Nano/Micro-Enabled Modification and Innovation of Conventional Adjuvants for Next-Generation Vaccines
Abstract
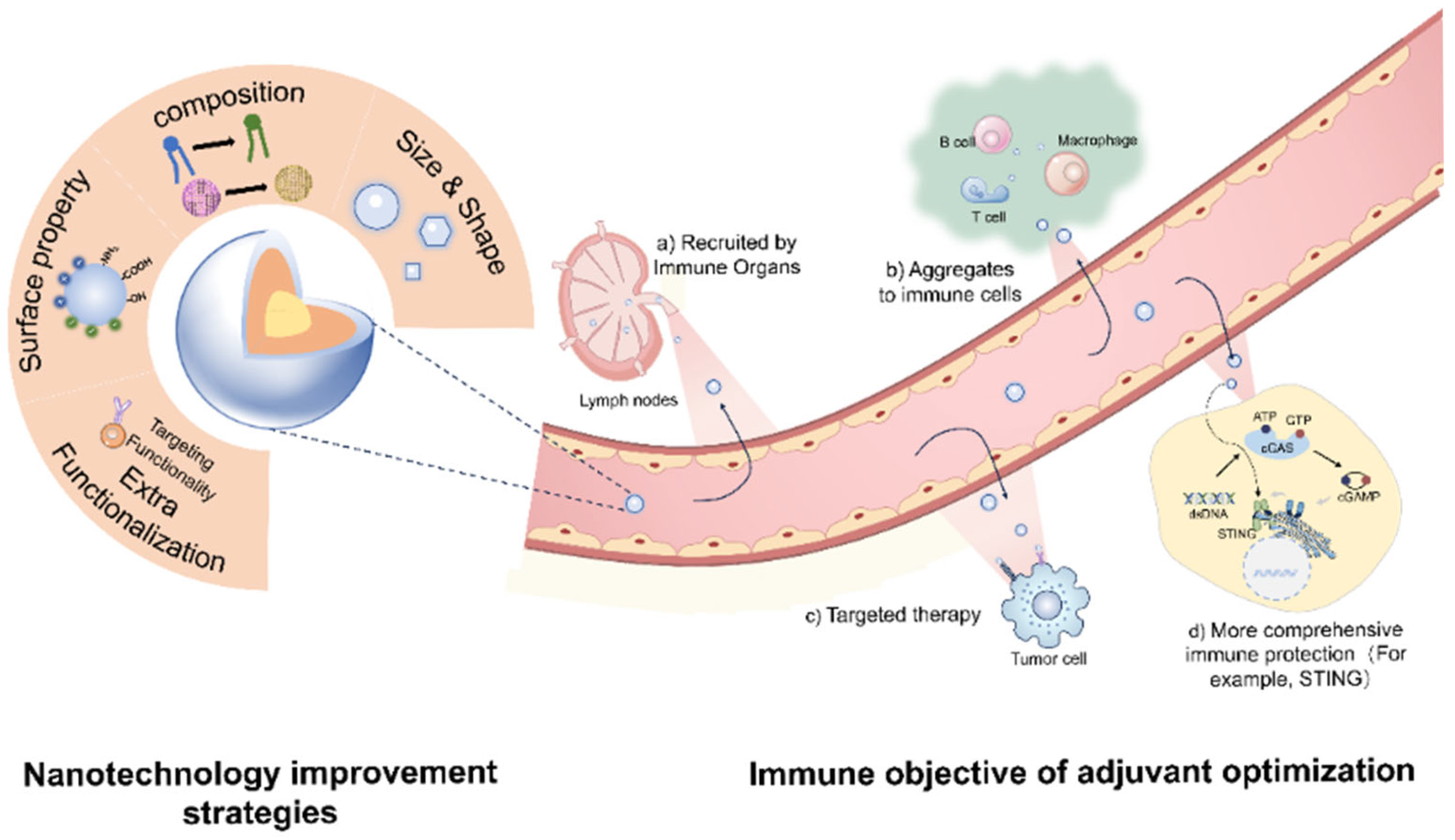
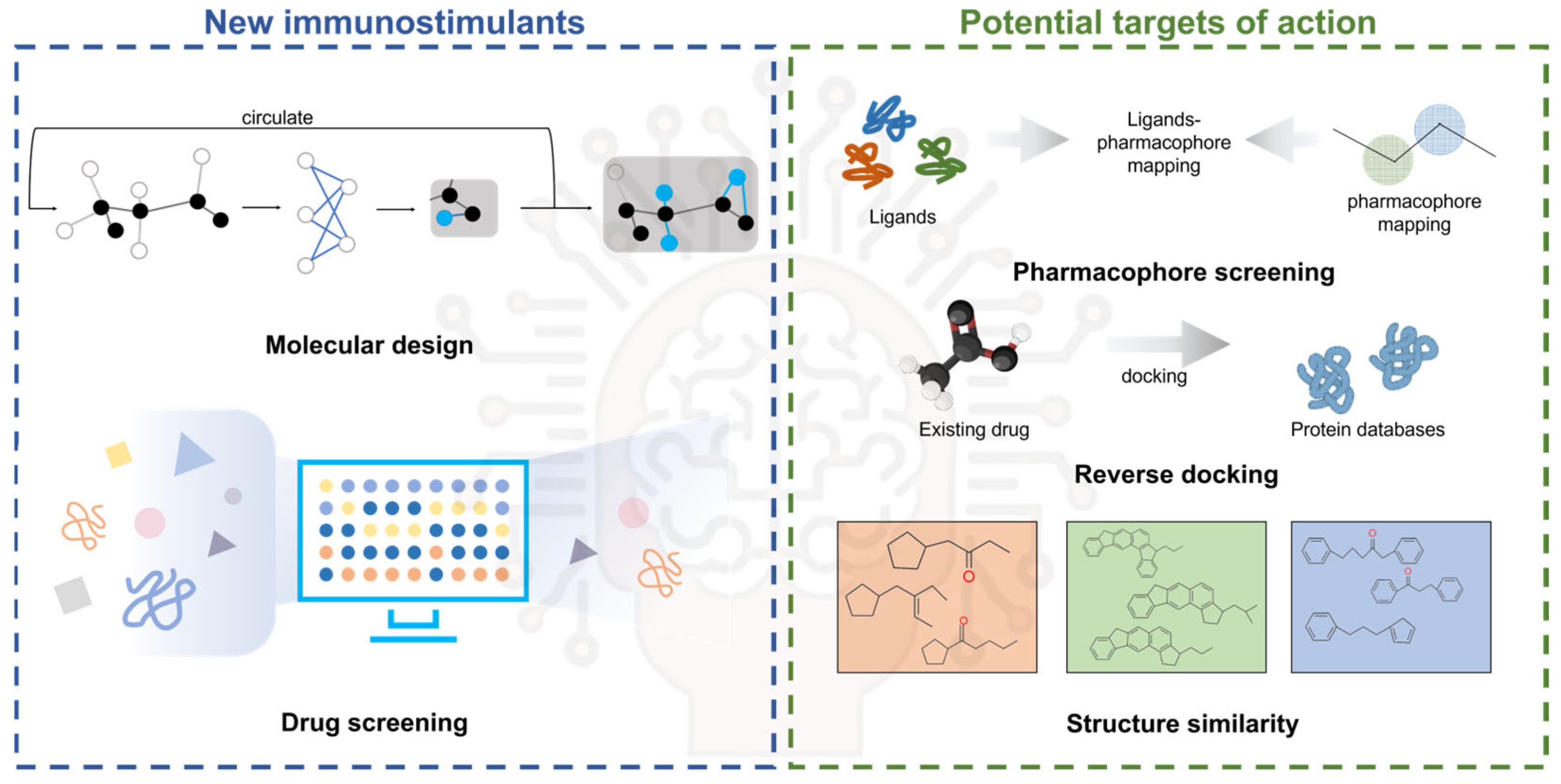
:1. Introduction
2. Aluminum
2.1. Overview of Current Research
2.2. Nanoalum
2.3. Composite Adjuvant Nano-Aluminum Salts
2.4. Surface Modification
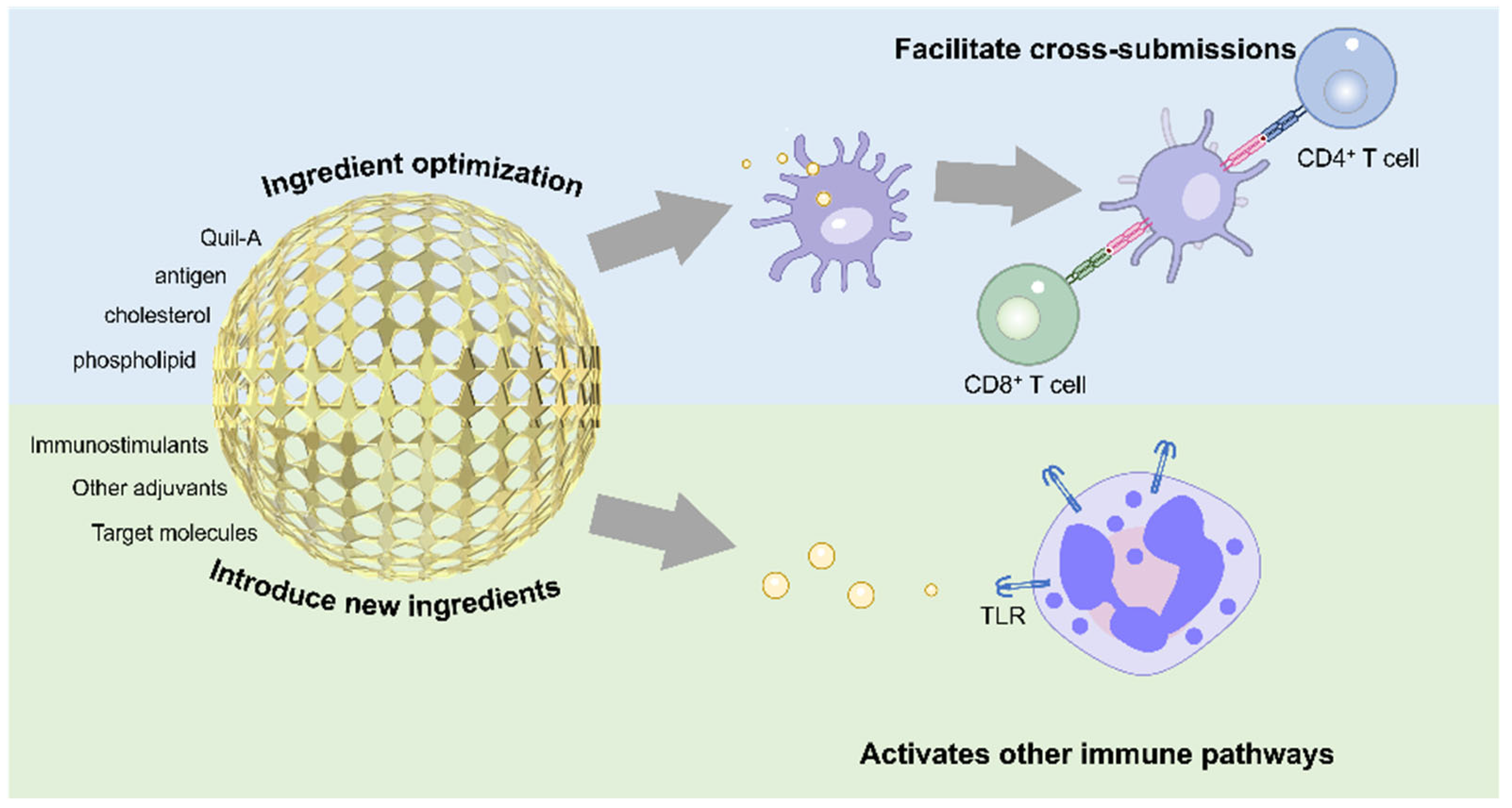
3. ISCOMs
3.1. Overview of Current Research
3.2. Nanostructure Modification Improvement
3.3. Nano-Functionalized Modification
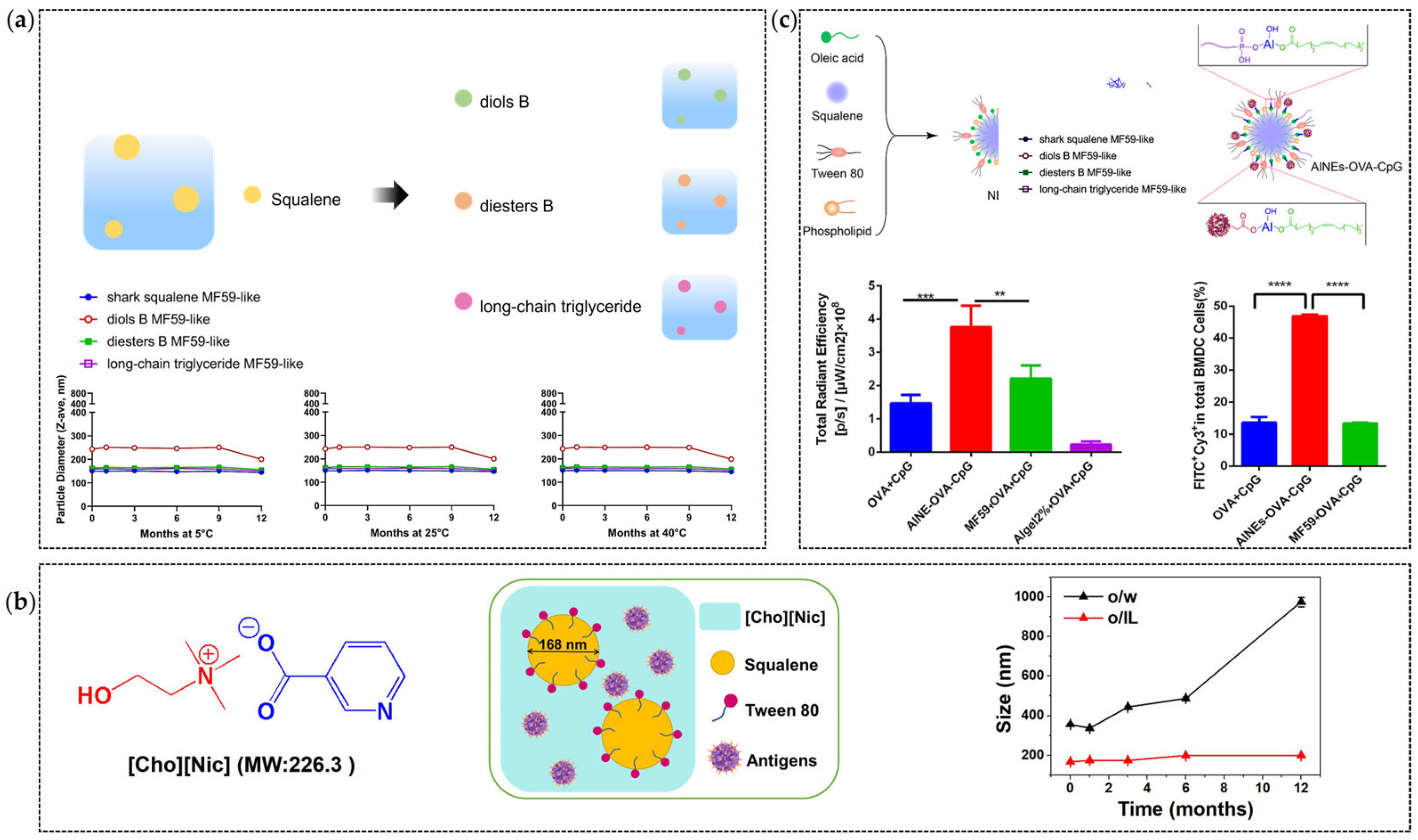
4. MF59
4.1. Overview of Current Research
4.2. Nano-Structural Adjustment
4.3. Optimization of Antigen Delivery Strategies
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trautmann, C.; Brüchle, W.; Spohr, R.; Vetter, J.; Angert, N. Pore geometry of etched ion tracks in polyimide. Nucl. Instrum. Methods Phys. Res. Sect. B 1996, 111, 70–74. [Google Scholar] [CrossRef]
- Gerhard, W.; Mozdzanowska, K.; Zharikova, D. Prospects for Universal Influenza Virus Vaccine. Emerg. Infect. Dis. 2006, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Sterner, E. Analyses of the 2023 Nobel Prize in Physiology or Medicine: Nucleoside Base Modifications and Effective mRNA Vaccines. Sci. Technol. Libr. 2024, 43, 1–17. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.-G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Xue, J.-B.; Lai, D.-Y.; Jiang, H.-W.; Qi, H.; Guo, S.-J.; Zhu, Y.-S.; Xu, H.; Zhou, J.; Tao, S.-C. Landscape of the RBD-specific IgG, IgM, and IgA responses triggered by the inactivated virus vaccine against the Omicron variant. Cell Discov. 2022, 8, 15. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Azman, A.S.; Sun, R.; Lu, W.; Zheng, N.; Zhou, J.; Wu, Q.; Deng, X.; Zhao, Z.; et al. Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants Induced by Natural Infection or Vaccination: A Systematic Review and Pooled Analysis. Clin. Infect. Dis. 2021, 74, 734–742. [Google Scholar] [CrossRef]
- Klein, N.P.; Fireman, B.; Yih, W.K.; Lewis, E.; Kulldorff, M.; Ray, P.; Baxter, R.; Hambidge, S.; Nordin, J.; Naleway, A.; et al. Measles-Mumps-Rubella-Varicella Combination Vaccine and the Risk of Febrile Seizures. Pediatrics 2010, 126, e1–e8. [Google Scholar] [CrossRef]
- Schink, T.; Holstiege, J.; Kowalzik, F.; Zepp, F.; Garbe, E. Risk of febrile convulsions after MMRV vaccination in comparison to MMR or MMR+V vaccination. Vaccine 2014, 32, 645–650. [Google Scholar] [CrossRef]
- Semenzato, L.; Le Vu, S.; Botton, J.; Bertrand, M.; Jabagi, M.-J.; Drouin, J.; Cuenot, F.; Zores, F.; Dray-Spira, R.; Weill, A.; et al. Long-Term Prognosis of Patients With Myocarditis Attributed to COVID-19 mRNA Vaccination, SARS-CoV-2 Infection, or Conventional Etiologies. JAMA 2024, 332, 1367–1377. [Google Scholar] [CrossRef]
- Pires-Marczeski, F.C.; Martinez, V.P.; Nemirovsky, C.; Padula, P.J. Intrathecal antibody production in two cases of yellow fever vaccine associated neurotropic disease in Argentina. J. Med. Virol. 2011, 83, 2208–2212. [Google Scholar] [CrossRef]
- Schillie, S.; Vellozzi, C.; Reingold, A.; Harris, A.; Haber, P.; Ward, J.W.; Nelson, N.P. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2018, 67, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Ren, Z.; Zhang, B.; Mao, L.; Zhu, G.; Gao, L.; Su, J.; Ye, J.; Long, Z.; Zhu, Y.; et al. Insufficient epitope-specific T cell clones are responsible for impaired cellular immunity to inactivated SARS-CoV-2 vaccine in older adults. Nat. Aging 2023, 3, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-E.; Jardine, L.; Gottgens, B.; Teichmann, S.A.; Haniffa, M. Prenatal development of human immunity. Science 2020, 368, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Exp. Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Foged, C. Subunit Vaccines of the Future: The Need for safe, Customized and Optimized Particulate Delivery Systems. Ther. Deliv. 2011, 2, 1057–1077. [Google Scholar] [CrossRef]
- Heidegger, S.; Gößl, D.; Schmidt, A.; Niedermayer, S.; Argyo, C.; Endres, S.; Bein, T.; Bourquin, C. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 938–948. [Google Scholar] [CrossRef]
- Kim, C.-G.; Lee, J.-C.; Ju, D.-B.; Kim, S.-K.; Yun, C.-H.; Cho, C.-S. Enhancement of Immune Responses Elicited by Nanovaccines through a Cross-Presentation Pathway. Tissue Eng. Regener. Med. 2023, 20, 355–370. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A Review on Biomedical Application of Polysaccharide-Based Hydrogels with a Focus on Drug Delivery Systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R. Insights into vaccines for elderly individuals: From the impacts of immunosenescence to delivery strategies. NPJ Vaccines 2024, 9, 77. [Google Scholar] [CrossRef]
- Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016, 16, 35–50. [Google Scholar] [CrossRef]
- Carroll, S.L.; Pasare, C.; Barton, G.M. Control of adaptive immunity by pattern recognition receptors. Immunity 2024, 57, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Martiñón, S.; Cisneros, A.; Villicaña, S.; Hernández-Miramontes, R.; Mixcoha, E.; Calderón-Vargas, P. Chemical and Immunological Characteristics of Aluminum-Based, Oil-Water Emulsion, and Bacterial-Origin Adjuvants. J. Immunol. Res. 2019, 2019, 3974127. [Google Scholar] [CrossRef]
- Yuan, L.; Gao, X.-D.; Xia, Y. Optimising the oil phases of aluminium hydrogel-stabilised emulsions for stable, safe and efficient vaccine adjuvant. Front. Chem. Sci. Eng. 2022, 16, 973–984. [Google Scholar] [CrossRef]
- Martina, B.E.E.; van de Bildt, M.W.G.; Kuiken, T.; van Amerongen, G.; Osterhaus, A.D.M.E. Immunogenicity and efficacy of recombinant subunit vaccines against phocid herpesvirus type 1. Vaccine 2003, 21, 2433–2440. [Google Scholar] [CrossRef]
- Ulmer, J. Vaccine Adjuvants: Mode of Action. Front. Immunol. 2013, 4, 214. [Google Scholar]
- Pedersen, G.K.; Wørzner, K.; Andersen, P.; Christensen, D. Vaccine Adjuvants Differentially Affect Kinetics of Antibody and Germinal Center Responses. Front. Immunol. 2020, 11, 579761. [Google Scholar] [CrossRef]
- Fox, C.B.; Kramer, R.M.; Barnes, V.L.; Dowling, Q.M.; Vedvick, T.S. Working together: Interactions between vaccine antigens and adjuvants. Ther. Adv. Vaccines 2013, 1, 7–20. [Google Scholar] [CrossRef]
- Cui, Y.; Ho, M.; Hu, Y.; Shi, Y. Vaccine adjuvants: Current status, research and development, licensing, and future opportunities. J. Mater. Chem. B 2024, 12, 4118–4137. [Google Scholar] [CrossRef]
- Qiao, D.; Li, L.; Liu, L.; Chen, Y. Universal and Translational Nanoparticulate CpG Adjuvant. ACS Appl. Mater. Interfaces 2022, 14, 50592–50600. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Ma, L.; Shao, Y.; Zhao, Y.; Chen, C. Morphologically Virus-Like Fullerenol Nanoparticles Act as the Dual-Functional Nanoadjuvant for HIV-1 Vaccine. Adv. Mater. 2013, 25, 5928–5936. [Google Scholar] [CrossRef]
- Moni, S.S.; Abdelwahab, S.I.; Jabeen, A.; Elmobark, M.E.; Aqaili, D.; Gohal, G.; Oraibi, B.; Farasani, A.M.; Jerah, A.A.; Alnajai, M.M.A.; et al. Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations. Vaccines 2023, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Barbateskovic, M.; Klingenberg, S.L.; Krauss, S.R.; Kong, D.; Wu, Z.; Petersen, S.B.; Kenfelt, M.; Gluud, C. Concentrations, Number of Doses, and Formulations of Aluminium Adjuvants in Vaccines: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Clinical Trials. Vaccines 2023, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Laera, D.; HogenEsch, H.; O’Hagan, D.T. Aluminum Adjuvants—‘Back to the Future’. Pharmaceutics 2023, 15, 1884. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; Van Nest, G. Recent advances in vaccine adjuvants: The development of MF59 emulsion and polymeric microparticles. Mol. Med. Today 1997, 3, 69–75. [Google Scholar] [CrossRef]
- Cohet, C.; van der Most, R.; Bauchau, V.; Bekkat-Berkani, R.; Doherty, T.M.; Schuind, A.; Tavares Da Silva, F.; Rappuoli, R.; Garçon, N.; Innis, B.L. Safety of AS03-adjuvanted influenza vaccines: A review of the evidence. Vaccine 2019, 37, 3006–3021. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Exp. Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Hoxie, I.; Vasilev, K.; Clark, J.J.; Bushfield, K.; Francis, B.; Loganathan, M.; Campbell, J.D.; Yu, D.; Guan, L.; Gu, C.; et al. A recombinant N2 neuraminidase-based CpG 1018® adjuvanted vaccine provides protection against challenge with heterologous influenza viruses in mice and hamsters. Vaccine 2024, 42, 126269. [Google Scholar] [CrossRef]
- Li, Y.; Wu, W.; Liu, Q.; Wu, Q.; Ren, P.; Xi, X.; Liu, H.; Zhao, J.; Zhang, W.; Wang, Z.; et al. Specific surface-modified iron oxide nanoparticles trigger complement-dependent innate and adaptive antileukaemia immunity. Nat. Commun. 2024, 15, 10400. [Google Scholar] [CrossRef]
- Croitoru, G.-A.; Pîrvulescu, D.-C.; Niculescu, A.-G.; Epistatu, D.; Rădulescu, M.; Grumezescu, A.M.; Nicolae, C.-L. Nanomaterials in Immunology: Bridging Innovative Approaches in Immune Modulation, Diagnostics, and Therapy. J. Funct. Biomater. 2024, 15, 225. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xia, H.; Zou, J.; Weaver, S.C.; Swanson, K.A.; Cai, H.; Cutler, M.; Cooper, D.; Muik, A.; et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature 2021, 596, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, J.; Wang, Z.; Liu, C.; Wang, T.; Kim, C.-J.; Durikova, H.; Fernandes, S.; Johnson, D.N.; De Rose, R.; et al. mRNA delivery enabled by metal–organic nanoparticles. Nat. Commun. 2024, 15, 9664. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Estapé Senti, M.; Labonia, M.C.I.; Papadopoulou, P.; Brans, M.A.D.; Dokter, I.; Fens, M.H.; van Mil, A.; Sluijter, J.P.G.; Schiffelers, R.M.; et al. Fusogenic Coiled-Coil Peptides Enhance Lipid Nanoparticle-Mediated mRNA Delivery upon Intramyocardial Administration. ACS Nano 2023, 17, 23466–23477. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakopoulos, L.; Moulia, D.L.; Godfrey, M.; Link-Gelles, R.; Roper, L.; Havers, F.P.; Taylor, C.A.; Stokley, S.; Talbot, H.K.; Schechter, R.; et al. Use of COVID-19 Vaccines for Persons Aged ≥6 Months: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024–2025. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 819–824. [Google Scholar] [CrossRef]
- Olshefsky, A.; Richardson, C.; Pun, S.H.; King, N.P. Engineering Self-Assembling Protein Nanoparticles for Therapeutic Delivery. Bioconjugate Chem. 2022, 33, 2018–2034. [Google Scholar] [CrossRef]
- Ikwuagwu, B.; Tullman-Ercek, D. Virus-like particles for drug delivery: A review of methods and applications. Curr. Opin. Biotechnol. 2022, 78, 102785. [Google Scholar] [CrossRef]
- Yang, J.-X.; Tseng, J.-C.; Yu, G.-Y.; Luo, Y.; Huang, C.-Y.F.; Hong, Y.-R.; Chuang, T.-H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Mochida, Y.; Uchida, S. mRNA vaccine designs for optimal adjuvanticity and delivery. RNA Biol. 2024, 21, 422–448. [Google Scholar] [CrossRef]
- Wang, S.H.; Smith, D.; Cao, Z.; Chen, J.; Acosta, H.; Chichester, J.A.; Yusibov, V.; Streatfield, S.J.; Fattom, A.; Baker, J.R. Recombinant H5 hemagglutinin adjuvanted with nanoemulsion protects ferrets against pathogenic avian influenza virus challenge. Vaccine 2019, 37, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, P.; Guo, D.; Chen, K.; Hu, Z.; Zou, Y. Research Progress of Aluminum Phosphate Adjuvants and Their Action Mechanisms. Pharmaceutics 2023, 15, 1756. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, H.; Rajesh, M.; Bhatnagar, R. Re-inventing traditional aluminum-based adjuvants: Insight into a century of advancements. Int. Rev. Immunol. 2025, 44, 58–81. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.F.M.; Haider, M.; Fahmy, S.A. From antigen uptake to immune modulation: The multifaceted potential of peptide nanofibers as vaccine nanocarriers. Mater. Adv. 2024, 5, 4112–4130. [Google Scholar] [CrossRef]
- Sun, B.; Ji, Z.; Liao, Y.-P.; Chang, C.H.; Wang, X.; Ku, J.; Xue, C.; Mirshafiee, V.; Xia, T. Enhanced Immune Adjuvant Activity of Aluminum Oxyhydroxide Nanorods through Cationic Surface Functionalization. ACS Appl. Mater. Interfaces 2017, 9, 21697–21705. [Google Scholar] [CrossRef]
- Büyükbayraktar, H.K.; Pelit Arayıcı, P.; Ihlamur, M.; Gökkaya, D.; Karahan, M.; Abamor, E.Ş.; Topuzoğulları, M. Effect of polycation coating on the long-term pulsatile release of antigenic ESAT-61–20 peptide from PLGA nanoparticles. Colloids Surf. B 2023, 228, 113421. [Google Scholar] [CrossRef]
- Liang, Z.; Bao, H.; Yao, Z.; Li, M.; Chen, C.; Zhang, L.; Wang, H.; Guo, Y.; Ma, Y.; Yang, X.; et al. The orientation of CpG conjugation on aluminum oxyhydroxide nanoparticles determines the immunostimulatory effects of combination adjuvants. Biomaterials 2024, 308, 122569. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Lee, D.; Huntoon, K.; Lux, J.; Kim, B.Y.S.; Jiang, W. Engineering nanomaterial physical characteristics for cancer immunotherapy. Nat. Rev. Bioeng. 2023, 1, 499–517. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Chea, E.K.; George, C.; Pillarsetty, N.; Gardner, J.R.; Livingston, P.O.; Ragupathi, G.; Lewis, J.S.; Tan, D.S.; Gin, D.Y. Development of a minimal saponin vaccine adjuvant based on QS-21. Nat. Chem. 2014, 6, 635–643. [Google Scholar] [CrossRef]
- Cruz-Bustos, T.; González-González, G.; Morales-Sanfrutos, J.; Megía-Fernández, A.; Santoyo-González, F.; Osuna, A. Functionalization of immunostimulating complexes (ISCOMs) with lipid vinyl sulfones and their application in immunological techniques and therapy. Int. J. Nanomed. 2012, 7, 5941–5956. [Google Scholar]
- Glenny, A.T. The antigenic value of toxoid precipitated by potassium alum. J. Pathol. Bacteriol. 1926, 29, 38–40. [Google Scholar]
- HogenEsch, H. Mechanism of Immunopotentiation and Safety of Aluminum Adjuvants. Front. Immunol. 2013, 3, 406. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.S.; Arunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Hem, S.L.; Johnston, C.T. Production and Characterization of Aluminum-Containing Adjuvants. In Vaccine Development and Manufacturing; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 319–346. [Google Scholar]
- Shardlow, E.; Mold, M.; Exley, C. Unraveling the enigma: Elucidating the relationship between the physicochemical properties of aluminium-based adjuvants and their immunological mechanisms of action. Allergy Asthma Clin. Immunol. 2018, 14, 80. [Google Scholar] [CrossRef]
- Rinella, J.V.; White, J.L.; Hem, S.L. Effect of Anions on Model Aluminum-Adjuvant-Containing Vaccines. J. Colloid. Interface Sci. 1995, 172, 121–130. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, G. Nano alum: A new solution to the new challenge. Hum. Vaccines Immunother. 2022, 18, 2060667. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, X.; Yu, G.; Li, M.; Shi, S.; Bao, H.; Chen, C.; Fu, D.; Ma, W.; Xue, C.; et al. Mechanistic understanding of the aspect ratio-dependent adjuvanticity of engineered aluminum oxyhydroxide nanorods in prophylactic vaccines. Nano Today 2022, 43, 101445. [Google Scholar] [CrossRef]
- Chen, W.; Zuo, H.; Li, B.; Duan, C.; Rolfe, B.; Zhang, B.; Mahony, T.J.; Xu, Z.P. Clay Nanoparticles Elicit Long-Term Immune Responses by Forming Biodegradable Depots for Sustained Antigen Stimulation. Small 2018, 14, 1704465. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, B.; Mahony, T.; Gu, W.; Rolfe, B.; Xu, Z.P. Efficient and Durable Vaccine against Intimin β of Diarrheagenic E. coli Induced by Clay Nanoparticles. Small 2016, 12, 1627–1639. [Google Scholar] [CrossRef]
- Ruwona, T.B.; Xu, H.; Li, X.; Taylor, A.N.; Shi, Y.-c.; Cui, Z. Toward understanding the mechanism underlying the strong adjuvant activity of aluminum salt nanoparticles. Vaccine 2016, 34, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.T.; Khandhar, A.P.; Seydoux, E.; Liang, H.; Gage, E.; Mikasa, T.; Beebe, E.L.; Rintala, N.D.; Persson, K.H.; Ahniyaz, A.; et al. Reprogramming the adjuvant properties of aluminum oxyhydroxide with nanoparticle technology. NPJ Vaccines 2019, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.G.; Xu, H.; Li, X.; Cui, Z. Uric acid and the vaccine adjuvant activity of aluminium (oxy)hydroxide nanoparticles. J. Drug Target. 2018, 26, 474–480. [Google Scholar] [CrossRef]
- Vrieling, H.; Espitia Ballestas, M.; Hamzink, M.; Willems, G.-J.; Soema, P.; Jiskoot, W.; Kersten, G.; Metz, B. Stabilised aluminium phosphate nanoparticles used as vaccine adjuvant. Colloids Surf. B 2019, 181, 648–656. [Google Scholar] [CrossRef]
- Lebre, F.; Pedroso de Lima, M.C.; Lavelle, E.C.; Borges, O. Mechanistic study of the adjuvant effect of chitosan-aluminum nanoparticles. Int. J. Pharm. 2018, 552, 7–15. [Google Scholar] [CrossRef]
- Wang, X.; Cao, F.; Yan, M.; Liu, Y.; Zhu, X.; Sun, H.; Ma, G. Alum-functionalized graphene oxide nanocomplexes for effective anticancer vaccination. Acta Biomater. 2019, 83, 390–399. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Li, L.; Zeng, Q.; Li, H.; Gong, T.; Zhang, Z.; Sun, X. Turning the Old Adjuvant from Gel to Nanoparticles to Amplify CD8+ T Cell Responses. Adv. Sci. 2018, 5, 1700426. [Google Scholar] [CrossRef]
- Li, D.; Xu, M.; Li, G.; Zheng, Y.; Zhang, Y.; Xia, D.; Wang, S.; Chen, Y. Mg/Al-LDH as a nano-adjuvant for pertussis vaccine: A evaluation compared with aluminum hydroxide adjuvant. Nanotechnology 2022, 33, 235102. [Google Scholar] [CrossRef]
- Sun, B.; Ji, Z.; Liao, Y.-P.; Wang, M.; Wang, X.; Dong, J.; Chang, C.H.; Li, R.; Zhang, H.; Nel, A.E.; et al. Engineering an Effective Immune Adjuvant by Designed Control of Shape and Crystallinity of Aluminum Oxyhydroxide Nanoparticles. ACS Nano 2013, 7, 10834–10849. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, R.; Nie, G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccines Immunother. 2014, 10, 2761–2774. [Google Scholar] [CrossRef]
- Mehdi, K.; Mohsen, M.; Naser Mohammadpour, D.; Mohsen, M.; Alireza, M. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef] [PubMed]
- Nazarizadeh, A.; Staudacher, A.H.; Wittwer, N.L.; Turnbull, T.; Brown, M.P.; Kempson, I. Aluminium Nanoparticles as Efficient Adjuvants Compared to Their Microparticle Counterparts: Current Progress and Perspectives. Int. J. Mol. Sci. 2022, 23, 4707. [Google Scholar] [CrossRef]
- Ghahary, M.; Taheri, R.A.; Fasihi-Ramandi, M.J.J.o.M.U.o.M.S. Protective Efficacy of the Killed Toxoplasma gondii Vaccine in Nano-alum Adjuvant. J. Maz. Univ. Med. Sci. 2019, 29, 11–23. [Google Scholar]
- Bi, S.; Li, M.; Liang, Z.; Li, G.; Yu, G.; Zhang, J.; Chen, C.; Yang, C.; Xue, C.; Zuo, Y.Y.; et al. Self-assembled aluminum oxyhydroxide nanorices with superior suspension stability for vaccine adjuvant. J. Colloid. Interface Sci. 2022, 627, 238–246. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nikkhoo, E.; Hussain, C.M. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coord. Chem. Rev. 2022, 451, 214262. [Google Scholar] [CrossRef]
- Ma, D.; Wang, G.; Lu, J.; Zeng, X.; Cheng, Y.; Zhang, Z.; Lin, N.; Chen, Q. Multifunctional nano MOF drug delivery platform in combination therapy. Eur. J. Med. Chem. 2023, 261, 115884. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, Y.; Tan, L.; Zheng, T.; Hou, Y.; Hong, X.; Du, G.; Chen, X.; Zhang, Y.; Sun, X. An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. J. Control. Release 2019, 300, 81–92. [Google Scholar] [CrossRef]
- Liu, J.; Guo, S.; Jin, Z.; Zhao, K. Adjuvanted quaternized chitosan composite aluminum nanoparticles-based vaccine formulation promotes immune responses in chickens. Vaccine 2023, 41, 2982–2989. [Google Scholar] [CrossRef]
- Sokal, E.M.; Hoppenbrouwers, K.; Vandermeulen, C.; Moutschen, M.; Léonard, P.; Moreels, A.; Haumont, M.; Bollen, A.; Smets, F.; Denis, M. Recombinant gp350 Vaccine for Infectious Mononucleosis: A Phase 2, Randomized, Double- Blind, Placebo-Controlled Trial to Evaluate the Safety, Immunogenicity, and Efficacy of an Epstein- Barr Virus Vaccine in Healthy Young Adults. J. Infect. Dis. 2007, 196, 1749–1753. [Google Scholar] [CrossRef]
- Garçon, N.; Morel, S.; Didierlaurent, A.; Descamps, D.; Wettendorff, M.; Van Mechelen, M. Development of an AS04-Adjuvanted HPV Vaccine with the Adjuvant System Approach. BioDrugs 2011, 25, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- He, C.; He, P.; Tang, X.; Bai, S.; Qin, M.; Zhang, Y.; Guo, Z.; Du, G.; Sun, X. Zoledronate-loaded aluminum salt nanovaccines amplify cellular immune response by enhancing cross-presentation. Nano Res. 2025, 18, 94907010. [Google Scholar] [CrossRef]
- Callahan, P.M.; Shorter, A.L.; Hem, S.L. The Importance of Surface Charge in the Optimization of Antigen–Adjuvant Interactions. Pharm. Res. 1991, 8, 851–858. [Google Scholar] [CrossRef]
- Yu, G.; Liang, Z.; Yu, Z.; Li, M.; Yang, W.; Zhang, Y.; Zhao, Y.; Yang, C.; Xue, C.; Shi, L.; et al. Engineering the hydroxyl content on aluminum oxyhydroxide nanorod for elucidating the antigen adsorption behavior. NPJ Vaccines 2022, 7, 62. [Google Scholar] [CrossRef]
- Chen, D.; Ling, X.; Wang, Y.; Zhang, Q.; He, X.; Dong, Z.; Li, M.; He, Q. Autophagy-activating aluminum hydroxide nanovaccine for enhanced antigen presentation and antitumor immunity. J. Control. Release 2025, 377, 223–235. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen–host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Chapter Seven—Metalloimmunology: The metal ion-controlled immunity. In Advances in Immunology; Dong, C., Jiang, Z., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 145, pp. 187–241. [Google Scholar]
- Li, Y.; Wang, C.; Lv, H.; Li, J.; Zhang, X.; Zhang, S.; Shen, Q.; Wu, Q.; Liu, Y.; Peng, R.; et al. Manganese-Modified Aluminum Adjuvant Enhances both Humoral and Cellular Immune Responses. Adv. Healthc. Mater. 2024, 13, 2401675. [Google Scholar] [CrossRef]
- Liu, D.; Liang, S.; Ma, K.; Meng, Q.-F.; Li, X.; Wei, J.; Zhou, M.; Yun, K.; Pan, Y.; Rao, L.; et al. Tumor Microenvironment-Responsive Nanoparticles Amplifying STING Signaling Pathway for Cancer Immunotherapy. Adv. Mater. 2024, 36, 2304845. [Google Scholar] [CrossRef]
- Sjölander, A.; Cox, J.C. Uptake and adjuvant activity of orally delivered saponin and ISCOM™ vaccines. Adv. Drug Deliv. Rev. 1998, 34, 321–338. [Google Scholar] [CrossRef]
- Barr, I.G.; Sjölander, A.; Cox, J.C. ISCOMs and other saponin based adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 247–271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Tuo, W. QS-21: A Potent Vaccine Adjuvant. Nat. Prod. Chem. Res. 2016, 3, e113. [Google Scholar]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.; Lu, S.; et al. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. [Google Scholar] [CrossRef]
- Pal, S.; Nath, S.; Meininger, C.J.; Gashev, A.A. Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology. Front. Immunol. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Silva, M.; Kato, Y.; Melo, M.B.; Phung, I.; Freeman, B.L.; Li, Z.; Roh, K.; Van Wijnbergen, J.W.; Watkins, H.; Enemuo, C.A.; et al. A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity. Sci. Immunol. 2021, 6, eabf1152. [Google Scholar] [CrossRef]
- Morein, B.; Lövgren, K.; Höglund, S.; Sundquist, B. The ISCOM: An immunostimulating complex. Immunol. Today 1987, 8, 333–338. [Google Scholar] [CrossRef]
- Morein, B.; Lövgren, K.; Rönnberg, B.; Sjölander, A.; Villacrés-Eriksson, M. Immunostimulating Complexes. Clin. Immunother. 1995, 3, 461–475. [Google Scholar] [CrossRef]
- Nielsen, H.M.; Hübschmann, H.B.; Rades, T. ISCOMs as a Vaccine Delivery System. In Subunit Vaccine Delivery; Foged, C., Rades, T., Perrie, Y., Hook, S., Eds.; Springer: New York, NY, USA, 2015; pp. 141–158. [Google Scholar]
- Cuevas-Romero, J.S.; Cerriteño-Sánchez, J.L.; Lara-Romero, R.; Vega-López, M.A.; Ramírez-Estudillo, C.; Ramírez-Mendoza, H.; Berg, M.; Lövgren-Bengtsson, K. Immunogenicity of a recombinant hemagglutinin neuraminidase-Porcine rubulavirus produced by Escherichia coli of Porcine rubulavirus gives protective immunity of litter after challenge. J. Vet. Med. Sci. 2022, 84, 1595–1604. [Google Scholar] [CrossRef]
- Chen, K.; Wang, N.; Zhang, X.; Wang, M.; Liu, Y.; Shi, Y. Potentials of saponins-based adjuvants for nasal vaccines. Front. Immunol. 2023, 14, 1153042. [Google Scholar] [CrossRef]
- Zhu, W.; Park, J.; Pho, T.; Wei, L.; Dong, C.; Kim, J.; Ma, Y.; Champion, J.A.; Wang, B.-Z. ISCOMs/MPLA-Adjuvanted SDAD Protein Nanoparticles Induce Improved Mucosal Immune Responses and Cross-Protection in Mice. Small 2023, 19, 2301801. [Google Scholar] [CrossRef]
- Pires, I.S.; Ni, K.; Melo, M.B.; Li, N.; Ben-Akiva, E.; Maiorino, L.; Dye, J.; Rodrigues, K.A.; Yun, D.; Kim, B.; et al. Controlled lipid self-assembly for scalable manufacturing of next-generation immune stimulating complexes. Chem. Eng. J. 2023, 464, 142664. [Google Scholar] [CrossRef] [PubMed]
- White, K.; Rades, T.; Kearns, P.; Toth, I.; Hook, S. Immunogenicity of Liposomes Containing Lipid Core Peptides and the Adjuvant Quil A. Pharm. Res. 2006, 23, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Myschik, J.; McBurney, W.T.; Hennessy, T.; Phipps-Green, A.; Rades, T.; Hook, S. Immunostimulatory biodegradable implants containing the adjuvant Quil-A—Part II: In vivo evaluation. J. Drug Target. 2008, 16, 224–232. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.A.; Galloway, J.; et al. Safety and Efficacy of the NVX-CoV2373 Coronavirus Disease 2019 Vaccine at Completion of the Placebo-Controlled Phase of a Randomized Controlled Trial. Clin. Infect. Dis. 2022, 76, 398–407. [Google Scholar] [CrossRef]
- Kersten, G.F.; Teerlink, T.; Derks, H.J.; Verkleij, A.J.; Wezel, T.L.v.; Crommelin, D.J.; Beuvery, E.C. Incorporation of the major outer membrane protein of Neisseria gonorrhoeae in saponin-lipid complexes (iscoms): Chemical analysis, some structural features, and comparison of their immunogenicity with three other antigen delivery systems. Infect Immun. 1988, 56, 432–438. [Google Scholar] [CrossRef]
- Demana, P.H.; Fehske, C.; White, K.; Rades, T.; Hook, S. Effect of incorporation of the adjuvant Quil A on structure and immune stimulatory capacity of liposomes. Immunol. Cell Biol. 2004, 82, 547–554. [Google Scholar] [CrossRef]
- Lövgren Bengtsson, K.; Morein, B.; Osterhaus, A.D.M.E. ISCOM technology-based Matrix M™ adjuvant: Success in future vaccines relies on formulation. Exp. Rev. Vaccines 2011, 10, 401–403. [Google Scholar] [CrossRef]
- Stertman, L.; Palm, A.-K.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-M™ adjuvant: A critical component of vaccines for the 21st century. Hum. Vaccines Immunother. 2023, 19, 2189885. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Hernández, A.Q.B.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.; Rades, T. Immune Stimulating Complexes (ISCOMs) and Quil-A Containing Particulate Formulations as Vaccine Delivery Systems. In Immunomic Discovery of Adjuvants and Candidate Subunit Vaccines; Flower, D.R., Perrie, Y., Eds.; Springer: New York, NY, USA, 2013; pp. 233–261. [Google Scholar]
- Li, I.A.; Popov, A.M.; Sanina, N.M.; Kostetskii, E.Y.; Novikova, O.D.; Reunov, A.V.; Nagorskaya, V.P.; Portnyagina, O.Y.; Khomenko, V.A.; Shnyrov, V.L. Physicochemical and Immune Properties of Glycoglycerolipids from Laminaria japonicain Immunostimulating Complexes (ISCOMs). Biol. Bull. Russ. Acad. Sci. 2004, 31, 244–249. [Google Scholar] [CrossRef]
- Lendemans, D.G.; Egert, A.M.; Hook, S.; Rades, T. Cage-like complexes formed by DOTAP, Quil-A and cholesterol. Int. J. Pharm. 2007, 332, 192–195. [Google Scholar] [CrossRef]
- Lendemans, D.G.; Myschik, J.; Hook, S.; Rades, T. Cationic cage-like complexes formed by DC-cholesterol, Quil-A, and phospholipid. J. Pharm. Sci. 2005, 94, 1794–1807. [Google Scholar] [CrossRef]
- Höglund, S.; Dalsgaard, K.; Lövgren, K.; Sundquist, B.; Osterhaus, A.; Morein, B. ISCOMs and Immunostimulation with Viral Antigens. In Virally Infected Cells; Harris, J.R., Ed.; Springer: Boston, MA, USA, 1989; pp. 39–68. [Google Scholar]
- Morein, B.; Bengtsson, K.L. Immunomodulation by Iscoms, Immune Stimulating Complexes. Methods 1999, 19, 94–102. [Google Scholar] [CrossRef]
- Morein, B.; Bengtsson, K.L. Functional aspects of iscoms. Immunol. Cell Biol. 1998, 76, 295–299. [Google Scholar] [CrossRef]
- Qiao, X.; Qu, L.; Guo, Y.; Hoshino, T. Secondary Structure and Conformational Stability of the Antigen Residues Making Contact with Antibodies. J. Phys. Chem. B 2021, 125, 11374–11385. [Google Scholar] [CrossRef]
- Qu, L.; Qiao, X.; Qi, F.; Nishida, N.; Hoshino, T. Analysis of Binding Modes of Antigen–Antibody Complexes by Molecular Mechanics Calculation. J. Chem. Inf. Model. 2021, 61, 2396–2406. [Google Scholar] [CrossRef]
- Le, T.T.T.; Drane, D.; Malliaros, J.; Cox, J.C.; Rothel, L.; Pearse, M.; Woodberry, T.; Gardner, J.; Suhrbier, A. Cytotoxic T cell polyepitope vaccines delivered by ISCOMs. Vaccine 2001, 19, 4669–4675. [Google Scholar] [CrossRef]
- Buglione-Corbett, R.; Pouliot, K.; Marty-Roix, R.; Li, W.; West, K.; Wang, S.; Morelli, A.B.; Lien, E.; Lu, S. Reduced MyD88 dependency of ISCOMATRIX™ adjuvant in a DNA prime-protein boost HIV vaccine. Hum. Vaccines Immunother. 2014, 10, 1078–1090. [Google Scholar] [CrossRef]
- De Libero, G.; Mori, L. Recognition of lipid antigens by T cells. Nat. Rev. Immunol. 2005, 5, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Alving, C.R. Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen-presenting cells. Adv. Drug Deliv. Rev. 2000, 41, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Moody, D.B.; Zajonc, D.M.; Wilson, I.A. Anatomy of CD1–lipid antigen complexes. Nat. Rev. Immunol. 2005, 5, 387–399. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Weeratna, R.D.; Evans, D.M.; Makinen, S.; Drane, D.; Davis, H.L. CpG ODN and ISCOMATRIX adjuvant: A synergistic adjuvant combination inducing strong T-Cell IFN-γ responses. Biomed. Res. Int. 2013, 2013, 636847. [Google Scholar] [CrossRef]
- Ko, E.-J.; Kang, S.-M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccines Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Toledo, E.; Gaforio, J.J. Squalene Stimulates a Key Innate Immune Cell to Foster Wound Healing and Tissue Repair. Evid. Based Complement. Altern. Med. 2018, 2018, 9473094. [Google Scholar] [CrossRef]
- Ho, H.-M.; Huang, C.-Y.; Cheng, Y.-J.; Shen, K.-Y.; Tzeng, T.-T.; Liu, S.-J.; Chen, H.-W.; Huang, C.-H.; Huang, M.-H. Assessment of adjuvantation strategy of lipid squalene nanoparticles for enhancing the immunogenicity of a SARS-CoV-2 spike subunit protein against COVID-19. Int. J. Pharm. 2021, 607, 121024. [Google Scholar] [CrossRef]
- Valenzuela, P.; Medina, A.; Rutter, W.J.; Ammerer, G.; Hall, B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 1982, 298, 347–350. [Google Scholar] [CrossRef]
- Noel Masihi, K.; Lange, W.; Brehmer, W.; Ribi, E. Immunobiological activities of nontoxic lipid A: Enhancement of nonspecific resistance in combination with trehalose dimycolate against viral infection and adjuvant effects. Int. J. Immunopharmacol. 1986, 8, 339–345. [Google Scholar] [CrossRef]
- Allison, A.C.; Byars, N.E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J. Immunol. Methods 1986, 95, 157–168. [Google Scholar] [CrossRef]
- Shi, S.; Liang, Z.; Sun, B. Response to comment on: Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2020, 38, 2759. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Seubert, A.; Monaci, E.; Pizza, M.; O’Hagan, D.T.; Wack, A. The Adjuvants Aluminum Hydroxide and MF59 Induce Monocyte and Granulocyte Chemoattractants and Enhance Monocyte Differentiation toward Dendritic Cells1. J. Immunol. 2008, 180, 5402–5412. [Google Scholar] [CrossRef]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Exp. Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- Chappell, K.J.; Mordant, F.L.; Li, Z.; Wijesundara, D.K.; Ellenberg, P.; Lackenby, J.A.; Cheung, S.T.M.; Modhiran, N.; Avumegah, M.S.; Henderson, C.L.; et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2021, 21, 1383–1394. [Google Scholar] [CrossRef]
- Amarowicz, R. Squalene: A natural antioxidant? Eur. J. Lipid Sci. Technol. 2009, 111, 411–412. [Google Scholar] [CrossRef]
- Lin, X.; Sheng, Y.; Zhang, X.; Li, Z.; Yang, Y.; Wu, J.; Su, Z.; Ma, G.; Zhang, S. Oil-in-ionic liquid nanoemulsion-based intranasal delivery system for influenza split-virus vaccine. J. Control. Release 2022, 346, 380–391. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, P.; Li, S.; Wei, D.; Zhang, Z.; Hao, S.; Li, W.; Wang, X.; Xu, J. Artificial Nanolipid Droplets with Monolayer Lecithin Membranes and Vitamin E Cores as Vaccine Adjuvants. ACS Appl. Nano Mater. 2022, 5, 15011–15020. [Google Scholar] [CrossRef]
- Fisher, K.J.; Kinsey, R.; Mohamath, R.; Phan, T.; Liang, H.; Orr, M.T.; Lykins, W.R.; Guderian, J.A.; Bakken, J.; Argilla, D.; et al. Semi-synthetic terpenoids with differential adjuvant properties as sustainable replacements for shark squalene in vaccine emulsions. NPJ Vaccines 2023, 8, 14. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, X.; Wang, H.; Zhong, X.; Chen, X.; Zhao, Y.; Zhang, Y.; Du, G.; Sun, X. Smart combination of aluminum hydroxide and MF59 to induce strong cellular immune responses. J. Control. Release 2022, 349, 699–711. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, J.; Wei, W.; Du, Y.; Wan, T.; Ma, X.; An, W.; Guo, A.; Miao, C.; Yue, H.; et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat. Mater. 2018, 17, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Big family of nano- and microscale drug delivery systems ranging from inorganic materials to polymeric and stimuli-responsive carriers as well as drug-conjugates. J. Drug Deliv. Sci. Technol. 2021, 66, 102790. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Xu, J.; Gattacceca, F.; Amiji, M. Biodistribution and Pharmacokinetics of EGFR-Targeted Thiolated Gelatin Nanoparticles Following Systemic Administration in Pancreatic Tumor-Bearing Mice. Mol. Pharm. 2013, 10, 2031–2044. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Ott, G.; Barchfeld, G.L.; Nest, G.V. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine 1995, 13, 1557–1562. [Google Scholar] [CrossRef]
- Dupuis, M.; McDonald, D.M.; Ott, G. Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine 1999, 18, 434–439. [Google Scholar] [CrossRef]
- Iyer, S.; HogenEsch, H.; Hem, S.L. Relationship between the degree of antigen adsorption to aluminum hydroxide adjuvant in interstitial fluid and antibody production. Vaccine 2003, 21, 1219–1223. [Google Scholar] [CrossRef]
- Gao, X.; Gong, J.; Cai, Y.; Wang, J.; Wen, J.; Peng, L.; Ji, H.; Jiang, S.; Guo, D. Chitosan modified squalene nanostructured lipid carriers as a promising adjuvant for freeze-dried ovalbumin vaccine. Int. J. Biol. Macromol. 2021, 188, 855–862. [Google Scholar] [CrossRef]
- Bravo, L.; Smolenov, I.; Han, H.H.; Li, P.; Hosain, R.; Rockhold, F.; Clemens, S.A.C.; Roa, C., Jr.; Borja-Tabora, C.; Quinsaat, A.; et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: A phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2022, 399, 461–472. [Google Scholar] [CrossRef]
- Gorse, G.J.; Grimes, S.; Buck, H.; Mulla, H.; White, P.; Hill, H.; May, J.; Frey, S.E.; Blackburn, P. A phase 1 dose-sparing, randomized clinical trial of seasonal trivalent inactivated influenza vaccine combined with MAS-1, a novel water-in-oil adjuvant/delivery system. Vaccine 2022, 40, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Schuind, A.E.; Adjei, S.; Antony, K.; Aponte, J.J.; Buabeng, P.B.Y.; Qadri, F.; Kemp, T.J.; Hossain, L.; Pinto, L.A.; et al. Safety and immunogenicity of Innovax bivalent human papillomavirus vaccine in girls 9–14 years of age: Interim analysis from a phase 3 clinical trial. Vaccine 2024, 42, 2290–2298. [Google Scholar] [CrossRef]
- Dayan, G.H.; Rouphael, N.; Walsh, S.R.; Chen, A.; Grunenberg, N.; Allen, M.; Antony, J.; Bhate, A.S.; Beresnev, T.; Bonaparte, M.I.; et al. Efficacy of a monovalent (D614) SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant in adults: A phase 3, multi-country study. eClinicalMedicine 2023, 64, 102168. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Popa, X.; Martínez, O.; Mendoza, S.; Santiesteban, E.; Crespo, T.; Amador, R.M.; Fleytas, R.; Acosta, S.C.; Otero, Y.; et al. A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvax-EGF as Switch Maintenance Therapy in Advanced Non–Small Cell Lung Cancer Patients. Clin. Cancer Res. 2016, 22, 3782–3790. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, J.; Guo, L.; Zhu, S.; Hou, X.; Cao, S.; Zhou, M.; Shi, J.; Li, J.; Liu, K.; et al. DNA-Engineered Degradable Invisibility Cloaking for Tumor-Targeting Nanoparticles. J. Am. Chem. Soc. 2024, 146, 25253–25262. [Google Scholar] [CrossRef]
- Mohammapdour, R.; Ghandehari, H. Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv. Drug Deliv. Rev. 2022, 180, 114022. [Google Scholar] [CrossRef]
- Heneweer, C.; Gendy, S.E.M.; Peñate-Medina, O. Liposomes and Inorganic Nanoparticles for Drug Delivery and Cancer Imaging. Ther. Deliv. 2012, 3, 645–656. [Google Scholar] [CrossRef]
- Kisby, T.; Yilmazer, A.; Kostarelos, K. Reasons for success and lessons learnt from nanoscale vaccines against COVID-19. Nat. Nanotechnol. 2021, 16, 843–850. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef]
- Ahmed, M.; Kurungottu, P.; Swetha, K.; Atla, S.; Ashok, N.; Nagamalleswari, E.; Bonam, S.R.; Sahu, B.D.; Kurapati, R. Role of NLRP3 inflammasome in nanoparticle adjuvant-mediated immune response. Biomater. Sci. 2025, 13, 2164–2178. [Google Scholar] [CrossRef] [PubMed]

| Name | Composition | Activation Mechanisms and Interactions with the Immune System | Vaccine Application | Limitations | Reference |
|---|---|---|---|---|---|
| Aluminum Adjuvant | Aluminum phosphate, aluminum hydroxide, aluminum potassium sulfate, amorphous aluminum phosphate, etc. | Th2 humoral immune response induction by activating NLRP3 inflammasome | Widely used in vaccines for diphtheria, tetanus, pertussis, Haemophilus influenza type B, pneumococcal, hepatitis A, hepatitis B, etc. | Th2-biased, low antigen-binding capacity, prone to aggregation | [32] |
| AS04 | Combination of 3-O-deacyl-4′-monophosphoryl lipid A (MPL) and aluminum salt | Th1/Th2 immune response induction by activating NLRP3 inflammasome | Cervarix for preventing HPV and Fendrix for preventing hepatitis B | Weak Th1 activation, poor receptor synergy | [33] |
| MF59 | Oil-in-water emulsion made of squalene, polysorbate 80 (Tween 80), and sorbitan trioleate (Span 85) | Th1 and Th2 induction via ATP release from muscle fibers, initiating CD4⁺ T-cell priming and NLRP3 inflammasome activation | Influenza vaccines, particularly for seasonal and pandemic flu in the elderly | Low antigen affinity, poor stability | [34] |
| AS03 | Oil-in-water emulsion containing DL-α-tocopherol, squalene, and polysorbate 80 (Tween 80) | Th1 and Th2 induction via NF-κB pathway and upregulation of inflammatory cytokines such as IL-6 and TNF-α | H1N1 influenza vaccines and RTS, S malaria vaccine | Harsh prep, unstable, local toxicity | [35] |
| AS01b | Liposome containing 3-O-deacyl-4′-monophosphoryl lipid A (MPL) and saponin QS-21 | Th1 induction via TLR4 stimulation on dendritic cells (DCs) | Shingrix for shingles and Mosquirix for malaria | Local/systemic toxicity, QS-21-linked risk, challenging for large-scale manufacturing | [36] |
| CpG1018 | CpG oligodeoxynucleotide (TLR9 agonist) | Th1 induction via TLR9-triggered DC maturation | Heplisav-B for hepatitis B | No self-delivery, carrier-dependent | [37] |
| Antigen Type | Intrinsic Characteristics | Primary Benefits of Nano-Adjuvant Modification | Representative Nano-Adjuvants | Representative Applications | References |
|---|---|---|---|---|---|
| Protein Antigens | Large molecular weight, naturally immunogenic but prone to degradation | Stabilizes antigen structure, enhances uptake, prolongs immune persistence | Nano-aluminum salts (e.g., nano-Al(OH)3), ISCOMs | Influenza vaccines, Hepatitis B vaccines, DTaP vac-cines | [51,52,53] |
| Peptide Antigens | Small molecules with weak immunogenicity | Promotes cellular uptake, enhances cross-presentation, activates CTL responses | ISCOMs, PLGA nanoparticles, cationic nanoemulsions | Tuberculosis vac-cines, malaria vaccines | [54,55,56] |
| Nucleic Acid Antigens | Easily degraded, requires intracellular expression | Improves stability, facilitates intracellular delivery and translational expression | LNPs, polymeric carriers (e.g., PEI, chitosan), nanoemulsions | COVID-19 mRNA vaccines, DNA vaccines | [57,58] |
| Name | Adjuvant Used | Trial Phase | Reference |
|---|---|---|---|
| SCB-2019 | CpG/Alum adjuvants | Phase III Clinical Trial | [165] |
| MAS-1 | A novel emulsion adjuvant | Phase I Clinical Trial | [166] |
| ChAdOx1 nCoV-19 | Matrix-M | Phase II Clinical Trial | [167] |
| Cecolin | ALOOH | Phase III Clinical Trial | [168] |
| CoV2 preS dTM-AS03 Monovalent (D614) | AS03 | Phase III Clinical Trial | [169] |
| CIMAVAX-EGF | Montanide ISA 51 | Phase III Clinical Trial | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, X.; Tao, L.; Li, X.; Chen, G.; Liu, Q. Nano/Micro-Enabled Modification and Innovation of Conventional Adjuvants for Next-Generation Vaccines. J. Funct. Biomater. 2025, 16, 185. https://doi.org/10.3390/jfb16050185
Liu X, Yang X, Tao L, Li X, Chen G, Liu Q. Nano/Micro-Enabled Modification and Innovation of Conventional Adjuvants for Next-Generation Vaccines. Journal of Functional Biomaterials. 2025; 16(5):185. https://doi.org/10.3390/jfb16050185
Chicago/Turabian StyleLiu, Xingchi, Xu Yang, Lu Tao, Xuanchen Li, Guoqiang Chen, and Qi Liu. 2025. "Nano/Micro-Enabled Modification and Innovation of Conventional Adjuvants for Next-Generation Vaccines" Journal of Functional Biomaterials 16, no. 5: 185. https://doi.org/10.3390/jfb16050185
APA StyleLiu, X., Yang, X., Tao, L., Li, X., Chen, G., & Liu, Q. (2025). Nano/Micro-Enabled Modification and Innovation of Conventional Adjuvants for Next-Generation Vaccines. Journal of Functional Biomaterials, 16(5), 185. https://doi.org/10.3390/jfb16050185







