A Novel Self-Expanding Transcatheter Mitral Valve with Dual Annulus/Valve Diameter
Abstract
1. Introduction
- -
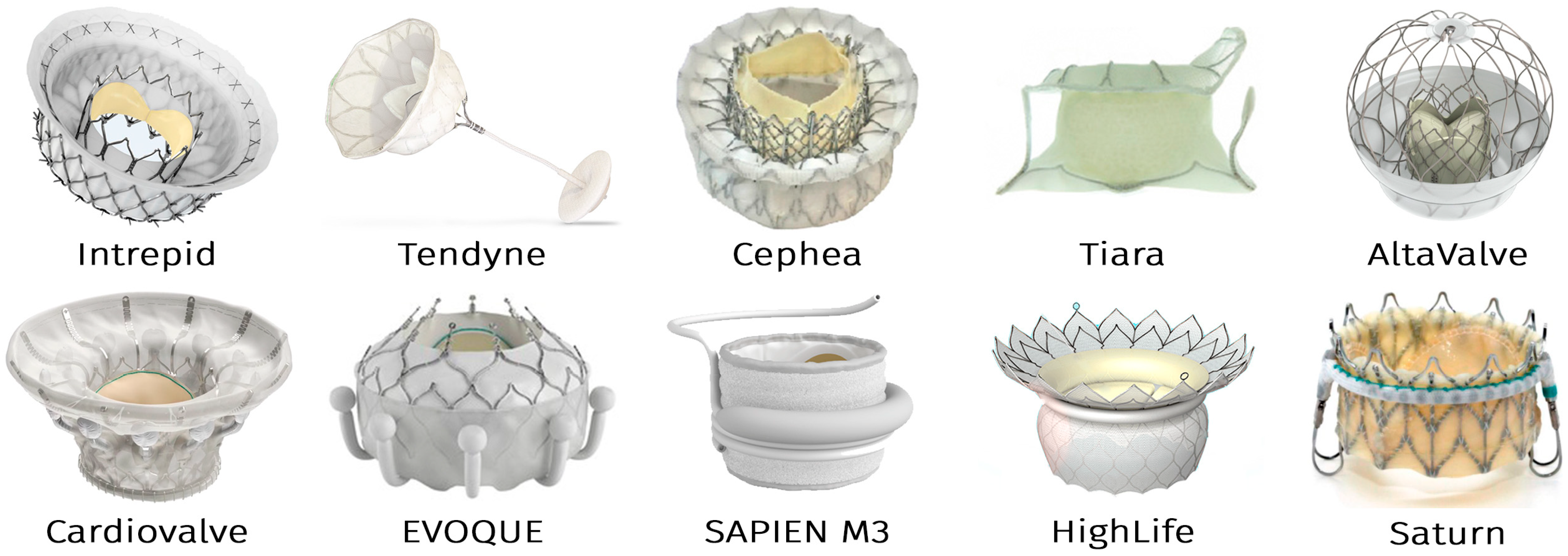
- Intrepid (Medtronic)
- -
- Tendyne and Cephea (Abbott Vascular)
- -
- Tiara (NeoVasc)
- -
- AltaValve (4C Medical)
- -
- Cardiovalve (Cardiovalve Ltd.)
- -
- EVOQUE (formerly CardiAQ) and SAPIEN M3 (Edwards Lifesciences)
- -
- HighLife (HighLife Medical)
- -
- Saturn (InnovHeart)
- Apical tethering with an epicardial pad;
- Atrial winglets or subannular piercing hooks;
- Stent elements grasping native mitral leaflets;
- Atrial and ventricular segments clamping leaflets/annulus;
- Two-component systems with external docking elements;
- Internal anchoring along the left atrial circumference (AltaValve).
- -
- Single-component stent laser-cut from a monolithic segment of the NiTi tube;
- -
- Dual-diameter central zone: a valve containing a smaller-diameter segment and a larger-diameter segment anchoring within the mitral annulus.
- -
- This design aims to balance the competing demands of:
- -
- Low-profile crimping (enabling transseptal delivery via reduced delivery system dimensions);
- -
- Secure annular anchoring (ensuring prosthesis stability under dynamic cardiac forces).
2. Materials and Methods
2.1. Stent Design Requirements
- -
- The bioprosthesis can be implanted using the transapical or endovascular transseptal approach; therefore, it must be packaged in a delivery system with an external diameter of no more than 24 Fr.
- -
- To prevent bioprosthetic dislocation to both the left ventricle and left atrium, its stent should have 3 reliable anchoring zones: in the patient’s mitral valve annulus, in the LV, and in the LA.
- -
- In order to reduce the cross-sectional area of the valve, the stent must have two concentric elements in the mitral annulus area. The outer one, of larger diameter, fixing in the mitral valve annulus, must have elastic deformability to match annular dynamics during the cardiac cycle. The inner concentric element must carry the valve itself and maintain valve circularity with a diameter of 28–30 mm, regardless of the deformations of the mitral annulus.
- -
- The design of the bioprosthesis as a whole should not provoke the left ventricle outflow tract (LVOT) obstruction, regardless of the anatomy of the patient’s left heart; the laser-cut template must be unified but adaptable to annular sizes (40–48 mm) with minimal post-shaping deviations of critical dimensional parameters for all stent sizes.
2.2. Stent Engineering and Manufacturing
2.3. Biomaterial Mounting
2.4. Crimping and Loading into the Delivery System
2.5. Pulsatile-Flow Testing
3. Results
3.1. Implementation of Design Parameters
3.2. Valve Modeling and Biomaterial Optimization
3.3. Implementation of TMV Prototypes
- Laser cutting of nitinol tubes;
- Thermal shaping and post-processing of stents;
- Biomaterial fixation onto stents (Figure 5).
3.4. Delivery System Compatibility Assessment
3.5. Hemodynamic Performance Evaluation
4. Discussion
- -
- The Tendyne valve is the most often used by surgeons and interventional cardiologists. There are two reasons for this. Firstly, this valve was the first to receive the CE Mark. Secondly, it seems more reliable to surgeons due to its additional anchoring mechanism: apical tethering with an epicardial pad.
- -
- Technical success of the implantation procedure is quite high and amounts to 95–100%, and this indicator depends to a lesser extent on the valve model and to a greater extent on the experience of the clinic. Such high indicators indicate good engineering of the entire “valve + delivery device” system.
- -
- Despite the fact that all dedicated transcatheter valves correct MR well, 1-year cardiovascular mortality remains high: 17–20%. According to Ludwig et al. [36], 2-year all-cause mortality is 38%, with similar values for Tendyne and other studied valves.
5. Study Limitations
6. Conclusions
- Our findings confirm the feasibility of a single-component, dual-diameter TMV stent, offering a promising solution for high-risk patients with mitral regurgitation.
- Biological material can be fixed to these stents with no risk of deformation or excessive suture-line stress during valve straightening into a tubular shape for 22 Fr delivery system loading.
- The developed valves have a dimensional geometry suitable for implantation in the mitral position and demonstrate adequate opening/closing function in the pulse duplicator.
- Before the next iteration of testing, further stent architecture optimization is required:
- -
- Lengthening of ventricular fixation elements, improving intra-LV anchoring;
- -
- Reduction in the cuff width to facilitate crimping of valves > 44 mm.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DS | Delivery system |
| LA | Left atrium |
| LV | Left ventricle |
| LVOT | Left ventricle outflow tract |
| MR | Mitral regurgitation |
| NiTi | Nitinol |
| TAV | Transcatheter aortic valve |
| TAVI | Transcatheter aortic valve implantation |
| TMV | Transcatheter mitral valve |
| TMVI | Transcatheter mitral valve implantation |
| VHD | Valvular heart disease |
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- d’Arcy, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur. Heart J. 2016, 37, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Solomon, S.D. Valvular heart disease in older adults: Seeking an ounce of prevention. Eur. Heart J. 2016, 37, 3523–3524. [Google Scholar] [CrossRef] [PubMed]
- Trichon, B.H.; Felker, G.M.; Shaw, L.K.; Cabell, C.H.; O’Connor, C.M. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am. J. Cardiol. 2003, 9, 538–543. [Google Scholar] [CrossRef]
- Mirabel, M.; Iung, B.; Baron, G.; Messika-Zeitoun, D.; Détaint, D.; Vanoverschelde, J.L.; Butchart, E.G.; Ravaud, P.; Vahanian, A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 2007, 28, 1358–1365. [Google Scholar] [CrossRef]
- Sannino, A.; Smith, R.L.; Schiattarella, G.G.; Trimarco, B.; Esposito, G.; Grayburn, P.A. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: A systematic review and meta-analysis. JAMA Cardiol. 2017, 2, 1130–1139. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Avierinos, J.F.; Messika-Zeitoun, D.; Detaint, D.; Capps, M.; Nkomo, V.; Scott, C.; Schaff, H.V.; Tajik, A.J. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N. Engl. J. Med. 2005, 352, 875–883. [Google Scholar] [CrossRef]
- Mehta, R.H.; Eagle, K.A.; Coombs, L.P.; Peterson, E.D.; Edwards, F.H.; Pagani, F.D.; Deeb, G.M.; Bolling, S.F.; Prager, R.L.; STS National Cardiac Registry. Influence of age on outcomes in patients undergoing mitral valve replacement. Ann. Thorac. Surg. 2002, 74, 1459–1467. [Google Scholar] [CrossRef]
- Fiorilli, P.N.; Herrmann, H.C.; Szeto, W.Y. Transcatheter mitral valve replacement: Latest advances and future directions. Ann. Cardiothorac. Surg. 2021, 10, 85–95. [Google Scholar] [CrossRef]
- Santangelo, G.; Bursi, F.; Faggiano, A.; Moscardelli, S.; Simeoli, P.S.; Guazzi, M.; Lorusso, R.; Carugo, S.; Faggiano, P. The Global Burden of Valvular Heart Disease: From Clinical Epidemiology to Management. J. Clin. Med. 2023, 12, 2178. [Google Scholar] [CrossRef]
- Meco, M.; Miceli, A.; Montisci, A.; Donatelli, F.; Cirri, S.; Ferrarini, M.; Lio, A.; Glauber, M. Sutureless aortic valve replacement versus transcatheter aortic valve implantation: A meta-analysis of comparative matched studies using propensity score matching. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Grossi, B.; Luraghi, G.; Barati, S.; Forte, C.; Gerosa, L.; Cozzi, O.; D’Ascenzo, F.; Condorelli, G.; Migliavacca, F.; Stefanini, G. The impact of bicuspid valve morphology on the selection of transcatheter aortic valve implantation devices: An in silico study. Eur. Heart J. Imaging Methods Pract. 2025, 3, qyaf018. [Google Scholar] [CrossRef]
- Piazza, N.; Overtchouk, P.; Ben-Shoshan, J. Predicting TMVR outcomes—The Tendyne experience. EuroIntervention 2019, 15, e1033–e1034. [Google Scholar] [CrossRef] [PubMed]
- Hensey, M.; Brown, R.A.; Lal, S.; Sathananthan, J.; Ye, J.; Cheung, A.; Blanke, P.; Leipsic, J.; Moss, R.; Boone, R.; et al. Transcatheter Mitral Valve Replacement: An Update on Current Techniques, Technologies, and Future Directions. JACC Cardiovasc. Interv. 2021, 14, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.; Galasso, M.; Margonato, A.; Godino, C. Patient selection for trans-catheter mitral valve Repair versus Replacement: Ongoing indications and glimpse to the future. Vessel. Plus 2020, 5, 6. [Google Scholar] [CrossRef]
- Tom, S.K.; Kalra, K.; Perdoncin, E.; Tully, A.; Devireddy, C.M.; Inci, E.; Greenbaum, A.; Grubb, K.J. Transcatheter Treatment Options for Functional Mitral Regurgitation: Which Device for Which Patients? Interv. Cardiol. 2024, 19, e10. [Google Scholar] [CrossRef]
- Dahle, G. Current Devices in TMVI and Their Limitations: Focus on Tendyne. Front. Cardiovasc. Med. 2020, 7, 592909. [Google Scholar] [CrossRef]
- Wyler von Ballmoos, M.C.; Kalra, A.; Reardon, M.J. Complexities of transcatheter mitral valve replacement (TMVR) and why it is not transcatheter aortic valve replacement (TAVR). Ann. Cardiothorac. Surg. 2018, 7, 724–730. [Google Scholar] [CrossRef]
- Bartorelli, A.L.; Monizzi, G.; Mastrangelo, A.; Grancini, L.; Fabbiocchi, F.; Conte, E.; Moltrasio, M.; Andreini, D. Transcatheter mitral valve replacement: There is still work to be done. Eur. Heart J. Suppl. 2022, 24 (Suppl. I), I16–I21. [Google Scholar] [CrossRef]
- Hinohara, T.T.; Reardon, M.J.; Goel, S.S. Latest Advances in Transcatheter Mitral Valve Replacement. Heart Int. 2021, 15, 79–83. [Google Scholar] [CrossRef]
- Regueiro, A.; Granada, J.F.; Dagenais, F.; Rodés-Cabau, J. Transcatheter Mitral Valve Replacement: Insights From Early Clinical Experience and Future Challenges. J. Am. Coll. Cardiol. 2017, 69, 2175–2192. [Google Scholar] [CrossRef] [PubMed]
- ASTM F2063-18; Standard Specification for Wrought Nickel-Titanium Shape Memory Alloys for Medical Devices and Surgical Implants. ASTM Volume 13.01: Medical and Surgical Materials and Devices (I): E667–F2503. ASTM: West Conshohocken, PA, USA, 2018. Available online: https://store.astm.org/f2063-18.html (accessed on 17 February 2025). [CrossRef]
- Zhuravleva, I.Y.; Karpova, E.V.; Oparina, L.A.; Poveschenko, O.V.; Surovtseva, M.A.; Titov, A.T.; Ksenofontov, A.L.; Vasilieva, M.B.; Kuznetsova, E.V.; Bogachev-Prokophiev, A.V.; et al. Cross-linking method using pentaepoxide for improving bovine and porcine bioprosthetic pericardia: A multiparametric assessment study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111473. [Google Scholar] [CrossRef] [PubMed]
- ISO 5840-3:2021; Cardiovascular Implants—Cardiac Valve Prostheses. Part 3: Heart Valve Substitutes Implanted by Transcatheter Techniques. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/67606.html (accessed on 17 February 2025).
- Ovcharenko, E.A.; Klyshnikov, K.U.; Yuzhalin, A.E.; Savrasov, G.V.; Kokov, A.N.; Batranin, A.V.; Ganyukov, V.I.; Kudryavtseva, Y.A. Modeling of transcatheter aortic valve replacement: Patient specific vs general approaches based on finite element analysis. Comput. Biol. Med. 2016, 69, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kobelev, E.; Bergen, T.A.; Tarkova, A.R.; Krestyaninov, O.V.; Bobrikova, E.E.; Safro, I.K.; Chernyavsky, A.M.; Zhuravleva, I.Y. A New Look at Structural Changes in the Aortic Root in Aortic Valve Stenosis. Sovrem. Tekhnologii Med. 2022, 14, 51–56. [Google Scholar] [CrossRef]
- Urena, M.; Lurz, P.; Sorajja, P.; Himbert, D.; Guerrero, M. Transcatheter mitral valve implantation for native valve disease. EuroIntervention 2023, 19, 720–738. [Google Scholar] [CrossRef]
- Ktenopoulos, N.; Katsaros, O.; Apostolos, A.; Drakopoulou, M.; Tsigkas, G.; Tsioufis, C.; Davlouros, P.; Toutouzas, K.; Karanasos, A. Emerging Transcatheter Therapies for Valvular Heart Disease: Focus on Mitral and Tricuspid Valve Procedures. Life 2024, 14, 842. [Google Scholar] [CrossRef]
- Sisinni, A.; Barreiro-Pérez, M.; Calvo-Iglesias, F.; Estévez-Loureiro, R. Mitral Regurgitation and Left Ventricular Outflow Tract Obstruction: Confluence of Challenges for Transcatheter Treatment. Rev. Cardiovasc. Med. 2024, 25, 134. [Google Scholar] [CrossRef]
- Khoffi, F.; Heim, F. Mechanical degradation of biological heart valve tissue induced by low diameter crimping: An early assessment. J. Mech. Behav. Biomed. Mater. 2015, 44, 71–75. [Google Scholar] [CrossRef]
- Sazzad, F.; Hon, J.K.F.; Ramanathan, K.; Nah, J.H.; Ong, Z.X.; Ti, L.K.; Foo, R.; Tay, E.; Kofidis, T. Design Variation, Implantation, and Outcome of Transcatheter Mitral Valve Prosthesis: A Comprehensive Review. Front. Cardiovasc. Med. 2022, 8, 782278. [Google Scholar] [CrossRef]
- Camaj, A.; Thourani, V.H.; Gillam, L.D.; Stone, G.W. Heart Failure and Secondary Mitral Regurgitation: A Contemporary Review. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101195. [Google Scholar] [CrossRef]
- Alharbi, Y.; Otton, J.; Muller, D.W.M.; Geelan-Small, P.; Lovell, N.H.; Al Abed, A.; Dokos, S. Predicting the outcome of transcatheter mitral valve implantation using image-based computational models. J. Cardiovasc. Comput. Tomogr. 2020, 14, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Surovtseva, M.A.; Poveschenko, O.V.; Kuzmin, O.S.; Kim, I.I.; Kozhukhov, A.S.; Bondarenko, N.A.; Chepeleva, E.V.; Kolodin, A.N.; Lykov, A.P.; Shcheglov, D.V.; et al. Titanium oxide– and oxynitride–coated nitinol: Effects of surface structure and composition on interactions with endothelial cells. Appl. Surf. Sci. 2022, 578, 152059. [Google Scholar] [CrossRef]
- Zhuravleva, I.Y.; Surovtseva, M.A.; Vaver, A.A.; Suprun, E.A.; Kim, I.I.; Bondarenko, N.A.; Kuzmin, O.S.; Mayorov, A.P.; Poveshchenko, O.V. Effect of the Nanorough Surface of TiO2 Thin Films on the Compatibility with Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 6699. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Perrin, N.; Coisne, A.; Weimann, J.; Duncan, A.; Akodad, M.; Scotti, A.; Kalbacher, D.; Bleiziffer, S.; Nickenig, G.; et al. Clinical outcomes of transcatheter mitral valve replacement: Two-year results of the CHOICE-MI Registry. EuroIntervention 2023, 19, 512–525. [Google Scholar] [CrossRef]
- Conradi, L.; Ludwig, S.; Sorajja, P.; Duncan, A.; Bethea, B.; Dahle, G.; Babaliaros, V.; Guerrero, M.; Thourani, V.; Dumonteil, N.; et al. Clinical outcomes and predictors of transapical transcatheter mitral valve replacement: The Tendyne Expanded Clinical Study. EuroIntervention 2024, 20, e887–e897. [Google Scholar] [CrossRef]
- Hell, M.M.; Wild, M.G.; Baldus, S.; Rudolph, T.; Treede, H.; Petronio, A.S.; Modine, T.; Andreas, M.; Coisne, A.; Duncan, A.; et al. Transapical Mitral Valve Replacement: 1-Year Results of the Real-World Tendyne European Experience Registry. JACC Cardiovasc. Interv. 2024, 17, 648–661. [Google Scholar] [CrossRef]
- Zahr, F.; Song, H.K.; Chadderdon, S.; Gada, H.; Mumtaz, M.; Byrne, T.; Kirshner, M.; Sharma, S.; Kodali, S.; George, I.; et al. 1-Year Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement: Intrepid TMVR Early Feasibility Study Results. JACC Cardiovasc. Interv. 2023, 16, 2868–2879. [Google Scholar] [CrossRef]
- Saxon, J.T.; Genereux, P.; Ninios, V.; Waggoner, T.; Tahirkheli, N.; Grygier, M.; Wrobel, K.; Adam, M.; Nickenig, G.; Kaneko, T.; et al. Transcatheter Mitral Valve Replacement With Atrial Fixation for Treatment of Atrial Functional Mitral Regurgitation. Circ. Cardiovasc. Interv. 2025, 18, e014985. [Google Scholar] [CrossRef]
- Généreux, P.; Wróbel, K.; Rinaldi, M.J.; Modine, T.; Bapat, V.; Ninios, V.; Sorajja, P. AltaValve Atrial Fixation System for the Treatment of Severe Mitral Regurgitation and Mitral Annular Calcification. Struct. Heart. 2024, 8, 100294. [Google Scholar] [CrossRef]
- Aoun, J.; Reardon, M.J.; Goel, S.S. Transcatheter Mitral Valve Replacement with Dedicated Devices. Methodist Debakey Cardiovasc. J. 2023, 19, 50–56. [Google Scholar] [CrossRef]
- Le, N.K.; Chervu, N.; Mallick, S.; Vadlakonda, A.; Kim, S.; Curry, J.; Benharash, P. Mortality and resource utilization in surgical versus transcatheter repeat mitral valve replacement: A national analysis. PLoS ONE 2024, 19, e0301939. [Google Scholar] [CrossRef]






| Parameters | Size, mm | ||||
| Recipient’s mitral annulus = outer diameter of implanted device | 40–41 | 42–43 | 44–45 | 46–47 | 48–49 |
| Real outer diameter of central anchoring zone | 45.0 | 47.0 | 49.0 | 51.0 | 53.0 |
| Valve diameter | 28.0 | 28.0 | 28.0 | 30.0 | 30.0 |
| Valve stent height | 15.6 | 15.6 | 15.6 | 15.1 | 15.1 |
| Total height, no more than | 20.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuravleva, I.Y. A Novel Self-Expanding Transcatheter Mitral Valve with Dual Annulus/Valve Diameter. J. Funct. Biomater. 2025, 16, 250. https://doi.org/10.3390/jfb16070250
Zhuravleva IY. A Novel Self-Expanding Transcatheter Mitral Valve with Dual Annulus/Valve Diameter. Journal of Functional Biomaterials. 2025; 16(7):250. https://doi.org/10.3390/jfb16070250
Chicago/Turabian StyleZhuravleva, Irina Yu. 2025. "A Novel Self-Expanding Transcatheter Mitral Valve with Dual Annulus/Valve Diameter" Journal of Functional Biomaterials 16, no. 7: 250. https://doi.org/10.3390/jfb16070250
APA StyleZhuravleva, I. Y. (2025). A Novel Self-Expanding Transcatheter Mitral Valve with Dual Annulus/Valve Diameter. Journal of Functional Biomaterials, 16(7), 250. https://doi.org/10.3390/jfb16070250





