zhang, and Li Wang. 2025. "Photothermally Responsive Biomimetic Composite Scaffolds Based on Polydopamine-Functionalized Nanoparticles/Polyurethane for Bone Repair" Journal of Functional Biomaterials 16, no. 8: 294. https://doi.org/10.3390/jfb16080294
APA StyleBai, R., Chen, J., Zhang, T., Chen, T., Liu, X., Yang, W., Wong, T.-W., Zhang, J., & Wang, L. (2025). Photothermally Responsive Biomimetic Composite Scaffolds Based on Polydopamine-Functionalized Nanoparticles/Polyurethane for Bone Repair. Journal of Functional Biomaterials, 16(8), 294. https://doi.org/10.3390/jfb16080294




