Accompanying Titanium Meshes and Titanium-Reinforced Membranes with Collagen Membranes in Vertical Alveolar Ridge Augmentations: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Types of Studies
2.1.2. Population
2.1.3. Intervention
2.1.4. Types of Outcome Measures
Primary Outcome
Secondary Outcomes
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Collection
2.4. Data Items
2.5. Quality Assessment
2.6. Synthesis Methods
3. Results
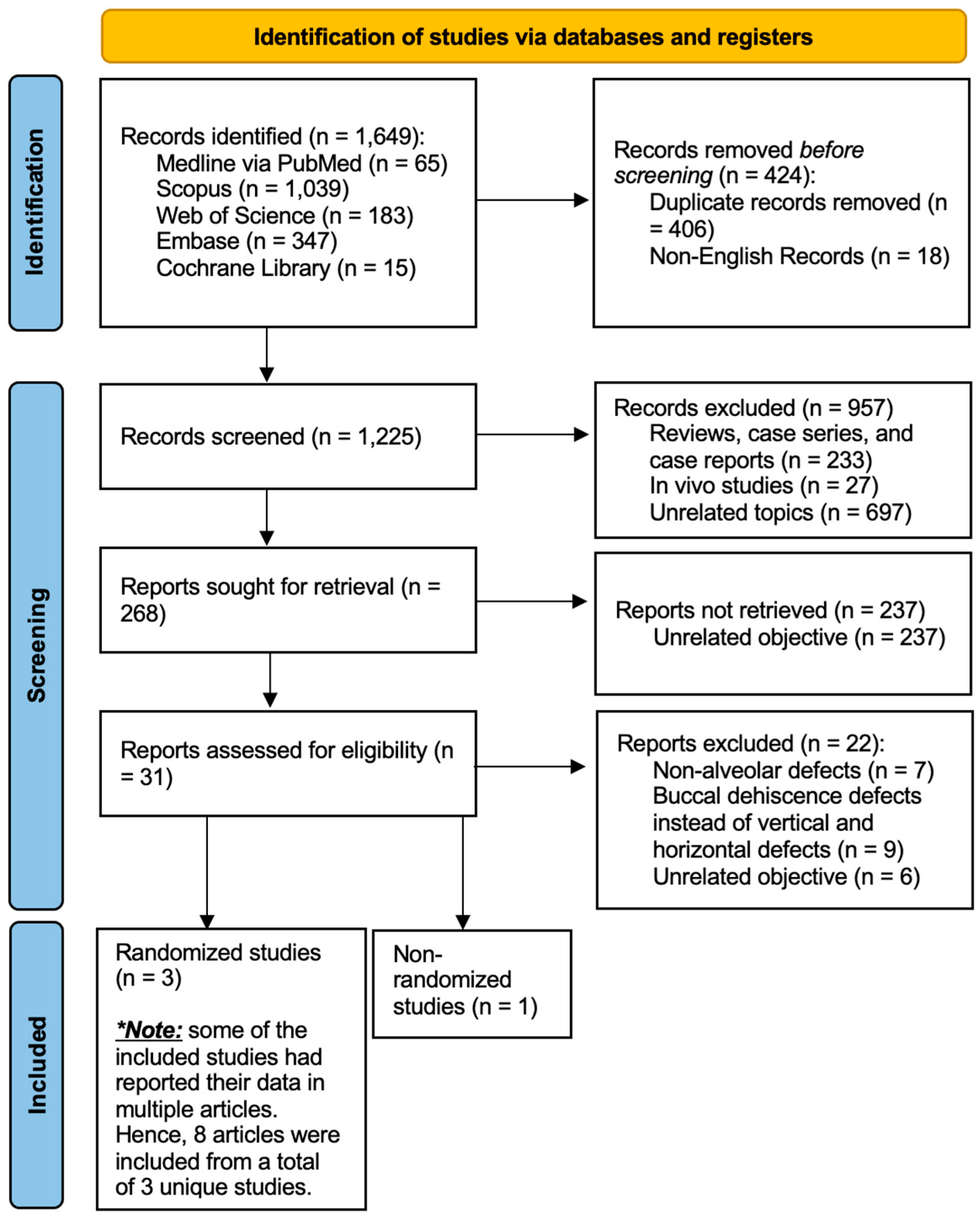
3.1. Study Selection
3.2. Results of Individual Studies
3.3. Study Characteristics
3.3.1. Study Design
3.3.2. Demographics
3.3.3. Patient Enrollment Exclusion Criteria
3.3.4. Surgical Rationale and Procedure
3.3.5. Alveolar Defects
3.3.6. Dental Implantation
3.3.7. Study Variables
3.4. Outcomes and Complications
3.4.1. Surgical and Healing Complications
3.4.2. Vertical Bone Gain and Regeneration Rate
3.4.3. Bone Density and Quality
3.4.4. Dental Implant Osseointegration and Stability
3.5. Quality Assessments
4. Discussion
4.1. Study Limitations and Suggestions
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alotaibi, F.F.; Rocchietta, I.; Buti, J.; D’Aiuto, F. Comparative Evidence of Different Surgical Techniques for the Management of Vertical Alveolar Ridge Defects in Terms of Complications and Efficacy: A Systematic Review and Network Meta-analysis. J. Clin. Periodontol. 2023, 50, 1487–1519. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Monje, A.; Tomasi, C. Long-term Biological Complications of Dental Implants Placed Either in Pristine or in Augmented Sites: A Systematic Review and Meta-analysis. Clin. Oral Implant. Res. 2018, 29, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of Vertical Ridge Augmentation Interventions: A Systematic Review and Meta-analysis. J. Clin. Periodontol. 2019, 46, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Boyapati, L. “PASS” Principles for Predictable Bone Regeneration. Implant. Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Rocchietta, I.; Fontana, F.; Simion, M. Clinical Outcomes of Vertical Bone Augmentation to Enable Dental Implant Placement: A Systematic Review. J. Clin. Periodontol. 2008, 35, 203–215. [Google Scholar] [CrossRef]
- Jepsen, S.; Schwarz, F.; Cordaro, L.; Derks, J.; Hämmerle, C.H.F.; Heitz-Mayfield, L.J.; Hernández-Alfaro, F.; Meijer, H.J.A.; Naenni, N.; Ortiz-Vigón, A.; et al. Regeneration of Alveolar Ridge Defects. Consensus Report of Group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 277–286. [Google Scholar] [CrossRef]
- Tay, J.R.H.; Ng, E.; Lu, X.J.; Lai, W.M.C. Healing Complications and Their Detrimental Effects on Bone Gain in Vertical-guided Bone Regeneration: A Systematic Review and Meta-analysis. Clin. Implant. Dent. Relat. Res. 2022, 24, 43–71. [Google Scholar] [CrossRef]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical Classification of Complications in Guided Bone Regeneration Procedures by Means of a Nonresorbable Membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265–273. [Google Scholar]
- Sanz-Sánchez, I.; Sanz-Martín, I.; Ortiz-Vigón, A.; Molina, A.; Sanz, M. Complications in Bone-grafting Procedures: Classification and Management. Periodontology 2000 2022, 88, 86–102. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Z.; Yun, J.; Liu, R.; Li, J.; Chen, Y.; Cai, H.; Jiang, H.B.; Lee, E.-S.; Han, J.; et al. Effect of Different Membranes on Vertical Bone Regeneration: A Systematic Review and Network Meta-Analysis. BioMed Res. Int. 2022, 2022, 7742687. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological Principle and Therapeutic Applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Moscatelli, M.; Mariotti, G.; Rotundo, R.; Bernardelli, F.; Nieri, M. Bone Level Variation After Vertical Ridge Augmentation: Resorbable Barriers Versus Titanium-Reinforced Barriers. A 6-Year Double-Blind Randomized Clinical Trial. Int. J. Oral Maxillofac. Implant. 2014, 29, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Moscatelli, M.; Mazzoni, A.; Mazzoni, S.; Pagliaro, U.; Breschi, L.; Motroni, A.; Nieri, M. Fence Technique: Guided Bone Regeneration for Extensive Three- Dimensional Augmentation. Int. J. Periodontics Restor. Dent. 2013, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Nagursky, H.; Lozada, J.L.; Nagy, K. Horizontal Ridge Augmentation with a Collagen Membrane and a Combination of Particulated Autogenous Bone and Anorganic Bovine Bone–Derived Mineral: A Prospective Case Series in 25 Patients. Int. J. Periodontics Restor. Dent. 2013, 33, 299–307. [Google Scholar] [CrossRef]
- Urban, I.A.; Mirsky, N.; Serroni, M.; Tovar, N.; Nayak, V.V.; Witek, L.; Marin, C.; Saleh, M.; Ravidà, A.; Baczko, I.; et al. Elucidating the Benefit of Perforated vs Nonperforated Membranes in Guided Bone Regeneration: An in Vivo Histologic Evaluation and Histomorphometric Analysis. Int. J. Periodontics Restor. Dent. 2024, 45, 341–355. [Google Scholar] [CrossRef]
- Dahlin, C.; Sennerby, L.; Lekholm, U.; Linde, A.; Nyman, S. Generation of New Bone around Titanium Implants Using a Membrane Technique: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implant. 1989, 4, 19–25. [Google Scholar]
- Schenk, R.K.; Buser, D.; Hardwick, W.R.; Dahlin, C. Healing Pattern of Bone Regeneration in Membrane-Protected Defects: A Histologic Study in the Canine Mandible. Int. J. Oral Maxillofac. Implant. 1994, 9, 13–29. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Funato, A.; Ishikawa, T.; Kitajima, H.; Yamada, M.; Moroi, H. A Novel Combined Surgical Approach to Vertical Alveolar Ridge Augmentation with Titanium Mesh, Resorbable Membrane, and RhPDGF-BB: A Retrospective Consecutive Case Series. Int. J. Periodontics Restor. Dent. 2013, 33, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of Complication Rates and Vertical Bone Gain after Guided Bone Regeneration with Non-resorbable Membranes versus Titanium Meshes and Resorbable Membranes. A Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and Histomorphometric Analysis of Bone Tissue after Guided Bone Regeneration with Non-resorbable Membranes vs Resorbable Membranes and Titanium Mesh. Clin. Implant. Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Franceschi, D.; Randellini, E.; Lizio, G.; Fiorino, A.; Corinaldesi, G. Vertical and Horizontal Ridge Augmentation Using Customized CAD/CAM Titanium Mesh with versus without Resorbable Membranes. A Randomized Clinical Trial. Clin. Oral Implant. Res. 2021, 32, 1411–1424. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Fiorino, A.; Pellegrino, G.; Corinaldesi, G. Vertical Ridge Augmentation (VRA) with Ti-reinforced D-PTFE Membranes or Ti Meshes and Collagen Membranes: 1-year Results of a Randomized Clinical Trial. Clin. Oral Implant. Res. 2021, 32, 1–14. [Google Scholar] [CrossRef]
- Cucchi, A.; Bettini, S.; Ghensi, P.; Fiorino, A.; Corinaldesi, G. Vertical Ridge Augmentation with Ti-reinforced Dense Polytetrafluoroethylene (D-PTFE) Membranes or Ti-meshes and Collagen Membranes: 3-year Results of a Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2023, 25, 352–369. [Google Scholar] [CrossRef]
- Cucchi, A.; Marchiori, G.; Sartori, M.; Fini, M.; Fiorino, A.; Donati, R.; Corinaldesi, G.; Maglio, M. A 3D Micro-CT Assessment of Composition and Structure of Bone Tissue after Vertical and Horizontal Alveolar Ridge Augmentation Using CAD/CAM-customized Titanium Mesh. Clin. Oral Implant. Res. 2024, 35, 1546–1559. [Google Scholar] [CrossRef]
- Cucchi, A.; Bettini, S.; Fiorino, A.; Maglio, M.; Marchiori, G.; Corinaldesi, G.; Sartori, M. Histological and Histomorphometric Analysis of Bone Tissue Using Customized Titanium Meshes with or without Resorbable Membranes: A Randomized Clinical Trial. Clin. Oral Implant. Res. 2024, 35, 114–130. [Google Scholar] [CrossRef]
- Urban, I.A.; Serroni, M.; Dias, D.R.; Baráth, Z.; Forster, A.; Araújo, T.G.; Saleh, M.H.A.; Cucchi, A.; Ravidà, A. Impact of Collagen Membrane in Vertical Ridge Augmentation Using Ti-Reinforced PTFE Mesh: A Randomised Controlled Trial. J. Clin. Periodontol. 2025, 52, 575–588. [Google Scholar] [CrossRef]
- Cucchi, A.; Sartori, M.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. A Proposal of Pseudo-Periosteum Classification After GBR by Means of Titanium-Reinforced d-PTFE Membranes or Titanium Meshes Plus Cross-Linked Collagen Membranes. Int. J. Periodontics Restor. Dent. 2019, 39, e157–e165. [Google Scholar] [CrossRef]
- Hartmann, A.; Hildebrandt, H.; Schmohl, J.U.; Kämmerer, P.W. Evaluation of Risk Parameters in Bone Regeneration Using a Customized Titanium Mesh: Results of a Clinical Study. Implant. Dent. 2019, 28, 543–550. [Google Scholar] [CrossRef]
- Ciocca, L.; Lizio, G.; Baldissara, P.; Sambuco, A.; Scotti, R.; Corinaldesi, G. Prosthetically CAD-CAM–Guided Bone Augmentation of Atrophic Jaws Using Customized Titanium Mesh: Preliminary Results of an Open Prospective Study. J. Oral Implantol. 2018, 44, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Herr, Y.; Kwon, Y.; Chung, J. Effect of a Collagen Membrane Combined with a Porous Titanium Membrane on Exophytic New Bone Formation in a Rabbit Calvarial Model. J. Periodontol. 2013, 84, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Paeng, K.; Cha, J.; Thoma, D.S.; Jung, R.E.; Jung, U.; Benic, G.I. Effect of Collagen Membrane and of Bone Substitute on Lateral Bone Augmentation with Titanium Mesh: An Experimental in Vivo Study. Clin. Oral Implant. Res. 2022, 33, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Gutta, R.; Baker, R.A.; Bartolucci, A.A.; Louis, P.J. Barrier Membranes Used for Ridge Augmentation: Is There an Optimal Pore Size? J. Oral Maxillofac. Surg. 2009, 67, 1218–1225. [Google Scholar] [CrossRef]
- Barber, H.D.; Lignelli, J.; Smith, B.M.; Bartee, B.K. Using a Dense PTFE Membrane Without Primary Closure to Achieve Bone and Tissue Regeneration. J. Oral Maxillofac. Surg. 2007, 65, 748–752. [Google Scholar] [CrossRef]
- Gu, C.; Xu, L.; Shi, A.; Guo, L.; Chen, H.; Qin, H. Titanium Mesh Exposure in Guided Bone Regeneration Procedures: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2022, 37, e29–e40. [Google Scholar] [CrossRef]
- Urban, I.A.; Saleh, M.H.A.; Ravidà, A.; Forster, A.; Wang, H.; Barath, Z. Vertical Bone Augmentation Utilizing a Titanium-reinforced PTFE Mesh: A Multi-variate Analysis of Influencing Factors. Clin. Oral Implant. Res. 2021, 32, 828–839. [Google Scholar] [CrossRef]
| Data Base | Date | Search Query | Results |
|---|---|---|---|
| Medline via PubMed | 5 June 2025 | (“Alveolar Ridge Augmentation” [MeSH] OR “Ridge Augmentation” OR “Bone Regeneration” [MeSH] OR “Bone Regeneration”) AND (“Collagen Membrane” OR (“Collagen” AND “Membrane”) OR “Resorbable Membrane” OR (“Resorbable” AND “Membrane”)) AND (“Surgical Mesh” [MeSH] OR “Mesh” OR “Titanium Mesh” OR (“Titanium” AND “Mesh”) OR “Ti-Reinforced Mesh” OR (“Ti-Reinforced” AND “Mesh”)) | 65 |
| Scopus | 5 June 2025 | TITLE-ABS-KEY (bone AND augmentation OR alveolar AND ridge AND augmentation) AND (collagen) AND (titanium AND mesh OR titanium OR mesh OR membrane) | 1039 |
| Web of Science | 5 June 2025 | (ALL = (alveolar ridge augmentation) OR ALL = (bone regeneration) OR ALL = (ridge augmentation) OR ALL = (guided bone regeneration)) AND (ALL = (collagen membrane) OR ALL = (resorbable membrane)) AND (ALL = (titanium mesh) OR ALL = (Ti-reinforced mesh) OR ALL = (Ti-reinforced membrane) OR ALL = (mesh)) | 183 |
| Embase | 5 June 2025 | (Alveolar ridge) AND (Collagen) AND (Titanium OR Mesh OR Membrane) | 347 |
| Cochrane Library | 5 June 2025 | Alveolar Ridge Augmentation [MeSH] AND Surgical Mesh [MeSH] AND Collagen | 15 |
| Groups | Meshes and Membranes | Patients | Studies |
|---|---|---|---|
| 1 | Titanium mesh | 15 | 1 [24,27,28] |
| 2 | Titanium mesh + cross-linked collagen | 35 | 2 [22,23,24,25,26,27,28] |
| 3 | Non-perforated Ti-reinforced dPTFE | 20 | 1 [22,23,25,26] |
| 4 | Perforated Ti-reinforced dPTFE | 15 | 1 [29] |
| 5 | Perforated Ti-reinforced dPTFE + non-cross-linked collagen | 15 | 1 [29] |
| 6 | Titanium mesh + cross-linked collagen (Case Series) | 19 | 1 [21] |
| Number | Study Variable | Evaluation Method | Evaluation Period | References |
|---|---|---|---|---|
| 1 | Surgical and healing complications | The Fontana et al.’s classification | T6 | 1 [24,27,28] |
| T9 | 2 [22,23,25,26,29] | |||
| 2 | Pseudo-periosteum | Clinical examination | T6 | 1 [24,27,28] |
| T9 | 1 [29] | |||
| 3 | Vertical bone gain | CBCT | T6 | 1 [24,27,28] |
| T9 | 2 [22,23,25,26,29] | |||
| 4 | Regeneration rate | CBCT | T6 | 1 [24,27,28] |
| T9 | 1 [29] | |||
| 5 | Bone quality | Histology and micro CT (sampling at least 4 mm of depth with a trephine bur) | T9 | 2 [22,23,24,25,26,27,28] |
| 6 | Implant osseointegration | Reverse (counter) torque at 25 N/cm | T9: 9 months after bone augmentation and 3 months after implantation | 1 [24,27,28] |
| T9: 9 months after bone augmentation and implantation | 1 [22,23,25,26] | |||
| 7 | Implant stability and survival | Resonance frequency analysis in ISQ | T9: 9 months after bone augmentation and 3 months after implantation | 1 [24,27,28] |
| T9: 9 months after bone augmentation and implantation | 1 [22,23,25,26] |
| Groups | Meshes and Membranes | Surgical Complications ‡ | Healing Complications ‡ | Pseudo-Periosteum § | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Class A | Class B | Class C | Class I | Class II | Class III | Class IV | Type 1 | Type 2 | Type 3 | ||
| 1 | Titanium mesh | 1/15 | 1/15 | - | - | 2/15 | 1/15 | 2/15 | 7/15 | 4/15 | 4/15 |
| 2 | Titanium mesh + cross-linked collagen | 2/34 | 6/34 | - | - | 2/34 | 3/34 | 1/34 | 10/15 † | 4/15 † | 1/15 † |
| 3 | Non-perforated Ti-reinforced dPTFE | - | 1/20 | - | - | 1/20 | 1/20 | 1/20 | NS | NS | NS |
| 4 | Perforated Ti-reinforced dPTFE | - | - | - | 1/15 | - | - | - | 4/15 * | 10/15 | 1/15 |
| 5 | Perforated Ti-reinforced dPTFE + non-cross-linked collagen | - | - | - | 1/15 | - | - | - | 11/15 * | 4/15 | 0/15 |
| 6 | Titanium mesh + cross-linked collagen (Case Series) | - | - | - | - | 1/19 | - | 1/19 | NS | NS | NS |
| Groups | Meshes and Membranes | Vertical Bone Gain (Mean ± SD (mm)) * | Regeneration Rate | References | ||||
|---|---|---|---|---|---|---|---|---|
| Mesial | Distal | Buccal | Lingual | Overall | ||||
| 1 | Titanium mesh | NS | NS | NS | NS | 4.74 ± 2.56 | 74.32 ± 22.10% | [24,27,28] |
| 2 | Titanium mesh + cross-linked collagen | NS | NS | NS | NS | 6.36 ± 2.31 | 82.30 ± 17.98% | |
| 3.3 ± 1.0 | 4.0 ± 1.0 | 5.1 ± 1.4 | 3.8 ± 0.8 | 4.1 ± 1.0 | 42.1 ± 18.1% † | [22,23,25,26] | ||
| 3 | Non-perforated Ti-reinforced dPTFE | 3.6 ± 1.2 | 4.1 ± 1.0 | 5.0 ± 1.0 | 4.2 ± 1.2 | 4.20 ± 1.00 | 39.7 ± 11.4% † | [22,23,25,26] |
| 4 | Perforated Ti-reinforced dPTFE | NS | NS | NS | NS | 4.47 ± 2.05 | 69.30 ± 17.90% | [29] |
| 5 | Perforated Ti-reinforced dPTFE + non-cross-linked collagen | NS | NS | NS | NS | 4.11 ± 2.69 | 72.3 ± 16.4% | |
| 6 | Titanium mesh + cross-linked collagen (Case Series) | NS | NS | NS | NS | 8.6 ± 4.0 | 85.8 ± 25.6% | [21] |
| Groups | Meshes and Membranes | Survival Rates | Osseointegration * | Resonance Frequency Analysis ** | References |
|---|---|---|---|---|---|
| 1 | Titanium mesh | 94.11% | >35 N/cm: 66.66% <35 N/cm: 33.33% | NS | [24,27,28] ‡ |
| 2 | Titanium mesh + cross-linked collagen | 97.29% | >35 N/cm: 60% <35 N/cm: 40% | NS | |
| 100% † | >35 N/cm: 100% | 66.5 ± 10.00 ISQ | [22,23,25,26] † | ||
| 3 | Non-perforated Ti-reinforced dPTFE | 100% † | >35 N/cm: 100% | 71.00 ± 8.00 ISQ | [22,23,25,26] † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi-Koma, A.-A.; Amid, R.; Moscowchi, A.; Nokhbatolfoghahaei, H.; Kadkhodazadeh, M. Accompanying Titanium Meshes and Titanium-Reinforced Membranes with Collagen Membranes in Vertical Alveolar Ridge Augmentations: A Systematic Review. J. Funct. Biomater. 2025, 16, 246. https://doi.org/10.3390/jfb16070246
Yousefi-Koma A-A, Amid R, Moscowchi A, Nokhbatolfoghahaei H, Kadkhodazadeh M. Accompanying Titanium Meshes and Titanium-Reinforced Membranes with Collagen Membranes in Vertical Alveolar Ridge Augmentations: A Systematic Review. Journal of Functional Biomaterials. 2025; 16(7):246. https://doi.org/10.3390/jfb16070246
Chicago/Turabian StyleYousefi-Koma, Amir-Ali, Reza Amid, Anahita Moscowchi, Hanieh Nokhbatolfoghahaei, and Mahdi Kadkhodazadeh. 2025. "Accompanying Titanium Meshes and Titanium-Reinforced Membranes with Collagen Membranes in Vertical Alveolar Ridge Augmentations: A Systematic Review" Journal of Functional Biomaterials 16, no. 7: 246. https://doi.org/10.3390/jfb16070246
APA StyleYousefi-Koma, A.-A., Amid, R., Moscowchi, A., Nokhbatolfoghahaei, H., & Kadkhodazadeh, M. (2025). Accompanying Titanium Meshes and Titanium-Reinforced Membranes with Collagen Membranes in Vertical Alveolar Ridge Augmentations: A Systematic Review. Journal of Functional Biomaterials, 16(7), 246. https://doi.org/10.3390/jfb16070246










