Diagnostic Value of Superparamagnetic Iron Oxide Nanoparticles as a Tracer for Sentinel Lymph Node Mapping in Early-Stage Cervical Cancer: The Preliminary Clinical Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study
2.2. Patients
2.3. Surgical Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Polish Cancer Registry. Available online: https://onkologia.org.pl (accessed on 10 March 2025).
- Margioula-Siarkou, C.; Almperis, A.; Gullo, G.; Almperi, E.A.; Margioula-Siarkou, G.; Nixarlidou, E.; Mponiou, K.; Papakotoulas, P.; Sardeli, C.; Guyon, F.; et al. Sentinel Lymph Node Staging in Early-Stage Cervical Cancer: A Comprehensive Review. J. Clin. Med. 2023, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Guani, B.; Dorez, M.; Magaud, L.; Buenerd, A.; Lecuru, F.; Mathevet, P. Impact of micrometastasis or isolated tumor cells on recurrence and survival in patients with early cervical cancer: SENTICOL Trial. Int. J. Gynecol. Cancer 2019, 29, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135, Erratum in Int. J. Gynaecol. Obstet. 2019, 147, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.F., Jr.; Elliott, N.; Kosloff, C.; Hoskins, W.J.; Lewis, J.L., Jr. Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage IB and IIA carcinoma of the cervix. Gynecol. Oncol. 1989, 33, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Ramirez, P.T.; Levenback, C.F.; Munsell, M.F.; Euscher, E.D.; Soliman, P.T.; Frumovitz, M. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol. Oncol. 2017, 145, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Holman, L.L.; Levenback, C.F.; Frumovitz, M. Sentinel lymph node evaluation in women with cervical cancer. J. Minim. Invasive Gynecol. 2014, 21, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Levenback, C.; Coleman, R.L.; Burke, T.W.; Lin, W.M.; Erdman, W.; Deavers, M.; Delpassand, E.S. Lymphatic mapping and sentinel node identification in patients with cervix cancer undergoing radical hysterectomy and pelvic lymphadenectomy. J. Clin. Oncol. 2002, 20, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Kawagoe, T.; Toki, N.; Tanaka, M.; Kashimura, M. Long-standing complications after treatment for cancer of the uterine cervix—Clinical significance of medical examination at 5 years after treatment. Int. J. Gynecol. Cancer 2006, 16, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Biglia, N.; Librino, A.; Ottino, M.C.; Panuccio, E.; Daniele, A.; Chahin, A. Lower limb lymphedema and neurological complications after lymphadenectomy for gynecological cancer. Int. J. Gynecol. Cancer 2015, 25, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Bodurtha Smith, A.J.; Fader, A.N.; Tanner, E.J. Sentinel lymph node assessment in endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; McCluggage, W.G. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol. Oncol. 2019, 152, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Kocian, R.; Plaikner, A.; Jarkovsky, J.; Klat, J.; Zapardiel, I.; Pilka, R.; Torne, A.; Sehnal, B.; Ostojich, M.; et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: The SENTIX trial. Eur. J. Cancer 2020, 137, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Mathevet, P.; Lécuru, F.; Uzan, C.; Boutitie, F.; Magaud, L.; Guyon, F.; Querleu, D.; Fourchotte, V.; Baron, M.; Bats, A.S.; et al. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentrerandomised trial (SENTICOL-2). Eur. J. Cancer 2021, 148, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Lecuru, F.R.; McCormack, M.; Hillemanns, P.; Anota, A.; Leitao, M.; Mathevet, P.; Zweemer, R.; Fujiwara, K.; Zanagnolo, V.; Zahl Eriksson, A.G.; et al. SENTICOL III: An international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int. J. Gynecol. Cancer 2019, 29, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Dusek, J.; Jarkovsky, J.; Dundr, P.; Querleu, D.; van der Zee, A.; Kucukmetin, A.; Kocian, R. A prospective multicenter trial on sentinel lymph node biopsy in patients with early-stage cervical cancer (SENTIX). Int. J. Gynecol. Cancer 2019, 29, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Lührs, O.; Ekdahl, L.; Lönnerfors, C.; Geppert, B.; Persson, J. Combining Indocyanine Green and Tc99-nanocolloid does not increase the detection rate of sentinel lymph nodes in earlystage cervical cancer compared to Indocyanine Green alone. Gynecol. Oncol. 2020, 156, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Olawaiye, A.B.; Baker, T.P.; Washington, M.K.; Mutch, D.G. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J. Clin. 2021, 71, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Kurylcio, A.; Welter, R.; vanHaasteren, V.; Grosse, B.; Berclaz, G.; Polkowski, W.; Hauser, N. The Central-European SentiMag study: Sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast 2014, 23, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Douek, M.; Klaase, J.; Monypenny, I.; Kothari, A.; Zechmeister, K.; Brown, D.; Wyld, L.; Drew, P.; Garmo, H.; Agbaje, O.; et al. SentiMAG Trialists Group. Sentinel node biopsy using a magnetic tracer versus standard technique: The SentiMAGMulticentre Trial. Ann. Surg. Oncol. 2014, 21, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Kowald, T.; Paulo, T.S.; Goos, P.; Engels, S.; Gerullis, H.; Schiffmann, J.; Chavan, A.; Wawroschek, F. Magnetic resonance sentinel lymph node imaging and magnetometer-guided intraoperative detection in prostate cancer using superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2018, 13, 6689–6698. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, M.; Lefor, A.T.; Hozumi, Y.; Kurihara, K.; Sata, N.; Yasuda, Y.; Kusakabe, M. Sentinel lymph node biopsy in patients with breast cancer using superparamagnetic iron oxide and a magnetometer. Breast Cancer 2013, 20, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Diaz-Botero, S.; Esgueva, A.; Rodriguez, R.; Cortadellas, T.; Cordoba, O.; Espinosa-Bravo, M. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur. J. Surg. Oncol. 2015, 41, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Houpeau, J.L.; Chauvet, M.P.; Guillemin, F.; Bendavid-Athias, C.; Charitansky, H.; Kramar, A.; Giard, S. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: The French Sentimag feasibility trial. J. Surg. Oncol. 2016, 113, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Engels, S.; Goos, P.; Süykers, M.C.; Gudenkauf, S.; Henke, R.P.; Wawroschek, F. Accuracy of Magnetometer-Guided Sentinel Lymphadenectomy after Intraprostatic Injection of Superparamagnetic Iron Oxide Nanoparticles in Prostate Cancer: The SentiMag Pro II Study. Cancers 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Jedryka, M.A.; Klimczak, P.; Kryszpin, M.; Poprawski, T.; Czekanski, A.; Lepka, P.; Matkowski, R. Superparamagnetic Iron Oxide Nanoparticles as a Tracer for Sentinel Lymph Node Mapping in Endometrial Cancer. Int. J. Mol. Sci. 2025, 26, 781. [Google Scholar] [CrossRef] [PubMed]
- Jedryka, M.A.; Klimczak, P.; Kryszpin, M.; Matkowski, R. Superparamagnetic iron oxide: A novel tracer for sentinel lymph node detection in vulvar cancer. Int. J. Gynecol. Cancer 2020, 30, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.; Ruiz, R.; Lekuona, A.; Cobas, P.; Jaunarena, I.; Gorostidi, M.; Cespedes, J. Superparamagnetic iron oxide (SPIO) for sentinel lymph node detection in vulvar cancer. Gynecol. Oncol. 2024, 21, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kotani, Y.; Suzuki, A.; Takaya, H.; Nakai, H.; Matsuki, M.; Sato, T.; Mandai, M.; Matsumura, N. Superparamagnetic iron oxide as a tracer for sentinel lymph node detection in uterine cancer: A pilot study. Sci. Rep. 2020, 10, 7945. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Cervical cancer Version 4.2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 18 April 2025).
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Magtrace® Product Brochure. Available online: https://www.endomag.com/resources/downloads/magtrace/?product=magtrace (accessed on 10 January 2025).
- Cousins, A.; Balalis, G.L.; Thompson, S.K.; Forero Morales, D.; Mohtar, A.; Wedding, A.B.; Thierry, B. Novel handheld magnetometer probe based on magnetic tunneling junction sensors for intraoperative sentinel lymph node identification. Sci. Rep. 2015, 5, 10842. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Giuliano, A.E.; Somerfield, M.R.; Benson, A.B., 3rd; Bodurka, D.C.; Burstein, H.J.; Cochran, A.J.; Cody, H.S., 3rd; Edge, S.B.; Galper, S.; et al. Amercican Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J. Clin. Oncol. 2005, 23, 7703–7720. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bouchard-Fortier, G.; Popa, I.; Grégoire, J.; Renaud, M.C.; Têtu, B.; Plante, M. Value of sentinel node mapping in cancer of the cervix. Gynecol. Oncol. 2011, 122, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Rob, L.; Robova, H.; Halaska, M.J.; Hruda, M.; Skapa, P. Current status of sentinel lymph node mapping in the management of cervical cancer. Expert Rev. Anticancer Ther. 2013, 13, 861–870, Erratum in Expert Rev. Anticancer Ther. 2014, 14, 1537. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Ivanova, A.; Boggess, J.F. Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: A feasibility study. Gynecol. Oncol. 2012, 124, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Lührs, O.; Bollino, M.; Ekdahl, L.; Lönnerfors, C.; Geppert, B.; Persson, J. Similar distribution of pelvic sentinel lymph nodes and nodal metastases in cervical and endometrial cancer. A prospective study based on lymphatic anatomy. Gynecol. Oncol. 2022, 165, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Obermair, A.; Hsu, H.C.; Chacon, E.; Collins, A.; Tsibulak, I.; Mutombo, A.; Abu-Rustum, N.R.; Balaya, V.; Buda, A.; et al. Consensus on surgical technique for sentinel lymph node dissection in cervical cancer. Int. J. Gynecol. Cancer 2024, 34, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Lécuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Daraï, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: Results of the SENTICOL study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Bresset, A.; Guani, B.; Magaud, L.; Montero Macias, R.; Delomenie, M.; Bonsang-Kitzis, H.; Ngô, C.; Bats, A.S.; Mathevet, P.; et al. Risk factors for failure of bilateral sentinel lymph node mapping in early-stage cervical cancer. Gynecol. Oncol. 2020, 156, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Slama, J.; Zikán, M.; Zaal, A.; Sevcik, L.; Kenter, G.; Querleu, D.; Jach, R.; et al. Bilateral ultrastaging of sentinel lymph node in cervical cancer: Lowering the false-negative rate and improving the detection of micrometastasis. Gynecol. Oncol. 2012, 127, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Tax, C.; Rovers, M.M.; de Graaf, C.; Zusterzeel, P.L.; Bekkers, R.L. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol. Oncol. 2015, 139, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Borcinova, M. Sentinel lymph node mapping in cervical cancer. In Clinical Lymphatic Mapping in Gynecologic Cancers, 2nd ed.; Levenback, C.E., van der Zee, A.G.J., Coleman, R.L., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: London, UK, 2022; pp. 71–81. [Google Scholar]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]

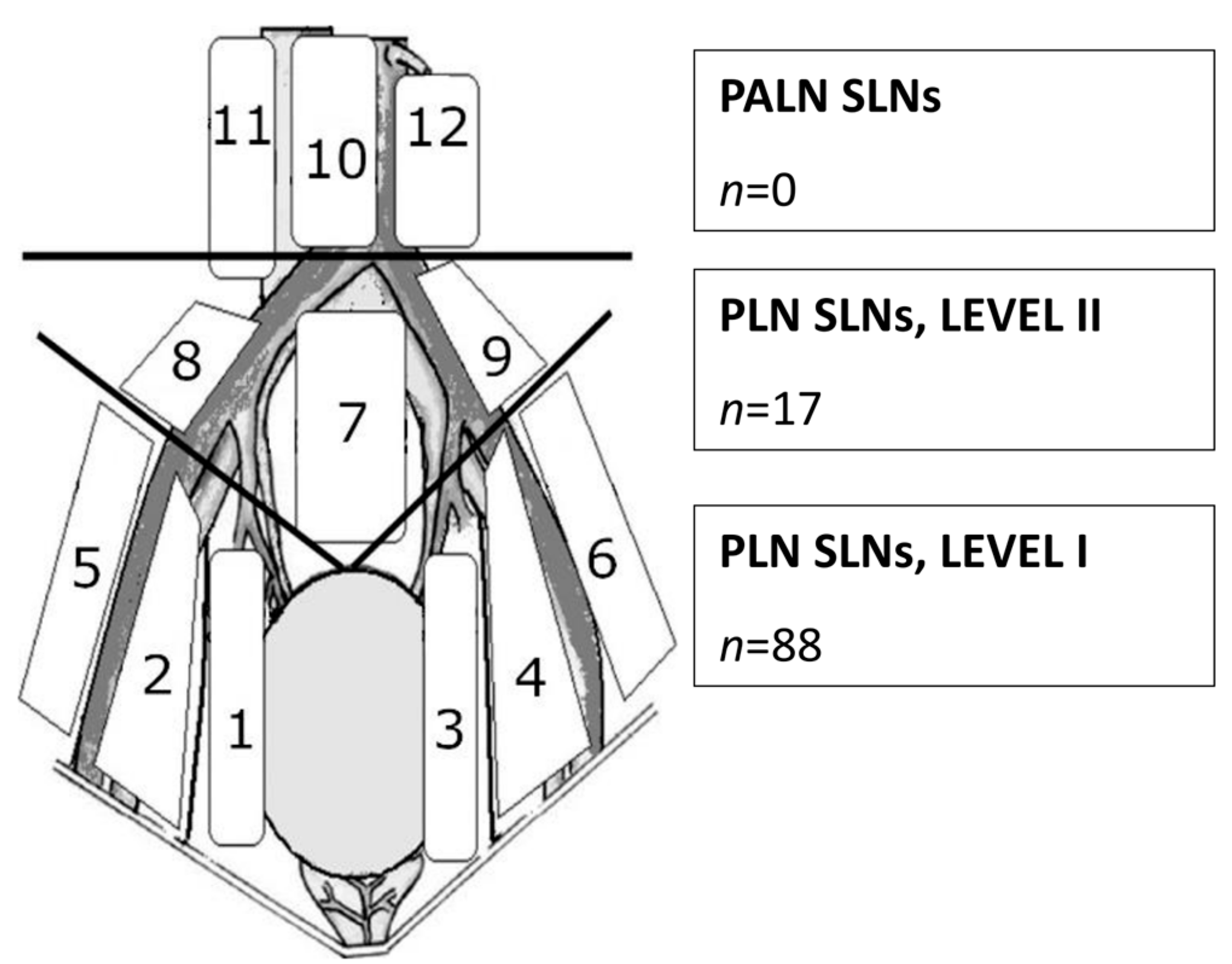
| Age (years) (mean (range)) | 46.9 (31–76) |
| BMI (kg/m2) (mean (range)) | 25.1 (17.3–40.9) |
| Squamous cancer (n) | 26 |
| Adenocarcinoma (n) | 4 |
| Tumor size (mm) (mean (range)) | 18.0 (3–45) |
| Interstitial cervical infiltration (mm) (mean (range)) | 7.5 (1–25) |
| Stage pT1A2 (n) | 2 |
| Stage pT1B1 (n) | 16 |
| Stage pT1B2 (n) | 7 |
| Stage pT1B3 (n) | 4 |
| Stage pT2A1 (n) | 1 |
| Low grade tumor (n) | 26 |
| High grade tumor (n) | 4 |
| LVSI positive (n) | 9 |
| LVSI negative (n) | 21 |
| Statistical Analysis | SLN_ALL Count | SLN_OBT Count | SLN_INT_ILIAC Count | SLN_EXT_ILIAC Count | SLN_COM_ILIAC Count | SLN_MET Count | LND Count |
|---|---|---|---|---|---|---|---|
| Mean | 3148 | 3513 | 2666 | 2244 | 3212 | 4270 | 21 |
| SD | 2991 | 3019 | 2972 | 2112 | 3089 | 3316 | 11 |
| Median | 2000 | 2500 | 1398 | 1600 | 2000 | 4700 | 20 |
| 25% centile | 650 | 650 | 370 | 680 | 650 | 1490 | 10 |
| 75% centile | 5300 | 5800 | 4500 | 3500 | 6100 | 7050 | 30 |
| IQR | 4735 | 5150 | 4373 | 2820 | 5450 | 5560 | 20 |
| Clinical Features | SLN | LND | Tumor Type | Tumor Size (mm) | Interstitial Infiltration Depth (mm) | Grade | LVSI |
|---|---|---|---|---|---|---|---|
| CASE 1 | |||||||
| Nodal metastasis detection method | n = 1 | n = 0 | |||||
| Metastatic LN localization | right obturator | ||||||
| Metastasis type | MIC | ||||||
| Pathological assessment | squamous | 33 | 15 | LG | positive | ||
| CASE 2 | |||||||
| Nodal metastasis detection method | n = 1 | n = 0 | |||||
| Metastatic LN localization | right obturator | ||||||
| Metastasis type | MIC | ||||||
| Pathological assessment | adenocarcinoma | 10 | 3 | LG | positive | ||
| CASE 3 | |||||||
| Nodal metastasis detection method | n = 1 | n = 0 | |||||
| Metastatic LN localization | right internal iliac | ||||||
| Metastasis type | MAC | ||||||
| Pathological assessment | squamous | 20 | 10 | LG | positive | ||
| CASE 4 | |||||||
| Nodal metastasis detection method | n = 1 | n = 1 | |||||
| Metastatic LN localization | left obturator | left parametrium | |||||
| Metastasis type | MIC | MAC | |||||
| Pathological assessment | squamous | 10 | 6 | LG | positive | ||
| Study | Patients (n) | Tracer | Sensitivity (%) | Bilateral Detection Rate (%) | Negative Predictive Value (%) | False Negative Rate (%) |
|---|---|---|---|---|---|---|
| SENTICOL [41] | 136 | Tc99+BD | 92.0 | 76.5 | 98.2 | 1.5 |
| SENTIX [14] | 395 | Tc99+BD; BD; ICG | N/A | 91.0 | N/A | N/A |
| Lührs et al. [18] | 65 | ICG vs. Tc99 | N/A | 98.5(ICG) vs. 60(Tc99) | N/A | N/A |
| Lührs et al. [39] | 145 | ICG | 100.0 | 97.9 | 100.0 | 0.0 |
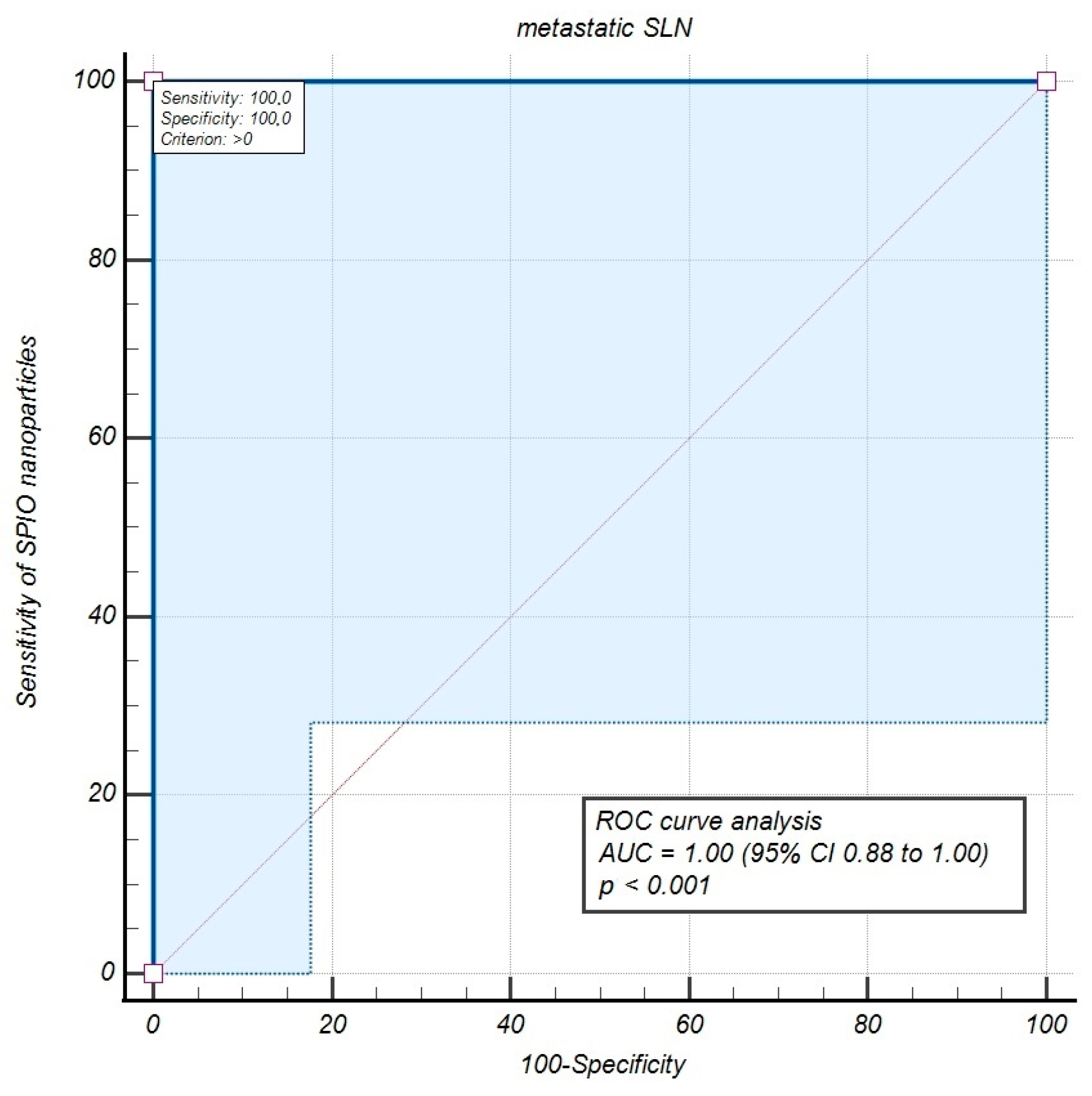
| SPIO | 30 | SPIO | 100.0 | 90.0 | 100.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedryka, M.A.; Czekanski, A.; Kryszpin, M.; Poprawski, T.; Grobelak, K.; Lepka, P.; Matkowski, R. Diagnostic Value of Superparamagnetic Iron Oxide Nanoparticles as a Tracer for Sentinel Lymph Node Mapping in Early-Stage Cervical Cancer: The Preliminary Clinical Experience. J. Funct. Biomater. 2025, 16, 196. https://doi.org/10.3390/jfb16060196
Jedryka MA, Czekanski A, Kryszpin M, Poprawski T, Grobelak K, Lepka P, Matkowski R. Diagnostic Value of Superparamagnetic Iron Oxide Nanoparticles as a Tracer for Sentinel Lymph Node Mapping in Early-Stage Cervical Cancer: The Preliminary Clinical Experience. Journal of Functional Biomaterials. 2025; 16(6):196. https://doi.org/10.3390/jfb16060196
Chicago/Turabian StyleJedryka, Marcin A., Andrzej Czekanski, Marcin Kryszpin, Tymoteusz Poprawski, Krzysztof Grobelak, Piotr Lepka, and Rafał Matkowski. 2025. "Diagnostic Value of Superparamagnetic Iron Oxide Nanoparticles as a Tracer for Sentinel Lymph Node Mapping in Early-Stage Cervical Cancer: The Preliminary Clinical Experience" Journal of Functional Biomaterials 16, no. 6: 196. https://doi.org/10.3390/jfb16060196
APA StyleJedryka, M. A., Czekanski, A., Kryszpin, M., Poprawski, T., Grobelak, K., Lepka, P., & Matkowski, R. (2025). Diagnostic Value of Superparamagnetic Iron Oxide Nanoparticles as a Tracer for Sentinel Lymph Node Mapping in Early-Stage Cervical Cancer: The Preliminary Clinical Experience. Journal of Functional Biomaterials, 16(6), 196. https://doi.org/10.3390/jfb16060196








