Abstract
The purpose of current study was the green synthesis of bioactive silver nanoparticles (AgNPs) using Brevibacillus sp. MAHUQ-41 and the exploration of their role in controlling drug-resistant bacterial pathogens Listeria monocytogenes and Yersinia enterocolitica. The culture supernatant of strain MAHUQ-41 was employed for a simple and eco-friendly synthesis of biofunctional silver nanoparticles (AgNPs). The resulting nanoparticles were analyzed using several techniques, including UV–Visible spectroscopy, XRD, FE-TEM, FTIR, and DLS. The UV–Vis spectral analysis of the AgNPs synthesized via Brevibacillus sp. MAHUQ-41 revealed a prominent absorption peak at 400 nm. FE-TEM results confirmed spherical-shaped 15–60 nm sized nanoparticles. XRD results indicated that the synthesized AgNPs were crystalline in nature. The FTIR spectrum determined various functional groups on the surface of synthesized nanoparticles. Potent antibacterial properties were observed in green-synthesized AgNPs against tested pathogens. The MIC value of extracellular synthesized AgNPs for both pathogenic bacteria was 6.2 µg/mL, and the MBCs were 25.0 µg/mL and 12.5 µg/mL for L. monocytogenes and Y. enterocolitica, respectively. Treatment by synthesized AgNPs resulted in morphological alterations and structural damages in both L. monocytogenes and Y. enterocolitica. These alterations can interfere with regular cellular activities, potentially resulting in cell death. This study is the first to report the antimicrobial properties of silver nanoparticles synthesized using Brevibacillus sp. MAHUQ-41. The findings obtained in the present study supported the role of Brevibacillus sp. MAHUQ-41-mediated synthesized AgNPs in controlling drug-resistant bacterial pathogens L. monocytogenes and Y. enterocolitica.
1. Introduction
Biomaterials are natural or artificial substances engineered to interface with biological systems for medical treatment or diagnostic use [1,2]. Bio-nanotechnology plays an important role in the creation of functional biomaterials by leveraging the distinctive characteristics of biological building blocks at the nanoscale. A key advantage of bio-nanotechnology is its ability to produce bioactive nanoparticles. Nanoparticles are functional nanomaterials and are the powerful therapeutic agents due to their unique characteristics, such as biocompatibility, non-toxicity, and multifunctional efficacy. Green-synthesized nanoparticles are attractive functional biomaterials because they offer several advantages over conventionally synthesized nanoparticles, including biocompatibility, improved properties, and environmentally friendly production methods [3,4,5]. This approach utilizes biological resources like plants or microorganisms, resulting in nanoparticles with specific biological functions and reduced toxicity. Nanomedicine has emerged as a promising agent for the safe and effective treatment of various deadly diseases, such as cancer and other infectious and non-infectious diseases. Nanoparticles and nanoconjugates have gained significant global attention due to their multifunctional applications in biomedical sectors [3,6]. Silver nanoparticles (AgNPs) are among the most extensively researched functional nanomaterials due to their diverse applications in biomedical fields, including antimicrobial activity, cancer treatment, targeted drug delivery, catalysis, and biomolecular sensing [7,8,9].
Nanoparticles can be synthesized using a range of approaches, such as chemical, physical, and biological methods [10,11,12]. Chemical and physical techniques are frequently employed to produce well-defined nanoparticles; however, they often require high energy input and involve harmful chemicals, resulting in toxic byproducts that limit their suitability [13,14]. The biosynthesis of nanoparticles is an emerging field of research due to their ease of use and environment friendly nature [15]. The green synthesis of nanoparticles has proven to be a safe, simple, non-hazardous, and biocompatible technique for developing nanomedicine using plants and microbes. Plant extracts contain various phytochemicals and microorganisms secreting numerous metabolites, which play a crucial role in the synthesis and stabilization of nanoparticles [4,16]. Green synthesis using microorganisms such as bacteria, yeast, actinomyces, and fungi has received increasing attention due to its simplicity and eco-friendliness [4,17,18,19]. Nanoparticle synthesis using bacterial biotransformation systems offers several benefits and has been recognized as an environmentally friendly approach [20,21]. Several bacterial species, including Bacillus safensis [22], Escherichia coli [23], Sphingobium sp. MAH-11 [9], Acinetobacter baumannii [24], Terrabacter humi [25], Bacillus subtilis [26], Microbacterium resistens [27], Lactobacillus Plantarum [28], Pseudomonas deceptionensis [29], and Weissella oryzae [30] have been studied for their role in the biosynthesis of AgNPs. In this study, we biologically synthesized novel AgNPs using Brevibacillus sp. MAHUQ-41 and evaluated their antimicrobial effects.
The lower effectiveness and side effects of available drugs as well as the emergence of drug-resistant microorganisms are serious concerns for public health globally. Consequently, there is a critical need to create new antimicrobial agents that are both safe and effective in fighting against drug-resistant pathogens. Listeria monocytogenes is a bacterial species responsible for listeriosis, an infection that can affect the brain, spinal cord membranes, and bloodstream of the host. This pathogen is commonly transmitted through the consumption of contaminated food, such as raw produce or unpasteurized dairy products. L. monocytogenes ranks as the third leading cause of fatal foodborne illness in humans, primarily affecting high-risk populations such as infants, the elderly, pregnant women, and individuals with weakened immune systems [31,32]. Similar to numerous other harmful pathogens, this bacterium has the potential to resist various antibiotics. There are many studies where the antimicrobial resistance in L. monocytogenes has been reported [33,34]. Yersinia enterocolitica is a Gram-negative, rod-shaped pathogen responsible for the zoonotic disease, yersiniosis. This infection typically presents with symptoms such as acute diarrhea, mesenteric lymphadenitis, terminal ileitis, and conditions mimicking appendicitis [35,36,37]. In many regions, infections caused by Y. enterocolitica pose a considerable public health challenge and lead to notable socioeconomic impacts [38]. Numerous studies have investigated antimicrobial resistance in Y. enterocolitica [38,39,40]. The development of safe and effective antibacterial agents is essential to control these pathogenic bacteria for protecting public health. Green-synthesized AgNPs appear to be a promising option for managing these bacterial pathogens. In the current study, a novel bacterial strain, Brevibacillus sp. MAHUQ-41, was isolated from the rhizosphere of the persimmon tree, and the culture supernatant of the strain MAHUQ-41 was utilized for the green, simple, and ecofriendly synthesis of bioactive AgNPs. Different instruments were used to analyze the properties of the synthesized AgNPs, including UV–Vis, TEM, EDX, XRD, DLS, and FTIR, and applied for the control of pathogenic L. monocytogenes and Y. enterocolitica. This is the first study on the green, simple synthesis of AgNPs using Brevibacillus sp. MAHUQ-41. It is also the first report on the antimicrobial application of Brevibacillus sp. MAHUQ-41-mediated synthesized AgNPs against human pathogens L. monocytogenes and Y. enterocolitica.
2. Materials and Methods
2.1. Chemicals and Materials
Silver nitrate (AgNO3) and culture media for bacteria were obtained from Sigma-Aldrich (St. Louis, MO, USA). The pathogenic bacteria L. monocytogenes [ATCC 19114] and Y. enterocolitica [ATCC 9610] were sourced from the American Type Culture Collection (ATCC, Manassas, VA, USA).
2.2. Isolation of AgNP-Producing Strain
The AgNP-producing strain Brevibacillus sp. MAHUQ-41 was isolated from the rhizosphere of a persimmon tree located in Anseong, South Korea, using the serial dilution method reported by Siddiqi et al. [41]. In brief, 1 g of each soil sample was combined with 9 mL of sterile 0.8% NaCl solution, shaken using a rotary shaker, and subsequently subjected to serial dilution. Each dilution (approximately 100 µL) was evenly spread over R2A agar plates. Following a 72 h incubation at 30 °C, individual colonies were selected based on their morphological characteristics. Each selected colony was then grown independently in 5 mL of R2A broth for 72 h at 30 °C. After incubation, 5 µL of a 1.5 M silver nitrate solution was introduced into the culture supernatant and further incubated in a shaking incubator at 35 °C for four days. Based on silver nitrate reduction efficacy, strain MAHUQ-41 was selected for further study. Strain MAHUQ-41 was deposited into the KACC.
2.3. 16S rRNA Gene Sequencing and Phylogenetic Analysis
Strain MAHUQ-41 was identified at the molecular level through 16S rRNA gene sequencing. Genomic DNA was extracted using a commercially available DNA isolation kit (Solgent, Republic of Korea). The 16S rRNA gene amplification was conducted using primers 27F and 1492R, followed by sequencing at Solgent Co., Ltd., in Daejeon, Republic of Korea. Identification of strain MAHUQ-41 was achieved by comparing the obtained 16S rRNA sequence with entries in the GenBank database using the BLASTn tool. To determine the phylogenetic relationship of MAHUQ-41, a phylogenetic tree was generated employing the neighbor-joining approach via MEGA6 software [42,43].
2.4. Cultural, Physiological, and Biochemical Characterization of Strain MAHUQ-41
To evaluate its growth characteristics, strain MAHUQ-41 was incubated at 30 °C for three days on different agar media, including trypticase soy agar, nutrient agar, Luria-Bertani agar, and R2A agar [44]. To determine the optimal growth temperature, the strain was cultured on R2A agar and incubated at temperatures ranging from 5 °C to 50 °C in 5 °C intervals [45]. For pH optimization, strain MAHUQ-41 was grown in R2A broth adjusted to different pH levels [46]. Transmission electron microscopy was used to observe the cell’s shape and size. Using API 20NE and API ZYM kits (bioMérieux), the physiological and biochemical properties of strain MAHUQ-41 were evaluated following the provided protocols.
2.5. Biosynthesis of AgNPs Using Strain MAHUQ-41
The culture supernatant of the potent strain MAHUQ-41 was employed to synthesize novel AgNPs. The isolated strain MAHUQ-41 was cultured in 100 mL R2A broth for 3 days at 30 °C under shaking at 180 rpm. Afterward, the supernatant was separated by centrifuging at 8000 rpm for 10 min. AgNPs were produced by combining the culture supernatant with silver nitrate solution, achieving a final concentration of 1.5 mM. The reaction was carried out in the dark at 35 °C with continuous shaking at 180 rpm in an orbital shaker for four days. The synthesis of bioactive AgNPs was regularly observed, and the completion of synthesis was confirmed using UV–Visible spectroscopy. The resulting AgNPs were harvested by centrifugation at 14,000 rpm for 20 min, followed by washing with deionized water. Afterward, the nanoparticles were air-dried and used for further characterization and antibacterial testing.
2.6. Characterization of Green-Synthesized AgNPs
The synthesized AgNPs were analyzed using a UV–Visible spectrophotometer (Optizen POP, Mecasys) to study their kinetic properties. Spectral scanning of the biosynthesized AgNPs was performed over the wavelength range of 300–800 nm, and the absorption data were recorded and graphed. Morphology, particle size, purity, elemental composition, distribution, and selected area electron diffraction (SAED) patterns of the green-synthesized AgNPs were examined using a field emission transmission electron microscope (FE-TEM) equipped with an energy-dispersive X-ray (EDX) detector. For TEM imaging, a drop of AgNP suspension was placed on a carbon-coated copper grid, air-dried, and then examined with a FE-TEM (TEM-2100F, Joel, Tokyo, Japan) operated at 200 kV. The crystalline structure of AgNPs was further analyzed through X-ray diffraction (XRD) using a D8 Advance diffractometer (Bruker, Germany). The diffractometer operates using CuKα radiation at 40 mA and 40 kV over the range of 30° to 80° (2θ). The surface chemistry of the biosynthesized AgNPs was investigated using FTIR (Fourier transform-infrared) spectroscopy (Spectrum One System, Perkin-Elmer, Waltham, MA, USA) with a resolution of 4 cm−1 in the range of 500–4000 cm−1. For FTIR analysis, the air-dried powder form of synthesized AgNPs was used. The hydrodynamic diameters and polydispersity index (PDI) of the AgNPs were measured using Dynamic Light Scattering (DLS) with a Malvern Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK). Deionized water, with a viscosity of 0.8878 and a refractive index of 1.3328, was used as the dispersing medium, and measurements were performed at a controlled temperature of 25.0 °C.
2.7. Antimicrobial Activity
The antimicrobial activity of the biosynthesized AgNPs was assessed against L. monocytogenes and Y. enterocolitica using the disc diffusion technique [47,48]. In brief, both bacterial strains were grown overnight in Mueller–Hinton (MH) broth. Each culture (100 µL) was evenly distributed across MH agar plates. A suspension was prepared by dissolving 1 mg of the synthesized AgNPs in 2 mL of sterile distilled water. Sterile paper discs were impregnated with either 50 µL or 100 µL of this AgNP solution and placed on the inoculated agar surfaces. As controls, six antibiotics—penicillin G (10 µg/disc), novobiocin (30 µg/disc), oleandomycin (15 µg/disc), erythromycin (15 µg/disc), vancomycin (30 µg/disc), and lincomycin (15 µg/disc)—were tested against both bacterial strains. Following a 24 h incubation at 37 °C, the diameter of the inhibition zones (ZOI) on the agar plates was recorded in millimeters [48,49].
2.8. MIC and MBC
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of AgNPs were assessed against the pathogenic strains L. monocytogenes and Y. enterocolitica. Using 96-well ELISA plates, the MIC was determined through the broth microdilution method. Both strains were cultured in MH broth overnight. A 100 µL aliquot of bacterial suspension (approximately 1 × 106 CFU/mL) was dispensed into each well of a 96-well ELISA plate, followed by the addition of 100 µL of AgNP solutions at concentrations ranging from 1.56 to 100 µg/mL. The minimum concentration of AgNPs that prevented microbial growth was determined as the MIC. The MBC was determined by transferring 10 µL from each well of the 96-well plate onto MH agar plates, followed by incubation at 37 °C for 24 h. No visible bacterial growth at the lowest AgNP concentration was noted in the MBC. The experimental procedures were conducted in accordance with previously published protocols [48,49].
2.9. Morphological Evaluation
To investigate the antibacterial action of synthesized AgNPs against L. monocytogenes and Y. enterocolitica, both bacterial strains (approximately 1 × 107 CFU/mL) were incubated at 37 °C in the presence and absence of nanoparticles at the MBC concentration. Following incubation, cells were pelleted by centrifuging at 8000 rpm for 5 min. After washing with buffer, the cells were fixed with 2.5% glutaraldehyde followed by 1% osmium tetroxide. The fixed samples were subsequently dehydrated using a graded ethanol series and dried in a desiccator. Morphological and structural changes in L. monocytogenes and Y. enterocolitica were examined via FE-SEM (field emission scanning electron microscopy) [48,50].
3. Results and Discussion
3.1. Molecular Identification of AgNP-Producing Bacteria
Strain MAHUQ-41’s 16S rRNA gene sequence, measuring 1210 bp, was submitted to the GenBank/EMBL/DDBJ database under accession number MK680117. Comparisons based on 16S rRNA gene sequences revealed that strain MAHUQ-41 was closely related to Brevibacillus choshinensis DSM 8552T, Brevibacillus formosus DSM 9885T, and Brevibacillus brevis NBRC 15304T a with sequence similarity of 98.7%. Phylogenetic analysis using 16S rRNA gene sequence also showed that strain MAHUQ-41 clusters with the members of the genus Brevibacillus (Figure 1). Strain MAHUQ-41 was deposited into the KACC (Deposition number KACC 21235).
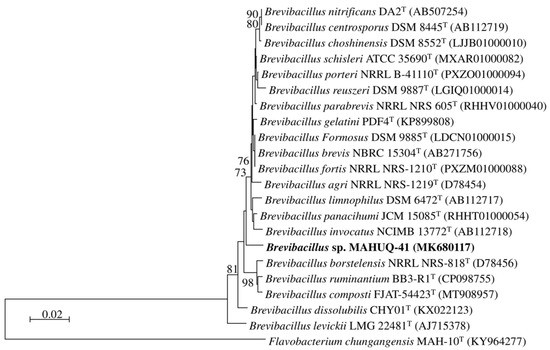
Figure 1.
A neighbor-joining (NJ) phylogenetic tree was constructed using 16S rRNA gene sequences to illustrate the evolutionary relationships between Brevibacillus sp. MAHUQ-41 and closely related type strains.
3.2. Cultural, Physiological, and Biochemical Characterization of Strain MAHUQ-41
The cells of strain MAHUQ-41 are rod shaped (Figure 2). Colonies grow well on R2A agar medium with an optimum temperature of 30 °C and pH of 7.0–7.5. Strain MAHUQ-41 shows positive activity for the hydrolysis of gelatin. The strain MAHUQ-41 shows the following enzyme activities: acid phosphatase, alkaline phosphatase, napthol-AS-BI-phosphohydrolase, esterase lipase (C8), and leucine arylamidase.
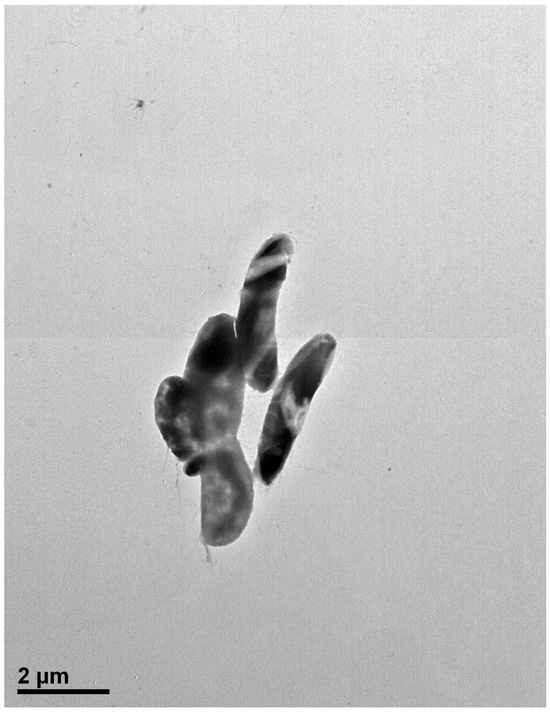
Figure 2.
Transmission electron microscopy image of Brevibacillus sp. MAHUQ-41 cells following negative staining with uranyl acetate. Scale bar represents 2.0 μm.
3.3. Green Synthesis of AgNPs
The bioactive AgNPs were synthesized using the culture supernatant of isolated strain Brevibacillus sp. MAHUQ-41. Strain MAHUQ-41 showed strong ability in the reduction of silver ion, leading to the synthesis of AgNPs. The synthesis of AgNPs was visually confirmed by a distinct color change in the reaction mixture, shifting from pale yellow to brown, a transformation attributed to the surface plasmon resonance (SPR) typically observed in green-synthesized AgNPs (Figure 3A,B) [48,51]. No noticeable color change occurred in the control sample containing R2A broth with AgNO3 (Figure 3A). The color transition occurred within four days of incubation (Figure 3B), serving as an initial visual indicator of successful AgNP formation mediated by Brevibacillus sp. MAHUQ-41 [48,52]. Similar findings were reported by Wang et al. [27], who observed a deep brown coloration in the reaction mixture containing the culture supernatant of Bacillus sonorensis and AgNO3. Hamouda et al. further documented a comparable brown color change, confirming AgNP formation [52]. In the present work, an extracellular synthesis method was employed to facilitate a straightforward and efficient production of AgNPs using the culture supernatant of Brevibacillus sp. MAHUQ-41. Although the exact mechanism behind AgNP synthesis remains unclear, several studies suggest the involvement of microbial enzymes and biomolecules such as proteins, pigments, flavonoids, enzymes, and amino acids in nanoparticle formation [4,53]. Microbial culture supernatants and biomass possess various bioactive substances, such as enzymes, proteins, amino acids, and other biomolecules, which function as reducing, capping, and stabilizing agents in the nanoparticle synthesis process [4,9]. In particular, microbial reductase enzymes are believed to play a pivotal role in reducing metal ions to their nanoparticle form [4,9,54,55].
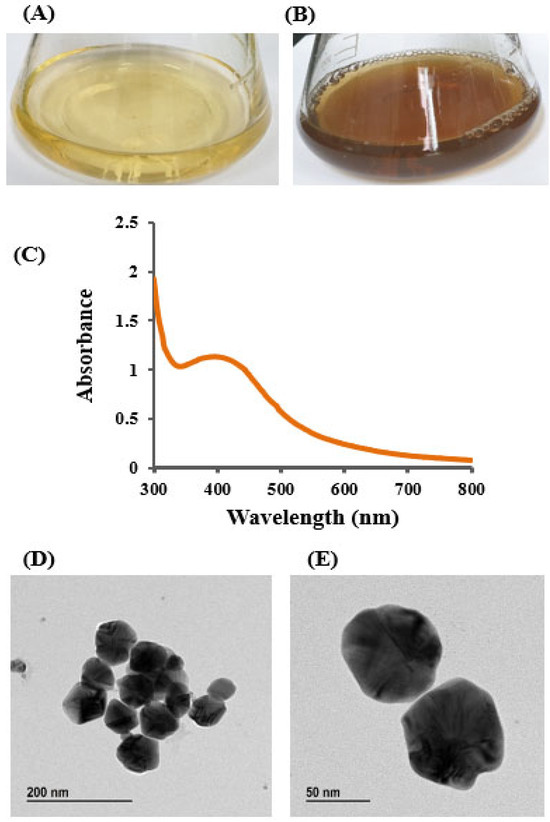
Figure 3.
R2A broth with AgNO3 as control (A), biosynthesized AgNPs (B), UV–Vis spectra (C), and FE-TEM images of AgNPs (D,E) synthesized by Brevibacillus sp. MAHUQ-41.
3.4. Characterization of Synthesized AgNPs
The appearance of a brown coloration in the reaction mixture served as an initial visual indication of AgNP synthesis. This observation was further validated using UV–Vis spectroscopy, which is commonly employed to monitor the surface plasmon resonance (SPR) associated with nanoparticle formation. The UV–Vis analysis confirmed the successful synthesis of AgNPs by the culture supernatant of Brevibacillus sp. MAHUQ-41, as evidenced by a characteristic broad absorption peak around 400 nm (Figure 3C), typical of AgNPs. This coincided with the plasmon resonance characteristic of AgNPs [21,52]. Hamouda et al. found the absorption peak of cyanobacterium Oscillatoria limnetica-mediated synthesized AgNPs at 426 nm [52]. An absorption peak at 413 nm was observed in AgNPs synthesized from the culture supernatant of Terrabacter humi [25]. The distinct UV–Vis absorption peak observed between 400 and 500 nm during the formation of silver nanoparticles (AgNPs) arises from surface plasmon resonance (SPR). This effect results from the collective oscillation of conduction electrons on the nanoparticle surface in response to incident light. The exact position of this resonance peak is influenced by factors such as the nanoparticle’s size, shape, and surrounding dielectric medium [56,57]. TEM was used to analyze the morphology, size, and distribution of AgNPs synthesized by Brevibacillus sp. MAHUQ-41. As shown in Figure 3D,E, the AgNPs were predominantly spherical, with particle sizes ranging from 15 to 60 nm. The TEM images also indicated a uniform distribution of nanoparticles, with no visible signs of aggregation, suggesting that the biosynthesized AgNPs possess good stability. These findings are in agreement with those of Singh et al. [58], who reported AgNP sizes between 10 and 40 nm when synthesized using the culture supernatant of Pseudomonas sp. THG-LS1.4. Similarly, Paenarthrobacter nicotinovorans MAHUQ-43 was shown to produce spherical AgNPs with sizes ranging from 13 to 27 nm [21]. Differences in average particle size among studies may be attributed to variations in the metabolites secreted by different biological sources used as reducing, capping, and stabilizing agents.
Elemental compositions and their distribution were examined by an EDX detector. The data revealed the main element in the green-synthesized nanomaterial was silver (Figure 4A–C). The EDX spectrum of the synthesized nanoparticles exhibited a prominent peak at 3 keV, characteristic of silver, confirming the presence of AgNPs (Figure 4A). Additional elemental signals detected in the spectrum were attributed to the TEM grid used during FE-TEM imaging [58]. The elemental composition obtained from the EDX analysis is presented in Table 1. Similar EDX profiles were observed in AgNPs produced by the culture supernatant of Pseudomonas sp. THG-LS1.4 [58] and Massilia sp. MAHUQ-52 [59]. SAED patterns further confirmed the crystalline nature of the AgNPs, showing distinct rings indicative of metallic and crystalline features (Figure 4D). The crystalline nature of the green-synthesized AgNPs was also verified by XRD analysis, with results displayed in Figure 4E. The XRD pattern displayed four distinct peaks at 2θ values of 38.51°, 44.38°, 64.69°, and 77.86°, corresponding to the (111), (200), (220), and (311) planes, respectively, which are characteristic of metallic silver (Figure 4E) [58]. Similar results were reported in earlier studies, where Pseudomonas sp. THG-LS1.4-mediated and Paenarthrobacter nicotinovorans MAHUQ-43-derived AgNPs exhibited similar XRD patterns [21,58].
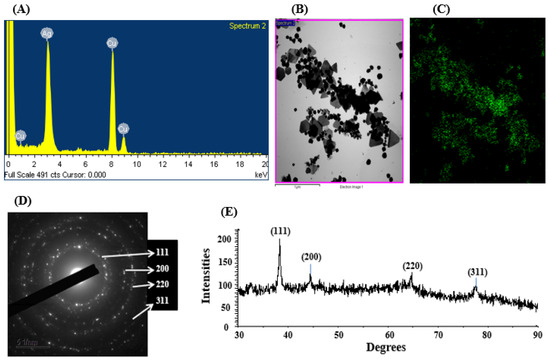
Figure 4.
EDX pattern of synthesized AgNPs (A), TEM image utilized for mapping (B), silver distribution in elemental mapping (C), SAED pattern (D), and X-ray diffraction pattern (E) of Brevibacillus sp. MAHUQ-41-mediated green-synthesized AgNPs.

Table 1.
The number and percentage of chemical elements present in Brevibacillus sp. MAHUQ-41-derived AgNPs.
FT-IR analysis was conducted to detect the presence of functional groups involved in the reduction and stabilization of silver ions during the green synthesis of AgNPs mediated by Brevibacillus sp. MAHUQ-41. The FT-IR spectrum displayed several prominent transmission peaks at 3445, 2918, 2845, 2359, 2342, and 1652 cm−1 (Figure 5A). The strong absorption band at 3445 cm−1 is indicative of O–H stretching vibrations from alcohols and/or N–H stretching from amine groups. The bands found at 2918 and 2845 cm−1 in Brevibacillus sp. MAHUQ-41-mediated green-synthesized AgNPs are due to the C-H bonds. The stretching peaks at 2359 and 2342 cm−1 corresponded to the O=C=O (carbonyl bond group) stretching. The peak observed at 1652 cm−1 corresponded to the C=O, –C=C, or N–H functional group. The presence of multiple functional groups in the FTIR spectrum suggests that a variety of biomolecules may play a role in both the reduction of silver ions and the stabilization of the synthesized AgNPs. These findings align with earlier research indicating that biomolecular components in microbial cell extracts contribute significantly to nanoparticle synthesis and stability [9,27,52]. DLS analysis revealed that the Brevibacillus sp. MAHUQ-41-mediated AgNPs had an average hydrodynamic diameter of 129.5 nm, with a polydispersity index (PDI) of 0.235, indicating that the green-synthesized AgNPs had a polydisperse standard (Figure 5B–D). The DLS analysis revealed the larger size of AgNPs than the actual size identified by FE-TEM analysis could be due to the presence of water molecules on the surface of AgNPs.
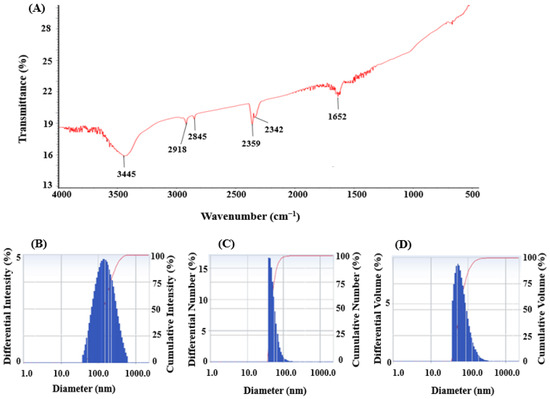
Figure 5.
FT-IR spectra (A) and particle size distribution of green-synthesized AgNPs mediated by Brevibacillus sp. MAHUQ-41, presented by intensity (B), number (C), and volume (D).
3.5. Antibacterial Activity
The rise of multidrug-resistant microbes presents a significant challenge to global health. Due to the limited effectiveness of existing antibiotics, resistant strains have become more prevalent, highlighting the pressing demand for novel and potent antimicrobial solutions. AgNPs synthesized through biological methods have shown promise as powerful antimicrobial agents capable of targeting harmful pathogens. This study assessed the antibacterial properties of AgNPs produced using Brevibacillus sp. MAHUQ-41 against the antibiotic-resistant bacteria L. monocytogenes and Y. enterocolitica. The biologically derived AgNPs demonstrated marked antibacterial activity against both strains. As shown in Figure 6, a distinct zone of inhibition (ZOI) was observed. The ZOI diameters for the AgNPs synthesized by Brevibacillus sp. MAHUQ-41 were measured at 19.1 ± 1.5 mm for L. monocytogenes and 18.9 ± 1.3 mm for Y. enterocolitica when exposed to a 100 µL solution at a concentration of 500 ppm (Table 2). These results indicate that biosynthesized AgNPs have the potential to effectively suppress these pathogenic bacteria.
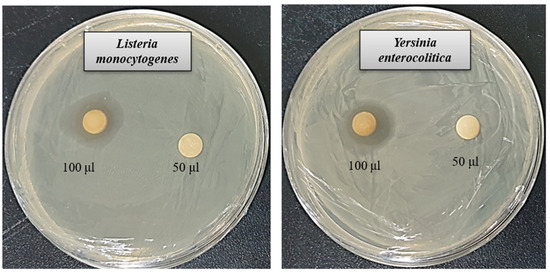
Figure 6.
Antibacterial activity of the synthesized AgNPs. Inhibition zones observed against L. monocytogenes and Y. enterocolitica using 50 µL and 100 µL of 500 ppm concentrations.

Table 2.
Antibacterial impact of Brevibacillus sp. MAHUQ-41-mediated green-synthesized AgNPs against L. monocytogenes and Y. enterocolitica.
In this investigation, the antibacterial performance of six commercially available antibiotics (penicillin G, novobiocin, erythromycin, vancomycin, oleandomycin, and lincomycin) was evaluated and compared with that of biosynthesized AgNPs against L. monocytogenes and Y. enterocolitica. The findings revealed that most of the tested antibiotics showed little to no effectiveness against either bacterial strain. Notably, only vancomycin displayed limited activity against L. monocytogenes, while novobiocin exhibited mild effects on Y. enterocolitica. The diameter of the ZOI of vancomycin against L. monocytogenes was 8.9 ± 1.2, and the diameter of the ZOI of novobiocin against Y. enterocolitica was 10.2 ± 1.0 (Table 3). These results are consistent with earlier research highlighting the potent antimicrobial capabilities of biosynthesized AgNPs [58,59]. The observed antibacterial efficacy of the synthesized AgNPs suggests their promise as alternative therapeutic agents for combating multidrug-resistant pathogens. A detailed presentation of the results is shown in Figure 7 and Table 3.

Table 3.
Antimicrobial impact of tested antibiotics against L. monocytogenes and Y. enterocolitica. -, No ZOI.
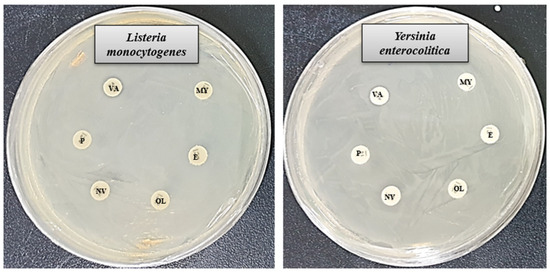
Figure 7.
ZOI of tested antibiotics against L. monocytogenes and Y. enterocolitica. Abbreviation: P (penicillin G), NV (novobiocin), E (erythromycin), VA (vancomycin), OL (oleandomycin), and MY (lincomycin).
3.6. MIC and MBC
The MIC of AgNPs synthesized by Brevibacillus sp. MAHUQ-41 was determined using the standard microdilution method. Various concentrations of the biosynthesized AgNPs (1.56, 3.12, 6.25, 12.5, 25, 50, and 100 µg/mL) were tested against L. monocytogenes and Y. enterocolitica. The results showed that both bacterial strains were inhibited at a MIC of 6.25 µg/mL. This indicates that the AgNPs synthesized by Brevibacillus sp. MAHUQ-41 are highly effective in suppressing the growth of these pathogens (Figure 8A,B). Notably, the MIC values observed in this study were considerably lower than those reported for other antimicrobial agents used against L. monocytogenes and Y. enterocolitica [60,61,62], underscoring the superior antimicrobial potential of the biosynthesized AgNPs.
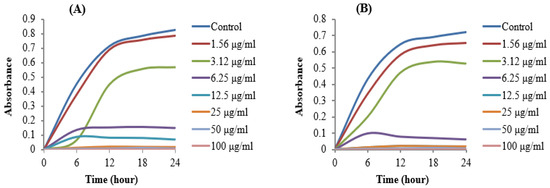
Figure 8.
MICs of biosynthesized AgNPs against L. monocytogenes (A) and Y. enterocolitica (B).
The MBC of AgNPs synthesized by Brevibacillus sp. MAHUQ-41 were determined to be 25 µg/mL for L. monocytogenes and 12.5 µg/mL for Y. enterocolitica (Figure 9A,B). These findings confirm the strong bactericidal efficacy of the Brevibacillus sp. MAHUQ-41-mediated synthesized AgNPs in suppressing the growth of both pathogenic L. monocytogenes and Y. enterocolitica.
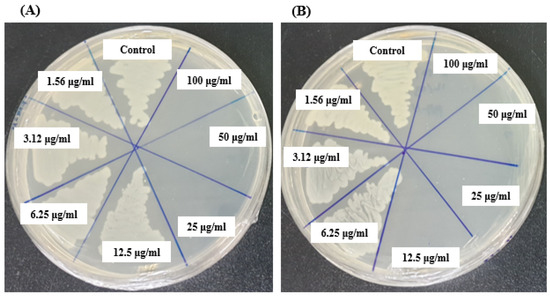
Figure 9.
MBCs of biosynthesized AgNPs against L. monocytogenes (A) and Y. enterocolitica (B).
3.7. Morphological Evaluation
FE-SEM was employed to examine the morphological alterations in L. monocytogenes and Y. enterocolitica following treatment with AgNPs synthesized by Brevibacillus sp. MAHUQ-41 (Figure 10). Untreated L. monocytogenes cells maintained their typical rod-like shape and displayed smooth, intact surfaces (Figure 10A). In contrast, cells exposed to a 1 × MBC concentration of the biosynthesized AgNPs exhibited significant surface irregularities, structural damage, and deformation, resulting in complete membrane collapse (Figure 10B). A similar pattern was observed in Y. enterocolitica. While the untreated cells appeared structurally intact, with their characteristic rod shape (Figure 10C), those treated with AgNPs showed notable surface damage, deformation, and loss of structural integrity (Figure 10D). These morphological disruptions suggest that the AgNPs interfere with cellular architecture and may lead to the loss of vital cell functions, ultimately causing bacterial cell death. Numerous antimicrobial actions of AgNPs were identified. Among the most recognized are the disruption of microbial cell membranes, the production of reactive oxygen species (ROS), and interactions with cellular DNA and proteins, all of which contribute to microbial cell death [4,63]. AgNPs compromise the structure of bacterial cell walls and membranes, increasing membrane permeability, which leads to the leakage of intracellular contents and ultimately causes cell death. They also disrupt the respiratory chain by binding to sulfhydryl groups, triggering lipid peroxidation and oxidative damage to DNA and proteins, resulting in cell destruction [4,64,65]. Furthermore, AgNPs attach to sulfur and phosphorus groups in DNA, causing DNA aggregation and damage, which interferes with transcription and translation processes. These nanoparticles can also promote the dephosphorylation of phosphotyrosine residues, thereby disturbing cellular signal transduction pathways and leading to cell death. AgNPs may release silver ions (Ag+) from their surfaces. These ions play a critical antimicrobial role by interacting with bacterial cell wall and membrane structures, representing a key mechanism in AgNP-induced toxicity [4,66,67].
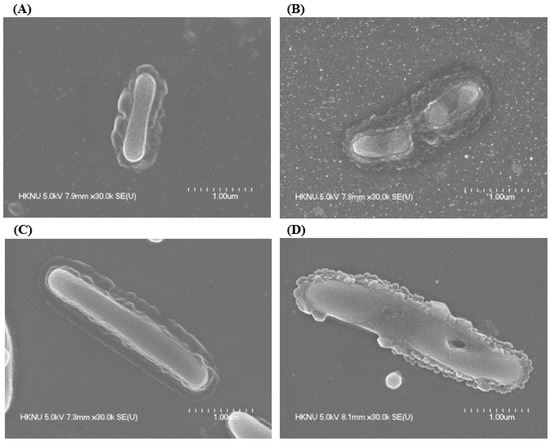
Figure 10.
Normal L. monocytogenes (A), AgNP-treated L. monocytogenes (B), normal Y. enterocolitica (C), AgNP-treated Y. enterocolitica (D).
4. Conclusions
This study is the first to report the green and straightforward synthesis of AgNPs utilizing Brevibacillus sp. MAHUQ-41. It also marks the initial application of these biosynthesized AgNPs in targeting the pathogenic bacteria L. monocytogenes and Y. enterocolitica. The culture supernatant of strain MAHUQ-41 functioned efficiently for the eco-friendly synthesis of AgNPs. The extracellular methodology was used for nanoparticle synthesis, which is a simple, fast, reproducible, and eco-friendly approach. The AgNPs produced through green synthesis were analyzed using techniques such as UV–Vis spectroscopy, FE-TEM, XRD, DLS, and FTIR. The UV–Vis spectrum of Brevibacillus sp. MAHUQ-41-mediated green-synthesized AgNPs showed a strong peak at 400 nm. FE-TEM results confirmed a spherical shape 15–60 nm in size. XRD analysis confirmed the crystalline nature of the synthesized AgNPs. FTIR analysis detected multiple biomolecules on the AgNPs’ surface, indicating their biological origin. The AgNPs synthesized using Brevibacillus sp. MAHUQ-41 exhibited strong antimicrobial efficacy against drug-resistant pathogenic L. monocytogenes and Y. enterocolitica. The nanoparticles created distinct inhibition zones measuring 19.1 ± 1.5 mm for L. monocytogenes and 18.9 ± 1.3 mm for Y. enterocolitica. The MIC for both bacterial strains was found to be 6.2 µg/mL, while the MBCs were 25.0 µg/mL for L. monocytogenes and 12.5 µg/mL for Y. enterocolitica. FE-SEM revealed significant morphological and structural disruptions in L. monocytogenes and Y. enterocolitica cells following treatment with the synthesized AgNPs. These observed alterations are likely to impair essential cellular functions in these multi-drug-resistant L. monocytogenes and Y. enterocolitica, ultimately resulting in cell death. The findings of this study suggest that the culture supernatant of Brevibacillus sp. MAHUQ-41 offers a straightforward and eco-friendly approach for synthesizing AgNPs, which demonstrate strong potential as effective antimicrobial agents against drug-resistant human pathogens such as L. monocytogenes and Y. enterocolitica.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflict of interest.
References
- Lele, M.; Kapur, S.; Hargett, S.; Sureshbabu, N.M.; Gaharwar, A.K. Global trends in clinical trials involving engineered biomaterials. Sci. Adv. 2024, 10, eabq0997. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, B.; Carson, L. Bioactive biomaterials for controlling biofilms. In Biomaterials and Medical Device-Associated Infections; Woodhead Publishing: Sawston, UK, 2014; Chapter 8. [Google Scholar]
- Datkhile, K.D.; Durgawale, P.P.; Jagdale, N.J.; More, A.L.; Patil, S.R. Biosynthesized silver nanoparticles of Cissus woodrowii inhibit proliferation of cancer cells through induction of apoptosis pathway. Cancer Nanotechnol. 2024, 15, 43. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: A review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Huq, M.A.; Rana, M.R.; Samad, A.; Rahman, M.S.; Rahman, M.M.; Ashrafudoulla, M.; Akter, S.; Park, J.-W. Green Synthesis, Characterization, and Potential Antibacterial and Anticancer Applications of Gold Nanoparticles: Current Status and Future Prospects. Biomedicines 2025, 13, 1184. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Huq, M.A. Biologically rapid synthesis of silver nanoparticles by Sphingobium sp. MAH-11T and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif. Cells Nanomed. Biotechnol. 2020, 48, 672–682. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Huq, M.A.; Apu, M.A.I.; Ashrafudoulla, M.; Rahman, M.M.; Parvez, M.A.K.; Balusamy, S.R.; Akter, S.; Rahman, M.S. Bioactive ZnO nanoparticles: Biosynthesis, characterization and potential antimicrobial applications. Pharmaceutics 2023, 15, 2634. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Binupriya, A.; Sathishkumar, M.; Yun, S.-I. Biocrystallization of silver and gold ions by inactive cell filtrate of Rhizopus stolonifer. Colloids Surf. B Biointerfaces 2010, 79, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ikram, S. Biosynthesis of gold nanoparticles: A green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Bukhari, A.; Ijaz, I.; Gilani, E.; Nazir, A.; Zain, H.; Saeed, R.; Alarfaji, S.S.; Hussain, S.; Aftab, R.; Naseer, Y. Green synthesis of metal and metal oxide nanoparticles using different plants’ parts for antimicrobial activity and anticancer activity: A review article. Coatings 2021, 11, 1374. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.S.; Ong, H.C. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 2022, 204, 111967. [Google Scholar] [CrossRef]
- Sedefoglu, N.; Zalaoglu, Y.; Bozok, F. Green synthesized ZnO nanoparticles using Ganoderma lucidum: Characterization and in vitro nanofertilizer effects. J. Alloys Compd. 2022, 918, 165695. [Google Scholar] [CrossRef]
- Sedefoglu, N.; Er, S.; Veryer, K.; Zalaoglu, Y.; Bozok, F. Green synthesized CuO nanoparticles using macrofungi extracts: Characterization, nanofertilizer and antibacterial effects. Mater. Chem. Phys. 2023, 309, 128393. [Google Scholar] [CrossRef]
- Gopinath, V.; Velusamy, P. Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusarium oxysporum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 170–174. [Google Scholar] [CrossRef]
- Huq, M.A.; Akter, S. Bacterial mediated rapid and facile synthesis of silver nanoparticles and their antimicrobial efficacy against pathogenic microorganisms. Materials 2021, 14, 2615. [Google Scholar] [CrossRef]
- Lateef, A.; Adelere, I.A.; Gueguim-Kana, E.B.; Asafa, T.B.; Beukes, L.S. Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13. Int. Nano Lett. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Saeed, S.; Iqbal, A.; Ashraf, M.A. Bacterial-mediated synthesis of silver nanoparticles and their significant effect against pathogens. Environ. Sci. Pollut. Res. 2020, 27, 37347–37356. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.A.; Shaaban, M.I. Synthesis of silver nanoparticles with antimicrobial and anti-adherence activities against multidrug-resistant isolates from Acinetobacter baumannii. J. Taibah Univ. Med. Sci. 2017, 12, 291–297. [Google Scholar] [CrossRef]
- Akter, S.; Lee, S.-Y.; Siddiqi, M.Z.; Balusamy, S.R.; Ashrafudoulla, M.; Rupa, E.J.; Huq, M.A. Ecofriendly synthesis of silver nanoparticles by Terrabacter humi sp. nov. and their antibacterial application against antibiotic-resistant pathogens. Int. J. Mol. Sci. 2020, 21, 9746. [Google Scholar] [CrossRef] [PubMed]
- Alsamhary, K.I. Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.-Y.; Akter, S.; Huq, M.A. Probiotic-mediated biosynthesis of silver nanoparticles and their antibacterial applications against pathogenic strains of Escherichia coli O157:H7. Polymers 2022, 14, 1834. [Google Scholar] [CrossRef]
- Mohd-Yusof, H.; Rahman, A.; Mohamad, R.; Zaidan, U.H. Microbial mediated synthesis of silver nanoparticles by Lactobacillus plantarum TA4 and its antibacterial and antioxidant activity. Appl. Sci. 2020, 10, 6973. [Google Scholar] [CrossRef]
- Jo, J.H.; Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Jin, C.-G.; Yang, D.C. Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1576–1581. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. Weissella oryzae DC6-facilitated green synthesis of silver nanoparticles and their antimicrobial potential. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1569–1575. [Google Scholar] [CrossRef]
- Luque-Sastre, L.; Arroyo, C.; Fox, E.M.; McMahon, B.J.; Bai, L.; Li, F.; Fanning, S. Antimicrobial resistance in Listeria species. Microbiol. Spectr. 2018, 6, 01–23. [Google Scholar] [CrossRef]
- Byun, K.-H.; Han, S.H.; Choi, M.W.; Kim, B.-H.; Ha, S.-D. Efficacy of disinfectant and bacteriophage mixture against planktonic and biofilm state of Listeria monocytogenes to control in the food industry. Int. J. Food Microbiol. 2024, 413, 110587. [Google Scholar] [CrossRef] [PubMed]
- Hanes, R.M.; Huang, Z. Investigation of Antimicrobial Resistance Genes in Listeria monocytogenes from 2010 through to 2021. Int. J. Environ. Res. Public Health 2022, 19, 5506. [Google Scholar] [CrossRef]
- Rippa, A.; Bilei, S.; Peruzy, M.F.; Marrocco, M.G.; Leggeri, P.; Bossù, T.; Murru, N. Antimicrobial resistance of Listeria monocytogenes strains isolated in food and food-processing environments in Italy. Antibiotics 2024, 13, 525. [Google Scholar] [CrossRef]
- Delibato, E.; Luzzi, I.; Pucci, E.; Proroga, Y.T.; Capuano, F.; De Medici, D. Fresh produce and microbial contamination: Persistence during the shelf life and efficacy of domestic washing methods. Ann. Dell’istituto Super. Sanità 2018, 54, 358–363. [Google Scholar]
- Raymond, P.; Houard, E.; Denis, M.; Esnault, E. Diversity of Yersinia enterocolitica isolated from pigs in a French slaughterhouse over 2 years. Microbiologyopen 2019, 8, e00751. [Google Scholar] [CrossRef]
- Porras, E.E.H.; Torres, L.E.R.; Barrera, E.L.P.; Montilla, J.A.G.; Rubio, A.L.G.; Castañeda, J.E.M. Foodborne disease outbreaks studied by molecular techniques. Rev. Salúd Publica 2017, 19, 671. [Google Scholar]
- Seakamela, E.M.; Diseko, L.; Malatji, D.; Makhado, L.; Motau, M.; Jambwa, K.; Magwedere, K.; Ntushelo, N.; Matle, I. Characterisation and antibiotic resistance of Yersinia enterocolitica from various meat categories, South Africa. Onderstepoort J. Vet. Res. 2022, 89, 2006. [Google Scholar] [CrossRef] [PubMed]
- Angelovska, M.; Zaharieva, M.M.; Dimitrova, L.L.; Dimova, T.; Gotova, I.; Urshev, Z.; Ilieva, Y.; Kaleva, M.D.; Kim, T.C.; Naydenska, S. Prevalence, genetic homogeneity, and antibiotic resistance of pathogenic Yersinia enterocolitica strains isolated from slaughtered pigs in Bulgaria. Antibiotics 2023, 12, 716. [Google Scholar] [CrossRef]
- Gkouletsos, T.; Patas, K.; Lambrinidis, G.; Neubauer, H.; Sprague, L.; Ioannidis, A.; Chatzipanagiotou, S. Antimicrobial resistance of Yersinia enterocolitica and presence of plasmid pYV virulence genes in human and animal isolates. New Microbes New Infect. 2019, 32, 100604. [Google Scholar] [CrossRef]
- Siddiqi, M.Z.; Shah, S.; Choi, K.D.; Lee, S.Y.; Kim, S.Y.; Im, W.T. Mesorhizobium hankyongi sp. nov. Isolated from Soil of Ginseng Cultivating Field. Curr. Microbiol. 2018, 75, 1453–1459. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Z.; Yeon, J.M.; Choi, H.; Lee, J.H.; Kim, S.Y.; Wee, J.H.; Im, W.T. Luteimonas granuli sp. nov., Isolated from Granules of the Wastewater Treatment Plant. Curr. Microbiol. 2020, 77, 2002–2007. [Google Scholar] [CrossRef]
- Quan, X.T.; Liu, Q.Z.; Siddiqi, M.Z.; Im, W.T. Caballeronia ginsengisoli sp. nov., isolated from ginseng cultivating soil. Arch. Microbiol. 2019, 201, 443–449. [Google Scholar] [CrossRef]
- Quan, X.T.; Siddiqi, M.Z.; Liu, Q.Z.; Lee, S.M.; Im, W.T. Devosia ginsengisoli sp. nov., isolated from ginseng cultivation soil. Int. J. Syst. Evol. Microbiol. 2020, 70, 1489–1495. [Google Scholar] [CrossRef]
- Jain, D.; Daima, H.K.; Kachhwaha, S.; Kothari, S.L. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig. J. Nanomater. Biostruct. 2009, 4, 557–563. [Google Scholar]
- Huq, M.A. Green synthesis of silver nanoparticles using Pseudoduganella eburnea MAHUQ-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R.; Mashwani, Z.-U.-R.; Quave, C.L. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Front. Pharmacol. 2018, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Baykal, A.; Asiri, S.; Rehman, S. Synthesis and characterization of antibacterial activity of spinel chromium-substituted copper ferrite nanoparticles for biomedical application. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2316–2327. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Mohamedin, A.; Hamza, S.S.; Sherief, A.-D. Extracellular Biofabrication, Characterization, and Antimicrobial Efficacy of Silver Nanoparticles Loaded on Cotton Fabrics Using Newly Isolated Streptomyces sp. SSHH-1E. J. Nanomater. 2016, 2016, 3257359. [Google Scholar] [CrossRef]
- Mahdieh, M.; Zolanvari, A.; Azimee, A. Green biosynthesis of silver nanoparticles by Spirulina platensis. Sci. Iran. 2012, 19, 926–929. [Google Scholar] [CrossRef]
- El-Batal, A.; Amin, M.; Shehata, M.; Hallol, M. Synthesis of silver nanoparticles by Bacillus stearothermophilus using gamma radiation and their antimicrobial activity. World Appl. Sci. J. 2013, 22, 1–16. [Google Scholar]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci. Rep. 2021, 11, 770. [Google Scholar] [CrossRef]
- Zannotti, M.; Piras, S.; Magnaghi, L.R.; Biesuz, R.; Giovannetti, R. Silver nanoparticles from orange peel extract: Colorimetric detection of Pb2+ and Cd2+ ions with a chemometric approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 323, 124881. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1. 4 and their antimicrobial application. J. Pharm. Anal. 2018, 8, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A.; Akter, S. Biosynthesis, characterization and antibacterial application of novel silver nanoparticles against drug resistant pathogenic Klebsiella pneumoniae and Salmonella enteritidis. Molecules 2021, 26, 5996. [Google Scholar] [CrossRef]
- Perveen, K.; Husain, F.M.; Qais, F.A.; Khan, A.; Razak, S.; Afsar, T.; Alam, P.; Almajwal, A.M.; Abulmeaty, M.M. Microwave-assisted rapid green synthesis of gold nanoparticles using seed extract of Trachyspermum ammi: ROS mediated biofilm inhibition and anticancer activity. Biomolecules 2021, 11, 197. [Google Scholar] [CrossRef]
- Moshtaghi, H.; Abbasvali, M. Effects of magnesium oxide and copper oxide nanoparticles on biofilm formation of Escherichia coli and Listeria monocytogenes. Nanotechnology 2023, 34, 155102. [Google Scholar]
- Polinarski, M.A.; Beal, A.L.; Silva, F.E.; Bernardi-Wenzel, J.; Burin, G.R.; de Muniz, G.I.; Alves, H.J. New perspectives of using chitosan, silver, and chitosan–silver nanoparticles against multidrug-resistant bacteria. Part. Part. Syst. Charact. 2021, 38, 2100009. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Emara, M.; Shawky, R.M.; El-domany, R.A.; Youssef, T. Silver nanoparticles: Antimicrobial activity, cytotoxicity, and synergism with N-acetyl cysteine. J. Basic Microbiol. 2017, 57, 659–668. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Mishra, D.; Ramalingam, C. Thermal Co-reduction engineered silver nanoparticles induce oxidative cell damage in human colon cancer cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Chem.-Biol. Interact. 2018, 295, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Marcato, P.D.; Conti, R.D.; Alves, O.L.; Costa, F.; Brocchi, M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 2010, 21, 949–959. [Google Scholar] [CrossRef]
- Xiu, Z.-m.; Zhang, Q.-b.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).