Enhancing the Physical Properties of Calcium Silicate Cement Modified with Elastin-like Polypeptides and Bioactive Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of V125E8
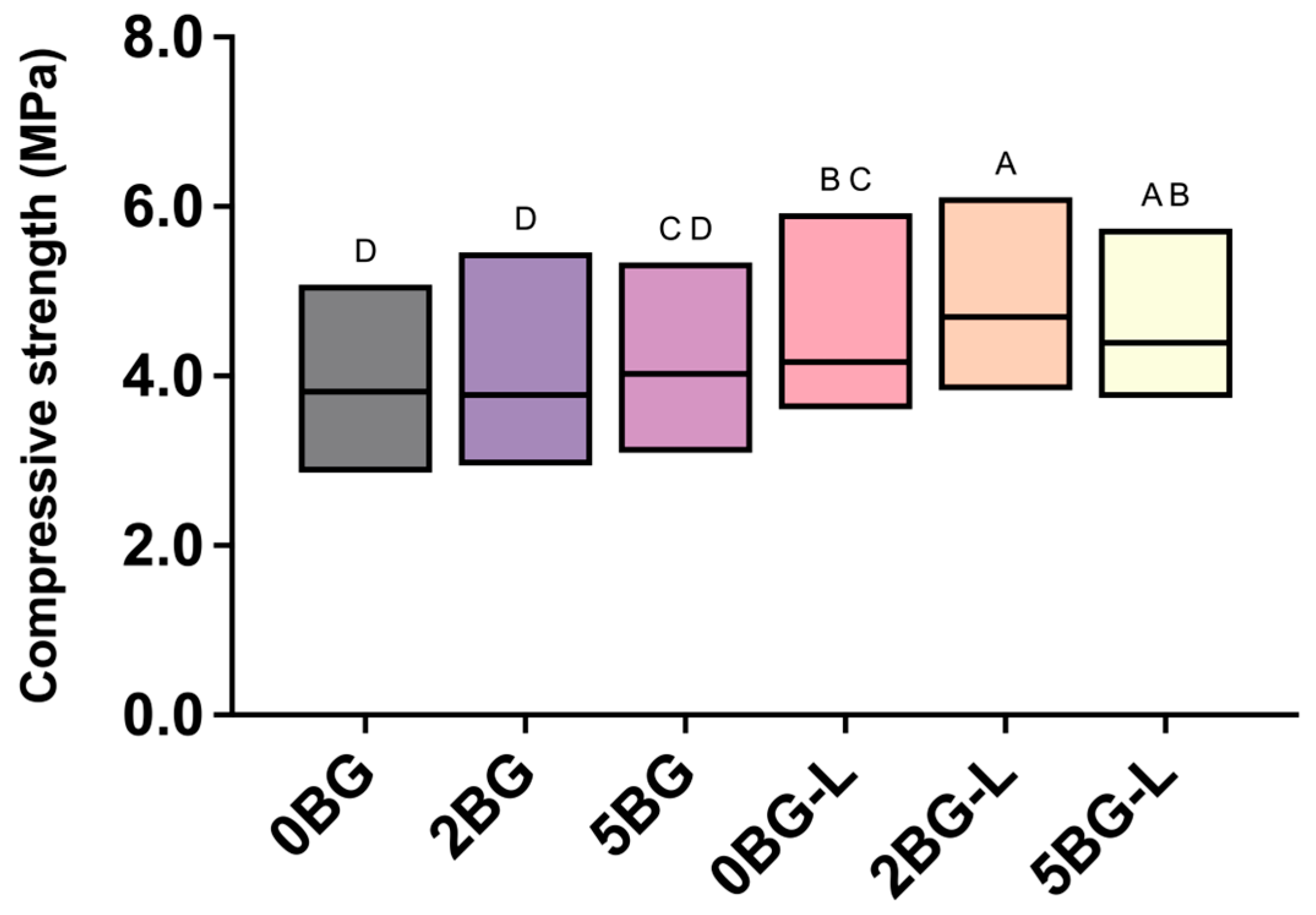
2.3. Compressive Strength Test
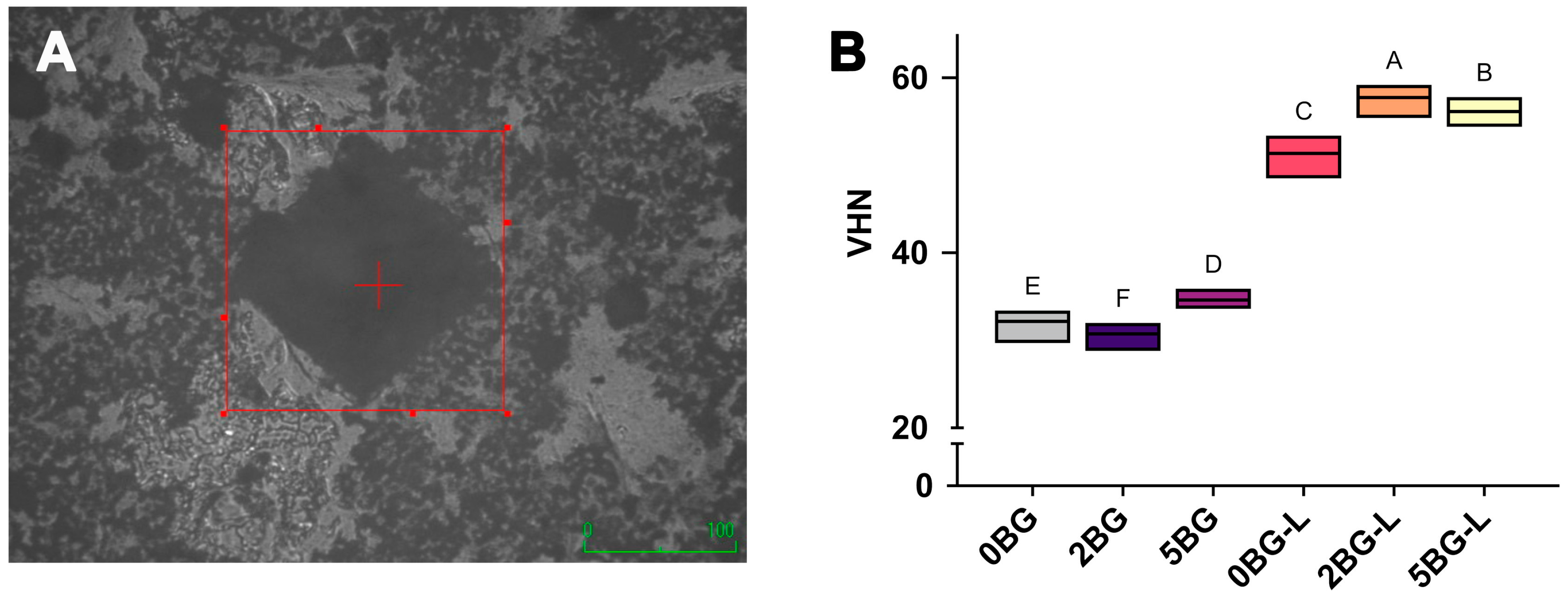
2.4. Microhardness
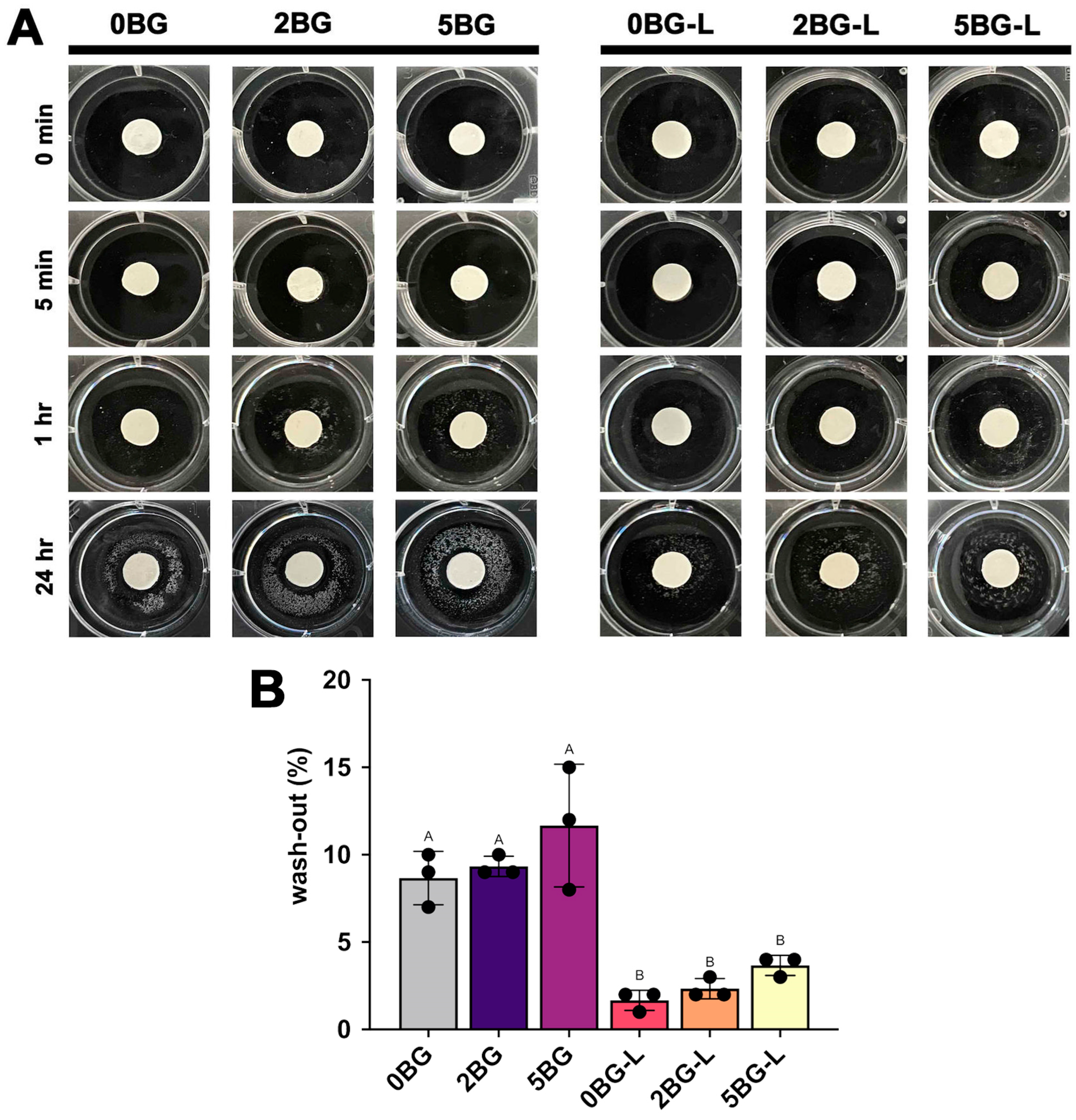
2.5. Wash-Out Resistance
2.6. Statistical Analysis
3. Results
3.1. Compressive Strength
3.2. Microhardness
3.3. Wash-Out Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eskandari, F.; Razavian, A.; Hamidi, R.; Yousefi, K.; Borzou, S. An Updated Review on Properties and Indications of Calcium Silicate-Based Cements in Endodontic Therapy. Int. J. Dent. 2022, 2022, 6858088. [Google Scholar] [CrossRef]
- Li, Q.; Coleman, N.J. The Hydration Chemistry of ProRoot MTA. Dent. Mater. J. 2015, 34, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Moshary, A.; Almasi, M.; Bitaraf, T. Evaluation of Cytotoxicity of ProRoot MTA and Endocem at Different Times and Con- Centrations on Human Gingival Fibroblasts. J. Res. Dent. Maxillofac. Sci. 2021, 6, 1–7. [Google Scholar]
- Kim, B.; Lee, Y.-H.; Kim, I.-H.; Lee, K.E.; Kang, C.-M.; Lee, H.-S.; Choi, H.-J.; Cheon, K.; Song, J.S.; Shin, Y. Biocompatibility and Mineralization Potential of New Calcium Silicate Cements. J. Dent. Sci. 2023, 18, 1189–1198. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part III: Clinical Applications, Drawbacks, and Mechanism of Action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, K.B.; Schwartz, S.A.; Schindler, W.G. Effect of Selected Accelerants on the Physical Properties of Mineral Trioxide Aggregate and Portland Cement. J. Endod. 2007, 33, 1235–1238. [Google Scholar] [CrossRef]
- Ber, B.S.; Hatton, J.F.; Stewart, G.P. Chemical Modification of ProRoot MTA to Improve Handling Characteristics and Decrease Setting Time. J. Endod. 2007, 33, 1231–1234. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ohtsuki, C. Design of Bioactive Bone Cement Based on Organic–Inorganic Hybrids. In Orthopaedic Bone Cements; Elsevier: Amsterdam, The Netherlands, 2008; pp. 358–376. ISBN 978-1-84569-376-3. [Google Scholar]
- Minet, J.; Abramson, S.; Bresson, B.; Franceschini, A.; Van Damme, H.; Lequeux, N. Organic Calcium Silicate Hydrate Hybrids: A New Approach to Cement Based Nanocomposites. J. Mater. Chem. 2006, 16, 1379. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; González, B. Medical Applications of Organic–Inorganic Hybrid Materials within the Field of Silica-Based Bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/Inorganic Hybrid Network Materials by the Sol−Gel Approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Kickelbick, G. Concepts for the Incorporation of Inorganic Building Blocks into Organic Polymers on a Nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar] [CrossRef]
- Baba, T.; Tsujimoto, Y. Examination of Calcium Silicate Cements with Low-Viscosity Methyl Cellulose or Hydroxypropyl Cellulose Additive. BioMed Res. Int. 2016, 2016, 4583854. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.-M.; Collar, E.P. Organic–Inorganic Hybrid Materials. Polymers 2020, 13, 86. [Google Scholar] [CrossRef]
- García-Martínez, J.-M.; Collar, E.P. On the Combined Effect of Both the Reinforcement and a Waste Based Interfacial Modifier on the Matrix Glass Transition in iPP/a-PP-pPBMA/Mica Composites. Polymers 2020, 12, 2606. [Google Scholar] [CrossRef] [PubMed]
- Gantar, A.; Drnovšek, N.; Casuso, P.; Vicente, A.P.-S.; Rodriguez, J.; Dupin, D.; Novak, S.; Loinaz, I. Injectable and Self-Healing Dynamic Hydrogel Containing Bioactive Glass Nanoparticles as a Potential Biomaterial for Bone Regeneration. RSC Adv. 2016, 6, 69156–69166. [Google Scholar] [CrossRef]
- Wang, E.; Lee, S.-H.; Lee, S.-W. Elastin-Like Polypeptide Based Hydroxyapatite Bionanocomposites. Biomacromolecules 2011, 12, 672–680. [Google Scholar] [CrossRef][Green Version]
- Meyer, D.E.; Chilkoti, A. Purification of Recombinant Proteins by Fusion with Thermally-Responsive Polypeptides. Nat. Biotechnol. 1999, 17, 1112–1115. [Google Scholar] [CrossRef]
- Desai, M.S.; Wang, E.; Joyner, K.; Chung, T.W.; Jin, H.-E.; Lee, S.-W. Elastin-Based Rubber-Like Hydrogels. Biomacromolecules 2016, 17, 2409–2416. [Google Scholar] [CrossRef]
- Kaou, M.H.; Furkó, M.; Balázsi, K.; Balázsi, C. Advanced Bioactive Glasses: The Newest Achievements and Breakthroughs in the Area. Nanomaterials 2023, 13, 2287. [Google Scholar] [CrossRef]
- Nettles, D.L.; Chilkoti, A.; Setton, L.A. Applications of Elastin-like Polypeptides in Tissue Engineering. Adv. Drug Deliv. Rev. 2010, 62, 1479–1485. [Google Scholar] [CrossRef]
- Bandiera, A.; Colomina-Alfaro, L.; Sist, P.; Gomez d’Ayala, G.; Zuppardi, F.; Cerruti, P.; Catanzano, O.; Passamonti, S.; Urbani, R. Physicochemical Characterization of a Biomimetic, Elastin-Inspired Polypeptide with Enhanced Thermoresponsive Properties and Improved Cell Adhesion. Biomacromolecules 2023, 24, 5277–5289. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, S.R.; Chilkoti, A. Elastin-like Polypeptides: Biomedical Applications of Tunable Biopolymers. Biopolymers 2010, 94, 60–77. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Vigneshwaran, M.; Qi, Y.; Zhou, Y.; Fu, G.; Li, Z.; Wang, J. Effect of Different Contents of 63s Bioglass on the Performance of Bioglass-PCL Composite Bone Scaffolds. Inventions 2023, 8, 138. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Salehi, R.; Fathi, M.H.; Seyedjafari, E.; Shafiee, A.; Soleimani, M. Cytotoxicity evaluation of 63s bioactive glass and bone-derived hydroxyapatite particles using human bone-marrow stem cells. Biomed. Pap. 2011, 155, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Biomaterials: A Forecast for the Future. Biomaterials 1998, 19, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Oliveira, A.A.R.; Lemos, E.M.F.; Pereira, M.M. Bioactive Glass Nanoparticles for Periodontal Regeneration and Applications in Dentistry. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 299–322. ISBN 978-1-4557-3127-5. [Google Scholar]
- Choi, M.; Kwon, J.; Jang, J.-H.; Kim, D.-S.; Kim, H.-J. Enhancing the Biological Properties of Organic–Inorganic Hybrid Calcium Silicate Cements: An In Vitro Study. J. Funct. Biomater. 2024, 15, 337. [Google Scholar] [CrossRef]
- Flores-Ledesma, A.; Barceló Santana, F.; Bucio, L.; Arenas-Alatorre, J.A.; Faraji, M.; Wintergerst, A.M. Bioactive Materials Improve Some Physical Properties of a MTA-like Cement. Mater. Sci. Eng. C 2017, 71, 150–155. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.-H.; Perez, R.A.; Kim, H.-W. Novel Bioactive Nanocomposite Cement Formulations with Potential Properties: Incorporation of the Nanoparticle Form of Mesoporous Bioactive Glass into Calcium Phosphate Cements. J. Mater. Chem. B 2015, 3, 1321–1334. [Google Scholar] [CrossRef]
- Chang, S.-W. Chemical Characteristics of Mineral Trioxide Aggregate and Its Hydration Reaction. Restor. Dent. Endod. 2012, 37, 188. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Lee, C.-O.; Kim, H.-J.; Kim, S.G.; Lee, S.-W.; Kim, S.-Y. Enhancing Effect of Elastinlike Polypeptide-Based Matrix on the Physical Properties of Mineral Trioxide Aggregate. J. Endod. 2018, 44, 1702–1708. [Google Scholar] [CrossRef]
- Jang, J.-H.; Shin, S.; Kim, H.-J.; Jeong, J.; Jin, H.-E.; Desai, M.S.; Lee, S.-W.; Kim, S.-Y. Improvement of Physical Properties of Calcium Phosphate Cement by Elastin-like Polypeptide Supplementation. Sci. Rep. 2018, 8, 5216. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.-J.; Chang, S.W.; Oh, S.; Kim, S.-Y.; Choi, K.-K.; Kim, D.-S.; Jang, J.-H. Effect of Bioactive Glass Addition on the Physical Properties of Mineral Trioxide Aggregate. Biomater. Res. 2021, 25, 39. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, S.; Jing, D.; Liu, N.; Luo, X. The Construction of Elastin-like Polypeptides and Their Applications in Drug Delivery System and Tissue Repair. J. Nanobiotechnol. 2023, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Orangi, J.; Asatourian, A.; Gutmann, J.L.; Garcia-Godoy, F.; Lotfi, M.; Sheibani, N. Calcium Silicate-Based Cements and Functional Impacts of Various Constituents. Dent. Mater. J. 2017, 36, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ledesma, A.; Tejeda-Cruz, A.; Bucio, L.; Wintergerst, A.M.; Rodríguez-Chávez, J.A.; Moreno-Vargas, Y.A.; Arenas-Alatorre, J.A. Hydration Products and Bioactivity of an Experimental MTA-like Cement Modified with Wollastonite and Bioactive Glass. Ceram. Int. 2020, 46, 15963–15971. [Google Scholar] [CrossRef]
- Kim, H.-J.; Bae, H.E.; Lee, J.-E.; Park, I.-S.; Kim, H.-G.; Kwon, J.; Kim, D.-S. Effects of Bioactive Glass Incorporation into Glass Ionomer Cement on Demineralized Dentin. Sci. Rep. 2021, 11, 7016. [Google Scholar] [CrossRef]
- Falkowska, J.; Chady, T.; Dura, W.; Droździk, A.; Tomasik, M.; Marek, E.; Safranow, K.; Lipski, M. The Washout Resistance of Bioactive Root-End Filling Materials. Materials 2023, 16, 5757. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Higa, R.K.; McKendry, D.J.; Pitt Ford, T.R. Dye Leakage of Four Root End Filling Materials: Effects of Blood Contamination. J. Endod. 1994, 20, 159–163. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Dhareshwar, V.; Sowmya, S.V.; Augustine, D.; Vinothkumar, T.S.; Renugalakshmi, A.; Shaiban, A.; Kakti, A.; Bhandi, S.H.; Dubey, A.; et al. Modified Mineral Trioxide Aggregate—A Versatile Dental Material: An Insight on Applications and Newer Advancements. Front. Bioeng. Biotechnol. 2022, 10, 941826. [Google Scholar] [CrossRef] [PubMed]
| Groups | Liquid | Powder | |
|---|---|---|---|
| Positive control | 0BG | DW | 100% MTA |
| Inorganic hybrid CSC | 2BG | DW | 98% MTA + 2% BG |
| 5BG | DW | 95% MTA + 5% BG | |
| Organic–inorganic hybrid CSC | 0BG-L | ELP | 100% MTA |
| 2BG-L | ELP | 98% MTA + 2% BG | |
| 5BG-L | ELP | 95% MTA + 5% BG |
| Component | Description | Composition/Details |
|---|---|---|
| Base Material | ProRoot MTA (Dentsply Sirona, Tulsa, OK, USA) | -75% White Portland cement (Ca3SiO5, Ca2SiO4, Ca3Al2O6) -20% Bi2O3 -5% CaSO4 |
| Inorganic Additive | 63S Bioactive Glass (Bonding Chemical, Katy, TX, USA) | -63% SiO2 -28% CaO -9% P2O5 -Particle size < 40 μm, purity 99.9% |
| Organic Additive | Elastin-like Polypeptide (V125E8) | -10 wt% aqueous solution -Synthesized via recombinant protein expression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Kim, H.-J. Enhancing the Physical Properties of Calcium Silicate Cement Modified with Elastin-like Polypeptides and Bioactive Glass. J. Funct. Biomater. 2025, 16, 188. https://doi.org/10.3390/jfb16050188
Kwon J, Kim H-J. Enhancing the Physical Properties of Calcium Silicate Cement Modified with Elastin-like Polypeptides and Bioactive Glass. Journal of Functional Biomaterials. 2025; 16(5):188. https://doi.org/10.3390/jfb16050188
Chicago/Turabian StyleKwon, Jiyoung, and Hyun-Jung Kim. 2025. "Enhancing the Physical Properties of Calcium Silicate Cement Modified with Elastin-like Polypeptides and Bioactive Glass" Journal of Functional Biomaterials 16, no. 5: 188. https://doi.org/10.3390/jfb16050188
APA StyleKwon, J., & Kim, H.-J. (2025). Enhancing the Physical Properties of Calcium Silicate Cement Modified with Elastin-like Polypeptides and Bioactive Glass. Journal of Functional Biomaterials, 16(5), 188. https://doi.org/10.3390/jfb16050188







