Histological Processing of Scaffolds: Challenges and Solutions
Abstract
1. Introduction
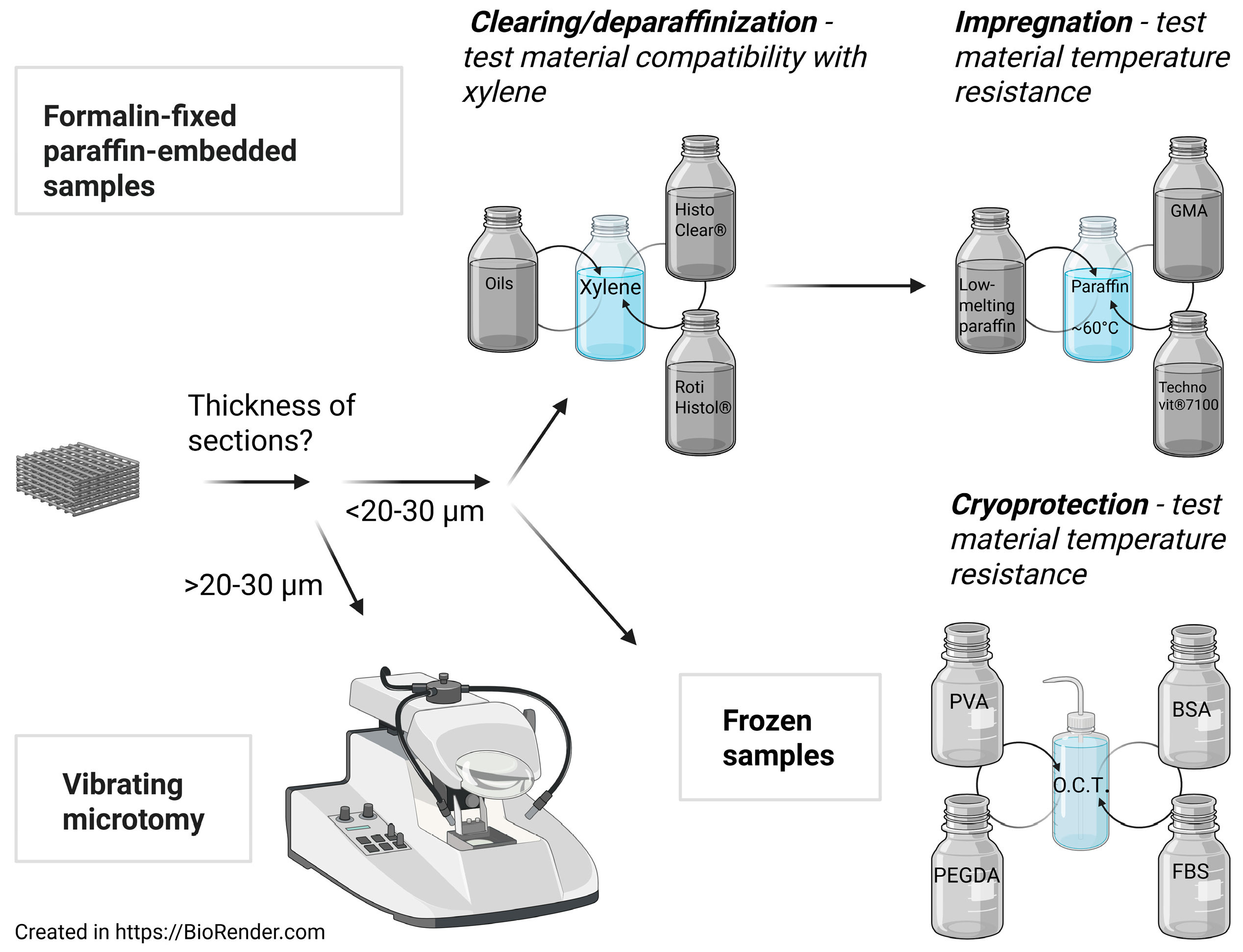
2. Sample Fixation for Further Processing
3. Cryomicrotomy
3.1. Cryopreservation
3.2. Cryosectioning
4. Vibrating Microtomy
5. Formalin-Fixed Paraffin-Embedded Histology
5.1. Dehydration
5.2. Clearing
5.3. Impregnation and Embedding
5.4. Sectioning
5.5. Deparaffinization
5.6. Staining and Visualization
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin; |
| DMSO | dimethyl sulfoxide; |
| FBS | fetal bovine serum; |
| FFPE | formalin-fixed paraffin-embedded |
| Gluc | glucose; |
| GMA | glycol methacrylate; |
| HA | hyaluronic acid; |
| HOPE | Hepes-glutamic acid buffer-mediated Organic solvent Protection Effect; |
| NBF | neutral-buffered formalin |
| O.C.T. | optimal cutting temperature; |
| PCL | polycaprolactone; |
| PEG | polyethylene glycol; |
| PEGDA | polyethylene glycol diacrylate; |
| PEGDMA | poly (ethylene glycol) dimethacrylate; |
| PEO | poly (ethylene)oxide; |
| PGA | polyglycolic acid; |
| PLA | polylactic acid; |
| PLGA | poly (lactide-co-glycolide); |
| PLLA | poly-L-lactic acid; |
| PPy | polypyrrole |
| PVA | polyvinyl alcohol; |
| SF | silk fibroin. |
References
- Zhan, L.; Zhou, Y.; Liu, R.; Sun, R.; Li, Y.; Tian, Y.; Fan, B. Advances in growth factor-containing 3D printed scaffolds in orthopedics. Biomed. Eng. OnLine 2025, 24, 14. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Lei, C. The Delivery and Activation of Growth Factors Using Nanomaterials for Bone Repair. Pharmaceutics 2023, 15, 1017. [Google Scholar] [CrossRef]
- Wong, S.K.; Yee, M.M.F.; Chin, K.-Y.; Ima-Nirwana, S. A Review of the Application of Natural and Synthetic Scaffolds in Bone Regeneration. J. Funct. Biomater. 2023, 14, 286. [Google Scholar] [CrossRef]
- Niu, X.; Li, N.; Du, Z.; Li, X. Integrated gradient tissue-engineered osteochondral scaffolds: Challenges, current efforts and future perspectives. Bioact. Mater. 2023, 20, 574–597. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, P.; Sang, S.; Xiang, C.; An, Y.; Wei, X.; Yan, Y.; Li, P. 3D printed PCL/GelMA biphasic scaffold boosts cartilage regeneration using co-culture of mesenchymal stem cells and chondrocytes: In vivo study. Mater. Des. 2021, 210, 110065. [Google Scholar] [CrossRef]
- Tan, J.; Chen, Z.; Xu, Z.; Huang, Y.; Qin, L.; Long, Y.; Wu, J.; Luo, W.; Liu, X.; Yi, W.; et al. A 3D-printed scaffold composed of Alg/HA/SIS for the treatment of diabetic bone defects. J. Orthop. Transl. 2024, 48, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Ok, M.; Halis Cerci, M.; Demirhan, R.; Surucu, S.; Mahirogullari, M. A comparative analysis of 3D bioprinted gelatin-hyaluronic acid-alginate scaffold and microfracture for the management of osteochondral defects in the rabbit knee joint. Jt. Dis. Relat. Surg. 2024, 35, 361–367. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Ga, X.; Gao, G.; Guo, T. Total meniscus replacement with a 3D printing of network hydrogel composite scaffold in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Cole, A.; Strachan, F. Biocomposite Scaffolds for Tissue Engineering: Materials, Fabrication Techniques and Future Directions. Materials 2024, 17, 5577. [Google Scholar] [CrossRef]
- Percival, K.M.; Paul, V.; Husseini, G.A. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. IJMS 2024, 25, 6012. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-Y.; Lee, W.-K.; Hsu, J.-T.; Shih, J.-Y.; Ma, T.-L.; Vo, T.T.T.; Lee, C.-W.; Cheng, M.-T.; Lee, I.-T. Polycaprolactone in Bone Tissue Engineering: A Comprehensive Review of Innovations in Scaffold Fabrication and Surface Modifications. JFB 2024, 15, 243. [Google Scholar] [CrossRef] [PubMed]
- James, R.; Jenkins, L.; Ellis, S.E.; Burg, K.J.L. Histological processing of hydrogel scaffolds for tissue-engineering applications. J. Histotechnol. 2004, 27, 133–139. [Google Scholar] [CrossRef]
- Holy, C.E.; Yakubovich, R. Processing cell-seeded polyester scaffolds for histology. J. Biomed. Mater. Res. 2000, 50, 276–279. [Google Scholar] [CrossRef]
- Fuchs, J.; Mueller, M.; Daxböck, C.; Stückler, M.; Lang, I.; Leitinger, G.; Bock, E.; El-Heliebi, A.; Moser, G.; Glasmacher, B.; et al. Histological processing of un-/cellularized thermosensitive electrospun scaffolds. Histochem. Cell Biol. 2019, 151, 343–356. [Google Scholar] [CrossRef]
- Dębski, T.; Wysocki, J.; Siennicka, K.; Jaroszewicz, J.; Szlązak, K.; Święszkowski, W.; Pojda, Z. Modified Histopathological Protocol for Poly-ε-Caprolactone Scaffolds Preserving Their Trabecular, Honeycomb-like Structure. Materials 2022, 15, 1732. [Google Scholar] [CrossRef]
- Bhat, A.H.; Hussein, S. Fixation and different types of fixatives: Their role and functions: A review. Int. J. Clin. Diagn. Pathol. 2021, 4, 113–119. [Google Scholar] [CrossRef]
- Olert, J.; Wiedorn, K.H.; Goldmann, T.; Kühl, H.; Mehraein, Y.; Scherthan, H.; Niketeghad, F.; Vollmer, E.; Müller, A.M.; Müller-Navia, J. HOPE fixation: A novel fixing method and paraffin-embedding technique for human soft tissues. Pathol. Res. Pract. 2001, 197, 823–826. [Google Scholar] [CrossRef]
- Blaschitz, A.; Gauster, M.; Dohr, G. Application of cryo-compatible antibodies to human placenta paraffin sections. Histochem. Cell Biol. 2008, 130, 595–599. [Google Scholar] [CrossRef]
- Short, A.R.; Czeisler, C.; Stocker, B.; Cole, S.; Otero, J.J.; Winter, J.O. Imaging Cell-Matrix Interactions in Three-Dimensional Collagen Hydrogel Culture Systems. Macromol. Biosci. 2017, 17, 1600478. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Negrini, N.C.; Farè, S.; Draghi, L. Cross-linking strategies for electrospun gelatin scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Pablos, J.L.; Jiménez-Holguín, J.; Salcedo, S.S.; Salinas, A.J.; Corrales, T.; Vallet-Regí, M. New Photocrosslinked 3D Foamed Scaffolds Based on GelMA Copolymers: Potential Application in Bone Tissue Engineering. Gels 2023, 9, 403. [Google Scholar] [CrossRef]
- Pazhouhnia, Z.; Noori, A.; Farzin, A.; Khoshmaram, K.; Hoseinpour, M.; Ai, J.; Ebrahimi, M.; Lotfibakhshaiesh, N. 3D-bioprinted GelMA/gelatin/amniotic membrane extract (AME) scaffold loaded with keratinocytes, fibroblasts, and endothelial cells for skin tissue engineering. Sci. Rep. 2024, 14, 12670. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.A.; Gvazava, N.; Putra Wendi, I.; Guinea, R.; García Giménez, F.; Stegmayr, J.; Klementieva, O.; Wagner, D.E. Formalin-free fixation and xylene-free tissue processing preserves cell-hydrogel interactions for histological evaluation of 3D calcium alginate tissue engineered constructs. Front. Biomater. Sci. 2023, 2, 1155919. [Google Scholar] [CrossRef]
- McGowan, B.H.; Nagatomi, J. Histological techniques for preservation of alginate bead structural integrity using glycolmethacrylate. J. Histotechnol. 2013, 36, 100–105. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Wang, H.; Song, Z.; Yu, D.; Li, G.; Liu, X.; Liu, W. Progress in Research on Metal Ion Crosslinking Alginate-Based Gels. Gels 2024, 11, 16. [Google Scholar] [CrossRef]
- Shindo, T.; Ihashi, S.; Sakamoto, Y.; Okuno, T.; Tomikawa, J.; Miyamoto, K. Visualization of endogenous nuclear F-actin in mouse embryos reveals abnormal actin assembly after somatic cell nuclear transfer. J. Biochem. 2021, 169, 303–311. [Google Scholar] [CrossRef]
- Nie, X.; Chuah, Y.J.; He, P.; Wang, D.A. Engineering a multiphasic, integrated graft with a biologically developed cartilage-bone interface for osteochondral defect repair. J. Mater. Chem. B 2019, 7, 6515–6525. [Google Scholar] [CrossRef]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef]
- McCullen, S.D.; Autefage, H.; Callanan, A.; Gentleman, E.; Stevens, M.M. Anisotropic Fibrous Scaffolds for Articular Cartilage Regeneration. Tissue Eng. Part A 2012, 18, 2073–2083. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, T.H.; Lee, J.H. Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials 2011, 32, 8254–8260. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-R.; Shi, Y.; Taylor, C.R. Antigen Retrieval Immunohistochemistry. J. Histochem. Cytochem. 2011, 59, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Ripper, D.; Schwarz, H.; Stierhof, Y. Cryo-section immunolabelling of difficult to preserve specimens: Advantages of cryofixation, freeze-substitution and rehydration. Biol. Cell 2008, 100, 109–123. [Google Scholar] [CrossRef]
- Jarošová, R.; Ondráčková, P.; Patočka, Z.; Sládek, Z. Comparison of cryoprotective methods for histological examination of rat and porcine lung tissue. Acta Vet. Brno 2021, 90, 225–231. [Google Scholar] [CrossRef]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R.T. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant. 2021, 30, 0963689721999617. [Google Scholar] [CrossRef]
- Ruan, J.L.; Tulloch, N.L.; Muskheli, V.; Genova, E.E.; Mariner, P.D.; Anseth, K.S.; Murry, C.E. An improved cryosection method for polyethylene glycol hydrogels used in tissue engineering. Tissue Eng.—Part C Methods 2013, 19, 794–801. [Google Scholar] [CrossRef]
- Heo, S.J.; Song, K.H.; Thakur, S.; Miller, L.M.; Cao, X.; Peredo, A.P.; Seiber, B.N.; Qu, F.; Driscoll, T.P.; Shenoy, V.B.; et al. Nuclear softening expedites interstitial cell migration in fibrous networks and dense connective tissues. Sci. Adv. 2020, 6, eaax5083. [Google Scholar] [CrossRef]
- Cocco, C.; Melis, G.V.; Ferri, G.-L. Embedding Media for Cryomicrotomy. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 274–280. [Google Scholar] [CrossRef]
- Fink, S. A solvent-free coating-procedure for the improved preparation of cryostat sections in light microscope histochemistry. Histochemistry 1992, 97, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Banigo, A.T.; Konings, I.B.M.; Nauta, L.; Zoetebier, B.; Karperien, M. Synthesis and Engineering of Hyaluronic Acid-Gelatin Hydrogels with Improved Cellular Attachment and Growth. Polymers 2024, 16, 3410. [Google Scholar] [CrossRef]
- Altay, G.; Tosi, S.; García-Díaz, M.; Martínez, E. Imaging the Cell Morphological Response to 3D Topography and Curvature in Engineered Intestinal Tissues. Front. Bioeng. Biotechnol. 2020, 8, 294. [Google Scholar] [CrossRef]
- Yang, C.C.; Jenkins, L.; Burg, K.J.L. Adapted cryosectioning method for hydrogels used in regenerative medicine. J. Histotechnol. 2007, 30, 185–191. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Keskin, D.; Bat, E.; Vrana, N.E.; Tezcaner, A. Dual growth factor delivery using PLGA nanoparticles in silk fibroin/PEGDMA hydrogels for articular cartilage tissue engineering. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2020, 108, 2041–2062. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.; Woods, I.; Hibbitts, A.; Dervan, A.; O’Brien, F.J. The Manufacture and Characterization of Biomimetic, Biomaterial-Based Scaffolds for Studying Physicochemical Interactions of Neural Cells in 3D Environments. Curr. Protoc. 2023, 3, e688. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.; Kumbar, S.G.; Nukavarapu, S.P. Engineered Osteochondral Scaffolds with Bioactive Cartilage Zone for Enhanced Articular Cartilage Regeneration. Ann. Biomed. Eng. 2025, 53, 597–611. [Google Scholar] [CrossRef]
- Brown, D.A.; Yu, F.C.; Beygui, R.E.; Dunn, J.C.Y.; Wu, B.M. Gelatin-embedded cell-polymer constructs for histological cryosectioning. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2005, 72, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Siwczak, F.; Hiller, C.; Pfannkuche, H.; Schneider, M.R. Culture of vibrating microtome tissue slices as a 3D model in biomedical research. J. Biol. Eng. 2023, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Giavaresi, G.; Sartori, M.; Baleani, M.; Brogini, S.; Erani, P.; Dallari, D.; Del Piccolo, N.; Ghezzi, C.E.; Martini, L.; Parrilli, A.; et al. Assessment of the advantages and limitations of an innovative silk fibroin scaffold for the reconstruction of the anterior cruciate ligament with preclinical in vitro and in vivo evaluations. Biomater. Adv. 2025, 166, 214029. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, M.; Xu, B.; Zhang, J.; Zhao, Y.; Ji, S.; Wang, L.; Wang, L.; Li, X.; Kong, D.; et al. Integrated trilayered silk fibroin scaffold for osteochondral differentiation of adipose-derived stem cells. ACS Appl. Mater. Interfaces 2014, 6, 16696–16705. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Cuy, J.L.; Hauch, K.D.; Ratner, B.D. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials 2007, 28, 2978–2986. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Murray, B.; Carnachan, R.; Przyborski, S. Alvetex®: Polystyrene Scaffold Technology for Routine Three Dimensional Cell Culture. In 3D Cell Culture; Haycock, J.W., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 695, pp. 323–340. [Google Scholar] [CrossRef]
- Romero, I.S.; Schurr, M.L.; Lally, J.V.; Kotlik, M.Z.; Murphy, A.R. Enhancing the interface in silk-polypyrrole composites through chemical modification of silk fibroin. ACS Appl. Mater. Interfaces 2013, 5, 553–564. [Google Scholar] [CrossRef]
- Pei, Y.; Zheng, Y.; Li, Z.; Liu, J.; Zheng, X.; Tang, K.; Kaplan, D.L. Ethanol-induced coacervation in aqueous gelatin solution for constructing nanospheres and networks: Morphology, dynamics and thermal sensitivity. J. Colloid Interface Sci. 2021, 582, 610–618. [Google Scholar] [CrossRef]
- Mohanty, B.; Bohidar, H.B. Systematic of alcohol-induced simple coacervation in aqueous gelatin solutions. Biomacromolecules 2003, 4, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.; Pertici, G.; Moscato, S.; Rita Metelli, M.; Danti, S.; Nesti, C.; Berrettini, S.; Petrini, M.; Danti, S. Processing large-diameter poly(L-lactic acid) microfiber mesh/mesenchymal stromal cell constructs via resin embedding: An efficient histologic method. Biomed. Mater. 2014, 9, 045007. [Google Scholar] [CrossRef]
- Bordes, C.; Fréville, V.; Ruffin, E.; Marote, P.; Gauvrit, J.Y.; Briançon, S.; Lantéri, P. Determination of poly(ε-caprolactone) solubility parameters: Application to solvent substitution in a microencapsulation process. Int. J. Pharm. 2010, 383, 236–243. [Google Scholar] [CrossRef]
- Vonbrunn, E.; Mueller, M.; Pichlsberger, M.; Sundl, M.; Helmer, A.; Wallner, S.A.; Rinner, B.; Tuca, A.C.; Kamolz, L.P.; Brislinger, D.; et al. Electrospun PCL/PLA Scaffolds Are More Suitable Carriers of Placental Mesenchymal Stromal Cells Than Collagen/Elastin Scaffolds and Prevent Wound Contraction in a Mouse Model of Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 604123. [Google Scholar] [CrossRef]
- Langman, J.M. D-Limonene: Is it a safe, effective alternative to xylene? J. Histotechnol. 1995, 18, 131–137. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, T.H.; Im, G.I.; Lee, J.H. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold. Biomacromolecules 2010, 11, 1948–1955. [Google Scholar] [CrossRef]
- Chen, M.; Michaud, H.; Bhowmick, S. Controlled vacuum seeding as a means of generating uniform cellular distribution in electrospun polycaprolactone (PCL) scaffolds. J. Biomech. Eng. 2009, 131, 074521. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Suaidi, N.A.; Alshawsh, M.A.; Hoe, S.-Z.; Mokhtar, M.H.; Zin, S.R. Toxicological Effects of Technical Xylene Mixtures on the Female Reproductive System: A Systematic Review. Toxics 2022, 10, 235. [Google Scholar] [CrossRef]
- Indu, S.; Ramesh, V.; Indu, P.C.; Prashad, K.V.; Premalatha, B.; Ramadoss, K. Comparative efficacy of cedarwood oil and xylene in hematoxylin and eosin staining procedures: An experimental study. J. Nat. Sci. Biol. Med. 2014, 5, 284–287. [Google Scholar] [CrossRef]
- Thamilselvan, S.; Sherlin, H.; Jayaraj, G.; Don, K.; Santhanam, A. Cedarwood oil as an alternative to xylene as a clearing agent in histopathological tissue processing—A comparative study. J. Oral Maxillofac. Pathol. 2021, 25, 299–305. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Hjort, K.N.; Mellerup, I.; Sether, G.; Christensen, N. Vegetable oils instead of xylene in tissue processing. Apmis 1992, 100, 827–831. [Google Scholar] [CrossRef]
- Madhura, M.G.; Bhavana, V.S.; Kumar, B.V.; Suma, S.; Sarita, Y. Bleached vegetable oil as a suitable bio-safe alternative to xylene: An exploratory study. J. Adv. Clin. Res. Insights 2016, 3, 185–189. [Google Scholar] [CrossRef]
- Lyon, H.; Holm, I.; Prentø, P.; Balslev, E. Non-hazardous organic solvents in the paraffin-embedding technique: A rational approach—Aliphatic monoesters for clearing and dewaxing: Butyldecanoate. Histochem. Cell Biol. 1995, 103, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Sermadi, W.; Prabhu, S.; Acharya, S.; Javali, S.B. Comparing the efficacy of coconut oil and xylene as a clearing agent in the histopathology laboratory. J. Oral Maxillofac. Pathol. 2014, 18, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Swamy, S.R.G.; Nandan, S.R.K.; Kulkarni, P.G.; Rao, T.M.; Palakurthy, P. Bio-friendly alternatives for xylene—Carrot oil, olive oil, pine oil, rose oil. J. Clin. Diagn. Res. 2015, 9, ZC16–ZC18. [Google Scholar] [CrossRef] [PubMed]
- Andre, G.G.; Wenger, J.B.; Rebolloso, D.; Arrington, J.B.; Mehm, W.J. Evaluation of clearing and infiltration mixtures (cims) as xylene substitutes for tissue processing. J. Histotechnol. 1994, 17, 137–142. [Google Scholar] [CrossRef]
- Stockert, J.C.; López-Arias, B.; Del Castillo, P.; Romero, A.; Blázquez-Castro, A. Replacing xylene with n-heptane for paraffin embedding. Biotech. Histochem. 2012, 87, 464–467. [Google Scholar] [CrossRef]
- Chen, C.Y.; He, T.; Mao, X.L.; Friis, T.E.; Qin, R.H.; Jian, Y.T. A novel xylene substitute for histotechnology and histochemistry. Biotech. Histochem. 2010, 85, 231–240. [Google Scholar] [CrossRef]
- Buesa, R.J.; Peshkov, M.V. Histology without xylene. Ann. Diagn. Pathol. 2009, 13, 246–256. [Google Scholar] [CrossRef]
- Adediran, O.; Ibikunle, D. Evaluation of Isopropanol as a Better Alternative to Xylene in Tissue Processing by the Paraffin Wax Method. Afr. J. Cell. Pathol. 2016, 6, 54–59. [Google Scholar]
- Metgud, R.; Astekar, M.S.; Soni, A.; Naik, S.; Vanishree, M. Conventional xylene and xylene-free methods for routine histopathological preparation of tissue sections. Biotech. Histochem. 2013, 88, 235–241. [Google Scholar] [CrossRef]
- Ninan, N.; Joseph, B.; Visalakshan, R.M.; Bright, R.; Denoual, C.; Zilm, P.; Dalvi, Y.B.; Priya, P.V.; Mathew, A.; Grohens, Y.; et al. Plasma assisted design of biocompatible 3D printed PCL/silver nanoparticle scaffolds: In vitro and in vivo analyses. Mater. Adv. 2021, 2, 6620–6630. [Google Scholar] [CrossRef]
- Choi, K.H.; Choi, B.H.; Park, S.R.; Kim, B.J.; Min, B.H. The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials 2010, 31, 5355–5365. [Google Scholar] [CrossRef]
- Erisken, C.; Kalyon, D.M.; Wang, H.; Örnek-Ballanco, C.; Xu, J. Osteochondral tissue formation through adipose-derived stromal cell differentiation on biomimetic polycaprolactone nanofibrous scaffolds with graded insulin and beta-glycerophosphate concentrations. Tissue Eng.—Part A 2011, 17, 1239–1252. [Google Scholar] [CrossRef]
- Loebsack, A.B.; Halberstadt, C.R.; Holder, W.D.; Culberson, C.R.; Beiler, R.J.; Greene, K.G.; Roland, W.D.; Burg, K.J.L. The development of an embedding technique for polylactide sponges. J. Biomed. Mater. Res. 1999, 48, 504–510. [Google Scholar] [CrossRef]
- Burg, K.J.L.; Jenkins, L.; Powers, D.L.; Shalaby, S.W. Special considerations in embedding a lactide absorbable polymer. J. Histotechnol. 1996, 19, 39–43. [Google Scholar] [CrossRef]
- Balguid, A.; Mol, A.; Van Marion, M.H.; Bank, R.A.; Bouten, C.V.C.; Baaijens, F.P.T. Tailoring fiber diameter in electrospun poly(ε-Caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng.—Part A 2009, 15, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Joiner, K.S.; Spangler, E.A. Evaluation of HistoGelTM-embedded specimens for use in veterinary diagnostic pathology. J. Vet. Diagn. Investig. 2012, 24, 710–715. [Google Scholar] [CrossRef]
- Kmecick, M.; Vieira Da Costa, M.C.; Ferreira, E.D.C.; Prodocimo, M.M.; Ortolani-Machado, C.F. Critical Evaluation of Embedding Media for Histological Studies of Early Stages of Chick Embryo Development. Methods Protoc. 2023, 6, 38. [Google Scholar] [CrossRef]
- Kiernan, J.A. Strategies for Preventing Detachment of Sections from Glass Slides. Microsc. Today 1999, 7, 20–24. [Google Scholar] [CrossRef][Green Version]
- Leitão, C.A.E. Working Optimally with Serial Sections in Glycol Methacrylate Resin. Braz. Arch. Biol. Technol. 2018, 61, e18180103. [Google Scholar] [CrossRef]
- Marudova, M.; Viraneva, A.; Antova, G.; Nikolova, K.; Petkova, Z.; Teneva, O. Physico-Chemical Characterization of Sesame/Rapeseed Oil Mixtures. Appl. Sci. 2025, 15, 704. [Google Scholar] [CrossRef]
- Yang, K.C.; Chen, I.H.; Yang, Y.T.; Hsiao, J.K.; Wang, C.C. Effects of scaffold geometry on chondrogenic differentiation of adipose-derived stem cells. Mater. Sci. Eng. C 2020, 110, 110733. [Google Scholar] [CrossRef] [PubMed]
- Sithara, K.; Prasad, B.G.; Desai, D. A Comparative Study of the Efficacy of Cedarwood Oil, Coconut Oil and Dish Wash Liquid As Alternatives To Xylene As Deparaffinizing Agents. Paripex Indian J. Res. 2021, 10, 33–35. [Google Scholar]
- Lozano, M.D.; Argueta, A.; Robledano, R.; García, J.; Ocon, V.; Gómez, N.; Fernandez, N. Practical issues related to immunocytochemistry on cytological smears: Tips and recommendations. Cytopathology 2024, 35, 761–769. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S. V Gelatin Based Scaffolds for Tissue Engineering—A Review. Polym. Res. J. 2014, 9, 15–32. [Google Scholar]
- Liu, J.; Nie, H.; Xu, Z.; Niu, X.; Guo, S.; Yin, J.; Guo, F.; Li, G.; Wang, Y.; Zhang, C. The effect of 3d nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PLoS ONE 2014, 9, e111566. [Google Scholar] [CrossRef] [PubMed]
- Čiužas, D.; Krugly, E.; Petrikaitė, V. Fibrous 3D printed poly(ε)caprolactone tissue engineering scaffold for in vitro cell models. Biochem. Eng. J. 2022, 185, 108531. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Y.; Xuan, C.; Liu, L.; Lai, C.; Chai, M.; Zhang, Z.; Wang, L.; Shi, X. A Biomimetic Biphasic Osteochondral Scaffold with Layer-Specific Release of Stem Cell Differentiation Inducers for the Reconstruction of Osteochondral Defects. Adv. Healthc. Mater. 2020, 9, 2000076. [Google Scholar] [CrossRef]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Puppi, D.; Morelli, A.; Chiellini, F. Additive manufacturing of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) blend scaffolds for tissue engineering. Bioengineering 2017, 4, 49. [Google Scholar] [CrossRef]
- Li, W.J.; Danielson, K.G.; Alexander, P.G.; Tuan, R.S. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(ε-caprolactone) scaffolds. J. Biomed. Mater. Res.—Part A 2003, 67, 1105–1114. [Google Scholar] [CrossRef] [PubMed]

| Processing Step | Commonly Used Reagent | Alternative Reagents/Means | Scaffold Material | References |
|---|---|---|---|---|
| Frozen samples | ||||
| Cryoprotection | Gluc, sucrose | 5% Gluc, 20% Gluc, 2:1 Gluc:O.C.T., 1:1 Gluc:O.C.T., 1:2 Gluc:O.C.T. | Collagen | [43] |
| 15% sucrose, 30% sucrose | PEGDMA | [44] | ||
| 100% FBS | PEG | [36] | ||
| 30% BSA | PEG | [36] | ||
| 1% PVA | PEG | [36] | ||
| 10% PVA + vacuum | PLGA | [14] | ||
| O.C.T. | PEG | [36] | ||
| PCL/PEO | [37] | |||
| Fisher cryogel | PEG | [36] | ||
| Low-molecular-weight PEGDA | PEGDA | [42] | ||
| Formalin-Fixed Paraffin-Embedded samples | ||||
| Dehydration | Ethanol | Acetone | PLLA | [57] |
| Clearing | Xylene | Automatic tissue processor | PCL/PLA | [59] |
| HistoClear® | PCL | [16] | ||
| Impregnation/embedding | Paraffin (melting point ~60 °C) | GMA + vacuum | [13] | |
| GMA | PCL | [80] | ||
| PLA | [81,82] | |||
| PLLA | [57] | |||
| Technovit® 7100 | PCL | [83] | ||
| Low-melting paraffin | PCL/PLA | [15] | ||
| 10% gelatin, 25% gelatin | PCL/PLA | [15] | ||
| Deparaffinization | Xylene | HistoClear® | PCL | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragauskas, T.; Uzieliene, I.; Bernotiene, E. Histological Processing of Scaffolds: Challenges and Solutions. J. Funct. Biomater. 2025, 16, 279. https://doi.org/10.3390/jfb16080279
Ragauskas T, Uzieliene I, Bernotiene E. Histological Processing of Scaffolds: Challenges and Solutions. Journal of Functional Biomaterials. 2025; 16(8):279. https://doi.org/10.3390/jfb16080279
Chicago/Turabian StyleRagauskas, Tomas, Ilona Uzieliene, and Eiva Bernotiene. 2025. "Histological Processing of Scaffolds: Challenges and Solutions" Journal of Functional Biomaterials 16, no. 8: 279. https://doi.org/10.3390/jfb16080279
APA StyleRagauskas, T., Uzieliene, I., & Bernotiene, E. (2025). Histological Processing of Scaffolds: Challenges and Solutions. Journal of Functional Biomaterials, 16(8), 279. https://doi.org/10.3390/jfb16080279








