Abstract
Introduction: Osteoporosis results from the dysregulation of osteoclast activation mechanisms. The subsequent inflammation in osteoporotic environments further hampers bone healing and impedes osseointegration. Therefore, developing treatments that can modulate osteoclast activity and regulate immune responses is essential for effectively treating osteoporotic bone defects. Methods: In this study, silver nanoparticle-decorated TiO2 nanotubes (Ag@TiO2-NTs) were synthesized through an electrochemical anodization technique for surface modification. The morphology and elemental composition of the Ag@TiO2-NTs structures were characterized using scanning electron microscopy (SEM) and related methods. Subsequently, a series of in vitro and in vivo experiments were conducted to investigate the regenerative potential of Ag@TiO2-NTs in osteoporotic bone defects. In vitro assays focused on evaluating cell viability and osteoclast function, while in vivo assessments employed osteoporotic rat models to monitor bone healing via histological examination and micro-computed tomography (micro-CT) imaging. Results: Our results demonstrated that Ag@TiO2, through the controlled release of trace amounts of silver ions, significantly suppressed osteoclast activity and consequently alleviated bone resorption under osteoporotic conditions. In addition, Ag@TiO2-NTs facilitated the polarization of macrophages toward the M2 phenotype. These biological effects were associated with the stimulation of autophagy, a fundamental mechanism involved in cellular repair. Moreover, the activation of autophagy contributed to the suppression of RANKL-induced NF-κB signaling, a pathway essential for the regulation of bone metabolism Conclusion: These results suggest that this surface modification strategy has the potential to be an ideal implant biomaterial for treating osteoporotic bone defects and a promising strategy for future implant surgeries.
1. Introduction
Osteoporosis is one of the most common diseases in aging people, characterized by significant bone loss and increased bone fragility [1,2,3]. With the aging population, osteoporosis has become a global health issue [4,5]. Dysregulated osteoclast generation is a major cause of osteoporosis [6,7]. Under osteoporotic conditions, excessive osteoclast activity leads to delayed bone defect healing [8]. On the other hand, the macrophages activated in osteoporotic conditions secrete a range of inflammatory cytokines, disrupting the local bone immune balance and further impairing implant osseointegration [9,10]. Therefore, the development of biomaterials that regulate osteoclast activity and macrophage-related immune responses is critical for treating osteoporotic bone defects.
Osteoclasts are bone-specific multinucleated cells originating from the monocyte–macrophage hematopoietic lineage, and they play a central role in bone resorption, being essential for bone remodeling, repair, and mineral homeostasis [11]. Osteoclasts and macrophages are key regulators of bone metabolism. Osteoclasts, originating from the monocyte/macrophage lineage, are multinucleated cells primarily involved in bone resorption [12]. The interplay between osteoblasts and osteoclasts is essential for preserving bone homeostasis and regulating bone remodeling. While osteoblasts drive new bone formation, osteoclasts are responsible for its resorption, and their coordinated activity maintains the dynamic balance of bone tissue renewal [13]. Macrophages participate in regulating bone metabolism through their diverse functions. Studies have shown that the polarization state of macrophages can influence the generation and function of osteoclasts. M1-type macrophages typically promote the formation of osteoclasts, whereas M2-type macrophages facilitate the osteoblastic process [14]. During bone remodeling, osteoclasts dissolve the bone matrix by secreting enzymes and acidic substances, while macrophages clear residual bone resorption debris through phagocytosis and release signaling molecules to regulate osteoblast activity [15]. The receptor activator of nuclear factor κB ligand (RANKL), by binding to its receptor RANK, plays a pivotal role in regulating osteoclast differentiation and survival [16]. Moreover, osteoclastogenesis relies on the transcriptional activities of NFATc1, cathepsin K (CTSK), and c-Fos, all of which are triggered by the nuclear factor-κB (NF-κB) signaling pathway, partially attributable to RANK recruitment [17,18]. Hence, osteoclast activity can be suppressed by inhibiting the downstream signaling pathways induced by RANKL [19]. Targeting RANKL-induced osteoclastogenesis to modulate excessive bone resorption may be an effective strategy for treating osteoporotic bone defects.
Titanium dioxide nanotubes (TiO2-NTs) have demonstrated significant potential in enhancing bone formation and regulating immune activity [20,21]. The strategies applied in are considered novel surface strategies for controlled drug delivery systems. Silver nanoparticles (AgNPs) have garnered particular attention due to their well-known antimicrobial properties and potential osteoinductive effects [22,23]. Additionally, AgNPs have been shown to modulate the proliferation and differentiation of the mesenchymal stem cells (MSCs) involved in bone regeneration [23]. Interestingly, recent studies have reported that biomaterials loaded with AgNPs, which release ultra-low doses of Ag ions (Ag+), can enhance cell adhesion, diffusion, proliferation, reactive oxygen species (ROS) clearance, and new bone formation through their unique controlled release and topographic features [24,25,26,27]. However, few reports have addressed the role of AgNPs in osteoclast differentiation.
Despite extensive research, only a few biomaterials have successfully been translated into clinical practice due to the complexity of the immune microenvironment network. Host immune responses can lead to the formation of surrounding inflammatory fibrous tissue around some implants, resulting in the loss of osseointegration efficiency [28]. Macrophages play a key role in the early host immune response by recognizing foreign materials and differentiating into two major phenotypes: pro-inflammatory M1 and wound-healing M2 phenotypes [29,30]. Recent studies have shown that the presence of chronic M1 macrophages at the bone–implant interface inhibits new bone formation and promotes the inflammatory encapsulation of the implant [31,32]. Furthermore, a higher ratio of M1/M2 macrophages has been found to be strongly correlated with the failure of joint replacement surgeries [33]. Therefore, modulating the M1/M2 macrophage ratio and promoting macrophage polarization toward the M2 phenotype may be crucial for inhibiting local adverse inflammation, achieving an appropriate immune response, and promoting tissue regeneration [34,35].
Autophagy is a highly conserved and ubiquitous cellular activity that maintains homeostasis by degrading excess and damaged intracellular components [36]. In recent years, increasing evidence has shown a close relationship between autophagy and bone metabolism, garnering significant attention [37]. Studies have indicated that calcium and vitamin D3 can induce autophagy, promoting osteoblastic differentiation [38,39]. Ha et al. discovered that silica nanoparticles can enhance osteoblast differentiation by inducing autophagy [40]. Song et al. demonstrated that different surface morphologies of titanium implants can trigger autophagic responses in various cell lines via the rapamycin target of the mTOR signaling pathway [41]. Thus, leveraging biomaterials, especially nanomaterials, to trigger autophagy is an attractive research direction, as it may offer therapeutic properties through the molecular/drug loading of the materials or their inherent characteristics (e.g., shape, roughness, and surface chemistry, etc.) [42,43]. This holds significant clinical implications for discovering autophagy-related signals, pathways, mechanisms, and therapies in bone diseases.
In this study, we aimed to explore the potential of Ag@TiO2-NTs as a therapeutic material for enhancing bone regeneration in osteoporotic conditions. Specifically, we fabricated Ag@TiO2-NTs through an electrochemical anodization process to enable the controlled release of ultra-low doses of silver ions (Ag+). The hypothesis driving this research was that the controlled release of silver ions would not only inhibit osteoclastogenesis, but also modulate immune responses by promoting M2 macrophage polarization, thereby facilitating bone regeneration in osteoporotic bone defects.
The primary objective of this study was to investigate the immunomodulatory effects of Ag@TiO2-NTs on RANKL-induced osteoclastogenesis and macrophage polarization in vitro. By understanding how Ag@TiO2-NTs influences these cellular processes, we aimed to shed light on its mechanisms of action at the cellular level. Additionally, we sought to evaluate the impact of Ag@TiO2-NTs on the regeneration of osteoporotic bone defects in ovariectomized (OVX) rats, which is a well-established model for postmenopausal osteoporosis. Our findings may provide valuable insights into the therapeutic potential of Ag@TiO2-NTs for treating osteoporotic bone defects, offering a novel approach to stimulating bone regeneration through immune modulation and controlled silver ion release.
2. Materials and Methods
The study design is illustrated in Figure 1.
Figure 1.
Flow sheet demonstrating the study design.
2.1. Ag@TiO2-NTs Preparation
The materials and titanium sheets (10 mm × 10 mm × 1 mm, Thermo Fisher Scientific Inc.,Waltham, MA, USA) were sequentially polished with 400#, 1000#, 1500#, and 2000# sandpaper to remove surface scratches and oxide layers. The polished sheets were sonicated in acetone, anhydrous ethanol, and distilled water for 5 min each to remove impurities. Finally, they were dried with nitrogen for later use. A polishing solution was prepared by mixing 200 mL distilled water, 200 mL nitric acid, 50 mL hydrofluoric acid, and 50 mL distilled water. The pretreated titanium sheets were immersed in the solution, sonicated for 1 min and 26 s, then rinsed twice with distilled water for 5 min. The polished sheets were dried with nitrogen. Graphite and pretreated titanium sheets served as the cathode and anode, respectively, with a 1 cm distance between them. The electrolyte bath was maintained at 17–18 °C under stirring. A voltage ramp from 0 V to 5 V (0.1 V/s) was applied for 3 min, followed by an increase to 60 V (0.1 V/s), and held for 30 min. After anodization, the titanium sheets turned yellow, indicating the formation of an oxide layer. The sheets were rinsed with distilled water and dried with nitrogen. The samples were then heated at 500 °C for 3 h to crystallize the oxide layer. After cooling, the residual electrolyte was removed by washing with a water–ethanol mixture (1:1) and sonicated until no white precipitates appeared. Finally, the samples were dried with nitrogen. The electrolyte was prepared by dissolving 1.6374 g NH4F, 15 mL distilled water, 15 mL methanol, and 270 mL ethylene glycol in a plastic bottle, followed by 3 h of stirring. The TiO2-NTs were immersed in 20 mL of 0.1 M AgNO3 solution for 15 min and irradiated with UV light for 30 min to obtain Ag-loaded TiO2-NTs (Ag@TiO2-NTs). Our immersion method was designed to ensure uniform AgNP distribution by maintaining an adequate liquid phase for redox reactions during UV irradiation, as supported by previous studies [22,44].
2.2. Characterization
The surface morphology of the samples (Ti, TiO2-NTs, and Ag@TiO2-NTs) was examined using scanning electron microscopy (SEM, FEI Nova400 Nano, Waltham, MA, USA). Energy-dispersive X-ray spectroscopy (EDS; Tecnai G20, FEI, Waltham, MA, USA) was used to analyze the chemical composition. The hydrophilicity of the samples was measured using a contact angle goniometer (DSA1, Kruss, Hamburg, Germany).
In this study, the size of AgNPs was estimated to be ~20 nm based on the synthesis protocol adapted from prior methodologies [22,44]. For the release of Ag+ ions, 0.01 mol/L PBS (pH 7.4) was used to immerse the Ag@TiO2-NTs at various time points. After incubation, the Ag+ concentration of the PBS solution was analyzed using flame atomic absorption spectroscopy (TAS-990, Beijing Pusix, China).
2.3. Cell Culture and Proliferation
RAW 264.7 macrophages and MC3T3-E1 preosteoblasts (Chinese Academy of Sciences, Shanghai, China) were cultured in DMEM (Gibco, Grand Island, NY, USA) with 10% FBS and 1% penicillin/streptomycin in a 5% CO2 atmosphere at 37 °C. Cell proliferation was assessed using a CCK-8 assay (Beyotime, Shanghai, China) after 24, 48, and 72 h. Absorbance was measured at 450 nm using a microplate reader (Nikon, Tokyo, Japan).
2.4. Cell Inflammatory Response
RAW 264.7 cells were polarized to M1 macrophages using LPS. RAW 264.7 is a widely recognized murine macrophage cell line extensively utilized to study osteoclastic differentiation due to its ability to differentiate into functional osteoclasts under RANKL stimulation [45,46]. The inflammatory response was assessed by culturing RAW 264.7 cells on Ti, TiO2-NTs, and Ag@TiO2-NTs (1 × 1 cm2) for 24 h. Supernatants were collected for ELISA analysis. The expression levels of pro-inflammatory (TNF-α, iNOS) and anti-inflammatory (TGF-β, Arginase1) biomarkers were observed via confocal microscopy (Olympus, Tokyo, Japan). ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to quantify TNF-α and TGF-β in the supernatants.
2.5. Macrophage–Preosteoblast Co-Culture Assay
RAW 264.7 cell-conditioned medium (CM) was collected after culturing for 24 h on different samples (Ti, TiO2-NTs, and Ag@TiO2-NTs) and co-cultured with MC3T3-E1 preosteoblasts for 72 h. Osteogenic differentiation was assessed via qPCR for ALP, OCN, OPN, and RUNX2 gene expression.
2.6. Osteoclastogenesis Assay
RAW 264.7 cells and bone marrow-derived macrophages (BMMs) were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin, and induced with M-CSF (30 ng/mL) and RANKL (50 ng/mL). Osteoclastogenesis was assessed after 24 and 72 h for RAW 264.7 cells and 3 and 7 days for BMMs. TRAP staining was used to test osteoclast differentiation, and F-actin immunofluorescence staining was used to identify mature osteoclasts.
2.7. In Vivo Animal Model
In this study, six ovariectomized 6–8-week-old female Sprague Dawley rats per group were used to establish an osteoporosis model for the in vivo experiments. A 2.0 × 6.0 mm defect was created in the iliac bone after 12 weeks of ovariectomy, and Ti, TiO2-NTs, and Ag@TiO2-NTs were implanted. The defects were randomly divided into three groups: Ti, TiO2-NTs, and Ag@TiO2-NTs. The rats were euthanized at 2 and 8 weeks post surgery for further analysis, including micro-CT and histological examination.
2.8. Micro-CT and Histological Analysis
Micro-CT scans were performed using a Bruker MicroCT Skyscan 1176 system to evaluate the bone structure and new bone formation. For histological analysis, iliac bones were decalcified in 10% EDTA, embedded in paraffin, and sectioned at a 4 μm thickness. Hematoxylin and eosin (H&E), Masson’s trichrome, and TRAP staining were performed, along with immunohistochemistry for macrophage markers (CD68, TNF-α, and TGF-β).
2.9. Gene and Protein Expression
Gene expression was quantified via RT-qPCR using a PrimeScript RT Reagent Kit (Takara, Kusatsu, Japan). The primers used for reverse transcription quantitative PCR in this study are shown in Table A1. Protein expression was analyzed via Western blotting with primary antibodies against LC3, p-IκBα, p65, p-p65, and β-actin, followed by incubation with secondary antibodies.
2.10. Statistical Analysis
GraphPad Prism software, version 9.0 was used for statistical analyses. All experiments were repeated three times, and the data are presented as mean ± standard deviation. Statistical significance was determined via one-way ANOVA with the Student–Newman–Keuls post hoc test. In this study, 6 rats per group were used for the in vivo experiments, and the sample size was statistically justified based on effect sizes from prior studies and in compliance with institutional animal ethics guidelines [47,48].
Differences were considered significant at p < 0.05 (* p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001).
3. Results
3.1. Surface Characterization, Ag+ Release, and Proliferation
SEM images showed that the ordered nanotube arrays on the titanium surface, with a diameter of approximately 120 nm, were fabricated via conventional anodization (Figure 2A). In this study, Ag+ was reduced using high-pressure Hg lamps to prepare AgNPs. This method does not require reducing agents or heat treatment. Figure 2B shows the corresponding EDX spectra, which indicate the chemical composition of the surface of the samples. Compared to TiO2-NTs, Ag@TiO2-NTs exhibit the presence of Ag, indirectly confirming the successful loading of AgNPs onto the TiO2-NT layer. Figure 2C presents the water contact angles of Ti, TiO2-NTs, and Ag@TiO2-NTs, which were 75.4° (left) and 73.0° (right), 67.9° (left) and 68.0° (right), and 67.3° (left) and 66.4° (right), respectively. This shows that the nanotube structures on the Ti surface increase the hydrophilicity of the material. Additionally, the Ag+ release kinetics are shown in Figure 2D, where the concentration of Ag+ was analyzed using flame atomic absorption spectroscopy at day 1, 3, 5, 6, 7, 8, 11, and month 2. The release curve indicates that, except for the first day, the concentrations of Ag+ were relatively stable, with the highest concentration on day one, and the levels remaining within a low range thereafter. After two months of immersion, the Ag+ concentration reached a saturation point in PBS solution and did not significantly increase with a prolonged immersion time.
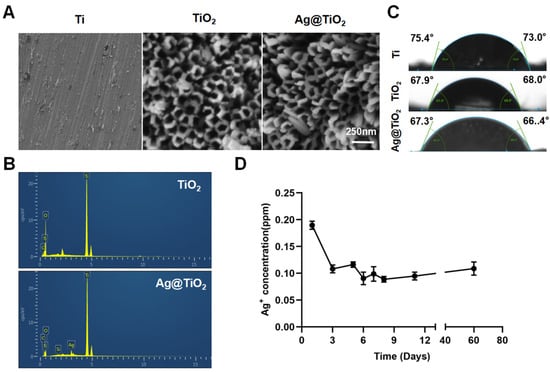
Figure 2.
Surface characterization and Ag+ release of implant. (A) Representative scanning electron microscopy (SEM) images depict the surface morphology of the different sample groups, highlighting the distinct structural characteristics of Ti, TiO2-NTs, and Ag@TiO2-NTs substrates. (B) Energy-dispersive X-ray (EDX) spectra for TiO2-NTs and Ag@TiO2-NTs confirm the successful incorporation of silver within the Ag@TiO2-NTs composite. (C) Measurements of water contact angles for Ti, TiO2-NTs, and Ag@TiO2-NTs reveal notable differences in surface wettability among the materials. (D) The release profile of Ag⁺ ions at various intervals (days 1, 3, 5, 6, 7, 8, 11, and 2 months) demonstrates a controlled and sustained release behavior over time.
To eliminate the potential toxicity of AgNPs on cell proliferation, ultra-low concentrations of AgNPs (releasing about 0.2 ppm in the first 24 h) were loaded onto TiO2-NTs (Figure 2D). CCK-8 assays (Figure A1) showed no significant differences between the groups. In summary, Ag@TiO2-NTs with ultra-low-dose AgNPs exhibited no significant cytotoxicity.
3.2. Osteo-Immunoregulatory Ability In Vitro
The LPS-treated macrophages were seeded onto Ti, TiO2-NTs, and Ag@TiO2-NT surfaces, and after two days of culture, the expression of M1 (iNOS and TNF-α) and M2 (Arginase1 and TGF-β) markers was analyzed via immunofluorescence staining and ELISA. As shown in Figure 3A,B, compared to Ti and TiO2-NTs, the fluorescence signal of iNOS and TGF-α in the Ag@TiO2-NTs group was much lower, while Arginase1 and TGF-β followed an opposite trend. Furthermore, the ELISA of TGF-α and TGF-β showed a similar trend (Figure 3C). We used a traditional co-culture method with macrophage-conditioned medium (CM) to treat MC3T3-E1 to investigate the effects of Ag@TiO2-NTs on osteogenic gene expression in MC3T3-E1 cells. The results showed that the CM from the Ag@TiO2-NTs group significantly promoted the expression of osteogenic genes ALP, Runx2, OCN, and OPN (Figure 3D).
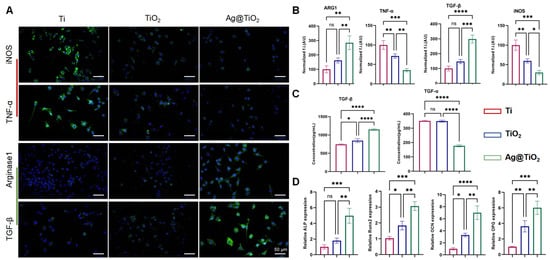
Figure 3.
Osteo-immunoregulatory ability in vitro. (A) Representative fluorescence images showing the expression of iNOS, TGF-α, Arginase 1, and TGF-β in different groups, illustrating the immunomodulatory effects on macrophage polarization and related cytokine production. (B) Quantitative analysis of iNOS, TGF-α, Arginase 1, and TGF-β levels in different groups, providing a comparison of their expression to assess the immune response and modulation by the treatments. (C) ELISA analysis of TGF-α and TGF-β levels in the groups, offering further quantification of these key cytokines involved in the osteo-immunoregulatory process. (D) mRNA expression of osteogenic genes ALP, Runx2, OCN, and OPN, evaluating the potential of the materials to promote osteogenesis and bone healing by influencing the expression of critical markers in osteoblast differentiation and function. Significance levels: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; ns = not significant. Error bars represent SEM (standard error of the mean).
3.3. Ag@TiO2-NTs Inhibited RANKL-Induced Osteoclastogenesis In Vitro
To evaluate osteoclast differentiation, tartrate-resistant acid phosphatase (TRAP), a well-known marker for osteoclasts, staining was performed, and F-actin immunofluorescence staining was used to reveal mature osteoclast formation. The TRAP activity and F-actin formation of BMMs under RANKL and m-CSF induction were evaluated to assess the level of osteoclast differentiation. The inhibitory effects of Ti, TiO2-NTs, and Ag@TiO2-NTs on RANKL-induced osteoclast differentiation was assessed using TRAP and F-actin immunofluorescence staining (Figure 4A,B). Quantitative analysis revealed that Ag@TiO2-NTs significantly inhibited osteoclast differentiation and maturation compared to Ti and TiO2-NTs groups (Figure 4C,D). qRT-PCR analysis identified the downregulated expression of osteoclast-specific genes such as Ctsk, NFATc1, c-Fos, and TRAP in the Ag@TiO2-NTs group upon RANKL induction (Figure 4E).
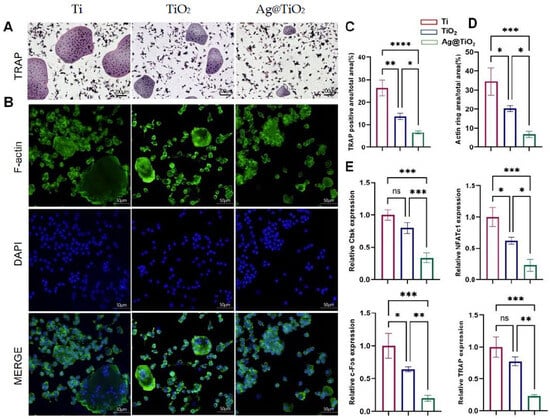
Figure 4.
Ag@TiO2-NTs inhibited RANKL-induced osteoclastogenesis in vitro. (A) Representative TRAP staining images of osteoclasts in different groups, highlighting the effect of Ag@TiO2-NTs on osteoclast differentiation. (B) Representative fluorescence images of F-actin in different groups, illustrating the cytoskeletal changes associated with osteoclast activity and differentiation. (C) Quantitative analysis of TRAP activity in different groups, providing a measure of osteoclast differentiation and function. (D) Quantitative analysis of F-actin in different groups, reflecting the impact of Ag@TiO2-NTs on the cytoskeletal organization of osteoclasts. (E) mRNA expression of osteoclast-specific genes NFATc1, c-Fos, TRAP, and Ctsk, assessing the molecular regulation of osteoclastogenesis and confirming the inhibitory effect of Ag@TiO2-NTs on osteoclast differentiation. Significance levels: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; ns = not significant. Error bars represent SEM (standard error of the mean).
3.4. Ag@TiO2-NTs Inhibited RANKL-Induced Osteoclastogenesis Through Modulation of Autophagy and NF-κB Pathways
Autophagy serves as a fundamental cellular mechanism for maintaining homeostasis and regulating immune responses, partly by mitigating oxidative stress through the removal of reactive oxygen species (ROS). To investigate autophagy activity, the expression levels of key markers in RANKL-stimulated RAW 264.7 cells were assessed via Western blot analysis, focusing on LC3, a core protein associated with autophagosome formation. Quantitative analysis revealed that Ag@TiO2-NTs significantly enhanced autophagy in RANKL-induced RAW 264.7 cells compared to the Ti and TiO2-NTs groups (Figure 5A,B). The NF-κB signaling pathway is a classic pathway involved in osteoclast activation. Western blot analysis was performed to assess the inhibition of the NF-κB pathway in RANKL-induced osteoclasts. Quantitative analysis revealed that Ag@TiO2-NTs had a significantly stronger inhibitory effect on the NF-κB signaling pathway in RANKL-induced osteoclasts compared to the Ti and TiO2-NTs groups (Figure 5A,B). In summary, Ag@TiO2-NTs inhibited RANKL-induced osteoclastogenesis by enhancing autophagy and suppressing the NF-κB pathway.
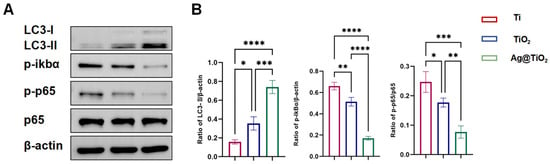
Figure 5.
Ag@TiO2-NTs inhibited RANKL-induced osteoclastogenesis through modulation of autophagy and NF-κB pathways. (A) Western blot analysis of autophagy and NF-κB protein expression in different groups, showing the impact of Ag@TiO2-NTs on key signaling pathways involved in osteoclastogenesis. (B) Quantitative analysis of autophagy and NF-κB protein expression, providing a comparison of protein levels in the different groups to assess the modulation of these pathways by Ag@TiO2-NTs. Significance levels: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Error bars represent SEM (standard error of the mean).
3.5. Ag@TiO2-NTs Implant Stimulated Bone Regeneration in OVX Rats
We evaluated the effects of Ag@TiO2-NTs implants on in vivo bone immune microenvironment modulation, bone resorption inhibition, and osteoporotic bone defect regeneration. An osteoporotic model was established in OVX rats (Figure A2), and the implants were placed into the iliac bone defects to create the osteoporotic iliac bone defect model. After 2 weeks, the osteo-immune microenvironment was assessed through inflammatory biomarkers. Immunohistochemical staining showed higher expression of TGF-β around the Ag@TiO2-NTs implant group (Figure 6A), indicating more M2 macrophages. Simultaneously, lower levels of TNF-α and CD68 expression were detected around the Ag@TiO2-NTs implant group, suggesting reduced M1 polarization and macrophage numbers (Figure 6B). The decrease in TRAP indicated that the number of osteoclasts was the lowest in the Ag@TiO2-NTs implant group (Figure 6C).
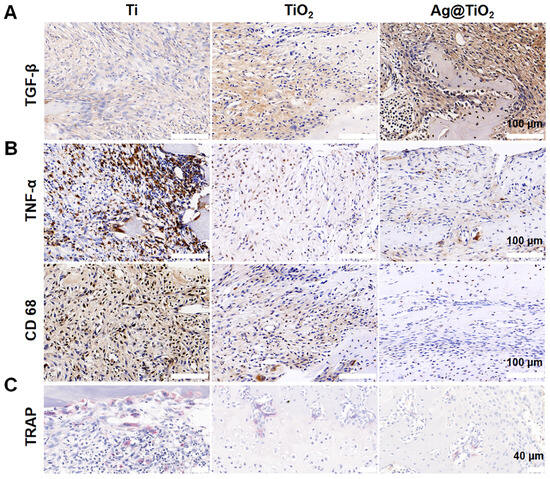
Figure 6.
Ag@TiO2-NTs implant created an osteo-immune microenvironment in OVX rats. (A) Representative immunohistochemical staining of TGF-β in different groups after 2 weeks, illustrating the expression of this key cytokine involved in osteogenesis and inflammation. (B) Representative immunohistochemical staining of TNF-α and CD68 in different groups after 2 weeks, showing the inflammatory response and macrophage activity in the treated groups. (C) Representative images of TRAP staining in different groups after 2 weeks, highlighting the osteoclast differentiation and activity in response to the treatments.
At 8 weeks post iliac bone defect induction, H&E and Masson staining indicated a small amount of new bone tissue in the defect of the Ti implant group (Figure 7A,B). The TiO2-NTs implant group showed a moderate amount of new bone tissue around the defect area, while large empty spaces still remained in the defect area. In contrast, the Ag@TiO2-NTs implant group exhibited uniformly distributed new bone tissue throughout the defect. Additionally, 3D images at week 8 showed more new regenerating bone in the Ag@TiO2-NTs implant group compared to the Ti and TiO2-NTs implant groups (Figure 7C). The percent bone volume (BV/TV)), trabecular thickness (Tb.Th), and trabecular number (Tb.N) in the Ag@TiO2-NTs implant group were significantly higher, and the trabecular separation (Tb.Sp) was significantly lower compared to the Ti and TiO2-NTs implant groups (Figure 7D). Moreover, the decrease in TRAP indicated that osteoclastogenesis around the Ag@TiO2-NTs implants was suppressed (Figure 7E).
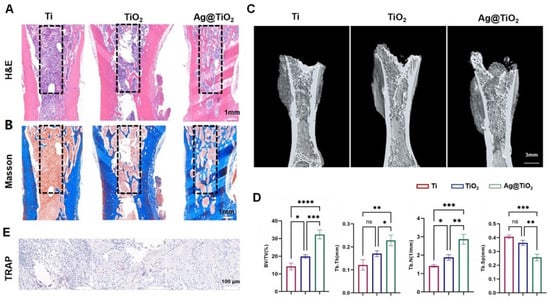
Figure 7.
In vivo results at 8 weeks post iliac bone defect induction. (A) Representative H&E staining in different groups, providing histological details on tissue morphology and bone healing after treatment. (B) Representative Masson staining in different groups, assessing collagen deposition and the extent of bone matrix formation in the treated groups. (C) Representative μ-CT images in different groups, showing 3D reconstructions of bone regeneration within the iliac defects, highlighting differences in bone formation and healing. (D) Micro-CT parameters of bone regeneration within iliac defects in different groups, quantitatively evaluating bone volume, density, and other key structural aspects. (E) Representative images of TRAP staining in different groups, illustrating osteoclast differentiation and activity, which is critical for understanding bone resorption and healing dynamics. Significance levels: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; ns = not significant. Error bars represent SEM (standard error of the mean).
4. Discussion
The dynamic equilibrium of the skeletal system relies on continuous remodeling orchestrated by osteoblasts and osteoclasts [49]. An imbalance between osteoblast-driven bone formation and osteoclast-mediated bone resorption results in bone loss and increases the risk of osteoporotic fractures, with osteoclast hyperactivation playing a central role in the underlying pathogenesis [7,50]. In this study, inhibiting osteoclast differentiation may be an effective strategy for treating osteoporosis. Furthermore, regulating macrophage M2 polarization to reduce inflammation could help create a favorable immune microenvironment around implants. In this study, we identified and clarified the role of Ag@TiO2-NTs with the controlled release of ultra-low-dose Ag+, which inhibits osteoclastogenesis and promotes the regeneration of osteoporotic bone defects by activating autophagy and suppressing RANKL-induced NF-κB signaling. Additionally, Ag@TiO2-NTs can promote M2 polarization of macrophages, thus modulating the immune microenvironment to facilitate osteogenesis.
This study successfully developed Ag@TiO2-NTs with the controlled release of ultra-low-dose Ag+ ions, using TiO2-NTs as a matrix. Notably, the AgNP size serves as an important factor of cytotoxic behavior. Chen et al. demonstrated that at around 20 nm, AgNPs had no statistical cytotoxicity in their experimental model [22,44]. Although some nanoparticles have been used in orthopedics, very few are suitable for treating osteoporosis. Over the recent decades, GNPs (gold nanoparticles) have been promising candidates for inhibiting osteoclast differentiation [51,52,53,54,55]. However, there are few reports on the effects of SNPs (silver nanoparticles) in inhibiting osteoclasts. Lee et al. reported the inhibitory effects of SNPs on osteoclast differentiation and function, but only conducted in vitro experiments at corresponding concentrations [56]. In this study, we developed an Ag-incorporated implant using TiO2 nanotubes (TiO2-NTs) as the supporting matrix, enabling the stable and sustained release of ultra-low levels of Ag⁺ ions to satisfy the prolonged ionic requirements for osteoporotic bone regeneration. Our findings demonstrated that Ag@TiO2-NTs suppress osteoclastogenesis and bone resorption by downregulating the expression of genes and markers specific to osteoclast activity. Additionally, compared with Ti and TiO2-NTs, Ag@TiO2-NTs significantly enhanced the expression of osteogenesis-associated genes in MC3T3-E1 cells, confirming their role in promoting osteogenic differentiation. Furthermore, in vivo experiments revealed that, relative to the Ti and TiO2-NTs implant groups, Ag@TiO2-NTs markedly improved bone defect repair in osteoporotic rat models. Collectively, these results indicate that silver possesses therapeutic potential for osteoporosis treatment, and that Ag@TiO2-NTs with controlled, ultra-low-dose Ag⁺ release represent a promising strategy for regenerating osteoporotic bone defects.
Autophagy is a ubiquitous cellular process that regulates cellular homeostasis and immunity and alleviates oxidative stress by removing reactive oxygen species (ROS) [57,58]. It plays a crucial role in maintaining cellular homeostasis in response to environmental stimuli. Previous studies have confirmed [44] that Ag@TiO2-NTs with the controlled release of ultra-low-dose Ag+ ions can regulate macrophage autophagy to clear ROS, promote M2 polarization, and create an osteogenic immune microenvironment rather than inhibiting the host response. This was also validated in our study, where compared to Ti and TiO2-NTs, Ag@TiO2-NTs significantly enhanced M2 polarization and the expression of osteogenic genes. Additionally, increased ROS in bone marrow-derived monocytes or macrophages can accelerate osteoclastogenesis through the activation of TNFα, TRAF6, and Rac1 [59]. Our data suggest that Ag@TiO2-NTs may inhibit RANKL-induced osteoclast differentiation by enhancing ROS clearance through macrophage autophagy. The NF-κB signaling pathway is one of the most important pathways for osteoclastogenesis. The transcription of target genes such as CTSK and cFos is stimulated by the phosphorylation of NF-κB signaling proteins. In RAW 264.7 cells, we observed that Ag@TiO2-NTs treatment significantly inhibited the phosphorylation of IκBα and p65. These results indicate that the potential molecular mechanisms through which Ag inhibits osteoclast formation may be identified. Indeed, our findings demonstrate that Ag@TiO2-NTs significantly enhance autophagy in macrophages under RANKL stimulation. In the context of osteoclastogenesis, autophagy has been shown to regulate the differentiation and function of osteoclast precursors [60]. Our study highlights that Ag@TiO2 induces autophagy in macrophages, which may contribute to the inhibition of osteoclast differentiation. The activation of autophagy was confirmed by the increased expression of LC3-II, a marker of autophagosome formation [61]. These findings suggest that Ag@TiO2-NTs promotes autophagy, which may serve as a protective mechanism to counteract the pro-inflammatory and osteoclastogenic signals induced by RANKL. The interplay between autophagy and the NF-κB signaling pathway is a critical aspect of this study. Autophagy has been shown to negatively regulate the NF-κB pathway by degrading IκBα and preventing its ubiquitination and subsequent degradation, thereby reducing the activation of NF-κB [62]. This mechanism may explain how Ag@TiO2-NTs suppresses osteoclastogenesis through the autophagy-mediated inhibition of the NF-κB pathway. Therefore, Ag@TiO2-NTs-induced bone regeneration in osteoporotic rats is considered to be associated with Ag+-mediated immune modulation, promoting osteogenesis and the autophagy-activated inhibition of RANKL-induced NF-κB signaling and leading to the suppression of osteoclast formation and resorption (Figure 8).
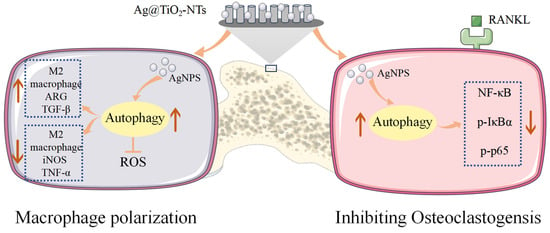
Figure 8.
Illustration of Ag@TiO2-NTs’ modulatory role in macrophage polarization and inhibiting osteoclastogensis.
Unfortunately, there are some limitations in this study. First, the causes and progression of osteoporosis are complex, involving multiple factors. However, in our investigation, we only examined autophagy and NF-κB signaling pathways, without further exploring their relationship. Second, it remains unclear whether Ag directly acts on ROS through autophagy and NF-κB pathways or if there are certain molecular targets connected to autophagy and NF-κB. Additionally, we created and used a rat iliac bone osteoporotic bone defect model for the first time in this study, but some rats in the model experienced bone fractures. Further improvements may be needed for the creation of iliac bone defects and the implantation of materials. The study focuses primarily on an osteoporosis-related bone defect model, limiting its generalizability to other bone diseases such as fractures or infections. Short-term observations were made, but long-term studies are required to assess the sustained efficacy and safety of Ag@TiO2-NTs in clinical settings. The fabrication method used for Ag@TiO2-NTs nanotube arrays may not be scalable or suitable for the industrial production of implants, and the potential toxicological effects of AgNPs remain unexplored, despite showing no significant cytotoxicity at ultra-low concentrations in the study [63]. Moreover, the study uses rat models, which may not fully translate to human physiology, limiting its direct applicability to clinical practice; therefore, future studies should involve larger animal models to better replicate human conditions [64]. The study used a specific concentration of AgNPs, but the optimal concentration and delivery method for clinical applications require further investigation. Although this study offers valuable mechanistic insights into the effects of Ag@TiO2-NTs, further investigations are required to comprehensively elucidate the molecular and cellular interactions at play. The clinical use of AgNPs in implants requires rigorous testing to ensure their safety, biocompatibility, and absence of adverse immune responses, which may require extensive preclinical and clinical trials. The fabrication method may not be cost-effective or scalable for large-scale production, and a more reproducible manufacturing process is needed for clinical adoption. Moreover, the success of titanium implants in clinical settings depends on their integration with bone tissue, requiring further investigation into mechanical stability and long-term performance [65]. Individual patient variations, including health, immune responses, and bone quality, may affect the efficacy of Ag@TiO2-NTs implants, necessitating personalized medicine approaches to optimize outcomes. Additionally, patients with osteoporosis often have comorbidities that require medications, so potential interactions between AgNPs and other drugs must be evaluated. Overall, our work provides insights for future osteoporosis research and therapy.
5. Conclusions
In summary, Ag@TiO2-NTs with controlled release of ultra-low-dose AgNPs were fabricated and applied to regenerate bone defects in osteoporotic rats. The incorporation of AgNPs not only endowed TiO2-NTs with the ability to regulate macrophage polarization, but also inhibited osteoclast formation and function in vitro. Furthermore, in vivo, a satisfactory bone immune microenvironment was generated by the increased expression of wound-healing M2 macrophages around Ag@TiO2-NTs. Additionally, Ag@TiO2-NTs reduced osteoclast numbers and accelerated bone defect regeneration in osteoporotic rats by activating autophagy and inhibiting the RANKL-induced NF-κB pathway. Our study indicates that Ag@TiO2-NTs modified with ultra-low-dose AgNPs exhibit both immune modulation and excellent osteogenic capabilities for osteoporosis, making them a promising biomaterial for implant applications (Table 1).

Table 1.
A summary table of key results.
Author Contributions
Conceptualization, Z.W., P.X., R.Z.and C.Y.; methodology, Z.W., P.X., Z.X., M.G., R.Z., F.X. and C.Y.; software, Z.W.; validation, P.X., Z.X., M.G., R.Z., Y.L. and C.Y.; formal analysis, Z.W.; investigation, Z.W., P.X., M.G., Y.L., F.X. and C.Y.; resources, Z.W., P.X., M.G., R.Z., Y.L. and F.X.; data curation, Z.W. and Y.L.; writing—original draft preparation, Z.W., P.X., Z.X., M.G., R.Z., Y.L., F.X. and C.Y.; writing—review and editing, Z.W., F.X. and C.Y.; visualization, Z.W. and C.Y.; supervision, R.Z., F.X. and C.Y.; project administration, Z.W., Z.X., R.Z., Y.L. and C.Y.; funding acquisition, Z.W. and C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFC2504303); Innovation Foundation of National Clinical Research Center for Orthopaedics, Sports Medicine and Rehabilitation (2021-NCRC-CXJJ-ZH-24); and Medical Innovation and Transformation Incubation Project of Tongji Hospital (2022ZHFY10).
Institutional Review Board Statement
The animal study protocol was approved by the Ethical Review Committee for Experimental Animal Welfare (IACUC) (SZHY No. 2024041003), approval date 10th April, 2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Primers used for real-time PCR.
Table A1.
Primers used for real-time PCR.
| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| iNOS | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Arg1 | CTCCAAGCCAAAGTCCTTAGAG | GGAGCTGTCATTAGGGACATCA |
| TGFa | CACTCTGGGTACGTGGGTG | CACAGGTGATAATGAGGACAGC |
| TGFb | CCACCTGCAAGACCATCGAC | CTGGCGAGCCTTAGTTTGGAC |
| ALP | GGCCTTTTACCTTTCACGGTG | TACGGCATTGTGGCTTCTCAA |
| OPG | ACCCAGAAACTGGTCATCAGC | CTGCAATACACACACTCATCACT |
| OCN | CTGAAAAGCCCACAGATACCAG | TGGAGAGGGTTGTTAGTGTGTC |
| RUNX2 | ATGCTTCATTCGCCTCACAAA | GCACTCACTGACTCGGTTGG |
| NFATc1 | GACCCGGAGTTCGACTTCG | TGACACTAGGGGACACATAACTG |
| c-Fos | CGGGTTTCAACGCCGACTA | TTGGCACTAGAGACGGACAGA |
| MMP9 | CTGGACAGCCAGACACTAAAG | CTCGCGGCAAGTCTTCAGAG |
| TRAP | CACTCCCACCCTGAGATTTGT | CATCGTCTGCACGGTTCTG |
| Ctsk | GAAGAAGACTCACCAGAAGCAG | TCCAGGTTATGGGCAGAGATT |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Figure A1.
CCK-8 assays for MC3T3-E1 of different groups at 24 h, 48 h, and 72 h.
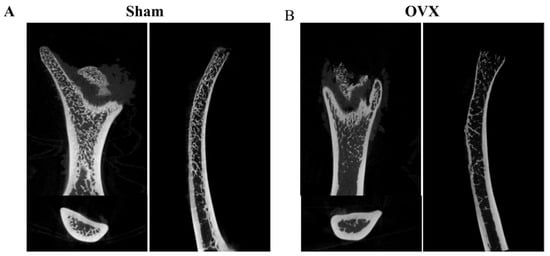
Figure A2.
The osteoporotic model was established in OVX rats. (A) Representative μ-CT images in sham model. (B) Representative μ-CT images in OVX model.
References
- Yang, T.L.; Shen, H.; Liu, A.; Dong, S.S.; Zhang, L.; Deng, F.Y.; Zhao, Q.; Deng, H.W. A road map for understanding molecular and genetic determinants of osteoporosis. Nat. Rev. Endocrinol. 2020, 16, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ge, K.; Jin, Y.; Han, Y.; Zhang, H.; Zhou, G.; Yang, X.; Liu, D.; Liu, H.; Liang, X.J.; et al. Bone-Targeted Nanoplatform Combining Zoledronate and Photothermal Therapy To Treat Breast Cancer Bone Metastasis. ACS Nano 2019, 13, 7556–7567. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Binding, C.; Bjerring Olesen, J.; Abrahamsen, B.; Staerk, L.; Gislason, G.; Nissen Bonde, A. Osteoporotic Fractures in Patients With Atrial Fibrillation Treated With Conventional Versus Direct Anticoagulants. J. Am. Coll. Cardiol. 2019, 74, 2150–2158. [Google Scholar] [CrossRef]
- Yoo, J.E.; Shin, D.W.; Han, K.; Kim, D.; Yoon, J.W.; Lee, D.Y. Association of Female Reproductive Factors With Incidence of Fracture Among Postmenopausal Women in Korea. JAMA Netw. Open 2021, 4, e2030405. [Google Scholar] [CrossRef]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef]
- Anesi, A.; Generali, L.; Sandoni, L.; Pozzi, S.; Grande, A. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int. J. Mol. Sci. 2019, 20, 4925. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Stem cells and osteoporosis therapy. Cell Stem Cell 2010, 7, 553–554. [Google Scholar] [CrossRef]
- Rao, S.S.; Hu, Y.; Xie, P.L.; Cao, J.; Wang, Z.X.; Liu, J.H.; Yin, H.; Huang, J.; Tan, Y.J.; Luo, J.; et al. Omentin-1 prevents inflammation-induced osteoporosis by downregulating the pro-inflammatory cytokines. Bone Res. 2018, 6, 9. [Google Scholar] [CrossRef]
- Wang, D.; Chen, M.W.; Wei, Y.J.; Geng, W.B.; Hu, Y.; Luo, Z.; Cai, K.Y. Construction of Wogonin Nanoparticle-Containing Strontium-Doped Nanoporous Structure on Titanium Surface to Promote Osteoporosis Fracture Repair. Adv. Healthc. Mater. 2022, 11, e2201405. [Google Scholar] [CrossRef]
- Gao, Y.; Ge, W. The histone methyltransferase DOT1L inhibits osteoclastogenesis and protects against osteoporosis. Cell Death Dis. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yang, W.; Cui, W.; Li, C.; Ma, C.; Ji, X.; Hong, J.; Qu, Z.; Chen, J.; Liu, A.; et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J. Nanobiotechnol 2023, 21, 14. [Google Scholar] [CrossRef]
- Yates, A.G.; Pink, R.C.; Erdbrügger, U.; Siljander, P.R.; Dellar, E.R.; Pantazi, P.; Akbar, N.; Cooke, W.R.; Vatish, M.; Dias-Neto, E.; et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology: Part I: Health and Normal Physiology. J. Extracell. Vesicles 2022, 11, e12151. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, H.; Zhu, R.; Zhang, Z. Contribution of macrophage polarization in bone metabolism: A literature review. Cytokine 2024, 184, 156768. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Luo, F.; Du, X.; Xian, F.; Li, X. The immune cells in modulating osteoclast formation and bone metabolism. Int. Immunopharmacol. 2024, 133, 112151. [Google Scholar] [CrossRef]
- Maria, S.; Samsonraj, R.M.; Munmun, F.; Glas, J.; Silvestros, M.; Kotlarczyk, M.P.; Rylands, R.; Dudakovic, A.; van Wijnen, A.J.; Enderby, L.T.; et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 2018, 64, e12465. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, L.; Zheng, Y.; Li, W. Involvement of Noncoding RNAs in the Differentiation of Osteoclasts. Stem Cells Int. 2020, 2020, 4813140. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Pan, Y.J.; Shi, X.F.; Yang, L.; Lou, Y.L. Orientin suppresses osteoclastogenesis and ameliorates ovariectomy-induced osteoporosis via suppressing ROS production. Food Sci. Nutr. 2023, 11, 5582–5595. [Google Scholar] [CrossRef]
- Liang, W.; Feng, R.; Li, X.; Duan, X.; Feng, S.; Chen, J.; Li, Y.; Chen, J.; Liu, Z.; Wang, X.; et al. A RANKL-UCHL1-sCD13 negative feedback loop limits osteoclastogenesis in subchondral bone to prevent osteoarthritis progression. Nat. Commun. 2024, 15, 8792. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef]
- Xu, W.C.; Dong, X.; Ding, J.L.; Liu, J.C.; Xu, J.J.; Tang, Y.H.; Yi, Y.P.; Lu, C.; Yang, W.; Yang, J.S.; et al. Nanotubular TiO(2) regulates macrophage M2 polarization and increases macrophage secretion of VEGF to accelerate endothelialization via the ERK1/2 and PI3K/AKT pathways. Int. J. Nanomed. 2019, 14, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chen, Y.; Wei, Y.; Song, H.; Gao, C.; Cheng, H.; Li, Y.; Huo, K.; Fu, J.; Xiong, W. Long-lasting bactericidal activity through selective physical puncture and controlled ions release of polydopamine and silver nanoparticles-loaded TiO(2) nanorods in vitro and in vivo. Int. J. Nanomed. 2019, 14, 2903–2914. [Google Scholar] [CrossRef] [PubMed]
- Damle, A.; Sundaresan, R.; Rajwade, J.M.; Srivastava, P.; Naik, A. A concise review on implications of silver nanoparticles in bone tissue engineering. Bio Adv. 2022, 141, 213099. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Cheng, H.; Xu, J.; Li, F.; Gao, B.; Li, Z.; Gao, C.; Huo, K.; Fu, J.; Xiong, W. Silver-loaded nanotubular structures enhanced bactericidal efficiency of antibiotics with synergistic effect in vitro and in vivo. Int. J. Nanomed. 2017, 12, 731–743. [Google Scholar] [CrossRef]
- Gao, C.; Cheng, H.; Xu, N.; Li, Y.; Chen, Y.; Wei, Y.; Gao, B.; Fu, J.; Huo, K.; Xiong, W. Poly(dopamine) and Ag nanoparticle-loaded TiO(2) nanotubes with optimized antibacterial and ROS-scavenging bioactivities. Nanomedicine 2019, 14, 803–818. [Google Scholar] [CrossRef]
- Zeng, X.; Xiong, S.; Zhuo, S.; Liu, C.; Miao, J.; Liu, D.; Wang, H.; Zhang, Y.; Wang, C.; Liu, Y. Nanosilver/poly (dl-lactic-co-glycolic acid) on titanium implant surfaces for the enhancement of antibacterial properties and osteoinductivity. Int. J. Nanomed. 2019, 14, 1849–1863. [Google Scholar] [CrossRef]
- Xue, Y.; Hong, X.; Gao, J.; Shen, R.; Ye, Z. Preparation and biological characterization of the mixture of poly(lactic-co-glycolic acid)/chitosan/Ag nanoparticles for periodontal tissue engineering. Int. J. Nanomed. 2019, 14, 483–498. [Google Scholar] [CrossRef]
- Saleh, L.S.; Bryant, S.J. The Host Response in Tissue Engineering: Crosstalk Between Immune cells and Cell-laden Scaffolds. Cur OpinBiomed Eng. 2018, 6, 58–65. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, Y.J.; Jang, J.H.; Park, J.W. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology 2016, 27, 085101. [Google Scholar] [CrossRef]
- Coronel, M.M.; Geusz, R.; Stabler, C.L. Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials 2017, 129, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Guan, M.; Jin, J.; Luo, Y.; Luo, Z.; Shi, T.; Liu, T.; Zhang, C.; Wang, Y. Mechanochemically Reprogrammed Interface Orchestrates Neutrophil Bactericidal Activity and Apoptosis for Preventing Implant-Associated Infection. Adv. Mater. 2024, 36, e2311855. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.J.; Gibon, E.; Ma, T.; Yao, Z.; Smith, R.L.; Goodman, S.B. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater 2012, 8, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef]
- Jin, J.; Xia, X.; Ruan, C.; Luo, Z.; Yang, Y.; Wang, D.; Qin, Y.; Li, D.; Zhang, Y.; Hu, Y.; et al. GAPDH-Silence Microsphere via Reprogramming Macrophage Metabolism and eradicating Bacteria for Diabetic infection bone regeneration. J. Nanobiotechnol. 2024, 22, 517. [Google Scholar] [CrossRef]
- Lahiri, V.; Hawkins, W.D.; Klionsky, D.J. Watch What You (Self-) Eat: Autophagic Mechanisms that Modulate Metabolism. Cell Metabol. 2019, 29, 803–826. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cao, J.; Wang, Y.; Anwar, N.; Zhang, Z.; Zhang, D.; Ma, Y.; Xiao, Y.; Xiao, L.; et al. The role of autophagy in bone metabolism and clinical significance. Autophagy 2023, 19, 2409–2427. [Google Scholar] [CrossRef]
- Høyer-Hansen, M.; Bastholm, L.; Szyniarowski, P.; Campanella, M.; Szabadkai, G.; Farkas, T.; Bianchi, K.; Fehrenbacher, N.; Elling, F.; Rizzuto, R.; et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell 2007, 25, 193–205. [Google Scholar] [CrossRef]
- Høyer-Hansen, M.; Nordbrandt, S.P.; Jäättelä, M. Autophagy as a basis for the health-promoting effects of vitamin D. Trends Mol. Med. 2010, 16, 295–302. [Google Scholar] [CrossRef]
- Ha, S.W.; Weitzmann, M.N.; Beck, G.R., Jr. Bioactive silica nanoparticles promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano 2014, 8, 5898–5910. [Google Scholar] [CrossRef]
- Song, W.; Shi, M.; Dong, M.; Zhang, Y. Inducing Temporal and Reversible Autophagy by Nanotopography for Potential Control of Cell Differentiation. ACS Appl. Mater. Inter. 2016, 8, 33475–33483. [Google Scholar] [CrossRef] [PubMed]
- Florance, I.; Cordani, M.; Pashootan, P.; Moosavi, M.A.; Zarrabi, A.; Chandrasekaran, N. The impact of nanomaterials on autophagy across health and disease conditions. Cell Mol. Life Sci. 2024, 81, 184. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yuan, L.; XuHong, J.; Tian, H.; Zhang, Y.; Deng, J.; Qi, X. Chiral nanomaterials for tumor therapy: Autophagy, apoptosis, and photothermal ablation. J. Nanobiotechnol. 2021, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guan, M.; Ren, R.; Gao, C.; Cheng, H.; Li, Y.; Gao, B.; Wei, Y.; Fu, J.; Sun, J.; et al. Improved Immunoregulation of Ultra-Low-Dose Silver Nanoparticle-Loaded TiO(2) Nanotubes via M2 Macrophage Polarization by Regulating GLUT1 and Autophagy. Int. J. Nanomed. 2020, 15, 2011–2026. [Google Scholar] [CrossRef]
- Zeng, X.Z.; He, L.G.; Wang, S.; Wang, K.; Zhang, Y.Y.; Tao, L.; Li, X.J.; Liu, S.W. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol. Sin. 2016, 37, 255–263. [Google Scholar] [CrossRef]
- Si, Y.; Li, Y.; Gu, K.; Yin, H.; Ma, Y. Icariin ameliorates osteoporosis in ovariectomized rats by targeting Cullin 3/Nrf2/OH pathway for osteoclast inhibition. Biomed. Pharmacother. 2024, 173, 116422. [Google Scholar] [CrossRef]
- Wang, Y.; Che, L.; Chen, X.; He, Z.; Song, D.; Yuan, Y.; Liu, C. Repurpose dasatinib and quercetin: Targeting senescent cells ameliorates postmenopausal osteoporosis and rejuvenates bone regeneration. Bioact. Mater. 2023, 25, 13–28. [Google Scholar] [CrossRef]
- Li, C.; Xu, W.; Li, L.; Zhou, Y.; Yao, G.; Chen, G.; Xu, L.; Yang, N.; Yan, Z.; Zhu, C.; et al. Concrete-Inspired Bionic Bone Glue Repairs Osteoporotic Bone Defects by Gluing and Remodeling Aging Macrophages. Adv. Sci. 2024, 11, e2408044. [Google Scholar] [CrossRef]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef]
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.K.; Moon, H.J.; Kim, H.J.; Lee, S.J.; Lee, J.B.; Bae, M.S.; Yi, J.K.; Hwang, Y.S.; Bang, J.B.; et al. Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes. ACS Nano 2014, 8, 12049–12062. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Heo, D.N.; Kim, H.J.; Ko, W.K.; Lee, S.J.; Heo, M.; Bang, J.B.; Lee, J.B.; Hwang, D.S.; Do, S.H.; et al. Inhibition of Osteoclast Differentiation and Bone Resorption by Bisphosphonate-conjugated Gold Nanoparticles. Sci. Rep. 2016, 6, 27336. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Pan, W.; Liu, Y.; Liu, J.; Xu, G.; Su, Y.; Chen, D.; Ye, X. α-Lipoic acid loaded hollow gold nanoparticles designed for osteoporosis treatment: Preparation, characterization and in vitro evaluation. Artif. Cell Nanomed. B 2023, 51, 131–138. [Google Scholar]
- Ko, W.K.; Heo, D.N.; Moon, H.J.; Lee, S.J.; Bae, M.S.; Lee, J.B.; Sun, I.C.; Jeon, H.B.; Park, H.K.; Kwon, I.K. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. Colloid. Inter. Sci. 2015, 438, 68–76. [Google Scholar] [CrossRef]
- Liang, H.; Jin, C.; Ma, L.; Feng, X.; Deng, X.; Wu, S.; Liu, X.; Yang, C. Accelerated Bone Regeneration by Gold-Nanoparticle-Loaded Mesoporous Silica through Stimulating Immunomodulation. ACS Appl. Mater. Inter. 2019, 11, 41758–41769. [Google Scholar] [CrossRef]
- Lee, D.; Ko, W.K.; Kim, S.J.; Han, I.B.; Hong, J.B.; Sheen, S.H.; Sohn, S. Inhibitory Effects of Gold and Silver Nanoparticles on the Differentiation into Osteoclasts In Vitro. Pharmaceutics 2021, 13, 462. [Google Scholar] [CrossRef]
- Keegan, G.M.; Learmonth, I.D.; Case, C.P. A systematic comparison of the actual, potential, and theoretical health effects of cobalt and chromium exposures from industry and surgical implants. Crit. Rev. Toxicol. 2008, 38, 645–674. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale 2017, 9, 706–718. [Google Scholar] [CrossRef]
- Yan, L.; Liang, M.; Yang, T.; Ji, J.; Jose Kumar Sreena, G.S.; Hou, X.; Cao, M.; Feng, Z. The Immunoregulatory Role of Myeloid-Derived Suppressor Cells in the Pathogenesis of Rheumatoid Arthritis. Front. Immunol. 2020, 11, 568362. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Z.; Tan, J.; Zhong, J.; Chen, L. TRAF6/ERK/p38 pathway is involved in interleukin-17-mediated autophagy to promote osteoclast precursor cell differentiation. Zhejiang Da Xue Xue Bao Zhejiang Da Xue Xue Bao Yi Xue Ban 2021, 50, 162–170. [Google Scholar] [CrossRef]
- Xing, Y.; Huang, L.; Jian, Y.; Zhang, Z.; Zhao, X.; Zhang, X.; Fu, T.; Zhang, Y.; Wang, Y.; Zhang, X. GORASP2 promotes phagophore closure and autophagosome maturation into autolysosomes. Autophagy 2025, 21, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, B.; Li, J.; Li, H.; Wang, G.; Cai, X.; Zhang, Q.; Liu, X.; Kan, C.; Wang, L.; et al. Alternative mRNA polyadenylation regulates macrophage hyperactivation via the autophagy pathway. Cell Mol. Immunol. 2024, 21, 1522–1534. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnol 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, D.; Qian, H.; Ruan, C.; Yang, Y.; Li, D.; Wang, G.; Zhu, X.; Hu, Y.; Lei, P. Precision pore structure optimization of additive manufacturing porous tantalum scaffolds for bone regeneration: A proof-of-concept study. Biomaterials 2025, 313, 122756. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Wang, S.; Fan, W.; Yang, Y.; Gao, P.; Chen, M.; Yang, W.; Cai, K. Promoting osseointegration by in situ biosynthesis of metal ion-loaded bacterial cellulose coating on titanium surface. Carbohydr. Polym. 2022, 297, 120022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).