A Comprehensive Literature Review for Total Hip Arthroplasty (THA): Part 2—Material Selection Criteria and Methods
Abstract
1. Introduction
2. Literature Analysis and Methodology
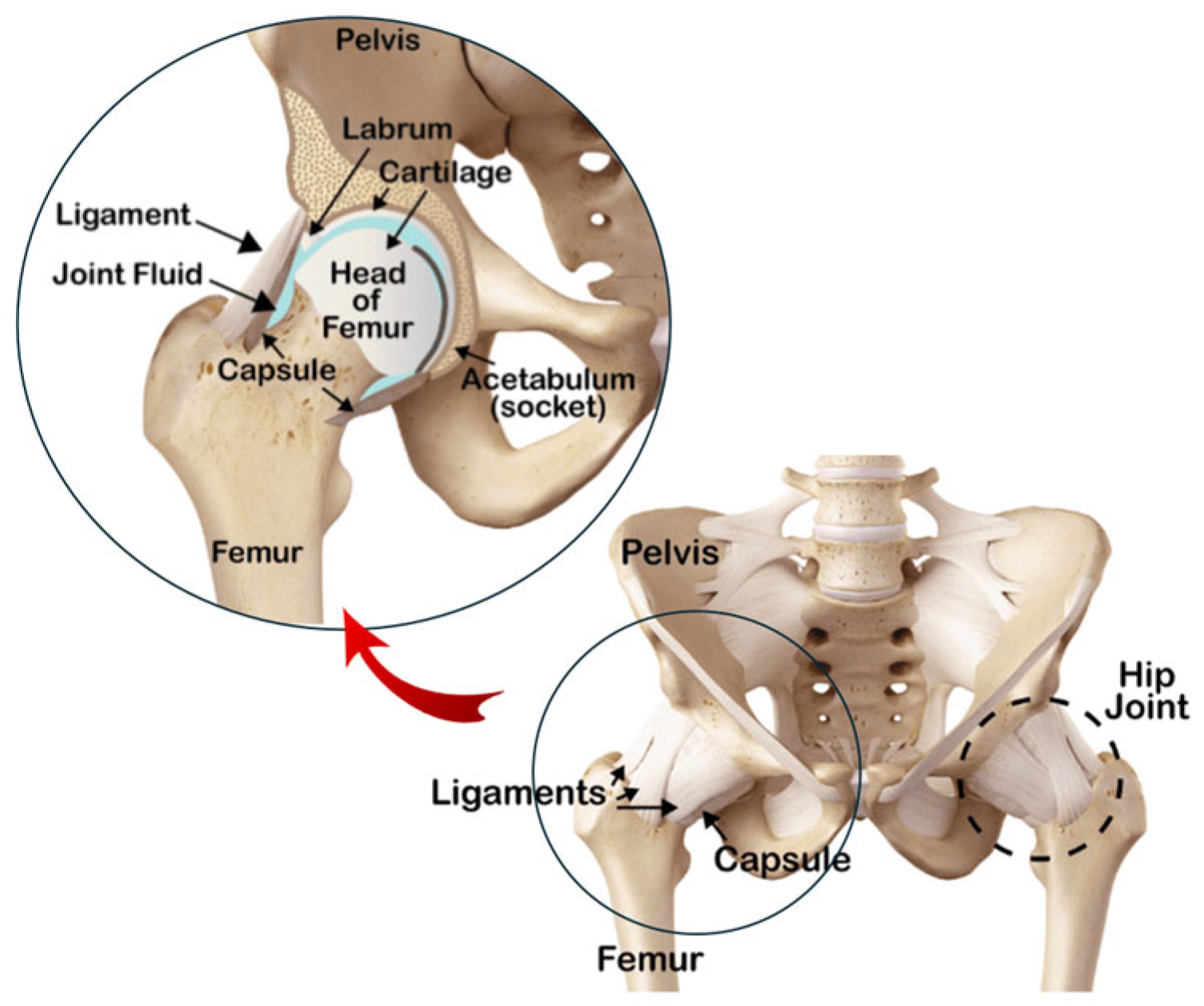
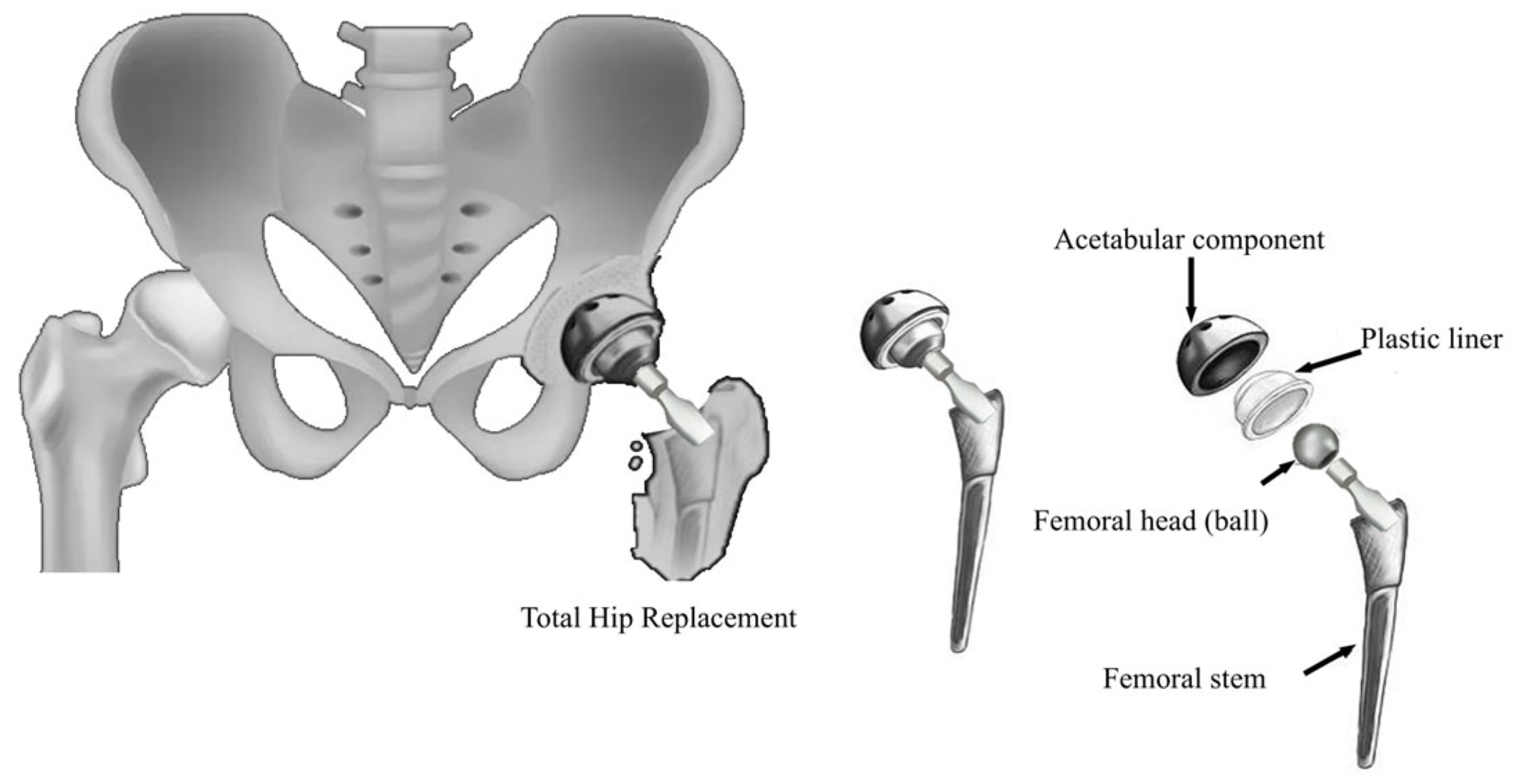
3. Hemiarthroplasty and Total Hip Arthroplasty
4. Longevity and Failure Mechanics of THA
- Aseptic loosening [42] often arises from inadequate interaction between the implant and the surrounding bone. This issue can be worsened by cement fractures, which weaken the bond between the implant and bone, leading to micromotion and stress-related loosening. Discrepancies in the elastic modulus between implant materials and the surrounding bone further contribute to loosening, highlighting the need for materials with suitable mechanical properties and biocompatibility. A major factor in aseptic loosening is bone mass reduction due to stress shielding [43]. The significant stiffness difference between the prosthesis and cortical bone causes the implant to absorb most of the mechanical stress, leaving only a small portion of the load for the surrounding cortical bone. Consequently, the stress is unevenly distributed between the prosthesis and femur, reducing the mechanical load on the bone.
- Dislocation or Instability [44] can result from a combination of factors, including improper alignment, faulty implant design, or inadequate surgical technique. Dislocation may occur due to a mismatch between the femoral head and the acetabular socket, leading to instability. Poor alignment or an incorrect size of the components can disrupt the natural biomechanics of the hip joint, increasing the risk of dislocation. Additionally, inadequate surgical technique or suboptimal implant design can compromise joint stability. Addressing these factors through precise surgical planning, careful component selection, and proper alignment can help minimize the risk of dislocation and ensure long-term joint stability.
- Periprosthetic femoral fractures [45] can result from trauma or surgical errors, but patient-specific factors such as bone density and overall bone health also play a significant role. High-impact activities or falls can lead to fractures around the prosthesis, particularly in patients with compromised bone quality or those who have undergone multiple surgeries. Surgical errors, such as improper placement of the implant or inadequate fixation, can weaken the surrounding bone and increase the risk of fracture. Furthermore, bone quality around the implant can deteriorate over time due to stress shielding or osteopenia, making the area more susceptible to fractures. Effective prevention strategies include careful surgical planning, proper implant placement, and addressing bone health issues through preoperative assessments and postoperative management.
- Infections [46] can result from non-sterile surgical techniques or complications during the procedure. Cemented implants are at risk if the adhesive cement is improperly applied or if there is a breach in sterility. The choice and effectiveness of the antimicrobial agents used in the cement are crucial in preventing infections, highlighting the importance of material quality and biocompatibility in infection control.
- Pain [49] experienced by the patient may result from a combination of surgical technique, implant adequacy, and individual patient response.
- 7.
- Implant malalignment [50] generally stems from errors in surgical technique or preoperative planning. Malalignment can lead to abnormal wear patterns and increased stress on certain parts of the implant, potentially accelerating wear and causing early failure. Precise alignment is critical to ensure even load distribution and proper joint mechanics, reducing the risk of complications such as dislocation, impingement, and irregular wear of the implant surfaces.
- 8.
- Lysis [51] is often due to an inflammatory reaction to implant debris, and is influenced by implant quality and design as well as surgical technique. The material properties of the implant, such as wear resistance and biocompatibility, are critical in mitigating inflammatory reactions.
- 9.
- Implant wear [52,53] is linked to the quality and material of the implant, affecting the longevity of the prosthetic components. The material’s durability, resistance to wear, and biocompatibility are decisive for maintaining implant function over time. Poor material quality can lead to increased wear particles, which can cause inflammatory reactions and contribute to osteolysis and aseptic loosening. Ensuring the use of high-quality, biocompatible materials helps to reduce wear rates, prevent adverse tissue reactions, and enhance the overall lifespan of the implant.
- 10.
- 11.
- Head/socket size mismatch [57] relates to implant design and selection during surgery. An improper match between the size of the femoral head and the acetabular socket can lead to increased wear, instability, and a higher risk of dislocation. Ensuring a proper fit is essential to maintain joint stability and function, as well as to minimize wear and prolong the lifespan of the implant.
5. Material Role, Selection Criteria, and Methods in THA
5.1. Material Significance in THA Failure
- The quality of the implant material directly impacts the durability of the prosthetic components. Longevity, wear resistance, and biocompatibility are essential for ensuring long-term functionality. Poor-quality materials may generate more wear particles, leading to inflammatory responses, osteolysis, and aseptic loosening [18,52,53].
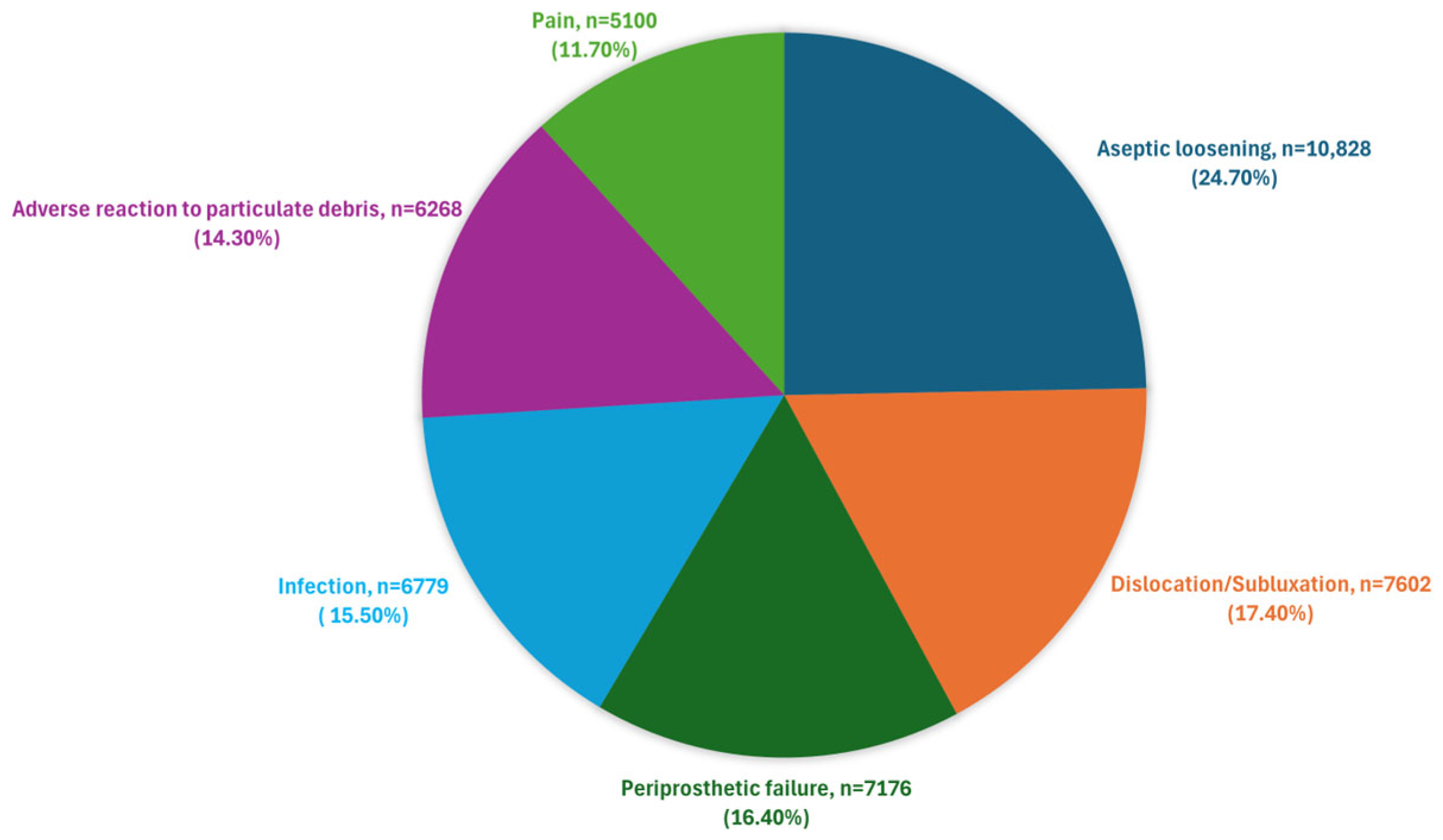
- Aseptic loosening (accounting for 24.7% of failures) is partially influenced by the material, alongside other factors.
- Adverse reactions to particulate debris (14.3%) are directly related to the material.
- Infections (15.5%) are largely caused by reactions to wear debris and partly influenced by the quality of antimicrobial materials.
- Implant wear (5.6%) and implant fracture (5.6%) are directly related to the material’s quality and strength.
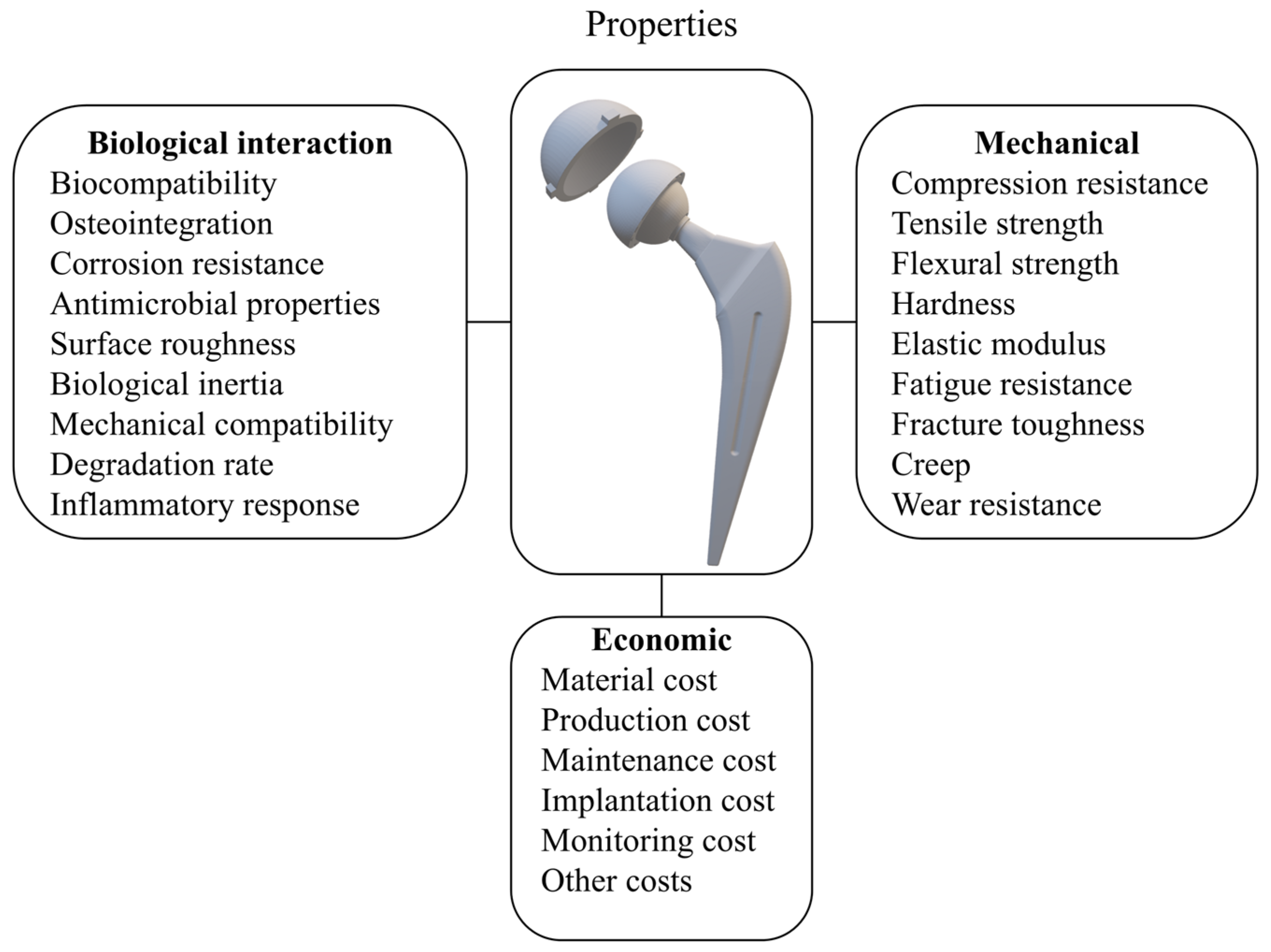
5.2. Criteria for Material Selection
- Biological interaction properties reflect how materials interact with biological tissue and are crucial for the compatibility and success of implants. The most important ones are as follows:
- ○
- ○
- Osteointegration: the artificial implant must fuse with the surrounding bone tissue for stability and long-term success. The aim is for bone to adhere to the implant surface, creating a strong bond resembling the natural connection, thus reducing the risk of failure. The osteointegration process involves the femoral stem and acetabular shell [64].
- ○
- Corrosion resistance: the materials must withstand the corrosive effects of body fluids and the challenging conditions of the organism, such as prolonged exposure to heat and moisture, while maintaining structural integrity and preventing the release of metal ions [65].
- ○
- Antimicrobial properties: the ability of the material to resist or inhibit microbial growth, reducing the risk of infections [61].
- ○
- ○
- Biological inertia: the property of remaining relatively neutral and non-reactive in the body.
- ○
- Mechanical compatibility: matching mechanical properties with surrounding tissue to ensure proper load transfer. Additionally, the rate at which the material breaks down in the body should align with the tissue-healing process for optimal integration.
- ○
- Degradation rate: the rate at which a material breaks down in the body, which should be compatible with the intended function and integration time of the implant.
- ○
- Inflammatory response: the degree to which the material induces or mitigates inflammatory responses, affecting overall implant acceptance and function.
- The mechanical properties of the biomaterials used for implants include the following [68]:
- ○
- Compression resistance: the material’s ability to withstand forces that tend to compress it without deforming or breaking.
- ○
- Tensile strength: the material’s ability to resist forces that tend to pull or stretch it.
- ○
- Flexural strength: the material’s capacity to resist deformation when bent.
- ○
- Hardness: the material’s resistance to penetration or abrasion, affecting the durability and wear of the implant.
- ○
- Elastic modulus: the stiffness of the femoral stem is crucial for load bearing. Since its stiffness impacts stress distribution, it should ideally have an elastic modulus comparable to that of the surrounding cortical bone (10 ÷ 30 GPa) to minimize stress shielding [58]. However, in practice, many femoral stems are made from materials with significantly higher elastic moduli, such as titanium alloys or cobalt-chromium alloys (100 ÷ 200 GPa). To counteract this mismatch, these implants are designed with specific geometries or surface treatments to reduce stress shielding. When the prosthesis is implanted, part of the load is transferred to the stem: the higher the stiffness of the implant, the more load it absorbs. This leads to a proportional reduction in the stress exerted on the bone, resulting in decreased bone density, which can accelerate osteoporosis progression and potentially cause aseptic loosening [59]. Emerging digital workflows and AM technologies—such as the 3D printing of porous titanium alloys—offer new possibilities for tailoring implant stiffness to match patient-specific biomechanics. By customizing internal architectures (e.g., graded porosity, lattice structures), it becomes feasible to engineer implants with site-specific elastic moduli that promote optimal load transfer and minimize stress shielding [59,69]. Moreover, the integration of biomechanical simulation tools with medical imaging enables the design of geometry- and load-optimized implants. These innovations are likely to drive the next generation of THA materials and designs, offering better personalization and long-term outcomes.
- ○
- Fatigue resistance: the material’s ability to withstand repeated cyclic loads without fracture or failure [70].
- ○
- Fracture toughness: the ability of the material to absorb energy and deform without breaking, which is essential for withstanding impacts and varying loads [71].
- ○
- Creep: the slow and progressive deformation of the material under constant load over time, which can affect the long-term stability of the implant.
- ○
- Wear Resistance: materials must resist mechanical wear from daily activities. Hence, components serving as bearing surfaces should possess appropriate coefficients of friction and minimize the generation of wear debris to prevent osteolysis and aseptic loosening. The release of toxic particles may induce adverse effects, such as allergic reactions and skin conditions [72,73]. Since surface wear is related to the types of materials that are mutually in contact, it might be interesting to analyze the types of bearing surfaces.
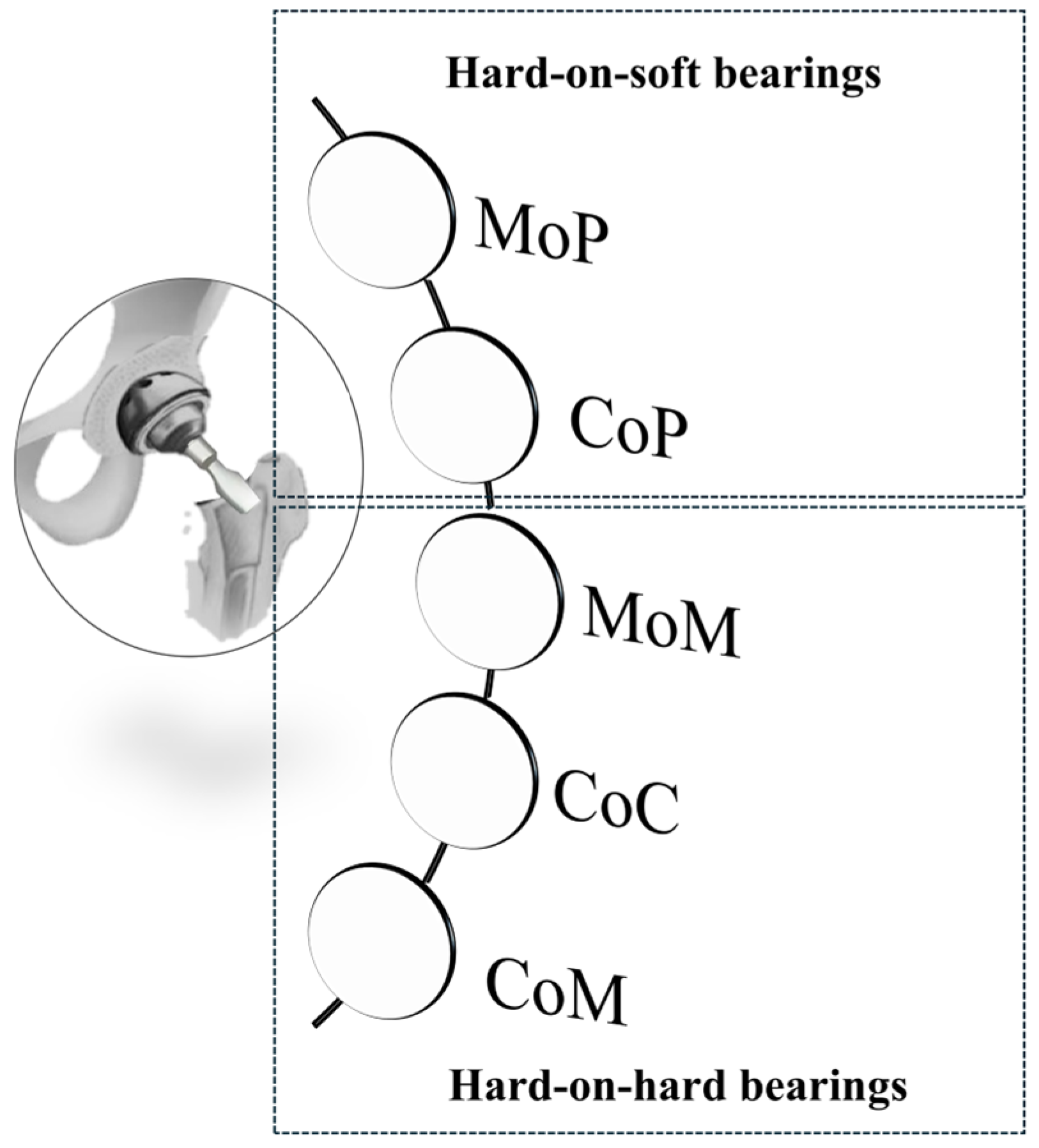
Bearing Surfaces
- ○
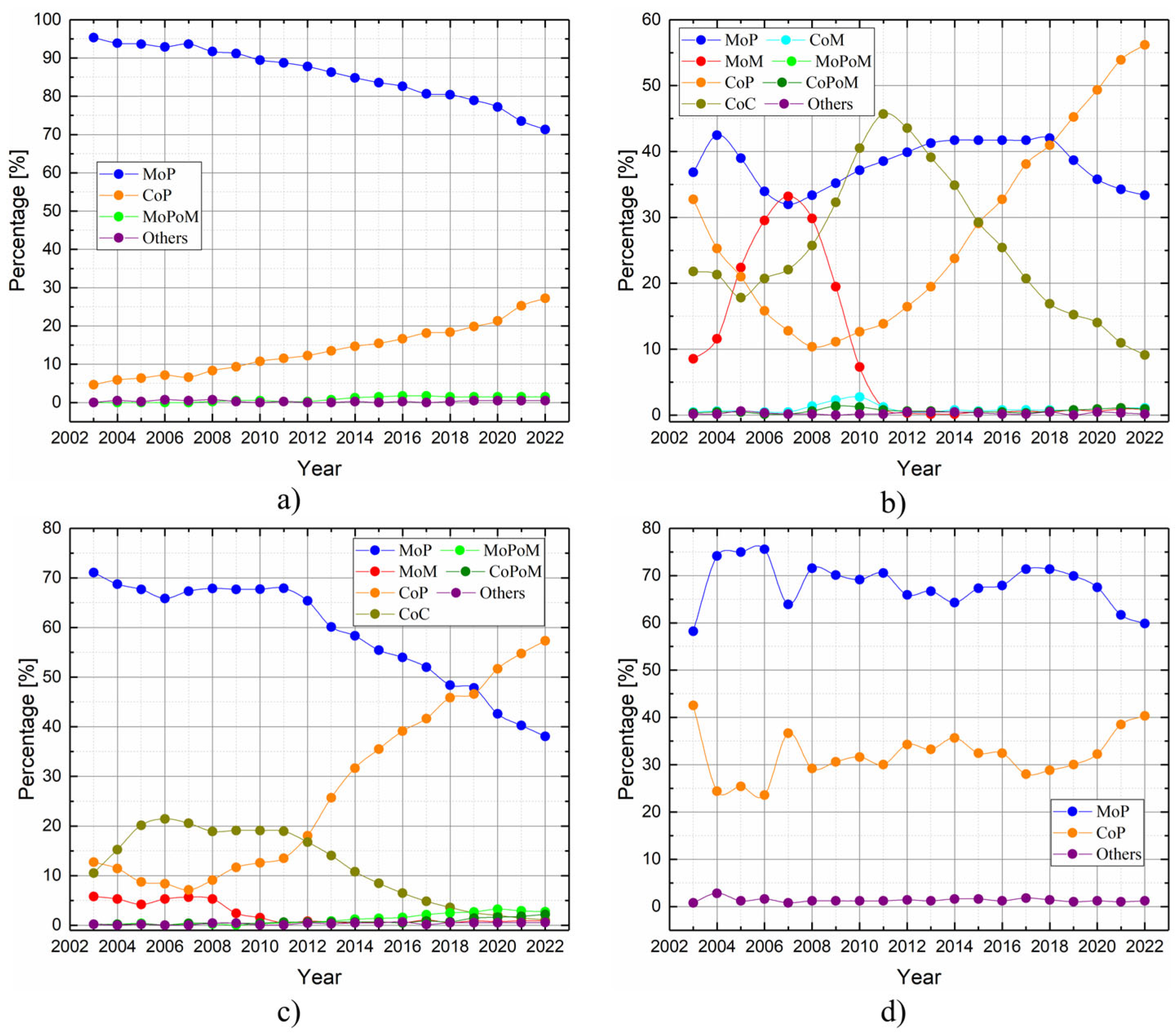
- Metal-on-Polyethylene (MoP): since their initial introduction by Charnley in the 1960s, metal femoral heads and polyethylene acetabular liners have become the most commonly used materials in hip replacements. They are widely favored due to their proven safety and cost-effectiveness. Owing to its affordability and effective impact absorption, polyethylene and its derivatives are frequently employed in orthopedic applications, and are particularly suitable for elderly patients. Nevertheless, notable challenges arise from their susceptibility to high wear rates and osteolysis [60,75].
- ○
- Ceramic-on-Polyethylene (CoP): these prosthetic systems feature a ceramic femoral head paired with a polyethylene acetabular liner. The ceramic head offers enhanced mechanical properties and superior resistance to scratching and deformation. Compared to other configurations, CoP systems demonstrate better wear characteristics thanks to the reduced friction coefficients. This is because worn ceramic heads retain smoother surfaces compared to their metal counterparts, particularly those made of cobalt–chromium alloys [60,75,76]. Despite its advantages, this approach has faced criticism due to its high cost and the potential risk of fractures in the ceramic femoral head;
- ○
- Metal-on-Metal (MoM): during the late 1990s, MoM prostheses emerged as a popular trend among arthroplasty surgeons, particularly for younger, physically active patients. The literature data report lower osteolysis and wear rates, attributed to diminished particle diameters and friction values. However, the heightened levels of metal ions in the bloodstream may induce hypersensitivity reactions in soft tissues, leading to systemic complications, including pseudotumor formation. Consequently, MoM implants have been supplanted [60,75,77].
- ○
- Ceramic-on-Ceramic (CoC): ceramic bearing surfaces combine high scratch resistance and low friction coefficients. Additionally, they release the smallest wear particles among tribological couples, reducing the risks of osteolysis and implant loosening. However, the limitations of CoC include squeaking, caused by head vibrations over worn surfaces, and ceramic fracture, a major complication arising from imperfections or misalignment of the acetabular cup. These risks, however, have been significantly reduced in newer generations of ceramic materials [60,75,78,79].
- ○
- Ceramic-on-Metal (CoM): in CoM prostheses, a ceramic femoral head articulates with a metal acetabular liner. This design aims to eliminate the ion generation and catastrophic failure associated with MoM bearings, while also preventing fractures and squeaking, which can occur with CoC bearings [60,75]. This bearing configuration aimed to blend the durability of ceramics with the strength of metals [79]. Nonetheless, a controlled trial conducted by Higgins et al. [77] recently highlighted concerns regarding the overall safety of this combination. In comparing CoM with MoM systems, researchers found no significant differences in functional and radiological outcomes between the two groups. Initially, chromium ion levels were significantly lower in the CoM group for up to three years, but these levels increased after five years. The study noted some revisions in the CoM group due to metal debris. The researchers determined that CoM systems continued to produce metal ions, which suggested a different wear pattern compared to MoM systems. Consequently, they recommended further investigation into the safety and efficacy of CoM bearings.
- Economic properties reflect the financial and systemic impact of material selection, influencing the total cost of treatment and implant maintenance over the implant’s lifetime. As highlighted in the literature [80,81], material cost, manufacturing complexity, and revision-related expenses are increasingly important in decision-making. The following economic aspects are commonly considered:
- ○
- Material cost: the price of the biomaterial or the implant itself, which can vary based on quality, the complexity of processing, and availability;
- ○
- Production cost: the expenses associated with the production and manufacturing of the implant, including processing, the technologies used, and labor;
- ○
- Maintenance cost: the costs anticipated for the preservation or replacement of the implant over time, including any necessary repairs or adjustments;
- ○
- Implantation cost: the expenses related to the surgical procedure for implant placement, including medical staff fees, hospitalization, and equipment;
- ○
- Monitoring cost: the costs associated with post-operative monitoring and follow-up visits to ensure the proper functioning of the implant;
- ○
- Other costs: additional expenses that may arise related to the implant’s use and maintenance.
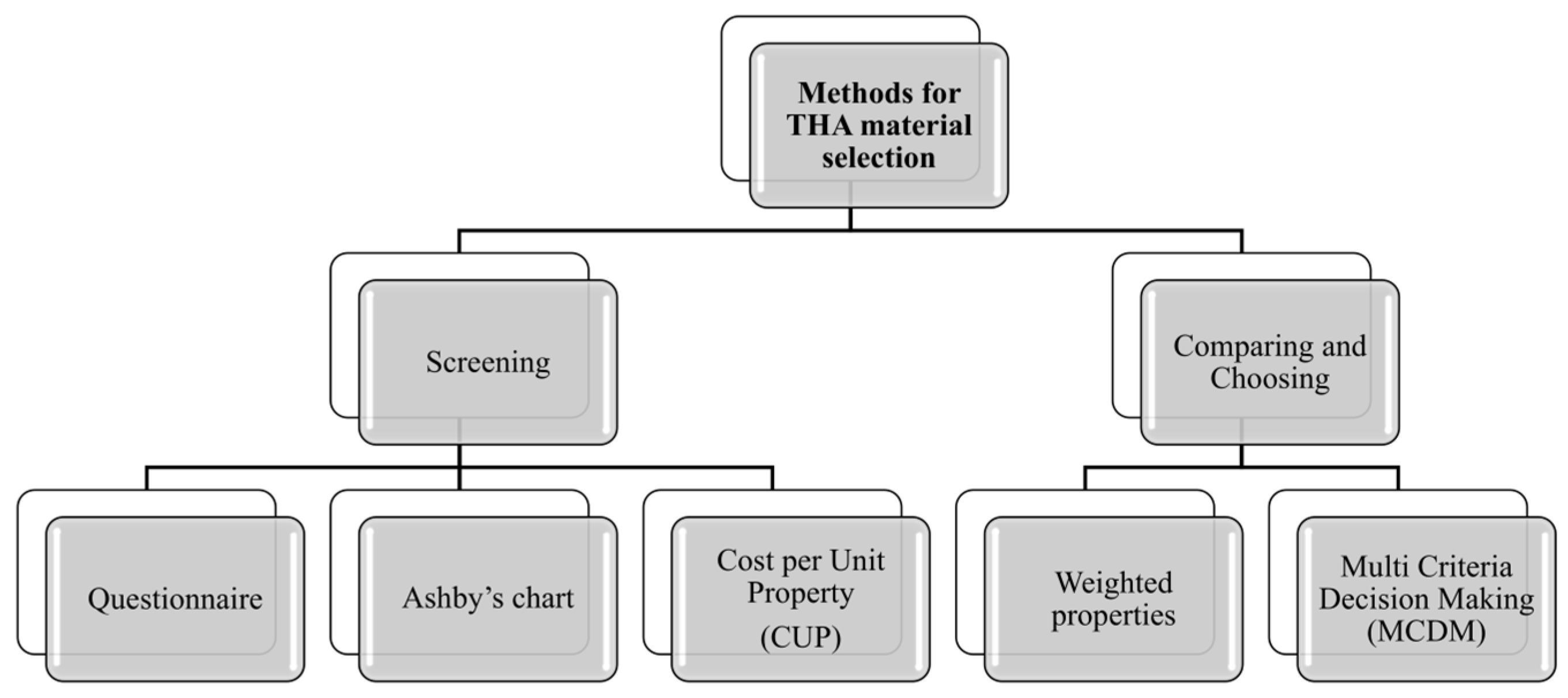
5.3. Methods for Material Selection
- Questionnaire method: this conventional method, which is the oldest and simplest approach to material selection, involves sourcing essential information about materials, including their physical properties and performance characteristics, from manufacturers, suppliers, and standards. The questionnaire method, although utilized in material selection, does not precisely fit within traditional screening methods. It acts as a tool for gathering information and directing the decision-making process through a set of structured questions designed to evaluate the compatibility and characteristics of materials for specific applications. This approach can be seen as a complementary methodology rather than an independent screening method. Referring to THA, consulting reports from national registries that collect data on surgical interventions and joint prostheses is highly beneficial for monitoring and enhancing treatment quality, as well as selecting appropriate materials. Numerous such registries exist globally, and some of the most significant ones are listed in Table 4.
- Ashby’s chart method: this method allows for the easy and intuitive visualization and comparison of material properties. Ashby’s material selection technique utilizes scatter plots, known as Ashby Charts, to display material properties on cartesian axes. Each point on the chart represents a specific material, with its properties plotted accordingly. In a study by Scholz et al. [69], Ashby diagrams were used to compare the mechanical properties of materials used in orthopedic and prosthetic applications. These diagrams were essential for understanding the trade-offs between different material properties, such as stiffness, strength, and fracture toughness, in relation to bone. The authors compared the properties of bone with those of metals, ceramics, composites, and fiber-reinforced plastics (Figure 12). However, a notable limitation of these charts is that they can only consider two or three criteria simultaneously.
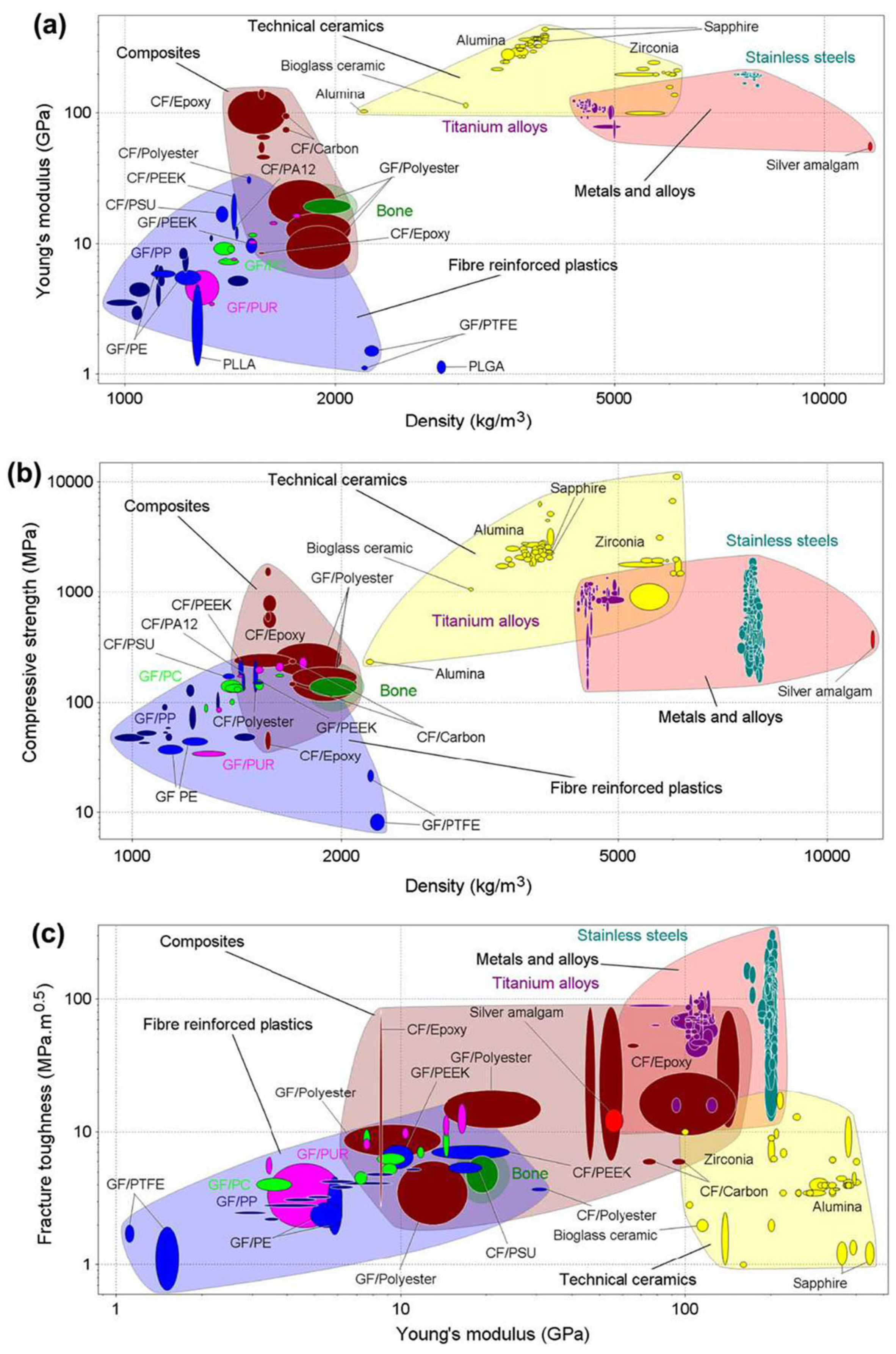
- Cost per Unit Property (CUP) method: this is an economical approach to material selection based on the ratio of cost to a specific material property. This method is useful when the goal is to optimize cost in relation to a particular material characteristic, such as strength, stiffness, or density. Generally, it is suitable for an initial screening where one property stands out as the most critical service requirement. Moreover, a target cost is set to remove expensive materials. To the best of the authors’ knowledge, this method was not applied in the context of this study.
- Weighted Properties Method (WPM): this method provides a systematic and quantitative approach to material selection, ensuring that all relevant properties are considered according to their relative importance. In the method, property “scoring” and weighting are typically performed by a multidisciplinary team of experts (biomedical engineers, orthopedic surgeons, materials scientists, and biomechanics experts). A typical application of this approach can be found in [34], where Hamidi et al. employed WPM to select the appropriate materials for hip prosthesis;
- Multi Criteria Decision Making (MDCM) technique: this approach aims to offer a well-founded recommendation to decision-makers by evaluating a finite set of alternatives against multiple criteria or objectives. It is particularly valuable in complex decision-making scenarios where trade-offs between different factors must be considered. By systematically comparing alternatives across various dimensions, MCDM helps ensure that the selected option aligns with the overall goals and constraints of the project. This method supports a balanced and comprehensive evaluation, leading to more informed and justifiable decisions [22].
- ○
- Arian and Ashkan Hafezalkotob [25] developed an advanced method called MULTIMOORA (Multi Objective Optimization by Ratio Analysis plus the Full Multiplicative Form), which integrates target-based attributes and significant coefficients for material selection in biomedical applications. Using this method, they identified the best candidate materials for the femoral component of hip and knee prostheses. The MULTIMOORA method evaluates multiple criteria simultaneously, providing a comprehensive and robust decision-making framework that ensures optimal material performance and compatibility in medical implants.
- ○
- Aherwar et al. [85] focused on the preliminary evaluation of new materials for the femoral head of the hip joint, applying an advanced MCDM technique to identify the best material. The combined use of the AHP (Analytic Hierarchy Process) and VIKOR (Vise Kriterijumska Optimizacija Kompromisno Resenjemeaning) allowed for a systematic and well-justified material selection, simultaneously considering multiple performance criteria.
- ○
- In the study by Gul et al. [86], a method called Fuzzy PROMETHEE (Preference Ranking Organization Method for Enrichment Evaluation) is developed to effectively address material selection problems by incorporating the uncertainty and vagueness inherent in MCDM. This methodology is adaptable to various applications, not limited to biomedical fields such as THA.
- Artificial Intelligence (AI): to the best of the authors’ knowledge, there are no applications of AI for material selection in THA, but developments in this area are anticipated. The study by Jodeiri et al. [87] on estimating Pelvic Sagittal Inclination (PSI) using AI highlights a promising future application in material selection for THA. In fact, by optimizing the positioning of the acetabular component based on patient-specific anatomical data, AI can help to ensure that the selected materials are ideally suited to withstand the specific stresses and movements of the hip joint, thereby enhancing the performance and longevity of the implant. This approach, although currently limited to the acetabular component, demonstrates the potential for AI to refine and improve material selection processes in orthopedic implants.
- Various material options: the table highlights a variety of materials, including metals (e.g., titanium alloys and cobalt–chromium alloys), ceramics, polymers, and composites, with each evaluated for their suitability in hip prosthesis components.
- Selection methods: a range of selection methods have been applied, from Finite Element Analysis (FEA) and Ashby Charts to more advanced techniques like MULTIMOORA and CES Selector. This heterogeneity underscores the complexity of the material selection process and the need to consider multiple criteria.
- Properties to optimize: the optimization of properties such as biocompatibility, mechanical strength, corrosion resistance, and wear resistance is critical for ensuring the longevity and performance of the implant. Different studies emphasize different properties based on the specific requirements of the component being designed.
- Consistency across studies: some materials, like Ti-6Al-4V and Co-Cr alloys, are consistently identified as optimal choices for various components, reflecting their well-established performance in biomedical applications.
| Evaluated Materials | Selection Method | Hip Component | Properties to Optimize | Reference |
|---|---|---|---|---|
| Co-Cr-Mo alloy Ti-6Al-4V Stainless Steel 316L Pure titanium | AHP | General * | Fatigue strength Corrosion resistance Wear resistance Cost Biocompatibility Elastic modulus | [23] |
| Stainless steel 316, 317, 321, 347 Co-Cr alloy (castable) Co-Cr alloy (wrought) Pure titanium Ti-6Al-4V Epoxy-70% glass Epoxy-63% carbon Epoxy-62% aramid | MULTIMOORA | Hip femoral component | Elastic modulus Corrosion resistance Tensile strength Fatigue strength Toughness Wear resistance Density Cost Tissue tolerance | [25] |
| 316L St Steel (cold worked) Co-28Cr-6Mo (cast, ASTM F75) Ti-6Al-4V (hot forged, ASTM F620), Zirconia (ceramic, 3Y-TZP) Alumina (ceramic, ZTA) | WPM | General * | Elastic modulus Yield strength Tensile strength Fatigue strength Corrosion rate Density | [34] |
| Fiber-reinforced composite | Ashby Diagrams | General * | Young’s modulus Compressive strength Fracture toughness | [69] |
| NiTi alloy wire, annealed, austenitic NiTi alloy, austenitic Polyarylamide (50% glass fiber) Stainless steel, austenitic, AISI 301 Stainless steel, austenitic, BioDur 108 Stainless steel, martensitic, AISI 410 Pure titanium Ti-6Al-1V | CES Selector integrated with FEA | General * | Density Yield strength Tensile strength Elastic modulus Hardness Biocompatibility Corrosion resistance | [84] |
| Co-30Cr-4Mo-1Ni with various tungsten percentages (0÷4 W%) | AHP integrated with VIKOR | Femoral head | Wear resistance Corrosion resistance Mechanical strength Density | [85] |
| Particulate composite Fiber composite Ti-6Al-4V Ti-6Al-4V (porous coat) | FEA | Femoral component of cemented THA | Stiffness Strength Fatigue limit Biocompatibility | [91] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| AHP | Analytic Hierarchy Process |
| AI | Artificial Intelligence |
| AISI | American Iron and Steel Institute |
| AJRR | American Joint Replacement Registry |
| AM | Additive Manufacturing |
| AOANJRR | Australian Orthopedic Association National Joint Replacement Registry |
| ASTM | American Society for Testing and Materials |
| CES Selector | Cambridge Engineering Selector |
| CJRR | Canadian Joint Replacement Registry |
| CoC | Ceramic-on-Ceramic |
| CoM | Ceramic-on-Metal |
| CoP | Ceramic-on-Polyethylene |
| Co-Cr | Cobalt–Chromium alloy |
| Co-Cr-Mo | Cobalt–Chromo–Molybdenum alloy |
| CUP | Cost per Unit Property |
| EduPack | Name of an educational tool for material science |
| EPRD | German Arthroplasty Register |
| FDM | Fusion Deposition Modeling |
| FEA | Finite Element Analysis |
| FGMs | Functionally Graded Materials |
| HDPE | High-Density-PolyEthylene |
| ISHKS | Indian Society of Hip and Knee Surgeons |
| JOANR | Japanese Orthopedic Association National Registry |
| KHRSE | Korea Hip Replacement Surgery Registry |
| LROI | Dutch Arthroplasty Register |
| MCDM | Multi-Criteria Decision-Making |
| MoM | Metal-on-Metal |
| MoP | Metal-on-Polyethylene |
| MULTIMOORA | Multi Objective Optimization by Ratio Analysis plus the Full Multiplicative Form |
| NiTi | Nickel Titanium alloy |
| NJR | National Joint Registry |
| NZOA | New Zealand Orthopedic Association |
| OA | Osteoarthritis |
| PCL | Poly(ε-caprolactone) |
| PLA | Poly(lactic acid) |
| PROMETHEE | Preference Ranking Organization Method for Enrichment Evaluation |
| PSI | Pelvic Sagittal Inclination |
| REBEC | Brazilian Registry of Clinical Trials |
| RIAP | Italian Arthroplasty Registry |
| RFNC | Spanish National Hip Fracture Registry |
| RPA | Portuguese Arthroplasty Register |
| SAAS | South African Arthroplasty Society |
| SAR | Swedish Arthroplasty Register |
| SOFCOT | French Society for Orthopedic Surgery and Traumatology |
| ST Steel | Stainless Steel |
| THA | Total Hip Arthroplasty |
| THR | Total Hip Replacement |
| Ti-6Al-4V | Titanium alloy (6% Aluminum, 4% Vanadium) |
| VIKOR | Vise Kriterijumska Optimizacija Kompromisno Resenjemeaning |
| VR | Virtual Reality |
| WPM | Weighted Properties Methods |
| ZTA | Zirconia-Toughened Alumina |
References
- Crawford, D.A.; Adams, J.B.; Hobbs, G.R.; Morris, M.J.; Berend, K.R.; Lombardi, A.V. Does Activity Level After Primary Total Hip Arthroplasty Affect Aseptic Survival? Arthroplast. Today 2021, 11, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yan, L.; Liu, H.; Li, X.; Fan, K.; Liu, Q.; Li, J.J.; Wang, B. The Prevalence of Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Res. Ther. 2023, 25, 51. [Google Scholar] [CrossRef] [PubMed]
- This Common Form of Arthritis Is Projected to Impact Nearly 1 Billion People by 2050. Available online: https://www.health.com/billion-people-with-osteoarthritis-2050-7852650 (accessed on 3 March 2025).
- American Joint Replacement Registry Surpasses 4.3 Million Captured Hip and Knee Arthroplasty Procedures. Available online: https://www.aaos.org/aaosnow/2024/dec/research/research04/ (accessed on 3 March 2025).
- Number of Hip Replacements in OECD-Countries 2021. Available online: https://www.statista.com/statistics/283234/number-of-knee-replacements-in-selected-countries/ (accessed on 3 March 2025).
- Tokgöz, E. Total Hip Arthroplasty: Medical and Biomedical Engineering and Science Concepts; Corrected Publication; Springer: Cham, Switzerland, 2023; ISBN 978-3-031-08926-8. [Google Scholar]
- Pabinger, C.; Lothaller, H.; Portner, N.; Geissler, A. Projections of Hip Arthroplasty in OECD Countries up to 2050. HIP Int. 2018, 28, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Erivan, R.; Villatte, G.; Dartus, J.; Reina, N.; Descamps, S.; Boisgard, S. Progression and Projection for Hip Surgery in France, 2008–2070: Epidemiologic Study with Trend and Projection Analysis. Orthop. Traumatol. Surg. Res. 2019, 105, 1227–1235. [Google Scholar] [CrossRef]
- Rasmussen, M.B.; El-Galaly, A.; Daugberg, L.; Nielsen, P.T.; Jakobsen, T. Projection of Primary and Revision Hip Arthroplasty Surgery in Denmark from 2020 to 2050. Acta Orthop. 2022, 93, 849–853. [Google Scholar] [CrossRef]
- Rupp, M.; Lau, E.; Kurtz, S.M.; Alt, V. Projections of Primary TKA and THA in Germany From 2016 Through 2040. Clin. Orthop. Relat. Res. 2020, 478, 1622–1633. [Google Scholar] [CrossRef]
- Nemes, S.; Gordon, M.; Rogmark, C.; Rolfson, O. Projections of Total Hip Replacement in Sweden from 2013 to 2030. Acta Orthop. 2014, 85, 238–243. [Google Scholar] [CrossRef]
- Shichman, I.; Askew, N.; Habibi, A.; Nherera, L.; Macaulay, W.; Seyler, T.; Schwarzkopf, R. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2040–2060. Arthroplast. Today 2023, 21, 101152. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Ang, S.B.; Chadha, M.; Chow, E.S.-L.; Chung, Y.-S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.-H.; et al. An Updated Hip Fracture Projection in Asia: The Asian Federation of Osteoporosis Societies Study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef]
- Matsuoka, H.; Nanmo, H.; Nojiri, S.; Nagao, M.; Nishizaki, Y. Projected Numbers of Knee and Hip Arthroplasties up to the Year 2030 in Japan. J. Orthop. Sci. 2023, 28, 161–166. [Google Scholar] [CrossRef]
- Park, J.-W.; Won, S.-H.; Moon, S.-Y.; Lee, Y.-K.; Ha, Y.-C.; Koo, K.-H. Burden and Future Projection of Revision Total Hip Arthroplasty in South Korea. BMC Musculoskelet. Disord. 2021, 22, 375. [Google Scholar] [CrossRef] [PubMed]
- Inacio, M.C.S.; Graves, S.E.; Pratt, N.L.; Roughead, E.E.; Nemes, S. Increase in Total Joint Arthroplasty Projected from 2014 to 2046 in Australia: A Conservative Local Model With International Implications. Clin. Orthop. Relat. Res. 2017, 475, 2130–2137. [Google Scholar] [CrossRef]
- Bucholz, R.W. Indications, Techniques and Results of Total Hip Replacement in the United States. Rev. Médica Clínica Las Condes 2014, 25, 756–759. [Google Scholar] [CrossRef][Green Version]
- Evans, J.T.; Evans, J.P.; Walker, R.W.; Blom, A.W.; Whitehouse, M.R.; Sayers, A. How Long Does a Hip Replacement Last? A Systematic Review and Meta-Analysis of Case Series and National Registry Reports with More than 15 Years of Follow-Up. Lancet 2019, 393, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kenney, C.; Dick, S.; Lea, J.; Liu, J.; Ebraheim, N.A. A Systematic Review of the Causes of Failure of Revision Total Hip Arthroplasty. J. Orthop. 2019, 16, 393–395. [Google Scholar] [CrossRef]
- Boubaker, O. (Ed.) Medical and Healthcare Robotics: New Paradigms and Recent Advances; Medical Robots and Devices; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2023; ISBN 978-0-443-18460-4. [Google Scholar]
- Achakri, H.; Ben-Shlomo, Y.; Blom, A.; Boulton, C.; Bridgens, J.; Brittain, R.; Clark, E.; Dawson-Bowling, S.; Deere, K.; Esler, C.; et al. The National Joint Registry 20th Annual Report 2023; National Joint Registry Annual Reports; National Joint Registry: London, UK, 2022. [Google Scholar]
- Rahim, A.A.; Musa, S.N.; Ramesh, S.; Lim, M.K. A Systematic Review on Material Selection Methods. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2020, 234, 1032–1059. [Google Scholar] [CrossRef]
- Çelik, İ.; Eroğlu, H. Selection Application of Material to Be Used in Hip Prosthesis Production with Analytic Hierarchy Process. Mater. Werkst. 2017, 48, 1125–1132. [Google Scholar] [CrossRef]
- Poovathikkal, S.; Nair, N. Biomaterials and Biocompatibility. World J. Pharm. Res. 2018, 7, 161–171. [Google Scholar] [CrossRef]
- Hafezalkotob, A.; Hafezalkotob, A. Comprehensive MULTIMOORA Method with Target-Based Attributes and Integrated Significant Coefficients for Materials Selection in Biomedical Applications. Mater. Des. 2015, 87, 949–959. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Rohatgi, Ankit WebPlotDigitizer—Copyright 2010–2024. Available online: https://apps.automeris.io/wpd4/ (accessed on 7 April 2025).
- OriginLab—Origin and OriginPro—Data Analysis and Graphing Software. Available online: https://www.originlab.com/ (accessed on 1 May 2025).
- Understanding the Hip Joint Anatomy and Hip Joint Related Pain. Available online: https://hippainhelp.com/understanding-the-hip-joint-anatomy-and-hip-joint-related-pain/ (accessed on 7 April 2025).
- Gomez, P.F.; Morcuende, J.A. Early Attempts at Hip Arthroplasty—1700s to 1950s. Iowa Orthop. J. 2005, 25, 25–29. [Google Scholar]
- Mulcahy, H.; Chew, F.S. Current Concepts of Hip Arthroplasty for Radiologists: Part 1, Features and Radiographic Assessment. Am. J. Roentgenol. 2012, 199, 559–569. [Google Scholar] [CrossRef]
- Learmonth, I.D.; Young, C.; Rorabeck, C. The Operation of the Century: Total Hip Replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty—Over 100 Years of Operative History. Orthop. Rev. 2011, 3, e16. [Google Scholar] [CrossRef]
- Hamidi, E.; Fazeli, A.; Mat Yajid, M.A.; Che Sidik, N.A. Materials Selection for Hip Prosthesis by the Method of Weighted Properties. J. Teknol. 2015, 75, 1–9. [Google Scholar] [CrossRef]
- Charnley, J. Arthroplasty of the Hip a New Operation. Lancet 1961, 277, 1129–1132. [Google Scholar] [CrossRef]
- Shon, W.Y.; Park, B.-Y.; R, R.N.; Park, P.S.; Im, J.T.; Yun, H.H. Total Hip Arthroplasty: Past, Present, and Future. What Has Been Achieved? Hip Pelvis 2019, 31, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Fontalis, A.; Epinette, J.-A.; Thaler, M.; Zagra, L.; Khanduja, V.; Haddad, F.S. Advances and Innovations in Total Hip Arthroplasty. SICOT-J. 2021, 7, 26. [Google Scholar] [CrossRef]
- Soliman, M.M.; Islam, M.T.; Chowdhury, M.E.H.; Alqahtani, A.; Musharavati, F.; Alam, T.; Alshammari, A.S.; Misran, N.; Soliman, M.S.; Mahmud, S.; et al. Advancement in Total Hip Implant: A Comprehensive Review of Mechanics and Performance Parameters across Diverse Novelties. J. Mater. Chem. B 2023, 11, 10507–10537. [Google Scholar] [CrossRef]
- Ulrich, S.D.; Seyler, T.M.; Bennett, D.; Delanois, R.E.; Saleh, K.J.; Thongtrangan, I.; Kuskowski, M.; Cheng, E.Y.; Sharkey, P.F.; Parvizi, J.; et al. Total Hip Arthroplasties: What Are the Reasons for Revision? Int. Orthop. 2008, 32, 597–604. [Google Scholar] [CrossRef]
- Kobayashi, N.; Yukizawa, Y. Causes of Failure after Total Hip Arthroplasty: A Narrative Review of Literatures. J. Jt. Surg. Res. 2023, 1, 56–61. [Google Scholar] [CrossRef]
- Colic, K.; Sedmak, A. The Current Approach to Research and Design of the Artificial Hip Prosthesis: A Review. Rheumatol. Orthop. Med. 2016, 1. [Google Scholar] [CrossRef]
- Feng, X.; Gu, J.; Zhou, Y. Primary Total Hip Arthroplasty Failure: Aseptic Loosening Remains the Most Common Cause of Revision. Am. J. Transl. Res. 2022, 14, 7080–7089. [Google Scholar]
- Huiskes, R.; Weinans, H.; van Rietbergen, B. The Relationship between Stress Shielding and Bone Resorption around Total Hip Stems and the Effects of Flexible Materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Duplantier, N.L.; McCulloch, P.C.; Nho, S.J.; Mather, R.C.; Lewis, B.D.; Harris, J.D. Hip Dislocation or Subluxation After Hip Arthroscopy: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 1428–1434. [Google Scholar] [CrossRef]
- Van Den Kieboom, J.; Tirumala, V.; Xiong, L.; Klemt, C.; Kwon, Y.-M. Periprosthetic Joint Infection Is the Main Reason for Failure in Patients Following Periprosthetic Fracture Treated with Revision Arthroplasty. Arch. Orthop. Trauma. Surg. 2021, 142, 3565–3574. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Whitehouse, M.R.; Beswick, A.D.; Kunutsor, S.K.; Burston, B.; Porter, M.; Blom, A.W. Risk Factors Associated with Revision for Prosthetic Joint Infection after Hip Replacement: A Prospective Observational Cohort Study. Lancet Infect. Dis. 2018, 18, 1004–1014. [Google Scholar] [CrossRef]
- Miller, R.A.; Ro, J.Y.; Schwartz, M.R. Adverse Tissue Reactions after Total Hip Arthroplasty. Ann. Diagn. Pathol. 2017, 27, 83–87. [Google Scholar] [CrossRef] [PubMed]
- French, J.M.R.; Bramley, P.; Scattergood, S.; Sandiford, N.A. Adverse Reaction to Metal Debris Due to Fretting Corrosion between the Acetabular Components of Modular Dual-Mobility Constructs in Total Hip Replacement: A Systematic Review and Meta-Analysis. EFORT Open Rev. 2021, 6, 343–353. [Google Scholar] [CrossRef]
- Zhang, B.; Rao, S.; Mekkawy, K.L.; Rahman, R.; Sarfraz, A.; Hollifield, L.; Runge, N.; Oni, J.K. Risk Factors for Pain after Total Hip Arthroplasty: A Systematic Review. Arthroplasty 2023, 5, 19. [Google Scholar] [CrossRef]
- Myers, C.A.; Laz, P.J.; Shelburne, K.B.; Judd, D.L.; Huff, D.N.; Winters, J.D.; Stevens-Lapsley, J.E.; Rullkoetter, P.J. The Impact of Hip Implant Alignment on Muscle and Joint Loading during Dynamic Activities. Clin. Biomech. 2018, 53, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, H.D. Femoral Lysis after Cemented Hip Arthroplasty. J. Arthroplast. 1988, 3, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chiu, K.; Tang, W. Review Article: Polyethylene Wear and Osteolysis in Total Hip Arthroplasty. J. Orthop. Surg. 2001, 9, 91–99. [Google Scholar] [CrossRef]
- Shahemi, N.; Liza, S.; Abbas, A.A.; Merican, A. Long-Term Wear Failure Analysis of Uhmwpe Acetabular Cup in Total Hip Replacement. J. Mech. Behav. Biomed. Mater. 2018, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Schils, J. The Pathology of Total Joint Arthroplasty. Skelet. Radiol. 1999, 28, 483–497. [Google Scholar] [CrossRef]
- Bäcker, H.C.; Wu, C.H.; Kienzle, A.; Perka, C.; Gwinner, C. Mechanical Failure of Total Hip Arthroplasties and Associated Risk Factors. Arch. Orthop. Trauma. Surg. 2022, 143, 1061–1069. [Google Scholar] [CrossRef]
- Babić, M.; Verić, O.; Božić, Ž.; Sušić, A. Fracture Analysis of a Total Hip Prosthesis Based on Reverse Engineering. Eng. Fract. Mech. 2019, 215, 261–271. [Google Scholar] [CrossRef]
- McTighe, T. Modular Head Mismatch in THA. ReconRev 2015, 5. [Google Scholar] [CrossRef]
- Sun, C.; Wang, L.; Kang, J.; Li, D.; Jin, Z. Biomechanical Optimization of Elastic Modulus Distribution in Porous Femoral Stem for Artificial Hip Joints. J. Bionic Eng. 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Yamako, G.; Janssen, D.; Hanada, S.; Anijs, T.; Ochiai, K.; Totoribe, K.; Chosa, E.; Verdonschot, N. Improving Stress Shielding Following Total Hip Arthroplasty by Using a Femoral Stem Made of β Type Ti-33.6Nb-4Sn with a Young’s Modulus Gradation. J. Biomech. 2017, 63, 135–143. [Google Scholar] [CrossRef]
- Zagra, L.; Gallazzi, E. Bearing Surfaces in Primary Total Hip Arthroplasty. EFORT Open Rev. 2018, 3, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Getzlaf, M.A.; Lewallen, E.A.; Kremers, H.M.; Jones, D.L.; Bonin, C.A.; Dudakovic, A.; Thaler, R.; Cohen, R.C.; Lewallen, D.G.; Van Wijnen, A.J. Multi-disciplinary Antimicrobial Strategies for Improving Orthopaedic Implants to Prevent Prosthetic Joint Infections in Hip and Knee. J. Orthop. Res. 2016, 34, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Aherwar, A.; K Singh, A.; Patnaik, A. Current and Future Biocompatibility Aspects of Biomaterials for Hip Prosthesis. AIMS Bioeng. 2015, 3, 23–43. [Google Scholar] [CrossRef]
- Learmonth, I.D. Biocompatibility: A Biomechanical and Biological Concept in Total Hip Replacement. Surg. 2003, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McCutchen, J.W.; Collier, J.P.; Mayor, M.B. Osseointegration of Titanium Implants in Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 1990, 261, 114–125. [Google Scholar] [CrossRef]
- Mueller, U.; Bormann, T.; Schroeder, S.; Renkawitz, T.; Kretzer, J.P. Taper Corrosion in Total Hip Arthroplasty—How to Assess and Which Design Features Are Crucial? J. Mech. Behav. Biomed. Mater. 2022, 133, 105307. [Google Scholar] [CrossRef]
- Cubillos, P.O.; Dos Santos, V.O.; Pizzolatti, A.L.A.; Moré, A.D.O.; Roesler, C.R.M. Surface Finish of Total Hip Arthroplasty Implants: Are We Evaluating and Manufacturing Them Appropriately? J. Test. Eval. 2021, 49, 4550–4559. [Google Scholar] [CrossRef]
- Ghosh, S.; Abanteriba, S. Status of Surface Modification Techniques for Artificial Hip Implants. Sci. Technol. Adv. Mater. 2016, 17, 715–735. [Google Scholar] [CrossRef]
- Kenney, S.; Garner, C. Mechanical Properties of Biomaterials Used in Total Hip and Knee Arthroplasty. 2021. Available online: https://shareok.org/server/api/core/bitstreams/b24a4cb8-76a3-4ad7-851e-d31090feb3cb/content (accessed on 9 April 2025).
- Scholz, M.-S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Gilbert, M.E.; Muflahi, S.A.; Pernice, M.F.; Rae, S.I.; et al. The Use of Composite Materials in Modern Orthopaedic Medicine and Prosthetic Devices: A Review. Compos. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar] [CrossRef]
- Corda, J.; Chethan, K.N.; Shenoy, S.; Shetty, S.; Bhat, S.; Zuber, M. Fatigue Life Evaluation of Different Hip Implant Designs Using Finite Element Analysis. J. Appl. Eng. Sci. 2023, 21, 896–907. [Google Scholar] [CrossRef]
- Choroszyński, M.; Choroszyński, M.R.; Skrzypek, S.J. Biomaterials for Hip Implants—Important Considerations Relating to the Choice of Materials. Bio-Algorithms Med-Syst. 2017, 13, 133–145. [Google Scholar] [CrossRef]
- Buford, A.; Goswami, T. Review of Wear Mechanisms in Hip Implants: Paper I—General. Mater. Des. 2004, 25, 385–393. [Google Scholar] [CrossRef]
- Ghadirinejad, K.; Day, C.W.; Milimonfared, R.; Taylor, M.; Solomon, L.B.; Hashemi, R. Fretting Wear and Corrosion-Related Risk Factors in Total Hip Replacement: A Literature Review on Implant Retrieval Studies and National Joint Replacement Registry Reports. Prosthesis 2023, 5, 774–791. [Google Scholar] [CrossRef]
- Khalifa, A.A.; Bakr, H.M. Updates in Biomaterials of Bearing Surfaces in Total Hip Arthroplasty. Arthroplasty 2021, 3, 32. [Google Scholar] [CrossRef]
- Seyyed Hosseinzadeh, H.R.; Eajazi, A.; Sina, A. The Bearing Surfaces in Total Hip Arthroplasty—Options, Material Characteristics and Selection. In Recent Advances in Arthroplasty; Fokter, S., Ed.; InTech: London, UK, 2012; ISBN 978-953-307-990-5. [Google Scholar]
- Kothiyal, G.P.; Srnivasan, A.B.; Kothiyal, G.P.; Srinivasan, A. (Eds.) Trends in Biomaterials; Pan Stanford Publishing: Singapore, 2016; ISBN 978-981-4613-98-9. [Google Scholar]
- Higgins, J.E.; Conn, K.S.; Britton, J.M.; Pesola, M.; Manninen, M.; Stranks, G.J. Early Results of Our International, Multicenter, Multisurgeon, Double-Blinded, Prospective, Randomized, Controlled Trial Comparing Metal-on-Metal With Ceramic-on-Metal in Total Hip Arthroplasty. J. Arthroplast. 2020, 35, 193–197.e2. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Jaber, S.A.; Taddei, P. Ceramics for Hip Joint Replacement. In Biomaterials in Clinical Practice; Zivic, F., Affatato, S., Trajanovic, M., Schnabelrauch, M., Grujovic, N., Choy, K.L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 167–181. ISBN 978-3-319-68024-8. [Google Scholar]
- Piconi, C.; Maccauro, G.; Muratori, F.; Brach Del Prever, E. Alumina and Zirconia Ceramics in Joint Replacements. J. Appl. Biomater. Biomech. 2003, 1, 19–32. [Google Scholar]
- Kurtz, S.M.; Ong, K.L.; Schmier, J.; Mowat, F.; Saleh, K.; Dybvik, E.; Kärrholm, J.; Garellick, G.; Havelin, L.I.; Furnes, O.; et al. Future Clinical and Economic Impact of Revision Total Hip and Knee Arthroplasty. J. Bone Jt. Surg. 2007, 89, 144–151. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kamath, A.F.; Ong, K.; Lau, E.; Kurtz, S.; Chan, V.; Vail, T.P.; Rubash, H.; Berry, D.J. Comparative Epidemiology of Revision Arthroplasty: Failed THA Poses Greater Clinical and Economic Burdens Than Failed TKA. Clin. Orthop. Relat. Res. 2015, 473, 2131–2138. [Google Scholar] [CrossRef]
- Ansys Granta Selector|Materials Selection Software. Available online: https://www.ansys.com/it-it/products/materials/granta-selector (accessed on 3 October 2024).
- Ansys Granta EduPack|Software for Materials Education. Available online: https://www.ansys.com/it-it/products/materials/granta-edupack (accessed on 3 October 2024).
- Şensoy, A.T.; Çolak, M.; Kaymaz, I.; Findik, F. Optimal Material Selection for Total Hip Implant: A Finite Element Case Study. Arab. J. Sci. Eng. 2019, 44, 10293–10301. [Google Scholar] [CrossRef]
- Aherwar, A.; Patnaik, A.; Bahraminasab, M.; Singh, A. Preliminary Evaluations on Development of New Materials for Hip Joint Femoral Head. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 885–899. [Google Scholar] [CrossRef]
- Gul, M.; Celik, E.; Gumus, A.T.; Guneri, A.F. A Fuzzy Logic Based PROMETHEE Method for Material Selection Problems. Beni-Suef Univ. J. Basic. Appl. Sci. 2018, 7, 68–79. [Google Scholar] [CrossRef]
- Jodeiri, A.; Zoroofi, R.A.; Hiasa, Y.; Takao, M.; Sugano, N.; Sato, Y.; Otake, Y. Fully Automatic Estimation of Pelvic Sagittal Inclination from Anterior-Posterior Radiography Image Using Deep Learning Framework. Comput. Methods Programs Biomed. 2020, 184, 105282. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G. Biomaterials: A Basic Introduction; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; ISBN 978-1-4822-2770-3. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical Evaluation of Porous Titanium (Ti6Al4V) Structures with Electron Beam Melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef]
- Fagan, M.J.; Lee, A.J.C. Material Selection in the Design of the Femoral Component of Cemented Total Hip Replacements. Clin. Mater. 1986, 1, 151–167. [Google Scholar] [CrossRef]


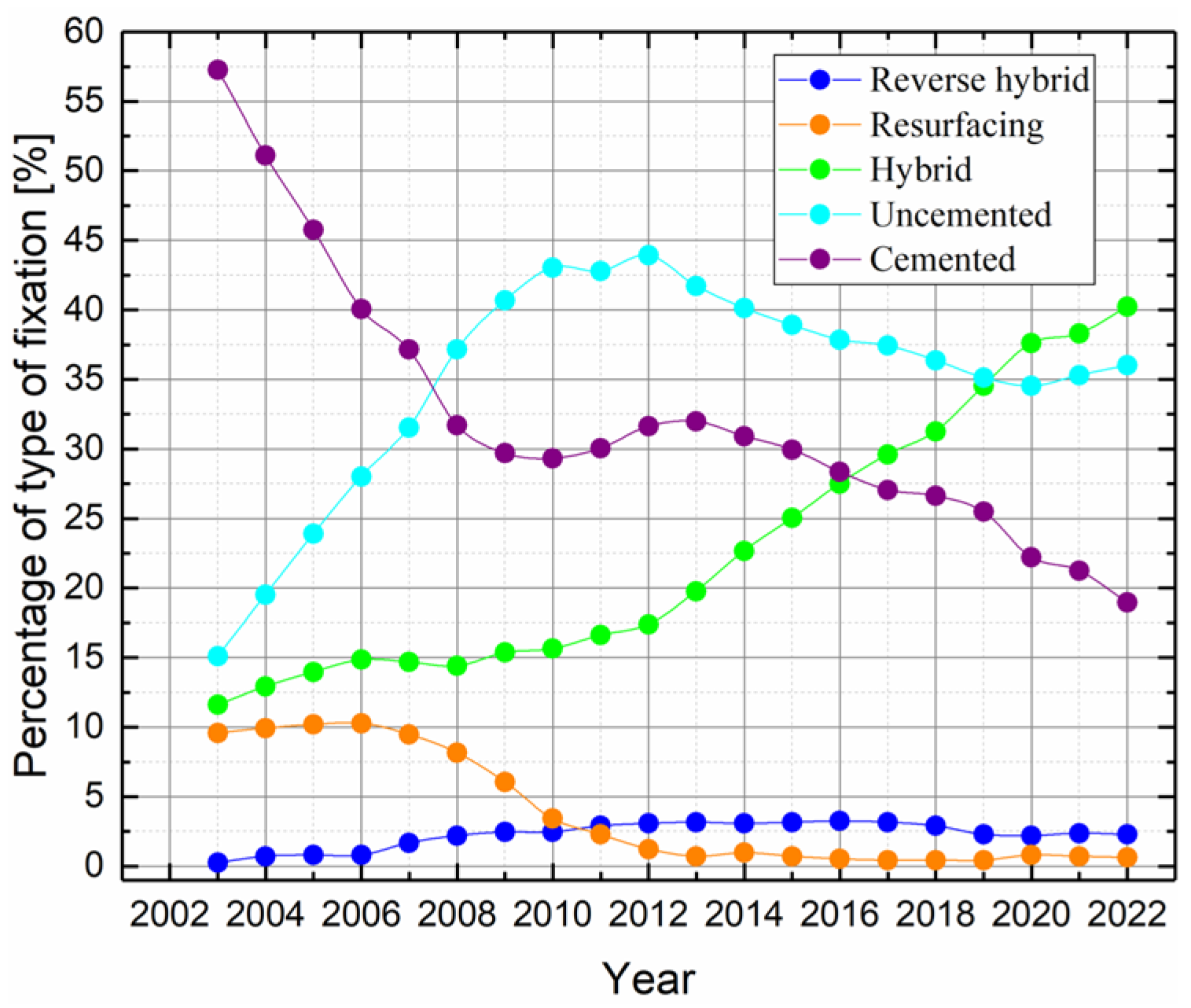
| Implant Type | Hybrid | Uncemented | Cemented | Reverse Hybrid | Resurfacing | Unclassified |
| Absolute value | 39,914 | 35,854 | 18,917 | 2278 | 693 | 1387 |
| Percentage | 40.3% | 36.2% | 19.1% | 2.3% | 0.7% | 1.4% |
| Indication for Revision | Percentage |
|---|---|
| Aseptic loosening | 24.7% |
| Dislocation/subluxation | 17.4% |
| Periprosthetic fracture | 16.4% |
| Infection | 15.5% |
| Adverse reaction to particulate debris | 14.3% |
| Pain * | 11.7% |
| More specific indications | |
| Malalignment | 6.6% |
| Lysis | 6.1% |
| Implant fracture | 5.6% |
| Implant wear ** | 5.6% |
| Head/socket size mismatch | 0.7% |
| Failure Mechanism | Early Failures (First Year) | Later Failures (Over Time) |
|---|---|---|
| Aseptic loosening | Fairly constant until five years | Steadily increases after five years |
| Pain | Increases over the first seven years | Declines after seven years |
| Subluxation/Dislocation | Higher in the first year | Decreases after the first year |
| Infection | Higher in the first year | Decreases after the first year |
| Malalignment | Higher in the first year | Decreases after the first year |
| Periprosthetic fracture | Highest in the first year, declines markedly | Begins to rise again at seven years |
| Adverse reaction to particulate debris | Increases until 15 years | Declines after 15 years |
| Lysis | - | Continues to rise with time |
| Registry Name | Country | Website |
|---|---|---|
| National Joint Registry (NJR) | United Kingdom | The National Joint Registry-Working for patients, committed to excellence. (https://www.njrcentre.org.uk/, accessed on 5 May 2025) |
| German Arthroplasty Register (EPRD) | Germany | Home|EPRD (https://www.eprd.de/en, accessed on 5 May 2025) |
| American Joint Replacement Registry (AJRR) | United States | AAOS Registry Program-American Academy of Orthopaedic Surgeons (https://www.aaos.org/registries, accessed on 5 May 2025) |
| Australian Orthopedic Association National Joint Replacement Registry (AOANJRR) | Australia | Home-AOANJRR (https://aoanjrr.sahmri.com/, accessed on 5 May 2025) |
| Japanese Orthopedic Association National Registry (JOANR) | Japan | トップページ|JOANR (https://www.joanr.org/, accessed on 5 May 2025) |
| Canadian Joint Replacement Registry (CJRR) | Canada | Canadian Joint Replacement Registry (CJRR)|CIHI (https://www.cihi.ca/en/canadian-joint-replacement-registry-cjrr, accessed on 5 May 2025) |
| Swedish Arthroplasty Register (SAR) | Sweden | The Swedish Arthroplasty Register (https://sar.registercentrum.se/, accessed on 5 May 2025) |
| Dutch Arthroplasty Register (LROI) | Netherlands | LROI (https://www.lroi.nl/, accessed on 5 May 2025) |
| Brazilian Registry of Clinical Trials (REBEC) | Brazil | REBEC (https://ensaiosclinicos.gov.br/, accessed on 5 May 2025) |
| Korea Hip Replacement Surgery Registry (KHRSR) | South Korea | Korea Hip Replacement Surgery Registry|TrialScreen (https://app.trialscreen.org/trials/korea-hip-replacement-surgery-registry-study-nct06307145, accessed on 5 May 2025) |
| French Society for Orthopedic Surgery and Traumatology (SOFCOT) | France | Accueil|SOFCOT (https://www.sofcot.fr/, accessed on 5 May 2025) |
| Italian Arthroplasty Registry (RIAP) | Italy | RIAP-Italian Arthroplasty Registry (https://riap.iss.it/riap/en/, accessed on 5 May 2025) |
| Spanish National Hip Fracture Registry (RNFC) | Spain | rnfc.es (https://rnfc.es/, accessed on 5 May 2025) |
| South African Arthroplasty Society (SAAS) | South Africa | South African Arthroplasty Society|SAAS (https://arthroplastysociety.org.za/, accessed on 5 May 2025) |
| Indian Society of Hip & Knee Surgeons (ISHKS) | India | ISHKS (https://www.ishks.com/ijr.html, accessed on 5 May 2025) |
| New Zealand Orthopedic Association (NZOA) | New Zealand | NZOA Joint Registry|New Zealand Orthopaedic Association (https://www.nzoa.org.nz/nzoa-joint-registry, accessed on 5 May 2025) |
| Portuguese Arthroplasty Register (RPA) | Portugal | R.P.A-Home (http://www.rpa.spot.pt/?lang=en-GB, accessed on 5 May 2025) |
| Other arthroplasty registries | – | Arthroplasty Registries-NORE-EFORT (https://nore.efort.org/arthroplasty-registries, accessed on 5 May 2025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garofalo, S.; Morano, C.; Bruno, L.; Pagnotta, L. A Comprehensive Literature Review for Total Hip Arthroplasty (THA): Part 2—Material Selection Criteria and Methods. J. Funct. Biomater. 2025, 16, 184. https://doi.org/10.3390/jfb16050184
Garofalo S, Morano C, Bruno L, Pagnotta L. A Comprehensive Literature Review for Total Hip Arthroplasty (THA): Part 2—Material Selection Criteria and Methods. Journal of Functional Biomaterials. 2025; 16(5):184. https://doi.org/10.3390/jfb16050184
Chicago/Turabian StyleGarofalo, Salvatore, Chiara Morano, Luigi Bruno, and Leonardo Pagnotta. 2025. "A Comprehensive Literature Review for Total Hip Arthroplasty (THA): Part 2—Material Selection Criteria and Methods" Journal of Functional Biomaterials 16, no. 5: 184. https://doi.org/10.3390/jfb16050184
APA StyleGarofalo, S., Morano, C., Bruno, L., & Pagnotta, L. (2025). A Comprehensive Literature Review for Total Hip Arthroplasty (THA): Part 2—Material Selection Criteria and Methods. Journal of Functional Biomaterials, 16(5), 184. https://doi.org/10.3390/jfb16050184







