Evaluation of Two Alloplastic Biomaterials in a Critical-Size Rat Calvarial Defect Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Biomaterials
2.3. Study Design and Sample
2.4. Surgical Procedures
2.5. Postsurgery Procedures
2.6. Radiographic Evaluation
2.6.1. Mineralized Tissue Volume/Total Volume and Biomaterial Volume/Total Volume
2.6.2. Biomaterial Displacement
2.7. Histological Preparation
2.8. Histomorphometric Analysis
- Defect width: distance between the defect margins;
- Defect closure: fraction (%) of accumulated length of new bone formation between the defect margins;
- Defect area: area of regeneration including new bone formation, residual biomaterial and other tissue limited by the defect margins;
- Defect fill: total area of newly formed bone between the defect margins;
- Bone area fraction: fraction (%) of newly formed bone within the defect area;
- Residual biomaterial: total area of residual biomaterial between the defect margins;
- Biomaterial area fraction: fraction (%) of residual biomaterial within the defect area.
2.9. Statistical Analysis
3. Results
3.1. Surgical Outcomes
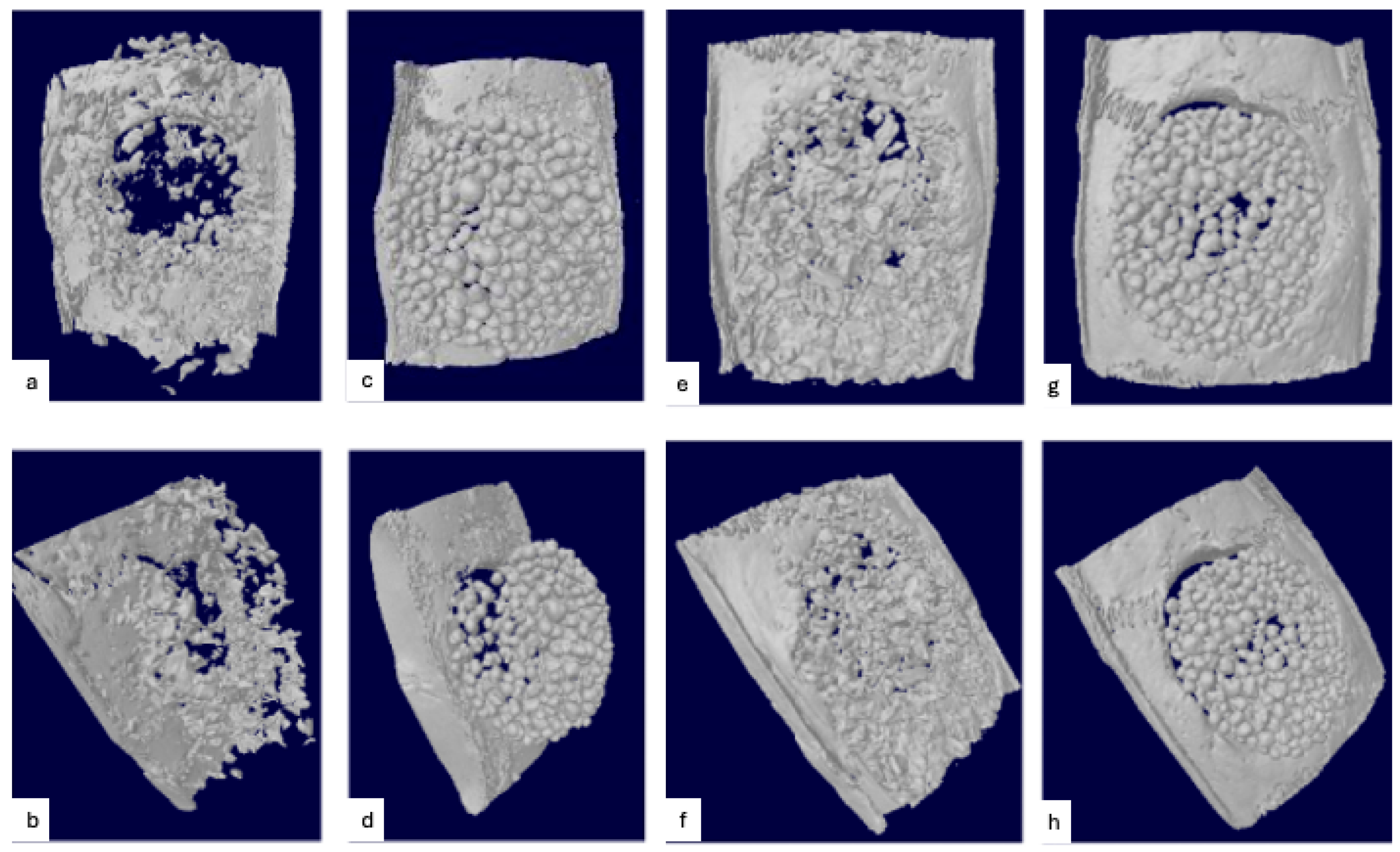
3.2. Radiographic Evaluation
Biomaterial Displacement
3.3. Histopathologic Observations
3.3.1. Sham Surgery
3.3.2. DBBM + CM
3.3.3. PLGA + β-TCP and PLGA + HA/β-TCP
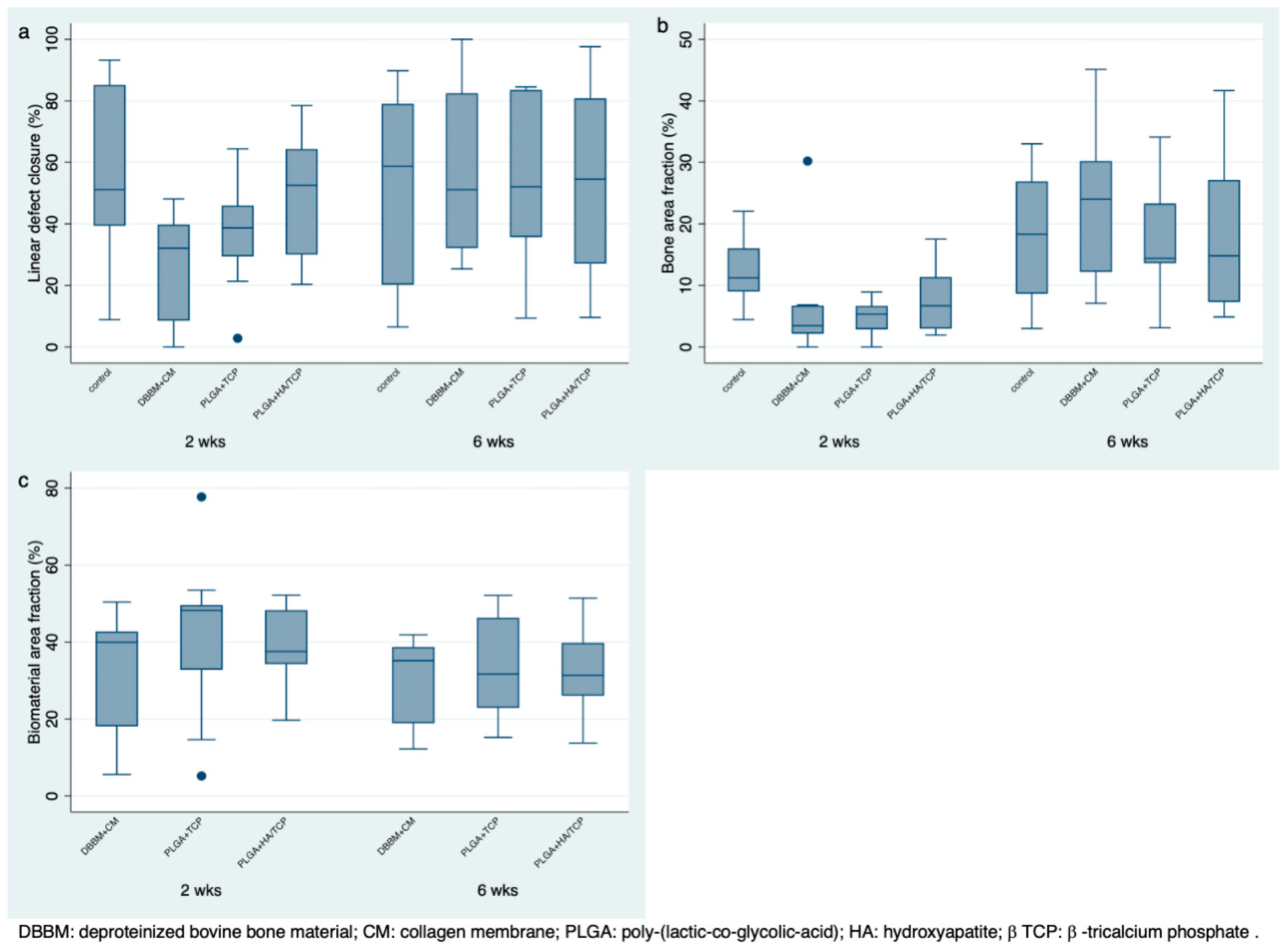
3.4. Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM | collagen membrane |
| PLGA | poly-(lactic-co-glycolic-acid) |
| DBBM | deproteinized bovine bone mineral |
| HA | hydroxyapatite |
| β-TCP | β-tricalcium phosphate |
| μCT | microcomputed tomography |
| MTV | mineralized tissue volume |
| BIO | biomaterial volume |
| ICC | intraclass correlation |
Appendix A
| Biomaterial | Composition | Particle Size (mm) | Source |
|---|---|---|---|
| Sham surgery | - | - | - |
| Bio-Oss | Bovine hydroxyapatite | 0.25–1.00 | Geistlich, Wolhusen, Switzerland |
| Easy-Graft Classic | Pure beta-tricalcium phosphate with poly(lactide-co-glycolide) + BioLinker (N-Methyl-2-pyrrolidone-solution) | 0.5–1.00 | Degradable Solutions AG, Schlieren, Switzerland |
| Easy-Graft Crystal | Biphasic calcium phosphate (60% hydroxyapatite, 40% beta-tricalcium phosphate) with poly(lactide-co-glycolide) + BioLinker (N-Methyl-2-pyrrolidone-solution) | 0.45–1.00 | Degradable Solutions AG, Schlieren, Switzerland |
Appendix B
References
- Araujo, M.G.; Lindhe, J. Dimensional Ridge Alterations Following Tooth Extraction. An Experimental Study in the Dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Elangovan, S.; Karimbux, N. Bone Graft Substitutes for Periodontal Use Available in the United States. Clin. Adv. Periodontol. 2013, 3, 187–190. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of Bone Regeneration Using the Rat Critical Size Calvarial Defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Herberg, S.; Susin, C.; Pelaez, M.; Howie, R.N.; Moreno de Freitas, R.; Lee, J.; Cray, J.J.; Johnson, M.H.; Elsalanty, M.E.; Hamrick, M.W.; et al. Low-Dose Bone Morphogenetic Protein-2/Stromal Cell-Derived Factor-1β Cotherapy Induces Bone Regeneration in Critical-Size Rat Calvarial Defects. Tissue Eng. Part A 2014, 20, 1444–1453. [Google Scholar] [CrossRef]
- Hyun, S.-J.; Han, D.-K.; Choi, S.-H.; Chai, J.-K.; Cho, K.-S.; Kim, C.-K.; Kim, C.-S. Effect of Recombinant Human Bone Morphogenetic Protein-2, -4, and -7 on Bone Formation in Rat Calvarial Defects. J. Periodontol. 2005, 76, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Poehling, S.; Pippig, S.D.; Hellerbrand, K.; Siedler, M.; Schütz, A.; Dony, C. Superior Effect of MD05, Beta-Tricalcium Phosphate Coated with Recombinant Human Growth/Differentiation Factor-5, Compared to Conventional Bone Substitutes in the Rat Calvarial Defect Model. J. Periodontol. 2006, 77, 1582–1590. [Google Scholar] [CrossRef]
- Susin, C.; Lee, J.; Fiorini, T.; Koo, K.-T.; Schüpbach, P.; Angst, P.D.M.; Finger Stadler, A.; Wikesjö, U.M. Screening of Candidate Biomaterials for Alveolar Augmentation Using a Critical-Size Rat Calvaria Defect Model. J. Clin. Periodontol. 2018, 45, 884–893. [Google Scholar] [CrossRef]
- Bizenjima, T.; Takeuchi, T.; Seshima, F.; Saito, A. Effect of Poly (Lactide-Co-Glycolide) (PLGA)-Coated Beta-Tricalcium Phosphate on the Healing of Rat Calvarial Bone Defects: A Comparative Study with Pure-Phase Beta-Tricalcium Phosphate. Clin. Oral Impl. Res. 2016, 27, 1360–1367. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Nicholls, F.; Kruse, A.; Zwahlen, R.A.; Weber, F.E. Evaluation of Moldable, in Situ Hardening Calcium Phosphate Bone Graft Substitutes. Clin. Oral Impl. Res. 2013, 24, 149–157. [Google Scholar] [CrossRef]
- Valdivia-Gandur, I.; Engelke, W.; Beltrán, V.; Borie, E.; Fuentes, R.; Manzanares-Céspedes, M.C. Novel Use of Cranial Epidural Space in Rabbits as an Animal Model to Investigate Bone Volume Augmentation Potential of Different Bone Graft Substitutes. Head. Face Med. 2016, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Kakar, A.; Kakar, K.; Sripathi Rao, B.H.; Lindner, A.; Nagursky, H.; Jain, G.; Patney, A. Lateral Alveolar Ridge Augmentation Procedure Using Subperiosteal Tunneling Technique: A Pilot Study. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 3. [Google Scholar] [CrossRef]
- Leventis, M.D.; Fairbairn, P.; Kakar, A.; Leventis, A.D.; Margaritis, V.; Lückerath, W.; Horowitz, R.A.; Rao, B.H.; Lindner, A.; Nagursky, H. Minimally Invasive Alveolar Ridge Preservation Utilizing an In Situ Hardening β-Tricalcium Phosphate Bone Substitute: A Multicenter Case Series. Int. J. Dent. 2016, 2016, 5406736. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef]
- Pelaez, M.; Susin, C.; Lee, J.; Fiorini, T.; Bisch, F.C.; Dixon, D.R.; McPherson, J.C.; Buxton, A.N.; Wikesjö, U.M.E. Effect of rhBMP-2 Dose on Bone Formation/Maturation in a Rat Critical-Size Calvarial Defect Model. J. Clin. Periodontol. 2014, 41, 827–836. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro-Computed Tomography. J. Bone Min. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural Graft Tissues and Synthetic Biomaterials for Periodontal and Alveolar Bone Reconstructive Applications: A Review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium Phosphate Cements: Brushite and Monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zuo, Y.; Li, J.; Ma, S.; Cheng, L. Biocompatibility and Osteogenesis of Biomimetic Nano-Hydroxyapatite/Polyamide Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2007, 28, 3338–3348. [Google Scholar] [CrossRef]
- Bagambisa, F.B.; Joos, U.; Schilli, W. Mechanisms and Structure of the Bond between Bone and Hydroxyapatite Ceramics. J. Biomed. Mater. Res. 1993, 27, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Wildburger, A.; Bubalo, V.; Magyar, M.; Nagursky, H.; Jakse, N.; Schmelzeisen, R.; Sauerbier, S. Sinus Floor Augmentation Comparing an In Situ Hardening Biphasic Calcium Phosphate (Hydroxyapatite/β-Tricalcium Phosphate) Bone Graft Substitute with a Particulate Biphasic Calcium Phosphate (Hydroxyapatite/β-Tricalcium Phosphate) Bone Graft Substitute: An Experimental Study in Sheep. Tissue Eng. Part C Methods 2017, 23, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Salem, D.; Natto, Z.; Elangovan, S.; Karimbux, N. Usage of Bone Replacement Grafts in Periodontics and Oral Implantology and Their Current Levels of Clinical Evidence—A Systematic Assessment. J. Periodontol. 2016, 87, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Caballe-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Penarrocha, M.; Hernandez-Alfaro, F. On the Search of the Ideal Barrier Membrane for Guided Bone Regeneration. J. Clin. Exp. Dent. 2018, 10, e477–e483. [Google Scholar] [CrossRef]
- Susin, C.; Fiorini, T.; Lee, J.; De Stefano, J.A.; Dickinson, D.P.; Wikesjö, U.M.E. Wound Healing Following Surgical and Regenerative Periodontal Therapy. Periodontol 2000 2015, 68, 83–98. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The Critical Size Defect as an Experimental Model for Craniomandibulofacial Nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Yip, I.; Ma, L.; Mattheos, N.; Dard, M.; Lang, N.P. Defect Healing with Various Bone Substitutes. Clin. Oral Impl. Res. 2015, 26, 606–614. [Google Scholar] [CrossRef]
- Naenni, N.; Sapata, V.; Bienz, S.P.; Leventis, M.; Jung, R.E.; Hämmerle, C.H.F.; Thoma, D.S. Effect of Flapless Ridge Preservation with Two Different Alloplastic Materials in Sockets with Buccal Dehiscence Defects—Volumetric and Linear Changes. Clin. Oral Investig. 2018, 22, 2187–2197. [Google Scholar] [CrossRef]
- Saito, H.; Couso-Queiruga, E.; Shiau, H.J.; Stuhr, S.; Prasad, H.; Allareddy, T.V.; Reynolds, M.A.; Avila-Ortiz, G. Evaluation of Poly Lactic-co-glycolic Acid-coated Β-tricalcium Phosphate for Alveolar Ridge Preservation: A Multicenter Randomized Controlled Trial. J. Periodontol. 2021, 92, 524–535. [Google Scholar] [CrossRef]



| Healing | Mean | Percentiles | Range | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Period | Group | n | Mean | SD | Median | 25 | 75 | Min | Max |
| 2 weeks | Sham | 10 | 56.08 A | 27.17 | 51.14 | 39.39 | 85.15 | 8.87 | 93.23 |
| DBBM + CM | 10 | 26.30 B | 16.73 | 32.09 | 8.53 | 39.80 | 0 | 48.1 | |
| PLGA + β-TCP | 9 | 37.48 AB | 18.54 | 38.70 | 29.43 | 45.98 | 2.84 | 64.39 | |
| PLGA + HA/β-TCP | 10 | 49.56 AB | 19.45 | 52.56 | 30.04 | 64.31 | 20.33 | 78.47 | |
| 6 weeks | Sham | 10 | 53.39 A | 30.94 | 58.67 | 20.20 | 79.07 | 6.52 | 89.79 |
| DBBM + CM | 10 | 57.50 A | 28.57 | 51.11 | 32.10 | 82.47 | 25.38 | 100 | |
| PLGA + β-TCP | 10 | 52.77 A | 26.91 | 52.04 | 35.70 | 83.54 | 9.42 | 84.52 | |
| PLGA + HA/β-TCP | 10 | 54.34 A | 32.02 | 54.51 | 27.05 | 80.82 | 9.61 | 97.61 | |
| Healing | Mean | Percentiles | Range | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Period | Group | n | Mean | SD | Median | 25 | 75 | Min | Max |
| 2 weeks | Sham | 10 | 12.17 A | 5.08 | 11.23 | 8.99 | 16.03 | 4.45 | 22.04 |
| DBBM + CM | 10 | 6.32 B | 8.72 | 3.46 | 2.16 | 6.74 | 0 | 30.21 | |
| PLGA + β-TCP | 9 | 4.71 B | 2.96 | 5.36 | 2.88 | 6.69 | 0 | 8.91 | |
| PLGA + HA/β-TCP | 10 | 7.64 AB | 5.60 | 6.69 | 2.97 | 11.37 | 1.93 | 17.51 | |
| 6 weeks | Sham | 10 | 17.84 A | 10.30 | 18.31 | 8.64 | 26.90 | 3.01 | 33.01 |
| DBBM + CM | 10 | 24.29 A | 12.94 | 24.01 | 12.18 | 30.21 | 7.08 | 45.11 | |
| PLGA + β-TCP | 10 | 17.84 A | 9.39 | 14.38 | 13.59 | 23.32 | 3.1 | 34.12 | |
| PLGA + HA/β-TCP | 10 | 18.11 A | 12.78 | 14.80 | 7.30 | 27.15 | 4.88 | 41.66 | |
| Healing | Mean | Percentiles | Range | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Period | Group | n | Mean | SD | Median | 25 | 75 | Min | Max |
| 2 weeks | Sham | 10 | - | - | - | - | - | - | - |
| DBBM + CM | 10 | 32.96 A | 14.45 | 39.96 | 18.08 | 42.71 | 5.56 | 50.41 | |
| PLGA + β-TCP | 9 | 40.82 A | 21.63 | 48.24 | 32.82 | 49.64 | 5.17 | 77.69 | |
| PLGA + HA/β-TCP | 10 | 38.47 A | 11.19 | 37.51 | 34.23 | 48.28 | 19.65 | 52.16 | |
| 6 weeks | Sham | 10 | - | - | - | - | - | - | - |
| DBBM + CM | 10 | 31.04 A | 10.88 | 35.15 | 18.89 | 38.67 | 12.18 | 41.86 | |
| PLGA + β-TCP | 10 | 34.05 A | 13.15 | 31.71 | 22.88 | 46.32 | 15.20 | 52.10 | |
| PLGA + HA/β-TCP | 10 | 32.71 A | 10.97 | 31.33 | 26.05 | 39.75 | 13.75 | 51.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finger Stadler, A.; Musskopf, M.L.; Gohel, V.; Reside, J.; Everett, E.; Miguez, P.; Susin, C. Evaluation of Two Alloplastic Biomaterials in a Critical-Size Rat Calvarial Defect Model. J. Funct. Biomater. 2025, 16, 214. https://doi.org/10.3390/jfb16060214
Finger Stadler A, Musskopf ML, Gohel V, Reside J, Everett E, Miguez P, Susin C. Evaluation of Two Alloplastic Biomaterials in a Critical-Size Rat Calvarial Defect Model. Journal of Functional Biomaterials. 2025; 16(6):214. https://doi.org/10.3390/jfb16060214
Chicago/Turabian StyleFinger Stadler, Amanda, Marta Liliana Musskopf, Vishal Gohel, Jonathan Reside, Eric Everett, Patricia Miguez, and Cristiano Susin. 2025. "Evaluation of Two Alloplastic Biomaterials in a Critical-Size Rat Calvarial Defect Model" Journal of Functional Biomaterials 16, no. 6: 214. https://doi.org/10.3390/jfb16060214
APA StyleFinger Stadler, A., Musskopf, M. L., Gohel, V., Reside, J., Everett, E., Miguez, P., & Susin, C. (2025). Evaluation of Two Alloplastic Biomaterials in a Critical-Size Rat Calvarial Defect Model. Journal of Functional Biomaterials, 16(6), 214. https://doi.org/10.3390/jfb16060214





