Impact of a Novel Pretreatment on Bond Strength of Universal Adhesive to Conventional and CAD/CAM Resin Composites: In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Study Design and Ethical Approval
2.3. Randomization, Grouping, and Blinding
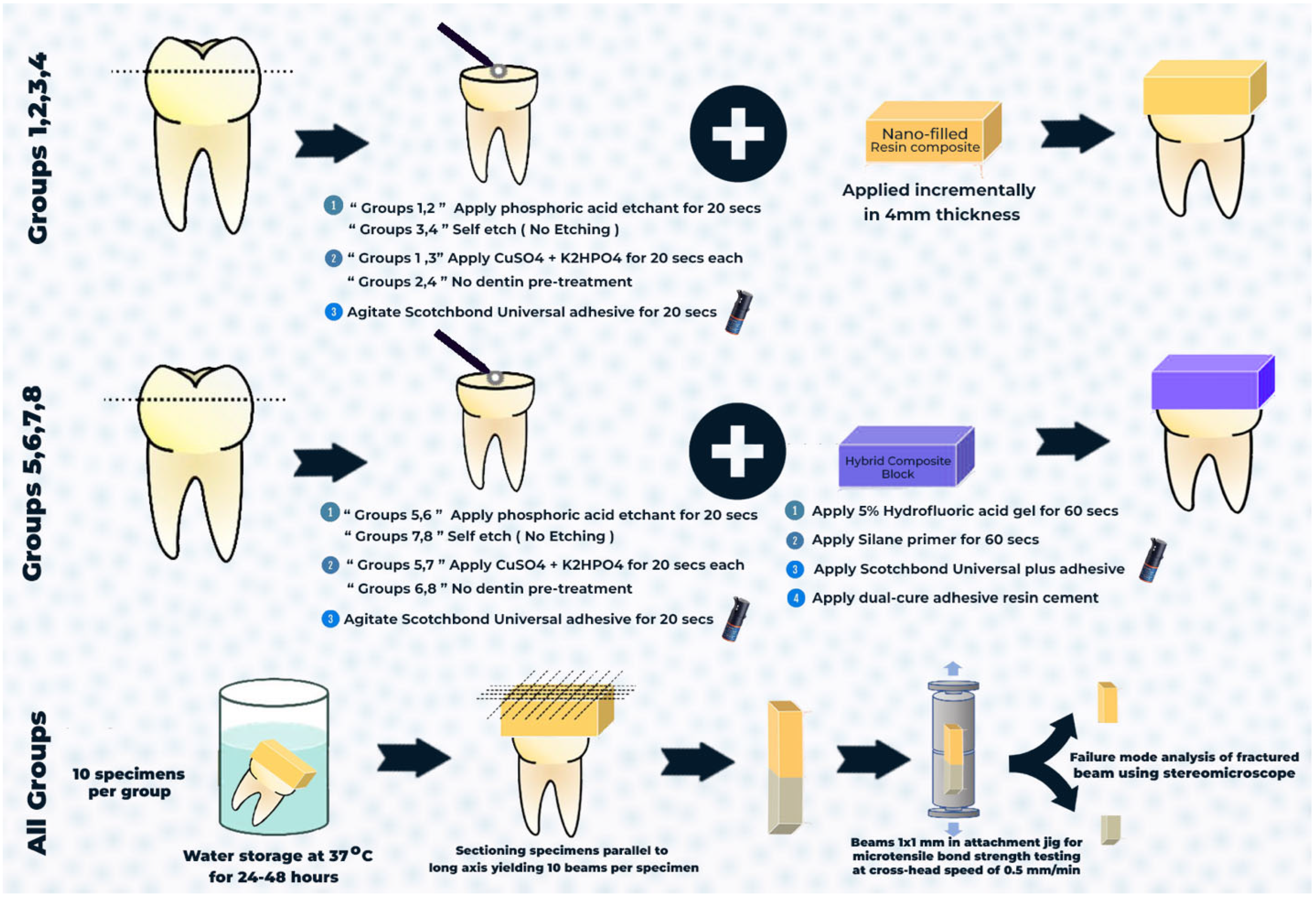
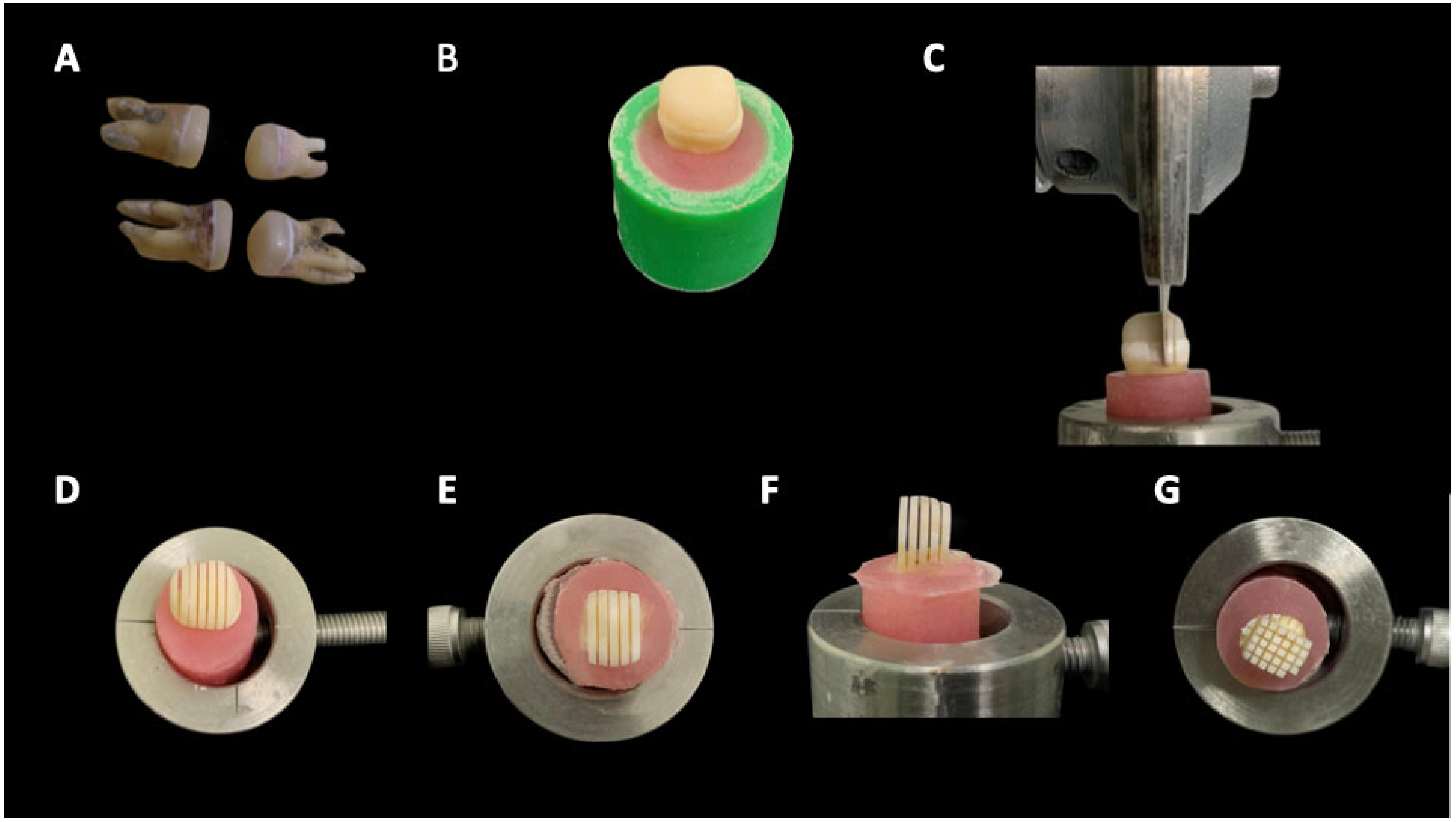
2.4. Specimen Preparation
2.5. Restorative Procedures
2.5.1. Conventional Composite
2.5.2. CAD/CAM Resin Blocks
2.6. Microtensile Bond Strength Test (μTBS)
2.7. Statistical Analysis
3. Results
3.1. Microtensile Bond Strength Values
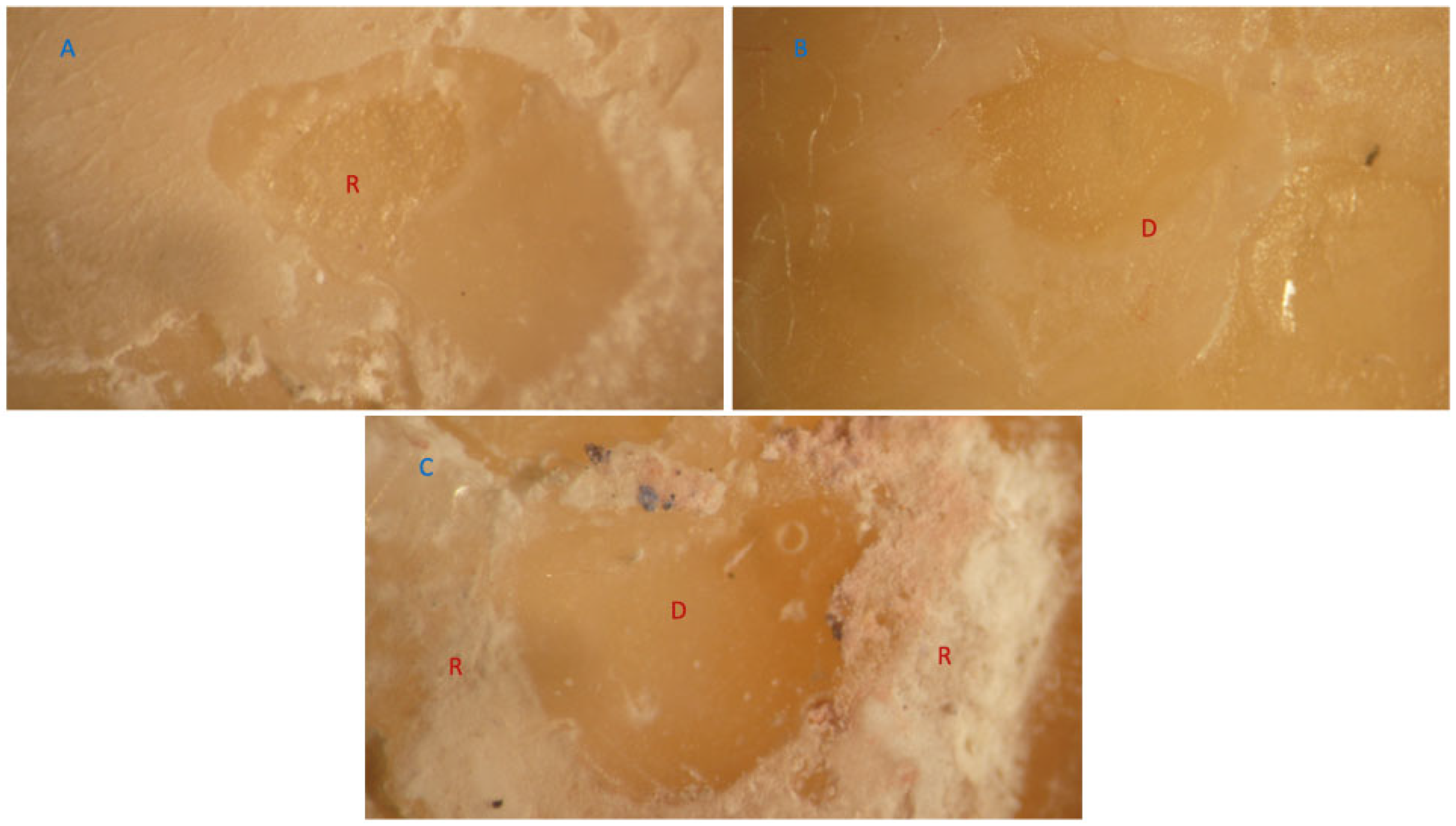
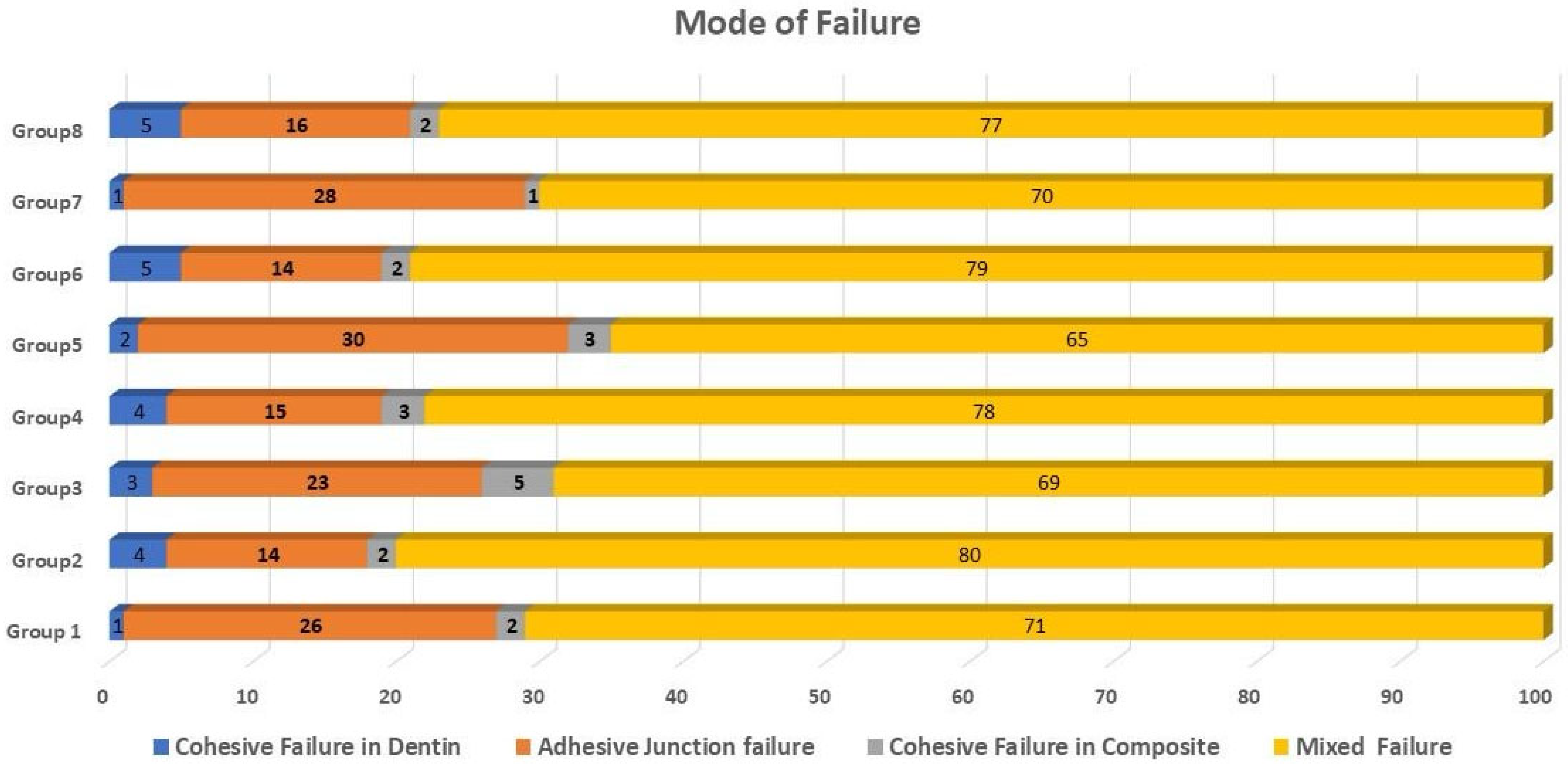
3.2. Failure Mode Analysis
4. Discussion
5. Conclusions
6. Clinical Relevance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Suárez, C.E.; da Rosa, W.L.O.; Lund, R.G.; da Silva, A.F.; Piva, E. Bonding Performance of Universal Adhesives: An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar] [PubMed]
- Jäggi, M.; Karlin, S.; Zitzmann, N.U.; Rohr, N. Shear bond strength of universal adhesives to human enamel and dentin. J. Esthet. Restor. Dent. 2024, 36, 804–812. [Google Scholar] [CrossRef]
- Betancourt, D.E.; Baldion, P.A.; Castellanos, J.E. Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer. Int. J. Biomater. 2019, 2019, 5268342. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Carvalho, R.M.; Rueggeberg, F.A.; Agee, K.A.; Carrilho, M.; Donnelly, A.; García-Godoy, F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am. J. Dent. 2007, 20, 7. [Google Scholar]
- Bottene Guarda, M.; Tulio Di Nizo, P.; Flores Abuna, G.; Catelan, A.; Coelho Sinhoreti, M.A.; Pino Vitti, R. Effect of Electric Current-assisted Application of Adhesives on their Bond Strength and Quality. J. Adhes. Dent. 2020, 22, 393–398. [Google Scholar]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.P.; Cecchin, D.; Vidal, C.M.; Leme-Kraus, A.A.; Bedran-Russo, A.K. Removal of water binding proteins from dentin increases the adhesion strength of low-hydrophilicity dental resins. Dent. Mater. 2020, 36, e302–e308. [Google Scholar] [CrossRef]
- Rucker, R.B.; Kosonen, T.; Clegg, M.S.; Mitchell, A.E.; Rucker, B.R.; Uriu-Hare, J.Y.; Keen, C.L. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am. J. Clin. Nutr. 1998, 67 (Suppl. S5), 996s–1002s. [Google Scholar] [CrossRef]
- Kubo, A.-L.; Vasiliev, G.; Vija, H.; Krishtal, J.; Tõugu, V.; Visnapuu, M.; Kisand, V.; Kahru, A.; Bondarenko, O.M. Surface carboxylation or PEGylation decreases CuO nanoparticles’ cytotoxicity to human cells in vitro without compromising their antibacterial properties. Arch. Toxicol. 2020, 94, 1561–1573. [Google Scholar] [CrossRef]
- Tiwari, M.; Narayanan, K.; Thakar, M.B.; Jagani, H.V.; Venkata Rao, J. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014, 8, 230–237. [Google Scholar] [CrossRef]
- Sabatini, C.; Mennito, A.S.; Wolf, B.J.; Pashley, D.H.; Renné, W.G. Incorporation of bactericidal poly-acrylic acid modified copper iodide particles into adhesive resins. J. Dent. 2015, 43, 546–555. [Google Scholar] [CrossRef]
- Grzebieluch, W.; Mikulewicz, M.; Kaczmarek, U. Resin composite materials for chairside CAD/CAM restorations: A comparison of selected mechanical properties. J. Healthc. Eng. 2021, 2021, 8828954. [Google Scholar] [CrossRef] [PubMed]
- Grzebieluch, W.; Kowalewski, P.; Grygier, D.; Rutkowska-Gorczyca, M.; Kozakiewicz, M.; Jurczyszyn, K. Printable and machinable dental restorative composites for CAD/CAM application—Comparison of mechanical properties, fractographic, texture and fractal dimension analysis. Materials 2021, 14, 4919. [Google Scholar] [CrossRef] [PubMed]
- Aladağ, S.; Ayaz, E.A. Bonding effectiveness of multi-step adhesive resin cements to CAD/CAM blocks: Impact of thermal cycling and surface treatment methods. BMC Oral Health 2024, 24, 1326. [Google Scholar] [CrossRef] [PubMed]
- Şenol, M.; Gürbüz, A.; Oyar, P. Bond strength of conventional resin-based adhesive cement and self-adhesive resin cement to CAD-CAM restorative materials. BMC Oral Health 2025, 25, 296. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, J.; Cai, X.; Li, X.; Wang, X. Effect of a novel pretreatment on the microtensile bond strength of universal adhesives with dentin. J. Dent. Sci. 2023, 18, 1148–1155. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 2010, 63, 834–840. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; Dos Santos, A.F.C.; da Cruz Santos, G.; da Silva Leite, L.S.; Lozada, J.C.; Silva-Concílio, L.R.; Baroudi, K.; Amaral, M. Effect of Cement Layer Thickness on the Immediate and Long-Term Bond Strength and Residual Stress between Lithium Disilicate Glass-Ceramic and Human Dentin. Materials 2021, 14, 5153. [Google Scholar] [CrossRef]
- Aker Sagen, M.; Dahl, J.E.; Matinlinna, J.P.; Tibballs, J.E.; Rønold, H.J. The influence of the resin-based cement layer on ceramic-dentin bond strength. Eur. J. Oral Sci. 2021, 129, e12791. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Li, X.; Lei, Y.; Shu, C.; Luo, Q.; Chen, L.; Li, X. Reconstruction of a demineralized dentin matrix via rapid deposition of CaF2 nanoparticles in situ promotes dentin bonding. ACS Appl. Mater. Interfaces 2021, 13, 51775–51789. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Gerlach, R.; Line, S. Inhibition of human gelatinases by metals released from dental amalgam. Biomaterials 2001, 22, 2025–2030. [Google Scholar] [CrossRef]
- Marelli, B.; Le Nihouannen, D.; Hacking, S.A.; Tran, S.; Li, J.; Murshed, M.; Doillon, C.J.; Ghezzi, C.E.; Zhang, Y.L.; Nazhat, S.N. Newly identified interfibrillar collagen crosslinking suppresses cell proliferation and remodelling. Biomaterials 2015, 54, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, J.-D.; Uzui, H.; Toyoda, K.; Geshi, T.; Yue, H.; Ueda, T. Effects of copper and zinc on the production of homocysteine-induced extracellular matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells. Acta Cardiol. 2005, 60, 353–359. [Google Scholar] [CrossRef]
- Sharma, V.; Nainan, M.T.; Shivanna, V. The effect of cavity disinfectants on the sealing ability of dentin bonding system: An in vitro study. J. Conserv. Dent. 2009, 12, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y.; Kondou, Y.; Suzuki, K.; Yatani, H.; Yamashita, A. Effect of dissolution of collagen on adhesion to dentin. Int. J. Prosthodont. 1994, 7, 302–306. [Google Scholar]
- Gutiérrez, M.F.; Malaquias, P.; Hass, V.; Matos, T.P.; Lourenço, L.; Reis, A.; Loguercio, A.D.; Farago, P.V. The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J. Dent. 2017, 61, 12–20. [Google Scholar] [CrossRef]
- Matos, T.P.; Gutiérrez, M.F.; Hanzen, T.A.; Malaquias, P.; de Paula, A.M.; de Souza, J.J.; Hass, V.; Fernández, E.; Reis, A.; Loguercio, A.D. 18-month clinical evaluation of a copper-containing universal adhesive in non-carious cervical lesions: A double-blind, randomized controlled trial. J. Dent. 2019, 90, 103219. [Google Scholar] [CrossRef]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Mazzitelli, C.; Josic, U.; Mancuso, E.; Cadenaro, M.; Pfeifer, C.S.; Mazzoni, A. The evolution of adhesive dentistry: From etch-and-rinse to universal bonding systems. Dent. Mater. 2024, 41, 141–158. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rosa, W.L.; Piva, E.; Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Elkaffas, A.A.; Hamama, H.H.; Mahmoud, S.H.; Fawzy, A.S. Effect of acid etching on dentin bond strength of ultra-mild self-etch adhesives. Int. J. Adhes. Adhes. 2020, 99, 102567. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zrow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suárez, C.E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef]
- Giannini, M.; Makishi, P.; Ayres, A.P.; Vermelho, P.M.; Fronza, B.M.; Nikaido, T.; Tagami, J. Self-etch adhesive systems: A literature review. Braz. Dent. J. 2015, 26, 3–10. [Google Scholar] [CrossRef]
- Costa, M.P.; Giacomini, M.C.; Zabeu, G.S.; Mosquim, V.; Dallavilla, G.G.; da Silva Santos, P.S.; Wang, L. Impact of functional monomers, bioactive particles, and HEMA, on the adhesive performance of self-etch adhesive systems applied to simulated altered dentin. J. Dent. 2024, 151, 105379. [Google Scholar] [CrossRef] [PubMed]
- Chersoni, S.; Suppa, P.; Grandini, S.; Goracci, C.; Monticelli, F.; Yiu, C.; Huang, C.; Prati, C.; Breschi, L.; Ferrari, M. In vivo and in vitro permeability of one-step self-etch adhesives. J. Dent. Res. 2004, 83, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Wendler, M.; Petschelt, A.; Belli, R.; Lohbauer, U. Bonding performance of universal adhesives in different etching modes. J. Dent. 2014, 42, 800–807. [Google Scholar] [CrossRef]
- Chen, C.; Niu, L.-N.; Xie, H.; Zhang, Z.-Y.; Zhou, L.-Q.; Jiao, K.; Chen, J.-H.; Pashley, D.H.; Tay, F. Bonding of universal adhesives to dentine–old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef]
- Brkanović, S.; Sever, E.K.; Vukelja, J.; Ivica, A.; Miletić, I.; Krmek, S.J. Comparison of different universal adhesive systems on dentin bond strength. Materials 2023, 16, 1530. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Meerbeek, B.V. Self-assembled nano-layering at the adhesive interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sezinando, A.; Linares Serrano, M.; Morales Perez, V.; Garcia Munoz, R.A.; Ceballos, L.; Perdigao, J. Chemical Adhesion of Polyalkenoate-based Adhesives to Hydroxyapatite. J. Adhes. Dent. 2016, 18, 257–265. [Google Scholar] [PubMed]
- Bourgi, R.; Kharouf, N.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Haikel, Y.; Hardan, L. A literature review of adhesive systems in dentistry: Key components and their clinical applications. Appl. Sci. 2024, 14, 8111. [Google Scholar] [CrossRef]
- Alomran, W.K.; Nizami, M.Z.I.; Xu, H.H.; Sun, J. Evolution of Dental Resin Adhesives—A Comprehensive Review. J. Funct. Biomater. 2025, 16, 104. [Google Scholar] [CrossRef]
- Muñoz, M.; Luque-Martinez, I.; Malaquias, P.; Hass, V.; Reis, A.; Campanha, N.; Loguercio, A. In vitro longevity of bonding properties of universal adhesives to dentin. Oper. Dent. 2015, 40, 282–292. [Google Scholar] [CrossRef]
- Turp, V.; Sen, D.; Tuncelli, B.; Özcan, M. Adhesion of 10-MDP containing resin cements to dentin with and without the etch-and-rinse technique. J. Adv. Prosthodont. 2013, 5, 226. [Google Scholar] [CrossRef]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability-A Systematic Review. Materials 2019, 12, 790. [Google Scholar] [CrossRef]
- Pimentel de Oliveira, R.; de Paula, B.L.; Ribeiro, M.E.; Alves, E.; Costi, H.T.; Silva, C. Evaluation of the Bond Strength of Self-Etching Adhesive Systems Containing HEMA and 10-MDP Monomers: Bond Strength of Adhesives Containing HEMA and 10-MDP. Int. J. Dent. 2022, 2022, 5756649. [Google Scholar] [CrossRef]
- Santander-Rengifo, F.; Carreras-Presas, C.M.; Aroste-Andía, R.; Hernández-Huamaní, E.; Gavilán-Chávez, P.; Cervantes-Ganoza, L.; Cayo-Rojas, C. Microtensile bond strength and failure mode of different universal adhesives on human dentin. Int. Dent. J. 2024, 74, 1239–1247. [Google Scholar] [CrossRef]
- Alam, A.; Yamauti, M.; Chowdhury, A.F.M.A.; Wang, X.; Álvarez-Lloret, P.; Zuñiga-Heredia, E.-E.; Cifuentes-Jiménez, C.; Dua, R.; Iijima, M.; Sano, H. Evaluating the advancements in a recently introduced universal adhesive compared to its predecessor. J. Dent. Sci. 2024, 19, 1609–1619. [Google Scholar] [CrossRef]
- Molla, K.; Park, H.J.; Haller, B. Bond strength of adhesive/composite combinations to dentin involving total- and self-etch adhesives. J. Adhes. Dent. 2002, 4, 171–180. [Google Scholar] [PubMed]
- Hussain, B.; Thieu, M.K.L.; Johnsen, G.F.; Reseland, J.E.; Haugen, H.J. Can CAD/CAM resin blocks be considered as substitute for conventional resins? Dent. Mater. 2017, 33, 1362–1370. [Google Scholar] [CrossRef] [PubMed]







| Groups | Dentin Pretreatment | Adhesive Strategy | Resin Composite |
|---|---|---|---|
| Group 1 | No dentin pretreatment | Etch-and-rinse | Nano-filled composite |
| Group 2 | CuSO4 + K2HPO4 | Etch-and-rinse | Nano-filled composite |
| Group 3 | No dentin pretreatment | Self-etching | Nano-filled composite |
| Group 4 | CuSO4 + K2HPO4 | Self-etching | Nano-filled composite |
| Group 5 | No dentin pretreatment | Etch-and-rinse | CAD/CAM resin blocks |
| Group 6 | CuSO4 + K2HPO4 | Etch-and-rinse | CAD/CAM resin blocks |
| Group 7 | No dentin pretreatment | Self-etching | CAD/CAM resin blocks |
| Group 8 | CuSO4 + K2HPO4 | Self-etching | CAD/CAM resin blocks |
| Material | Commercial Name and Manufacturer | Composition |
|---|---|---|
| CAD/CAM resin block | SHOFU Inc., Kyoto, Japan | Triethylene glycol dimethacrylate, urethane dimethacrylate, silica powder, zirconium silicate, fine particulate silica and colorant |
| Nano-filled resin compsite | Filtek Z350 XT, 3M Oral Care, St. Paul, MN, USA | Urethane dimethacrylate, Bisphenol-A-glycidyl methacrylate, ethoxylated bisphenol-A dimethacrylate, in addition to trace amounts of triethylene glycol dimethacrylate and nanoagglomerated nanoparticles of zirconium/silica particles with sizes ranging from 0.6 to 1.4 mm, accounting for 78.5% of the total weight (59.5% of the volume) |
| Adhesive resin cement | RelyX Ultimate, 3-M ESPE, St. Paul, MN, USA | Silane-treated glass powder, 2-methyl, 1,10-[1-(hydroxymethyl) 1,2-ethanediyl] ester, 2-propenoic acid, TEGDEMA, silane-treated silica, oxide glass chemicals, sodium persulfate, tert-butyl peroxy-3,5,5 trimethylhexanoate, reaction products with 2-hydroxy-1,3-propanediyl dimethacrylate and phosphorus oxide, copper (II) acetate monohydrate, silane-treated silica, substituted dimethacrylate, 1,12-dodecane dimethacrylate, -benzyl-5-phenyl-barbic-acid, sodium p-toluenesulfinate, 2-propenoic acid, calcium salt, calcium hydroxide, and titanium dioxide |
| Scotchbond Universal Plus Adhesive | Surefil® SDR™flow, Dentsply Caulk, Milford, DE, USA | 2-hydroxyethyl methacrylate, 2-propenoic acid, 10-methacryloyloxydecyl dihydrogen phosphate, 1,3-benzenediol 2-(2-hydroxyethoxy) ethyl 3-hydroxypropyl diethers, 2-methyl-, 3 triethoxysilyl) propyl ester, reaction products with silica and 3-aminopropyl triethoxysilane, ethanol, water, camphorquinone, a copolymer of acrylic and itaconic acid, and copper (II) acetate monohydrate |
| Source | Sum of Squares | df | Mean Squares | F | p-Value | Partial Eta Squared (Effect Size) |
|---|---|---|---|---|---|---|
| Corrected Model | 1497.787 a | 7 | 213.970 | 12.073 | 0.000 | 0.540 |
| Intercept | 61,217.113 | 1 | 61,217.113 | 3453.986 | 0.000 | 0.980 |
| Dentin pretreatment | 1386.113 | 1 | 1386.113 | 78.207 | 0.000 | 0.521 |
| Adhesive strategy | 82.012 | 1 | 82.012 | 4.627 | 0.035 | 0.060 |
| Resin composite | 6.613 | 1 | 6.613 | 0.373 | 0.543 | 0.005 |
| Dentin pretreatment * adhesive strategy | 0.113 | 1 | 0.113 | 0.006 | 0.937 | 0.000 |
| Dentin pretreatment * resin composite | 5.513 | 1 | 5.513 | 0.311 | 0.579 | 0.004 |
| Adhesive strategy * resin composite | 17.113 | 1 | 17.113 | 0.966 | 0.329 | 0.013 |
| Dentin pretreatment * Adhesive strategy * resin composite | 0.313 | 1 | 0.313 | 0.018 | 0.895 | 0.000 |
| Error | 1276.100 | 72 | 17.724 | |||
| Total | 63,991.000 | 80 | ||||
| Corrected Total | 2773.887 | 79 |
| Dentin Pretreatment + Adhesive Strategy | Resin Composite | ||
|---|---|---|---|
| Nano-Filled Resin Composite Mean ± SD | CAD/CAM Resin Block Mean ± SD | p-Value | |
| No dentin pretreatment | |||
| Etch-and-rinse | 24.20 ± 4.54 Ac | 24.71 ± 4.33 Ac | 1.000 |
| Self-etching | 21.20 ± 3.40 Ac | 23.89 ± 3.89 Ac | 1.000 |
| CuSO4 + K2HPO4 Dentin pretreatment | |||
| Etch-and-rinse | 33.66 ± 5.22 Aa | 32.49 ± 4.92 Aa | 1.000 |
| Self-etching | 30.31 ± 3.87 Ab | 31.22 ± 4.71 Aab | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkaffas, A.A.; Alshehri, A.; Alhalabi, F.; Bayoumi, R.; Alqahtani, A.A.; Almudahi, A.; Alsubaie, A.F.; Alharbi, A.F. Impact of a Novel Pretreatment on Bond Strength of Universal Adhesive to Conventional and CAD/CAM Resin Composites: In Vitro Study. J. Funct. Biomater. 2025, 16, 197. https://doi.org/10.3390/jfb16060197
Elkaffas AA, Alshehri A, Alhalabi F, Bayoumi R, Alqahtani AA, Almudahi A, Alsubaie AF, Alharbi AF. Impact of a Novel Pretreatment on Bond Strength of Universal Adhesive to Conventional and CAD/CAM Resin Composites: In Vitro Study. Journal of Functional Biomaterials. 2025; 16(6):197. https://doi.org/10.3390/jfb16060197
Chicago/Turabian StyleElkaffas, Ali A., Abdullah Alshehri, Feras Alhalabi, Rania Bayoumi, Abdullah Ali Alqahtani, Abdulellah Almudahi, Abdulaziz Fahd Alsubaie, and Abdulaziz Fahd Alharbi. 2025. "Impact of a Novel Pretreatment on Bond Strength of Universal Adhesive to Conventional and CAD/CAM Resin Composites: In Vitro Study" Journal of Functional Biomaterials 16, no. 6: 197. https://doi.org/10.3390/jfb16060197
APA StyleElkaffas, A. A., Alshehri, A., Alhalabi, F., Bayoumi, R., Alqahtani, A. A., Almudahi, A., Alsubaie, A. F., & Alharbi, A. F. (2025). Impact of a Novel Pretreatment on Bond Strength of Universal Adhesive to Conventional and CAD/CAM Resin Composites: In Vitro Study. Journal of Functional Biomaterials, 16(6), 197. https://doi.org/10.3390/jfb16060197






