What Are the Best Biocompatible Materials for Extracorporeal Membrane Oxygenation
Abstract
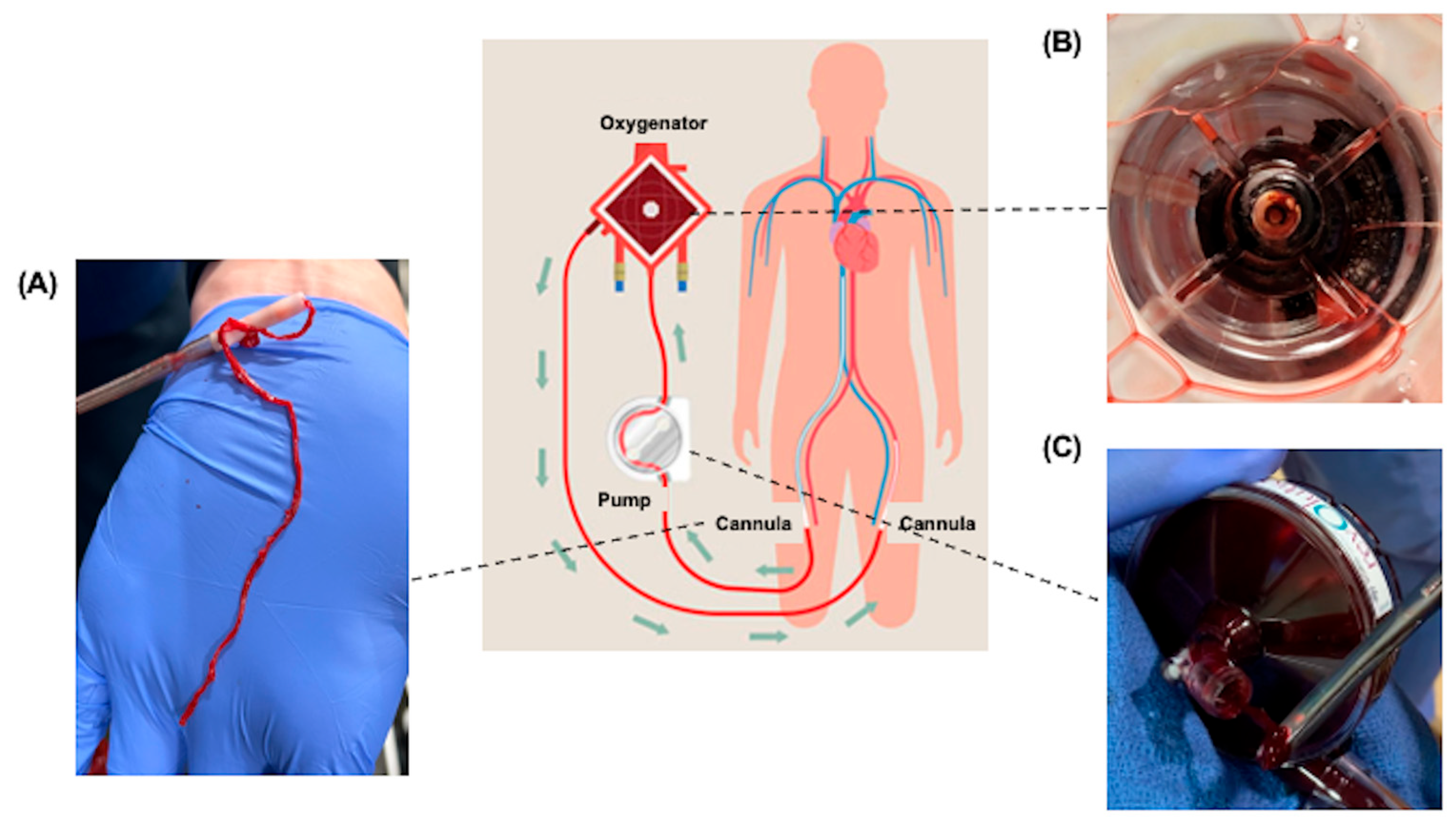
1. Introduction
2. Cannula
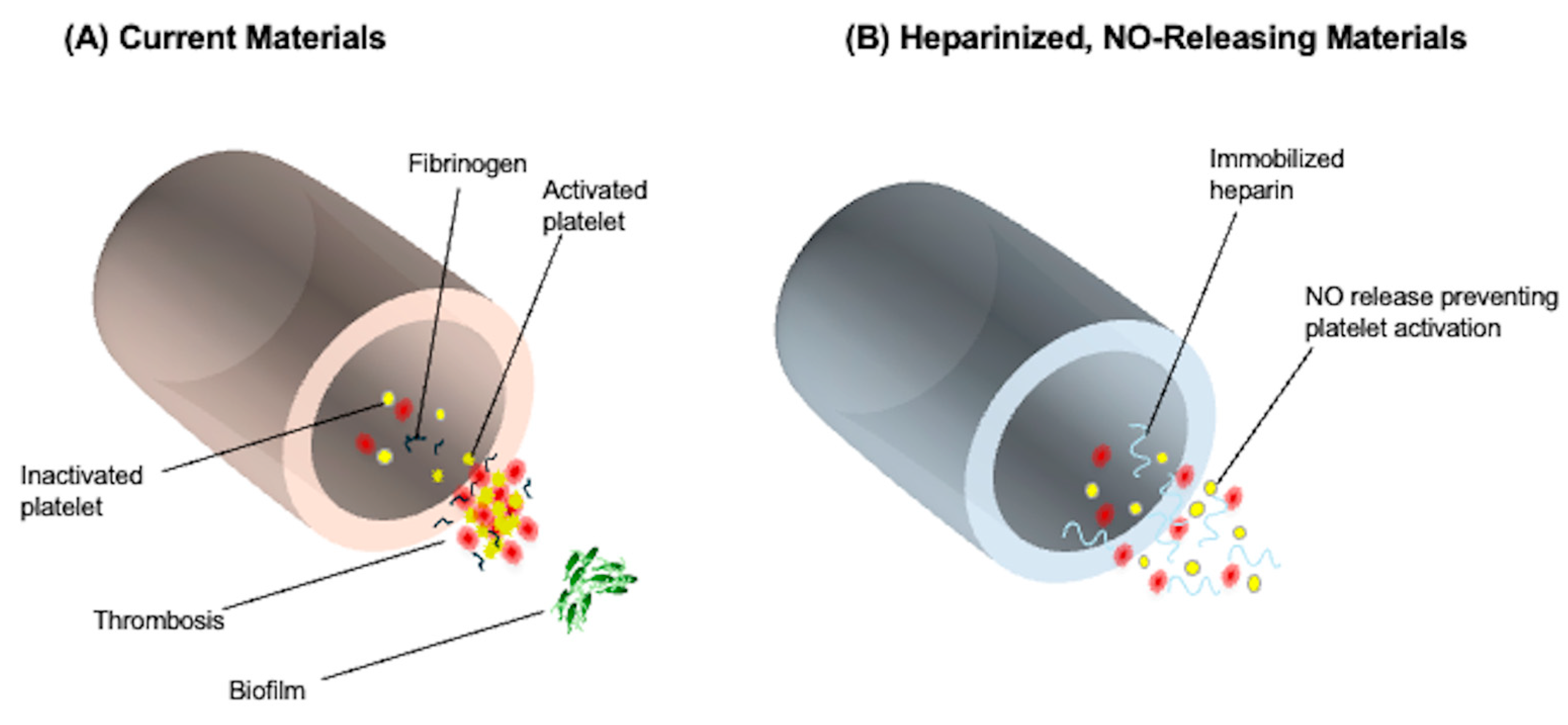
2.1. Heparin-Coated Surfaces
2.2. Poly-2-Methoxyethylacrylate (PMEA)-Coated Circuits
2.3. Phosphorylcholine (PC)-Based Coatings
2.4. Nitric Oxide (NO)-Releasing Biomimetic Surfaces
2.5. Fluid-Repellent Surfaces (Omniphobic)
| Mechanism | Metrics | Regulatory Consideration | |
|---|---|---|---|
| * Heparin-coated surfaces (Standard globally) |
|
|
|
| * PMEA-coated circuits (Asia, Europe, etc.) |
|
|
|
| * PC-based coatings (Europe, etc.) |
|
|
|
| NO biomimetic surfaces (Experimental) |
|
|
|
| Fluid-repellent surfaces (omniphobic) (Experimental) |
|
|
|
| Heparin plus NO-releasing surfaces (Experimental) |
|
|
|
3. Future Directions in ECMO Cannulas
4. Oxygenator
4.1. Physicochemical Surface Engineering
4.1.1. Surface Chemical Modification
4.1.2. Physical Absorption Modification
4.1.3. Plasma Deposition
4.1.4. Self-Assembly Technology
4.1.5. Surface Structure Design
4.2. Biomimetic Interface Design
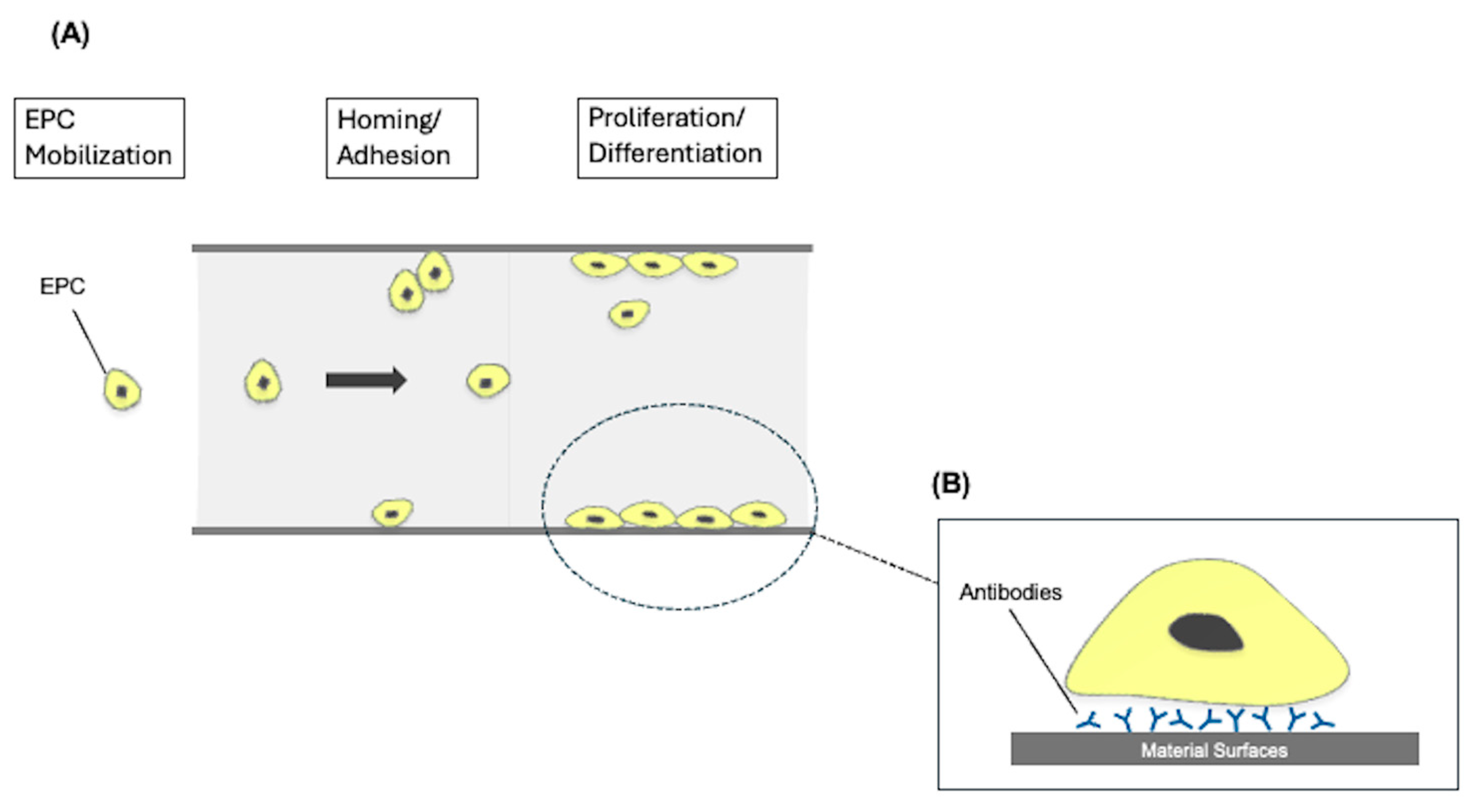
4.2.1. Surface Endothelialization
4.2.2. Graft Phosphorylcholine (PC)
4.2.3. Graft Protein
4.2.4. Graft Tissue Plasminogen Activator (tPA)
| Mechanism | Metrics | Regulatory Consideration | |
|---|---|---|---|
| Surface chemical modification |
|
|
|
| Physical absorption modification |
|
|
|
| Plasma deposition |
|
|
|
| Self-assembly technology |
|
|
|
| Surface structure design |
|
|
|
| Surface endothelialization |
|
|
|
| Graft phosphorylcholine (PC) |
|
|
|
| Graft protein |
|
|
|
| Graft tissue plasminogen activator (tPA) |
|
|
|
5. Future Directions in ECMO Oxygenators
6. Centrifugal Pump
6.1. Magnetic Levitation Systems
6.2. Hybrid Polymer-Composite Impellers
| Mechanism | Metrics | Regulatory Consideration | |
|---|---|---|---|
| Magnetic Levitation Systems |
|
|
|
| Hybrid Polymer -Composite Impellers |
|
|
|
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Combes, A.; Price, S.; Slutsky, A.S.; Brodie, D. Temporary circulatory support for cardiogenic shock. Lancet 2020, 396, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Garg, M. Intravascular Hemolysis and Complications During Extracorporeal Membrane Oxygenation. Neoreviews 2020, 21, e728–e740. [Google Scholar] [CrossRef] [PubMed]
- Materne, L.A.; Hunsicker, O.; Menk, M.; Graw, J.A. Hemolysis in patients with Extracorporeal Membrane Oxygenation therapy for severe Acute Respiratory Distress Syndrome—A systematic review of the literature. Int. J. Med. Sci. 2021, 18, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Willers, A.; Swol, J.; Buscher, H.; McQuilten, Z.; van Kuijk, S.M.J.; Ten Cate, H.; Rycus, P.T.; McKellar, S.; Lorusso, R.; Tonna, J.E. Longitudinal Trends in Bleeding Complications on Extracorporeal Life Support Over the Past Two Decades-Extracorporeal Life Support Organization Registry Analysis. Crit. Care Med. 2022, 50, e569–e580. [Google Scholar] [CrossRef]
- Sy, E.; Sklar, M.C.; Lequier, L.; Fan, E.; Kanji, H.D. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J. Crit. Care 2017, 39, 87–96. [Google Scholar] [CrossRef]
- Lorusso, R.; Gelsomino, S.; Parise, O.; Di Mauro, M.; Barili, F.; Geskes, G.; Vizzardi, E.; Rycus, P.T.; Muellenbach, R.; Mueller, T.; et al. Neurologic Injury in Adults Supported With Veno-Venous Extracorporeal Membrane Oxygenation for Respiratory Failure: Findings From the Extracorporeal Life Support Organization Database. Crit. Care Med. 2017, 45, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Barili, F.; Mauro, M.D.; Gelsomino, S.; Parise, O.; Rycus, P.T.; Maessen, J.; Mueller, T.; Muellenbach, R.; Belohlavek, J.; et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Crit. Care Med. 2016, 44, e964–e972. [Google Scholar] [CrossRef]
- Treml, B.; Breitkopf, R.; Bukumirić, Z.; Bachler, M.; Boesch, J.; Rajsic, S. ECMO Predictors of Mortality: A 10-Year Referral Centre Experience. J. Clin. Med. 2022, 11, 1224. [Google Scholar] [CrossRef]
- Olson, S.R.; Murphree, C.R.; Zonies, D.; Meyer, A.D.; McCarty, O.J.T.; Deloughery, T.G.; Shatzel, J.J. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. Asaio J. 2021, 67, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Wickramarachchi, A.; Khamooshi, M.; Burrell, A.; Pellegrino, V.A.; Kaye, D.M.; Gregory, S.D. The effect of drainage cannula tip position on risk of thrombosis during venoarterial extracorporeal membrane oxygenation. Comput. Methods Programs Biomed. 2023, 231, 107407. [Google Scholar] [CrossRef]
- Bidar, F.; Lancelot, A.; Lebreton, G.; Pineton de Chambrun, M.; Schmidt, M.; Hékimian, G.; Juvin, C.; Bréchot, N.; Schoell, T.; Leprince, P.; et al. Venous or arterial thromboses after venoarterial extracorporeal membrane oxygenation support: Frequency and risk factors. J. Heart Lung Transplant. 2021, 40, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, A.; Thibault, D.; Fugett, J.; Hayanga, H.K.; McCarthy, P.; Badhwar, V.; Awori Hayanga, J.W. Incremental effect of complications on mortality and hospital costs in adult ECMO patients. Perfusion 2022, 37, 461–469. [Google Scholar] [CrossRef]
- Fuchs, G.; Berg, N.; Broman, L.M.; Prahl Wittberg, L. Flow-induced platelet activation in components of the extracorporeal membrane oxygenation circuit. Sci. Rep. 2018, 8, 13985. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, W.H.; Son, J.; Lee, S.K.; Yeo, H.J. Catastrophic Mechanical Complications of Extracorporeal Membrane Oxygenation. Asaio J. 2021, 67, 1000–1005. [Google Scholar] [CrossRef]
- Vasques, F.; Sanderson, B.; Correa, G.; Collins, P.; Camarda, V.; Giosa, L.; Retter, A.; Meadows, C.; Barrett, N.A.; Camporota, L. Prevalence and Indications for Oxygenator Circuit Replacement in Patients Receiving Venovenous Extracorporeal Membrane Oxygenation. Asaio J. 2023, 69, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, L.H., Jr. Hastings lecture. Breaking the blood-biomaterial barrier. Asaio J. 1995, 41, 824–830. [Google Scholar]
- Annich, G.M. Extracorporeal life support: The precarious balance of hemostasis. J. Thromb. Haemost. 2015, 13 (Suppl. 1), S336–S342. [Google Scholar] [CrossRef]
- Frantzeskaki, F.; Konstantonis, D.; Rizos, M.; Kitsinelis, V.; Skyllas, G.; Renieris, I.; Doumani, M.; Kolias, V.; Kefalidi, E.; Angouras, D.; et al. Extracorporeal Membrane Oxygenation (ECMO)-Associated Coagulopathy in Adults. Diagnostics 2023, 13, 3496. [Google Scholar] [CrossRef] [PubMed]
- Gott, V.L.; Whiffen, J.D.; Dutton, R.C. Heparin Bonding on Colloidal Graphite Surfaces. Science 1963, 142, 1297–1298. [Google Scholar] [CrossRef]
- Mangoush, O.; Purkayastha, S.; Haj-Yahia, S.; Kinross, J.; Hayward, M.; Bartolozzi, F.; Darzi, A.; Athanasiou, T. Heparin-bonded circuits versus nonheparin-bonded circuits: An evaluation of their effect on clinical outcomes. Eur. J. Cardiothorac. Surg. 2007, 31, 1058–1069. [Google Scholar] [CrossRef]
- Ranucci, M.; Balduini, A.; Ditta, A.; Boncilli, A.; Brozzi, S. A systematic review of biocompatible cardiopulmonary bypass circuits and clinical outcome. Ann. Thorac. Surg. 2009, 87, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Kreuziger, L.B.; Rein, L.; Kleman, A.; Fraser, R.; Aster, R.H.; Hari, P.; Padmanabhan, A. Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: A population-based study. Lancet Haematol. 2018, 5, e220–e231. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Motomura, T.; Kawada, M.; Anzai, T.; Kasori, Y.; Shiroya, T.; Shimura, K.; Onishi, M.; Mochizuki, A. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)—Relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials 2000, 21, 1471–1481. [Google Scholar] [CrossRef]
- Gunaydin, S.; Farsak, B.; Kocakulak, M.; Sari, T.; Yorgancioglu, C.; Zorlutuna, Y. Clinical performance and biocompatibility of poly(2-methoxyethylacrylate)-coated extracorporeal circuits. Ann. Thorac. Surg. 2002, 74, 819–824. [Google Scholar] [CrossRef]
- Saito, N.; Motoyama, S.; Sawamoto, J. Effects of new polymer-coated extracorporeal circuits on biocompatibility during cardiopulmonary bypass. Artif. Organs 2000, 24, 547–554. [Google Scholar] [CrossRef]
- Itoh, H.; Ichiba, S.; Ujike, Y.; Douguchi, T.; Kasahara, S.; Arai, S.; Sano, S. A prospective randomized trial comparing the clinical effectiveness and biocompatibility of heparin-coated circuits and PMEA-coated circuits in pediatric cardiopulmonary bypass. Perfusion 2016, 31, 247–254. [Google Scholar] [CrossRef]
- Hosoyama, K.; Ito, K.; Kawamoto, S.; Kumagai, K.; Akiyama, M.; Adachi, O.; Kawatsu, S.; Sasaki, K.; Suzuki, M.; Sugawara, Y.; et al. Poly-2-methoxyethylacrylate-coated cardiopulmonary bypass circuit can reduce transfusion of platelet products compared to heparin-coated circuit during aortic arch surgery. J. Artif. Organs 2016, 19, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Izuha, H.; Hattori, M.; Igari, T.; Wakamatsu, D.; Watanabe, M.; Yokoyama, H. Changes in platelet aggregation during cardiopulmonary bypass: Comparison of poly-2-methoxyethylacrylate and heparin as a circuit coating material. J. Artif. Organs 2005, 8, 41–46. [Google Scholar] [CrossRef]
- Pieri, M.; Turla, O.G.; Calabrò, M.G.; Ruggeri, L.; Agracheva, N.; Zangrillo, A.; Pappalardo, F. A new phosphorylcholine-coated polymethylpentene oxygenator for extracorporeal membrane oxygenation: A preliminary experience. Perfusion 2013, 28, 132–137. [Google Scholar] [CrossRef]
- Ye, S.H.; Arazawa, D.T.; Zhu, Y.; Shankarraman, V.; Malkin, A.D.; Kimmel, J.D.; Gamble, L.J.; Ishihara, K.; Federspiel, W.J.; Wagner, W.R. Hollow fiber membrane modification with functional zwitterionic macromolecules for improved thromboresistance in artificial lungs. Langmuir 2015, 31, 2463–2471. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Shi, K.-H.; Jiang, H.-L.; Gong, Y.-K. Significantly reduced adsorption and activation of blood components in a membrane oxygenator system coated with crosslinkable zwitterionic copolymer. Acta Biomater. 2016, 40, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Pazzaglia, A.; Isgrò, G.; Cazzaniga, A.; Ditta, A.; Boncilli, A.; Cotza, M.; Carboni, G.; Brozzi, S.; Bonifazi, C. Closed, phosphorylcholine-coated circuit and reduction of systemic heparinization for cardiopulmonary bypass: The intraoperative ECMO concept. Int. J. Artif. Organs 2002, 25, 875–881. [Google Scholar] [CrossRef]
- Ranucci, M.; Isgrò, G.; Soro, G.; Canziani, A.; Menicanti, L.; Frigiola, A. Reduced systemic heparin dose with phosphorylcholine coated closed circuit in coronary operations. Int. J. Artif. Organs 2004, 27, 311–319. [Google Scholar] [CrossRef]
- Moussa, M.D.; Abou-Arab, O.; Staessens, S.; Jungling, M.; Labreuche, J.; Lamer, A.; Beyls, C.; Rousse, N.; Rauch, A.; Loobuyck, V.; et al. Comparison of the effects of phosphorylcholin versus heparin-based surface coating on clinical and histologic outcomes during veno-arterial extracorporeal membrane oxygenation support: A propensity score weighted analysis. J. Thromb. Haemost. 2025, 23, 1879–1892. [Google Scholar] [CrossRef]
- Reynolds, M.M.; Annich, G.M. The artificial endothelium. Organogenesis 2011, 7, 42–49. [Google Scholar] [CrossRef]
- Skrzypchak, A.M.; Lafayette, N.G.; Bartlett, R.H.; Zhou, Z.; Frost, M.C.; Meyerhoff, M.E.; Reynolds, M.M.; Annich, G.M. Effect of varying nitric oxide release to prevent platelet consumption and preserve platelet function in an in vivo model of extracorporeal circulation. Perfusion 2007, 22, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Handa, H.; Brisbois, E.J.; Major, T.C.; Refahiyat, L.; Amoako, K.A.; Annich, G.M.; Bartlett, R.H.; Meyerhoff, M.E. In vitro and in vivo study of sustained nitric oxide release coating using diazeniumdiolate-oped poly(vinyl chloride) matrix with poly(lactide-co-glycolide) additive. J. Mater. Chem. B 2013, 1, 3578–3587. [Google Scholar] [CrossRef]
- Devine, R.; Goudie, M.J.; Singha, P.; Schmiedt, C.; Douglass, M.; Brisbois, E.J.; Handa, H. Mimicking the Endothelium: Dual Action Heparinized Nitric Oxide Releasing Surface. ACS Appl. Mater. Interfaces 2020, 12, 20158–20171. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Movafaghi, S.; Wang, W.; Bark, D.L., Jr.; Dasi, L.P.; Popat, K.C.; Kota, A.K. Hemocompatibility of Super-Repellent surfaces: Current and Future. Mater. Horiz. 2019, 6, 1596–1610. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef]
- Faase, R.A.; Hummel, M.H.; Hasbrook, A.V.; Carpenter, A.P.; Baio, J.E. A biomimetic approach towards a universal slippery liquid infused surface coating. Beilstein J. Nanotechnol. 2024, 15, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Badv, M.; Jaffer, I.H.; Weitz, J.I.; Didar, T.F. An omniphobic lubricant-infused coating produced by chemical vapor deposition of hydrophobic organosilanes attenuates clotting on catheter surfaces. Sci. Rep. 2017, 7, 11639. [Google Scholar] [CrossRef] [PubMed]
- Amoako, K.; Ukita, R.; Cook, K.E. Antifouling Zwitterionic Polymer Coatings for Blood-Bearing Medical Devices. Langmuir 2025, 41, 2994–3006. [Google Scholar] [CrossRef] [PubMed]
- Crago, M.; Tan, R.; Hung, J.; Wise, S.G.; Akhavan, B.; Bilek, M.; Dehghani, F.; Talebian, S.; Naficy, S. Durable plasma-mediated zwitterionic grafting on polymeric surfaces for implantable medical devices. Commun. Mater. 2024, 5, 24. [Google Scholar] [CrossRef]
- Roberts, T.R.; Harea, G.T.; Singha, P.; Sieck, K.N.; Beely, B.M.; Wendorff, D.S.; Choi, J.H.; Ande, S.; Handa, H.; Batchinsky, A.I. Heparin-Free Extracorporeal Life Support Using Tethered Liquid Perfluorocarbon: A Feasibility and Efficacy Study. Asaio J. 2020, 66, 809–817. [Google Scholar] [CrossRef]
- Roberts, T.R.; Choi, J.H.; Wendorff, D.S.; Harea, G.T.; Beely, B.M.; Sieck, K.N.; Douglass, M.E.; Singha, P.; Dean, J.B.; Handa, H.; et al. Tethered Liquid Perfluorocarbon Coating for 72 Hour Heparin-Free Extracorporeal Life Support. Asaio J. 2021, 67, 798–808. [Google Scholar] [CrossRef]
- Gao, W.; Wang, H.; Liu, Y.; Tang, Q.; Wu, P.; Lin, T.; Li, T.; Sun, D. Sodium alginate-hydrogel coatings on extracorporeal membrane oxygenation for anticoagulation. Front. Cardiovasc. Med. 2022, 9, 966649. [Google Scholar] [CrossRef]
- Yao, M.; Wei, Z.; Li, J.; Guo, Z.; Yan, Z.; Sun, X.; Yu, Q.; Wu, X.; Yu, C.; Yao, F.; et al. Microgel reinforced zwitterionic hydrogel coating for blood-contacting biomedical devices. Nat. Commun. 2022, 13, 5339. [Google Scholar] [CrossRef]
- Avci-Adali, M.; Ziemer, G.; Wendel, H.P. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization—A review of current strategies. Biotechnol. Adv. 2010, 28, 119–129. [Google Scholar] [CrossRef]
- Roberts, T.R.; Garren, M.R.S.; Handa, H.; Batchinsky, A.I. Toward an artificial endothelium: Development of blood-compatible surfaces for extracorporeal life support. J. Trauma. Acute Care Surg. 2020, 89, S59–S68. [Google Scholar] [CrossRef] [PubMed]
- Quandt, J.; Garay-Sarmiento, M.; Witzdam, L.; Englert, J.; Rutsch, Y.; Stöcker, C.; Obstals, F.; Grottke, O.; Rodriguez-Emmenegger, C. Interactive Hemocompatible Nanocoating to Prevent Surface-Induced Coagulation in Medical Devices. Adv. Mater. Interfaces 2022, 9, 2201055. [Google Scholar] [CrossRef]
- Wallisch, M.; Lorentz, C.U.; Lakshmanan, H.H.S.; Johnson, J.; Carris, M.R.; Puy, C.; Gailani, D.; Hinds, M.T.; McCarty, O.J.T.; Gruber, A.; et al. Antibody inhibition of contact factor XII reduces platelet deposition in a model of extracorporeal membrane oxygenator perfusion in nonhuman primates. Res. Pract. Thromb. Haemost. 2020, 4, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Rim, G.; Hyun, K.; Cho, D.G.; Lim, Z.; Lee, B.; Kim, K.; Yoo, G.Y. Early thrombus detection in the extracorporeal membrane oxygenation circuit by noninvasive real-time ultrasonic sensors. Sci. Rep. 2024, 14, 10438. [Google Scholar] [CrossRef]
- Wang, S.; Kunselman, A.R.; Ündar, A. Evaluation of Capiox RX25 and Quadrox-i Adult Hollow Fiber Membrane Oxygenators in a Simulated Cardiopulmonary Bypass Circuit. Artif. Organs 2016, 40, E69–E78. [Google Scholar] [CrossRef]
- Ginther, R.M., Jr.; Gorney, R.; Cruz, R. A clinical evaluation of the Maquet Quadrox-i Neonatal oxygenator with integrated arterial filter. Perfusion 2013, 28, 194–199. [Google Scholar] [CrossRef]
- Mazzeffi, M.A.; Tanaka, K.; Roberts, A.; Rector, R.; Menaker, J.; Kon, Z.; Deatrick, K.B.; Kaczorowski, D.; Griffith, B.; Herr, D. Bleeding, Thrombosis, and Transfusion With Two Heparin Anticoagulation Protocols in Venoarterial ECMO Patients. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1216–1220. [Google Scholar] [CrossRef]
- He, T.; He, J.; Wang, Z.; Cui, Z. Modification strategies to improve the membrane hemocompatibility in extracorporeal membrane oxygenator (ECMO). Adv. Compos. Hybrid. Mater. 2021, 4, 847–864. [Google Scholar] [CrossRef]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Yuan, J. Design of hemocompatible and antifouling PET sheets with synergistic zwitterionic surfaces. J. Colloid. Interface Sci. 2016, 480, 205–217. [Google Scholar] [CrossRef]
- Obstals, F.; Vorobii, M.; Riedel, T.; de Los Santos Pereira, A.; Bruns, M.; Singh, S.; Rodriguez-Emmenegger, C. Improving Hemocompatibility of Membranes for Extracorporeal Membrane Oxygenators by Grafting Nonthrombogenic Polymer Brushes. Macromol. Biosci. 2018, 18, 1700359. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Brook, M.A.; Sheardown, H. Silicone elastomers for reduced protein adsorption. Biomaterials 2004, 25, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Z.; Chen, Y.; Brook, M.A.; Sheardown, H. Protein repellant silicone surfaces by covalent immobilization of poly(ethylene oxide). Biomaterials 2005, 26, 2391–2399. [Google Scholar] [CrossRef]
- Zhao, J.; Song, L.; Shi, Q.; Luan, S.; Yin, J. Antibacterial and hemocompatibility switchable polypropylene nonwoven fabric membrane surface. ACS Appl. Mater. Interfaces 2013, 5, 5260–5268. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Deppisch, R.M.; Forrestal, L.J.; Ritzau, G.H.; Oram, A.D.; Göhl, H.J.; Voorhees, M.E. Surface modifying additives for improved device-blood compatibility. Asaio J. 1994, 40, M619–M624. [Google Scholar] [CrossRef]
- Goushki, M.N.; Mousavi, S.A.; Abdekhodaie, M.J.; Sadeghi, M. Free radical graft polymerization of 2-hydroxyethyl methacrylate and acrylic acid on the polysulfone membrane surface through circulation of reaction media to improve its performance and hemocompatibility properties. J. Membr. Sci. 2018, 564, 762–772. [Google Scholar] [CrossRef]
- Wang, D.A.; Ji, J.; Gao, C.Y.; Yu, G.H.; Feng, L.X. Surface coating of stearyl poly(ethylene oxide) coupling-polymer on polyurethane guiding catheters with poly(ether urethane) film-building additive for biomedical applications. Biomaterials 2001, 22, 1549–1562. [Google Scholar] [CrossRef]
- Chen, J.Y.; Leng, Y.X.; Tian, X.B.; Wang, L.P.; Huang, N.; Chu, P.K.; Yang, P. Antithrombogenic investigation of surface energy and optical bandgap and hemocompatibility mechanism of Ti(Ta+5)O2 thin films. Biomaterials 2002, 23, 2545–2552. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Creating long-lived superhydrophobic polymer surfaces through mechanically assembled monolayers. Science 2000, 290, 2130–2133. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Xiang, T.; Wang, R.; Zhao, W.F.; Sun, S.D.; Zhao, C.S. Covalent deposition of zwitterionic polymer and citric acid by click chemistry-enabled layer-by-layer assembly for improving the blood compatibility of polysulfone membrane. Langmuir 2014, 30, 5115–5125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, X.; Zhou, T.; Wang, R.; Hou, J.; Tang, J.; Zhu, B.; Su, Y.; Zhu, X. Layer by layer assembled phosphorylcholine groups on paclitaxel/chitosan nanofibers coatings for hemocompatibility improvement. Surf. Coat. Technol. 2019, 357, 984–992. [Google Scholar] [CrossRef]
- Wang, T.; Handschuh-Wang, S.; Yang, Y.; Zhuang, H.; Schlemper, C.; Wesner, D.; Schönherr, H.; Zhang, W.; Jiang, X. Controlled surface chemistry of diamond/β-SiC composite films for preferential protein adsorption. Langmuir 2014, 30, 1089–1099. [Google Scholar] [CrossRef]
- Lee, J.H.; Khang, G.; Lee, J.W.; Lee, H.B. Platelet adhesion onto chargeable functional group gradient surfaces. J. Biomed. Mater. Res. 1998, 40, 180–186. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, J.; Peng, M.; Yang, Z.; Wan, Y.; Yao, F.; Zhou, J.; Ouyang, C.; Deng, X.; Luo, H. Laser-induced wettability gradient surface on NiTi alloy for improved hemocompatibility and flow resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110847. [Google Scholar] [CrossRef]
- Prudnikov, E.; Polishchuk, I.; Sand, A.; Hamad, H.A.; Massad-Ivanir, N.; Segal, E.; Pokroy, B. Self-assembled fatty acid crystalline coatings display superhydrophobic antimicrobial properties. Mater. Today Bio 2023, 18, 100516. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, M.K.; Whitesides, G.M. How to make water run uphill. Science 1992, 256, 1539–1541. [Google Scholar] [CrossRef]
- Dejana, E. Endothelial cell-cell junctions: Happy together. Nat. Rev. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef]
- Koenneker, S.; Teebken, O.E.; Bonehie, M.; Pflaum, M.; Jockenhoevel, S.; Haverich, A.; Wilhelmi, M.H. A biological alternative to alloplastic grafts in dialysis therapy: Evaluation of an autologised bioartificial haemodialysis shunt vessel in a sheep model. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 810–816. [Google Scholar] [CrossRef]
- Achneck, H.E.; Jamiolkowski, R.M.; Jantzen, A.E.; Haseltine, J.M.; Lane, W.O.; Huang, J.K.; Galinat, L.J.; Serpe, M.J.; Lin, F.H.; Li, M.; et al. The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells: EPC-seeded antithrombotic Ti implants. Biomaterials 2011, 32, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.H.; Farhatnia, Y.; Godarzi, F.; Tan, A.; Rajadas, J.; Cousins, B.G.; Seifalian, A.M. In situ Endothelialization: Bioengineering Considerations to Translation. Small 2015, 11, 6248–6264. [Google Scholar] [CrossRef] [PubMed]
- Pflaum, M.; Kühn-Kauffeldt, M.; Schmeckebier, S.; Dipresa, D.; Chauhan, K.; Wiegmann, B.; Haug, R.J.; Schein, J.; Haverich, A.; Korossis, S. Endothelialization and characterization of titanium dioxide-coated gas-exchange membranes for application in the bioartificial lung. Acta Biomater. 2017, 50, 510–521. [Google Scholar] [CrossRef]
- Chou, S.F.; Caltrider, B.A.; Azghani, A.; Neuenschwander, P.F. Inhibition of Platelet Adhesion from Surface Modified Polyurethane Membranes. Biomed. J. Sci. Tech. Res. 2020, 32, 24988–24993. [Google Scholar] [CrossRef]
- Ishihara, K. Blood-Compatible Surfaces with Phosphorylcholine-Based Polymers for Cardiovascular Medical Devices. Langmuir 2019, 35, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, J.N.; Faradji, A.; Oberling, F.; Cazenave, J.P. Surface passivation by human albumin of plasmapheresis circuits reduces platelet accumulation and thrombus formation. Experimental and clinical studies. J. Biomed. Mater. Res. 1990, 24, 155–163. [Google Scholar] [CrossRef]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces—A Review. Bioengineering 2021, 8, 215. [Google Scholar] [CrossRef]
- Burzava, A.L.S.; Jasieniak, M.; Cockshell, M.P.; Voelcker, N.H.; Bonder, C.S.; Griesser, H.J.; Moore, E. Attachment of endothelial colony-forming cells onto a surface bearing immobilized anti-CD34 antibodies: Specific CD34 binding versus nonspecific binding. Biointerphases 2022, 17, 031003. [Google Scholar] [CrossRef]
- Li, S.; Atkinson, H.M.; Fusch, G.; Rochow, N.; Fusch, C.; Selvaganapathy, P.R.; Brash, J.L.; Chan, A.K.C.; Sask, K.N. Dual surface modification of polydimethylsiloxane (PDMS) with antithrombin-heparin complex (ATH) and tissue plasminogen activator (t-PA) for enhanced antithrombotic activity. Biomater. Adv. 2025, 166, 214067. [Google Scholar] [CrossRef]
- Witzdam, L.; Sandhu, S.; Shin, S.; Hong, Y.; Kamal, S.; Grottke, O.; Cook, K.E.; Rodriguez-Emmenegger, C. Enhancing Hemocompatibility in ECMO Systems With a Fibrinolytic Interactive Coating: In Vitro Evaluation of Blood Clot Lysis Using a 3D Microfluidic Model. Macromol. Biosci. 2025, 25, e2400530. [Google Scholar] [CrossRef]
- Pratama, J.H.; Lestari, W.W.; Rofida, A.; Putri, A.K.; Widian, R.N.; Gunawan, T.; Hastuti, D.S.; Sulistiono, D.O.; Sari, K.P. Novel polymer composite coated with ethylcellulose nanoparticle from waste paper as an alternative material to extracorporeal oxygenation membrane. J. Polym. Res. 2023, 30, 220. [Google Scholar] [CrossRef]
- Yi, E.; Kang, H.S.; Lim, S.M.; Heo, H.J.; Han, D.; Kim, J.F.; Park, A.; Choi, D.H.; Park, Y.-I.; Park, H. Superamphiphobic blood-repellent surface modification of porous fluoropolymer membranes for blood oxygenation applications. J. Membr. Sci. 2022, 648, 120363. [Google Scholar] [CrossRef]
- Türkmen, M.; Lauwigi, T.; Fechter, T.; Gries, F.; Fischbach, A.; Gries, T.; Rossaint, R.; Bleilevens, C.; Winnersbach, P. Bioimpedance Analysis as Early Predictor for Clot Formation Inside a Blood-Perfused Test Chamber: Proof of Concept Using an In Vitro Test-Circuit. Biosensors 2023, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Fiusco, F.; Broman, L.M.; Prahl Wittberg, L. Blood Pumps for Extracorporeal Membrane Oxygenation: Platelet Activation During Different Operating Conditions. Asaio J. 2022, 68, 79–86. [Google Scholar] [CrossRef]
- Hastings, S.M.; Deshpande, S.R.; Wagoner, S.; Maher, K.; Ku, D.N. Thrombosis in centrifugal pumps: Location and composition in clinical and in vitro circuits. Int. J. Artif. Organs 2016, 39, 200–204. [Google Scholar] [CrossRef]
- Schibilsky, D.; Takatani, S.; Schibilsky, B.; Graf, T.; da Silva, D.M.; Wendel, H.P.; Avci-Adali, M.; Schlensak, C. Hemocompatibility of new magnetically-levitated centrifugal pump technology compared to the CentriMag adult pump. Sci. Rep. 2020, 10, 22055. [Google Scholar] [CrossRef]
- Zhou, M.; Qi, Z.; Xia, Z.; Li, Y.; Ling, W.; Yang, J.; Yang, Z.; Pei, J.; Wu, D.; Huo, W.; et al. Miniaturized soft centrifugal pumps with magnetic levitation for fluid handling. Sci. Adv. 2021, 7, eabi7203. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.; Wu, Y.; Lin, H.; Liang, Q.; Teng, G.; Liu, Z.; Huang, M. Application of a novel extracorporeal membrane oxygenation system in awake Hu sheep under various durations. BMC Anesthesiol. 2025, 25, 59. [Google Scholar] [CrossRef]
- Bansal, A.; Uriel, N.; Colombo, P.C.; Narisetty, K.; Long, J.W.; Bhimaraj, A.; Cleveland, J.C., Jr.; Goldstein, D.J.; Stulak, J.M.; Najjar, S.S.; et al. Effects of a fully magnetically levitated centrifugal-flow or axial-flow left ventricular assist device on von Willebrand factor: A prospective multicenter clinical trial. J. Heart Lung Transplant. 2019, 38, 806–816. [Google Scholar] [CrossRef]
- Abdul Samad, M. Recent Advances in UHMWPE/UHMWPE Nanocomposite/UHMWPE Hybrid Nanocomposite Polymer Coatings for Tribological Applications: A Comprehensive Review. Polymers 2021, 13, 608. [Google Scholar] [CrossRef]
- Ishihara, K. Biomimetic materials based on zwitterionic polymers toward human-friendly medical devices. Sci. Technol. Adv. Mater. 2022, 23, 498–524. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Shen, H.; Chang, Y.; Tang, Q.; Li, T.; Sun, D. Bivalirudin-hydrogel coatings of polyvinyl chloride on extracorporeal membrane oxygenation for anticoagulation. Front. Cardiovasc. Med. 2023, 10, 1301507. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagiwara, J.; DellaVolpe, J.D.; Matsuzaki, Y. What Are the Best Biocompatible Materials for Extracorporeal Membrane Oxygenation. J. Funct. Biomater. 2025, 16, 226. https://doi.org/10.3390/jfb16060226
Hagiwara J, DellaVolpe JD, Matsuzaki Y. What Are the Best Biocompatible Materials for Extracorporeal Membrane Oxygenation. Journal of Functional Biomaterials. 2025; 16(6):226. https://doi.org/10.3390/jfb16060226
Chicago/Turabian StyleHagiwara, Junya, Jeffrey D. DellaVolpe, and Yuichi Matsuzaki. 2025. "What Are the Best Biocompatible Materials for Extracorporeal Membrane Oxygenation" Journal of Functional Biomaterials 16, no. 6: 226. https://doi.org/10.3390/jfb16060226
APA StyleHagiwara, J., DellaVolpe, J. D., & Matsuzaki, Y. (2025). What Are the Best Biocompatible Materials for Extracorporeal Membrane Oxygenation. Journal of Functional Biomaterials, 16(6), 226. https://doi.org/10.3390/jfb16060226






