The Influence of the Machining Drill and Direction of Rotation on the Surfaces of Ti6Al4V Dental Implants Subjected to Implantoplasty
Abstract
1. Introduction
2. Materials and Methods
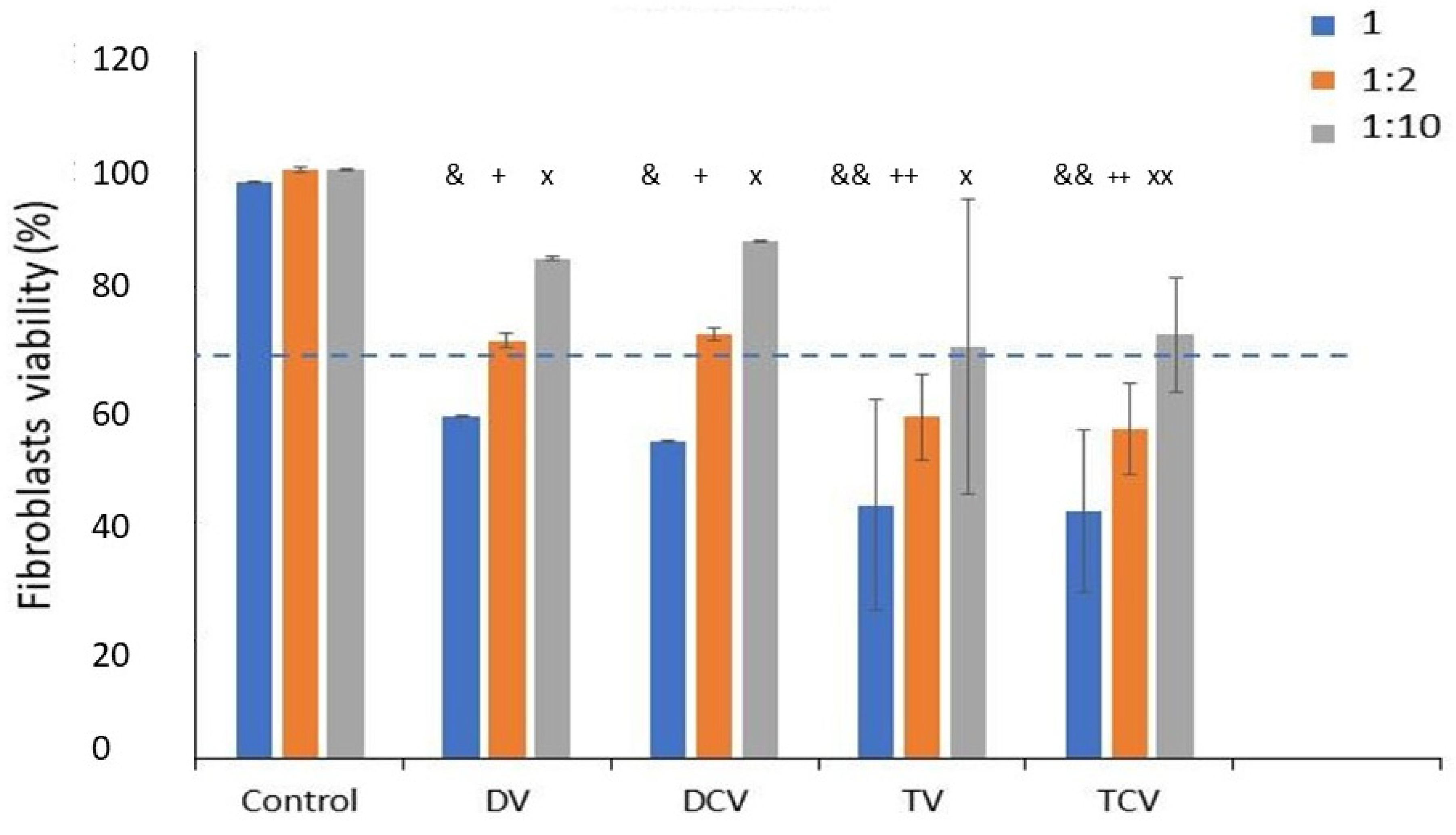
- Control: Ti6Al4V disks machined.
- DV: Diamond drill with clockwise rotation.
- DCV: Diamond drill with counterclockwise rotation.
- TV: Tungsten carbide with clockwise rotation.
- TCV: Tungsten carbide with counterclockwise rotation.
2.1. Morphology Charaterization
2.2. Roughness
2.3. Wettability
2.4. Nanoindentation
2.5. Residual Stress
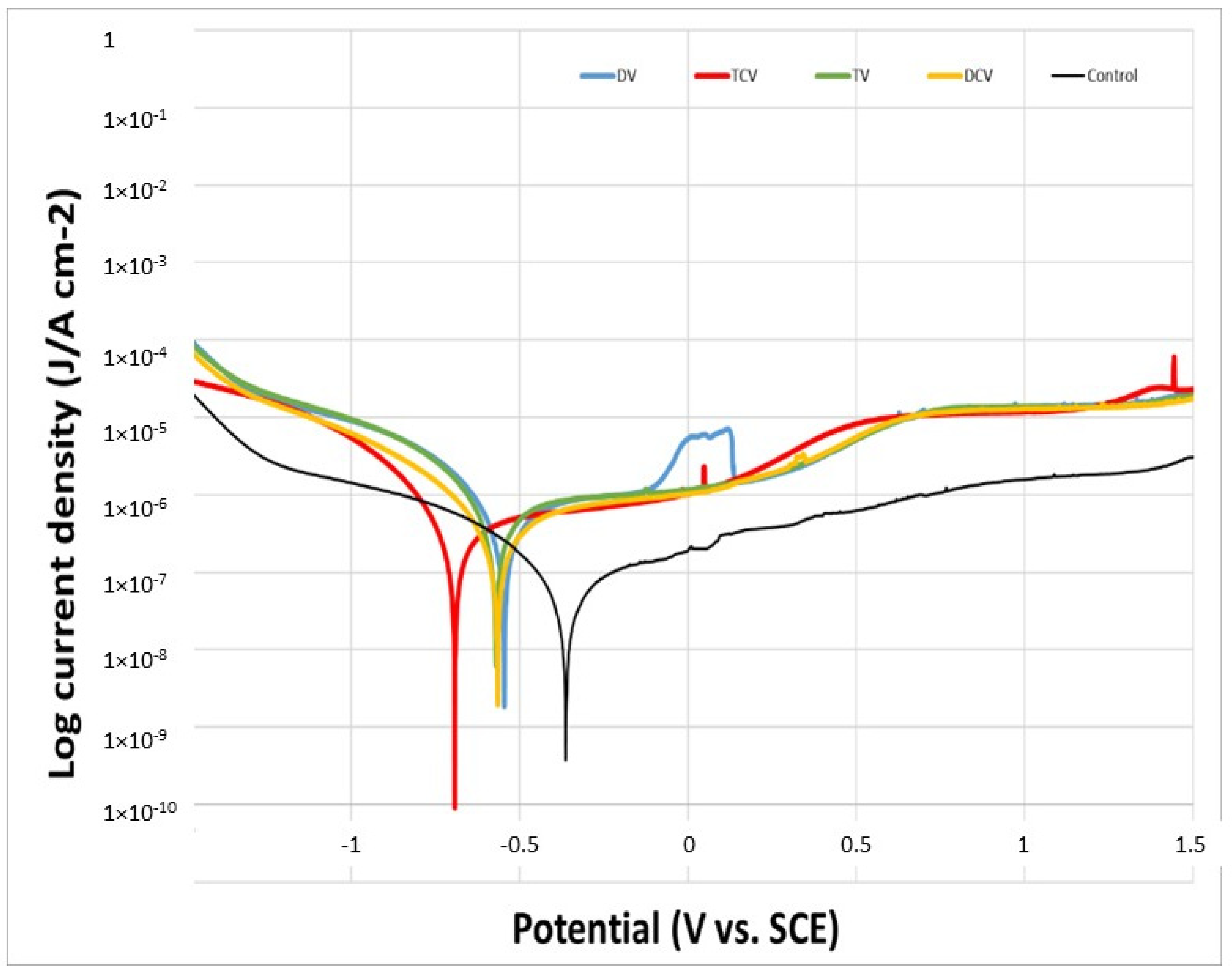
2.6. Corrosion Resistance
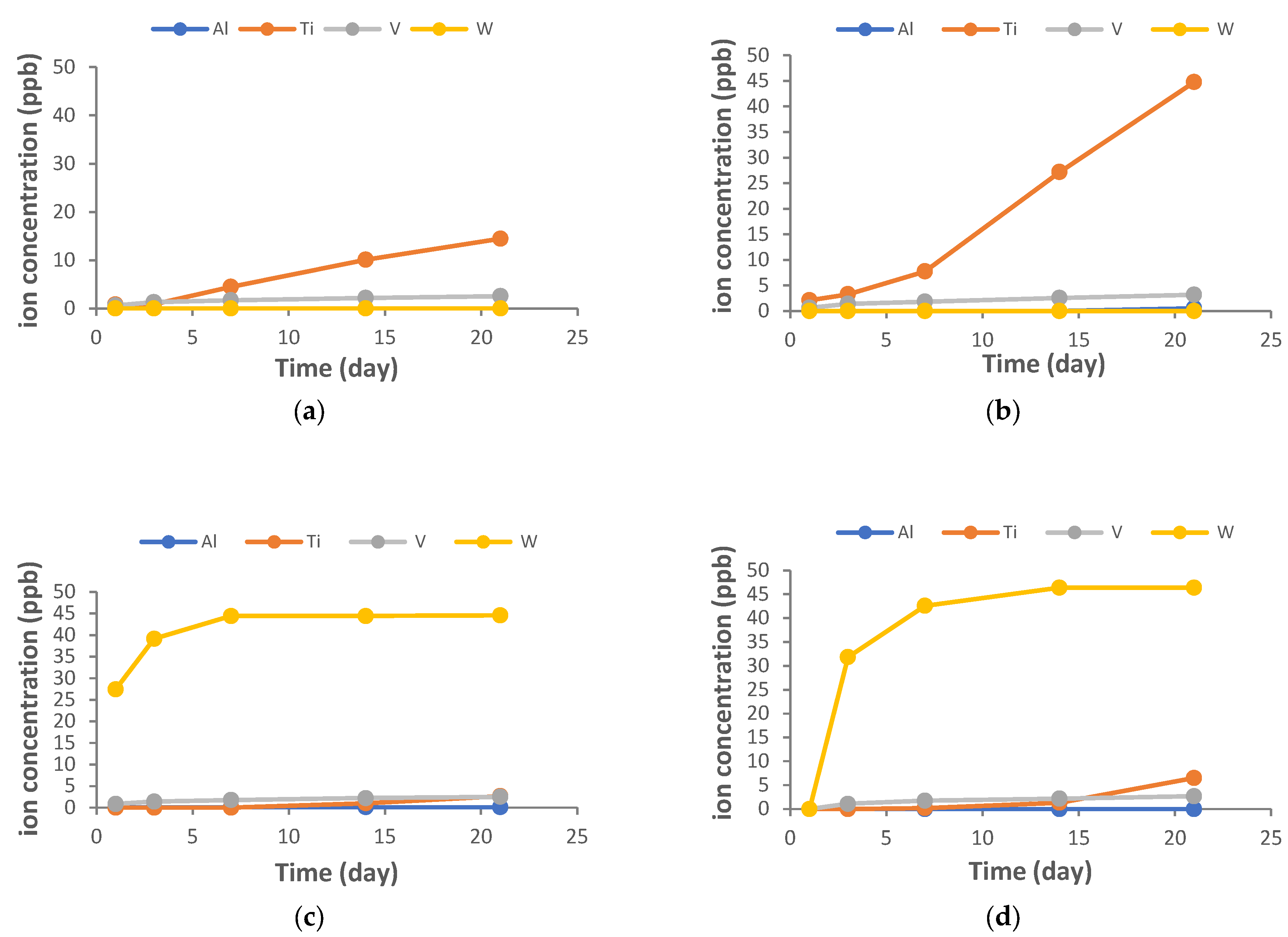
2.7. Assessment of Ion Release from Samples
2.8. Procedure for Cytotoxicity Testing
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef] [PubMed]
- Kensara, A.; Saito, H.; Mongodin, E.F.; Masri, R. Microbiological profile of peri-implantitis: Analyses of microbiome within dental implants. J. Prosthodont. 2023, 32, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Roos-Jansåker, A.M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2022, 35, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Peri-Implantitis Treatment Market Size & Share, Statistics Report 2024–2036. Research Nester. Available online: https://www.researchnester.com/reports/periimplantitis-treatment-market/3922 (accessed on 1 March 2025).
- Diaz, P.; Gonzalo, E.; Villagra, L.J.G.; Miegimolle, B.; Suarez, M.J. What is the prevalence of periimplantitis? A systematic review and meta-analysis. BMC Oral Health 2022, 22, 449. [Google Scholar] [CrossRef]
- Thakkar, R. Trends in Dental Implants 2022. American Academy of Implant Dentistry. Available online: https://connect.aaid-implant.org/blog/trends-in-dental-implants-2022 (accessed on 12 January 2025).
- Francis, S.; Iaculli, F.; Perrotti, V.; Piattelli, A.; Quaranta, A. Titanium Surface Decontamination: A Systematic Review of In Vitro Comparative Studies. Int. J. Oral Maxillofac. Implants 2022, 37, 76–84. [Google Scholar] [CrossRef]
- Tomasi, C.; Regidor, E.; Ortiz-Vigón, A.; Derks, J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 340–356. [Google Scholar] [CrossRef]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implants Dent. 2015, 1, 22. [Google Scholar] [CrossRef]
- Sadowsky, S.J. Peri-implantitis after 40 years: Evidence, mechanisms, and implications: A mapping review. J. Prosthet. Dent. 2024, 132, 1215–1225. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin. Oral Implant. Res. 2014, 25, 132–136. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Román, J.; Padilla, S.; Doadrio, J.C.; Gil, F.J. Bioactivity and mechanical properties of SiO2–CaO–P2O5 glassceramics. J. Mater. Chem. 2005, 15, 1353–1359. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol. 2022, 93, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Schwarz, F. Principles of Combined Surgical Therapy for the Management of Peri-Implantitis. Clin. Adv. Periodontics 2022, 12, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; Isidor, F.; von Steyern, P.V.; Stavropoulos, A. Does implantoplasty affect the failure strength of narrow and regular diameter implants? A laboratory study. Clin. Oral Investig. 2021, 25, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- Leitão-Almeida, B.; Camps-Font, O.; Correia, A.; Mir-Mari, J.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of bone loss on the fracture resistance of narrow dental implants after implantoplasty. An in vitro study. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e611–e618. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Xie, L.; Siddiqui, D.A.; Daubert, D.; Graham, D.J.; Gil, F.J. Dynamic assessment of titanium surface oxides following mechanical damage reveals only partial passivation under inflammatory conditions. NPJ Mater. Degrad. 2024, 8, 98. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A. Aplicaciones biomédicas del titanio v sus aleaciones. Biomecánica 1993, 1, 34–43. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.; Proubasta, I.; Vazquez, J. Fundamentos de biomecánica y biomateriales. In Fundamentos de Biomecánica y Biomateriales; Ergon: Barcelona, Spain, 1997; pp. 125–132. [Google Scholar]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.A.; Gay-Escoda, C.; Valmaseda-Castellon, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosión behavior of Ti6Al4V particles obtained by Implatoplasty: An in vivo study. Part. II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials 2021, 14, 6507. [Google Scholar] [CrossRef]
- Dasgupta, D.; Banerjee, S.; Parasrampuria, N.; Pal, D. Efficacy of implantoplasty in management of peri-implantitis: A systematic review. J. Indian Prosthodont. Soc. 2023, 23, 210–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martins, O.; Sahrmann, P.; Ramos, J.; Caramelo, F.; Matos, S.; Baptista, I.P. Implantoplasty Improves Clinical Parameters over a 2-Year Follow-Up: A Case Series. Medicina 2022, 58, 113. [Google Scholar] [CrossRef]
- Bianchini, M.A.; Galarraga-Vinueza, M.E.; Bedoya, K.A.; Correa, B.B.; de Souza Magini, R.; Schwarz, F. Implantoplasty Enhancing Peri-implant Bone Stability Over a 3-Year Follow-up: A Case Series. Int. J. Periodontics Restor. Dent. 2020, 40, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.A.; Marcela de Luna-Gomes, J.; Daltro Rosa, J.; de Oliveira-Limirio, P.; Dos Santos, D.; Coelho-Goiato, M.; Piza-Pellizzer, E. Steel drills versus zirconia drills on heat generation at the surgical site of dental implants: A systematic review and meta-analysis. Saudi Dent. J. 2024, 36, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsiao, C.K.; Ciou, J.S.; Tsai, Y.J.; Tu, Y.K. Effects of implant drilling parameters for pilot and twist drills on temperature rise in bone analog and alveolar bones. Med. Eng. Phys. 2016, 38, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Hannusch, S.; Stockmann, M.; Ihlemann, J. Experimental Method for Residual Stress Analysis with Fibre Bragg Grating Sensors. Mater. Today Proc. 2016, 3, 979–982. [Google Scholar] [CrossRef]
- ASTM E837-08; Standard Test Method for Determining Residual Stresses by the Hole-Drilling Strain-Gage Method. ASTM International: West Conshohocken, PA, USA, 2008.
- Tabatabaeian, A.; Ghasemi, A.R.; Shokrieh, M.M.; Marzbanrad, B.; Baraheni, M.; Fotouhi, M. Residual Stress in Engineering Materials: A Review. Adv. Eng. Mater. 2022, 24, 2100786. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Urban, R.M.; Jacobs, J.J.; Gilbert, J.L. In vivo severe corrosion and hydrogen embrittlement of retrieved modular body titanium alloy hip-implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 206–219. [Google Scholar] [CrossRef]
- Fonseca, D.; de Tapia, B.; Pons, R.; Aparicio, C.; Guerra, F.; Messias, A.; Gil, J. The Effect of Implantoplasty on the Fatigue Behavior and Corrosion Resistance in Titanium Dental Implants. Materials 2024, 17, 2944. [Google Scholar] [CrossRef]
- Tkacz, J.; Slouková, K.; Minda, J.; Drábiková, J.; Fintová, S.; Doležal, P.; Wasserbauer, J. Influence of the Composition of the Hank’s Balanced Salt Solution on the Corrosion Behavior of AZ31 and AZ61 Magnesium Alloys. Metals 2017, 7, 465. [Google Scholar] [CrossRef]
- ASTM G31-90; Standard Practice for Laboratory Immersion Corrosion Testing Metals. 1992 Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1992.
- ASTM G5; Potentiodynamic Anodic Polarization Measurements. 1992 Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1992.
- Padulles-Gaspar, E.; Padulles-Roig, E.; Cabanes, G.; Pérez, R.A.; Gil, J.; Bosch, B.M. Effects of Hypochlorous Acid and Hydrogen Peroxide Treatment on Bacterial Disinfection Treatments in Implantoplasty Procedures. Materials 2023, 16, 2953. [Google Scholar] [CrossRef]
- Subramani, K.; Wismeijer, D. Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: A literature review. Int. J. Oral Maxillofac. Implants 2012, 27, 1043–1054. [Google Scholar]
- Mas-Moruno, C.; Garrido, B.; Rodriguez, D.; Ruperez, E.; Gil, F.J. Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J. Mater. Sci. Mater. Med. 2015, 26, 109. [Google Scholar] [CrossRef] [PubMed]
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. 1999 Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1992.
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron–Nickel, or Cobalt-Based Alloys. 1992 Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1992.
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. Corrosion behaviour of commercially pure titanium shot blasted with different materials and sizes of shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 2003, 24, 263–273. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993—5 (55); Biological Evaluation of Medical Devices—Part 5, Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Afzali, P.; Ghomashchi, R.; Oskouei, R.H. On the Corrosion Behaviour of Low Modulus Titanium Alloys for Medical Implant Applications: A Review. Metals 2019, 9, 878. [Google Scholar] [CrossRef]
- Guerra-Yánez, H.; Rubén Florido-Suárez, N.; Voiculescu, I.; Mirza-Rosca, J.C. Corrosion behavior of new titanium alloys for medical applications. Mater. Today Proc. 2023, 72, 533–537. [Google Scholar] [CrossRef]
- Ramel, C.F.; Lussi, A.; Ozcan, M.; Jung, R.E.; Hammerle, C.H.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implants Res. 2016, 27, 776–781. [Google Scholar] [CrossRef]
- Costa-Berenguer, X.; Garcia-Garcia, M.; Sanchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellon, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implants Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tu, Y.K.; Tsai, Y.J.; Tsai, Y.S.; Yen, C.Y.; Yang, S.C.; Hsiao, C.K. Assessment of thermal necrosis risk regions for different bone qualities as a function of drilling parameters. Comput. Methods Programs Biomed. 2018, 162, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Tu, Y.K.; Zhuang, J.Y.; Tsai, Y.J.; Yen, C.Y.; Hsiao, C.K. Evaluation of the parameters affecting bone temperature during drilling using a three-dimensional dynamic elastoplastic finite element model. Med. Biol. Eng. Comput. 2017, 55, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Maia, P.; Rios-Santos, J.V.; Herrero-Climent, M.; Rios-Carrasco, B.; Aparicio, C.; Gil, J. Influence of Titanium Surface Residual Stresses on Osteoblastic Response and Bacteria Colonization. Materials 2024, 17, 1626. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Aparicio, C.; Manero, J.M.; Conde, F.; Pegueroles, M.; Planell, J.A.; Vallet-Regí, M.; Gil, F.J. Acceleration of apatite nucleation on microrough bioactive titanium for bone-replacing implants. J. Biomed. Mater. Res. 2007, 82, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; AramburúJúnior, J.S.; Dedavid, B.A.; Shibli, J.A. Analysis of implant strength after implantoplasty in three implant-abutment connection designs: An in vitro study. Int. J. Oral Maxillofac. Implants 2016, 31. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Gil-Mur, F.J.; Herrero-Climent, M.; Chávarri-Prado, D.; Chento-Valiente, Y.; Dieguez-Pereira, M. Relationship Between Insertion Torque and Resonance Frequency Measurements, Performed by Resonance Frequency Analysis, in Micromobility of Dental Implants: An In Vitro Study. Implant Dent. 2015, 24, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, A.H.; Bhlol, M.; Dawood, O.M.; Ghayad, I.M.; Elkady, O.A. Effect of tungsten carbide (WC) on electrochemical corrosion behavior, hardness, and microstructure of CrFeCoNi high entropy alloy. J. Eng. Appl. Sci. 2022, 69, 43. [Google Scholar] [CrossRef]
- Racz, A.S.; Kerner, Z.; Menyhard, M. Corrosion resistance of tungsten carbide-rich coating layers produced by noble gas ion mixing. Appl. Surf. Sci. 2022, 605, 154662. [Google Scholar] [CrossRef]
- El Sawy, A.A.; Shaarawy, M.A. Evaluation of metal ion release from Ti6Al4V and Co-Cr-Mo casting alloys: In vivo and in vitro study. J. Prosthodont. 2014, 23, 89–97. [Google Scholar] [CrossRef]
- Yao, J.; Zhou, P.; Zhang, X.; Yuan, B.; Pan, Y.; Jiang, J. The Cytotoxicity of Tungsten Ions Derived from Nanoparticles Correlates with Pulmonary Toxicity. Toxics 2023, 11, 528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bastian, S.; Busch, W.; Kühnel, D.; Springer, A.; Meissner, T.; Holke, R.; Scholz, S.; Iwe, M.; Pompe, W.; Gelinsky, M.; et al. Toxicity of tungsten carbide and cobalt-doped tungsten carbide nanoparticles in mammalian cells in vitro. Environ. Health Perspect. 2009, 117, 530–536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrak, F.N.; Li, S.; Muntane, A.M.; Jones, J.R. Particle release from implantoplasty of dental implants and impact on cells. Int. J. Implant Dent. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Messous, R.; Henriques, B.; Bousbaa, H.; Silva, F.S.; Teughels, W.; Souza, J.C.M. Cytotoxic effects of submicron- and nano-scale titanium debris released from dental implants: An integrative review. Clin. Oral Investig. 2021, 25, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.G.; Nalli, G.; Verdú, S.; Paparella, M.L.; Cabrini, R.L. Exfoliative Cytology and Titanium Dental Implants: A Pilot Study. J. Periodontol. 2013, 84, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, J.; Dai, K.; Huang, Y. Metallic wear debris collected from patients induces apoptosis in rat primary osteoblasts via reactive oxygen species-mediated mitochondrial dysfunction and endoplasmic reticulum stress. Mol. Med. Rep. 2019, 19, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Callejas, J.A.; Gil, J.; Brizuela, A.; Pérez, R.A.; Bosch, B.M. Effect of the Size of Titanium Particles released from Dental Implants on Immunological Response. Int. J. Mol. Sci. 2022, 23, 7333. [Google Scholar] [CrossRef]
- Wu, X.; Cai, C.; Gil, J.; Jantz, E.; Al Sakka, Y.; Padial-Molina, M.; Suárez-López Del Amo, F. Characteristics of Particles and Debris Released after Implantoplasty: A Comparative Study. Materials 2022, 15, 602. [Google Scholar] [CrossRef]
- Malik, S.; Waheed, Y. Emerging Applications of Nanotechnology in Dentistry. Dent. J. 2023, 11, 266. [Google Scholar] [CrossRef]
- Bourgi, R.; Doumandji, Z.; Cuevas-Suárez, C.E.; Ben Ammar, T.; Laporte, C.; Kharouf, N.; Haikel, Y. Exploring the Role of Nanoparticles in Dental Materials: A Comprehensive Review. Coatings 2025, 15, 33. [Google Scholar] [CrossRef]


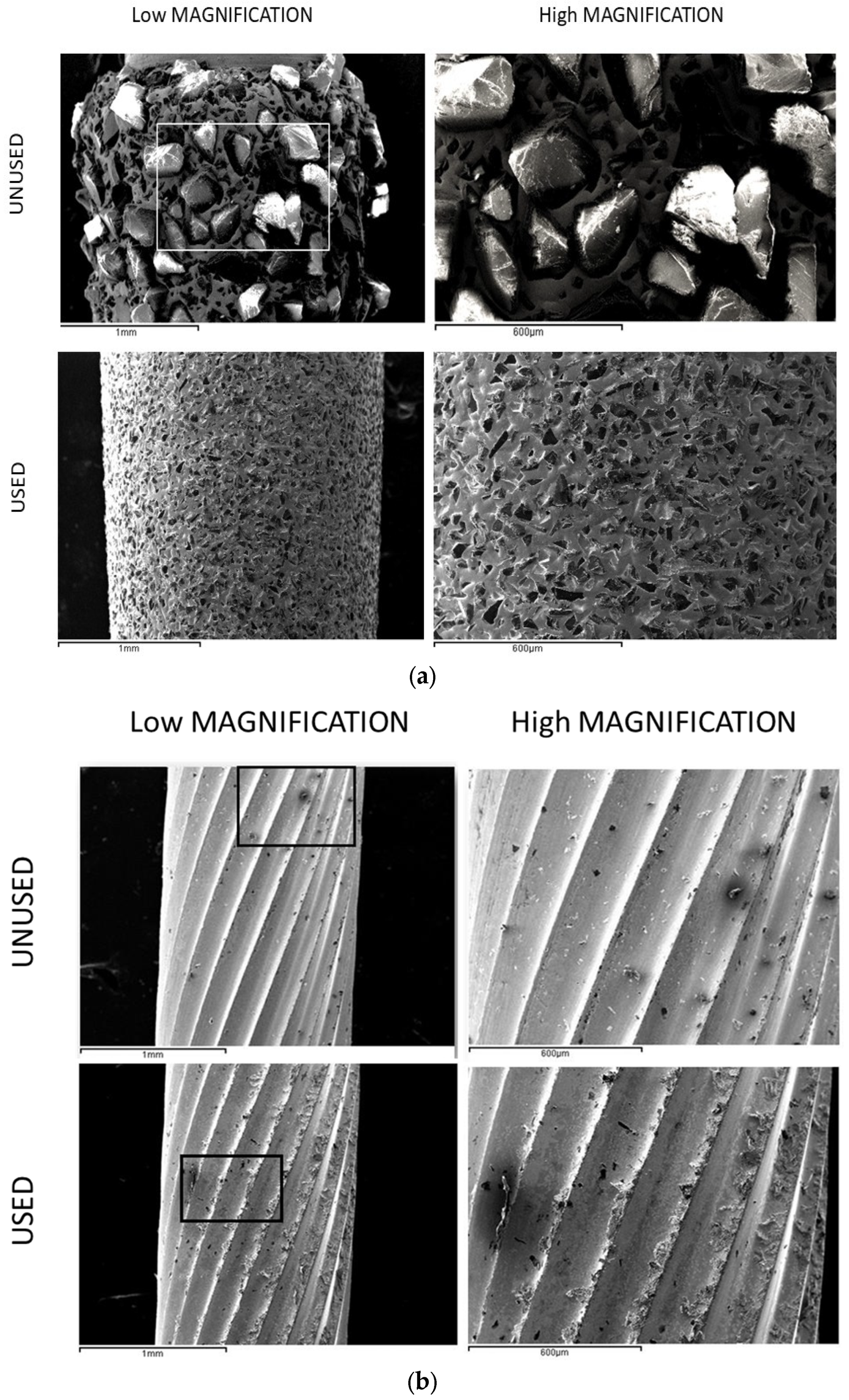
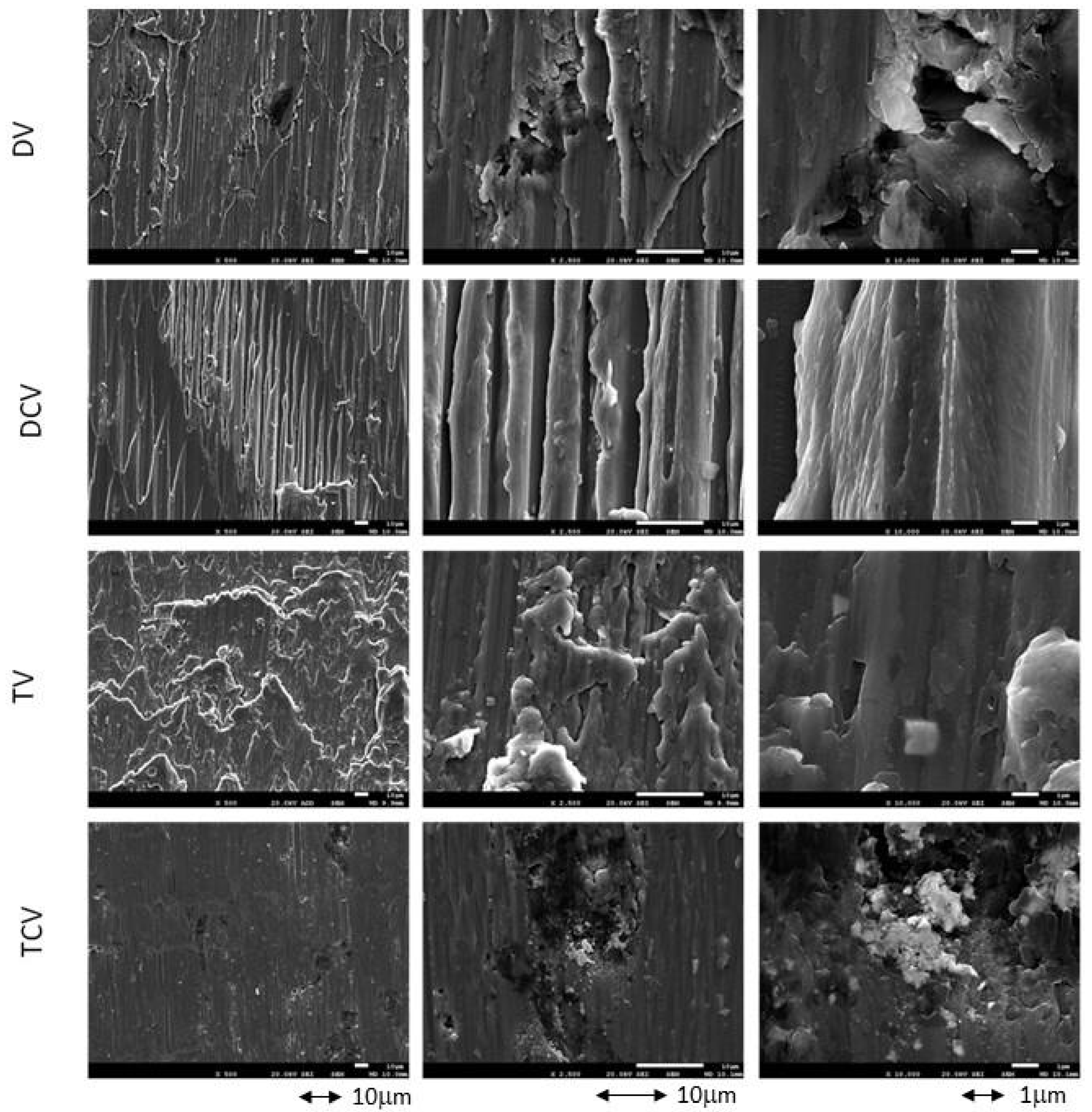

| Component | K2HPO4 | KCl | CaCl2 | Na2HPO4 | NaCl | NaHCO3 | MgSO4 | C6H12O6 |
| Composition | 0.44 | 5.4 | 1.3 | 0.25 | 137 | 4.2 | 1.0 | 5.5 |
| Scheme | Ra (μm) | Contact Angle (°) | Hardness (GPa) | Residual Stress (MPa) |
|---|---|---|---|---|
| Control | 0.22 ± 0.09 | 80 ± 7 | 2.2 ±1.2 | −26 ± 5 |
| DV | 0.73 ± 0.10 * | 54 ± 5 * | 4.8 ±1.0 * | −125 ± 12 * |
| DCV | 0.72 ± 0.08 * | 56 ± 5 * | 4.7 ±0.9 * | −124 ± 20 * |
| TV | 0.59 ± 0.09 ** | 62 ± 9 ** | 3.9 ±0.9 * | −111 ± 15 * |
| TCV | 0.57 ± 0.12 ** | 67 ± 8 ** | 3.9 ± 1.2 * | −112 ± 10 * |
| Sample | EOCP (V) | jcorr (µA/cm2) | Icorr (µA) | Ecorr (mV) | Rp (µΩ) | CR (mm/year) |
|---|---|---|---|---|---|---|
| Control | −0.0204 ± 0.0010 | 0.067 ± 0.018 | 0.051 ± 0.007 | −361 ± 14 | 1.142 × 1012 ± 1.135 × 1011 | 0.0044 ± 0.0009 |
| DV | −0.0798 ± 0.0010 * | 3.645 ± 0.654 * | 2.843 ± 0.453 * | −511 ± 30 * | 1.445 × 1011 ± 0.235 × 1010 * | 0.0323 ± 0.0032 * |
| DCV | −0.0638 ± 0.0011 * | 2.261 ± 0.123 ** | 1.764 ± 0.324 ** | −512 ± 22 * | 1.797 × 1011 ± 0.305 × 1010 * | 0.0263 ± 0.0024 * |
| TV | −0.2356 ± 0.0052 ** | 4.435 ± 0.013 * | 3.459 ± 0.202 * | −483 ± 83 * | 1.412 × 1011 ± 0.245 × 1010 * | 0.0518 ± 0.0046 ** |
| TCV | −0.3749 ± 0.0064 ** | 1.791 ± 0.328 ** | 1.392 ± 0.433 ** | −603 ± 134 * | 1.193 × 1011 ± 0.396 × 1010 * | 0.0515 ± 0.0085 ** |
| Property | Diamond Drill | Tungsten Carbide Drill |
|---|---|---|
| Roughness | ⇑⇑ | ⇑ |
| Nanohardness | ⇑⇑ | ⇑ |
| Residual Stress | ⇑⇑ | ⇑ |
| Wettability | ⇑⇑ | ⇑ |
| Corrosion | ⇑⇑ | ⇑ |
| Ion Release | ⇑ | ⇑⇑ (W) |
| Citotoxicity | ⇑ | ⇑⇑ |
| Where ⇑ = High; ⇑⇑ = Higher; W = Tungsten release | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padullés-Gaspar, E.; Real-Voltas, F.; Padullés-Roig, E.; Punset, M.; Cabanes, G.; Fernández, P.; Gil, J. The Influence of the Machining Drill and Direction of Rotation on the Surfaces of Ti6Al4V Dental Implants Subjected to Implantoplasty. J. Funct. Biomater. 2025, 16, 224. https://doi.org/10.3390/jfb16060224
Padullés-Gaspar E, Real-Voltas F, Padullés-Roig E, Punset M, Cabanes G, Fernández P, Gil J. The Influence of the Machining Drill and Direction of Rotation on the Surfaces of Ti6Al4V Dental Implants Subjected to Implantoplasty. Journal of Functional Biomaterials. 2025; 16(6):224. https://doi.org/10.3390/jfb16060224
Chicago/Turabian StylePadullés-Gaspar, Esteban, Francisco Real-Voltas, Esteban Padullés-Roig, Miguel Punset, Guillermo Cabanes, Pablo Fernández, and Javier Gil. 2025. "The Influence of the Machining Drill and Direction of Rotation on the Surfaces of Ti6Al4V Dental Implants Subjected to Implantoplasty" Journal of Functional Biomaterials 16, no. 6: 224. https://doi.org/10.3390/jfb16060224
APA StylePadullés-Gaspar, E., Real-Voltas, F., Padullés-Roig, E., Punset, M., Cabanes, G., Fernández, P., & Gil, J. (2025). The Influence of the Machining Drill and Direction of Rotation on the Surfaces of Ti6Al4V Dental Implants Subjected to Implantoplasty. Journal of Functional Biomaterials, 16(6), 224. https://doi.org/10.3390/jfb16060224








