Abstract
Osteosarcoma (OS), a prevalent primary malignant bone tumor in children and adolescents, has maintained consistent treatment protocols since the 1970s combining surgery, chemotherapy, and radiotherapy. While effective for localized tumors, these strategies show limited efficacy against metastatic or recurrent cases. Although emerging immunotherapies (PD-1 inhibitors, CAR-T-cell therapy) demonstrate therapeutic potential, their clinical impact remains constrained by the tumor’s low immunogenicity and immunosuppressive microenvironment, resulting in suboptimal response rates. The disease’s aggressive nature and propensity for pulmonary metastasis contribute to poor prognosis, with survival rates showing negligible improvement over five decades despite therapeutic advances, creating substantial clinical and socioeconomic challenges. Recent developments in nanomedicine offer promising solutions for OS treatment optimization. This review systematically examines nanomaterial applications in OS therapy through a materials science lens, analyzing mechanism-specific interventions and highlighting notable advancements from the past five years. We critically evaluate current strategies for enhancing therapeutic efficacy while reducing toxicity profiles, ultimately outlining translational pathways and key challenges in clinical adaptation. The analysis establishes a framework for developing next-generation nanotherapeutic platforms to address persistent limitations in OS management.
1. Introduction
Osteosarcoma (OS) is a primary malignant bone tumor arising from malignantly transformed mesenchymal stromal cells. The annual incidence of OS in the general population ranges from two to three cases per million individuals, with a distinct peak observed during adolescence [1]. Notably, the 15–19 age group demonstrates the highest disease burden, exhibiting an annual incidence rate of 8–11 cases per million population and it is the third most common cancer affecting children and adolescents, following lymphoma and brain tumors [2]. OS arises from genetic predispositions (TP53/RB1/BLM mutations), Paget’s disease-related bone remodeling, and radiation-induced DNA damage, with SV40 viral co-detection requiring further validation [3]. Pathologically, it manifests as metaphyseal lesions with aggressive invasion, malignant osteoid production, and characteristic molecular aberrations (chromosomes 6/8 anomalies). Treatment combines margin-negative surgery with platinum-based chemotherapy, supplemented by emerging immunotherapies (checkpoint inhibitors/CAR-T-cell therapy) and targeted agents against angiogenesis and mammalian target of rapamycin (mTOR) pathways [4]. Since the introduction of chemotherapy for OS treatment in the 1970s, the 5-year survival rate of OS patients has significantly improved, reaching 60–70% [5]. Currently, the standard clinical treatment approach involves preoperative neoadjuvant chemotherapy, surgical resection, and postoperative adjuvant radiotherapy [6]. However, these treatments are associated with numerous issues. For example, there is the problem of chemotherapy resistance, as well as systemic toxicities. Additionally, postoperative recurrence is a concern, along with poor prognosis and surgically induced bone defects. These problems severely impact patients’ quality of life. To make matters worse, approximately 15–20% of OS patients have metastases at the time of diagnosis, with the lungs being the most common site (85%) and bones accounting for 8–10% [2]. The presence of metastases is a clear indicator of a poor prognosis. Specifically, the 5-year survival rate for patients with worsening lung metastases is less than 20% [7]. Therefore, there is an urgent need for a treatment that is more effective and less toxic in order to improve OS outcomes and extend patients’ life expectancy.
With advancements in nanotechnology within the medical field, the emergence of nanomaterials (Figure 1) has introduced a new direction in the treatment of OS. Leveraging their unique small size, charge characteristics, and targeting capabilities, nanomaterials have found extensive applications in the treatment of various tumors, including both solid and hematologic malignancies [8]. Nanomaterials demonstrate several notable advantages in tumor therapy: (1) Nanomaterials leverage the enhanced permeability and retention (EPR) effect for passive tumor targeting, enabled by structural abnormalities in tumor vasculature. Tumor blood vessels exhibit enlarged endothelial gaps (100–1000 nm) compared to healthy tissues, permitting selective accumulation of optimally sized nanomaterials (10–200 nm) through vascular leakage. Prolonged retention arises from deficient lymphatic drainage in tumors. Key determinants include vascular permeability, interstitial pressure gradients, and nanomaterial characteristics: size extremes impair vascular extravasation or accelerate systemic clearance, while surface PEGylation enhances circulation time by evading reticuloendothelial uptake. Clinically exemplified by doxorubicin-loaded liposomes (Doxil), this strategy concentrates chemotherapeutics in malignancies like ovarian and breast cancers, optimizing therapeutic efficacy while minimizing systemic toxicity [9]. (2) Active targeting of nanomaterials integrates molecular recognition, environmental responsiveness, bioinspired engineering, and physical guidance to optimize therapeutic precision. Ligand-functionalized systems exploit tumor-specific biomarkers through antibody conjugation (e.g., HER2-targeting trastuzumab) or receptor-binding motifs (folic acid/RGD peptides) for selective accumulation. Smart nanomaterials achieve spatiotemporal control via pH-triggered drug release in acidic microenvironments or enzyme-activated payload deployment through MMP-responsive mechanisms. Biomimetic strategies employ tumor cell membrane coatings for homologous targeting or engineer immune cell-derived surfaces (e.g., T-cell membranes with PD-1 blockers) to modulate antitumor immunity. Magnetic guidance systems synergize with external fields to enhance deep-tissue penetration, exemplified by 10-fold drug concentration increases achieved in leukemia models through magnetically directed nanocarriers. These multimodal approaches collectively enhance tumor-specific delivery while circumventing systemic toxicity [10]. (3) Stimuli-responsive nanomaterials enable precise tumor therapy through microenvironmental or external activation. pH-sensitive carriers release drugs via acidic tumor-triggered structural changes, while redox-responsive systems exploit intracellular glutathione to cleave disulfide/selenium bonds. Enzyme-activated platforms utilize tumor-overexpressed proteases to degrade peptide linkers, exposing targeting ligands. Light-responsive designs combine photodynamic/photothermal effects with controlled drug release through light-induced reactive oxygen species (ROS) generation or nanostructural disruption. Temperature-sensitive materials achieve spatiotemporal control via thermal phase transitions in hydrogels or liposomes, synchronizing drug release with localized hyperthermia. These intelligent systems collectively enhance therapeutic specificity by coupling drug activation with pathological or physical triggers [11]. (4) Nanomaterials possess a large specific surface area, enabling them to carry a higher load of drug molecules and improve drug delivery. Additionally, they provide protection for encapsulated drugs, genes, and therapeutic agents, shielding them from degradation in complex physiological microenvironments and ensuring their stability and effectiveness [8]. (5) By adjusting their internal properties and applying surface modifications, nanomaterials can be loaded with functional molecules such as contrast agents, chemotherapeutic drugs, phototherapeutic agents, Fenton agents, fluorescent markers, and immunomodulators [10]. This enables the integration of diverse functions, including photothermal therapy, fluorescence imaging, and immunotherapy, facilitating multimodal synergistic treatments or combining diagnostics and therapy on a single platform. Nanomaterial-based targeting systems enhance OS treatment through controlled drug distribution and release yet face multidimensional challenges. Functionalized carriers like alendronate-conjugated liposomes significantly improve tumor accumulation (demonstrated by Morton’s orthogonal targeting system), reducing systemic exposure while enhancing local efficacy. Sustained-release platforms (e.g., silk fibroin composites) maintain therapeutic concentrations, and multifunctional theranostic systems integrate targeting, drug delivery, and imaging capabilities. However, clinical translation is hindered by manufacturing complexities, material instability, and biosafety concerns, including immunogenicity (silk fibroin) and cationic polymer cytotoxicity. Targeting efficiency is further compromised by off-organ sequestration and tumor microenvironment heterogeneity. While these systems drive advancements in personalized therapeutic strategies, critical gaps persist in understanding long-term biodistribution risks and optimizing biocompatibility for clinical implementation [12].
Figure 1.
Nanomaterials for the treatment of OS.
Despite advances in OS nanotherapeutics and multimodal strategies, clinical translation remains hindered by inadequate integration of nanomaterial innovations with OS pathobiology. While nanomaterials leverage enhanced permeability, active targeting, and stimulus responsiveness, their systematic alignment with OS-specific challenges—metastatic dissemination, immunosuppressive bone niches, and adaptive chemoresistance—lacks critical analysis. Current reviews disproportionately emphasize isolated nanomaterial functions (drug delivery, imaging) over multimodal synergy and neglect translational barriers: tumor heterogeneity-compromised targeting, undefined long-term biosafety, and scalability constraints. This gap necessitates frameworks bridging nanotherapeutic design with OS biological complexity and industrial-scale feasibility. This review establishes three translational objectives: (1) Mechanistic alignment of nanomaterial properties with OS pathobiology—hypoxic niches, aberrant angiogenesis, and metastatic pathways—to derive precision design principles; (2) systematic evaluation of pharmacological/immunological manufacturing barriers through safety-optimized production frameworks; (3) biocompatibility-driven integration of AI-optimized carriers, biomimetic interfaces, and in situ biosensing technologies, coupled with ethical precision medicine paradigms to accelerate clinical translation. The tripartite strategy bridges molecular insights with industrial-scale therapeutic development for localized and metastatic OS. This review aims to bridge the molecular mechanism/clinical reality gap, drive next-generation nanotherapeutic development, overcome traditional efficacy/toxicity trade-offs, and ultimately improve survival outcomes for localized and metastatic OS patients.
2. Functional Properties of Nanomaterials
2.1. Drug Delivery
2.1.1. Controlled-Release
The controlled release properties of nanomaterials enable precise spatiotemporal regulation of therapeutic agent delivery through material design, demonstrating significant potential in OS treatment through four primary mechanisms:
(1) Temporal release control: Nanocarriers modulate release kinetics through material degradation rates, drug encapsulation architectures, or chemical bond responsiveness. pH-sensitive hydrogels achieve gradual chemotherapeutic release in acidic tumor microenvironments. Fatema et al. pH-responsive hydrogel employs dual mechanisms for tumor-targeted curcumin release: PAA-based networks protonate in acidic tumor microenvironments (pH 5.0), contracting to expel drugs while deprotonating at physiological pH (7.4) to sustain release (Figure 2). The SA-reinforced dual-network architecture ensures mechanical stability. Acidic conditions significantly enhanced cumulative drug release (44% vs. 5% at 24 h), with GLF scaffolds showing 25% vs. 3% release between pH 5.0 and 7.4. This pH-dependent system enables sustained tumor-specific drug delivery for postoperative residual cancer suppression [13], while glutathione (GSH)-responsive nanomotors trigger payload release upon encountering elevated intracellular GSH levels [14].
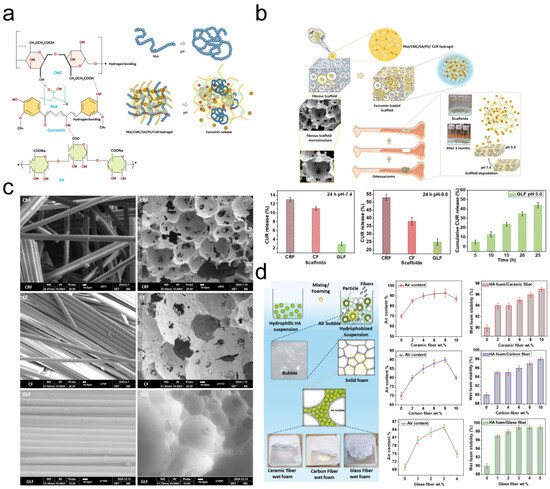
Figure 2.
(a) Proposed schematic representation of curcumin delivery from PAA/SA/CMC/PS/CUR hydrogel. * means hydrogen bonding. (b) Curcumin release from the double-network hydrogel loading CRF, CF, and GLF scaffolds. Experiments were performed in triplicate at pH 5.0 and 7.4. (c) SEM images of fiber-reinforced scaffolds. (d) Wet foam characterization. Adapted with permission from Ref. [13]. Copyright 2025 Elsevier.
(2) Stimulus-responsive release: As illustrated in Figure 3, endogenous-responsive nanomaterials are designed to react to intrinsic physiological conditions arising from normal biological processes or pathological alterations. These materials respond to inherent biological signals without requiring external stimulation, with representative categories including pH-responsive, redox-responsive, enzyme-responsive, and temperature-responsive systems. In contrast, exogenous-stimulus-responsive nanomaterials exhibit controlled behavioral modifications under artificially imposed physical, chemical, or biological interventions. These externally applied stimuli (typically operator-regulated) enable precise modulation of material properties and functions, with principal activation modalities encompassing optical irradiation, magnetic field application, ultrasonic excitation, and electric field manipulation [15]. Polydopamine nanoparticles release DOX under NIR irradiation, and Huang et al. bone-targeting system integrates triple stimuli-responsiveness: acidic pH-triggered DOX release via PDA protonation; reductive microenvironment-activated disulfide cleavage; and NIR-induced photothermal release. Experimental results demonstrated enhanced pharmacokinetics with prolonged circulation and amplified therapeutic efficacy through synergistic multi-stimuli drug liberation [16], while ROS-responsive carriers activated payload discharge in tumor-specific oxidative microenvironments [17].
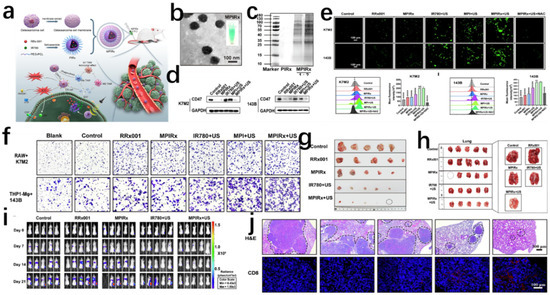
Figure 3.
(a) Construction of MPIRx nanodrugs for CD47 immune checkpoint/sonodynamic therapy of osteosarcoma and pulmonary metastasis. (b) TEM image of MPIRx nanodrugs. (c) Coomassie Brilliant Blue staining of MPIRx nanodrugs. MPIRx performed in the assay at mass ratio of 1:2. (d) Western blot detected the CD47 protein expression in OS cells. (e) Fluorescence microscopy images and flow cytometry analysis of ROS in K7M2 and 143B cells which were stained with DCFH-DA after different treatments, including control, RRx001 or MPIRx only, IR780,MPI with US or MPIRx with US activation. **** indicates significant difference, NS means no significant difference. (f) Transwell migration assay of macrophages (THP1-Mφ, RAW264.7) cocultured with OS cells (K7M2, 143B). (g) Photographs of tumor tissues from different treatments including control, RRx001 or MPIRx only, IR780 or MPIRx with US activation. (h) Photographs of lungs from each group showing the tumor metastasis. (i) Bioluminescence of mouse tumor cells showing the tumor developments at orthotopic right tibia and potential lungs at 0–21 days after the first treatment. (j) Representative H&E staining images of pulmonary metastasis (marked with black dotted line) from each group. Adapted with permission from Ref [15]. Copyright 2023 Elsevier.
(3) Zero-order sustained release: Structural engineering of polymer microgels [18] and nonporous coordination polymers [19] achieves constant release rates, eliminating initial burst effects and prolonging therapeutic duration. As shown in Figure 4, Murphy et al. developed therapeutic coordination polymers and nonporous materials enabling drug release via metal–ligand bond degradation rather than diffusion. With >62% drug loading and zero-order kinetics spanning three orders of magnitude (0.815–958 mg·cm−2·min−1), prolonged release is sustained. Release rates are tunable through (1) metal center selection (Mg2⁺, Mn2⁺, Zn2+; correlating with Lewis acidity), (2) bisimidazole ligand chain-length modifications altering crystal packing, and (3) competitive chelators (e.g., Na2EDTA) triggering accelerated stimulus-responsive release [19].
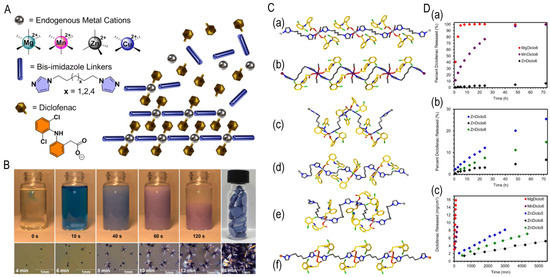
Figure 4.
(A) Components of TCPs (MDicloX): M = Mg2+, Mn2+, Zn2+, or Cu2+ metal cations. Diclo = diclofenac anions, and X = bisimidazole linkers with total carbon chain lengths of 5, 6, or 8 between imidazoles. Representation of drug release from TCPs via degradation of metal–ligand interactions. (B) Rapid synthesis of TCP CuDiclo6 upon addition of Cu(NO3)2 in MeOH with pressed monoliths depicted and formation of large single crystals at lower concentration in MeOH. (C) Single-crystal structures of TCPs: (a) MgDiclo6, (b) MnDiclo6, (c) ZnDiclo5, (d) ZnDiclo6, (e) ZnDiclo8, and (f) CuDiclo6. Mg = light grey, Mn = purple, Zn = grey, Cu = orange, N = blue, O = red, Cl = green; bisimidazole and diclofenac C-atoms are shown in silver and gold, respectively. (D) Drug release profiles from 5 mm TCP monoliths in 0.05 M phosphate buffer, pH 6.8 at 37 °C. (a) MgDiclo6 (red), MnDiclo6 (purple), and ZnDiclo6 (black) demonstrate effect of varying metal ion composition, (b) ZnDiclo5 (blue), ZnDiclo6 (black), and ZnDiclo8 (green) demonstrate effect of varying linker composition, (c) intrinsic dissolution release (IDR) rates of diclofenac from TCPs measured using a Woods apparatus, demonstrating prolonged zero-order release kinetics. Adapted from Ref. [19].
(4) Sequential multidrug release: Composite nanosystems like FSD-CHR@PPP [20] enable timed release of chemotherapeutics and genetic agents, amplifying synergistic effects between DNA damage and immune activation [21].
2.1.2. Target-Oriented
The targeting capability of nanomaterials refers to their ability to specifically deliver therapeutic agents to diseased tissues or cells, enhancing therapeutic efficacy while minimizing off-target effects. This targeting precision is achieved through three principal strategies:
(1) Active Targeting: Surface modification with targeting ligands (antibodies, peptides, or small molecules) enables specific recognition and accumulation at OS-specific surface markers or within tumor microenvironments. (1) Bone targeting: Phytic acid (PA)-functionalized nanoparticles (e.g., MnO2-PA) exploit high hydroxyapatite affinity for osseous tumor accumulation [22]. Bone-specific targeting is exemplified by saporin-boronated nanotherapeutics, which achieve ribosomal inactivation through hydroxyapatite binding, offering a targeted approach for bone malignancies [23] (Figure 5). (2) Tumor vascular targeting: iRGD peptide conjugation facilitates αvβ3 integrin-mediated tumor vascular endothelial binding and enhanced vascular penetration [24,25]. (3) Metabolic targeting: Nanoparticles targeting critical signaling molecules (STAT3, METTL3) disrupt tumor proliferation and chemoresistance pathways [26,27]. (2)Passive Targeting: Exploitation of the EPR effect enables selective nanoparticle accumulation in OS through leaky tumor vasculature [28]. (3) Bioinspired Targeting: Biomimetic nanocarriers (cell membrane-coated or bacterial outer membrane vesicles) evade immune clearance while enhancing tumor homing [17,29].
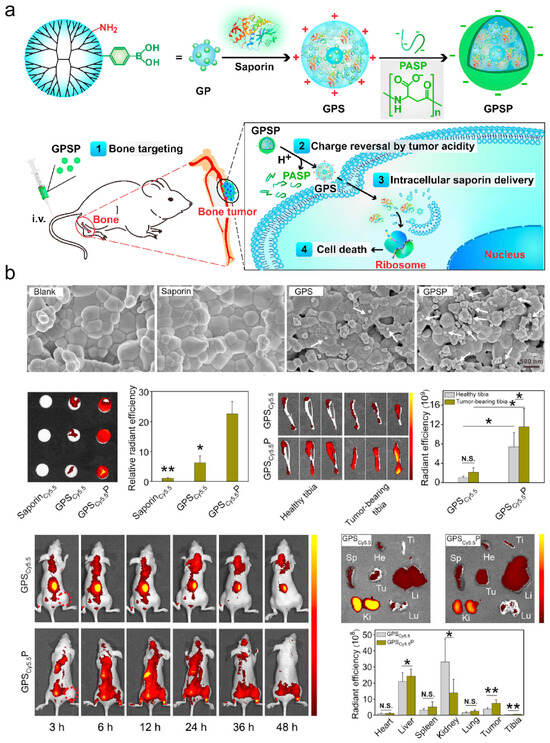
Figure 5.
(a) Design of bone-targeted protein nanomedicine for the treatment of malignant bone tumors. (b) Bone-targeting capability of GPSP. The white arrows indicate GPS or GPSP nanoparticles adsorbed on the hydroxyapatite tablets. (* p < 0.05 and ** p < 0.01 analyzed by student’s t-test, N.S. means no significance, one tailed). Adapted from Ref. [23].
2.2. Immune Regulation
2.2.1. Targeted Modulation of Immune Cell Activity
Nanomaterials can be engineered to specifically target immune cells within the OS tumor microenvironment (TME), including macrophages, dendritic cells, and T lymphocytes. For instance, aluminum-based nanosheets (nAl) enable co-delivery of chemotherapeutic agents and immunomodulators, driving macrophage polarization from pro-tumorigenic M2 to antitumor M1 phenotypes to amplify antitumor immunity [30]. Additionally, activating CD8+ T-cell infiltration through nanoplatforms can reprogram immunologically “cold” tumors into “hot” phenotypes [31]. Immunomodulatory approaches are exemplified by MPIRx nanomedicine, which co-delivers IR780 and RRx-001 in PEG-PCL nanocompartments to promote macrophage phagocytosis and M1 polarization via CD47 inhibition, offering a novel strategy for macrophage-associated immunotherapy (Figure 6) [32].
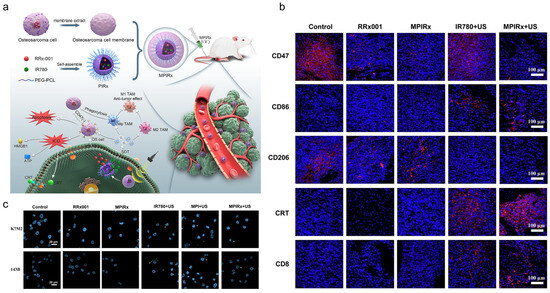
Figure 6.
(a) Construction of MPIRx nanodrugs for CD47 immune-checkpoint/sonodynamic therapy of OS and pulmonary metastasis. (b) Representative immunofluorescence images of tumor sections by different treatments showing CD47, CD86, CD206, and CRT expression. (c) Effect of MPIRx nanodrugs on ICD of OS cells. Calreticulin on the outer surface of OS cells was imaged by LSCM. Adapted with permission from Ref. [32]. Copyright 2023 Elsevier.
2.2.2. Remodeling the Immunosuppressive Microenvironment
The OS TME exhibits profound immunosuppression, characterized by elevated immune checkpoint molecules (e.g., PD-1/PD-L1) and inhibitory cells (e.g., regulatory T cells) [33]. Nanomaterials counteract immune evasion by delivering checkpoint inhibitors or disrupting immunosuppressive pathways such as Wnt/β-catenin signaling [34,35]. Select nanocarriers induce immunogenic cell death (ICD) in tumor cells, triggering damage-associated molecular pattern (DAMP) release to enhance antigen presentation and adaptive immune activation [36,37]. Lipid nanoparticles or biomimetic carriers deliver tumor-specific antigens to elicit antigen-specific T-cell responses or induce immune tolerance (for autoimmune applications). Bioinspired nanovehicles mimicking cellular membrane structures prolong circulation and enable lymph node-targeted antigen delivery, bolstering antitumor immunological memory [17].
2.3. Catalytic Properties
2.3.1. Enzyme-Mimetic Catalytic Activity
Nanomaterials can mimic natural enzymes such as peroxidase (POD) and catalase (CAT), catalyzing TME-specific substrates (e.g., H2O2, lactate) to generate cytotoxic ROS like hydroxyl radicals (•OH). For example, CuO@CeO2 heterojunction nanocatalysts synergize photothermal effects with chemodynamic therapy (CDT) under near-infrared (NIR) irradiation, enhancing H2O2 decomposition efficiency and ROS production [38,39]. RhRu/Ti3C2Tₓ nanozymes exhibit dual POD-like and CAT-like activities, generating •OH while decomposing H2O2 into O2 to alleviate tumor hypoxia and amplify photodynamic therapy (PDT) efficacy [40].
2.3.2. Cascade Catalytic Reactions
Nanomaterials can initiate multi-step catalytic reactions in response to tumor-specific conditions (e.g., high lactate, low pH, elevated GSH). Lactate-responsive CuO@CeO2 nanosystems activate cascade catalysis to deplete glutathione (GSH) and produce ROS, inducing apoptosis and ferroptosis [41]. Tong et al. bone-targeted silica nanoreactor achieved concentrated OS deposition and prolonged retention, utilizing laser-activated chlorin e6 for dual ROS generation and controlled DOX/doxycycline release, where doxycycline amplified oxidative stress to potentiate chemotherapy [42].
2.3.3. Exogenous Field-Enhanced Catalysis
External stimuli (e.g., light, heat, ultrasound) further amplify catalytic efficiency: NIR-II-driven FeS@CP NPs accelerate •OH generation and GSH depletion under irradiation, enabling synergistic photothermal/chemodynamic therapy [39]. Geng et al. synthesized W-doped TiO2 nanorods via high-temperature solution methods, where W5+/W6+ doping enhanced ROS generation for ultrasound-activated tumor ablation with rapid renal clearance (Figure 7) [43].
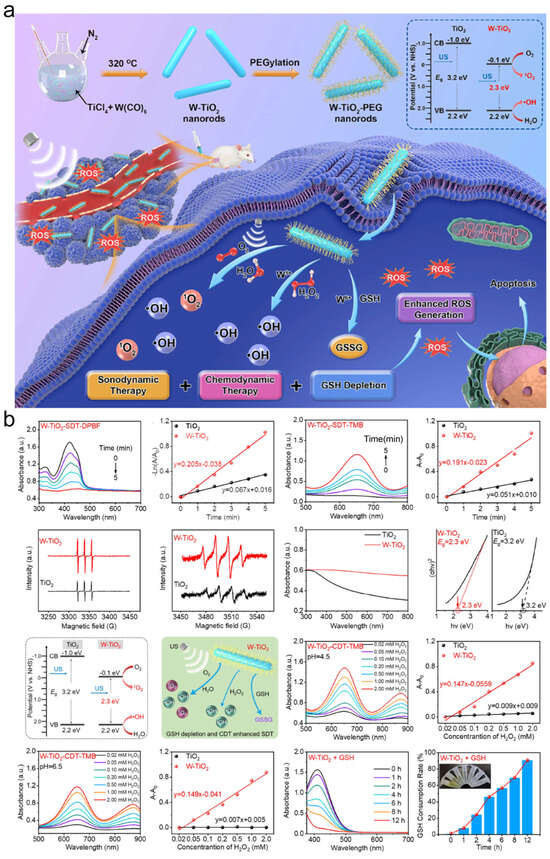
Figure 7.
(a) Overall scheme of the preparation procedure for ultrafine W-TiO2 nanorods with TME-regulating ability for CDT-enhanced SDT. (b) Schematic illustration of the enhanced ROS generation based on integrating SDT, CDT, and GSH depletion via W-TiO2 nanorods. Adapted with permission from Ref. [43]. Copyright 2021 American Chemical Society.
2.4. Thermal Effect
Thee thermal effects of nanomaterials refer to their capacity to absorb external energy (e.g., light, magnetic fields, electricity) and convert it into thermal energy.
2.4.1. Photothermal Effects
Under near-infrared (NIR) excitation, nanomaterials such as polydopamine, carbon-based materials, and metal sulfides convert light energy into localized hyperthermia (42–50 °C), inducing direct tumor cell ablation [44,45]. For instance, FePS3 nanosheets enable efficient OS ablation through photothermal conversion (Figure 8) [46].
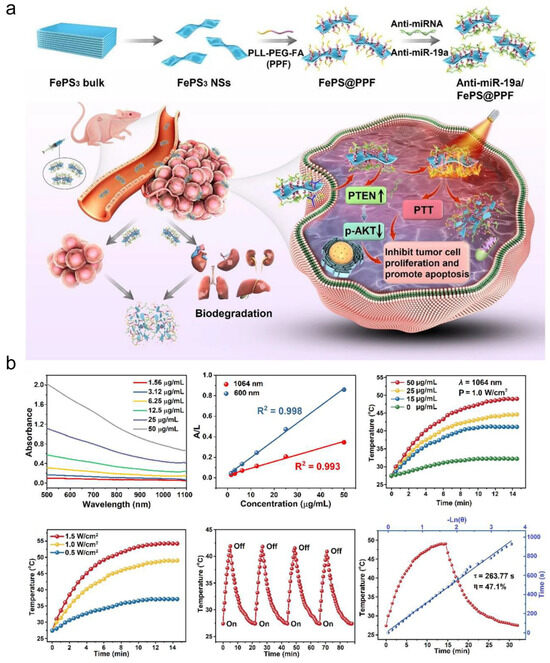
Figure 8.
(a) The fabrication of anti-miRNA-loaded FePS3 nanoplatform (anti-miR-19a/FePS@PPF) for combination treatment of OS. (b) NIR-II photothermal effects of FePS@PPF. Adapted from Ref. [46].
2.4.2. Magnetothermal Effects
Magnetic nanomaterials (e.g., iron oxides) generate eddy current-induced heat in alternating magnetic fields, facilitating temperature-modulated drug release or synergistic immunotherapy [47,48]. Notably, magnesium-based materials exhibit enhanced antitumor efficacy via thermal effects under low-intensity alternating magnetic fields [47]. Under electric field stimulation, nanomaterials generate therapeutic heat levels to enhance cellular endocytosis or drug delivery efficiency [49].
2.5. Tissue Engineering
2.5.1. Bioactivity Regulation
Nanomaterials can be engineered as multifunctional systems for precise delivery of immunomodulators, growth factors, or gene therapies. Surface modifications enable targeted release mechanisms, amplifying localized bioactivity while minimizing systemic toxicity [50]. Nanocarriers (e.g., mesoporous silica, metal-based materials) encapsulate natural agents like quercetin, achieving tumor microenvironment-responsive release via pH or ROS triggers to enhance therapeutic precision [51].
2.5.2. Tissue Regeneration and Biomimetic Support
Nanomaterials (e.g., hydroxyapatite, calcium phosphate composites) mimic native bone matrix composition and microstructure, promoting osteoblast adhesion, proliferation, and differentiation to accelerate bone defect repair [52,53]. As shown in Figure 9, Xu et al. designed a low-temperature 3D-printed tricalcium phosphate (TCP)-FePSe3 scaffold integrating FePSe3 nanosheets into α-TCP matrices, combining OS therapy and bone regeneration. This system enables efficient photothermal tumor ablation while releasing therapeutic ions: Se induces tumor apoptosis, while Fe/Ca/P promote angiogenesis and bone repair. The low-temperature fabrication preserves bioactivity and enables customized anatomical fitting for complex bone defects [48]. For example, neodymium/manganese co-doped whitlockite nanoparticles within alginate hydrogels simultaneously exhibit osteoinductive and antitumor properties [54]. Multifunctional scaffolds (e.g., drug-loaded silk fibroin/nano-hydroxyapatite fibers) eradicate residual tumor cells while providing structural support and bioactive cues for bone regeneration. This dual functionality significantly reduces postoperative recurrence and restores bone integrity [55].
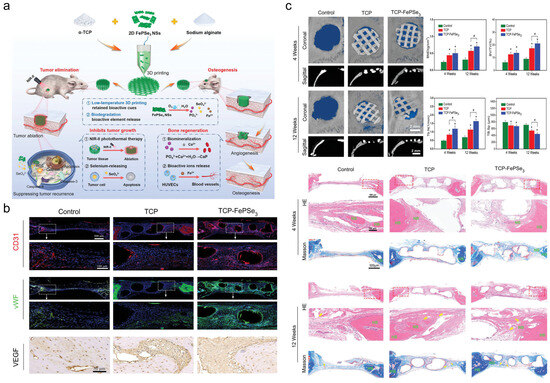
Figure 9.
(a) Schematic illustration of the 3D-printed TCP-FePSe3 scaffold as an all-in-one platform for bone regeneration and postsurgical suppression of OS recurrence. (b) Bone defect repair in vivo after scaffold implantation assessed by immunofluorescence and immunohistochemical labeling. (c) In vivo osteogenesis performance of TCP-FePSe3 scaffolds. (Yellow arrows as blood vessel, HB indicated as host bone, NB as new bone). (n = 5), * p < 0.05 when comparing TCP/TCP-FePSe3 scaffolds to the control group; # p < 0.05 in comparison of TCP-FePSe3 scaffolds to the TCP group.). Adapted with permission from Ref. [52]. Copyright 2023 John Wiley and Sons.
3. Nanomaterials for Treatment of OS
3.1. Nanovesicle
Nanovesicle materials (Table 1) represent a class of hollow or semi-hollow nanoscale carriers characterized by customizable functionality through molecular design. As shown in Figure 10, nanovesicles play a pivotal role in OS therapeutics and can be categorized into four classes: biologically derived, virus-derived, synthetic, and hybrid nanovesicles. Each variant demonstrates distinct functional characteristics: (1) Biologically derived nanovesicles originate from natural cellular sources including tumor cells and mesenchymal stem cells. These structures preserve native membrane composition and architecture while transporting diverse biomolecules. Their advantages include superior biocompatibility with minimal immunogenicity, inherent tumor-targeting capabilities through surface biomarkers, and multifactorial therapeutic effects via combined delivery of bioactive components. However, limitations encompass low production yield, compositional heterogeneity, and compromised in vivo stability [56]. (2) Virus-derived nanovesicles are engineered through pathogenic gene deletion while retaining viral transduction capacity. These vectors enable efficient delivery of therapeutic cargo (drugs/genes) to tumor cells, with modifiable tropism for targeted action. Challenges include residual immunogenicity potentially triggering adverse immune responses, latent pathogenicity risks, and complex manufacturing processes requiring substantial technical expertise [57]. (3) Synthetic nanovesicles, exemplified by liposomal and polymeric systems, feature programmable composition and architecture through controlled fabrication. Key advantages include precise engineering of physicochemical properties, enhanced storage stability, high drug-loading capacity, and capacity for combination therapies. Drawbacks involve reduced biological recognition requiring artificial targeting ligands and potential cytotoxicity from synthetic materials [58]. (4) Hybrid nanovesicles integrate biological and synthetic components to synergize therapeutic advantages. This chimeric design enables performance optimization through material hybridization while addressing individual system limitations [59]. Current technical barriers include intricate manufacturing protocols, stringent quality control requirements, and challenges in batch-to-batch reproducibility. Current nanovesicle systems for OS therapy primarily include liposomes, exosomes, virus-like particles, and cell membrane vesicles [60].
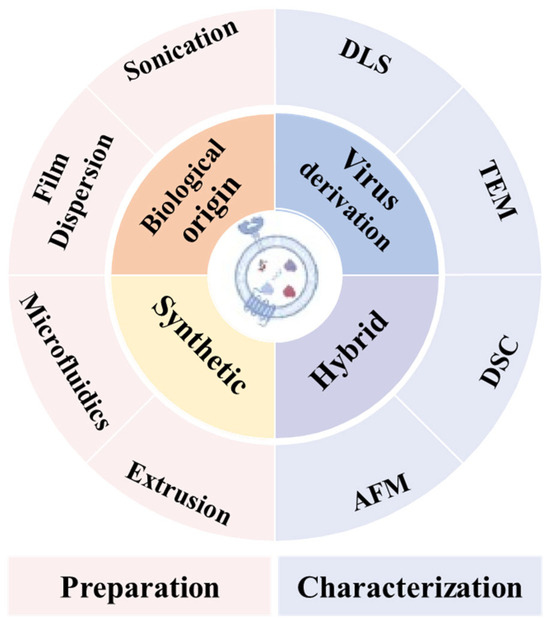


Figure 10.
Classification of nanovesicles and methods used for their preparation and characterization. Reprinted from Ref. [61]. (DLS: Dynamic Light Scattering; TEM: Transmission Electron Microscopy; DSC: Differential Scanning Calorimetry; AFM: Atomic Force Microscopy).

Table 1.
Representative paradigms of biovesicular nanomaterials for the treatment of OS.
Table 1.
Representative paradigms of biovesicular nanomaterials for the treatment of OS.
| Nanomaterial | Cell Line | Functional Properties | Tumor Model | Inhibition Rate | Innovation Points | Ref. |
|---|---|---|---|---|---|---|
| EXO-RIF | 143B, MG63 | Rifampicin delivery | Xenograft nude mice | 67.3% | BMSC exosomes, Drp1 agonist | [62] |
| EM-Dox | 143B | DOX delivery | carcinoma in situ | 82.9% | Exosome mimetics (EMs) | [63] |
| Exo-Dox | MG63, 143B | DOX nanocarrier | Xenograft nude mouse | 83.3% | SDF1-CXCR4 targeted therapy | [64] |
| BT-Exo-CAP | MG63, 143B | Carrying CAP | carcinoma in situ | 75% | Bone targeting, ferroptosis | [65] |
| DAELNs | HOS, MG63 | OS-targeted agents | Xenograft model | 70% | Dipsacus asperoides-derived P38/JNK signaling pathway | [66] |
| YSA-SPION-MV/MTX | MG63, 143B | MTX delivery | Orthotopic model | significantly inhibited | EphA2 magnetic targeting | [67] |
| ALN-LWMH-DOX-Lip | K7M2 | DOX delivery | Orthotopic model | 79.2% | Bone targeting, anti-metastasis | [68] |
| HA-DOPE@Lips/HNK | 143B | Honokiol delivery | Xenograft model | 63.7% | Hyaluronic acid HNK | [69] |
| FA-Res/Lps | 143B | Resveratrol delivery | Metastasis models | 82.9% | Lung metastasis JAK2/STAT3 pathway | [70] |
3.1.1. Liposomes
Liposomes, featuring phospholipid bilayer structures mimicking biological membranes, demonstrate superior biocompatibility and low toxicity. These nanostructures have emerged as prominent drug delivery systems in OS management [71]. To address platinum-based chemotherapy limitations, Yan et al. developed liposome-encapsulated Pt prodrugs (Lipo-OXA-ALN), enhancing tumor-specific accumulation while minimizing systemic toxicity [72]. Wei’s team employed liposomal miRNA delivery to reverse cisplatin resistance by targeting the METTL3/LINC00520 axis through glycolytic pathway modulation [26]. Surface engineering through polymeric or protein modifications enhances liposomal performance. Wu et al. constructed a dual—functional liposome system modified with alendronate (ALN) and low-molecular-weight heparin (LMWH) for targeted DOX delivery to bone tumors. ALN targets bone tissue and combats osteoporosis, while LMWH prolongs circulation and suppresses metastasis. Their synergistic action enhances drug enrichment and reduces side effects [68]. Zhang et al. developed a novel HA-DOPE@Lips/HNK liposome carrier. It uses hyaluronic acid (HA) to bind to CD44 receptors, enabling active targeting of OS. This approach addresses the challenges of honokiol (HNK)’s poor water solubility and lack of targeting [69]. Zhu et al. developed FA-Res/Lps for resveratrol delivery. FA binds to FR on tumor cells for active targeting, solving resveratrol’s solubility and bioavailability issues. This enhances tumor enrichment and antitumor efficacy. Mechanistic studies show it acts via JAK2/STAT3 inhibition, revealing a new molecular target [70]. Turánek et al. demonstrated that HPMA copolymer-modified liposomes exhibit prolonged circulation without immunogenicity compared to PEGylated counterparts [73]. SPI or 7S/11S globulin coatings significantly improve colloidal stability [74,75]. Chen’s team designed cationic liposomes with enhanced membrane interaction for improved vaccine adjuvant efficacy [76]. Multifunctional liposomal systems enable combination therapies. Song et al. developed catalytic liposomes (Au/PDA/HRP@DLP) integrating SDT and photothermal therapy (PTT) for dual tumor ablation and immune activation [77]. Zhang’s T-APR-246-loaded liposomes effectively remodeled the immunosuppressive microenvironment [78]. Despite promising preclinical results in toxicity reduction (e.g., DOX cardiotoxicity mitigation), clinical translation requires resolution of manufacturing scalability and long-term safety challenges [79]. Current research focuses on developing stimulus-responsive or actively targeted liposomes to overcome tumor penetration limitations [17,80].
3.1.2. Exosomes
Exosomes, naturally secreted nanoscale vesicles, serve as critical intercellular communicators by transferring biomolecules (proteins, nucleic acids, lipids) between cells. These membrane-bound structures are ubiquitously present in bodily fluids, including blood, urine, and saliva. Han et al. identified elevated plasma exosomal protein markers (CD63, VIM, EpCAM) in OS patients, suggesting diagnostic potential [81]. Almeida’s team engineered tumor-derived exosomes as PET imaging probes for detecting occult pulmonary metastases [82]. While BMSC-derived exosomes offer biocompatible drug delivery platforms, their intrinsic targeting limitations risk off-site effects [65,67]. Wei et al. leveraged MSC-derived exosomes’ intrinsic OS targeting via SDF1-CXCR4 axis chemotaxis to achieve tumor-specific DOX accumulation. The Exo-Dox system significantly mitigated DOX-induced cardiotoxicity while overcoming the dose-limiting cardiotoxicity in clinical applications [64]. Ji et al. engineered bone-targeting peptide (SDSSD)-modified BMSC exosomes (BT-EXO-CAP) delivering capreomycin, achieving synchronized bone/homing targeting for enhanced drug delivery. The system induced OS ferroptosis via ROS/Fe2⁺/lipid peroxidation cascades, mechanistically involving Keap1/Nrf2/GPX4 axis modulation [65]. Chen et al. developed exosomes from bone marrow mesenchymal stem cells to deliver rifampicin nanodrugs, discovering this anti-tuberculosis agent’s novel anti-OS effect. Their mechanistic study revealed rifampicin activates Drp1 protein, inducing excessive mitochondrial fission and triggering tumor cell apoptosis [62]. Du et al. enhanced OS targeting through NGR peptide-modified CAF exosomes, delivering circ_0004872-encoded peptides to induce autophagy-dependent ferroptosis [83]. Jiang’s group demonstrated exosomal miR-4443-mediated ferroptosis via ATF3 regulation [84]. Liu’s research revealed tumor-derived migrasomes as microenvironment modulators promoting metastasis [85]. Huang et al. identified tumor-suppressive effects of exosomal lncRNA MEG3 through oncogenic pathway inhibition [86]. Engineered exosomes demonstrate immunotherapeutic potential through inflammatory mediator delivery. Sun et al. characterized DC-derived exosomes mediating PD-L1-dependent immunosuppression [87]. Wang’s team proposed serum exosomal PD-L1 as both prognostic biomarker and therapeutic target for checkpoint inhibitor optimization [88]. Wang et al. developed bone marrow stem cell-derived exosome mimetics (EMs) via sequential extrusion for DOX delivery, yielding 20-fold more EMs than natural exosomes while maintaining tumor targeting. Using ammonium sulfate gradients, they achieved efficient DOX encapsulation (0.8 mg/mL). The EM-Dox system exhibited pH-responsive release, preferentially liberating drugs in acidic tumor microenvironments (pH 5.5) [63]. Lu et al. isolated exosome-like nanoparticles from Dipsacus asperoides, revealing their first-reported anti-OS activity through p38/JNK pathway activation. These plant-derived vesicles demonstrate precise targeting and low toxicity, advancing phytogenic nanomedicine applications [66].
3.1.3. Virus-Like Particles
Virus-like particles (VLPs), synthetic nanoscale vesicles mimicking viral morphology without containing genetic material, combine non-infectious properties with preserved antigenic surface features capable of eliciting immune responses. VLP-based strategies in OS management focus on two key applications: precision nanocarrier systems and immunotherapeutic enhancement. These engineered structures demonstrate exceptional drug/gene delivery potential through their inherent biocompatibility and nanoscale architecture. Suffian et al. developed VLP-encapsulated CRISPR-based gene-editing systems for modulating metastasis-related pathways [89]. The immunostimulatory capacity of VLPs enables tumor-specific antigen presentation. Yin’s team engineered DC-targeting VLPs displaying OS antigens to potentiate T-cell activation [90]. Alvaro et al. demonstrated synergistic antitumor effects through VLP/oncolytic virus combinations, achieving enhanced tumor lysis via dual mechanisms [91].
3.2. Polymer
3.2.1. Poly (Lactic-c–o-Glycolic Acid)
Poly (Lactic-co-Glycolic Acid) (PLGA) has emerged as a promising platform for OS therapy due to its biocompatibility, biodegradability, and controlled drug release capabilities. Recent advances demonstrate enhanced therapeutic efficacy through innovative nanoformulations (Table 2): Cai et al. created PTX-PLGA@[143B-RAW] nanoparticles by ultrasound-assisted paclitaxel loading on PLGA cores wrapped with hybrid OS/macrophage membranes. This dual-targeting system combines homologous tumor recognition and inflammatory chemotaxis, enhancing drug delivery. In vitro tests revealed pH-responsive release (90% at pH 5.3 vs. 70% neutral) and efficient tumor cell uptake, offering a biomimetic strategy for precise OS therapy [92]. Alsulays et al. developed chitosan-coated IVO-CS-PLGA-NPs to improve pharmacokinetic stability and tumor targeting of ivosidenib [93]. Biomimetic engineering approaches, exemplified by Wang’s IR780@PLGA@HM platform utilizing cancer cell membrane coating, enable microenvironment-specific targeting and immunomodulation [94]. Multimodal therapeutic strategies achieve synergistic effects through rational material design. Huang et al. combined DOX-loaded PLGA nanoparticles with polydopamine for NIR-triggered chemo-photothermal synergy [16], while Crisafulli’s layer-by-layer chitosan-PLGA system co-delivers miRNA-34a with chemotherapeutics to overcome drug resistance [95]. Advanced formulations like Wang’s MnO/Fe@PLGA nanoparticles leverage metal ion-mediated ROS generation and macrophage polarization to prevent postoperative recurrence. PLGA-based platforms also address antimicrobial challenges in cancer therapy through photothermally enhanced antibacterial peptide delivery. Furthermore, Sapino’s P-gp inhibitor-loaded nanoparticles demonstrate improved chemosensitivity by blocking drug efflux mechanisms, highlighting PLGA’s potential in overcoming conventional chemotherapy limitations. Li et al. designed a dual-responsive (redox/pH) mPEG-PaLA copolymer nanocarrier for co-delivering PTX and DOX in OS treatment. In K7 models, NP-PTX-DOX exhibited superior tumor suppression over single-drug and free-drug groups, confirming synergistic therapeutic efficacy [96]. Wang et al. developed MH-PLGA-IR780 NPs, a homologous-targeted nanoplatform using HOS membrane-coated PLGA nanoparticles encapsulating IR780. This system integrates dual-modality imaging (photoacoustic/fluorescence) with NIR-triggered photodynamic therapy, inducing concurrent apoptosis and ferroptosis in OS. Mechanistically, ROS accumulation triggers mitochondrial apoptosis and NCOA4-mediated ferritinophagy while suppressing GPX4, causing lethal lipid peroxidation. The HOS membrane coating enables immune evasion and deep tumor penetration, overcoming conventional nanoparticle limitations in OS therapy [97].

Table 2.
Representative paradigms of polymer nanomaterials for the treatment of OS.
3.2.2. Polydopamine
Polydopamine (PDA) nanomaterials, formed via oxidative self-polymerization of dopamine monomers under alkaline conditions, demonstrate significant potential in OS therapy due to their photothermal conversion and radical scavenging capabilities. Liu et al. developed a PDA-MOF-E-M nanocomposite integrating tumor-targeting ligands, anti-PD-1/PD-L1 immune-checkpoint inhibitors, and near-infrared (NIR) photothermal effects for multimodal therapy. This combination significantly enhanced tumor cell eradication through photothermal/chemotherapy synergy while suppressing bone destruction progression in vivo [25]. Ma’s team engineered PDA@MnS nanostructures that induce localized hyperthermia and catalyze ROS generation, establishing a dual PTT-CDT therapeutic mechanism. This strategy achieved enhanced antitumor efficacy with reduced systemic toxicity through low-dose chemotherapeutic synergy [110]. PDA-based platforms effectively deliver immunoadjuvants (e.g., CpG oligonucleotides) or chemotherapeutics, triggering immunogenic cell death (ICD) to activate T-cell responses and potentiate anti-PD-1 therapy [111]. Furthermore, Li et al. demonstrated that PDA@HA coatings mitigate oxidative stress in tumor microenvironments via ROS scavenging, effectively inhibiting osteoclast activation and bone degradation [112].
3.2.3. Hydrogel
Polymer hydrogels, three-dimensional networks formed through crosslinked polymer chains, exhibit exceptional water absorption and retention capacities. Characterized by high hydration, tunable mechanics, and biocompatibility, these materials demonstrate dual therapeutic and regenerative potential in OS management. Classified by origin, they encompass natural (e.g., collagen, alginate) and synthetic variants (e.g., poly(N-isopropylacrylamide), polyvinyl alcohol). Innovative in situ crosslinking systems like MOG hydrogel—comprising MgO nanoparticles and dopamine-conjugated gelatin—effectively fill post-resection defects while delivering localized electrostimulation via mild ultrasound. This approach achieved 67.6% tumor suppression in tibial models while stimulating bone regeneration [113,114]. Si et al. created an injectable thermoresponsive PLGA-PEG-PLGA hydrogel for localized co-delivery of DOX and CDDP. The material’s temperature-dependent phase transition enables sustained drug release at tumor sites, enhancing local drug concentration by 2.7-fold while reducing systemic exposure by 58–63%, with 96 h retention and synergistic cytotoxicity [107]. Freeman et al. demonstrated that localized miR-29b delivery concurrently inhibits OS growth and restores bone homeostasis. They engineered an injectable hyaluronic acid (HA)-based hydrogel enabling sustained release of miR-29b-encapsulated poly(β-amino ester) (pBAE) nanoparticles, achieving precision-controlled miRNA delivery as an adjunct to conventional chemotherapy during tumor resection [108]. Wang et al. engineered an injectable hydrogel for sequential Nox4i/L-Dox delivery. Nox4i inhibits CAF activation to reverse T-cell exclusion, while L-Dox induces immunogenic cell death, amplifying antitumor immunity. Clinical data link Nox4 overexpression to poor OS prognosis, validating this sequential therapy [109]. Luo’s FePSe nanocomposite hydrogel synergizes photothermal ablation with controlled chemotherapeutic release, demonstrating 35.6-fold tumor volume reduction and reduced recurrence [46]. Xie’s Mg2⁺/nHA-incorporated PH-GBS@CCP hydrogel sustains bioactive ion release for critical-size defect repair [115]. Electrically conductive variants like polyacrylic acid-based composites promote osteoblast differentiation while inhibiting tumor proliferation through electrostimulation [116,117]. Significantly, hydrogels modulate tumor immune microenvironments by reprogramming CAFs via TGF-β regulation, enhancing T-cell infiltration and suppressing metastasis [109,118]. Combinatorial systems co-delivering αPD-L1 antibodies and Hedgehog inhibitors demonstrate enhanced checkpoint blockade efficacy [119,120]. Clinically transformative applications include 3D-bioprinted models incorporating extracellular matrix mimics for personalized drug screening [121]. Key challenges include (1) mechanical optimization via nanofiber reinforcement or supramolecular crosslinking to balance flexibility and strength [122,123]; (2) overcoming chemoresistance through CRISPR-identified targets like RFWD3 in gene-silencing hydrogels [124]; and (3) infection control using antimicrobial formulations integrating silver nanoparticles or antibiotics [125].
3.3. Inorganic Nonmetal
3.3.1. Calcium-Based Nanomaterials
Calcium-based nanomaterials (e.g., hydroxyapatite, calcium phosphate) demonstrate unique advantages in OS treatment due to their inherent biocompatibility and osseointegration capacity. These materials degrade into metabolically compatible Ca2⁺ and PO43− ions, offering superior safety over conventional metallic or polymeric carriers. Major calcium-based variants (Table 3) include hydroxyapatite (HAp), tricalcium phosphate (TCP), calcium carbonate (CaCO3), calcium peroxide (CaO2), and perovskite-type nanomaterials.

Table 3.
Representative paradigms of inorganic nonmetallic nanomaterials for the treatment of OS.
As the principal inorganic component of bone, nano-hydroxyapatite exhibits exceptional biocompatibility and osteoconductivity. Its nanoparticulate form enhances osteoblast activity while providing high surface area for drug/growth factor loading. Wang et al. first demonstrated nano-HAPs concentration-dependently inhibit OS OS-732 cell viability, migration, and invasion via FAK/PI3K/Akt downregulation. Intratumoral injection suppressed tumor growth comparably to combined implantation [126]. Liu et al. developed a hydroxyapatite/BSA/paclitaxel ternary nanoparticle system for postoperative OS therapy, achieving 32.17% drug loading with sustained PTX/calcium release, low cytotoxicity, and enhanced osteogenic differentiation for bone repair [127]. Liu et al. developed HA nanoparticle/microparticle carriers for doxorubicin delivery, achieving pH-dependent drug release via OS cellular internalization. Mitochondrial-targeted DOX induced apoptosis through metabolic disruption, while the dual-particle system enhanced safety and sustained release [128]. Wu et al. demonstrated morphology/size-dependent HANPs selectively inhibit OS cells with low toxicity. Rod/needle-shaped variants showed optimal efficacy via calcium/ROS elevation and mitochondrial apoptosis while inducing immunogenic cell death. HANPs trigger immunogenic cell death by releasing DAMPs (CRT, ATP, HMGB1) to activate innate immunity and polarize macrophages toward the M1 phenotype while enhancing CD8+ T-cell infiltration, with the highest-aspect-ratio HANPs-3 exhibiting optimal immunomodulatory and antitumor effects [129,130]. Xie et al. developed 3D-printed polylactic acid scaffolds with surface-modified HAp (PH-GBS@CCP), achieving simultaneous bone regeneration and localized cisplatin release through hydrogel integration [115]. HAp’s tumor-suppressive mechanism involves calcium-mediated apoptosis induction via dense mineralization layers on OS cells, demonstrating superior efficacy to DOX in vitro [29]. Metal-doped HAp nanoparticles (ZnHAU, FeHAU) enable pH-responsive drug release through hydrogen bonding and metal–ligand interactions, minimizing systemic toxicity [144,145]. Antimicrobial HAp composites incorporating MgO/FeO nanosheets or antibacterial peptides effectively inhibit biofilm formation while promoting osseointegration [146,147].
Calcium phosphate nanomaterials enable tumor–bone dual therapy through functional composites. FePSe-integrated cryogenic-3D-printed scaffolds achieve synergistic osteogenesis and tumor suppression [52]. Germanium–selenium co-doped TCP bioceramic regulate neurogenesis and angiogenesis for combined tumor treatment and bone repair [148]. Tumor microenvironment-responsive calcium phosphonate nanoagents enable sustained drug release and immune activation, suppressing pulmonary metastasis through dendritic cell maturation and CD8⁺ T-cell recruitment [149]. Calcium phosphate nanoparticles disrupt tumor calcium homeostasis via mitochondrial dysfunction, while zinc-doped variants exhibit pH-dependent antitumor activity [150,151].
Calcium carbonate nanomaterials induce tumor-specific calcium overload through acidic microenvironment-responsive degradation. Gadolinium-stabilized amorphous calcium carbonate (ACC) achieves Magnetic Resonance Imaging (MRI)-trackable theranostics with enhanced stability [152,153]. Hyaluronic acid-coated ACC nanoparticles (H-CNP@pGMD) trigger pyroptosis and antitumor immunity via lysosomal degradation-activated gene delivery [154]. Organic solvent-stabilized ACC nanospheres show promise for controlled drug delivery [155,156]. Wang et al. developed europium-doped calcium fluoride (CaF2:Eu) nanoparticles for OS radiotherapy enhancement, demonstrating selective cytotoxicity, migration suppression, and X-ray sensitization via amplified radiation interactions to reduce proliferation and metastasis [132].
3.3.2. Carbon-Based Nanomaterials
Carbon-based nanomaterials, composed primarily of carbon atoms at nanoscale dimensions, have found extensive applications in oncotherapy. Representative materials include graphene, carbon nanotubes (CNTs), carbon dots (CDs), fullerene (C60), mesoporous carbon nanospheres (MCNs), and nanodiamonds (NDs).
Graphene derivatives, particularly graphene oxide nanosheets, exhibit exceptional surface-to-volume ratios, mechanical strength, and functionalizable surfaces, serving as efficient nanocarriers for chemotherapeutic agents like DOX through physical adsorption or covalent conjugation [157,158]. These platforms enable multimodal OS therapy by integrating targeting ligands (e.g., hyaluronic acid) with combinatorial chemo-photothermal-photodynamic approaches. Near-infrared (NIR)-responsive graphene composites generate localized hyperthermia and reactive oxygen species (ROS) under irradiation, achieving synergistic tumor ablation [159]. Notably, graphene-modified bone scaffolds regulate osteogenic differentiation via RUNX2 and OCN expression, facilitating post-resection bone regeneration [160,161]. Optimized parameters (<20 mg/kg dose, 50–1000 nm size, PEGylation) enhance biocompatibility while mitigating inflammatory responses [162]. Huang et al. fabricated PEG-GO-FA/ICG nanoparticles for chemo-photodynamic therapy. They enhanced photodynamic efficacy by inhibiting MTH1, inducing apoptosis via ER stress and JNK/p53/p21 pathways, with autophagy offering protection [139].
Carbon dots (CDs) enable dual diagnostic/therapeutic functionality through tunable fluorescence and surface engineering. NIR-emitting CDs demonstrate concurrent photothermal therapy and chemodynamic effects via Fenton-like •OH generation [163]. Their sub-10 nm dimensions enhance tumor penetration and multidrug resistance reversal. Mn-doped CDs achieve MRI-fluorescence bimodal imaging while catalyzing ROS production in tumor microenvironments [164]. Advanced applications include boron neutron capture therapy via B-enriched CDs [165] and antibacterial/osteogenic 3D hydrogels for infected bone defects [166]. Their favorable biosafety profile (<100 μg/mL cytotoxicity) and biodegradability support clinical translation [167,168]. Consoli et al. prepared phenol/carboxyl-containing Carbon-BNQDs via a DMF-based one-step method. These water-dispersible dots have pH-dependent fluorescence, high biocompatibility, and photothermal efficiency (60.7% in solution, 90% in solid state) [142].
Carbon nanotubes (CNTs), categorized as single-walled (SWCNTs) or multi-walled (MWCNTs), exhibit unique advantages in modulating tumor immune microenvironments. MWCNTs disrupt cytoskeletal dynamics, mimicking microtubule-targeting agents like paclitaxel [168]. Magnetic CNTs (mCNTs) implement mechanotherapeutic strategies by exerting mechanical stress to rupture tumor cells under magnetic guidance [169,170]. Gisbert-Garzarán et al. engineered pH-responsive CMK-3/1 mesoporous carbon nanoparticles coated with self-immolative polyurethane, demonstrating enhanced drug loading over MSNs and precise pH-dependent release validated by in vitro/vivo biocompatibility studies [138].
3.3.3. Silicon-Based Nanomaterials
Silicon-based nanomaterials constitute a diverse class of nanostructures with silicon as the core element, including silica nanoparticles (SiO2 NPs), mesoporous silica nanoparticles (MSNs), bioactive glass (BG), silicon quantum dots (Si QDs), and silicon nanowires (SiNWs).
Mesoporous silica nanoparticles (MSNs), characterized by ordered pore structures and tunable surface chemistry, serve as versatile platforms for OS therapy. He et al. engineered three selenium-loaded mesoporous silica nanoparticles (surface/pore-loaded, matrix-doped, core-loaded) demonstrating rapid controllable selenium release, enhanced under reductants, with Se-doped variants showing selective Saos-2 cytotoxicity and minimal hFOB 1.19 toxicity [134]. Ye et al. designed GSH/pH-responsive organic/inorganic hybrid MCD nanoparticles exhibiting photothermal/antibacterial activity, which combat OS via cuproptosis/ROS synergy while promoting osteogenic differentiation, amplified by NIR-II irradiation [135]. Metal co-doped MSNs (e.g., Cu&Ce-MSNs) enable NIR-II fluorescence/MRI dual-modal imaging while amplifying ROS generation and metabolic interference for enhanced tumor suppression [171]. Aptamer/antibody-functionalized MSNs improve tumor targeting via CD47 recognition, enhancing phagocytosis and drug accumulation [172]. The hierarchical porosity facilitates spatiotemporally controlled release of chemo-gene combination therapies (e.g., temozolomide/siHDGF co-delivery), effectively overcoming drug resistance [173]. Immunotherapeutic applications exploit mannose-modified MSNs to activate DC-mediated T-cell responses, significantly reducing pulmonary metastasis [174]. Lanthanide-incorporated MSNs (Eu/Gd-MSNs) provide real-time therapeutic monitoring through bimodal imaging [175]. Stimulus-responsive MSNs with pH/ROS-triggered drug release mechanisms enhance therapeutic precision [176]. Surface engineering strategies (PEGylation, Mn-O bond integration) address biodegradation concerns while maintaining biocompatibility [177,178].
Silicon nanowires (SiNWs) demonstrate tumor-selective internalization advantages, with 3 × 3 µm microdevices showing preferential uptake in Saos-2 OS cells versus normal osteoblasts [179]. Pre-lithiated porous SiNWs (LipSiNs) combine lithium/silicon co-delivery with bioresorbability, enabling innovative drug/gene loading approaches [180]. Multifunctional composites like Mn-doped silicon/hydroxyapatite nanowires regulate T-cell immunity via MnSOD/AMPK pathways, concurrently suppressing inflammation and promoting osseointegration [181]. Silicene-modified bone matrices (SNS@DBM) achieve photothermal ablation and osteogenic factor release through “thermal switch” mechanisms [182].
Bioactive glass (BG), first developed by Hench in the 1970s, exhibits dynamic biointeractivity through ionic dissolution/reprecipitation processes. Typical compositions comprise SiO2, CaO, P2O5 with optional additions of Na2O, K2O, or therapeutic metal ions. Modern BG scaffolds integrate dual osteogenic/antitumor functions through nanocomposite designs. SeNP/MgFe-LDH-modified BG systems eradicate residual tumors via ROS/Mg2⁺ release while stimulating osteogenesis [183]. Strontium-doped BG modulates macrophage polarization to attenuate fibrosis and enhance angiogenesis [184]. Zn/V co-doped BG nanoparticles regulate diabetic bone healing through antioxidant delivery and immunomodulation [185]. Advanced 3D-printed CeO2-BG scaffolds demonstrate sequential ROS scavenging, photothermal conversion, and osteoinduction capabilities [186]. Hu et al. synthesized CTAB-templated selenium-doped mesoporous bioactive glass nanospheres (400 nm) via sol–gel methods, exhibiting high surface area (>400 m2/g) and pH/Se-dependent drug release with selective MG63 cytotoxicity for bone engineering applications [136].
3.4. Metal Based
3.4.1. Noble Metal Nanoparticles
Noble metal nanoparticles (NMNs) comprise nanoscale particles of gold (Au), silver (Ag), platinum (Pt), palladium (Pd), and other noble metals. Major categories include gold nanoparticles (AuNPs), silver nanoparticles (AgNPs), platinum nanoparticles (PtNPs), and palladium nanoparticles (PdNPs), along with nanoparticles of rhodium, iridium, osmium, and related elements.
AuNPs (Table 4) exhibit diverse morphologies, including nanospheres (AuNSs), nanorods (AuNRs), nanostars (AuNSts), and hollow structures. Surface-modified AuNPs enable active tumor targeting in OS therapy. RGD-conjugated AuNPs demonstrate enhanced tumor penetration and retention in subcutaneous and pulmonary metastasis models. Mesenchymal stem cell-mediated delivery of AuNPs improves tumor targeting through cellular homing mechanisms [187]. CRISPR-Cas9 systems integrated with AuNPs achieve precise gene editing to enhance chemosensitivity in OS cells [188]. The surface-enhanced Raman scattering (SERS) properties of sub-5 nm excretable AuNPs facilitate intraoperative tumor imaging with reduced systemic retention risks [189]. AuNRs demonstrate near-infrared (NIR) photothermal conversion capabilities, with Janus-type AuNRs@MnO composites enabling multimodal photoacoustic imaging-guided chemo-photothermal therapy [190]. Biomimetic GNRs@SiO2@MnO platforms activate STING pathways through Mn2⁺ release, enhancing antitumor immunity against metastatic OS [191]. Chakraborty et al. studied the size-dependent toxicity of AuNPs on MG63 cells via a modified Frens method. Different sizes significantly affected cell viability at equal concentrations, inducing apoptosis via ROS elevation and mitochondrial membrane disruption. SERS technology was used to confirm apoptosis and monitor intracellular molecular changes [192]. Yang et al. assembled AuNR@CA hybrids via chlorogenic acid and gold nanorods. They regulated photothermal effects, enabling tumor cell inhibition and bone regeneration under near-infrared laser. Laser power was adjusted to control temperature for synergistic therapy [193]. Bu et al. developed anisotropic TOh Au@Pt nanoplatforms with edge-deposited Pt amplifying LSPR via spatially separated architecture, enabling synergistic PTT/PDT efficacy and H2O2-catalyzed oxygenation for hypoxic tumor therapy [194]. Feng et al. engineered core-shell ACZO nanoparticles (Au NRs-encapsulated Ce/Zn composites) enabling acid-triggered ROS generation, GSH depletion, and Zn2⁺-mediated cytotoxicity, with photothermal-enhanced CDT efficiency for synergistic antitumor efficacy [195].

Table 4.
Representative paradigms of metal-based nanomaterials for the treatment of OS.
AgNPs exhibit potent antimicrobial activity and SERS capabilities, though with comparatively lower biocompatibility. Dendrimer-complexed AgNPs significantly enhance antibacterial efficacy against postoperative infections [212]. Sodium alginate-coated AgNPs enable mitochondrial-targeted resveratrol delivery for improved anticancer effects [213]. Green synthesis approaches using microalgae or polysaccharides improve biocompatibility [214]. Morphological optimization (nanowires vs. nanospheres) balances bactericidal efficiency and cytotoxicity [215]. Despite progress, long-term biosafety assessments remain crucial for clinical translation [216,217]. Hu et al. developed smaller AgNPs through ultrasonic optimization, showing enhanced antitumor efficacy (outperforming cisplatin in vitro) by reprogramming glycolytic metabolism to induce apoptosis, with 68% fecal excretion within 1 week and minimal toxicity [196]. Wen et al. synthesized silver nanoparticles using tannin-rich mangrove extract via eco-friendly synthesis, demonstrating potent anti-OS activity (IC50 below standard thresholds) through ROS-mediated apoptosis with low cytotoxicity and therapeutic potential [197]. Cheng et al. engineered AgBiS2 theranostic nanoparticles integrating CT imaging (Bi/Ag-enhanced contrast) and NIR-triggered phototherapy (36.51% efficiency) with potent ROS generation and Staphylococcus aureus eradication, reducing infection risks [198].
PtNPs demonstrate catalytic versatility and chemotherapeutic potential. Acid/ROS-responsive Pt(IV) prodrug nanoparticles achieve tumor-specific drug release with reduced systemic toxicity [218,219]. Exosome-loaded ultrasmall PtNPs offer low-toxicity alternatives to conventional cisplatin [220]. Catalytic Pt-based nanomotors enhance drug diffusion in hypoxic tumor microenvironments [221]. Combinatorial strategies with RFWD3 inhibitors overcome platinum resistance through PHGDH-mediated metabolic reprogramming [128]. Immunogenic cell death induction by PtNPs synergizes with checkpoint inhibitors for enhanced therapeutic outcomes [222].
3.4.2. Metal Oxide
Metal oxide nanomaterials can be categorized by metallic elements into iron-, copper-, zinc-, titanium-, and manganese-based systems, each demonstrating distinct therapeutic mechanisms in OS management.
Iron-based oxides (Fe3O4, Fe2O3) leverage superparamagnetism for targeted accumulation and Fenton reaction-mediated ROS generation. FeS@CP NPs synergize chemotherapy and photothermal therapy while promoting bone regeneration [39]. FeOOH nanosheets enhance chemodynamic therapy through Fe2⁺/Fe3⁺ cycling and GSH depletion [223]. Three-dimensionally printed scaffolds incorporating single-atom iron catalysts demonstrate tumoricidal ferroptosis induction coupled with antibacterial activity [224]. Multimodal systems like Fe-TiO nanodots combine sonodynamic and chemodynamic therapies for enhanced efficacy [225]. Zhang et al. engineered FSR-Fin56 nanoparticles (Fe3O4 core/RGD-mesoporous silica shell) for dual ferroptosis induction: Fin56-triggered GPX4 degradation and Fe3⁺/GSH depletion-driven Fenton catalysis. Photothermal activation amplifies ROS/LPO accumulation, enabling targeted tumor therapy [202]. Liang et al. developed DOX/Fe3O4@PMMA bone cement enabling in situ phase-transition solidification, combining magnetothermal ablation (AMF-activated) with pH/magneto-thermal dual-responsive DOX release for synergistic tumor therapy via minimally invasive delivery and precise drug control [203].
Copper-based oxides (CuO, Cu2O) exploit cuproptosis and Fenton-like reactions for dual therapeutic action. CuH@HA nanoparticles enable tumor-specific copper release to activate mitochondrial respiration collapse [226]. CuO-MnO@PEG nanocomposites disrupt copper homeostasis through GSH depletion and ATP7A/B inhibition [227]. Immunomodulatory effects are achieved via HMGB1/ATP release, enhancing DC-CTL activation when combined with PD-L1 inhibitors [228]. Chu et al. engineered DOX-loaded hollow copper ferrite nanoparticles (DOX@HCFP) with tumor microenvironment-responsive trimodal therapy (chemodynamic/photothermal/chemo), achieving 35.25% photothermal efficiency and GSH depletion-enhanced synergistic antitumor efficacy [199].
Zinc-based oxides utilize Zn2⁺-mediated mitophagy and β-catenin degradation for metastasis suppression. ZnO NPs inhibit OS progression through Hypoxia-Inducible Factor 1 alpha(HIF-1α)/BNIP3/LC3B pathway modulation [229]. Biodegradable Zn-0.8Li scaffolds demonstrate PI3K/Akt pathway-mediated antitumor effects alongside osteogenic activity [230]. Cheng et al. synthesized ZnONPs via green synthesis using Rehmannia extract, inducing MG-63 apoptosis through ROS generation, MMP reduction, and Bax/caspase-3/9 activation while suppressing cell adhesion to limit metastatic potential [200].
Titanium-based oxides (TiO2) exhibit broad-spectrum ROS generation under ultrasound/light activation. Amorphous TiO2/polymer hybrids enhance sonodynamic efficiency [231]. RhRu/Ti3C2Tx nanozymes combine photothermal conversion and catalytic H2O2 decomposition for multimodal therapy [40].
Manganese-based oxides (MONs) address tumor hypoxia while enabling theranostics. HMnO nanoparticles achieve targeted drug delivery through biomimetic coating [232]. MnO nanospikes induce ICD and cGAS-STING pathway activation for enhanced immunotherapy [233]. MONs potentiate radiotherapy by oxygen generation and ROS amplification [234]. Zhang et al. engineered GCMMR nanocomposites (GOx/Cu2⁺ core, MET@MnO2 shell, RGD-PEG coating) for synergistic starvation/chemodynamic therapy through GSH depletion, Fenton-driven OH generation, and biomineralized enzyme protection [195]. Popov et al. engineered photochromic PVP-WO3 nanoparticles (∼1 nm) with selective OS cytotoxicity (IC50:5 mg/mL) and low hMSC toxicity, while RT-PCR profiling of 96 ROS/cell-death genes revealed molecular mechanisms [204].
3.4.3. Metal–Organic Framework
Metal–organic frameworks (MOFs) represent a class of porous crystalline materials formed through coordination-driven self-assembly of metal ions/clusters and organic ligands. Characterized by highly ordered networks, tunable pore sizes (0.3–20 nm), exceptional surface areas (up to 7000 m2/g), and abundant functional sites, their structural and functional properties can be precisely engineered through metal node and ligand selection. These attributes enable diverse applications in gas adsorption, catalysis, drug delivery, and biosensing, with particular therapeutic potential in oncology. Major MOF categories include ZIFs, MILs, UIO series, IRMOFs, and porphyrinic MOFs, differentiated by metal node composition. Du et al. engineered HA@MOF/D-Arg nanoparticles for dual-action OS radiotherapy enhancement: Fenton-mediated ROS generation and D-Arg-derived NO suppression of HIF-1α to alleviate hypoxia and boost radiosensitivity [205]. Li et al. designed Ta/Zr-co-doped TZM nanoMOFs with TCPP ligands, boosting radiotherapy via enhanced X-ray absorption and RDT efficacy through narrowed HOMO-LUMO gaps while activating antitumor immunity via ICD and enabling trimodal imaging [210].
ZIFs (Zeolitic Imidazolate Frameworks) exhibit zeolite-like topologies constructed from imidazolate ligands and divalent metal ions (Zn2⁺, Co2⁺). Their pH-responsive degradation in acidic tumor microenvironments enables controlled release of chemotherapeutics (e.g., 5-fluorouracil) and zinc ions, synergizing chemotherapy with microenvironment modulation [235,236]. Microwave-responsive ZnS@ZIF-8 composites demonstrate dual functionality in tumor ablation through thermo-chemotherapy and zinc-mediated osteogenesis [204]. SF-ZIF@NA hydrogels achieve selective osteoclast inhibition while promoting vascularized bone regeneration in osteoporotic defects [237]. Zinc ions released from ZIF-8 enhance osteogenic differentiation and angiogenesis, supporting its integration into polylactic acid bone scaffolds [238,239]. Glucose-deprivation strategies using GOx@ZIF-8/5-FU nanofibers demonstrate cascade therapeutic effects against OS [235]. Tantalum/zirconium co-doped MOFs (TZM) reverse immunosuppressive microenvironments in metastatic OS through radiosensitization and immunogenic cell death induction [210]. Ma et al. designed 3D-printed Ti scaffolds integrating ZIF-8 nanoparticles co-loading DOX and IDO inhibitor for microwave-triggered thermo-chemo-immunotherapy and Zn2⁺-mediated osteogenesis via immunogenic cell death activation and scaffold-guided bone regeneration [207]. Wu et al. engineered DOX-Fe3O4@ZIF-8 nanoparticles combining pH-responsive ZIF-8’s high porosity with Fe3O4’s magnetic guidability, enabling tumor-targeted DOX release under acidic conditions via external magnetic field guidance for precision chemotherapy [208]. Deng et al. engineered IrO2@ZIF-8/BSA-FA(Ce6) nanoplatform with catalase-mimicking IrO2 NPs and pH-responsive ZIF-8 for NIR/pH-triggered drug release, oxygen generation, and high-efficiency (62.1%) stable photothermal therapy [209].
MILs (Materials of Institute Lavoisier), developed using Fe/Cr clusters and carboxylate ligands, display superior thermal/chemical stability. Electronic modulation via ligand engineering enhances their nanozyme activity—NO-functionalized MIL-53(Fe) exhibits 10-fold increased oxidase activity compared to pristine counterparts, enabling ROS-mediated tumor cytotoxicity [240]. Co-doped MIL-101(Fe) improves Fenton-like catalysis for microenvironment-specific ROS generation [241]. Carbonized MIL-88C derivatives show potential as drug/gene carriers for OS targeting [242]. MIL-120(Al)-AP’s CO2 adsorption capacity may facilitate tumor pH modulation [243]. Wang et al. developed FA-BSA-targeted PDA-sealed Fe-MOF nanoparticles co-loaded with D-Arg/GOX/TPZ, enabling MRI-guided multimodal OS therapy through synergistic ST/CDT/GT/chemo under triple ROS/NO/free radical stress [206].
UiO frameworks, zirconium-based architectures with terephthalate linkers, demonstrate pharmaceutical stabilization capabilities. CA@UiO-66 enhances caffeic acid stability while inhibiting bacterial growth [244]. LysB/Rif@UiO-66 nanocomposites exhibit amplified antimicrobial effects through enzyme-antibiotic synergy [245]. Defect-engineered UiO-66 variants (Br-doped/aminated) improve drug loading efficiency and pollutant adsorption [246,247,248]. Current optimization focuses on hydrothermal stability enhancement for prolonged in vivo applications [249,250]. Emerging strategies combine UiO-66’s catalytic properties (H2/CO2 conversion) with antitumor functions through ROS-generating composites like UiO-66@Pt [251,252]. Chen et al. developed TPZ/PFA-loaded UiO-66@PDA nanoparticles via MOF encapsulation, utilizing high surface area/porosity for dual-drug delivery and PFA-enhanced hypoxia-triggered TPZ activation, inducing apoptosis through oxygen depletion [211].
4. Discussion
The rapid advancement of nanotechnology has revolutionized OS treatment by overcoming limitations of conventional therapies through multifunctional nanomaterials. This review systematically examines the functional characteristics and therapeutic applications of nanovesicles, polymers, and inorganic non-metallic/metallic materials in drug delivery, immunomodulation, catalytic therapy, thermal effects, and tissue engineering. For instance, stimulus-responsive nanocarriers enable spatiotemporal control of drug release, catalytic nanomaterials generate cytotoxic ROS via TME modulation, while biomimetic and bone-targeting strategies enhance site-specific accumulation. Immunomodulatory nanomaterials reshape immunosuppressive TME to activate antitumor immunity.
Notably, while most preclinical studies focus on OS models, emerging evidence reveals both shared efficacy and cancer-type-specific variations. Passive targeting via EPR effects, ligand-mediated active targeting, and stimulus-responsive mechanisms show broad applications in hepatocellular [253] and breast cancers [254]. Nanomaterial-mediated photothermal therapy achieves 78% tumor regression in OS xenografts, comparable to 72–80% efficacy in breast cancer PDX models [23]. However, OS-specific pathobiology fundamentally dictates unique therapeutic design requirements: Mineralized bone barriers: (1) OS requires bone-affinity strategies (e.g., hydroxyapatite-targeting ligands, polyphosphoinositol modifications) to penetrate calcified matrices, contrasting with vascular targeting in soft-tissue tumors. Hydroxyapatite-based systems exploit OS’s inherent mineral affinity, exemplified by Wu et al. HANPs showing 427% higher bone accumulation than conventional liposomes [129]. (2) Metastatic propensity management: With 85% pulmonary metastasis rates (vs. <30% in most solid tumors), nanoplatforms demand dual primary/metastatic targeting. The FePS3 nanoplatform ablates primary tumors via NIR-II photothermia while releasing anti-miR-19a to suppress lung metastasis pathways, demonstrating unparalleled “local therapy + systemic prevention” capacity [46]. (3) Immunosuppressive TME reprogramming: High Treg infiltration and PD-L1 expression necessitate nanomaterials integrating checkpoint blockade with immunogenic cell death induction. MPIRx nanoparticles co-delivering IR780/RRx-001 activate macrophage phagocytosis and M1 polarization, showing OS-specific “cold tumor” reprogramming efficacy [32]. Jiang’s IL-11Rα-targeted nanocomplex induces 3.2× greater T-cell infiltration in OS than hepatocellular models [100]. OS nanotherapy innovations thus center on bone targeting, antimetastasis, and osteoimmunomodulation synergies, contrasting melanoma’s reliance on antigen presentation [255] or leukemia’s focus on circulatory persistence [256].
Promising clinical candidates include (1) bone-targeted systems: ALN-LMWH-DOX-Lip reduces pulmonary nodules via dual targeting (ALN) and antimetastatic (LMWH) modifications, with 60% lower cardiotoxicity than free doxorubicin [68]. HA-DOPE@Lips/HNK achieves 83.8% tumor suppression with osteogenic differentiation, addressing post-resection regeneration needs [69]. (2) Stimulus-responsive platforms: pH/redox-dual responsive PTX-PLGA@[143B-RAW] achieves 90% tumor suppression via TME-triggered release and macrophage membrane camouflage [92]. (3) Immunomodulatory agents: MnO2@PA activates cGAS-STING, inducing 67.3% CD8+ T-cell infiltration in immunosuppressed models [22]. (4) Tissue-engineered systems: 3D-printed TCP-FePSe3 achieves 35.6-fold tumor reduction with concurrent bone regeneration [257]. Injectable MgO2-PEG hydrogel enables 67.6% tumor suppression via ultrasound-triggered cisplatin release and osteoinduction [113]. These platforms demonstrate mechanism specificity (targeting OS microenvironment/metastasis), validated efficacy (>70% suppression, >60% metastasis reduction), and clinical readiness (biocompatibility/degradability assessments).
Current challenges (Table 5) include scalable production of MOFs/biomimetic vesicles, long-term safety verification of metallic residues, and immunogenicity assessment of VLPs in primate models. Future directions should integrate AI-optimized nanomaterial design with TME-responsive probes, combining OS-specific targeting (bone ligands, metastasis inhibitors) with universal technologies (stimulus-responsiveness, immunomodulation) to accelerate clinical translation.

Table 5.
Comparison of advantages and limitations among different types of nanomaterials.
Author Contributions
Investigation, Z.Z.; writing—original draft preparation, Z.Z. and F.H.; writing—review and editing, C.D.; supervision, X.Q. and C.D.; funding acquisition, X.Q., C.D., W.L. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the National Natural Science Foundation of China (82200648), the Key Research and Development Program of Hubei (Grant Nos. 2022BCA050), the Open Research Fund of Hubei Province Key Laboratory of Precision Radiation Oncology (No. 2024ZLJZFL010), and the Nature Science Foundation of Hubei Province (2024AFB032).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The author thanks Xiaojuan Qin and Cheng Deng for their guidance and assistance and Fang He for their support in writing this paper. I completed the proofreading with my co-author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OS | Osteosarcoma |
| EPR | Enhanced Permeability and Retention |
| mTOR | Mammalian Target of Rapamycin |
| CAR-T | Chimeric Antigen Receptor T cell |
| PD-1 | Programmed Cell Death Protein 1 |
| DOX | Doxorubicin |
| miRNA | MicroRNA |
| HIF-1α | Hypoxia-Inducible Factor 1 alpha |
| pH | Power of Hydrogen |
| ROS | Reactive Oxygen Species |
| CDT | Chemodynamic Therapy |
| PDT | Photodynamic Therapy |
| PTT | Photothermal Therapy |
| MOFs | Metal–Organic Frameworks |
| PLGA | Poly(lactic-co-glycolic acid) |
| PDA | Polydopamine |
| TCP | Tricalcium Phosphate |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| CD47 | Cluster of Differentiation 47 |
| PD-L1 | Programmed Death-Ligand 1 |
| siRNA | Small Interfering RNA |
References
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21, vii320–vii325. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Van Tine, B.A. Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. J. Clin. Med. 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. Biomed. Eng. Online 2021, 20, 24. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef]
- Yu, S.; Yao, X. Advances on immunotherapy for osteosarcoma. Mol. Cancer 2024, 23, 192. [Google Scholar] [CrossRef]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef]
- Ji, T.; Zhao, Y.; Ding, Y.; Nie, G. Using functional nanomaterials to target and regulate the tumor microenvironment: Diagnostic and therapeutic applications. Adv. Mater. 2013, 25, 3508–3525. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Kong, H.; He, P.; Yang, G.; Zhu, D.; Guo, L.; Wei, G. Self-Assembled Peptide-Based Nanodrugs: Molecular Design, Synthesis, Functionalization, and Targeted Tumor Bioimaging and Biotherapy. Small 2023, 19, e2205787. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Song, S.; Zhang, H. Tumor Diagnosis and Therapy Mediated by Metal Phosphorus-Based Nanomaterials. Adv. Mater. 2021, 33, e2103936. [Google Scholar] [CrossRef]
- Xie, J.; Gong, L.; Zhu, S.; Yong, Y.; Gu, Z.; Zhao, Y. Emerging Strategies of Nanomaterial-Mediated Tumor Radiosensitization. Adv. Mater. 2019, 31, e1802244. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Wang, J.; Yao, F.; Tang, X.; Guo, W. Nanosized drug delivery strategies in osteosarcoma chemotherapy. APL Bioeng. 2023, 7, 011501. [Google Scholar] [CrossRef] [PubMed]
- Fatema, K.N.; Li, L.L.; Ahmad, K.; Kim, J.; Lee, D.W. Development of Multifunctional PAA-Alginate-Carboxymethyl Cellulose Hydrogel-Loaded Fiber-Reinforced Biomimetic Scaffolds for Controlled Release of Curcumin. Int. J. Biol. Macromol. 2025, 301, 140449. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hu, Z.; Zheng, W.; Qin, C.; Bai, X.; Yang, Q.; Zeng, T.; Mo, D.; Zhou, B.; Lu, C.; et al. GSH-Responsive Pt-Based Nanomotor with Improved Doxorubicin Delivery for Synergistic Osteosarcoma Chemotherapy. Acta Biomater. 2025, 195, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Huang, H.; Asghar, S.; Lin, L.; Chen, S.; Yuan, C.; Sang, M.; Xiao, Y. Design and evaluation of a multi-responsive dual-modality bone-targeted drug delivery vehicle for the treatment of osteosarcoma. Int. J. Pharm. 2025, 671, 125191. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, Y.; Li, B.; Wu, C.; Wang, D.; Xin, Y.; Xu, W.; Xiao, J. IL-11-Engineered Macrophage Membrane-Coated Reactive Oxygen Species-Responsive Nanoparticles for Targeted Delivery of Doxorubicin to Osteosarcoma. ACS Appl. Mater. Interfaces 2024, 16, 55981–55995. [Google Scholar] [CrossRef]
- Kaniewska, K.; Mackiewicz, M.; Smutok, O.; Gonchar, M.; Katz, E.; Karbarz, M. Enzymatically Triggered Drug Release from Microgels Controlled by Glucose Concentration. ACS Biomater. Sci. Eng. 2024, 10, 6415–6424. [Google Scholar] [CrossRef]
- Murphy, J.N.; Kobti, J.L.; Dao, M.; Wear, D.; Okoko, M.; Pandey, S.; Vukotic, V.N. Therapeutic coordination polymers: Tailoring drug release through metal-ligand interactions. Chem. Sci. 2024, 15, 7041–7050. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, C.; Cao, Z.; Ke, X. CPPs-modified chitosan as permeability-enhancing chemotherapeutic combined with gene therapy nanosystem by thermosensitive hydrogel for the treatment of osteosarcoma. Int. J. Biol. Macromol. 2024, 267 Pt 2, 130915. [Google Scholar] [CrossRef]
- Luo, S.; Zhao, C.; Wang, R.; Wu, D. Sequential drug release nanocomposites for synergistic therapy in disease treatment. J. Mater. Chem. B 2025, 13, 4313–4329. [Google Scholar] [CrossRef]
- Ju, Q.; Huang, R.; Hu, R.; Fan, J.; Zhang, D.; Ding, J.; Li, R. Phytic acid-modified manganese dioxide nanoparticles oligomer for magnetic resonance imaging and targeting therapy of osteosarcoma. Drug Deliv. 2023, 30, 2181743. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, L.; Sun, Z.; Song, D.; Cheng, Y. Targeted and intracellular delivery of protein therapeutics by a boronated polymer for the treatment of bone tumors. Bioact. Mater. 2022, 7, 333–340. [Google Scholar] [CrossRef]
- Liu, T.; Liang, X.; Liu, W.; Yang, S.; Cui, T.; Yan, F.; Li, Z. iRGD-Targeted Biosynthetic Nanobubbles for Ultrasound Molecular Imaging of Osteosarcoma. Int. J. Nanomed. 2025, 20, 791–805. [Google Scholar] [CrossRef]
- Liu, Y.J.; Dong, S.H.; Hu, W.H.; Chen, Q.L.; Zhang, S.F.; Song, K.; Han, Z.C.; Li, M.M.; Han, Z.T.; Liu, W.B.; et al. A multifunctional biomimetic nanoplatform for image-guideded photothermal-ferroptotic synergistic osteosarcoma therapy. Bioact. Mater. 2024, 36, 157–167. [Google Scholar] [CrossRef]
- Wei, X.; Feng, J.; Chen, L.; Zhang, C.; Liu, Y.; Zhang, Y.; Xu, Y.; Zhang, J.; Wang, J.; Yang, H.; et al. METTL3-Mediated m6A Modification of LINC00520 Confers Glycolysis and Chemoresistance in Osteosarcoma via Suppressing Ubiquitination of ENO1. Cancer Lett. 2024, 217194. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, T.; Zhou, W.; He, P.; Sun, Y.; Hu, S.; Chen, H.; Ma, X.; Peng, Y.; Yi, Z.; et al. Discovery of 2-Amino-3-Cyanothiophene Derivatives as Potent STAT3 Inhibitors for the Treatment of Osteosarcoma Growth and Metastasis. J. Med. Chem. 2022, 65, 6710–6728. [Google Scholar] [CrossRef]
- Liu, W.; Guo, M.; Hu, Y.; Chen, Y.; Wang, Y.; Nezamzadeh-Ejhieh, A.; Li, H.; Lu, C.; Liu, J. Recent advances in metal-based nanomaterials for malignant bone tumor therapy. Eur. J. Med. Chem. 2025, 288, 117427. [Google Scholar] [CrossRef]
- Yang, X.; Gao, S.; Yang, B.; Yang, Z.; Lou, F.; Huang, P.; Zhao, P.; Guo, J.; Fang, H.; Chu, B.; et al. Bioinspired Tumor-Targeting and Biomarker-Activatable Cell-Material Interfacing System Enhances Osteosarcoma Treatment via Biomineralization. Adv. Sci. 2023, 10, e2302272. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, Y.; Wang, Y.; Wu, Y.; Zhu, Y. Enhancing osteosarcoma therapy through aluminium hydroxide nanosheets-enabled macrophage modulation. Int. J. Pharm. 2024, 649, 123640. [Google Scholar] [CrossRef]
- Luo, T.; Fan, Z.; Zeng, A.; Wang, A.; Pan, Y.; Xu, Y.; Chen, H.; Chen, W.; Nie, D.; Lin, J.; et al. Biomimetic Targeted Co-Delivery System Engineered from Genomic Insights for Precision Treatment of Osteosarcoma. Adv. Sci. 2025, 12, e2410427. [Google Scholar] [CrossRef]
- Gong, M.; Huang, Y.; Feng, H.; Lin, J.; Huang, A.; Hu, J.; Tang, Q.; Zhu, X.; Han, S.; Lu, J.; et al. A Nanodrug Combining CD47 and Sonodynamic Therapy Efficiently Inhibits Osteosarcoma Deterioration. J. Control. Release 2023, 355, 68–84. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Ren, H.; Song, X.; Chen, L.; Yu, L.; Ren, J.; Chen, Y. An “Outer Piezoelectric and Inner Epigenetic” Logic-Gated PANoptosis for Osteosarcoma Sono-Immunotherapy and Bone Regeneration. Adv. Mater. 2025, 37, e2415814. [Google Scholar] [CrossRef]
- Lian, H.; Zhang, J.; Hou, S.; Ma, S.; Yu, J.; Zhao, W.; Zhao, D.; Zhang, Z. Immunotherapy of osteosarcoma based on immune microenvironment modulation. Front. Immunol. 2024, 15, 1498060. [Google Scholar] [CrossRef]
- Wei, Z.; Xia, K.; Liu, W.; Huang, X.; Wei, Z.; Guo, W. CGREF1 Modulates Osteosarcoma Proliferation by Regulating the Cell Cycle Through the Wnt/β-Catenin Signaling Pathway. Mol. Med. 2024, 30, 260. [Google Scholar] [CrossRef]
- Nie, J.J.; Zhang, B.; Luo, P.; Luo, M.; Luo, Y.; Cao, J.; Wang, H.; Mao, J.; Xing, Y.; Liu, W.; et al. Enhanced pyroptosis induction with pore-forming gene delivery for osteosarcoma microenvironment reshaping. Bioact. Mater. 2024, 38, 455–471. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samir, O.; Emam, H.E.; Soliman, A.; Abdelgalil, R.M.; Elmorshedy, Y.M.; Elkhodairy, K.A.; Nasr, M.L. Engineering nanomedicines for immunogenic eradication of cancer cells: Recent trends and synergistic approaches. Acta Pharm. Sin. B 2024, 14, 2475–2504. [Google Scholar] [CrossRef]
- Xia, J.; Hu, C.; Ji, Y.; Wang, M.; Jin, Y.; Ye, L.; Xie, D.; Jiang, S.; Li, R.; Hu, Z.; et al. Copper-Loaded Nanoheterojunction Enables Superb Orthotopic Osteosarcoma Therapy via Oxidative Stress and Cell Cuproptosis. ACS Nano. 2023, 17, 21134–21152. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Huang, J.; Tang, S.; Yang, J.; Xu, Z.; Xu, G.; Chen, X.; Liu, J.; Yang, C. High-performance pyrite nano-catalyst driven photothermal/chemodynamic synergistic therapy for Osteosarcoma. J. Nanobiotechnol. 2024, 22, 141. [Google Scholar] [CrossRef]
- Liang, Y.; Liao, C.; Guo, X.; Li, G.; Yang, X.; Yu, J.; Zhong, J.; Xie, Y.; Zheng, L.; Zhao, J. RhRu Alloy-Anchored MXene Nanozyme for Synergistic Osteosarcoma Therapy. Small 2023, 19, e2205511. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Chen, Y.; Yin, M.; Mäkilä, E.; Zhou, W.; Su, W.; Zhang, H. Metabolism-Regulating Nanozyme System for Advanced Nanocatalytic Cancer Therapy. Small 2024, 20, e2307794. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Ye, Y.; Chen, B.; Gao, J.; Liu, L.; Ou, J.; van Hest, J.C.M.; Liu, S.; Peng, F.; Tu, Y. Bone-Targeting Prodrug Mesoporous Silica-Based Nanoreactor with Reactive Oxygen Species Burst for Enhanced Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 34630–34642. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Yang, X.; Li, P.; Shi, W.; Pan, D.; Shen, L. W-Doped TiO2 Nanorods for Multimode Tumor Eradication in Osteosarcoma Models Under Single Ultrasound Irradiation. ACS Appl. Mater. Interfaces 2021, 13, 45325–45334. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.H.; Wang, D.D.; Qian, W.; Zhang, Z.L.; Ao, Y.W.; Li, J.M.; Huang, S.W. High-Efficiency Gold Nanoaggregates for NIR LED-Driven Sustained Mild Photothermal Therapy Achieving Complete Tumor Eradication and Immune Enhancement. Adv. Mater. 2025, 37, e2412191. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, C.; Yu, S.; Fan, Y.; Jiang, Y.; Zhou, R.; Yan, W.; Sun, Y. IOX1 Epigenetically Enhanced Photothermal Therapy of 3D-Printing Silicene Scaffolds Against Osteosarcoma with Favorable Bone Regeneration. Mater. Today Bio 2023, 23, 100887. [Google Scholar] [CrossRef]
- Luo, T.; Jiang, M.; Cheng, Z.; Lin, Y.; Chen, Y.; Zhang, Z.; Zhou, J.; Zhou, W.; Yu, X.F.; Li, S.; et al. Biodegradable FePS3 nanoplatform for efficient treatment of osteosarcoma by combination of gene and NIR-II photothermal therapy. J. Nanobiotechnol. 2023, 21, 224. [Google Scholar] [CrossRef]
- Ge, J.; Yang, N.; Yang, Y.; Yu, H.; Yang, X.; Wang, Y.; Wang, T.; Cheng, S.; Wang, Y.; Han, Z.; et al. The combination of eddy thermal effect of biodegradable magnesium with immune checkpoint blockade shows enhanced efficacy against osteosarcoma. Bioact. Mater. 2023, 25, 73–85. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, H.; Xu, Y.; Cao, Y.; Zheng, Y.; Liang, B. Engineering a triple-functional magnetic gel driving mutually-synergistic mild hyperthermia-starvation therapy for osteosarcoma treatment and augmented bone regeneration. J. Nanobiotechnol. 2023, 21, 201. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, G.; Guo, X.; Zhang, W.; Wu, C. Electrical Stimulation Promotes Endocytosis of Magnetic Nanoparticles by Cancer Cells. Small 2024, 20, e2403381. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Desimone, M.F.; Zhang, Y.S.A. Dolatshahi-Pirouz, and G. Orive, Nanomaterial-based drug delivery of immunomodulatory factors for bone and cartilage tissue engineering. Biomater. Adv. 2023, 154, 213637. [Google Scholar] [CrossRef]
- Shi, Q.; Lu, Y.; Zhang, G.; Yang, X.; Li, R.; Zhang, G.; Guo, X.; Song, J.; Ding, Q. Multifunctional Mesoporous Silica Nanoparticles for pH-Response and Photothermy Enhanced Osteosarcoma Therapy. Colloids Surf. B Biointerfaces 2022, 217, 112615. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xia, Y.; Zhuang, P.; Liu, W.; Mu, C.; Liu, Z.; Wang, J.; Chen, L.; Dai, H.; Luo, Z. FePSe3-Nanosheets-Integrated Cryogenic-3D-Printed Multifunctional Calcium Phosphate Scaffolds for Synergistic Therapy of Osteosarcoma. Small 2023, 19, e2303636. [Google Scholar] [CrossRef]
- Wang, L.; Dai, Z.; Bi, J.; Chen, Y.; Wang, Z.; Sun, Z.; Ji, Z.; Wang, H.; Zhang, Y.; Wang, L.; et al. Polydopamine-functionalized calcium-deficient hydroxyapatite 3D-printed scaffold with sustained doxorubicin release for synergistic chemo-photothermal therapy of osteosarcoma and accelerated bone regeneration. Mater. Today Bio 2024, 29, 101253. [Google Scholar] [CrossRef] [PubMed]
- Heng, C.; Zheng, X.; Hui, J.; Ma, X.; Fan, D. Neodymium and manganese ions co-doped whitlockite for temperature monitoring, photothermal therapy, and bone tissue repair in osteosarcoma. J. Colloid Interface Sci. 2024, 653 Pt B, 1488–1503. [Google Scholar] [CrossRef]
- Han, R.; Min, Y.; Li, G.; Chen, S.; Xie, M.; Zhao, Z. Supercritical CO2-Assisted Fabrication of CM-PDA/SF/nHA Nanofibrous Scaffolds for Bone Regeneration and Chemo-Photothermal Therapy Against Osteosarcoma. Biomater. Sci. 2023, 11, 5218–5231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Kang, Y.; Yao, B.; Dong, J.; Zhu, Y.; He, Y.; Ji, X. Genetically Engineered-Cell-Membrane Nanovesicles for Cancer Immunotherapy. Adv. Sci. 2023, 10, e2302131. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Su, Y.; Wang, Z. Engineering bacterial membrane nanovesicles for improved therapies in infectious diseases and cancer. Adv. Drug Deliv. Rev. 2022, 186, 114340. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Sun, J. Lipid Nanovesicles by Microfluidics: Manipulation, Synthesis, and Drug Delivery. Adv. Mater. 2019, 31, e1804788. [Google Scholar] [CrossRef]
- Sun, M.; Yang, J.; Fan, Y.; Zhang, Y.; Sun, J.; Hu, M.; Sun, K.; Zhang, J. Beyond Extracellular Vesicles: Hybrid Membrane Nanovesicles as Emerging Advanced Tools for Biomedical Applications. Adv. Sci. 2023, 10, e2303617. [Google Scholar] [CrossRef]
- Liu, J.; You, Q.; Liang, F.; Ma, L.; Zhu, L.; Wang, C.; Yang, Y. Ultrasound-nanovesicles interplay for theranostics. Adv. Drug Deliv. Rev. 2024, 205, 115176. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular vesicles: A rising star for therapeutics and drug delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, W.; Yu, N.; Zhang, L.; Wu, Z.; Chen, Y.; Li, Z.; Gong, F.; Li, N.; Chen, X.; et al. Activation of Dynamin-Related Protein 1 and Induction of Mitochondrial Apoptosis by Exosome-Rifampicin Nanoparticles Exerts Anti-Osteosarcoma Effect. Int. J. Nanomed. 2022, 17, 5431–5446. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Jin, L.; Guo, P.; Zhang, Z.; Zhanghuang, C.; Tan, X.; Mi, T.; Liu, J.; Wu, X.; et al. Exosome mimetics derived from bone marrow mesenchymal stem cells deliver doxorubicin to osteosarcoma in vitro and in vivo. Drug Deliv. 2022, 29, 3291–3303. [Google Scholar] [CrossRef]
- Wei, H.; Chen, F.; Chen, J.; Lin, H.; Wang, S.; Wang, Y.; Wu, C.; Lin, J.; Zhong, G. Mesenchymal Stem Cell Derived Exosomes as Nanodrug Carrier of Doxorubicin for Targeted Osteosarcoma Therapy via SDF1-CXCR4 Axis. Int. J. Nanomed. 2022, 17, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Z.; Yu, N.; Zhang, L.; Li, H.; Chen, Y.; Gong, F.; Lin, W.; He, X.; Wang, S.; et al. Bone-Targeting Exosome Nanoparticles Activate Keap1/Nrf2/GPX4 Signaling Pathway to Induce Ferroptosis in Osteosarcoma Cells. J. Nanobiotechnol. 2023, 21, 355. [Google Scholar] [CrossRef]
- Lu, J.; Chen, J.; Ye, J.; Shi, Z.; Gao, X.; Chen, P.; Chang, Y.; Lin, H.; Li, P. Dipsacus Asperoides-Derived Exosomes-Like Nanoparticles Inhibit the Progression of Osteosarcoma via Activating P38/JNK Signaling Pathway. Int. J. Nanomed. 2024, 19, 1097–1108. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Wan, J.; Chen, A.; Cheng, P.; Zhu, W. EphA2-specific microvesicles derived from tumor cells facilitate the targeted delivery of chemotherapeutic drugs for osteosarcoma therapy. J. Nanobiotechnol. 2024, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, Y.; Xu, D.; Ke, X.; Ci, T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromol. 2020, 164, 2583–2597. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Zhang, Y.; Huang, Q.; Feng, J.; Xing, H.; Fu, X.; Yan, X.; Zhang, Y.; Xu, Q.; et al. HA-DOPE-Modified Honokiol-Loaded Liposomes Targeted Therapy for Osteosarcoma. Int. J. Nanomed. 2022, 17, 5137–5151. [Google Scholar] [CrossRef]
- Zhu, W.T.; Zeng, X.F.; Yang, H.; Jia, M.L.; Zhang, W.; Liu, W.; Liu, S.Y. Resveratrol Loaded by Folate-Modified Liposomes Inhibits Osteosarcoma Growth and Lung Metastasis via Regulating JAK2/STAT3 Pathway. Int. J. Nanomed. 2023, 18, 2677–2691. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Zhang, C.; Yang, K.; Wang, Y.; Shan, S.; Yan, Y.; Dawson, K.A.; Wang, C.; Liang, X.-J. Transportation of AIE-visualized nanoliposomes is dominated by the protein corona. Natl. Sci. Rev. 2021, 8, nwab068. [Google Scholar] [CrossRef]
- Yan, J.; Wei, D.; Zhao, Z.; Sun, K.; Sun, Y. Osteosarcoma-targeting PtIV prodrug amphiphile for enhanced chemo-immunotherapy via Ca2+ trapping. Acta Biomater. 2025, 193, 474–483. [Google Scholar] [CrossRef]
- Turánek, J.; Kosztyu, P.; Turánek Knötigová, P.; Bartheldyová, E.; Hubatka, F.; Odehnalová, N.; Mikulík, R.; Vaškovicová, N.; Čelechovská, H.; Kratochvílová, I.; et al. Long circulating liposomal platform utilizing hydrophilic polymer-based surface modification: Preparation, characterisation, and biological evaluation. Int. J. Pharm. 2024, 661, 124465. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Cao, X.; Zheng, L.; Liu, S.; Li, L.; Cheng, L.; Tian, T.; Tong, X.; Wang, H.; Jiang, L. Liposomes decorated with β-conglycinin and glycinin: Construction, structure and in vitro digestive stability. Int. J. Biol. Macromol. 2024, 269, 131900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, C.; Wang, T.; Sun, L.; Wu, F.; Yu, D. Combined multispectral analysis and molecular docking to research the interaction of soybean isolate protein with different kinds of phospholipid liposomes and its effect on liposome properties. Food Chem. 2025, 474, 143160. [Google Scholar] [CrossRef]
- Chen, X.-A.; Chuang, C.-C.; Chen, C.-C.; Lee, C.-Y.; Chin, C.-Y.; Young, J.-J.; Bai, M.-Y.; Chuang, C.-C. Polyelectrolyte-coated liposomes microfluidically assembled in one-step for enhancing cell endocytosis and in-vivo immune responses. Colloids Surf. B Biointerfaces 2024, 241, 114030. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Yuan, Y.; Zhang, D.; Wang, Z.; Qi, B.; Jiang, P.; Yu, A. Synergistic photothermal-sonodynamic therapy for antibacterial and immune reprogramming in chronic osteomyelitis. J. Control. Release 2025, 381, 113612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Luo, Y.; Xu, J.; Zhang, B.; Feng, P.; Guo, C.; Wang, Y.; Huang, Z.; Kong, Q.; et al. Reactivating P53 to treat osteosarcoma: A tetrahedral framework nucleic acids-based approach. Int. J. Biol. Macromol. 2025, 304, 140765. [Google Scholar] [CrossRef]
- Kodel, H.d.A.C.; Alizadeh, P.; Ebrahimi, S.N.; Machado, T.O.X.; Oliveira, M.B.P.P.; Fathi, F.; Souto, E.B. Liposomes and Niosomes: New trends and applications in the delivery of bioactive agents for cancer therapy. Int. J. Pharm. 2025, 668, 124994. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Chi, X.; Yu, S.; He, W.; He, H.; Wang, G.; Hao, K.; Zhang, J. Modeling based dynamics mechanism and pathway of liposome penetration in multicellular tumor spheroid for liposome optimization. Int. J. Pharm. 2025, 671, 125237. [Google Scholar] [CrossRef]
- Han, Z.; Peng, X.; Yang, Y.; Yi, J.; Zhao, D.; Bao, Q.; Long, S.; Yu, S.-X.; Xu, X.-X.; Liu, B.; et al. Integrated microfluidic-SERS for exosome biomarker profiling and osteosarcoma diagnosis. Biosens. Bioelectron. 2022, 217, 114709. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.F.F.; Fonseca, A.; Sereno, J.; Ferreira, H.R.S.; Lapo-Pais, M.; Martins-Marques, T.; Rodrigues, T.; Oliveira, R.C.; Miranda, C.; Almeida, L.P.; et al. Osteosarcoma-Derived Exosomes as Potential PET Imaging Nanocarriers for Lung Metastasis. Small 2022, 18, e2203999. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Meng, X.; Yang, M.; Chen, G.; Li, J.; Zhu, Z.; Wu, X.; Hu, W.; Tian, M.; Li, T.; et al. NGR-Modified CAF-Derived exos Targeting Tumor Vasculature to Induce Ferroptosis and Overcome Chemoresistance in Osteosarcoma. Adv. Sci. 2025, 12, 2410918. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jike, Y.; Liu, K.; Gan, F.; Zhang, K.; Xie, M.; Zhang, J.; Chen, C.; Zou, X.; Jiang, X.; et al. Exosome-mediated miR-144-3p promotes ferroptosis to inhibit osteosarcoma proliferation, migration, and invasion through regulating ZEB1. Mol. Cancer 2023, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, L.; Bai, X.; Zhang, M.; Lv, W.; Ma, Y.; Sun, Y.; Zhang, H.; Jiang, Q.; Yao, Q.; et al. Osteosarcoma Cell-Derived Migrasomes Promote Macrophage M2 Polarization to Aggravate Osteosarcoma Proliferation and Metastasis. Adv. Sci. 2025, 12, 2409870. [Google Scholar] [CrossRef]
- Huang, X.; Wu, W.; Jing, D.; Yang, L.; Guo, H.; Wang, L.; Zhang, W.; Pu, F.; Shao, Z. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J. Control. Release Off. J. Control. Release Soc. 2022, 343, 107–117. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, J.; Yu, L.; Guo, T.; Wang, J.; Wang, X.; Chen, Y. PD-L1+ exosomes from bone marrow-derived cells of tumor-bearing mice inhibit antitumor immunity. Cell. Mol. Immunol. 2021, 18, 2402–2409. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Sun, X.; Wang, X.; Ren, T.; Huang, Y.; Zhang, R.; Zheng, B.; Guo, W. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J. Nanobiotechnology 2020, 18, 151. [Google Scholar] [CrossRef]
- Suffian, I.F.B.M.; Al-Jamal, K.T. Bioengineering of virus-like particles as dynamic nanocarriers for in vivo delivery and targeting to solid tumours. Adv. Drug Deliv. Rev. 2022, 180, 114030. [Google Scholar] [CrossRef]
- Yin, D.; Zhong, Y.; Ling, S.; Lu, S.; Wang, X.; Jiang, Z.; Wang, J.; Dai, Y.; Tian, X.; Huang, Q.; et al. Dendritic-cell-targeting virus-like particles as potent mRNA vaccine carriers. Nat. Biomed. Eng. 2025, 9, 185–200. [Google Scholar] [CrossRef]
- Morales-Molina, A.; Gambera, S.; Leo, A.; García-Castro, J. Combination immunotherapy using G-CSF and oncolytic virotherapy reduces tumor growth in osteosarcoma. J. Immunother. Cancer 2021, 9, e001703. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.X.; Liu, J.H.; Wu, J.Y.; Li, Y.J.; Qiu, X.H.; Xu, W.J.; Xu, P.; Xiang, D.-X. Hybrid Cell Membrane-Functionalized Biomimetic Nanoparticles for Targeted Therapy of Osteosarcoma. Int. J. Nanomedicine 2022, 17, 837–854. [Google Scholar] [CrossRef]
- Alsulays, B.B.; Aodah, A.H.; Ahmed, M.M.; Anwer, M.K. Preparation and Evaluation of Chitosan Coated PLGA Nanoparticles Encapsulating Ivosidenib with Enhanced Cytotoxicity Against Human Liver Cancer Cells. Int. J. Nanomedicine 2024, 19, 3461–3473. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, S.; Chen, S.; Ma, T.; Chen, M.; Niu, C.; Leng, Y.; Wang, L. Bacterial outer membrane vesicle-cancer cell hybrid membrane-coated nanoparticles for sonodynamic therapy in the treatment of breast cancer bone metastasis. J. Nanobiotechnology 2024, 22, 328. [Google Scholar] [CrossRef]
- Crisafulli, E.; Scalzone, A.; Tonda-Turo, C.; Girón-Hernández, J.; Gentile, P. Multimodal layer-by-layer nanoparticles: A breakthrough in gene and drug delivery for osteosarcoma. J. Mater. Chem. B 2024, 12, 12540–12552. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, H.; Zhang, P.; Zhang, Z. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Deliv. 2020, 27, 1044–1053. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhao, G.; Zhang, Y.; Zhan, F.; Chen, Z.; He, T.; Cao, Y.; Hao, L.; Wang, Z.; et al. Homologous targeting nanoparticles for enhanced PDT against osteosarcoma HOS cells and the related molecular mechanisms. J. Nanobiotechnology 2022, 20, 83. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wang, L.; Yu, J.; Lu, G.; Zhao, W.; Miao, C.; Zou, C.; Wu, J. Overcoming therapeutic failure in osteosarcoma via Apatinib-encapsulated hydrophobic poly(ester amide) nanoparticles. Biomater. Sci. 2020, 8, 5888–5899. [Google Scholar] [CrossRef]
- Qiu, R.; Sun, D.; Bai, Y.; Li, J.; Wang, L. Application of tumor-targeting peptide-decorated polypeptide nanoparticles with doxorubicin to treat osteosarcoma. Drug Deliv. 2020, 27, 1704–1717. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, R.; Yang, L.; Sha, Y.; Zhao, S.; Guo, J.; Chen, D.; Zhong, Z.; Meng, F. IL-11Rα-targeted nanostrategy empowers chemotherapy of relapsed and patient-derived osteosarcoma. J. Control. Release Off. J. Control. Release Soc. 2022, 350, 460–470. [Google Scholar] [CrossRef]
- Li, M.; Lin, Z.-I.; Yang, J.; Huang, H.; Liu, G.-L.; Liu, Q.; Zhang, X.; Zhang, Y.; Xu, Z.; Lin, H.; et al. Biodegradable Carbon Dioxide-Derived Non-Viral Gene Vectors for Osteosarcoma Gene Therapy. Adv. Healthc. Mater. 2023, 12, 2201306. [Google Scholar] [CrossRef] [PubMed]
- Mpekris, F.; Panagi, M.; Michael, C.; Voutouri, C.; Tsuchiya, M.; Wagatsuma, C.; Kinoh, H.; Osada, A.; Akinaga, S.; Yoshida, S.; et al. Translational nanomedicine potentiates immunotherapy in sarcoma by normalizing the microenvironment. J. Control. Release Off. J. Control. Release Soc. 2023, 353, 956–964. [Google Scholar] [CrossRef]
- Lou, H.; Ji, A.; Qu, C.; Liu, H.; Jiang, L.; Chen, H.; Cheng, Z. A Small-Molecule Based Organic Nanoparticle for Photothermal Therapy and Near-Infrared-IIb Imaging. ACS Appl. Mater. Interfaces 2022, 14, 35454–35465. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Diao, S.; Ni, X.; Zhang, D.; Yi, W.; Jian, C.; Hu, X.; Li, D.; Yu, A.; Zhou, W.; et al. Peptide-based semiconducting polymer nanoparticles for osteosarcoma-targeted NIR-II fluorescence/NIR-I photoacoustic dual-model imaging and photothermal/photodynamic therapies. J. Nanobiotechnology 2022, 20, 44. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Tong, S.; Sun, Y.; Zhang, Y.; Zhang, J.; Zhao, D.; Su, Y.; Ding, J.; Chen, X. Acidity-Triggered Transformable Polypeptide Self-Assembly to Initiate Tumor-Specific Biomineralization. Adv. Mater. 2023, 35, 2203291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, J.; Luo, W.; Wen, C.; Mao, W.; Yang, Y.; Liu, C.; Xu, Y.; Chen, W.; Wen, L. Mitochondria targeted biomimetic platform for chemo/photodynamic combination therapy against osteosarcoma. Int. J. Pharm. 2024, 652, 123865. [Google Scholar] [CrossRef]
- Si, M.; Xia, Y.; Cong, M.; Wang, D.; Hou, Y.; Ma, H. In situ Co-Delivery of Doxorubicin and Cisplatin by Injectable Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment. Int. J. Nanomedicine 2022, 17, 1309–1322. [Google Scholar] [CrossRef]
- Freeman, F.E.; Dosta, P.; Shanley, L.C.; Ramirez Tamez, N.; Riojas Javelly, C.J.; Mahon, O.R.; Kelly, D.J.; Artzi, N. Localized Nanoparticle-Mediated Delivery of miR-29b Normalizes the Dysregulation of Bone Homeostasis Caused by Osteosarcoma whilst Simultaneously Inhibiting Tumor Growth. Adv. Mater. 2023, 35, e2207877. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wei, R.; Zhang, J.; Zhu, J.; Wang, W.; Wang, Z.; Wupur, Z.; Li, Y.; Meng, H. Synergistic Chemoimmunotherapy Augmentation via Sequential Nanocomposite Hydrogel-Mediated Reprogramming of Cancer-Associated Fibroblasts in Osteosarcoma. Adv. Mater. 2024, 36, e2309591. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, X.; Zhao, K.; Zhang, S.; Ren, K.; Mu, M.; Wang, C.; Wang, X.; Liu, H.; Dong, J.; et al. Polydopamine Nanostructure-Enhanced Water Interaction with pH-Responsive Manganese Sulfide Nanoclusters for Tumor Magnetic Resonance Contrast Enhancement and Synergistic Ferroptosis-Photothermal Therapy. ACS Nano 2024, 18, 3369–3381. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, C.; Liu, J.; Yuan, Y.; Yao, C.; Liu, M.; Deng, L.; Sun, J.; Chen, Z.; Wang, L.; et al. Tumor specific in situ synthesis of therapeutic agent for precision cancer therapy. J. Nanobiotechnology 2024, 22, 612. [Google Scholar] [CrossRef]
- Li, B.; Liu, F.; Ye, J.; Cai, X.; Qian, R.; Zhang, K.; Zheng, Y.; Wu, S.; Han, Y. Regulation of Macrophage Polarization Through Periodic Photo-Thermal Treatment to Facilitate Osteogenesis. Small 2022, 18, e2202691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, L.; Wang, Z.; Nie, Y.; Yang, Y.; Li, C.; Zhang, Y.; Jiang, Y.; Kou, Y.; Zhang, W.; et al. Injectable and adhesive MgO2-potentiated hydrogel with sequential tumor synergistic therapy and osteogenesis for challenging postsurgical osteosarcoma treatment. Biomaterials 2025, 315, 122959. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, R.; Fu, R.; Yu, P.; Guo, J.; Li, G.; Wang, Z.; Wang, H.; Nie, J.; Liu, W.; et al. Piezo-enhanced near infrared photocatalytic nanoheterojunction integrated injectable biopolymer hydrogel for anti-osteosarcoma and osteogenesis combination therapy. Bioact. Mater. 2024, 34, 381–400. [Google Scholar] [CrossRef]
- Xie, D.; Hu, C.; Zhu, Y.; Yao, J.; Li, J.; Xia, J.; Ye, L.; Jin, Y.; Jiang, S.; Hu, T.; et al. Sequential Therapy for Osteosarcoma and Bone Regeneration via Chemodynamic Effect and Cuproptosis Using a 3D-Printed Scaffold with TME-Responsive Hydrogel. Small 2025, 21, 2406639. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.; Nie, Y.; Du, X.; Huang, C.; Li, L.; Long, J.; Wang, X.; Tong, W.; Qin, L.; et al. Time-Sequential and Multi-Functional 3D Printed MgO2/PLGA Scaffold Developed as a Novel Biodegradable and Bioactive Bone Substitute for Challenging Postsurgical Osteosarcoma Treatment. Adv. Mater. 2024, 36, e2308875. [Google Scholar] [CrossRef]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 2023, 14, 759. [Google Scholar] [CrossRef]
- Ma, Y.; Lai, P.; Sha, Z.; Li, B.; Wu, J.; Zhou, X.; He, C.; Ma, X. TME-responsive nanocomposite hydrogel with targeted capacity for enhanced synergistic chemoimmunotherapy of MYC-amplified osteosarcoma. Bioact. Mater. 2025, 47, 83–99. [Google Scholar] [CrossRef]
- Wu, H.; Chen, C.; Li, J.; Yu, D.; Wu, X.; Huang, H.; Tang, Z.; Wu, Q.; Yan, S.; Wang, N.; et al. Engineered Magneto-Piezoelectric Nanoparticles-Enhanced Scaffolds Disrupt Biofilms and Activate Oxidative Phosphorylation in Icam1+ Macrophages for Infectious Bone Defect Regeneration. ACS Nano 2024, 18, 35575–35594. [Google Scholar] [CrossRef]
- Chu, X.; Mi, B.; Xiong, Y.; Wang, R.; Liu, T.; Hu, L.; Yan, C.; Zeng, R.; Lin, J.; Fu, H.; et al. Bioactive nanocomposite hydrogel enhances postoperative immunotherapy and bone reconstruction for osteosarcoma treatment. Biomaterials 2025, 312, 122714. [Google Scholar] [CrossRef]
- Costa, S.; Rodrigues, J.; Vieira, C.; Dias, S.; Viegas, J.; Castro, F.; Sarmento, B.; Leite Pereira, C. Advancing osteosarcoma 3D modeling in vitro for novel tumor microenvironment-targeted therapies development. J. Control. Release 2024, 376, 1068–1085. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pang, X.; Li, Y.; Yu, X. Ultrafast Self-Healing, Superstretchable, and Ultra-Strong Polymer Cluster-Based Adhesive Based on Aromatic Acid Cross-Linkers for Excellent Hydrogel Strain Sensors. Small 2024, 20, 2305875. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, L.; Wang, C.; Wu, J.; Zhu, B.; Meng, X.; Qiu, D. Reinforcing Hydrogel by Nonsolvent-Quenching-Facilitated In Situ Nanofibrosis. Adv. Mater. 2023, 35, 2303728. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, C.; Qi, L.; Liu, Z.; Xu, R.; Tu, C.; Li, Z. RFWD3 Reprograms Nucleotide Metabolism Through PHGDH to Induce Chemoresistance In Osteosarcoma. Adv. Sci. 2025, 12, 2410937. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Li, H.; Niu, X.; Zhang, D.; Wang, K. Antimicrobial Hydrogels: Potential Materials for Medical Application. Small 2024, 20, 2304047. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Wang, Q.; Li, G.; Wan, B.; Sun, Y.; Niu, X.; Chen, D.; Tian, W. Anti-osteosarcoma effect of hydroxyapatite nanoparticles both in vitro and in vivo by downregulating the FAK/PI3K/Akt signaling pathway. Biomater. Sci. 2020, 8, 4426–4437. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, Z.; Gao, J.; Wu, F.; Sun, B.; Lian, M.; Qian, J.; Su, Y.; Zhu, X.; Zhu, B. Hydroxyapatite-Bovine Serum Albumin-Paclitaxel Nanoparticles for Locoregional Treatment of Osteosarcoma. Adv. Healthc. Mater. 2021, 10, e2000573. [Google Scholar] [CrossRef]
- Liu, Y.; Nadeem, A.; Sebastian, S.; Olsson, M.A.; Wai, S.N.; Styring, E.; Engellau, J.; Isaksson, H.; Tägil, M.; Lidgren, L.; et al. Bone mineral: A trojan horse for bone cancers. Efficient mitochondria targeted delivery and tumor eradication with nano hydroxyapatite containing doxorubicin. Mater. Today Bio 2022, 14, 100227. [Google Scholar] [CrossRef]
- Wu, H.; Liu, S.; Chen, S.; Hua, Y.; Li, X.; Zeng, Q.; Zhou, Y.; Yang, X.; Zhu, X.; Tu, C.; et al. A Selective Reduction of Osteosarcoma by Mitochondrial Apoptosis Using Hydroxyapatite Nanoparticles. Int. J. Nanomedicine 2022, 17, 3691–3710. [Google Scholar] [CrossRef]
- Wu, H.; Wang, R.; Li, S.; Chen, S.; Liu, S.; Li, X.; Yang, X.; Zeng, Q.; Zhou, Y.; Zhu, X.; et al. Aspect ratio-dependent dual-regulation of the tumor immune microenvironment against osteosarcoma by hydroxyapatite nanoparticles. Acta Biomater. 2023, 170, 427–441. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, Y.; Tan, S.; Zhu, K.; Cai, T.; Zhan, T.; He, S.; Chen, F.; Zhang, C. Selenium-doped calcium phosphate biomineral reverses multidrug resistance to enhance bone tumor chemotherapy. Nanomedicine Nanotechnol. Biol. Med. 2021, 32, 102322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Tsai, S.; Chen, M.-H.; Hsieh, F.-Y.; Chang, Y.-C.; Tung, F.-I.; Liu, T.-Y. Synthesis and Functionalization of Graphene Materials for Biomedical Applications: Recent Advances, rcoma. ACS Appl. Mater. Interfaces 2022, 14, 5586–5597. [Google Scholar] [CrossRef] [PubMed]
- AbouAitah, K.; Soliman, A.A.F.; Swiderska-Sroda, A.; Nassrallah, A.; Smalc-Koziorowska, J.; Gierlotka, S.; Lojkowski, W. Co-Delivery System of Curcumin and Colchicine Using Functionalized Mesoporous Silica Nanoparticles Promotes Anticancer and Apoptosis Effects. Pharmaceutics 2022, 14, 2770. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Habibovic, P.; Rijt, S. van Selenium-incorporated mesoporous silica nanoparticles for osteosarcoma therapy. Biomater. Sci. 2023, 11, 3828–3839. [Google Scholar] [CrossRef]
- Ye, L.; Yu, C.; Xia, J.; Ni, K.; Zhang, Y.; Ying, X.; Xie, D.; Jin, Y.; Sun, R.; Tang, R.; et al. Multifunctional nanomaterials via cell cuproptosis and oxidative stress for treating osteosarcoma and OS-induced bone destruction. Mater. Today Bio 2024, 25, 100996. [Google Scholar] [CrossRef]
- Hu, M.; Fang, J.; Zhang, Y.; Wang, X.; Zhong, W.; Zhou, Z. Design and evaluation a kind of functional biomaterial for bone tissue engineering: Selenium/mesoporous bioactive glass nanospheres. J. Colloid Interface Sci. 2020, 579, 654–666. [Google Scholar] [CrossRef]
- Irani, M.; Mir Mohamad Sadeghi, G.; Haririan, I. A novel biocompatible drug delivery system of chitosan/temozolomide nanoparticles loaded PCL-PU nanofibers for sustained delivery of temozolomide. Int. J. Biol. Macromol. 2017, 97, 744–751. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Berkmann, J.C.; Giasafaki, D.; Lozano, D.; Spyrou, K.; Manzano, M.; Steriotis, T.; Duda, G.N.; Schmidt-Bleek, K.; Charalambopoulou, G.; et al. Engineered pH-Responsive Mesoporous Carbon Nanoparticles for Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 14946–14957. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Wu, W.; Yang, W.; Zhong, B.; Qing, X.; Shao, Z. Delivery of MutT homolog 1 inhibitor by functionalized graphene oxide nanoparticles for enhanced chemo-photodynamic therapy triggers cell death in osteosarcoma. Acta Biomater. 2020, 109, 229–243. [Google Scholar] [CrossRef]
- Giusto, E.; Žárská, L.; Beirne, D.F.; Rossi, A.; Bassi, G.; Ruffini, A.; Montesi, M.; Montagner, D.; Ranc, V.; Panseri, S. Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy. Nanomaterials 2022, 12, 2372. [Google Scholar] [CrossRef]
- Xu, Y.; Du, L.; Han, B.; Wang, Y.; Fei, J.; Xia, K.; Zhai, Y.; Yu, Z. Black phosphorus quantum dots camouflaged with platelet-osteosarcoma hybrid membrane and doxorubicin for combined therapy of osteosarcoma. J. Nanobiotechnology 2023, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Consoli, G.M.L.; Maugeri, L.; Musso, N.; Gulino, A.; D’Urso, L.; Bonacci, P.; Buscarino, G.; Forte, G.; Petralia, S. One-Pot Synthesis of Luminescent and Photothermal Carbon Boron-Nitride Quantum Dots Exhibiting Cell Damage Protective Effects. Adv. Healthc. Mater. 2024, 13, e2303692. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.; Fasolino, I.; Borsacchi, S.; Valente, C.; Calucci, L.; Turacchio, G.; Pannico, M.; Serrano-Ruiz, M.; Ambrosio, L.; Raucci, M.G. A theragenerative bio-nanocomposite consisting of black phosphorus quantum dots for bone cancer therapy and regeneration. Bioact. Mater. 2024, 35, 99–121. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, M.; Rui, L.; Huan, X.; Li, Y.; Huang, Y.; Wei, W. Polystyrene microplastics as carriers for nano-hydroxyapatite particles: Impact of surface functionalization and mechanistic insights. J. Hazard. Mater. 2024, 479, 135680. [Google Scholar] [CrossRef]
- Verma, R.; Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Hydroxyapatite-based composites: Excellent materials for environmental remediation and biomedical applications. Adv. Colloid Interface Sci. 2023, 315, 102890. [Google Scholar] [CrossRef]
- Huang, H.; Qiang, L.; Fan, M.; Liu, Y.; Yang, A.; Chang, D.; Li, J.; Sun, T.; Wang, Y.; Guo, R.; et al. 3D-printed tri-element-doped hydroxyapatite/polycaprolactone composite scaffolds with antibacterial potential for osteosarcoma therapy and bone regeneration. Bioact. Mater. 2024, 31, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tan, J.; Xu, R.; Du, H.; Xie, J.; Peng, F.; Liu, X. Collaborative Design of MgO/FeOx Nanosheets on Titanium: Combining Therapy with Regeneration. Small 2023, 19, 2204852. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, C.; Song, H.; Feng, X.; Ma, L.; Zhang, X.; Li, G.; Mu, C.; Tan, L.; Zhang, Z.; et al. Biomimetic bone-periosteum scaffold for spatiotemporal regulated innervated bone regeneration and therapy of osteosarcoma. J. Nanobiotechnology 2024, 22, 250. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, L.; Ma, C.; Zhang, S.; Lin, S.; Zhang, L.W.; Wang, Y.; Gao, M. Chemotherapy-Sensitized In Situ Vaccination for Malignant Osteosarcoma Enabled by Bioinspired Calcium Phosphonate Nanoagents. ACS Nano 2023, 17, 6247–6260. [Google Scholar] [CrossRef]
- Parra-Torrejón, B.; Cáceres, A.; Sánchez, M.; Sainz, L.; Guzmán, M.; Bermúdez-Perez, F.J.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Multifunctional Nanomaterials for Biofortification and Protection of Tomato Plants. Environ. Sci. Technol. 2023, 57, 14950–14960. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, M.; Jiang, Y.; Zhang, X.; Li, J.; Zhu, Y.; Yao, Q. Calcium-based biomaterials: Unveiling features and expanding applications in osteosarcoma treatment. Bioact. Mater. 2024, 32, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wen, Z.; Liu, Z.; Yang, Y.; Wang, H.; Xia, X.; Ye, J.; Liu, Y. Unlocking the potential of amorphous calcium carbonate: A star ascending in the realm of biomedical application. Acta Pharm. Sin. B 2024, 14, 602–622. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, Y.-J.; Sui, C.; Zhao, Y.; Mao, L.-B.; Gebauer, D.; Rosenberg, R.; Avaro, J.; Wu, Y.-D.; Gao, H.-L.; et al. Highly hydrated paramagnetic amorphous calcium carbonate nanoclusters as an MRI contrast agent. Nat. Commun. 2022, 13, 5088. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, W.; Zhu, X.; Liu, Z.; Liu, M.; Liu, S.; Li, B.; Chen, Y.; Wang, Z.; et al. Biomimetic-gasdermin-protein-expressing nanoplatform mediates tumor-specific pyroptosis for cancer immunotherapy. J. Control. Release Off. J. Control. Release Soc. 2024, 367, 61–75. [Google Scholar] [CrossRef]
- Nicholas, T.C.; Stones, A.E.; Patel, A.; Michel, F.M.; Reeder, R.J.; Aarts, D.G.A.L.; Deringer, V.L.; Goodwin, A.L. Geometrically frustrated interactions drive structural complexity in amorphous calcium carbonate. Nat. Chem. 2024, 16, 36–41. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Zhao, Y.; Guan, Q.-F.; Yang, S.-Y.; Wang, W.; Yan, B.-B.; Meng, Y.-F.; Li, S.-C.; Tang, P.-P.; Mao, L.-B.; et al. Amorphous Calcium Carbonate Cluster Nanospheres in Water-Deficient Organic Solvents. Angew. Chem. Int. Ed. 2022, 61, e202211254. [Google Scholar] [CrossRef] [PubMed]
- Taheriazam, A.; Abad, G.G.Y.; Hajimazdarany, S.; Imani, M.H.; Ziaolhagh, S.; Zandieh, M.A.; Bayanzadeh, S.D.; Mirzaei, S.; Hamblin, M.R.; Entezari, M.; et al. Graphene oxide nanoarchitectures in cancer biology: Nano-modulators of autophagy and apoptosis. J. Control. Release Off. J. Control. Release Soc. 2023, 354, 503–522. [Google Scholar] [CrossRef]
- Rahimi, S.; Chen, Y.; Zareian, M.; Pandit, S.; Mijakovic, I. Cellular and subcellular interactions of graphene-based materials with cancerous and non-cancerous cells. Adv. Drug Deliv. Rev. 2022, 189, 114467. [Google Scholar] [CrossRef]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Luo, H.; Luo, B.; Zhao, F.; Kang, Y.; Zhang, Y.; Shao, L. Nanographene Oxide Promotes Angiogenesis by Regulating Osteoclast Differentiation and Platelet-Derived Growth Factor Secretion. ACS Nano 2024, 18, 22390–22403. [Google Scholar] [CrossRef]
- An, N.; Yan, X.; Qiu, Q.; Zhang, Z.; Zhang, X.; Zheng, B.; Zhao, Z.; Guo, J.; Liu, Y. Human periodontal ligament stem cell sheets activated by graphene oxide quantum dots repair periodontal bone defects by promoting mitochondrial dynamics dependent osteogenic differentiation. J. Nanobiotechnology 2024, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Pang, Y.X.; Yan, Y.; Qian, P.; Zhao, H.; Manickam, S.; Wu, T.; Pang, C.H. Synthesis and Functionalization of Graphene Materials for Biomedical Applications: Recent Advances, Challenges, and Perspectives. Adv. Sci. 2023, 10, 2205292. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Qu, H.; Huang, X.; Shi, Y.; Li, K.; Lin, W.; Xu, Z.; Li, D.; Chen, H.; Gao, L.; et al. Manganese Amplifies Photoinduced ROS in Toluidine Blue Carbon Dots to Boost MRI Guided Chemo/Photodynamic Therapy. Small 2024, 20, 2304968. [Google Scholar] [CrossRef] [PubMed]
- Cillari, R.; Acúrcio, R.C.; Barateiro, A.; Florindo, H.F.; Mauro, N.; Cavallaro, G. Harnessing sulfur-doped carbon nanodots conjugated with IDO inhibitors act as a dual-mode breast cancer immunotherapy. J. Controlled Release 2025, 381, 113575. [Google Scholar] [CrossRef]
- Zhong, T.; Yang, Y.; Pang, M.; Pan, Y.; Jing, S.; Qi, Y.; Huang, Y. Human Serum Albumin-Coated 10B Enriched Carbon Dots as Targeted “Pilot Light” for Boron Neutron Capture Therapy. Adv. Sci. 2024, 11, 2406577. [Google Scholar] [CrossRef]
- Tang, J.; Hu, J.; Bai, X.; Wang, Y.; Cai, J.; Zhang, Z.; Geng, B.; Pan, D.; Shen, L. Near-Infrared Carbon Dots With Antibacterial and Osteogenic Activities for Sonodynamic Therapy of Infected Bone Defects. Small 2024, 20, e2404900. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Gong, X. The Emerging Star of Carbon Luminescent Materials: Exploring the Mysteries of the Nanolight of Carbon Dots for Optoelectronic Applications. Small 2024, 20, 2400107. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Dheyab, M.A.; Nafchi, A.M.; Ghasemlou, M.; Ivanova, E.P.; Adhikari, B. Turning food waste into value-added carbon dots for sustainable food packaging application: A review. Adv. Colloid Interface Sci. 2023, 321, 103020. [Google Scholar] [CrossRef]
- García-Hevia, L.; Soltani, R.; González, J.; Chaloin, O.; Ménard-Moyon, C.; Bianco, A.; Fanarraga, M.L. Carbon nanotubes targeted to the tumor microenvironment inhibit metastasis in a preclinical model of melanoma. Bioact. Mater. 2024, 34, 237–247. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Z.; Wang, T.; Law, J.; Chen, X.; Wanggou, S.; Wang, J.; Ying, B.; Francisco, M.; Dong, W.; et al. Mechanical nanosurgery of chemoresistant glioblastoma using magnetically controlled carbon nanotubes. Sci. Adv. 2023, 9, eade5321. [Google Scholar] [CrossRef]
- Cheng, M.; Kong, Q.; Tian, Q.; Cai, W.; Wang, C.; Yuan, M.; Wang, W.; Wang, P.; Yan, W. Osteosarcoma-targeted Cu and Ce based oxide nanoplatform for NIR II fluorescence/magnetic resonance dual-mode imaging and ros cascade amplification along with immunotherapy. J. Nanobiotechnology 2024, 22, 151. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-Q.; Liu, R.; Chen, F.-M.; Zhang, J.-Y.; Zheng, S.-J.; Shao, D.; Du, J.-Z. Nanoparticle-Mediated CD47-SIRPα Blockade and Calreticulin Exposure for Improved Cancer Chemo-Immunotherapy. ACS Nano 2023, 17, 8966–8979. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Jin, Y.; Jiang, N.; Zhou, Y.; Wei, N.; Liu, Y.; Miao, J.; Zhang, L.; Li, R.; Zhang, A.; et al. Gint4.T-siHDGF chimera-capped mesoporous silica nanoparticles encapsulating temozolomide for synergistic glioblastoma therapy. Biomaterials 2024, 306, 122479. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ding, B.; Chen, H.; Meng, Q.; Li, J.; Yang, C.; Zhang, W.; Li, X.; Han, D.; Zheng, P.; et al. Effects of Skeleton Structure of Mesoporous Silica Nanoadjuvants on Cancer Immunotherapy. Small 2024, 20, 2305567. [Google Scholar] [CrossRef]
- Lee, Z.-H.; Lee, M.-F.; Chen, J.-H.; Tsou, M.-H.; Wu, Z.-Y.; Lee, C.-Z.; Huang, Y.-Y.; Lin, S.-M.; Lin, H.-M. Fucoidan with three functions extracted from Sargassum aquifolium integrated rice-husk synthesis dual-imaging mesoporous silica nanoparticle. J. Nanobiotechnology 2022, 20, 298. [Google Scholar] [CrossRef]
- Chen, Z.-A.; Wu, C.-H.; Wu, S.-H.; Huang, C.-Y.; Mou, C.-Y.; Wei, K.-C.; Yen, Y.; Chien, I.-T.; Runa, S.; Chen, Y.-P.; et al. Receptor Ligand-Free Mesoporous Silica Nanoparticles: A Streamlined Strategy for Targeted Drug Delivery across the Blood–Brain Barrier. ACS Nano 2024, 18, 12716–12736. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, F.; Cao, Y.; Zhang, F.; Sun, L.; Yang, C.; Xie, X.; Wu, Z.; Sun, M.; Ma, F.; et al. An Engineered Nanoplatform with Tropism Toward Irradiated Glioblastoma Augments Its Radioimmunotherapy Efficacy. Adv. Mater. 2024, 36, 2314197. [Google Scholar] [CrossRef]
- Liu, G.; Xia, R.; Gui, M.; Zhang, L.; Zhou, X.; Xue, J.; Cai, Y.; Cao, Y.; Xiao, Y.; Chen, Z. Turn Hood into Good: Recycling Silicon from Mesoporous Silica Nanoparticles through Magnesium Modification to Lower Toxicity and Promote Tissue Regeneration. ACS Nano 2024, 18, 32932–32949. [Google Scholar] [CrossRef]
- Lefaix, L.; Navarro, M.; Nogués, C.; Blanquer, A.; Murillo, G. Tailoring Piezoelectric Nanogenerators and Microdevices for Cellular Excitation: Impact of Size and Morphology. Adv. Sci. 2025, 2415028. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Zhang, R.; Vashisth, P.; Birjandi, A.A.; S’Ari, M.; Martella, D.A.; Isaacs, M.; Mäkilä, E.; Wang, C.; Moldenhauer, E.; et al. Lithiated porous silicon nanowires stimulate periodontal regeneration. Nat. Commun. 2024, 15, 487. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Z.; Zhang, B.; Jiang, T.; Zhu, C.; Mei, P.; Jin, Y.; Wang, R.; Li, Y.; Guo, W.; et al. Manganese Enhances the Osteogenic Effect of Silicon-Hydroxyapatite Nanowires by Targeting T Lymphocyte Polarization. Adv. Sci. 2024, 11, 2305890. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-X.; Luo, Y.-P.; Hou, X.-D.; Zhang, L.; Wang, T.-L.; Li, X.-F.; Liu, Z.-Q.; Zhao, J.-H.; Aierken, A.; Cai, Z.-Y.; et al. Natural Affinity Driven Modification by Silicene to Construct a “Thermal Switch” for Tumorous Bone Loss. Adv. Sci. 2024, 11, 2404534. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zhao, K.; Hu, T.; Tan, C.; Liang, R.; Weng, X. A Se Nanoparticle/MgFe-LDH Composite Nanosheet as a Multifunctional Platform for Osteosarcoma Eradication, Antibacterial and Bone Reconstruction. Adv. Sci. 2024, 11, 2403791. [Google Scholar] [CrossRef]
- Moses, J.C.; Sapkota, A.; Wu, Y.; Martinez, I.; Handa, H.; Brisbois, E.J. In Situ Nitric Oxide Generating Nano-Bioactive Glass-Based Coatings and Its Therapeutic Ion Release toward Attenuating Implant-Associated Fibrosis and Infection. Small 2025, 21, e2411984. [Google Scholar] [CrossRef]
- Li, L.; Qin, W.; Ye, T.; Wang, C.; Qin, Z.; Ma, Y.; Mu, Z.; Jiao, K.; Tay, F.R.; Niu, W.; et al. Bioactive Zn-V-Si-Ca Glass Nanoparticle Hydrogel Microneedles with Antimicrobial and Antioxidant Properties for Bone Regeneration in Diabetic Periodontitis. ACS Nano 2025, 19, 7981–7995. [Google Scholar] [CrossRef]
- Zhang, M.; Zhai, X.; Ma, T.; Huang, Y.; Jin, M.; Yang, H.; Fu, H.; Zhang, S.; Sun, T.; Jin, X.; et al. Sequential Therapy for Bone Regeneration by Cerium Oxide-Reinforced 3D-Printed Bioactive Glass Scaffolds. ACS Nano 2023, 17, 4433–4444. [Google Scholar] [CrossRef]
- Xu, L.; Xu, M.; Sun, X.; Feliu, N.; Feng, L.; Parak, W.J.; Liu, S. Quantitative Comparison of Gold Nanoparticle Delivery via the Enhanced Permeation and Retention (EPR) Effect and Mesenchymal Stem Cell (MSC)-Based Targeting. ACS Nano 2023, 17, 2039–2052. [Google Scholar] [CrossRef]
- Fu, R.; Xianyu, Y. Gold Nanomaterials-Implemented CRISPR-Cas Systems for Biosensing. Small 2023, 19, 2300057. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Jeong, M.S.; Cruz, E.O.; Alam, I.S.; Tumbale, S.K.; Zlitni, A.; Lee, S.Y.; Park, Y.I.; Ferrara, K.; Kwon, S.-H.; et al. Highly Excretable Gold Supraclusters for Translatable In Vivo Raman Imaging of Tumors. ACS Nano 2023, 17, 2554–2567. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, X.; Gao, S.; Song, J. Glutathione and transglutaminase responsive janus gold nanorods for photoacoustic imaging-guided radiotherapy and chemodynamic therapy of tumors. J. Control. Release Off. J. Control. Release Soc. 2025, 380, 751–759. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, Y.; Zou, K.; Lan, Z.; Cheng, G.; Mai, Q.; Cui, H.; Meng, Q.; Chen, T.; Rao, L.; et al. Biomimetic manganese-based theranostic nanoplatform for cancer multimodal imaging and twofold immunotherapy. Bioact. Mater. 2023, 19, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Das, A.; Raha, S.; Barui, A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J. Photochem. Photobiol. B 2020, 203, 111778. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Zheng, Y.; Liu, J.-J.; Chang, Z.-P.; Wang, Y.-H.; Shao, Y.-Y.; Hou, R.-G.; Zhang, X. Natural Chlorogenic Acid Planted Nanohybrids with Steerable Hyperthermia for Osteosarcoma Suppression and Bone Regeneration. Adv. Healthc. Mater. 2023, 12, e2300325. [Google Scholar] [CrossRef]
- Bu, Y.; Huang, R.; Li, Z.; Zhang, P.; Zhang, L.; Yang, Y.; Liu, Z.; Guo, K.; Gao, F. Anisotropic Truncated Octahedral Au with Pt Deposition on Arris for Localized Surface Plasmon Resonance-Enhanced Photothermal and Photodynamic Therapy of Osteosarcoma. ACS Appl. Mater. Interfaces 2021, 13, 35328–35341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, H.; Deng, X.; Song, Q.; Xing, X.; Liu, W.; Zhang, Y. Cascade-Enhanced Catalytic Nanocomposite with Glutathione Depletion and Respiration Inhibition for Effective Starving-Chemodynamic Therapy Against Hypoxic Tumor. Int. J. Nanomedicine 2022, 17, 5491–5510. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-K.; Rao, S.-S.; Tan, Y.-J.; Yin, H.; Luo, M.-J.; Wang, Z.-X.; Zhou, J.-H.; Hong, C.-G.; Luo, Z.-W.; Du, W.; et al. Fructose-coated Angstrom silver inhibits osteosarcoma growth and metastasis via promoting ROS-dependent apoptosis through the alteration of glucose metabolism by inhibiting PDK. Theranostics 2020, 10, 7710–7729. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Q.; Dai, T.; Shao, J.; Wu, X.; Jiang, Z.; Jacob, J.A.; Jiang, C. Identification of possible reductants in the aqueous leaf extract of mangrove plant Rhizophora apiculata for the fabrication and cytotoxicity of silver nanoparticles against human osteosarcoma MG-63 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111252. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, W.; Xu, X.; Lin, Z.; Xie, C.; Zhang, Y.; Zhang, T.; Li, L.; Lu, Y.; Li, Q. AgBiS2 nanoparticles with synergistic photodynamic and bioimaging properties for enhanced malignant tumor phototherapy. Mater. Sci. Eng. C 2020, 107, 110324. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, L.; Li, Y.; He, Y.; Zhang, Y.; Du, C. NIR Responsive Doxorubicin-Loaded Hollow Copper Ferrite @ Polydopamine for Synergistic Chemodynamic/Photothermal/Chemo-Therapy. Small 2023, 19, 2205414. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Qiu, L.; Li, Y.; Marraiki, N.; Elgorban, A.M.; Xue, L. Green synthesized zinc oxide nanoparticles regulates the apoptotic expression in bone cancer cells MG-63 cells. J. Photochem. Photobiol. B 2020, 202, 111644. [Google Scholar] [CrossRef]
- Seshadri, V.D. Zinc oxide nanoparticles from Cassia auriculata flowers showed the potent antimicrobial and in vitro anticancer activity against the osteosarcoma MG-63 cells. Saudi J. Biol. Sci. 2021, 28, 4046–4054. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Q.; Zhang, Y.; Xiao, J.; Deng, X.; Xing, X.; Hu, H.; Zhang, Y. Iron-Based Nanovehicle Delivering Fin56 for Hyperthermia-Boosted Ferroptosis Therapy Against Osteosarcoma. Int. J. Nanomedicine 2024, 19, 91–107. [Google Scholar] [CrossRef]
- Liang, B.; Zuo, D.; Yu, K.; Cai, X.; Qiao, B.; Deng, R.; Yang, J.; Chu, L.; Deng, Z.; Zheng, Y.; et al. Multifunctional bone cement for synergistic magnetic hyperthermia ablation and chemotherapy of osteosarcoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110460. [Google Scholar] [CrossRef]
- Popov, A.L.; Han, B.; Ermakov, A.M.; Savintseva, I.V.; Ermakova, O.N.; Popova, N.R.; Shcherbakov, A.B.; Shekunova, T.O.; Ivanova, O.S.; Kozlov, D.A.; et al. PVP-stabilized tungsten oxide nanoparticles: pH sensitive anti-cancer platform with high cytotoxicity. Mater. Sci. Eng. C 2020, 108, 110494. [Google Scholar] [CrossRef]
- Du, C.; Zhou, M.; Jia, F.; Ruan, L.; Lu, H.; Zhang, J.; Zhu, B.; Liu, X.; Chen, J.; Chai, Z.; et al. D-arginine-loaded metal-organic frameworks nanoparticles sensitize osteosarcoma to radiotherapy. Biomaterials 2021, 269, 120642. [Google Scholar] [CrossRef]
- Wang, Y.; Williams, G.R.; Zheng, Y.; Guo, H.; Chen, S.; Ren, R.; Wang, T.; Xia, J.; Zhu, L.-M. Polydopamine-cloaked Fe-based metal organic frameworks enable synergistic multidimensional treatment of osteosarcoma. J. Colloid Interface Sci. 2023, 651, 76–92. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, J.; Wu, Q.; Luo, G.; Zhao, M.; Zhong, G.; Zheng, Y.; Meng, X.; Cheng, S.; Zhang, Y. Multifunctional 3D-printed scaffolds eradiate orthotopic osteosarcoma and promote osteogenesis via microwave thermo-chemotherapy combined with immunotherapy. Biomaterials 2023, 301, 122236. [Google Scholar] [CrossRef]
- Wu, W.; Yu, X.; Sun, J.; Han, Y.; Ma, Y.; Zhang, G.; Ma, Q.; Li, Q.; Xiang, H. Zeolitic Imidazolate Framework (ZIF-8) Decorated Iron Oxide Nanoparticles Loaded Doxorubicin Hydrochloride for Osteosarcoma Treatment—In vitro and in vivo Preclinical Studies. Int. J. Nanomedicine 2023, 18, 7985–7999. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, R.; Song, Q.; Zhang, Y.; Zhao, H.; Hu, H.; Zhang, Z.; Liu, W.; Lin, W.; Wang, G. Synthesis of dual-stimuli responsive metal organic framework-coated iridium oxide nanocomposite functionalized with tumor targeting albumin-folate for synergistic photodynamic/photothermal cancer therapy. Drug Deliv. 2022, 29, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, M.; Wu, Z.; Yang, J.; Mo, B.; Yu, S.; Gong, X.; Liu, J.; Wang, W.; Luo, S.; et al. Tantalum-Zirconium Co-Doped Metal-Organic Frameworks Sequentially Sensitize Radio-Radiodynamic-Immunotherapy for Metastatic Osteosarcoma. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2023, 10, e2206779. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, Y.; Feng, K.; Zhou, Y.; Wang, X.; Huang, H.; Chen, Y.; Wang, W.; Xu, Y.; Tian, H.; et al. Polydopamine-coated UiO-66 nanoparticles loaded with perfluorotributylamine/tirapazamine for hypoxia-activated osteosarcoma therapy. J. Nanobiotechnology 2021, 19, 298. [Google Scholar] [CrossRef]
- Fu, X.; Rehman, U.; Wei, L.; Chen, Z.-S.; Abourehab, M.A.S.; Kesharwani, P.; Cheng, Z.-H. Silver-dendrimer nanocomposite as emerging therapeutics in anti-bacteria and beyond. Drug Resist. Updates 2023, 68, 100935. [Google Scholar] [CrossRef]
- Iqbal, Y.; Amin, F.; Aziz, M.H.; Wahab, R. In-situ fabrication of resveratrol loaded sodium alginate coated silver nanoparticles for in vitro studies of mitochondrial-targeted anticancer treatment against MCF-7 cell lines. Int. J. Biol. Macromol. 2024, 280, 135656. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Jian, M.; Pei, Y.; Liu, J.; Zheng, X.; Tang, K. Sustained-release, antibacterial, adhesive gelatin composite hydrogel with AgNPs double-capped with curdlan derivatives. Int. J. Biol. Macromol. 2024, 277, 134222. [Google Scholar] [CrossRef]
- Han, M.; Xia, Z.; Zou, Y.; Hu, P.; Zhang, M.; Yang, X.; Ma, M.-G.; Yang, R. Comparative Study and Transcriptomic Analysis on the Antifungal Mechanism of Ag Nanoparticles and Nanowires Against Trichosporon asahii. Int. J. Nanomedicine 2024, 19, 11789–11804. [Google Scholar] [CrossRef]
- Tan, Z.; Zhao, W.; Yin, Y.; Xu, M.; Pan, W.; Liu, Y.; Zhang, Q.; Gale, B.K.; Rui, Y.; Liu, J. Insight into the formation and biological effects of natural organic matter corona on silver nanoparticles in water environment using biased cyclical electrical field-flow fractionation. Water Res. 2023, 228, 119355. [Google Scholar] [CrossRef]
- Nõlvak, H.; Truu, M.; Tiirik, K.; Devarajan, A.K.; Peeb, A.; Truu, J. The effect of synthetic silver nanoparticles on the antibiotic resistome and the removal efficiency of antibiotic resistance genes in a hybrid filter system treating municipal wastewater. Water Res. 2023, 237, 119986. [Google Scholar] [CrossRef]
- Xiang, J.; Li, Y.; Zhang, Y.; Wang, G.; Xu, H.; Zhou, Z.; Tang, J.; Shen, Y. Polyphenol-cisplatin complexation forming core-shell nanoparticles with improved tumor accumulation and dual-responsive drug release for enhanced cancer chemotherapy. J. Control. Release Off. J. Control. Release Soc. 2021, 330, 992–1003. [Google Scholar] [CrossRef]
- Zhang, R.; You, X.; Luo, M.; Zhang, X.; Fang, Y.; Huang, H.; Kang, Y.; Wu, J. Poly(β-cyclodextrin)/platinum prodrug supramolecular nano system for enhanced cancer therapy: Synthesis and in vivo study. Carbohydr. Polym. 2022, 292, 119695. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Martín-Pardillos, A.; Lujan, L.; Sebastian, V.; Santamaria, J.; Martín-Duque, P. Exosomes loaded with ultrasmall Pt nanoparticles: A novel low-toxicity alternative to cisplatin. J. Nanobiotechnology 2022, 20, 473. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, K.; Yang, Z.; Qian, Z.; Zong, S.; Cui, Y.; Wang, Z. Chemically Powered Nanomotors with Magnetically Responsive Function for Targeted Delivery of Exosomes. Small 2024, 20, 2311207. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Jin, Q.; Zhang, L.; Zhang, H.; Montesdeoca, N.; Karges, J.; Xiao, H.; Mao, X.; Song, H.; Shang, K. Endowing Pt(IV) with Perfluorocarbon Chains and Human Serum Albumin Encapsulation for Highly Effective Antitumor Chemoimmunotherapy. ACS Nano 2024, 18, 13683–13695. [Google Scholar] [CrossRef]
- Jie, Z.; Xiong, B.; Shi, J. Allicin-Decorated FeO1-xOH Nanocatalytic Medicine for Fe2+/Fe3+ Cycling-Promoted Efficient and Sustained Tumor Regression. Adv. Sci. 2024, 11, 2402801. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Q.; Huo, M.; Lu, D.; Gao, Y.; Chen, Y.; Xu, H. Engineering Single-Atomic Iron-Catalyst-Integrated 3D-Printed Bioscaffolds for Osteosarcoma Destruction with Antibacterial and Bone Defect Regeneration Bioactivity. Adv. Mater. 2021, 33, 2100150. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yang, N.; Wang, X.; Gong, F.; Dong, Z.; Gong, Y.; Liu, Z.; Cheng, L. Ultrasmall Iron-Doped Titanium Oxide Nanodots for Enhanced Sonodynamic and Chemodynamic Cancer Therapy. ACS Nano 2020, 14, 15119–15130. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Pan, Y.; Zeng, F.; Qin, S.; Luan, X.; Lu, Q.; Xie, C.; Hu, P.; Gao, Y.; Yang, J.; et al. Microfluidic Synthesis of CuH Nanoparticles for Antitumor Therapy through Hydrogen-Enhanced Apoptosis and Cuproptosis. ACS Nano 2024, 18, 9031–9042. [Google Scholar] [CrossRef]
- Xie, L.; Gong, J.; He, Z.; Zhang, W.; Wang, H.; Wu, S.; Wang, X.; Sun, P.; Cai, L.; Wu, Z.; et al. A Copper-Manganese Based Nanocomposite Induces Cuproptosis and Potentiates Anti-Tumor Immune Responses. Small 2025, 21, 2412174. [Google Scholar] [CrossRef]
- Gu, L.; Sun, Y.; Bai, T.; Shao, S.; Tang, S.; Xue, P.; Cai, W.; Qin, X.; Zeng, X.; Yan, S. Functional nanozyme system for synergistic tumor immunotherapy via cuproptosis and ferroptosis activation. J. Nanobiotechnology 2025, 23, 212. [Google Scholar] [CrossRef]
- He, G.; Nie, J.-J.; Liu, X.; Ding, Z.; Luo, P.; Liu, Y.; Zhang, B.-W.; Wang, R.; Liu, X.; Hai, Y.; et al. Zinc oxide nanoparticles inhibit osteosarcoma metastasis by downregulating β-catenin via HIF-1α/BNIP3/LC3B-mediated mitophagy pathway. Bioact. Mater. 2023, 19, 690–702. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, A.; Jin, S.; Dai, J.; Yu, Y.; Wen, P.; Zheng, Y.; Xia, D. Additively Manufactured Biodegradable Zn-Based Porous Scaffolds to Suppress Osteosarcoma and Promote Osteogenesis. Adv. Mater. 2025, 37, 2410589. [Google Scholar] [CrossRef]
- Zlotver, I.; Sosnik, A. Glucosylated Hybrid TiO2/Polymer Nanomaterials for Actively Targeted Sonodynamic Therapy of Cancer. Small 2024, 20, e2305475. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, W.; Zhou, X.; Fu, J.; He, C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact. Mater. 2022, 17, 221–233. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, P.; Jiang, F.; Zhao, Y.; Wang, M.; Chang, M.; Ma, P.; Lin, J. MnOx Nanospikes as Nanoadjuvants and Immunogenic Cell Death Drugs with Enhanced Antitumor Immunity and Antimetastatic Effect. Angew. Chem. Int. Ed. 2020, 59, 16381–16384. [Google Scholar] [CrossRef]
- Deng, Z.; Xi, M.; Zhang, C.; Wu, X.; Li, Q.; Wang, C.; Fang, H.; Sun, G.; Zhang, Y.; Yang, G.; et al. Biomineralized MnO2 Nanoplatforms Mediated Delivery of Immune Checkpoint Inhibitors with STING Pathway Activation to Potentiate Cancer Radio-Immunotherapy. ACS Nano 2023, 17, 4495–4506. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Jiang, K.; Li, H.; Zhang, L.; Chen, B. Hierarchically Micro-, Meso-, and Macro-Porous MOF Nanosystems for Localized Cross-Scale Dual-Biomolecule Loading and Guest-Carrier Cooperative Anticancer Therapy. ACS Nano 2024, 18, 21911–21924. [Google Scholar] [CrossRef]
- Fang, C.; Cen, D.; Wang, Y.; Wu, Y.; Cai, X.; Li, X.; Han, G. ZnS@ZIF-8 core-shell nanoparticles incorporated with ICG and TPZ to enable H2S-amplified synergistic therapy. Theranostics 2020, 10, 7671–7682. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, W.; Tang, Y.; Dong, Q.; Huang, K.; Tan, J.; Zhang, J.; Cai, J.; Yu, Q.; Dai, Q.; et al. Trifunctional Sialylation-Based SF-ZIF@NA Hydrogel for Selective Osteoclast Inhibition and Enhanced Bone-Vessel Regeneration in Osteoporotic Bone Defects. Adv. Sci. 2025, 12, 2415895. [Google Scholar] [CrossRef]
- Tang, H.; Yu, Y.; Zhan, X.; Chai, Y.; Zheng, Y.; Liu, Y.; Xia, D.; Lin, H. Zeolite imidazolate framework-8 in bone regeneration: A systematic review. J. Control. Release Off. J. Control. Release Soc. 2024, 365, 558–582. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, Y.; Liu, S.; Zhou, M.; Fang, H.; Wang, Z.; Sun, J. ZIF-8 induced hydroxyapatite-like crystals enabled superior osteogenic ability of MEW printing PCL scaffolds. J. Nanobiotechnology 2023, 21, 264. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Jin, X.; Zhang, S.; Li, T.; Zhang, Y.; Xing, H.; Yu, Y.; Zhang, H.; Gao, X.; et al. Hammett Relationship in Oxidase-Mimicking Metal–Organic Frameworks Revealed through a Protein-Engineering-Inspired Strategy. Adv. Mater. 2021, 33, 2005024. [Google Scholar] [CrossRef]
- Liang, H.; Liu, R.; Hu, C.; An, X.; Zhang, X.; Liu, H.; Qu, J. Synergistic effect of dual sites on bimetal-organic frameworks for highly efficient peroxide activation. J. Hazard. Mater. 2021, 406, 124692. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.-H.; Xin, Y.; Cai, B.; Hu, P.-F.; Dai, H.-Y.; Liang, C.-M.; Meng, Y.-T.; Su, J.-H.; Zhang, X.-J.; et al. Designing Symmetric Gradient Honeycomb Structures with Carbon-Coated Iron-Based Composites for High-Efficiency Microwave Absorption. Nano-Micro Lett. 2024, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Fan, D.; Pinto, R.V.; Dovgaliuk, I.; Nandi, S.; Chakraborty, D.; García-Moncada, N.; Vimont, A.; McMonagle, C.J.; Bordonhos, M.; et al. A Scalable Robust Microporous Al-MOF for Post-Combustion Carbon Capture. Adv. Sci. 2024, 11, 2401070. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guo, M.; Wu, D.; Shen, M.; Liu, D.; Ding, T. Synthesis of UiO-66 loaded-caffeic acid and study of its antibacterial mechanism. Food Chem. 2023, 402, 134248. [Google Scholar] [CrossRef]
- Eldin, Z.E.; Dishisha, T.; Sayed, O.M.; Salama, H.M.; Farghali, A. A novel synergistic enzyme-antibiotic therapy with immobilization of mycobacteriophage Lysin B enzyme onto Rif@UiO-66 nanocomposite for enhanced inhaled anti-TB therapy; Nanoenzybiotics approach. Int. J. Biol. Macromol. 2024, 262, 129675. [Google Scholar] [CrossRef]
- Huang, H.; Heng, Y.; Yu, Z.; Zhang, X.; Zhu, X.; Fang, Z.; Li, J.; Guo, X. Solvent-free synthesis of defective Zr-based metal-organic framework from waste plastic bottles for highly efficient lomefloxacin removal. J. Colloid Interface Sci. 2024, 670, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xie, P.; Yu, Z.; Gu, R.; Su, Y. Enhanced adsorption performance of UiO-66 via modification with functional groups and integration into hydrogels. Environ. Res. 2022, 212, 113354. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Fu, M.; Ye, D.; Fan, L.; Hu, Y. Derivatives of Br-doped metal-organic framework for improved acetaldehyde adsorption-photocatalytic oxidation. Sci. Total Environ. 2024, 932, 172941. [Google Scholar] [CrossRef]
- Liu, T.; Wang, P.; Wang, Z.-L. A high-efficient and recyclable aged nanoscale zero-valent iron compound for V5+ removal from wastewater: Characterization, performance and mechanism. Chemosphere 2022, 302, 134833. [Google Scholar] [CrossRef]
- Li, R.; Zhuang, W.; Gao, Y.; Bai, Y.; Zhang, K.; Wang, Z. Interfacial modulation of hierarchically porous UIO-66 for the immobilization of Rhizomucor miehei lipase towards the efficient synthesis of 1,3-dioleic acid glycerol. Int. J. Biol. Macromol. 2025, 291, 138993. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Yang, H.; Li, C.; Wang, Z.; Xiong, J.; Xv, Y.; Wang, Z.; Shen, J.; Jiang, H. The effects of UiO-66 ultrafine particles on the rapid detection of sulfonamides in milk: Adsorption performance and mechanism. Food Chem. 2023, 417, 135878. [Google Scholar] [CrossRef]
- Wang, S.; Ai, Z.; Niu, X.; Yang, W.; Kang, R.; Lin, Z.; Waseem, A.; Jiao, L.; Jiang, H.L. Linker Engineering of Sandwich-Structured Metal-Organic Framework Composites for Optimized Photocatalytic H2 Production. Adv. Mater. 2023, 35, e2302512. [Google Scholar] [CrossRef]
- Emanet, M.; Lefevre, M.C.; Ceccarelli, M.C.; Battaglini, M.; Carmignani, A.; Schiavone, F.; Marino, A.; De Pasquale, D.; Prato, M.; De Boni, F.; et al. Polydopamine Nanoparticle-Based Combined Chemotherapy and Photothermal Therapy for the Treatment of Liver Cancer. ACS Appl. Mater. Interfaces 2024, 16, 40695–40713. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Cao, G.; Wang, R.; Cheng, L.; Huang, W.; Yin, Y.; Ma, H.; Ho, S.-H.; Wang, Z.; Zhu, M.; et al. Metal-ion-chelating phenylalanine nanostructures reverse immune dysfunction and sensitize breast tumour to immune checkpoint blockade. Nat. Nanotechnol. 2024, 19, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, C.; Wu, Y.; Wang, Y.; Meng, Y.; Wu, F.; Zhang, H.; Cheng, Y.Y.; Jiang, X.; Shi, J.; et al. Nutrient-delivery and metabolism reactivation therapy for melanoma. Nat. Nanotechnol. 2024, 19, 1399–1408. [Google Scholar] [CrossRef]
- Smith, T.T.; Stephan, S.B.; Moffett, H.F.; McKnight, L.E.; Ji, W.; Reiman, D.; Bonagofski, E.; Wohlfahrt, M.E.; Pillai, S.P.S.; Stephan, M.T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017, 12, 813–820. [Google Scholar] [CrossRef]
- Dutta, S.D.; Hexiu, J.; Moniruzzaman, M.; Patil, T.V.; Acharya, R.; Kim, J.S.; Lim, K.-T. Tailoring osteoimmunity and hemostasis using 3D-Printed nano-photocatalytic bactericidal scaffold for augmented bone regeneration. Biomaterials 2025, 316, 122991. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 1–31. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Li, W.; Jiang, L.; Li, X. Recent progress of rare earth doped hydroxyapatite nanoparticles: Luminescence properties, synthesis and biomedical applications. Acta Biomater. 2022, 148, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Han, Y.-H.; Na, J.; Lee, C.-H.; Sun, Z.; Wang, S.-B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.-Z.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yang, J.; Chhowalla, M.; Loh, K.P. Synthesis and reduction of large sized graphene oxide sheets. Chem. Soc. Rev. 2017, 46, 7306–7316. [Google Scholar] [CrossRef]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomedicine 2019, 14, 1633–1657. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).