Propolis as an Adjunct in Non-Surgical Periodontal Therapy: Current Clinical Perspectives from a Narrative Review
Abstract
1. Introduction
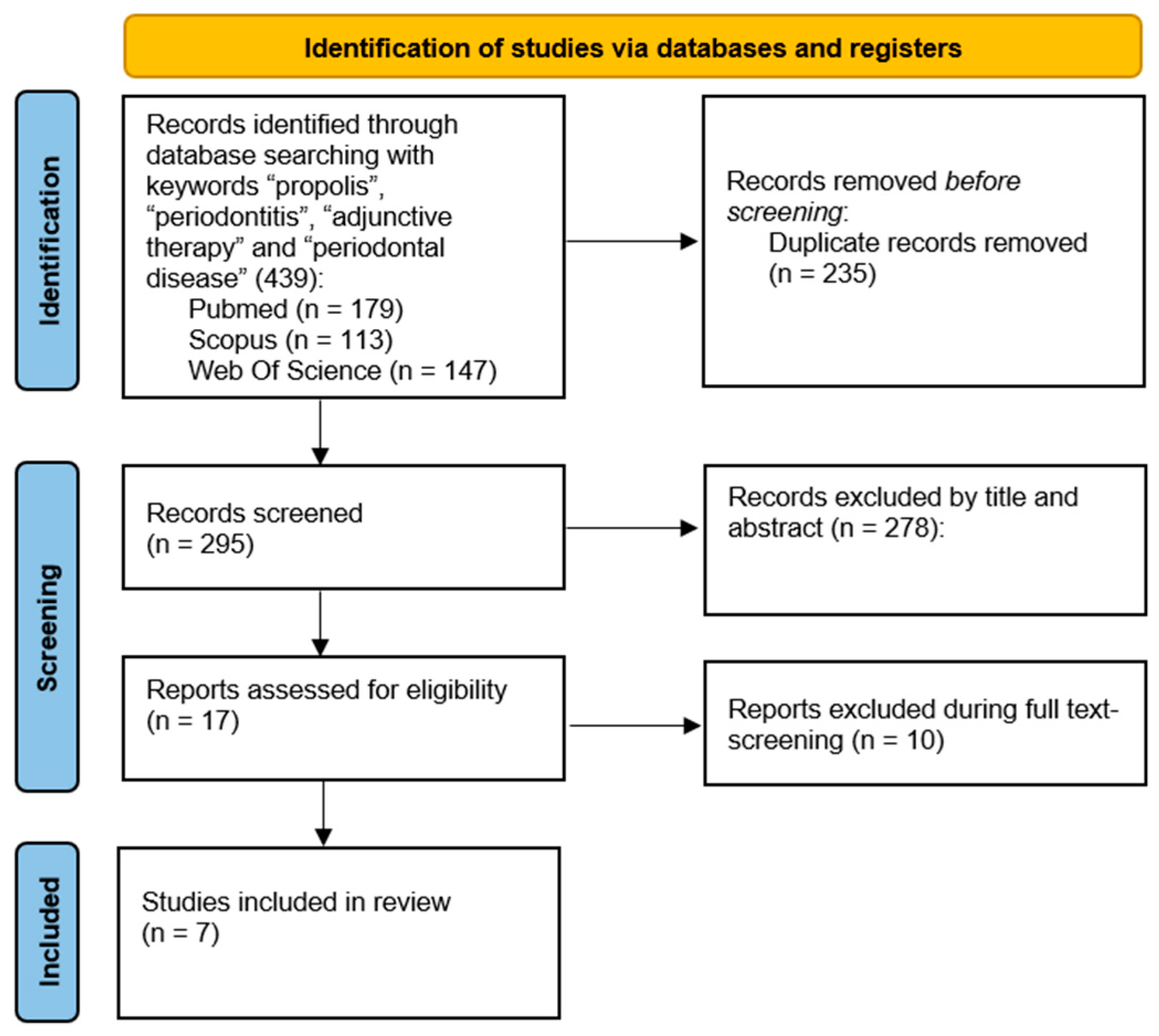
2. Materials and Methods
3. Results
| Authors (Year)—Country | Study Design | Setting | N. of Patients | Gender (M/F) | Mean Age | Type of Periodontitis | Treatment | Results (PD—CAL) | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Sanghani N. et al. (2014)—India [47] | RCT | University | 20 | 9 M/11 F | 35.60 ± 12.20 | Chronic Periodontitis | Test Group: SRP + subgingival administration of propolis (~5 mg) Control Group: SRP | Test Group PD Baseline: 5.35 ± 0.67 15 days: 4.60 ± 0.68 1 month: 3.60 ± 0.68 CAL Baseline: 3.35 ± 0.67 15 days: 2.10 ± 0.79 1 month: 1.60 ± 0.68 Control Group PD Baseline: 5.10 ± 0.55 15 days: 4.55 ± 0.83 1 month: 3.75 ± 0.79 CAL Baseline: 3.10 ± 0.55 15 days: 2.55 ± 0.83 1 month: 1.75 ± 0.79 | 15 days 1 month |
| De Andrade D.P. et al. (2017)—Brazil [43] | RCT | University | 16 | 10 M/6 F | Test group: 50.22 ± 7.75 Control group: 48.00 ± 9.10 | Chronic Periodontitis | Test Group: SRP + subgingival irrigation with a hydroalcoholic solution of propolis extract 20%; second irrigation after 15 days Control Group: SRP + subgingival irrigation with a saline solution; second irrigation after 15 days | Test Group PD change Baseline–45 days: 1.42 ± 1.37 Baseline–75 days: 1.48 ± 1.39 Baseline–90 days: 1.50 ± 1.40 45 days–75 days: 0.06 ± 0.72 45 days–90 days: 0.08 ± 0.78 75 days–90 days: 0.02 ± 0.55 Control Group PD change Baseline–45 days: 1.13 ± 1.29 Baseline–75 days: 1.23 ± 1.34 Baseline–90 days: 1.30 ± 1.41 45 days–75 days: 0.09 ± 0.69 45 days–90 days: 0.16 ± 0.83 75 days–90 days: 0.07 ± 0.57 | 45 days 75 days 90 days |
| Pundir A. et al. (2017)—India [44] | RCT | University | 30 | N.A. | 25–55 | Chronic Periodontitis | Test Group: SRP + one-stage full mouth disinfection (brushing the dorsum of the tongue for 60 s + rinsing the mouth twice for 1 min with propolis solution + subgingival irrigation with 20% propolis hydroalcoholic solution) 24 h after SRP Control Group: SRP | Test Group PD Baseline: 5.87 ± 0.92 4 weeks: 3.87 ± 0.92 12 weeks: 3.87 ± 0.92 CAL Baseline: 3.87 ± 0.92 4 weeks: 1.47 ± 1.51 12 weeks: 1.47 ± 1.51 Control Group PD Baseline: 5.53 ± 0.52 4 weeks: 4.53 ± 0.52 12 weeks: 4.53 ± 0.52 CAL Baseline: 3.53 ± 0.52 4 weeks: 2.53 ± 0.52 12 weeks: 2.53 ± 0.52 | 4 weeks 12 weeks |
| Seth T. et al. (2022)—India [46] | RCT | University | 20 | N.A. | 18–55 | Mild/Moderate Periodontitis | Test Group: Single-phase full-mouth SRP + subgingival irrigation for 30 s for 5 mL with 25% propolis extract. Subgingival irrigation repeated on the 7th and 15th day from the day of first application Control Group: Single-phase full-mouth SRP + subgingival irrigation for 30 s for 5 mL with 0.2% chlorhexidine. Subgingival irrigation repeated on the 7th and15th day from the day of first application | Test Group PD Baseline: 6.20 ± 1.00 15 days: 5.05 ± 1.14 30 days: 4.10 ± 1.16 Control Group PD Baseline 6.50 ± 1.05 15 days: 4.95 ± 1.09 30 days: 3.65 ± 1.18 | 15 days 30 days |
| Sahu S. et al. (2023)—India [45] | RCT | University | 40 | 30 M/10 F | Test group: 49.90 ± 11.60 Control group: 50.10 ± 12.20 | Generalised Stage II-III | Test Group: SRP + subgingival administration of propolis nanoparticle solution + pocket sealing with cyanoacrylate Control Group: SRP + subgingival administration of saline solution + pocket sealing with cyanoacrylate | Test Group * PD Baseline: 4.90 (4.00–6.65) 1 month: 2.90 (2.00–4.65) 3 months: 2.45 (2.00–3.20) CAL Baseline: 9.65 (8.95–11.30) 1 month: 7.65 (6.55–9.95) 3 months: 7.20 (6.75–9.95) Control Group * PD Baseline: 4.90 (4.00–6.00) 1 month: 3.30 (3.00–5.00) 3 months: 3.15 (2.00–3.00) CAL Baseline: 9.55 (8.00–11.55) 1 month: 7.95 (6.00–10.00) 3 months: 7.80 (6.00–9.00) | 1 month 3 months |
| Aggarwal R. et al. (2023)—India [48] | RCT | University | 30 | N.A. | N.A. | Moderate/Severe Chronic Periodontitis | Test group 1: SRP + application in periodontal pocket of propolis gel Test group 2: SRP + diode laser (set at 1.5 W, 940 nm, 30 s, continuous wave) into the periodontal pocket Control group: SRP | Test Group 1 PD 1 month: 4.22 ± 0.59 3 months: 3.93 ± 0.63 CAL 1 month: 1.92 ± 0.40 3 months: 1.67 ± 0.40 Test Group 2 PD 1 month: 4.04 ± 0.48 3 months: 2.77 ± 0.60 CAL 1 month: 0.97 ± 0.11 3 months: 1.26 ± 0.2 Control Group PD 1 month: 4.40 ± 0.54 3 months: 3.58 ± 0.53 CAL 1 month: 1.43 ± 0.29 3 months: 1.12 ± 0.50 | 1 month 3 months |
| Waqar et al. (2024)—Pakistan [49] | RCT | University | 100 | 0 M/100 F | N.A. | Chronic Periodontitis (Stage I–II) | Test Group: SRP + 20% propolis mouthwash twice daily for six weeks Control Group: SRP + 0.2% chlorhexidine mouthwash twice daily for six weeks | Test Group PD Baseline: 4.67 (4.56–4.89) 6 weeks: 4.01 (3.72–4.15) 12 weeks: 3.59 (3.28–3.92) CAL Baseline: 4.45 ± 0.73 6 weeks: 4.15 ± 0.57 12 weeks: 3.77 ± 0.51 Control Group PD Baseline: 4.65 (4.43–4.89) 6 weeks: 4.44 (4.16–4.63) 12 weeks: 4.25 (4.02–4.48) CAL Baseline: 4.80 ± 0.70 6 weeks: 4.50 ± 0.61 12 weeks: 4.19 ± 0.56 | 6 weeks 12 weeks |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSPT | Non-Surgical Periodontal Therapy |
| RCT | Randomised Controlled Trial |
| SRP | Scaling and Root Planing |
| PD | Probing Depth |
| CAL | Clinical Attachment Level |
| BoP | Bleeding on Probing |
| OSFMD | One-Stage Full Mouth Disinfection |
| GI | Gingival Index |
| PI | Plaque Index |
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Merete Aass, A.; Aimetti, M.; et al. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Tooth Loss: A Systematic Review and Meta-Analysis. J. Dent. Res. 2014, 93, 20S–28S. [Google Scholar] [CrossRef] [PubMed]
- Raittio, E.; Leite, F.R.M.; Machado, V.; Botelho, J.; Nascimento, G.G. Do All Individuals Benefit Equally from Non-Surgical Periodontal Therapy? Secondary Analyses of Systematic Review Data. J. Periodontal Res. 2024, 60, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Association between Periodontal Diseases and Cardiovascular Diseases, Diabetes and Respiratory Diseases: Consensus Report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European Arm of the World Organization of Family Doctors (WONCA Europe). J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Tartaglia, G.M.; Santonocito, S.; Polizzi, A.; Williams, R.C.; Iorio-Siciliano, V. Impact of N-Terminal pro-B-Type Natriuretic Peptide and Related Inflammatory Biomarkers on Periodontal Treatment Outcomes in Patients with Periodontitis: An Explorative Human Randomized-Controlled Clinical Trial. J. Periodontol. 2023, 94, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, G.A.; Dekkers, G.J.; Slot, D.E. Success of Non-Surgical Periodontal Therapy in Adult Periodontitis Patients: A Retrospective Analysis. Int. J. Dent. Hyg. 2019, 17, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival Instrumentation for Treatment of Periodontitis. A Systematic Review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilho, R.M.; Sanz, M.; Messora, M.R. New Tendencies in Non-Surgical Periodontal Therapy. Braz. Oral Res. 2021, 35, e095. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Siciliano, V.; Blasi, A.; Mauriello, L.; Salvi, G.E.; Ramaglia, L.; Sculean, A. Non-Surgical Treatment of Moderate Periodontal Intrabony Defects With Adjunctive Cross-Linked Hyaluronic Acid: A Single-Blinded Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2025, 52, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Kardaras, G.; Marcovici, I.; Rusu, D.; Dehelean, C.; Coricovac, D.; Iorio-Siciliano, V.; Sculean, A.; Stratul, S.-I. In-Vitro Safety Evaluation of Sodium Hypochlorite (NaOCl) as Part of Step 2 and Maintenance Therapy Protocols in Patients with Periodontitis Stages III-IV. Oral Health Prev. Dent. 2023, 21, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, V.; Boariu, M.I.; Rusu, D.; Roman, A.; Surlin, P.; Voicu, A.; Didilescu, A.C.; Jentsch, H.; Siciliano, V.I.; Ramaglia, L.; et al. Clinical and Microbiological Effects of a Single Application of Sodium Hypochlorite Gel during Subgingival Re-Instrumentation: A Triple-Blind Randomized Placebo-Controlled Clinical Trial. Clin. Oral Investig. 2022, 26, 6639–6652. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Siciliano, V.; Ramaglia, L.; Isola, G.; Blasi, A.; Salvi, G.E.; Sculean, A. Changes in Clinical Parameters Following Adjunctive Local Sodium Hypochlorite Gel in Minimally Invasive Nonsurgical Therapy (MINST) of Periodontal Pockets: A 6-Month Randomized Controlled Clinical Trial. Clin. Oral Investig. 2021, 25, 5331–5340. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Gou, J.; Yin, T.; He, H.; et al. Local Drug Delivery Systems as Therapeutic Strategies against Periodontitis: A Systematic Review. J. Control. Release 2021, 333, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Iorio-Siciliano, V.; Alibrandi, A.; Ramaglia, L.; Leonardi, R. Effectiveness of a Nutraceutical Agent in the Non-Surgical Periodontal Therapy: A Randomized, Controlled Clinical Trial. Clin. Oral Investig. 2021, 25, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Gawish, A.S.; ElMofty, M.S.; Jambi, S.; Felemban, D.; Ragheb, Y.S.E.; Elsayed, S.A. Phytotherapy in Periodontics as an Effective and Sustainable Supplemental Treatment: A Narrative Review. J. Periodontal Implant. Sci. 2024, 54, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Bonsignore, G.; Ranzato, E. Propolis: A Natural Substance with Multifaceted Properties and Activities. Int. J. Mol. Sci. 2025, 26, 1519. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- El-Sakhawy, M.; Salama, A.; Tohamy, H.A.S. Applications of Propolis-Based Materials in Wound Healing. Arch. Dermatol. Res. 2024, 316, 61. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-S.; Xie, H.-Q.; Li, C.-G.; You, M.-M.; Zheng, Y.-F.; Li, G.Q.; Chen, X.; Zhang, C.-P.; Hu, F.-L. Chinese Propolis Inhibits the Proliferation of Human Gastric Cancer Cells by Inducing Apoptosis and Cell Cycle Arrest. Evid. Based Complement. Altern. Med. 2020, 2020, 2743058. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, O.K.; Calder, P.C. The Effect of Propolis and Its Components on Eicosanoid Production during the Inflammatory Response. Prostaglandins Leukot. Essent. Fatty Acids 1996, 55, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Franchin, M.; Cólon, D.F.; Castanheira, F.V.S.; da Cunha, M.G.; Bueno-Silva, B.; Alencar, S.M.; Cunha, T.M.; Rosalen, P.L. Vestitol Isolated from Brazilian Red Propolis Inhibits Neutrophils Migration in the Inflammatory Process: Elucidation of the Mechanism of Action. J. Nat. Prod. 2016, 79, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S. Propolis as a Novel Antibacterial Agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Bouchelaghem, S. Propolis Characterization and Antimicrobial Activities against Staphylococcus Aureus and Candida Albicans: A Review. Saudi J. Biol. Sci. 2022, 29, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.S.; De Mendonça, S.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Lagareiro Netto, A.A.; Lira, I.S.; López, B.G.-C.; Negrão, V.; Marcucci, M.C. Artepillin C and Phenolic Compounds Responsible for Antimicrobial and Antioxidant Activity of Green Propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Zafar, M.S.; Najeeb, S.; Zohaib, S. Propolis: A Natural Biomaterial for Dental and Oral Healthcare. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 265–274. [Google Scholar] [CrossRef]
- Natarajan, K.; Singh, S.; Burke, T.R.; Grunberger, D.; Aggarwal, B.B. Caffeic Acid Phenethyl Ester Is a Potent and Specific Inhibitor of Activation of Nuclear Transcription Factor NF-Kappa B. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Orban, Z.; Mitsiades, N.; Burke, T.R., Jr.; Tsokos, M.; Chrousos, G.P. Caffeic Acid Phenethyl Ester Induces Leukocyte Apoptosis, Modulates Nuclear Factor-Kappa B and Suppresses Acute Inflammation. Neuroimmunomodulation 2000, 7, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cai, Y.; Chen, X.; Ji, T.; Sun, L. Optimized Extraction Based on the Terpenoids of Heterotrigona Itama Propolis and Their Antioxidative and Anti-inflammatory Activities. J. Food Biochem. 2020, 44, e13296. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Muninathan, N.; Megalatha, S.T.; Suresh, A.; Kumar, K.S.; Jhansi, N.; Kalaivani, K.; Krishnamoorthy, G. An Insight into Anticancer Effect of Propolis and Its Constituents: A Review of Molecular Mechanisms. Evid.-Based Complement. Altern. Med. 2022, 2022, 5901191. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Neto, E.M.; Valadas, L.A.R.; Lobo, P.L.D.; Fonseca, S.G.D.C.; Fechine, F.V.; Lotif, M.A.L.; Bandeira, M.A.M.; Mendonça, J.F.; de Mendonça, K.M.; Fonteles, M.M.D.F.; et al. Antimicrobial Efficacy of Propolis-Containing Varnish in Children: A Randomized and Double-Blind Clinical Trial. Evid.-Based Complement. Altern. Med. 2021, 2021, 5547081. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, Z.; Naseri, M.; Jalili, M.; Mansouri, Y.; Mashhadiabbas, F.; Torkaman, A. Effect of Propolis on Dentin Regeneration and the Potential Role of Dental Pulp Stem Cell in Guinea Pigs. Cell J. 2012, 13, 223–228. [Google Scholar] [PubMed]
- Alghutaimel, H. Endodontic Applications of Propolis in Primary and Permanent Teeth: A Scoping Review of Clinical Studies. Eur. Endod. J. 2024, 9, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Samet, N.; Laurent, C.; Susarla, S.M.; Samet-Rubinsteen, N. The Effect of Bee Propolis on Recurrent Aphthous Stomatitis: A Pilot Study. Clin. Oral Investig. 2007, 11, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Khabazian, A.; Mirhashemi, F.S.; Sadeghi, F. Investigating the Effect of Propolis-Containing Chewing Gum in Comparison with Propolis-Containing Mouthwash on Reducing Gingival Inflammation in Patients with Gingivitis. BMC Oral Health 2025, 25, 231. [Google Scholar] [CrossRef] [PubMed]
- Ballouk, M.A.-H.; Altinawi, M.; Al-Kafri, A.; Zeitounlouian, T.S.; Fudalej, P.S. Propolis Mouthwashes Efficacy in Managing Gingivitis and Periodontitis: A Systematic Review of the Latest Findings. BDJ Open 2025, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Gebara, E.C.E.; Lima, L.A.; Mayer, M.P.A. Propolis Antimicrobial Activity against Periodontopathic Bacteria. Braz. J. Microbiol. 2002, 33, 365–369. [Google Scholar] [CrossRef]
- Yoshimasu, Y.; Ikeda, T.; Sakai, N.; Yagi, A.; Hirayama, S.; Morinaga, Y.; Furukawa, S.; Nakao, R. Rapid Bactericidal Action of Propolis against Porphyromonas Gingivalis. J. Dent. Res. 2018, 97, 928–936. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, D.P.; Carvanho, I.C.; Gadoi, B.H.; Rosa, L.C.L.; Barreto, L.M.R.C.; Pallos, D. Subgingival Irrigation with a Solution of 20% Propolis Extract as an Adjunct to Non-Surgical Periodontal Treatment: A Preliminary Study. J. Int. Acad. Periodontol. 2017, 19, 145–151. [Google Scholar]
- Pundir, A.; Vishwanath, A.; Pundir, S.; Swati, M.; Banchhor, S.; Jabee, S. One-Stage Full Mouth Disinfection Using 20% Propolis Hydroalcoholic Solution: A Clinico-Microbiologic Study. Contemp. Clin. Dent. 2017, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.A.; Panda, S.; Das, A.C.; Mishra, L.; Rath, S.; Sokolowski, K.; Kumar, M.; Mohanty, R.; Nayak, R.; Satpathy, A.; et al. Efficacy of Sub-Gingivally Delivered Propolis Nanoparticle in Non-Surgical Management of Periodontal Pocket: A Randomized Clinical Trial. Biomolecules 2023, 13, 1576. [Google Scholar] [CrossRef] [PubMed]
- Seth, T.; Kale, T.; Lendhey, S.; Bhalerao, P. Comparative Evaluation of Subgingival Irrigation with Propolis Extract versus Chlorhexidine as an Adjunct to Scaling and Root Planing for the Treatment of Chronic Periodontitis: A Randomized Controlled Trial. J. Indian Soc. Periodontol. 2022, 26, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Sanghani, N.N.; Shivaprasad, B.M.; Savita, S. Health from the Hive: Propolis as an Adjuvant in the Treatment of Chronic Periodontitis—A Clinicomicrobiologic Study. J. Clin. Diagn. Res. 2014, 8, ZC41–ZC44. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Bawa, S.S.; Palwankar, P.; Kaur, S.; Choudhary, D.; Kochar, D. To Evaluate the Clinical Efficacy of 940 Nm Diode Laser and Propolis Gel (A Natural Product) in Adjunct to Scaling and Root Planing in Treatment of Chronic Periodontitis. J. Pharm. Bioallied Sci. 2023, 15, S1218–S1220. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.M.; Razi, A.; Qureshi, S.S.; Saher, F.; Zaidi, S.J.A.; Kumar, C. Comparative Evaluation of Propolis Mouthwash with 0.2% Chlorhexidine Mouthwash as an Adjunct to Mechanical Therapy in Improving the Periodontitis among Perimenopausal Women: A Randomized Controlled Trial. BMC Oral Health 2024, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, G. Clinical versus Statistical Significance as They Relate to the Efficacy of Periodontal Therapy. J. Am. Dent. Assoc. 2003, 134, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzella, V.; Cuozzo, A.; Mauriello, L.; Polizzi, A.; Iorio Siciliano, V.; Ramaglia, L.; Blasi, A. Propolis as an Adjunct in Non-Surgical Periodontal Therapy: Current Clinical Perspectives from a Narrative Review. J. Funct. Biomater. 2025, 16, 265. https://doi.org/10.3390/jfb16070265
Pezzella V, Cuozzo A, Mauriello L, Polizzi A, Iorio Siciliano V, Ramaglia L, Blasi A. Propolis as an Adjunct in Non-Surgical Periodontal Therapy: Current Clinical Perspectives from a Narrative Review. Journal of Functional Biomaterials. 2025; 16(7):265. https://doi.org/10.3390/jfb16070265
Chicago/Turabian StylePezzella, Vitolante, Alessandro Cuozzo, Leopoldo Mauriello, Alessandro Polizzi, Vincenzo Iorio Siciliano, Luca Ramaglia, and Andrea Blasi. 2025. "Propolis as an Adjunct in Non-Surgical Periodontal Therapy: Current Clinical Perspectives from a Narrative Review" Journal of Functional Biomaterials 16, no. 7: 265. https://doi.org/10.3390/jfb16070265
APA StylePezzella, V., Cuozzo, A., Mauriello, L., Polizzi, A., Iorio Siciliano, V., Ramaglia, L., & Blasi, A. (2025). Propolis as an Adjunct in Non-Surgical Periodontal Therapy: Current Clinical Perspectives from a Narrative Review. Journal of Functional Biomaterials, 16(7), 265. https://doi.org/10.3390/jfb16070265






