Histologic and Histomorphometric Evaluation of Bone Regeneration Using Human Allogeneic Bone Graft with or Without Mesenchymal Stem Cell–Conditioned Media in a Rabbit Calvarial Defect Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Materials
2.3. Experimental Group Settings
2.4. Tissue Preparation
2.5. Growth Factor Profiling of MSC-CM by Multiplex Immunoassay
2.6. Statistical Analysis
3. Results
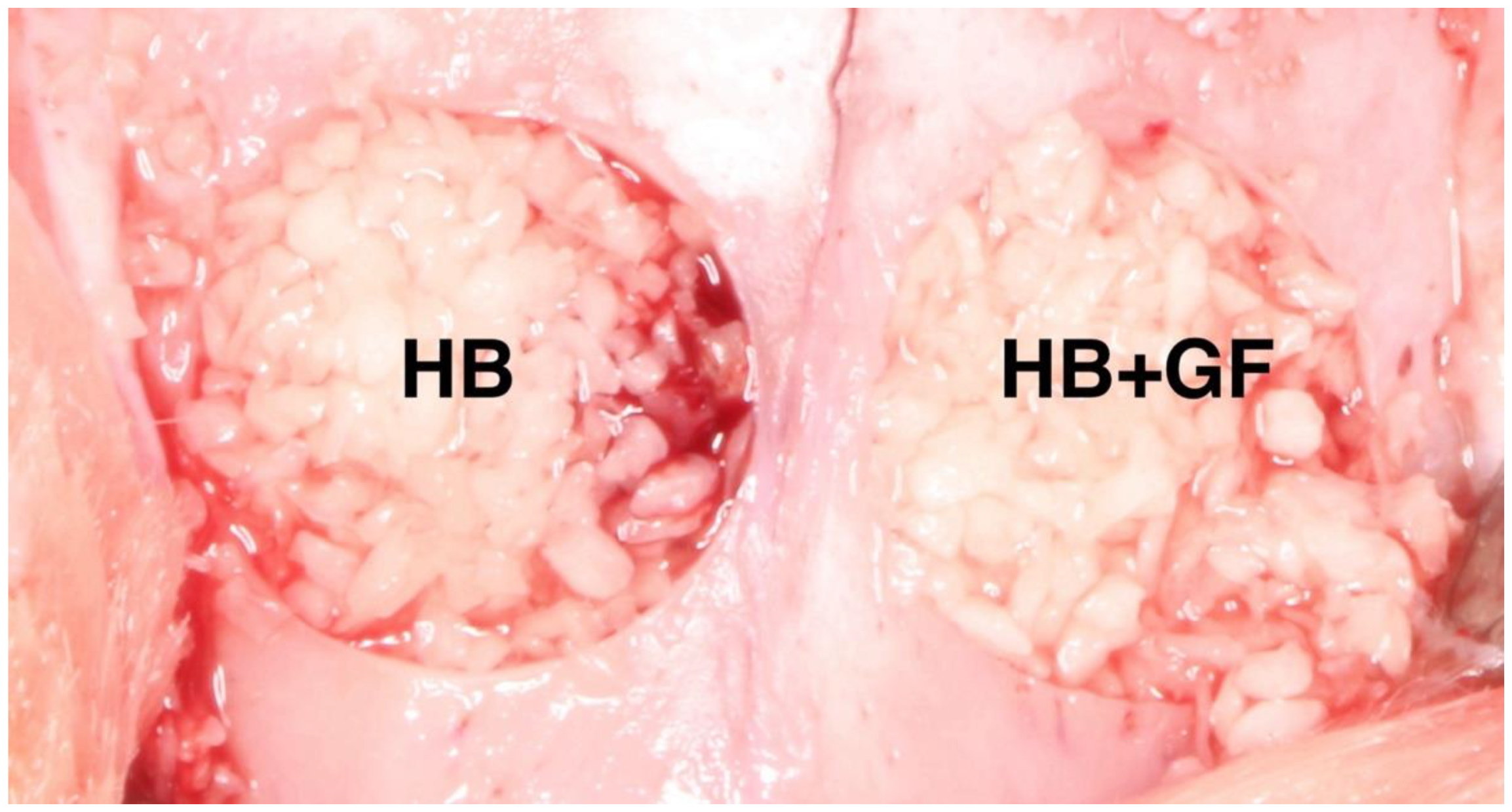
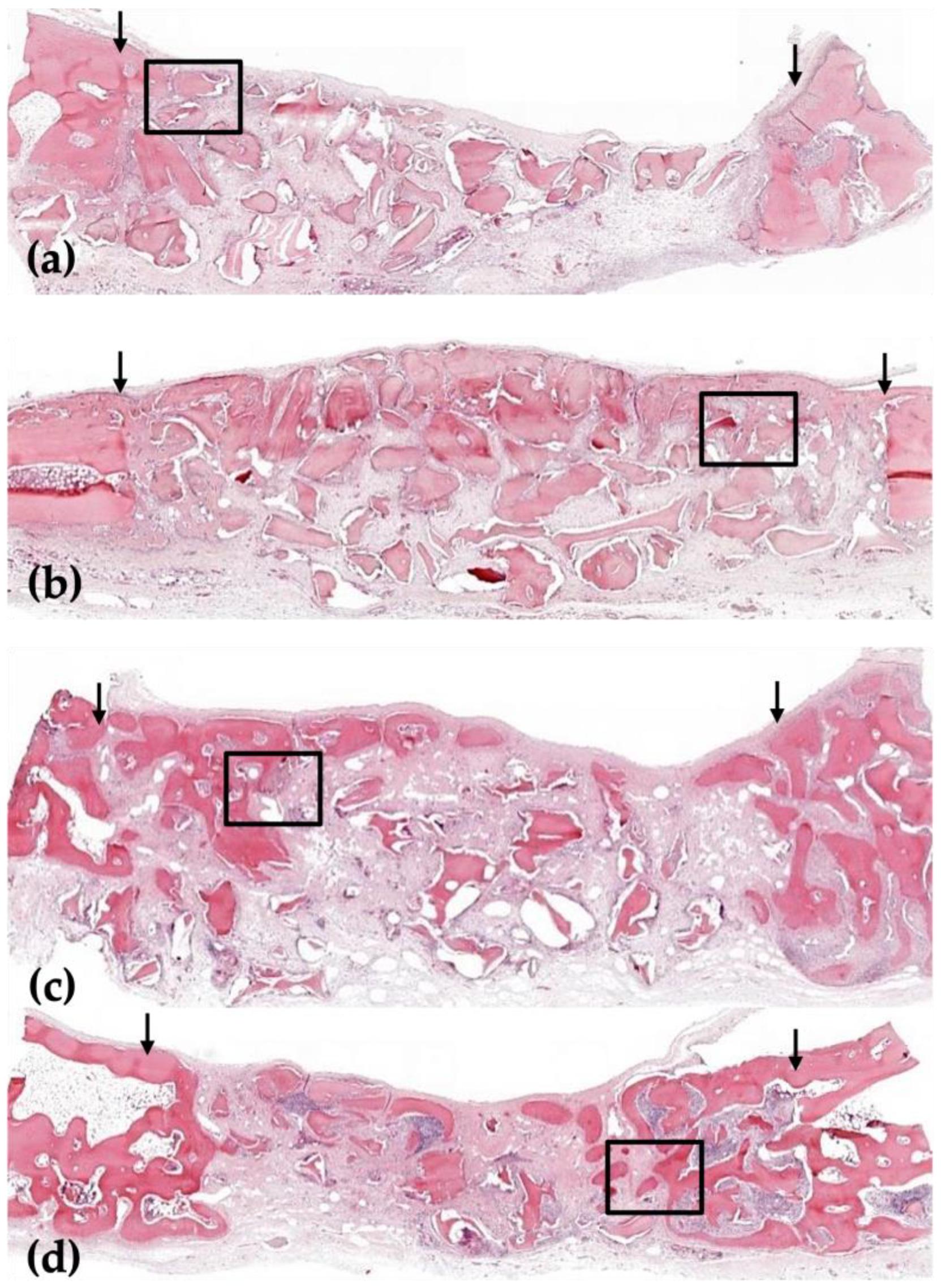
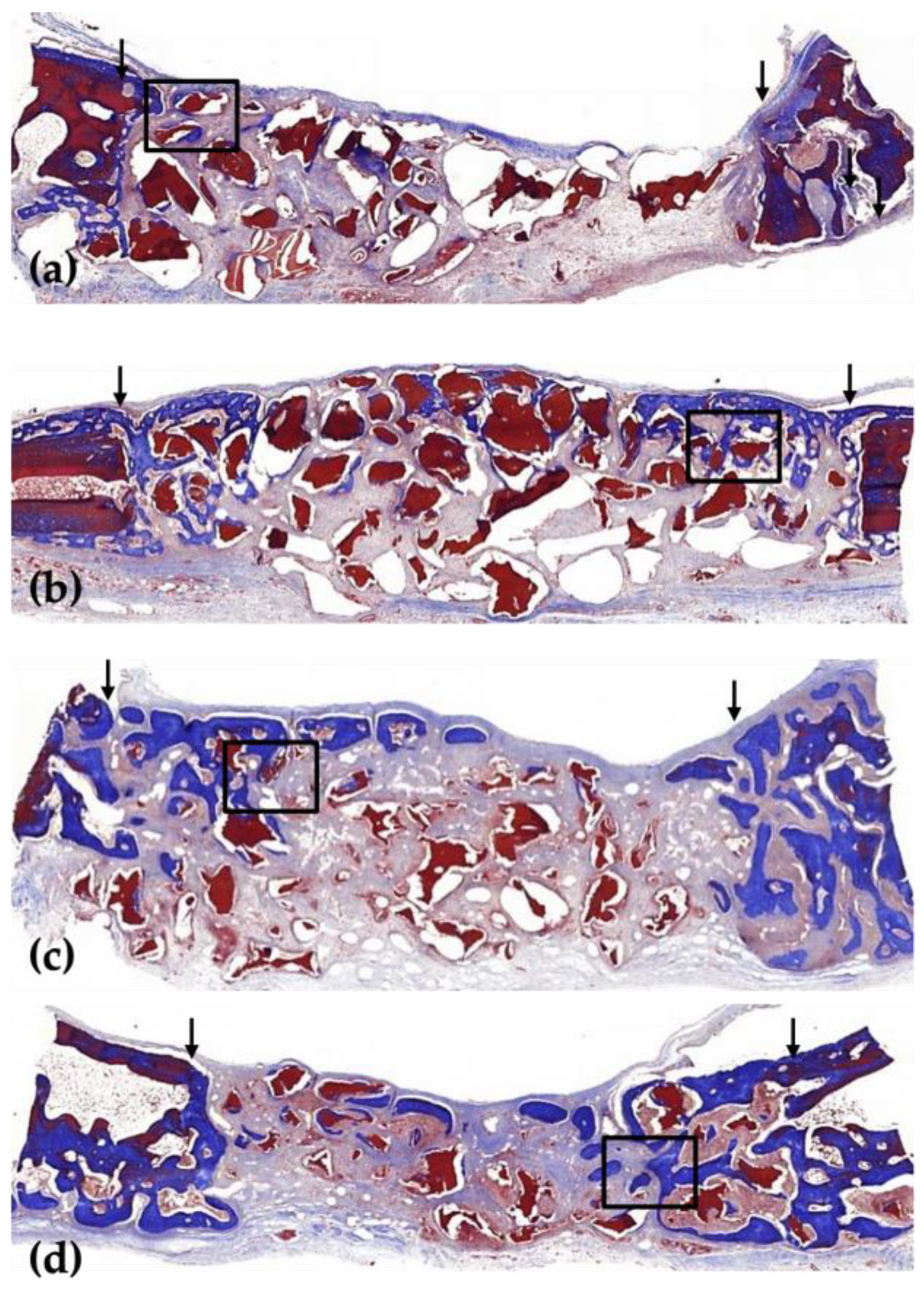
3.1. Histological Analysis
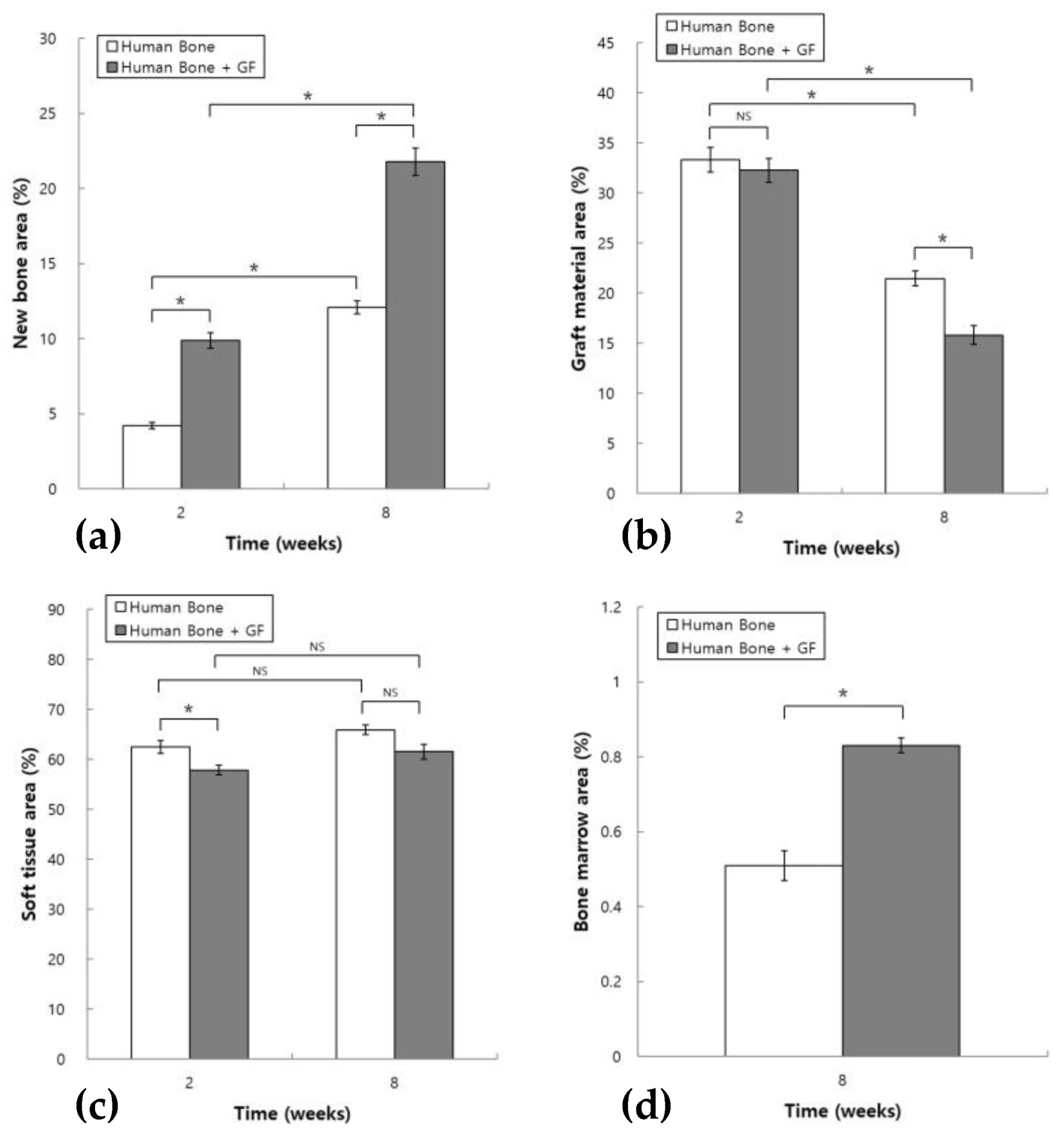
3.2. Histomorphometric Analysis
3.3. Quantitative Results of Growth Factors in MSC-CM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsuchida, S.; Nakayama, T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines 2023, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Materials 2021, 14, 1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Greenwell, H.; Fiorellini, J.; Giannobile, W.; Offenbacher, S.; Salkin, L.; Townsend, C.; Sheridan, P.; Genco, R.J. Periodontal regeneration. J. Periodontol. 2005, 76, 1601–1622. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Haben Fesseha, M. Bone grafting, its principle and application: A review. Osteol. Rheumatol. Open J. 2020, 1, 43–50. [Google Scholar]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L. Autogenous iliac crest bone graft. Complications and functional assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications. Nanomaterials 2018, 8, 337. [Google Scholar] [CrossRef]
- Olivos-Meza, A.; Mata-Miranda, M.M.; Robles-Rodríguez, M.; Vázquez-Zapién, G.J.; Guerrero-Ruiz, M.; Landa-Solís, C. Osteogenic Potential and Bone Matrix Maturity: Comparison of Demineralized Bone Matrix and P15 Polypeptide iFactor® in an In Vitro Study. Medicina 2025, 61, 914. [Google Scholar] [CrossRef]
- Khoshzaban, A.; Mehrzad, S.; Tavakoli, V.; Keshel, S.H.; Behrouzi, G.R.; Bashtar, M. The comparative effectiveness of demineralized bone matrix, beta-tricalcium phosphate, and bovine-derived anorganic bone matrix on inflammation and bone formation using a paired calvarial defect model in rats. Clin. Cosmet. Investig. Dent. 2011, 3, 69–78. [Google Scholar] [CrossRef]
- Padial-Molina, M.; O’Valle, F.; Lanis, A.; Mesa, F.; Dohan Ehrenfest, D.M.; Wang, H.L.; Galindo-Moreno, P. Clinical Application of Mesenchymal Stem Cells and Novel Supportive Therapies for Oral Bone Regeneration. BioMed Res. Int. 2015, 2015, 341327. [Google Scholar] [CrossRef]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Davies, L.C. MSCs-cells with many sides. Cytotherapy 2018, 20, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Klyushnenkova, E.; Mosca, J.D.; Zernetkina, V.; Majumdar, M.K.; Beggs, K.J.; Simonetti, D.W.; Deans, R.J.; McIntosh, K.R. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J. Biomed. Sci. 2005, 12, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: In vivo observations of cell kinetics. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Ide, C.; Nakai, Y.; Nakano, N.; Seo, T.B.; Yamada, Y.; Endo, K.; Noda, T.; Saito, F.; Suzuki, Y.; Fukushima, M.; et al. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010, 1332, 32–47. [Google Scholar] [CrossRef]
- Benavides-Castellanos, M.P.; Garzón-Orjuela, N.; Linero, I. Effectiveness of mesenchymal stem cell-conditioned medium in bone regeneration in animal and human models: A systematic review and meta-analysis. Cell Regen. 2020, 9, 5. [Google Scholar] [CrossRef]
- Katagiri, W.; Osugi, M.; Kinoshita, K.; Hibi, H. Conditioned Medium From Mesenchymal Stem Cells Enhances Early Bone Regeneration After Maxillary Sinus Floor Elevation in Rabbits. Implant. Dent. 2015, 24, 657–663. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Sakaguchi, K.; Kawai, T.; Wakayama, Y.; Osugi, M.; Hibi, H. A defined mix of cytokines mimics conditioned medium from cultures of bone marrow-derived mesenchymal stem cells and elicits bone regeneration. Cell Prolif. 2017, 50, e12333. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.-S.; Bae, J.-H.; Kim, S.-E.; Bae, E.-B.; Kim, S.-Y.; Choi, K.-H.; Moon, K.-O.; Jeong, C.-M.; Huh, J.-B. The Effect of Bisphasic Calcium Phosphate Block Bone Graft Materials with Polysaccharides on Bone Regeneration. Materials 2017, 10, 17. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Unursaikhan, O.; Yang, C.; Lee, J.-S.; Jung, U.-W.; Kim, C.-S.; Choi, S.-H. Comparative Study of Bone Regeneration in Rabbit Calvarial Defect Following Implantation with Demineralized Bone Matrix Gel, Bovine Bone, Synthetic Hydroxyapatite. Biomater. Res. 2012, 16, 140–146. [Google Scholar]
- Marquez, L.; de Abreu, F.A.; Ferreira, C.L.; Alves, G.D.; Miziara, M.N.; Alves, J.B. Enhanced bone healing of rat tooth sockets after administration of epidermal growth factor (EGF) carried by liposome. Injury 2013, 44, 558–564. [Google Scholar] [CrossRef]
- Krasilnikova, O.; Yakimova, A.; Ivanov, S.; Atiakshin, D.; Kostin, A.A.; Sosin, D.; Shegay, P.; Kaprin, A.D.; Klabukov, I. Gene-Activated Materials in Regenerative Dentistry: Narrative Review of Technology and Study Results. Int. J. Mol. Sci. 2023, 24, 16250. [Google Scholar] [CrossRef]
- Triplett, R.G.; Nevins, M.; Marx, R.E.; Spagnoli, D.B.; Oates, T.W.; Moy, P.K.; Boyne, P.J. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2009, 67, 1947–1960. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Vaca, A.; Vizoso, F.J. Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering 2023, 10, 4. [Google Scholar] [CrossRef]
- Dirzu, N.; Lucaciu, O.; Dirzu, D.S.; Soritau, O.; Cenariu, D.; Crisan, B.; Tefas, L.; Campian, R.S. BMP-2 Delivery through Liposomes in Bone Regeneration. Appl. Sci. 2022, 12, 1373. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, W.; Huang, J.; He, B.C.; Zuo, G.W.; Zhang, W.; Luo, Q.; Shi, Q.; Zhang, B.Q.; Wagner, E.R.; et al. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J. Bone Miner. Res. 2010, 25, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Nishimura, M.; Sekiya, K.; Suehiro, F.; Kanawa, M.; Nikawa, H.; Hamada, T.; Kato, Y. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007, 16, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Wislet-Gendebien, S.; Poulet, C.; Neirinckx, V.; Hennuy, B.; Swingland, J.T.; Laudet, E.; Sommer, L.; Shakova, O.; Bours, V.; Rogister, B. In vivo tumorigenesis was observed after injection of in vitro expanded neural crest stem cells isolated from adult bone marrow. PLoS ONE 2012, 7, e46425. [Google Scholar] [CrossRef]
- L., P.K.; Kandoi, S.; Misra, R.; S., V.; K., R.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Ando, Y.; Matsubara, K.; Ishikawa, J.; Fujio, M.; Shohara, R.; Hibi, H.; Ueda, M.; Yamamoto, A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014, 61, 82–90. [Google Scholar] [CrossRef]
- Rokn, A.; Moslemi, N.; Eslami, B.; Abadi, H.K.; Paknejad, M. Histologic Evaluation of Bone Healing Following Application of Anorganic Bovine Bone and β-tricalcium Phosphate in Rabbit Calvaria. J. Dent. 2012, 9, 35–40. [Google Scholar]
- Pripatnanont, P.; Nuntanaranont, T.; Vongvatcharanon, S. Proportion of deproteinized bovine bone and autogenous bone affects bone formation in the treatment of calvarial defects in rabbits. Int. J. Oral Maxillofac. Surg. 2009, 38, 356–362. [Google Scholar] [CrossRef]
- Xu, S.; Lin, K.; Wang, Z.; Chang, J.; Wang, L.; Lu, J.; Ning, C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar] [CrossRef]
- Sohn, J.Y.; Park, J.C.; Um, Y.J.; Jung, U.W.; Kim, C.S.; Cho, K.S.; Choi, S.H. Spontaneous healing capacity of rabbit cranial defects of various sizes. J. Periodontal Implant. Sci. 2010, 40, 180–187. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo Guirado, J.L.; Romanos, G.E. Bone grafting materials in critical defects in rabbit calvariae. A systematic review and quality evaluation using ARRIVE guidelines. Clin. Oral Implant. Res. 2018, 29, 620–634. [Google Scholar] [CrossRef]
- Toosi, S.; Behravan, J. Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. Biofactors 2020, 46, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, X.; Lin, S.; Li, X.; Zhang, S.; Song, Y.H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 356, 780–784. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.; Chen, X.; Wang, Z.; Zheng, S.; Cheng, Y.; Liu, S.; Hao, L. The role of insulin-like growth factor-1 in bone remodeling: A review. Int. J. Biol. Macromol. 2023, 238, 124125. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medina, T.; Vaquette, C.; Ivanovski, S. Systematic Comparison of the Effect of Four Clinical-Grade Platelet Rich Hemoderivatives on Osteoblast Behaviour. Int. J. Mol. Sci. 2019, 20, 6243. [Google Scholar] [CrossRef]
- Jann, J.; Gascon, S.; Roux, S.; Faucheux, N. Influence of the TGF-β Superfamily on Osteoclasts/Osteoblasts Balance in Physiological and Pathological Bone Conditions. Int. J. Mol. Sci. 2020, 21, 7597. [Google Scholar] [CrossRef]
- Trivedi, T.; Pagnotti, G.M.; Guise, T.A.; Mohammad, K.S. The Role of TGF-β in Bone Metastases. Biomolecules 2021, 11, 1643. [Google Scholar] [CrossRef]
- Krishnan, S.; Szabo, E.; Burghardt, I.; Frei, K.; Tabatabai, G.; Weller, M. Modulation of cerebral endothelial cell function by TGF-beta in glioblastoma: VEGF-dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget 2015, 6, 22480–22495. [Google Scholar] [CrossRef]
- Bahhnassy, A.; Mohanad, M.; Shaarawy, S.; Ismail, M.F.; El-Bastawisy, A.; Ashmawy, A.M.; Zekri, A.R. Transforming growth factor-β, insulin-like growth factor I/insulin-like growth factor I receptor and vascular endothelial growth factor-A: Prognostic and predictive markers in triple-negative and non-triple-negative breast cancer. Mol. Med. Rep. 2015, 12, 851–864. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Drosos, G.I.; Kazakos, K.I.; Kouzoumpasis, P.; Verettas, D.A. Safety and efficacy of commercially available demineralised bone matrix preparations: A critical review of clinical studies. Injury 2007, 38 (Suppl. 4), S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Rajagopal, A.; Shanmugasundaram, S. Role of Biomaterials Used for Periodontal Tissue Regeneration—A Concise Evidence-Based Review. Polymers 2022, 14, 3038. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-G.; Moon, Y.-S.; Sohn, D.-S. Histologic and Histomorphometric Evaluation of Bone Regeneration Using Human Allogeneic Bone Graft with or Without Mesenchymal Stem Cell–Conditioned Media in a Rabbit Calvarial Defect Model. J. Funct. Biomater. 2025, 16, 251. https://doi.org/10.3390/jfb16070251
Kim H-G, Moon Y-S, Sohn D-S. Histologic and Histomorphometric Evaluation of Bone Regeneration Using Human Allogeneic Bone Graft with or Without Mesenchymal Stem Cell–Conditioned Media in a Rabbit Calvarial Defect Model. Journal of Functional Biomaterials. 2025; 16(7):251. https://doi.org/10.3390/jfb16070251
Chicago/Turabian StyleKim, Hyung-Gyun, Yong-Suk Moon, and Dong-Seok Sohn. 2025. "Histologic and Histomorphometric Evaluation of Bone Regeneration Using Human Allogeneic Bone Graft with or Without Mesenchymal Stem Cell–Conditioned Media in a Rabbit Calvarial Defect Model" Journal of Functional Biomaterials 16, no. 7: 251. https://doi.org/10.3390/jfb16070251
APA StyleKim, H.-G., Moon, Y.-S., & Sohn, D.-S. (2025). Histologic and Histomorphometric Evaluation of Bone Regeneration Using Human Allogeneic Bone Graft with or Without Mesenchymal Stem Cell–Conditioned Media in a Rabbit Calvarial Defect Model. Journal of Functional Biomaterials, 16(7), 251. https://doi.org/10.3390/jfb16070251





