Influence of Stent Structure on Mechanical and Degradation Properties of Poly (Lactic Acid) Vascular Stent
Abstract
1. Introduction
2. Methods
2.1. Widening Strategy for the Ring Bend
2.2. Degradation Model of PLA
2.3. Finite Element Analysis
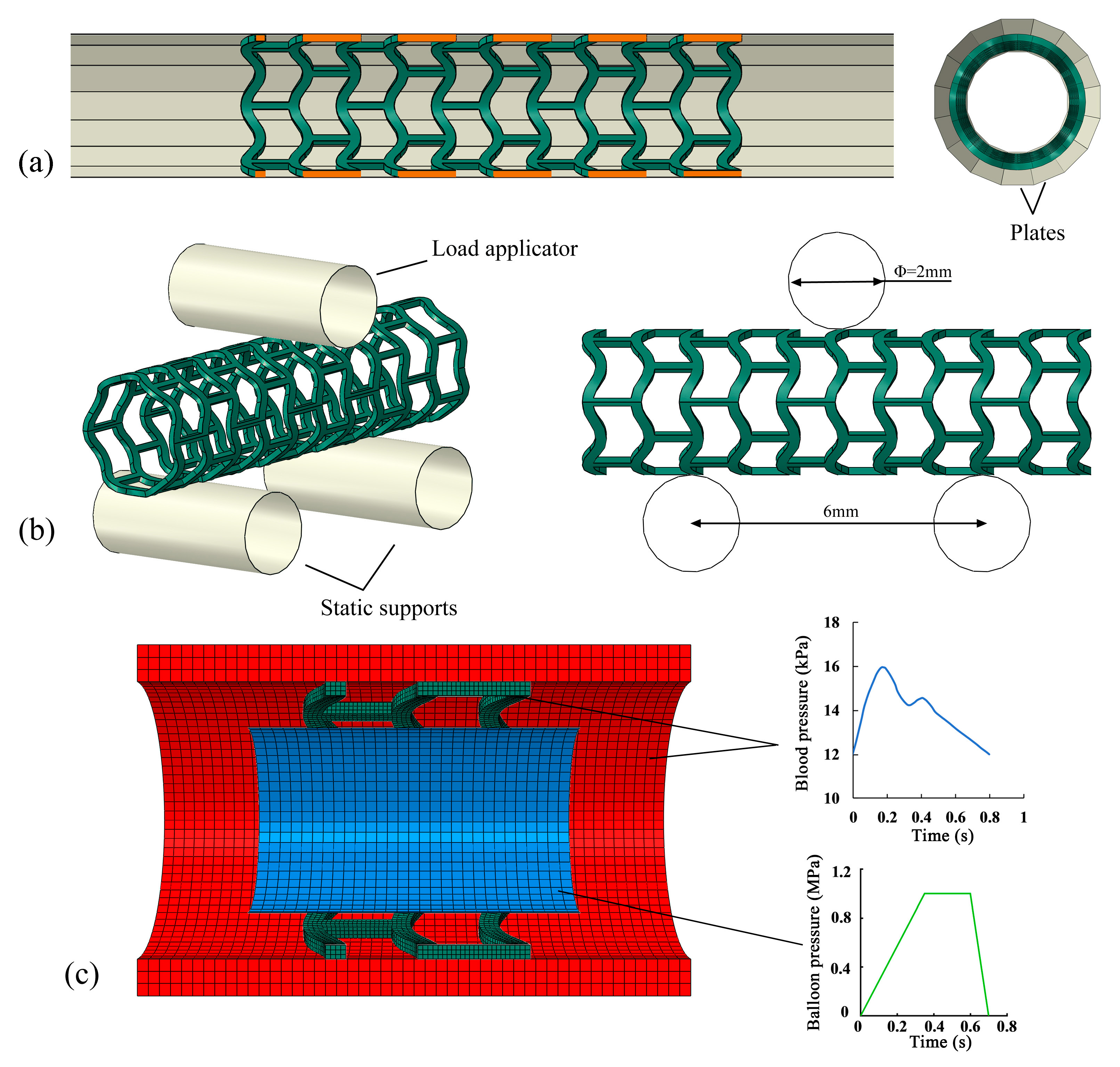
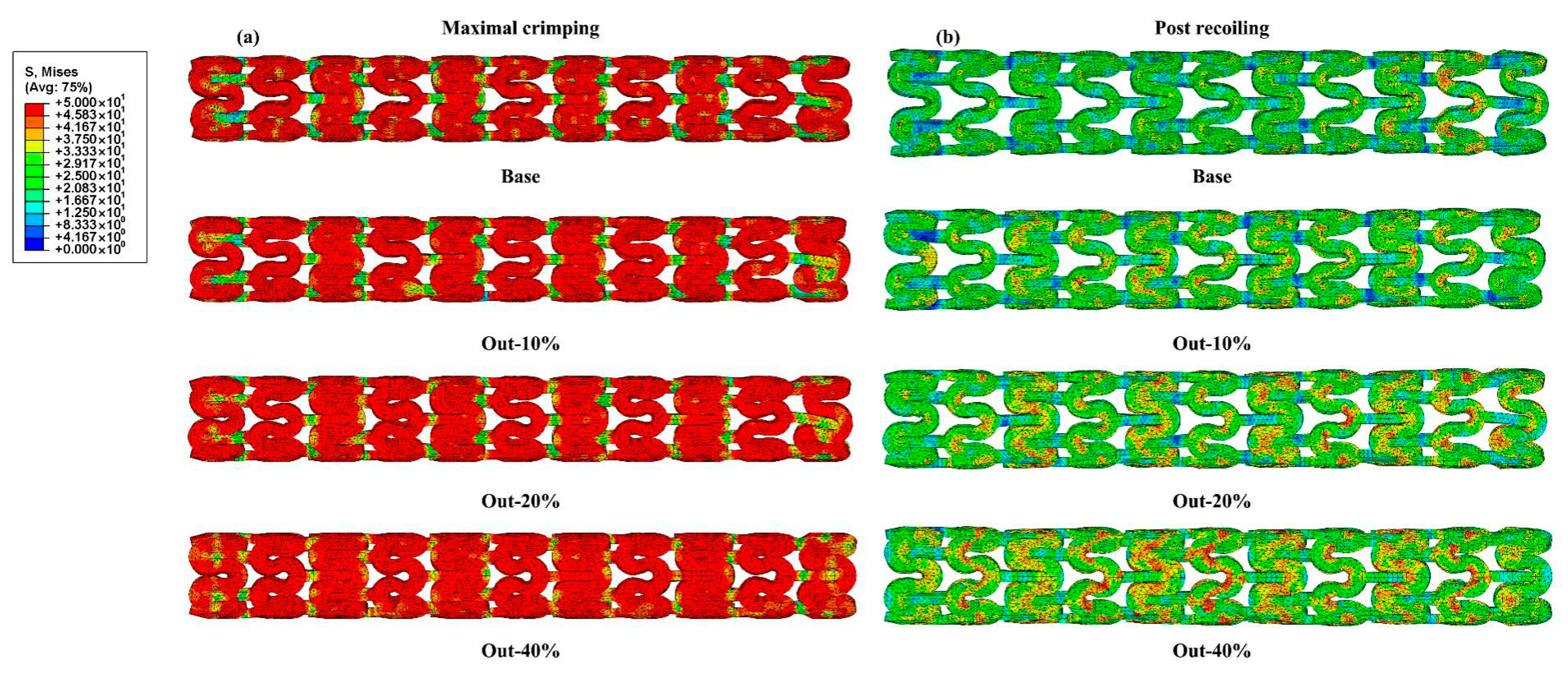
2.3.1. Simulation of Stent Crimping
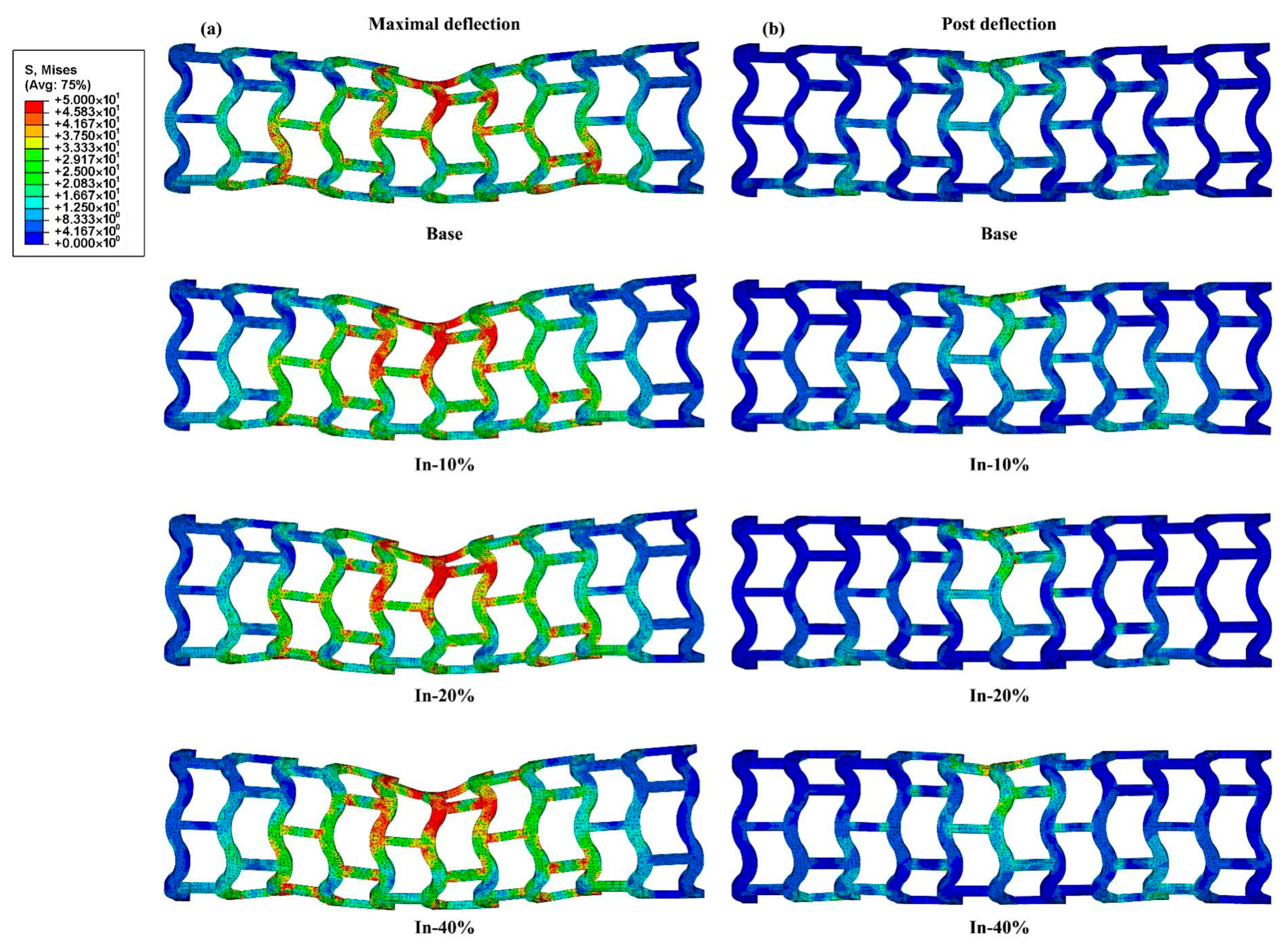
2.3.2. Simulation of Stent Bending Flexibility
2.3.3. Simulation of Stent Degradation
3. Results and Discussion
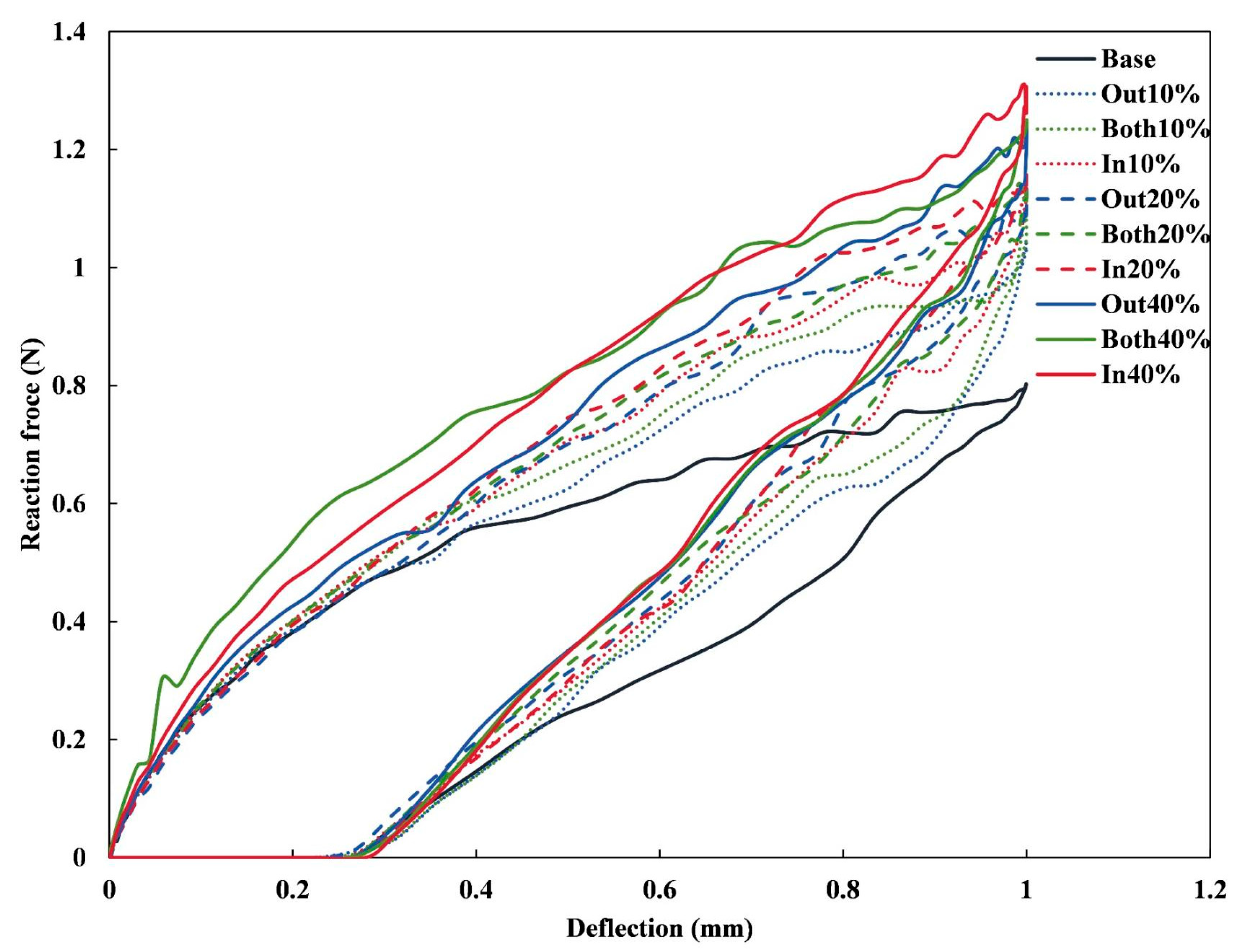
3.1. Radial Mechanical Performances of Stents
3.2. Bending Stiffness of Stents
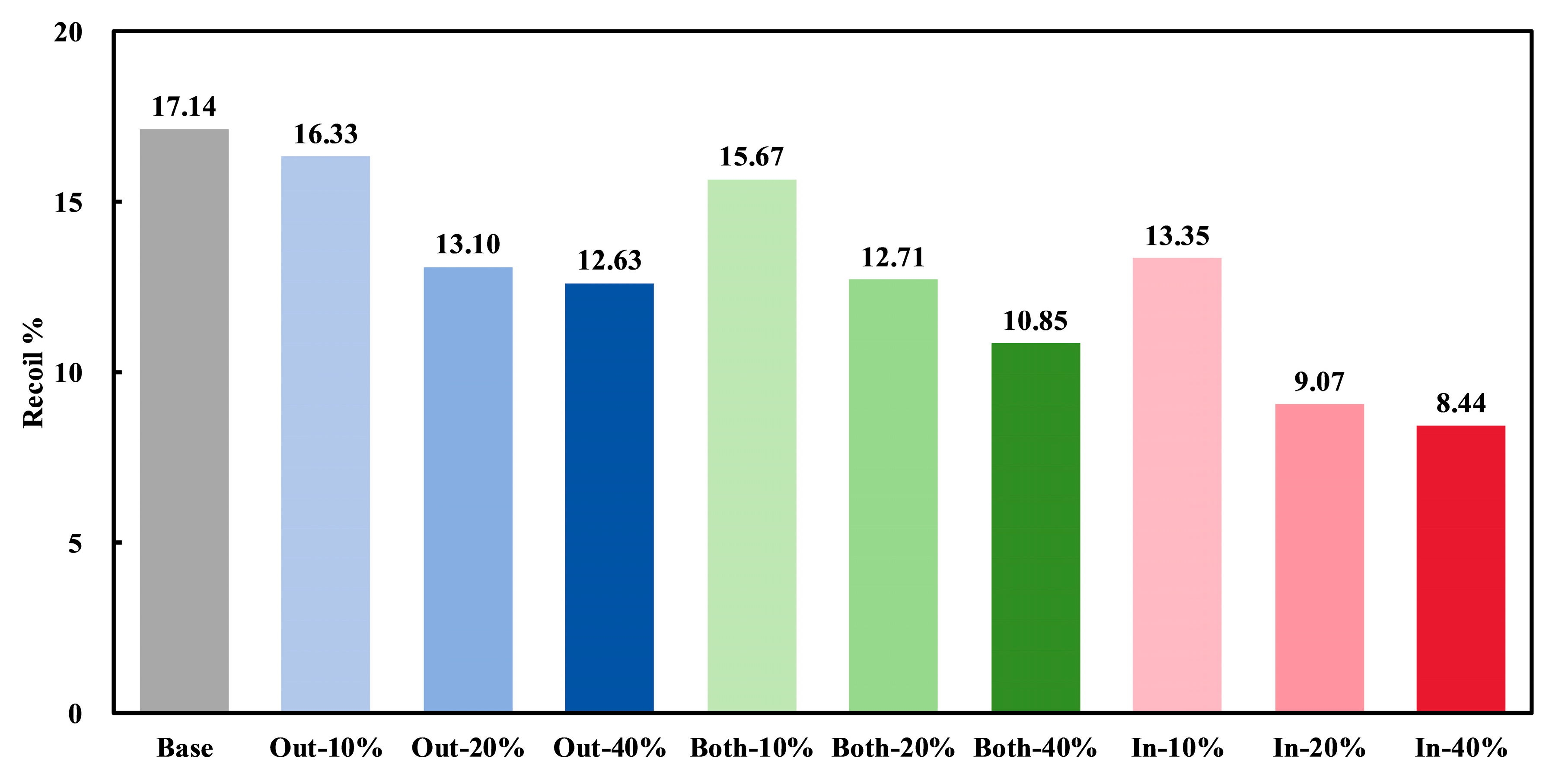
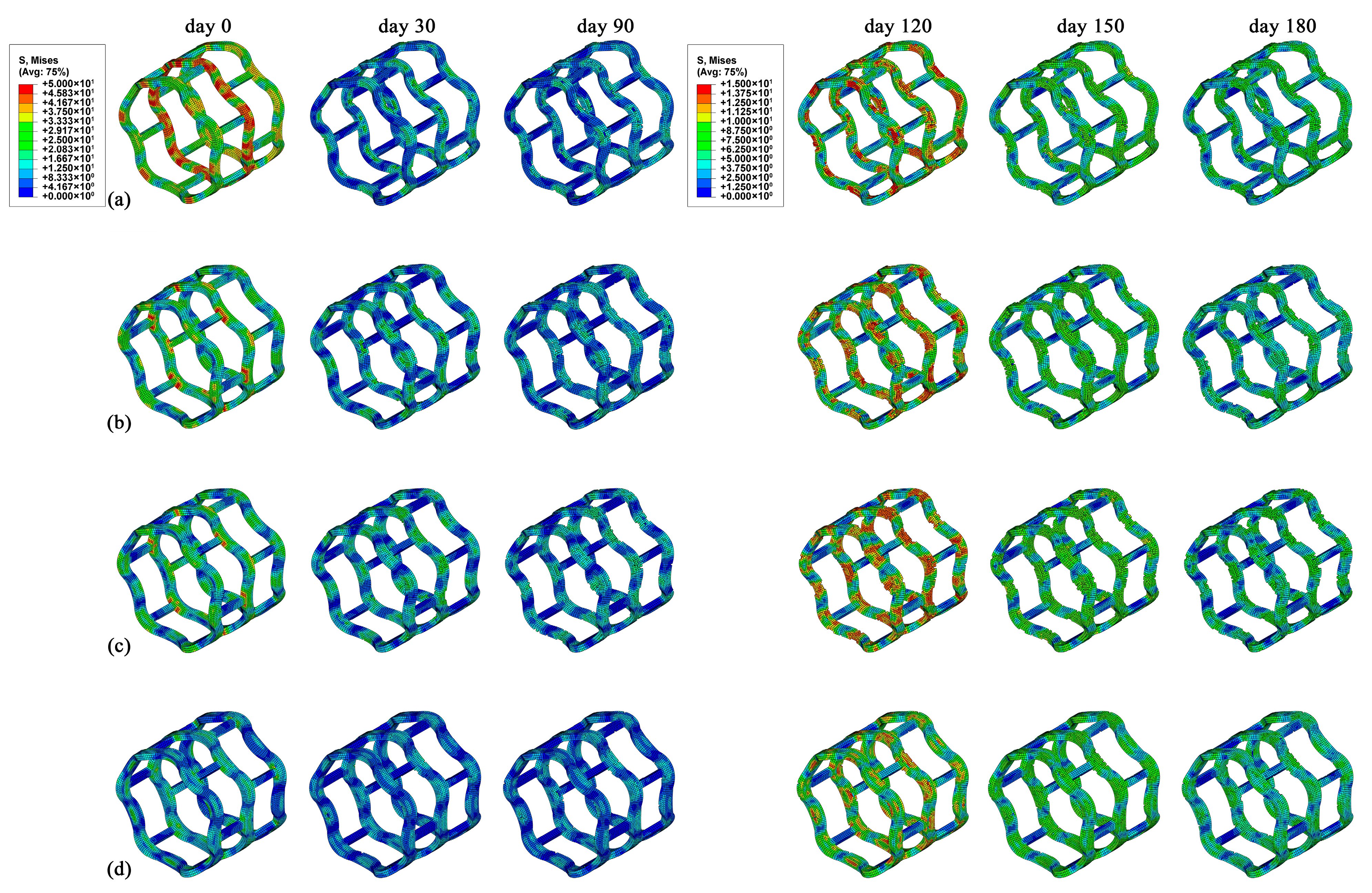
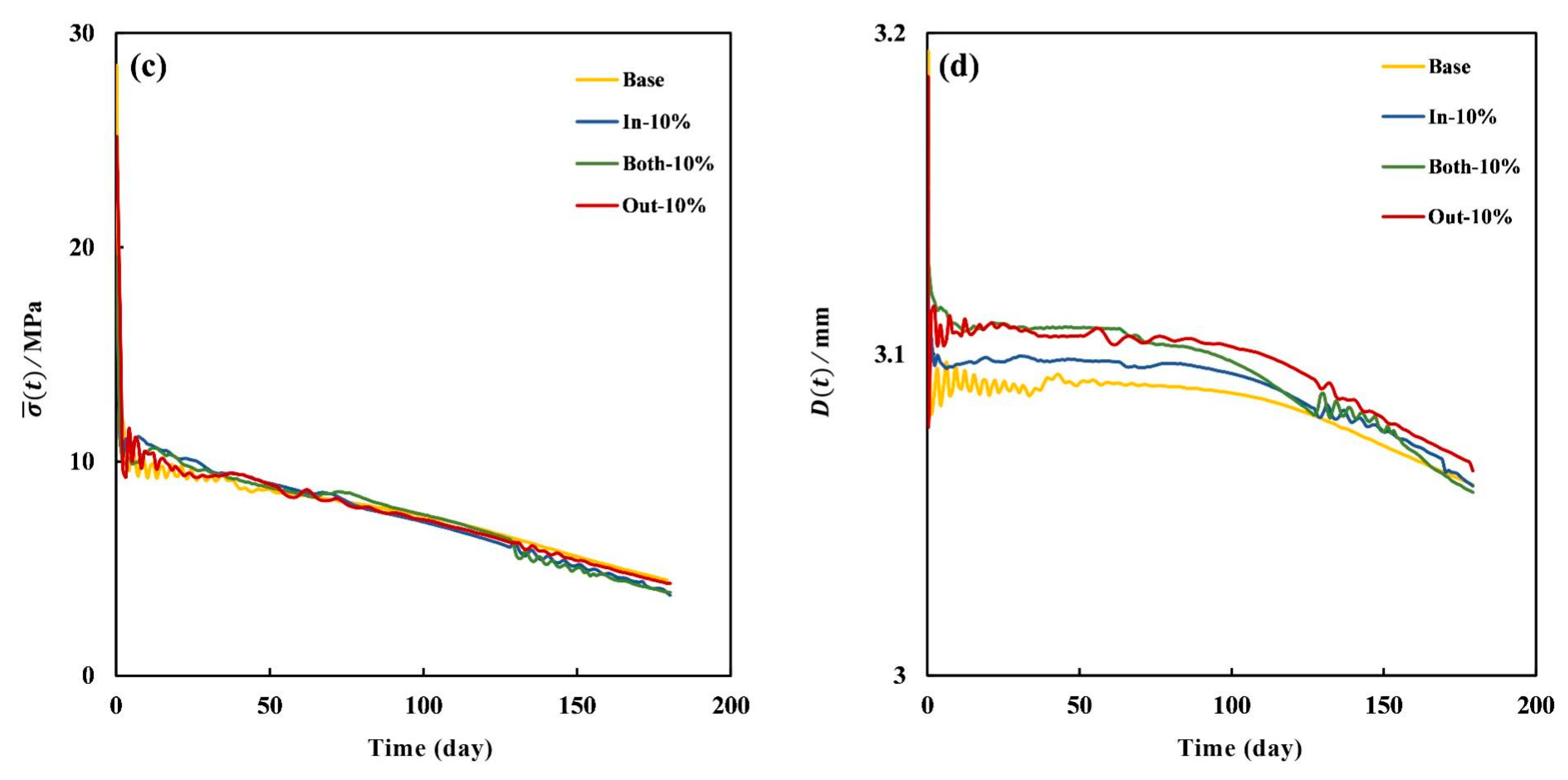
3.3. Degradation Properties of Stents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hytönen, J.P.; Taavitsainen, J.; Tarvainen, S.; Ylä-Herttuala, S. Biodegradable coronary scaffolds: Their future and clinical and technological challenges. Cardiovasc. Res. 2018, 114, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.L.; Shine, R.; Kelly, N.; Sweeney, C.A.; McHugh, P.E. A Review of Material Degradation Modelling for the Analysis and Design of Bioabsorbable Stents. Ann. Biomed. Eng. 2016, 44, 341–356. [Google Scholar] [CrossRef]
- Bian, D.; Zhou, W.; Deng, J.; Liu, Y.; Li, W.; Chu, X.; Xiu, P.; Cai, H.; Kou, Y.; Jiang, B.; et al. Development of magnesium-based biodegradable metals with dietary trace element germanium as orthopaedic implant applications. Acta Biomater. 2017, 64, 421–436. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, T.; Chen, K.; Canavese, F.; Huang, C.; Yang, H.; Shi, J.; He, W.; Zheng, Y.; Chen, S. Application of biodegradable implants in pediatric orthopedics: Shifting from absorbable polymers to biodegradable metals. Bioact. Mater. 2025, 50, 189–214. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A review on current research status of the surface modification of Zn-based biodegradable metals. Bioact. Mater. 2022, 7, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Q.; Gao, Y.; Wang, L.; Fan, Y. Multi-objective structural optimization and degradation model of magnesium alloy ureteral stent. Med. Nov. Technol. Devices 2024, 22, 100291. [Google Scholar] [CrossRef]
- Sousa, A.M.; Amaro, A.M.; Piedade, A.P. 3D Printing of Polymeric Bioresorbable Stents: A Strategy to Improve Both Cellular Compatibility and Mechanical Properties. Polymers 2022, 14, 1099. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Lu, Y.; Li, X.; Zhou, J.; Zadpoor, A.A.; Wang, L. Additive manufacturing of vascular stents. Acta Biomater. 2023, 167, 16–37. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, L. Research into biodegradable polymeric stents: A review of experimental and modelling work. Vessel. Plus 2018, 2, 12. [Google Scholar] [CrossRef]
- Singh, R.; Bathaei, M.J.; Istif, E.; Beker, L. A Review of Bioresorbable Implantable Medical Devices: Materials, Fabrication, and Implementation. Adv. Healthc. Mater. 2020, 9, e2000790. [Google Scholar] [CrossRef]
- Song, K.; Bi, Y.; Zhao, H.; Wu, T.; Xu, F.; Zhao, G. Structural optimization and finite element analysis of poly-l-lactide acid coronary stent with improved radial strength and acute recoil rate. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2754–2764. [Google Scholar] [CrossRef]
- Tenekecioglu, E.; Poon, E.K.W.; Collet, C.; Thondapu, V.; Torii, R.; Bourantas, C.V.; Zeng, Y.P.; Onuma, Y.; Ooi, A.S.H.; Serruys, P.W.; et al. The Nidus for Possible Thrombus Formation Insight from the Microenvironment of Bioresorbable Vascular Scaffold. JACC Cardiovasc. Interv. 2016, 9, 2167–2168. [Google Scholar] [CrossRef]
- Sotomi, Y.; Onuma, Y.; Collet, C.; Tenekecioglu, E.; Virmani, R.; Kleiman, N.S.; Serruys, P.W. Bioresorbable Scaffold The Emerging Reality and Future Directions. Circ. Res. 2017, 120, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Fan, S.; Wu, J.; Yang, S. Structure design and mechanical performance analysis of three kinds of bioresorbable poly-lactic acid (PLA) stents. Comput. Methods Biomech. Biomed. Eng. 2023, 26, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Torki, M.M.; Hassanajili, S.; Jalisi, M.M. Design optimizations of PLA stent structure by FEM and investigating its function in a simulated plaque artery. Math. Comput. Simul. 2020, 169, 103–116. [Google Scholar] [CrossRef]
- Blair, R.W.; Dunne, N.J.; Lennon, A.B.; Menary, G.H. Multi-objective optimisation of material properties and strut geometry for poly(L-lactic acid) coronary stents using response surface methodology. PLoS ONE 2019, 14, e0218768. [Google Scholar] [CrossRef]
- Abaei, A.R.; Shine, C.J.; Vaughan, T.J.; Ronan, W. An integrated mechanical degradation model to explore the mechanical response of a bioresorbable polymeric scaffold. J. Mech. Behav. Biomed. Mater. 2024, 152, 106419. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.B.; Wang, M.J.; Zhao, D.Y.; Li, H.X.; Jin, Y.F. Structural Design of Mechanical Property for Biodegradable Polymeric Stent. Adv. Mater. Sci. Eng. 2019, 2019, 2960435. [Google Scholar] [CrossRef]
- Kumar, A.; Bhatnagar, N. Finite element simulation and testing of cobalt-chromium stent: A parametric study on radial strength, recoil, foreshortening, and dogboning. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 245–259. [Google Scholar] [CrossRef]
- Sousa, A.M.; Amaro, A.M.; Piedade, A.P. Structural design optimization through finite element analysis of additive manufactured bioresorbable polymeric stents. Mater. Today Chem. 2024, 36, 101972. [Google Scholar] [CrossRef]
- Sanjari, S.; Saraeian, P.; Haghighi, S.E.; Alinia-ziazi, A. Modeling Degradation Behavior of Biodegradable Polymers for Medical Devices: A Comparative Review of Phenomenological and Stochastic Approaches. J. Polym. Environ. 2024, 32, 4794–4821. [Google Scholar] [CrossRef]
- He, S.C.; Wei, L.L.; Wang, G.X.; Pugno, N.M.; Chen, Q.; Li, Z.Y. In Silico Evaluation of In Vivo Degradation Kinetics of Poly(Lactic Acid) Vascular Stent Devices. J. Funct. Biomater. 2024, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, H.; Chen, S.; Du, T.; Zhang, Y.; Qiao, A. Numerical Simulation of Dynamic Degradation and Fatigue Damage of Degradable Zinc Alloy Stents. J. Funct. Biomater. 2023, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, T.; Chen, S.; Liu, Y.; Yang, Y.; Hou, Q.; Qiao, A. Finite Element Analysis of the Non-Uniform Degradation of Biodegradable Vascular Stents. J. Funct. Biomater. 2022, 13, 152. [Google Scholar] [CrossRef]
- Gogas, B.D.; Farooq, V.; Onuma, Y.; Serruys, P.W. The ABSORB bioresorbable vascular scaffold: An evolution or revolution in interventional cardiology? Hell. J. Cardiol. 2012, 53, 301–309. [Google Scholar]
- Qiu, T.Y.; Zhao, L.G.; Song, M. A Computational Study of Mechanical Performance of Bioresorbable Polymeric Stents with Design Variations. Cardiovasc. Eng. Technol. 2019, 10, 46–60. [Google Scholar] [CrossRef]
- He, S.; Liu, W.; Wei, L.; Chen, Q.; Li, Z. A phenomenological model of pulsatile blood pressure-affected degradation of polylactic acid (PLA) vascular stent. Med. Biol. Eng. Comput. 2024, 62, 1347–1359. [Google Scholar] [CrossRef]
- David Chua, S.N.; Mac Donald, B.J.; Hashmi, M.S.J. Finite element simulation of stent and balloon interaction. J. Mater. Process. Technol. 2003, 143–144, 591–597. [Google Scholar] [CrossRef]
- Grabow, N.; Schlun, M.; Sternberg, K.; Hakansson, N.; Kramer, S.; Schmitz, K.P. Mechanical properties of laser cut poly(L-lactide) micro-specimens: Implications for stent design, manufacture, and sterilization. J. Biomech. Eng. 2005, 127, 25–31. [Google Scholar] [CrossRef]
- Liu, S.; He, S.; Chen, C.; Li, C.; Luo, W.; Zheng, K.; Wang, J.; Li, Z.; He, H.; Chen, Q.; et al. A Versatile Disorder-to-Order Technology to Upgrade Polymers into High-Performance Bioinspired Materials. Adv. Healthc. Mater. 2023, 12, e2300068. [Google Scholar] [CrossRef]
- Qiu, T.; He, R.; Abunassar, C.; Hossainy, S.; Zhao, L.G. Effect of two-year degradation on mechanical interaction between a bioresorbable scaffold and blood vessel. J. Mech. Behav. Biomed. Mater. 2018, 78, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Abbaslou, M.; Hashemi, R.; Etemadi, E. Novel hybrid 3D-printed auxetic vascular stent based on re-entrant and meta-trichiral unit cells: Finite element simulation with experimental verifications. Mater. Today Commun. 2023, 35, 105742. [Google Scholar] [CrossRef]
- Antonini, L.; Poletti, G.; Mandelli, L.; Dubini, G.; Pennati, G.; Petrini, L. Comprehensive computational analysis of the crimping procedure of PLLA BVS: Effects of material viscous-plastic and temperature dependent behavior. J. Mech. Behav. Biomed. Mater. 2021, 123, 104713. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Hao, J.; Duan, F.; Mitchell, K.; Jin, Y.; Zhao, D. Mechanical analysis of radial performance in biodegradable polymeric vascular stents manufactured using micro-injection molding. J. Mech. Behav. Biomed. Mater. 2024, 150, 106362. [Google Scholar] [CrossRef]
- Bobel, A.C.; McHugh, P.E. Computational Analysis of the Utilisation of the Shape Memory Effect and Balloon Expansion in Fully Polymeric Stent Deployment. Cardiovasc. Eng. Technol. 2018, 9, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Khalaj Amnieh, S.; Mashayekhi, M.; Shahnooshi, E.; Tavafoghi, M.; Mosaddegh, P. Biodegradable performance of PLA stents affected by geometrical parameters: The risk of fracture and fragment separation. J. Biomech. 2021, 122, 110489. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Shen, Z.; Miao, F.; Wang, J.; Sun, Y.; Zhu, S.; Zheng, Y.; Guan, S. A biodegradable magnesium alloy vascular stent structure: Design, optimisation and evaluation. Acta Biomater. 2022, 142, 402–412. [Google Scholar] [CrossRef]
- Khalilimeybodi, A.; Alishzadeh Khoei, A.; Sharif-Kashani, B. Future Balloon-Expandable Stents: High or Low-Strength Materials? Cardiovasc. Eng. Technol. 2020, 11, 188–204. [Google Scholar] [CrossRef]
- Schmidt, W.; Lanzer, P.; Behrens, P.; Topoleski, L.D.; Schmitz, K.P. A comparison of the mechanical performance characteristics of seven drug-eluting stent systems. Catheter. Cardiovasc. Interv. 2009, 73, 350–360. [Google Scholar] [CrossRef]
- Pan, C.; Han, Y.; Lu, J. Structural Design of Vascular Stents: A Review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef]
- Tanaka, N.; Martin, J.B.; Tokunaga, K.; Abe, T.; Uchiyama, Y.; Hayabuchi, N.; Berkefeld, J.; Rufenacht, D.A. Conformity of carotid stents with vascular anatomy: Evaluation in carotid models. AJNR Am. J. Neuroradiol. 2004, 25, 604–607. [Google Scholar] [PubMed]
- Iantorno, M.; Lipinski, M.J.; Garcia-Garcia, H.M.; Forrestal, B.J.; Rogers, T.; Gajanana, D.; Buchanan, K.D.; Torguson, R.; Weintraub, W.S.; Waksman, R. Meta-Analysis of the Impact of Strut Thickness on Outcomes in Patients with Drug-Eluting Stents in a Coronary Artery. Am. J. Cardiol. 2018, 122, 1652–1660. [Google Scholar] [CrossRef]
- Nakatani, S.; Nishino, M.; Taniike, M.; Makino, N.; Kato, H.; Egami, Y.; Shutta, R.; Tanouchi, J.; Yamada, Y. Initial findings of impact of strut width on stent coverage and apposition of sirolimus-eluting stents assessed by optical coherence tomography. Catheter. Cardiovasc. Interv. 2013, 81, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, C.; Wei, Y.; Qi, C.; Bai, J.; Guo, C.; Xue, F.; Lin, P.; Chu, P.K. In vitro degradation kinetics of pure PLA and Mg/PLA composite: Effects of immersion temperature and compression stress. Acta Biomater. 2017, 48, 468–478. [Google Scholar] [CrossRef]
- Bergstrom, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.C.; Espinosa, F.M.; Garcia, R.; Foschi, G.; Tortorella, S.; Valle, F.; Dallavalle, M.; Zerbetto, F.; Biscarini, F. In situ nanomechanical characterization of the early stages of swelling and degradation of a biodegradable polymer. Nanoscale 2015, 7, 5403–5410. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, G.; Zhao, Y.H.; Wang, G.H.; Cai, T. Computational and experimental investigation into mechanical performances of Poly-L-Lactide Acid (PLLA) coronary stents. J. Mech. Behav. Biomed. 2017, 65, 415–427. [Google Scholar] [CrossRef]
- Alfonso, F.; Coughlan, J.J.; Giacoppo, D.; Kastrati, A.; Byrne, R.A. Management of in-stent restenosis. EuroIntervention 2022, 18, e103–e123. [Google Scholar] [CrossRef]
- Farooq, V.; Gogas, B.D.; Serruys, P.W. Restenosis: Delineating the numerous causes of drug-eluting stent restenosis. Circ. Cardiovasc. Interv. 2011, 4, 195–205. [Google Scholar] [CrossRef]
- Liu, W.; Huang, J.; He, S.; Du, R.; Shi, W.; Wang, Y.; Du, D.; Du, Y.; Liu, Q.; Wang, Y.; et al. Senescent endothelial cells’ response to the degradation of bioresorbable scaffold induces intimal dysfunction accelerating in-stent restenosis. Acta Biomater. 2023, 166, 266–277. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Jiang, Y.; Yuan, Y. Optimizing the compression resistance of low-nickel stainless steel coronary stents using finite element and response surface methodology. J. Biomech. 2024, 172, 112227. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.S.; Folgado, J.; Rodrigues, H.C. Surrogate-based multi-objective design optimization of a coronary stent: Altering geometry towards improved biomechanical performance. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3453. [Google Scholar] [CrossRef] [PubMed]
- Foin, N.; Lee, R.D.; Torii, R.; Guitierrez-Chico, J.L.; Mattesini, A.; Nijjer, S.; Sen, S.; Petraco, R.; Davies, J.E.; Di Mario, C.; et al. Impact of stent strut design in metallic stents and biodegradable scaffolds. Int. J. Cardiol. 2014, 177, 800–808. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Parameter Symbols | Value | Unit |
|---|---|---|---|
| Young’s modulus of PLA | E | 3000 [29] | MPa |
| Yield strength of PLA | 50 [30] | MPa | |
| Poisson’s ratio of PLA | 0.3 [31] | - | |
| Elastic constants of vessel | 1.02 [27] | MPa | |
| 0.71 [27] | MPa | ||
| Elastic constants of balloon | 1.07 [28] | MPa | |
| 0.71 [28] | MPa |
| Stent | Radial Stiffness (N/mm) | Radial Strength (N/mm) |
|---|---|---|
| Base | 34.32 | 0.24 |
| In-10% | 51.91 | 0.33 |
| In-20% | 57.57 | 0.36 |
| In-40% | 83.72 | 0.49 |
| Both-10% | 43.74 | 0.28 |
| Both-20% | 48.75 | 0.32 |
| Both-40% | 64.34 | 0.40 |
| Out-10% | 37.19 | 0.25 |
| Out-20% | 40.10 | 0.27 |
| Out-40% | 44.47 | 0.30 |
| Stent | Bending Stiffness (N·mm2) |
|---|---|
| Base | 3.61 |
| In-10% | 5.13 |
| In-20% | 5.27 |
| In-40% | 5.90 |
| Both-10% | 4.91 |
| Both-20% | 5.10 |
| Both-40% | 5.63 |
| Out-10% | 4.68 |
| Out-20% | 5.10 |
| Out-40% | 5.58 |
| Stent | Material | Dexpan (mm) | Dcrimp (mm) | Recoil% |
|---|---|---|---|---|
| Base | PLA | 3.19 | 3.11 | 2.50 |
| In-10% | 3.15 | 3.11 | 1.27 | |
| In-20% | 3.11 | 3.07 | 1.29 | |
| In-40% | 3.06 | 3.03 | 0.98 | |
| Wang et al. [47] | PLLA | 3.70 | - | ~2.00 |
| 3.92 | - | ~2.00 | ||
| 3.10 | - | 4.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Chen, Q.; Li, Z. Influence of Stent Structure on Mechanical and Degradation Properties of Poly (Lactic Acid) Vascular Stent. J. Funct. Biomater. 2025, 16, 248. https://doi.org/10.3390/jfb16070248
He S, Chen Q, Li Z. Influence of Stent Structure on Mechanical and Degradation Properties of Poly (Lactic Acid) Vascular Stent. Journal of Functional Biomaterials. 2025; 16(7):248. https://doi.org/10.3390/jfb16070248
Chicago/Turabian StyleHe, Shicheng, Qiang Chen, and Zhiyong Li. 2025. "Influence of Stent Structure on Mechanical and Degradation Properties of Poly (Lactic Acid) Vascular Stent" Journal of Functional Biomaterials 16, no. 7: 248. https://doi.org/10.3390/jfb16070248
APA StyleHe, S., Chen, Q., & Li, Z. (2025). Influence of Stent Structure on Mechanical and Degradation Properties of Poly (Lactic Acid) Vascular Stent. Journal of Functional Biomaterials, 16(7), 248. https://doi.org/10.3390/jfb16070248








