Prevention of Tooth Discoloration Using Fluoride Varnish Immediately After Bleaching
Abstract
1. Introduction
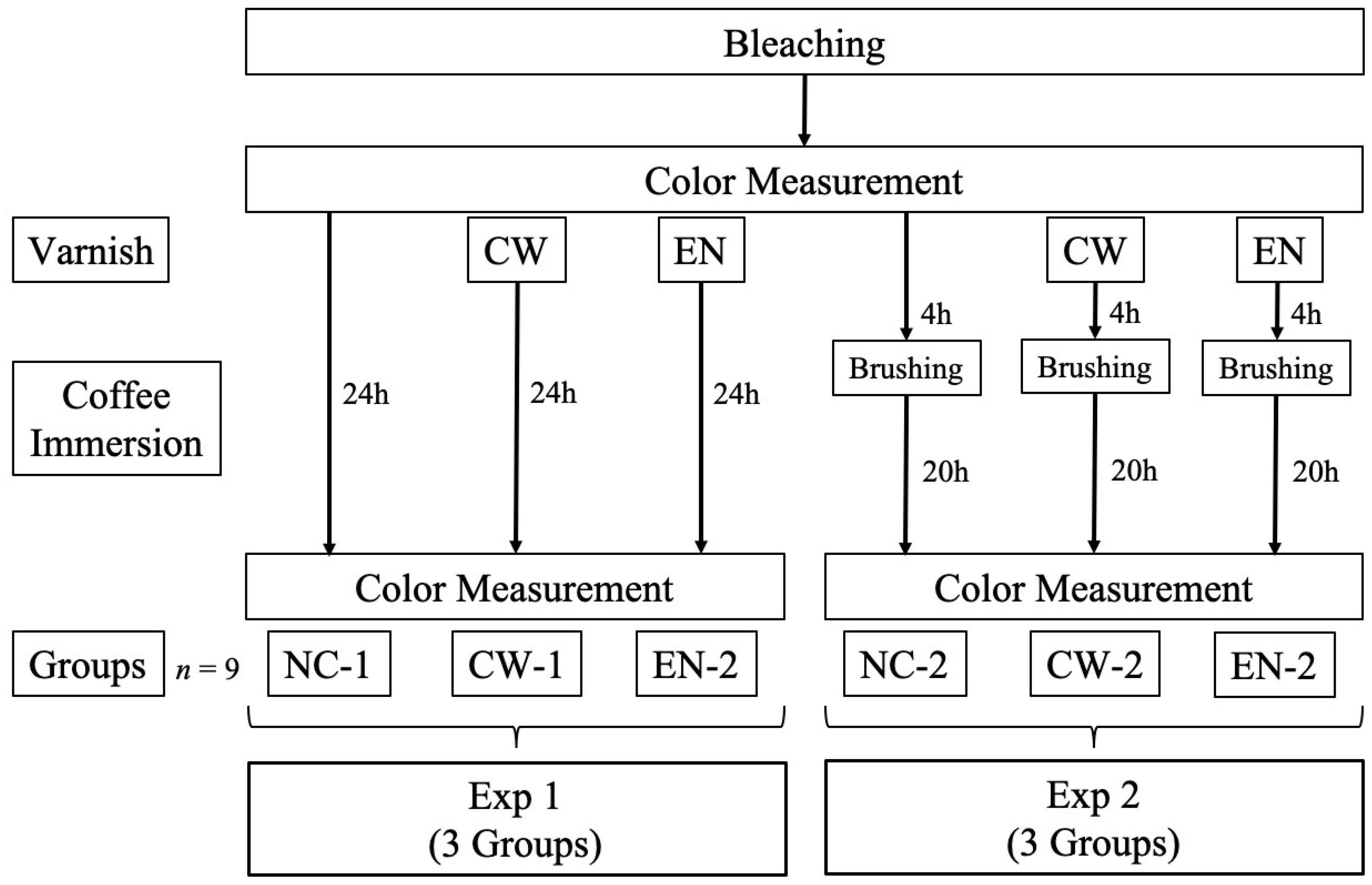
2. Materials and Methods
2.1. Experimental Materials
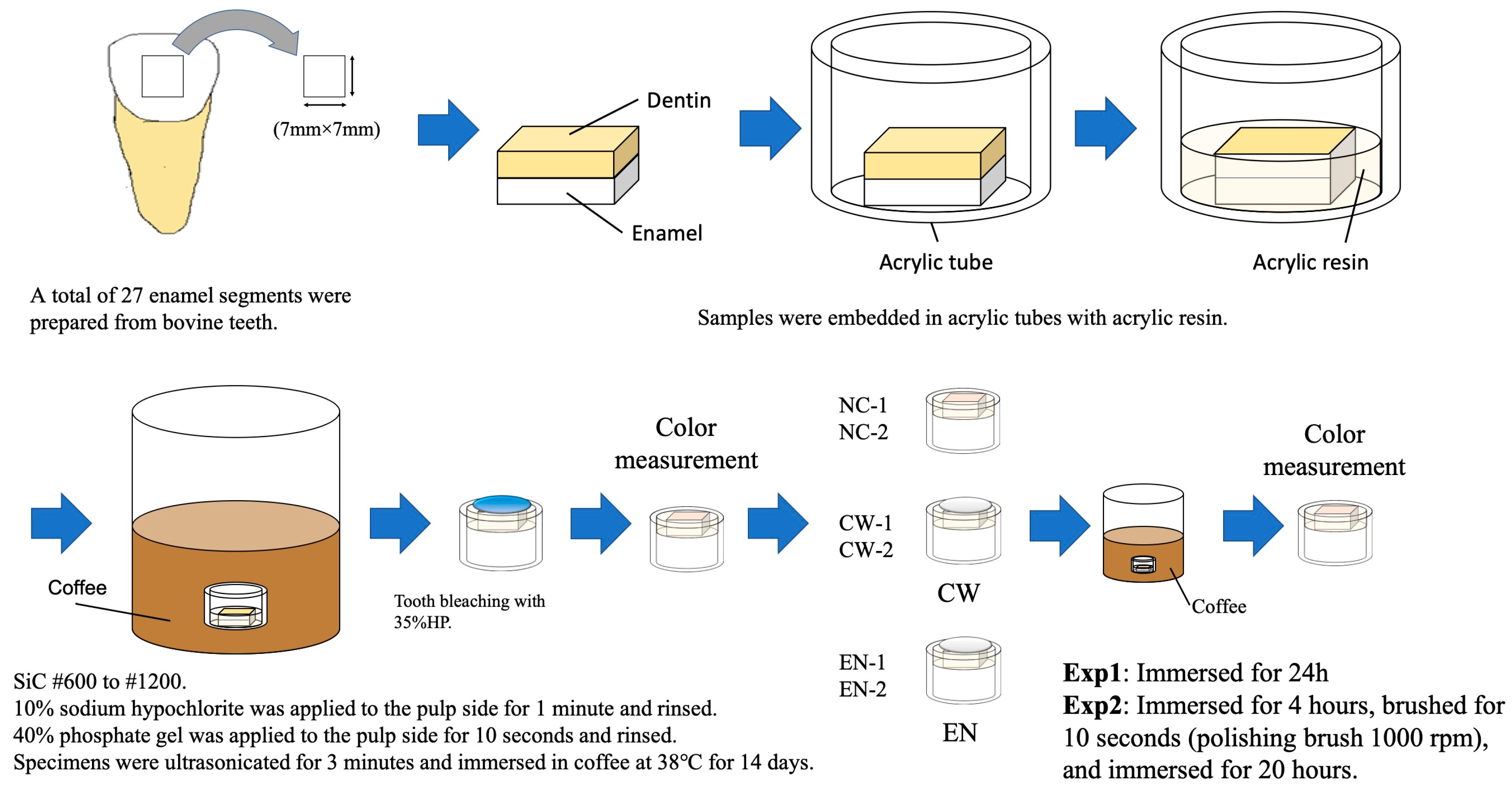
2.2. Preparation of Specimens
2.3. Color Measurement
- Exp 1: Immersion in coffee for 24 h after bleaching.
- Exp 2: Simulation of a clinical scenario in which patients brush their teeth after varnish application. The specimens were subsequently immersed in coffee for 24 h after bleaching. After 4 h of immersion, the samples were removed, brushed, and reimmersed. Brushing was performed for 10 s at 1000 rpm using a Robinson brush (Mersage brush, Shofu, Kyoto, Japan).
2.4. Scanning Electron Microscope (SEM) Observation
2.5. Statistical Analysis
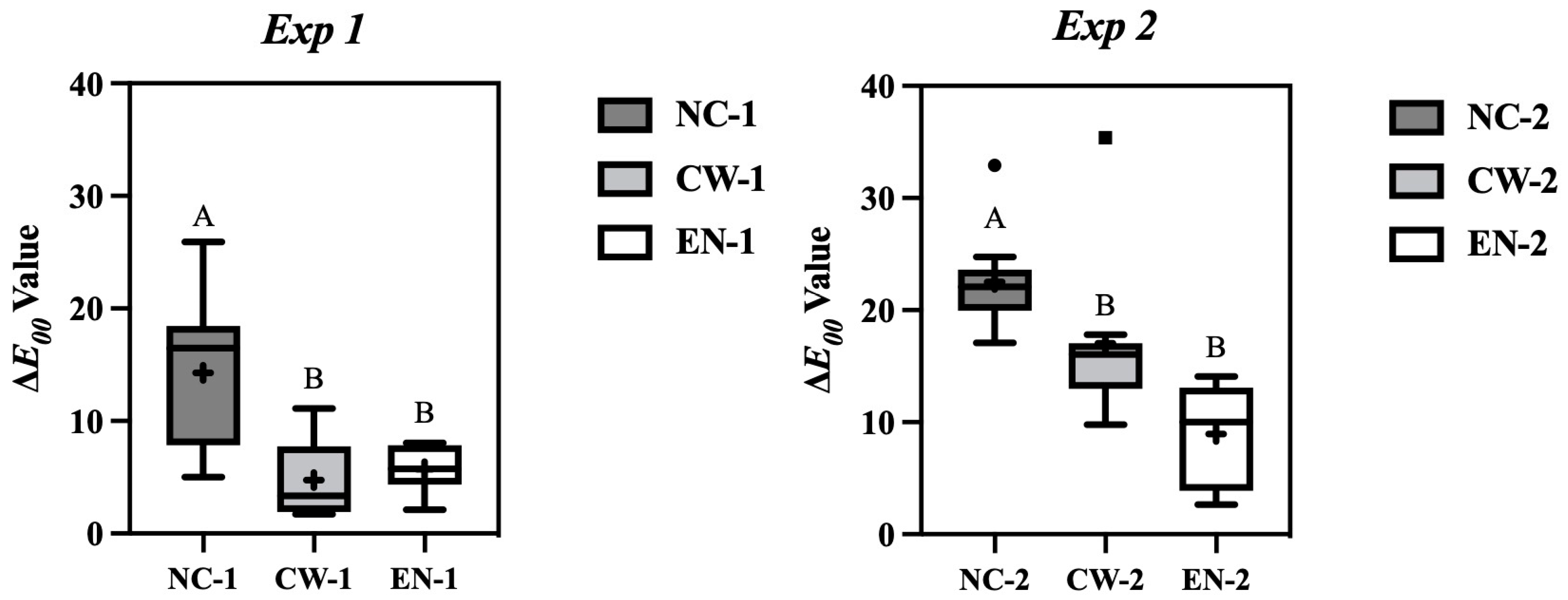
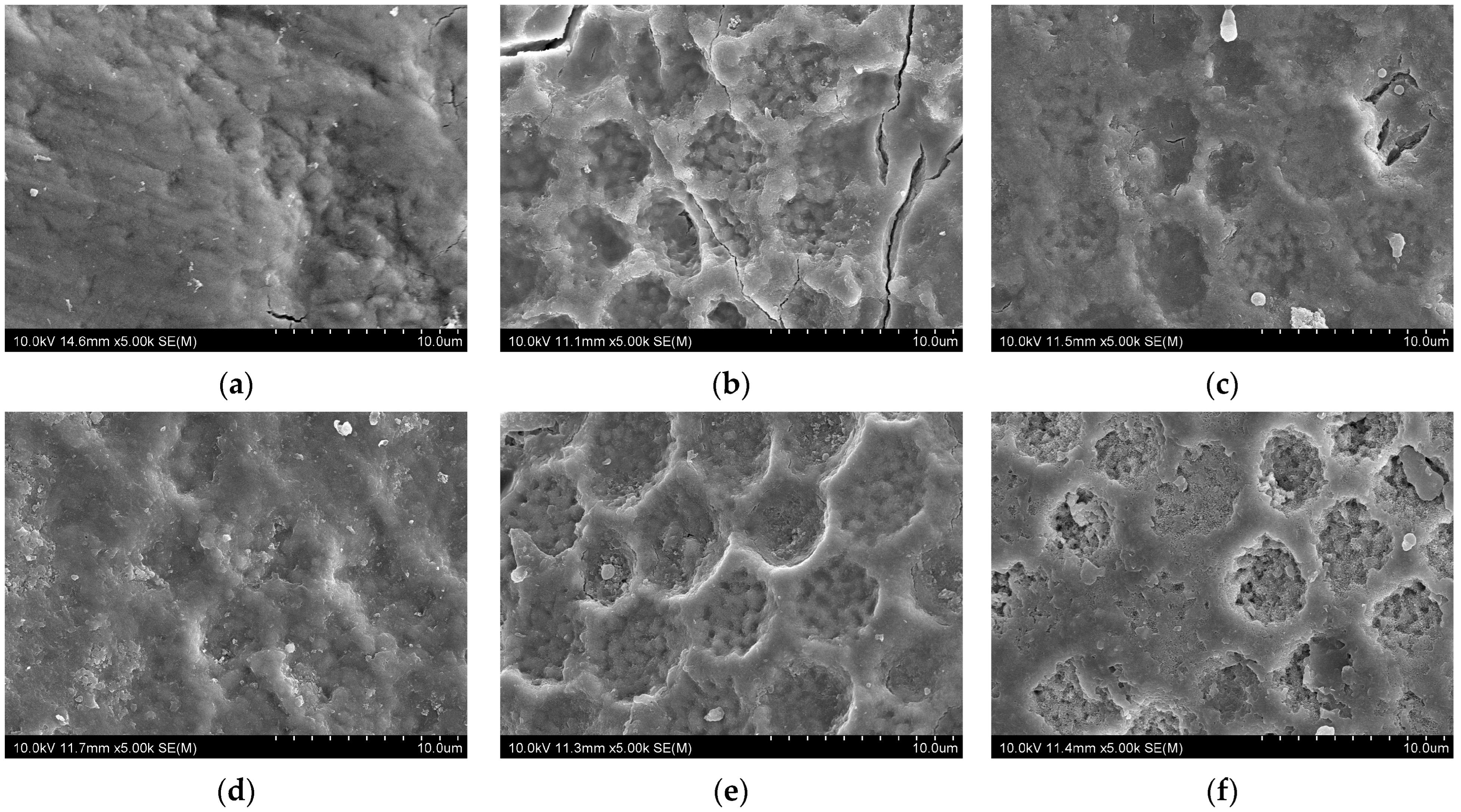
3. Results
3.1. Color Measurements
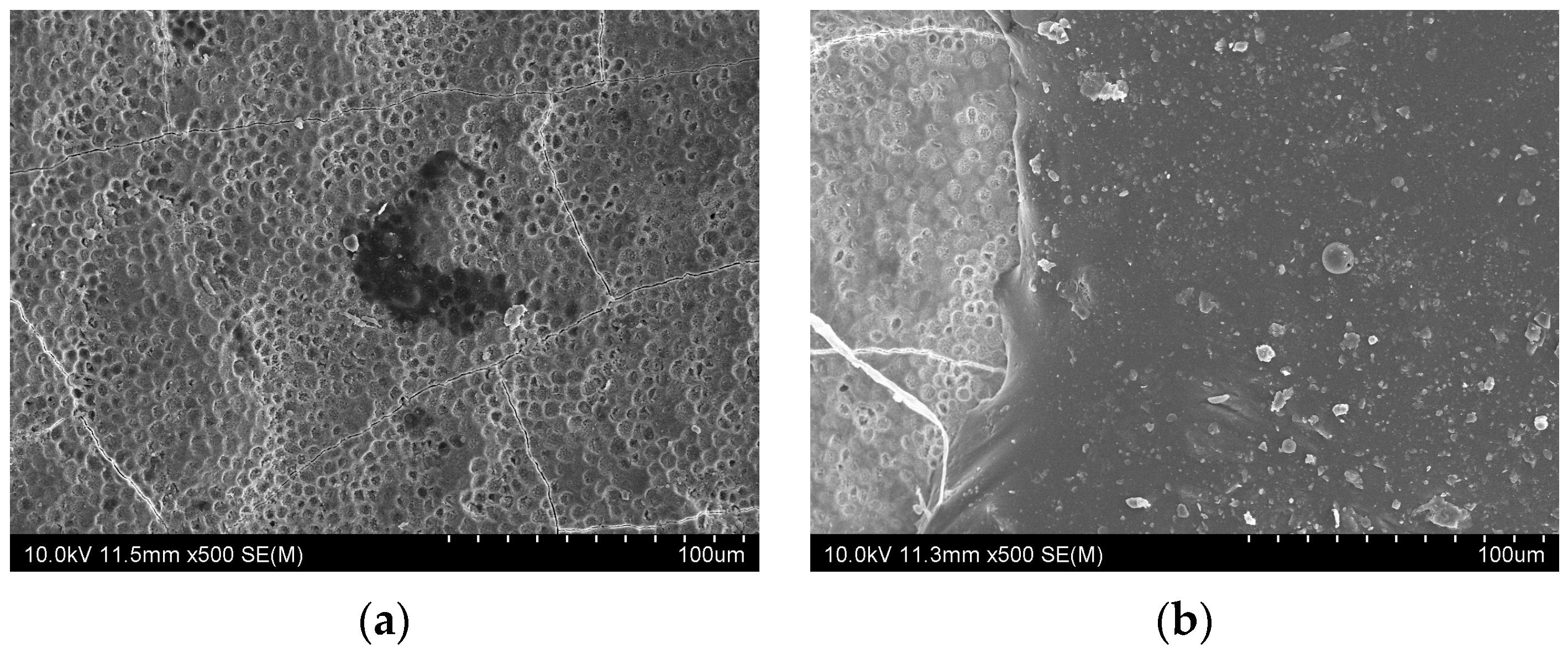
3.2. SEM Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lajnert, V.; Kovacevic Pavicic, D.; Pavlic, A.; Pokrajac-Bulian, A.; Spalj, S. Smile Aesthetics Satisfaction Scale: Development and validation of a new brief five-item measure of satisfaction with smile aesthetics in adults and the elderly. Int. Dent. J. 2018, 68, 162–170. [Google Scholar] [CrossRef]
- Féliz, M.L.; Hernández, L.M.; Abreu, N. Dental Bleaching Techniques; Hydrogen-carbamide Peroxides and Light Sources for Activation, an Update. Mini Review Article. Open Dent. J. 2015, 8, 264–268. [Google Scholar] [CrossRef]
- Al-Tarakemah, Y.; Darvell, B.W. On the permanence of tooth bleaching. Dent. Mater. 2016, 32, 1281–1288. [Google Scholar] [CrossRef]
- Moghadam, F.V.; Majidinia, S.; Chasteen, J.; Ghavamnasiri, M. The degree of color change, rebound effect and sensitivity of bleached teeth associated with at-home and power bleaching techniques: A randomized clinical trial. Eur. J. Dent. 2013, 7, 405–411. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.C.; de Souza, T.F.; Liporoni, P.C.; Pizi, E.C.; Matuda, L.A.; Catelan, A. Effect of bleaching agents on hardness, surface roughness and color parameters of dental enamel. J. Clin. Exp. Dent. 2020, 12, e670–e675. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.E.; Pallesen, U. Tooth Bleaching—A Critical Review of the Biological Aspects. Crit. Rev. Oral Biol. Med. 2003, 14, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Aragão, W.A.B.; Chemelo, V.S.; Alencar, C.M.; Silva, C.M.; Pessanha, S.; Reis, A.; Souza-Rodrigues, R.D.; Lima, R.R. Biological action of bleaching agents on tooth structure: A review. Histol. Histopathol. 2024, 39, 1229–1243. [Google Scholar] [CrossRef]
- Pinto, C.F.; Oliveira, R.; Cavalli, V.; Giannini, M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz. Oral Res. 2004, 18, 306–311. [Google Scholar] [CrossRef]
- Lopes, G.C.; Bonissoni, L.; Baratieri, L.N.; Vieira, L.C.; Monteiro, S., Jr. Effect of bleaching agents on the hardness and morphology of enamel. J. Esthet. Restor. Dent. 2002, 14, 24–30. [Google Scholar] [CrossRef]
- Sasaki, R.T.; Catelan, A.; Bertoldo Edos, S.; Venâncio, P.C.; Groppo, F.C.; Ambrosano, G.M.; Marchi, G.M.; Lima, D.A.; Aguiar, F.H. Effect of 7.5% hydrogen peroxide containing remineralizing agents on hardness, color change, roughness and micromorphology of human enamel. Am. J. Dent. 2015, 28, 261–267. [Google Scholar]
- AlShehri, A.; AlRefeai, M.H.; AlZamil, F.; AlOtaibi, N.; AlKinani, Y. Effect of Over-The-Counter Tooth-Whitening Products on Enamel Surface Roughness and Microhardness. Appl. Sci. 2022, 12, 6930. [Google Scholar] [CrossRef]
- Spalding, M.; Taveira, L.A.; de Assis, G.F. Scanning Electron Microscopy Study of Dental Enamel Surface Exposed to 35% Hydrogen Peroxide: Alone, With Saliva, and With 10% Carbamide Peroxide. J. Esthet. Restor. Dent. 2003, 15, 154–165. [Google Scholar] [CrossRef]
- Pharmaceuticals and Medical Devices Agency (PMDA). Hi-Lite [Package Insert]. Shofu. Available online: https://www.info.pmda.go.jp/downfiles/md/PDF/340177/340177_20900BZY01021000_A_03_01.pdf (accessed on 20 June 2025).
- Pharmaceuticals and Medical Devices Agency (PMDA). Pyrenees Medical Device Information. Nissin Dental Products. Available online: https://www.pmda.go.jp/PmdaSearch/kikiDetail/530104_21800BZZ10066000_A_01_03#HDR_ContraindicationAndProhibitions (accessed on 20 June 2025).
- Beltrán-Aguilar, E.D.; Goldstein, J.W.; Lockwood, S.A. Fluoride Varnishes: A Review of Their Clinical Use, Cariostatic Mechanism, Efficacy and Safety. J. Am. Dent. Assoc. 2000, 131, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.; Worthington, H.V.; Walsh, T.; Clarkson, J.E. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2013, 2013, Cd002279. [Google Scholar] [CrossRef] [PubMed]
- Veneri, F.; Vinceti, S.R.; Filippini, T. Fluoride and caries prevention: A scoping review of public health policies. Ann. Ig. 2024, 36, 270–280. [Google Scholar] [CrossRef]
- Zendron, M.P.; Rocha, A.O.; Simões, M.S.D.S.; Santana, C.M.; Bolan, M.; Cardoso, M. Fluoride Varnish in Dentistry: A Bibliometric Analysis of the 100 Most-Cited Papers. Caries Res. 2023, 57, 189–196. [Google Scholar] [CrossRef] [PubMed]
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; Lennon, M.A.; Petersen, P.E.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and Oral Health. Community Dent. Health 2016, 33, 69–99. [Google Scholar]
- Richards, D. Substantial reduction in caries from regular fluoride varnish application. Evid. Based Dent. 2013, 14, 72–73. [Google Scholar] [CrossRef]
- Jullien, S. Prophylaxis of caries with fluoride for children under five years. BMC Pediatr. 2021, 21 (Suppl. 1), 351. [Google Scholar] [CrossRef]
- Carey, C.M. Focus on fluorides: Update on the use of fluoride for the prevention of dental caries. J. Evid. Based Dent. Pract. 2014, 14, 95–102. [Google Scholar] [CrossRef]
- Qeli, E.; Toti, Ç.; Odorici, A.; Blasi, E.; Tragaj, E.; Tepedino, M.; Masedu, F.; Kaçani, G.; Hysi, D.; Meto, A.; et al. Effectiveness of Two Different Fluoride-Based Agents in the Treatment of Dentin Hypersensitivity: A Prospective Clinical Trial. Materials 2022, 15, 1266. [Google Scholar] [CrossRef]
- Roriz, V.M.; Santana, M.; Boaventura, V.L.; Zanotto, E.D.; Peitl, F.O.; Dias, D.R. Efficacy of Biosilicate Glass-ceramic and Fluoride Varnish in the Treatment of Dentin Hypersensitivity-A Randomized Controlled Clinical Trial. Oper. Dent. 2024, 49, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.V.; de L Dias, W.; Miguez, P.; Caplan, D.J.; Swift, E.J., Jr. Treating cervical dentin hypersensitivity with fluoride varnish: A randomized clinical study. J. Am. Dent. Assoc. 2006, 137, 1013–1020, quiz 1029. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, S.; Liu, P.; Sardana, D.; Peng, S.; Lo, E.C.; Yiu, C.K. Randomized clinical trial to compare three fluoride varnishes in preventing early childhood caries. J. Dent. 2024, 147, 105141. [Google Scholar] [CrossRef]
- Mashhour, A.; Allam, G.; Wassel, M. Comparative evaluation of prevention of demineralization of artificial enamel caries treated with two fluoride varnishes and 38% SDF in primary teeth: An in vitro study. BMC Oral Health 2023, 23, 110. [Google Scholar] [CrossRef] [PubMed]
- Wierichs, R.J.; Stausberg, S.; Lausch, J.; Meyer-Lueckel, H.; Esteves-Oliveira, M. Caries-Preventive Effect of NaF, NaF plus TCP, NaF plus CPP-ACP, and SDF Varnishes on Sound Dentin and Artificial Dentin Caries in vitro. Caries Res. 2018, 52, 199–211. [Google Scholar] [CrossRef]
- Matar, M.A.; Darwish, S.S.; Salma, R.S.; Lotfy, W.A. Correction to: Evaluation of the antibacterial activity of Enamelast(®) and Fluor defender(®) fluoride varnishes against Streptococcus mutans biofilm: An in vitro study in primary teeth. Eur. Arch. Paediatr. Dent. 2024, 25, 137–140. [Google Scholar] [CrossRef]
- Singh, V.; Naik, S.; Vashisth, P.; Sharma, S.; Chandak, A.; Murry, J.N. Comparative Evaluation of Longevity of Fluoride Release from Three Different Fluoride Varnishes: An Observational Study. Int. J. Clin. Pediatr. Dent. 2024, 17, 341–345. [Google Scholar] [CrossRef]
- Ito, Y.; Otsuki, M.; Tagami, J. Effect of pH conditioners on tooth bleaching. Clin. Exp. Dent. Res. 2019, 5, 212–218. [Google Scholar] [CrossRef]
- Kyaw, K.Y.; Otsuki, M.; Segarra, M.S.; Tagami, J. Effect of sodium fluoride pretreatment on the efficacy of an in-office bleaching agent: An in vitro study. Clin. Exp. Dent. Res. 2018, 4, 113–118. [Google Scholar] [CrossRef]
- Yago, R.; Kawamoto, C.; Wu, D.; Mirokuin, T.; Islam, R.; Yamauti, M.; Sano, H.; Tomokiyo, A. Visibility of Recurrent Caries Through Universal Shade Resin Composite Restorations. Materials 2024, 17, 5815. [Google Scholar] [CrossRef]
- Tejada, C.M.; Herrera, L.J.; Carrillo, P.F.; Ruiz, L.J.; Ghinea, R.I.; Pérez, M.M. Exploring the CIEDE2000 thresholds for lightness, chroma, and hue differences in dentistry. J. Dent. 2024, 150, 105327. [Google Scholar] [CrossRef]
- Castellano, J.B.; Donly, K.J. Potential remineralization of demineralized enamel after application of fluoride varnish. Am. J. Dent. 2004, 17, 462–464. [Google Scholar]
- Kim, H.N.; Kim, J.B.; Jeong, S.H. Remineralization effects when using different methods to apply fluoride varnish in vitro. J. Dent. Sci. 2018, 13, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.D.P.; Mora, V.S.A.; Dávila, M.; Montesinos-Guevara, C. Dental caries prevention in pediatric patients with molar incisor hypomineralization: A scoping review. J. Clin. Pediatr. Dent. 2023, 47, 9–15. [Google Scholar] [CrossRef]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- González, C.C.; Fernández, C.E. Recent Advances in Remineralization Therapies for Caries Lesions. Adv. Dent. Res. 2018, 29, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Flemming, J.; Hannig, C.; Hannig, M. Caries Management-The Role of Surface Interactions in De- and Remineralization-Processes. J. Clin. Med. 2022, 11, 7044. [Google Scholar] [CrossRef]
- Ramos, G.F.; Kinsler, J.; Askaryar, H. Understanding oral health disparities in children as a global public health issue: How dental health professionals can make a difference. J. Public. Health Policy 2020, 41, 114–124. [Google Scholar] [CrossRef]
- Melo, M.; Fioresta, R.; Sanz, J.L.; Pecci, L.M.P.; Llena, C. Effect of highly concentrated bleaching gels on enamel microhardness and superficial morphology, and the recovery action of four remineralizing agents. BMC Oral Health 2022, 22, 645. [Google Scholar] [CrossRef]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Research 2020, 9, 171. [Google Scholar] [CrossRef]
- Twetman, S.; Keller, M.K. Fluoride Rinses, Gels and Foams: An Update of Controlled Clinical Trials. Caries Res. 2016, 50 (Suppl. 1), 38–44. [Google Scholar] [CrossRef] [PubMed]
- Dall, A.M.A.; Battiston, C.; Tenuta, L.M.A.; Cury, J.A. Fluoride Formed on Enamel by Fluoride Varnish or Gel Application: A Randomized Controlled Clinical Trial. Caries Res. 2022, 56, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kayalidere, E.E.; Dorter, C. Effects of in-office bleaching agents on polished and unpolished nanofilled resin composite. J. Am. Dent. Assoc. 2023, 154, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Yu, M.; Jin, C.; Huang, C. Effect of aging and bleaching on the color stability and surface roughness of a recently introduced single-shade composite resin. J. Dent. 2024, 143, 104917. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Martini, T.; Lima, K.P.; Araújo, T.T.; Feitosa, C.; Marron, L.R.; Lavender, S.; Grizzo, L.T.; Magalhães, A.C.; Buzalaf, M.A.R. Xylitol associated or not with fluoride: Is the action the same on de- and remineralization? Arch. Oral Biol. 2024, 159, 105873. [Google Scholar] [CrossRef]
- Gkavela, G.; Kakouris, V.; Pappa, E.; Rahiotis, C. Effect of Bleaching Agents on Healthy Enamel, White Spots, and Carious Lesions: A Systematic Review and Meta-Analysis. Dent. J. 2024, 12, 140. [Google Scholar] [CrossRef]
- Mosayebi, N.; Toodehzaeim, M.H.; Zandi, H.; Joshan, N.; Haerian, A. Evaluation of the effects of fluoride mouth rinse and varnish on the early biofilm formation of Streptococcus mutans in two types of orthodontic adhesive resins: An in vitro study. Dent. Res. J. 2022, 19, 54. [Google Scholar] [CrossRef]
- Atteya, S.M.; Amer, H.A.; Saleh, S.M.; Safwat, Y. The effect of nano silver fluoride, self-assembling peptide and sodium fluoride varnish on salivary cariogenic bacteria: A randomized controlled clinical trial. Clin. Oral Investig. 2024, 28, 167. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, J.; Valiente, M.; Sánchez-Martín, M.-J. Tooth whitening: From the established treatments to novel approaches to prevent side effects. J. Esthet. Restor. Dent. 2019, 31, 431–440. [Google Scholar] [CrossRef]
- Yahya, G.; AlAlwi, A.; Shurayji, F.; Baroom, W.; Rajeh, M.; AbdelAleem, N. Effectiveness of sodium fluoride varnish and/or diode laser in decreasing post-bleaching hypersensitivity: A comparative study. Saudi Dent. J. 2022, 34, 62–67. [Google Scholar] [CrossRef] [PubMed]
| Material (Code) | ppm F | Composition | Percent per Weight | Manufacturer | Lot |
|---|---|---|---|---|---|
| Clinpro White Varnish (CW) | 22,600 | pentaerythritol glycerol ester of colophony resin | 30–75 | Solventum, Saint Paul, MN, USA | 9660455 |
| n-hexane | 10–15 | ||||
| ethyl alcohol | 1–15 | ||||
| sodium fluoride | 1–5 | ||||
| flavor enhancer | 1–5 | ||||
| thickener | 1–5 | ||||
| food-grade flavor | 1–5 | ||||
| modified tricalcium phosphate | <5 | ||||
| Enamelast Fluoride Varnish (EN) | 22,600 | synthetic resin | <50 | Ultradent Products, South Jordan, UT, USA | BWB4Y |
| ethyl alcohol | <15 | ||||
| sodium fluoride | <5 | ||||
| methyl ester of hydrogenated rosin | <5 | ||||
| citric acid | <3 | ||||
| xylitol | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yago, R.; Kawamoto, C.; Islam, R.; Kaneko, H.; Yamauti, M.; Otsuki, M.; Sano, H.; Tomokiyo, A. Prevention of Tooth Discoloration Using Fluoride Varnish Immediately After Bleaching. J. Funct. Biomater. 2025, 16, 245. https://doi.org/10.3390/jfb16070245
Yago R, Kawamoto C, Islam R, Kaneko H, Yamauti M, Otsuki M, Sano H, Tomokiyo A. Prevention of Tooth Discoloration Using Fluoride Varnish Immediately After Bleaching. Journal of Functional Biomaterials. 2025; 16(7):245. https://doi.org/10.3390/jfb16070245
Chicago/Turabian StyleYago, Ryotaro, Chiharu Kawamoto, Rafiqul Islam, Hirofumi Kaneko, Monica Yamauti, Masayuki Otsuki, Hidehiko Sano, and Atsushi Tomokiyo. 2025. "Prevention of Tooth Discoloration Using Fluoride Varnish Immediately After Bleaching" Journal of Functional Biomaterials 16, no. 7: 245. https://doi.org/10.3390/jfb16070245
APA StyleYago, R., Kawamoto, C., Islam, R., Kaneko, H., Yamauti, M., Otsuki, M., Sano, H., & Tomokiyo, A. (2025). Prevention of Tooth Discoloration Using Fluoride Varnish Immediately After Bleaching. Journal of Functional Biomaterials, 16(7), 245. https://doi.org/10.3390/jfb16070245






