Abstract
Tissue engineering represents a revolutionary approach to regenerating damaged bones and tissues. The most promising materials for this purpose are calcium phosphate-based bioactive ceramics (CaPs) and bioglasses, due to their excellent biocompatibility, osteoconductivity, and bioactivity. This review aims to provide a comprehensive and comparative analysis of different bioactive calcium phosphate derivatives and bioglasses, highlighting their roles and potential in both bone and soft tissue engineering as well as in drug delivery systems. We explore their applications as composites with natural and synthetic biopolymers, which can enhance their mechanical and bioactive properties. This review critically examines the advantages and limitations of each material, their preparation methods, biological efficacy, biodegradability, and practical applications. By summarizing recent research from scientific literature, this paper offers a detailed analysis of the current state of the art. The novelty of this work lies in its systematic comparison of bioactive ceramics and bioglasses, providing insights into their suitability for specific tissue engineering applications. The expected primary outcomes include a deeper understanding of how each material interacts with biological systems, their suitability for specific applications, and the implications for future research directions.
1. Introduction
Bioactive materials (bioglasses and ceramics) are commonly employed to restore and replace unhealthy and damaged hard and soft tissues [1]. Two major classes of bioactive materials are bioactive ceramics and bioglasses. They have gained widespread attention due to their ability to connect with biological tissues, promote cellular interactions, and regenerate tissue [2]. Thus, these novel materials are pivotal in a wide range of biomedical applications. According to the host tissue interactions, bioceramics can be classified as nearly bioinert, bioactive, and bioresorbable [3]. Among these, calcium phosphate-based ceramics are highly bioactive materials, and they are particularly significant due to their compositional similarity to natural bone minerals. Overall, the damaged tissues and bones can be restored or replaced using these types of biomaterials and other bioactive molecules. Theoretically, bioceramics and bioglasses bond to bone through a bone-like carbonated hydroxyapatite layer, facilitating effective biological interaction [4]. Similarly, bioglasses are also recognized for their high bioactivity and potential stimulation of osteogenesis [1]. The advantages of bioactive ceramics include their various chemical compositions and controlled degradation rates, whereas bioglasses excel in forming strong bonds with hard tissues and have enhanced versatility in composition. However, both materials present challenges such as limited flexibility, fragile texture, and low mechanical properties [5,6,7]. It is well known that the most bone-like bioactive ceramics are the different calcium phosphate phases, such as hydroxyapatite (HAp), dicalcium phosphate (DCP), and tricalcium phosphate (TCP), that are widely used in bone tissue engineering since they can support osteointegration and osteoconduction. On the other hand, bioglasses, such as 45S5 Bioglass, are known for their ability to bond with soft and hard tissues, making them versatile for widespread applications such as bone grafts, scaffolds, coatings for implants, and even in dentistry as bone fillers. However, their brittleness (in ceramics) and rapid degradation (in bioglasses) limit their standalone use or their applications in load-bearing conditions. Recent advancements have focused on combining these materials with biopolymers to overcome these limitations [8,9].
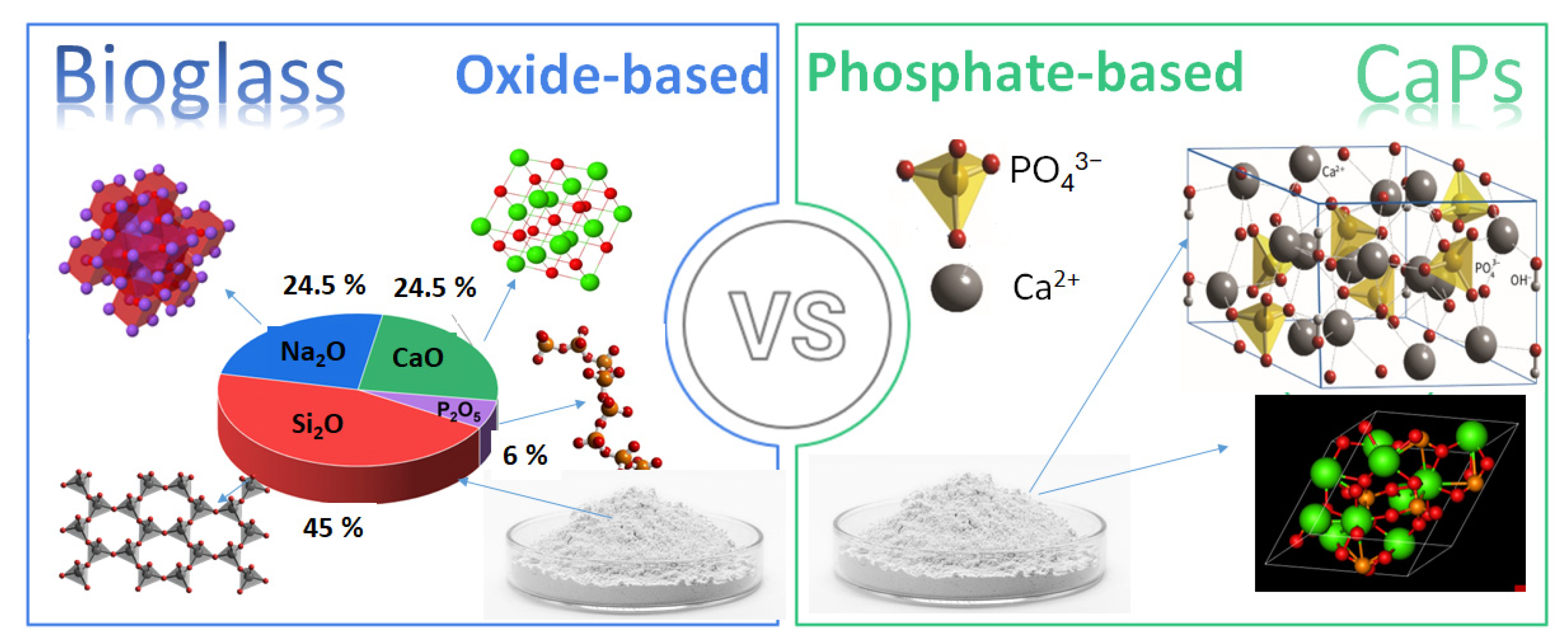
In addition, the demand for biomaterials in regenerative medicine has also significantly increased due to the growing need for functional tissue replacements. Consequently, recent research has expanded their use in soft tissue repair [10], wound healing [11,12], and drug delivery [13,14,15]. Despite extensive research on both material types, a detailed comparative analysis of calcium phosphate-based ceramics vs. bioglasses in tissue engineering is still missing. This review systematically compares their biological interactions, material properties, applications, and limitations to provide a clear understanding of their respective advantages and potential improvements. The review also explores composites with biopolymers, which offer enhanced mechanical and biological properties, making them attractive alternatives for clinical applications. Figure 1 illustrates the differences between the two materials in composition and main constituents.
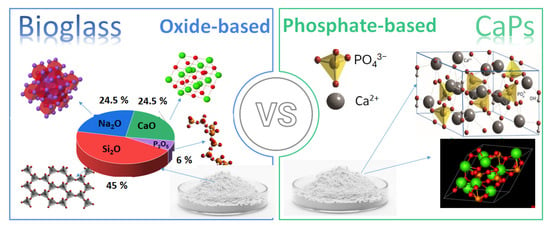
Figure 1.
Schematic illustration the differences in compositions of bioglasses and CaP-based ceramics.
2. Calcium Phosphate-Based Bioactive Ceramics
Calcium phosphate (CaPs)-based ceramics constitute a group of biomaterials which include many different calcium phosphate phases with varying Ca to P molar ratios (from 0.5 to 2) that cause different morphologies, particle sizes, and forms, thus diverse biological performance [16]. These materials have a chemical composition and structure like the mineral phase of bone. Properly manufactured CaP ceramics have an interconnected porous structure, and they are well known for their excellent osteoconductive properties. However, they do not have proper osteoinductivity, moderating the attachment and the differentiation of osteogenic cells [17]. Porous CaP ceramics possess a compressive strength corresponding to 10–25% of the compressive strength of long bones [18]. Thus, porous CaP ceramics need more mechanical support via osteosynthesis when used in weight-bearing bone parts [19].
Owing to their unique structures, CaP ceramics facilitate the growth of bones on their surface and into their three-dimensional structure, which means osseointegration. It has been shown that osseointegration depends on the pore size of the materials. The optimal osteoconductivity of biomaterials can be achieved with pore sizes ranging from 300 to 400 μm, while the minimum pore size that is required to generate mineralized bone is considered to be 50 μm [20].
The different calcium phosphate phases can be synthesized by mixing calcium and phosphate solutions under acid or alkaline conditions. The preparation parameters and conditions used strongly determine the formed phases [21]. Only particular CaP compounds are beneficial for implantation in the body, as those with a calcium-to-phosphorus ratio lower than 1 are not fit for biological use because they are highly soluble [22]. The high osteoinductive and bone regenerative capability of these materials mainly linked to their chemical and physical properties, such as surface roughness and porosity [23,24]. It is also reported [25,26] that the presence of calcium ions and inorganic phosphate activates specific signaling routes that facilitate bone generation. The calcium phosphate powder particles can be prepared in many ways, either at low or high temperatures and in dry or wet conditions. The main technologies are summarized in Table 1.

Table 1.
Most used preparation methods for CaPs, classified as wet chemical methods and dry methods [21,22,23,24,25,26].
CaP powders obtained from the above discussed methods can be processed further by integrating dopant elements, such as Sr, Zn, and Mg, or by forming composites with polymers or alternative bioceramics [27].
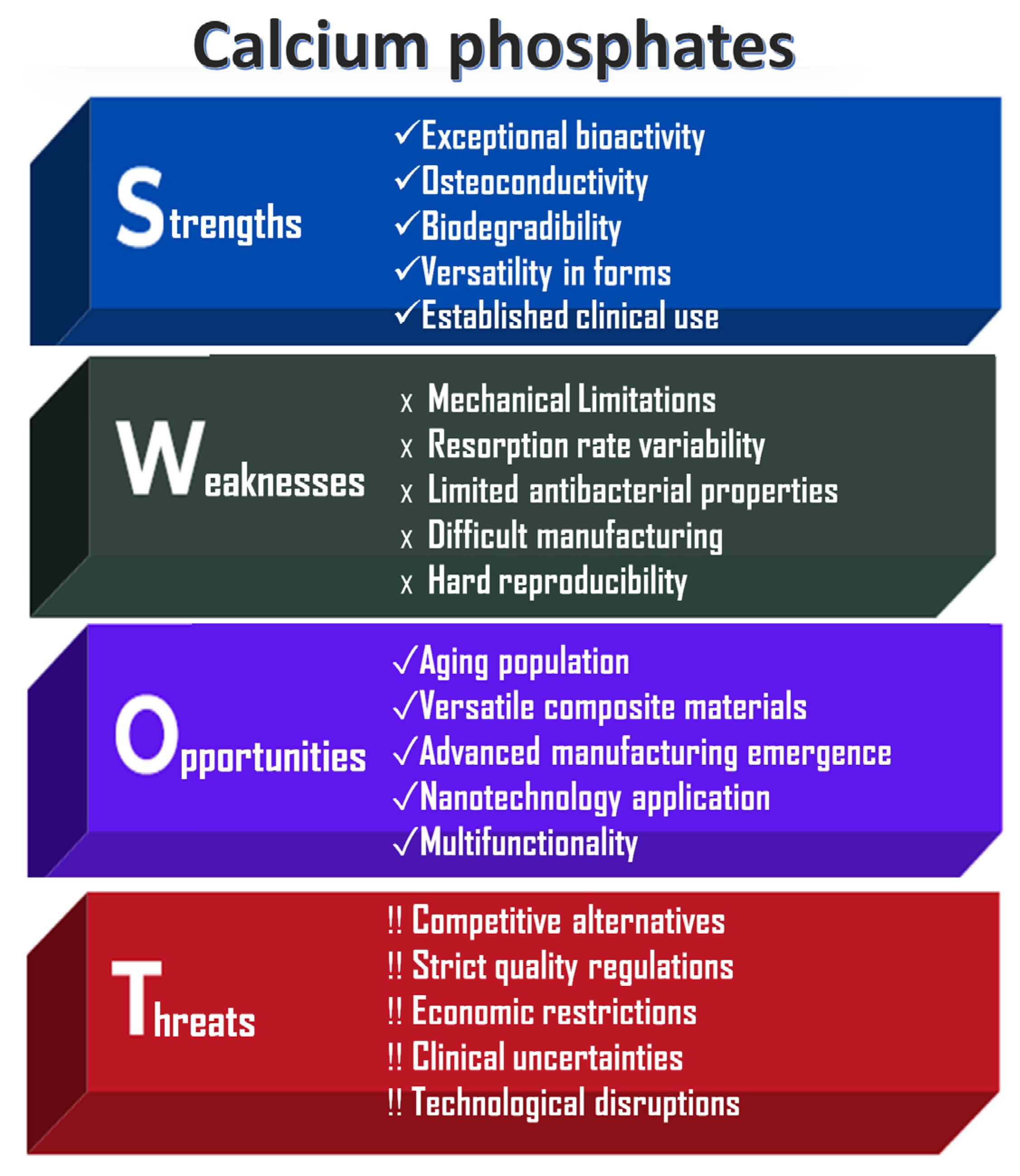
It is also important to assess the practical effectiveness of these CaP powders. As shown in Figure 2, a SWOT analysis has been conducted on the applications of calcium phosphate ceramics, which underscores both their significant advantages and the challenges they present to society.
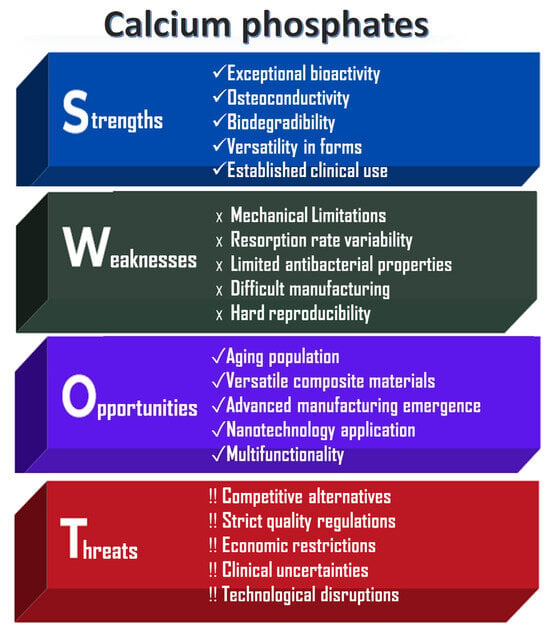
Figure 2.
SWOT analysis of CaP-based ceramics.
The main strengths of calcium phosphates (CaPs) lie in their exceptional chemical properties and morphology, which facilitate integration into the body through direct bonding with bones. When used as scaffolds, CaPs support bone cell migration and proliferation, thereby promoting natural bone regeneration. Their gradual degradation enables replacement by native bone; for example, β-tricalcium phosphate resorbs more rapidly than the hydroxyapatite phase [24,25,26]. Additionally, CaPs can be employed in various forms and applications, including dental, orthopedic, and craniofacial procedures.
However, several limitations affect their broader application. The inherent brittleness and limited fracture resistance of CaPs restrict their use in load-bearing regions [18,19]. Furthermore, the degradation rate may not always align with the rate of bone healing, potentially leading to premature scaffold failure or interference with tissue regeneration. Without additional surface modifications, these materials can also be prone to microbial colonization [25].
Other challenges include stringent FDA (Food and Drug Administration) and EMA (European Medicines Agency) regulatory requirements, which delay market entry and increase development costs. High production expenses and limited accessibility in resource-constrained regions further hinder widespread adoption. Moreover, there is a lack of long-term clinical studies addressing rare complications such as inflammation or inconsistent resorption behavior.
Despite these challenges, CaPs remain important biomaterials, particularly as the aging and growing population drives increased demand for bone grafts due to osteoporosis, trauma, and joint replacements. The use of advanced manufacturing technologies, such as 3D and 4D printing, offers significant potential for producing patient-specific scaffolds with complex geometries, thereby improving surgical outcomes [3].
3. Bioglasses
Bioglasses are a type of bioactive material that exhibit excellent biocompatibility and the ability to bond directly to bone tissue and have the function of enhancing the regeneration of bone tissues while gradually degrading [28]. The commercial composition of bioglasses is mainly an amorphous mix of oxides, such as silicon dioxide, sodium oxide, calcium oxide, and phosphorus pentoxide in different ratios. The most used composition is 45 Wt.% SiO2-24.5 Wt.% Na2O-24.5 Wt.% CaO-6 Wt.% P2O5, but many other variations, which are widely elaborated in detail in numerous reviews, are available [29,30,31].
Generally, these materials are typically amorphous silicate glasses made either by the melting method [32,33] or the sol–gel method [34]. In addition, researchers [35] compared the mechanical and physical properties of bioglass scaffolds made in two different ways. They found that the sol–gel-synthesized bioglasses are superior to melt-produced bioglasses because of their greater porosity and better bioactivity. According to the main constituent, there are three main types of bioglasses, such as silica-based glasses [36], borate-based glasses [12,37,38], and phosphate-based glasses [39,40]. Each type has its unique chemical composition and mechanical and structural properties; therefore, they can be utilized in various applications in biomedical fields. Their main applications are as bone grafts and fillers. Recent studies have demonstrated their effectiveness in promoting osteogenesis and angiogenesis [41]. The bioglasses can be prepared alkali-free, especially Na-free. Sodium is regarded as an essential component for bioactivity, since it effectively disintegrates the glass network. However, bioglasses without sodium revealed similar dissolution characteristics and bioactivity as traditional bioglasses with sodium [42,43,44]. In addition, some in vitro biocompatibility studies have proven that the bioglasses with higher Na2O content can be linked to cytotoxic effects [45].
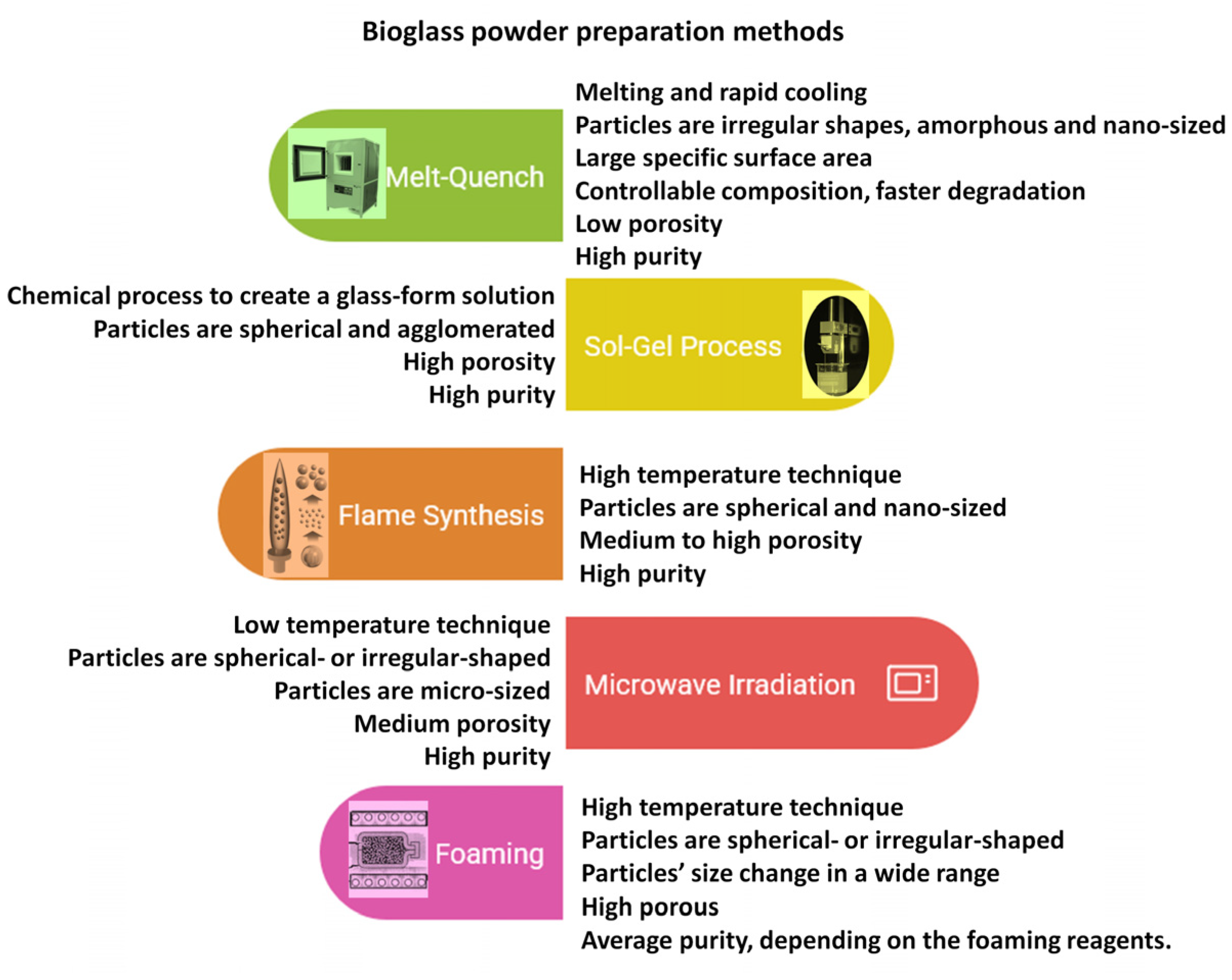
It has also been investigated that the apatite formation and the degradation rate are significantly affected by the glass network porosity and the amount of phosphate. The CaO, Na2O, and P2O5 components are network modifiers, and they can be integrated into the original SiO2-CaO-Na2O composition to obtain a more reactive surface [46,47]. The P2O5 content as a phosphate derivative is also necessary for bioactivity [48,49]. The different types of bioglass powders can be prepared in many ways, the most widely used ones are illustrated in Figure 3 [32,42,43,44,45,46,47,48,49].
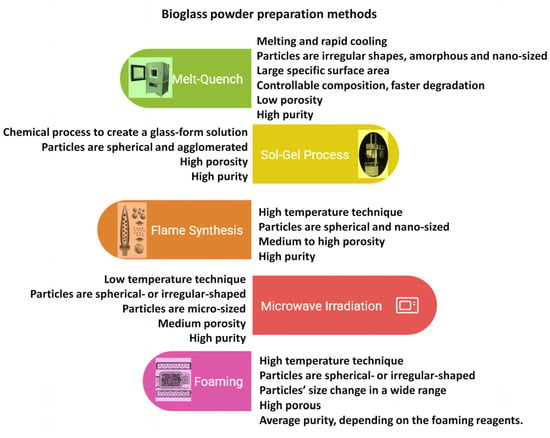
Figure 3.
Schematic illustration of bioglass preparation methods and their properties.
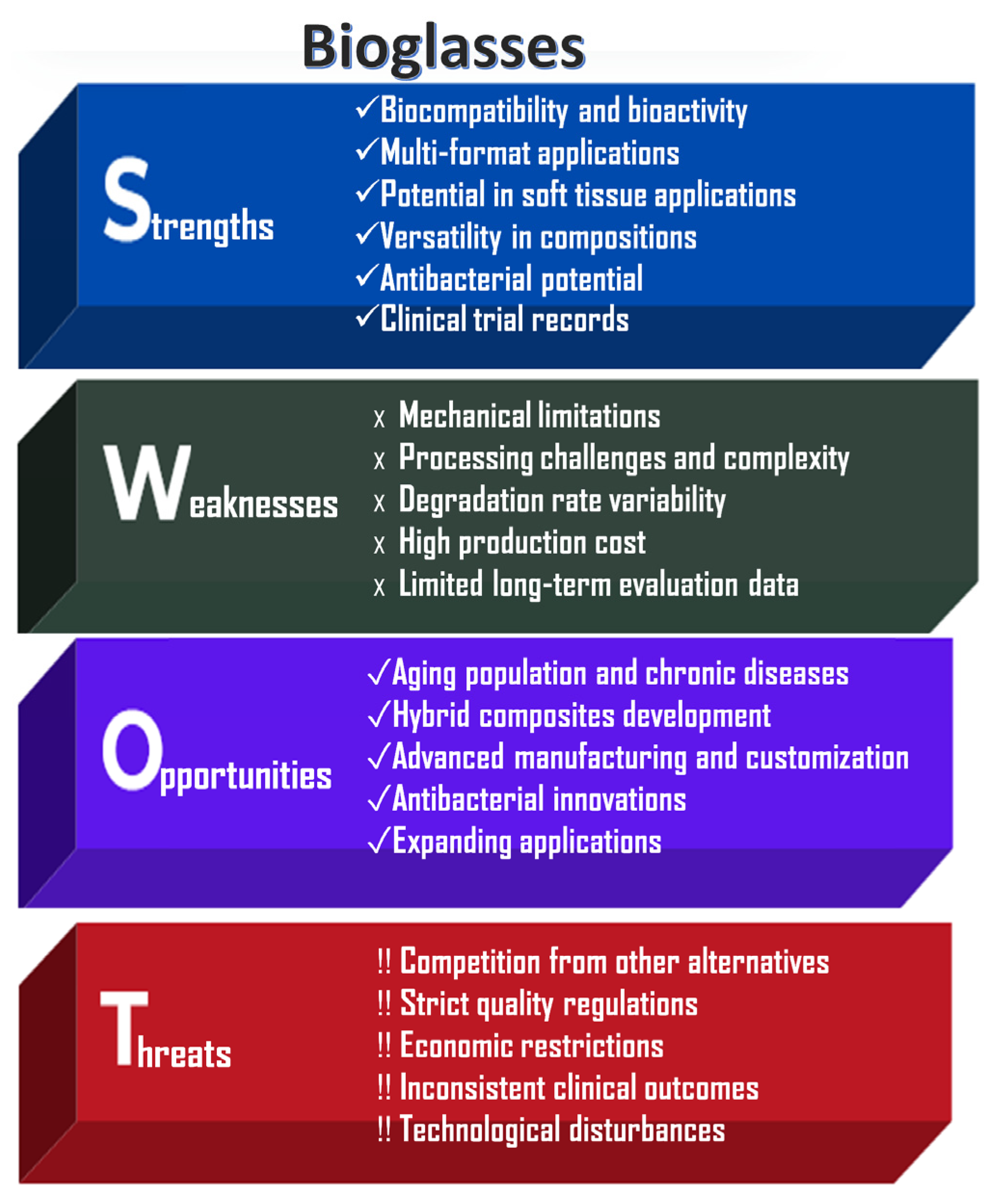
An analysis of the strengths, weaknesses, opportunities, and risks associated with bioglass powder applications, highlighting their significant societal benefits and drawbacks, is shown in Figure 4.
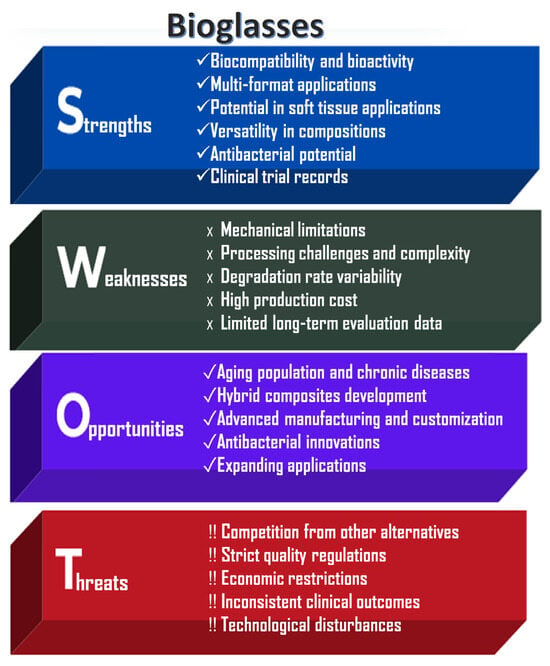
Figure 4.
SWOT analysis of bioglass powders.
The key strengths of bioglass powders are their excellent bioactivity and highly versatile composition. They are capable of stimulating both osteogenesis (bone formation) and angiogenesis (blood vessel formation). In physiological fluids, a hydroxycarbonate apatite (HCA) layer can form on their surface, enabling strong chemical bonding with both bone and soft tissue [1,2,3]. Bioglasses can be engineered with tunable degradation rates and controlled ion release profiles [11]. Another significant advantage is their processability into various forms, including powders, scaffolds, coatings, fibers (e.g., bioactive glass wool for wound healing), and injectable cements. Unlike other bioceramics, bioglasses have the unique ability to bond with soft tissues, making them particularly useful in periodontal and other soft tissue applications [10].
However, bioglasses also present notable limitations. Similarly to calcium phosphate ceramics, they exhibit poor mechanical properties, including brittleness, low tensile strength, and limited fracture toughness, which restricts their use in load-bearing applications [5,6]. Large-scale production is technically challenging due to high melting temperatures and the risk of unwanted crystallization during manufacturing. Furthermore, their degradation behavior and ion release are composition-dependent, potentially leading to inconsistent or unpredictable biological responses [19].
Like other bioactive materials, bioglasses are subject to stringent regulatory requirements by agencies such as the FDA and EMA, which can delay commercialization and increase development costs. Nevertheless, ongoing innovations, particularly in composite materials, 3D printing technologies, and antimicrobial doping, are expected to expand the applicability of bioglasses in emerging fields such as personalized regenerative medicine and chronic wound care [3].
4. Applications of Bioactive Ceramics
The main application areas of bioactive ceramics (CaPs and bioglasses alike) are versatile. These bioceramics can be utilized in both soft and hard tissue engineering, along with their application in drug delivery mechanisms and as coatings for metal implant surfaces.
4.1. Bioglasses and Calcium Phosphates in Bone Tissue Engineering (BTE)
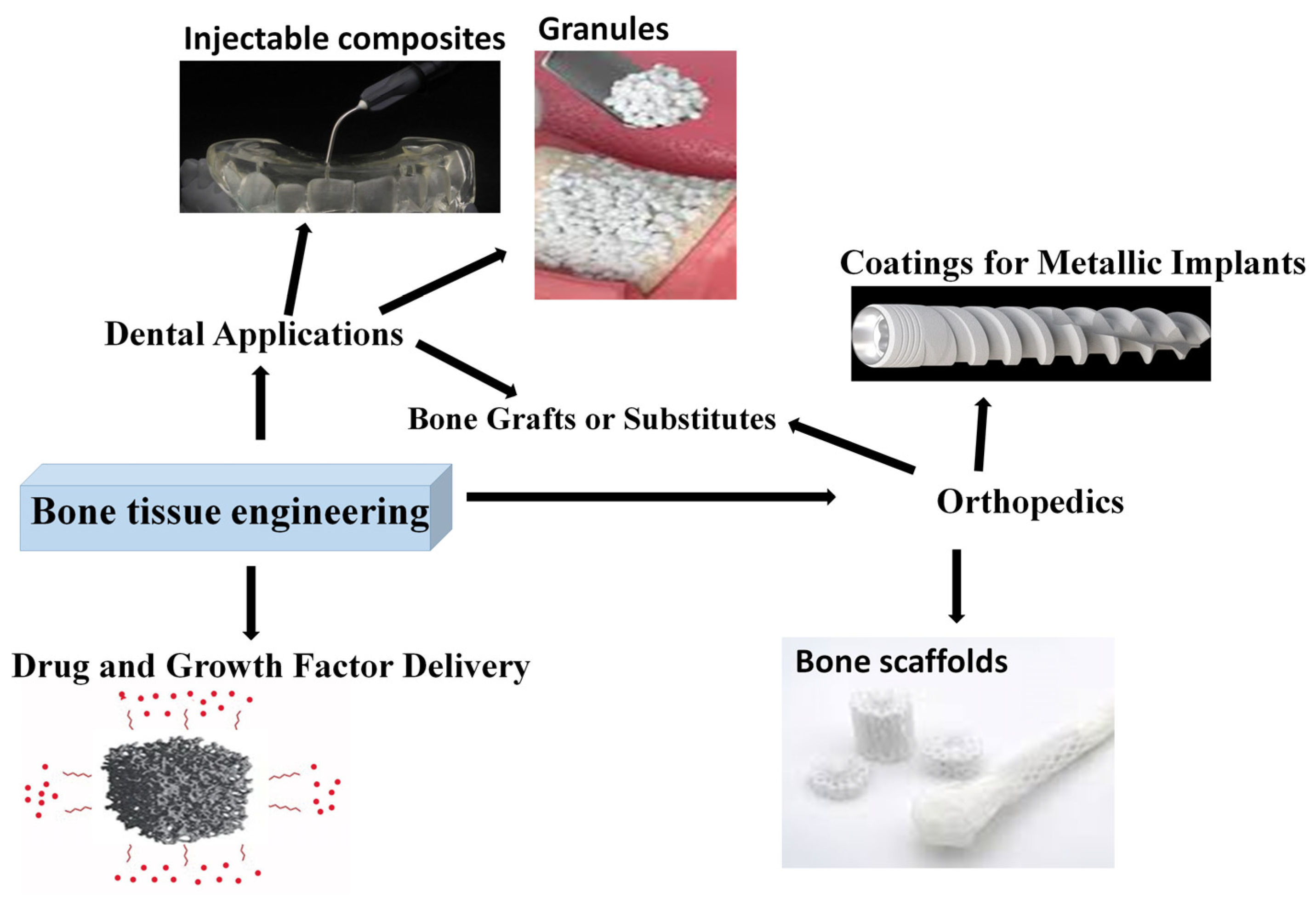
The most important applications are depicted in Figure 5. As presented, the main application fields in bone tissue engineering are orthopedics, where they can be used as scaffolds, bone grafts, or coatings, and dentistry, where the most common forms are granules, bone fillers, and injectable composites. Another emerging and developing field is drug delivery matrices.
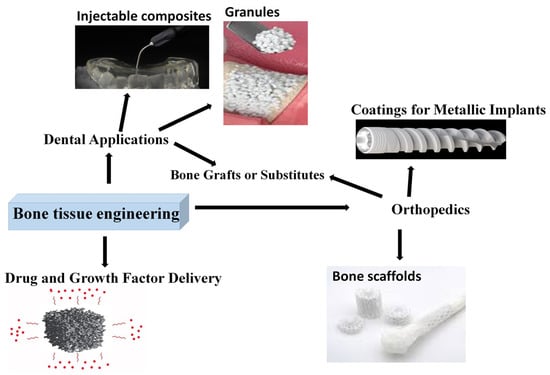
Figure 5.
Bioactive ceramic utilization in bone tissue engineering.
4.1.1. Scaffold Materials and Bone Substitutes
In clinical practice, bone irregularities are prevalent and impose a considerable burden on the families of patients, as well as on society and healthcare infrastructures. Scaffolds made from calcium phosphate with complex nano- and micro-level structures have proven to be highly effective in facilitating bone regeneration. These materials can be fabricated into porous scaffolds that support cell attachment, proliferation, and differentiation [50,51,52,53].
Calcium phosphate cements (CPCs), as unique forms of CaPs, are synthetic, self-assembling bone substitute materials. The main benefit of CPCs is that they are injectable, moldable, and hardened in situ, so the contact between the tissue and the implant will be optimal, even when the defect dimensions are irregular [49]. Calcium phosphate scaffolds provide stable properties and allow the control of porosity and biocompatibility. The pore size of the scaffold improves revascularization and bone remodeling, enabling the ingrowth of cells and proteins and enhancing biocompatibility, making them suitable for implant use [54].
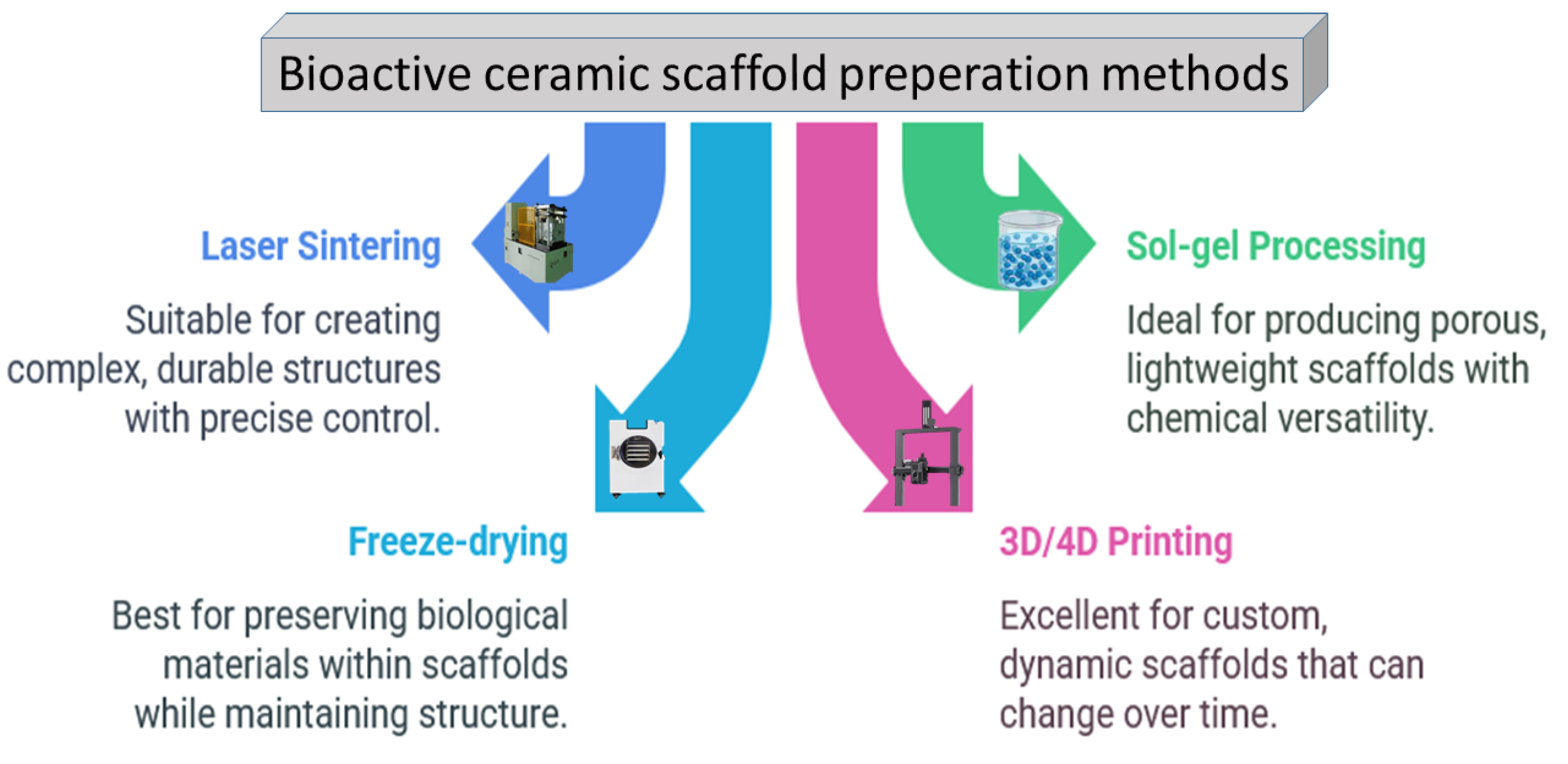
As depicted in Figure 6, a range of approaches is used to fabricate porous ceramic scaffolds, and each method has a unique impact on the mechanical properties and the suitability of the end products for specific applications [55,56].
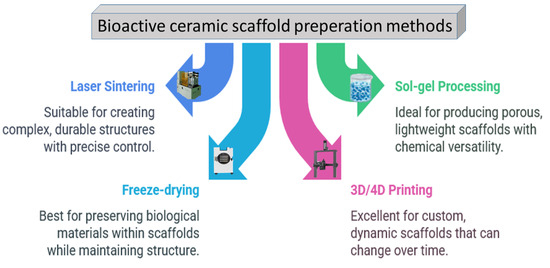
Figure 6.
The most utilized ceramic scaffold preparation methods so far.
However, numerous other methods and additives can be utilized to modify the porous characteristics of ceramic scaffolds. For example, adding a pore-forming agent [57], applying foam technology [58], or extrusion molding method [59]. Nonetheless, the connectivity of pores plays a crucial role in determining the biological performances, and at the same time, a three-dimensional arrangement of connected pores has been found to be more susceptible to a decrease in the mechanical properties of ceramic materials [60,61].
Bioglasses and CaPs have a similar role in bone tissue engineering thanks to their ability to directly adhere to bones. They function effectively as bone grafts, fillers, and restorative materials, promoting rapid integration and regeneration. Preparation methods like melt-quenching and sol–gel facilitate the development of porous structures with adjustable characteristics. Specifically, the ideal method for bioactive glass preparation is the sol–gel technique [62]. It helps to customize the properties of materials for bone tissue regeneration, because they can degrade at a controlled rate, transform into bone-like material, and connect well with tissues while promoting bone-forming cell growth. Adding bioactive elements or drugs into these bioglasses can further enhance their ability to heal bone and prevent infections. One of the most ideal structures for scaffold materials is the mesoporous system [63,64] that can be prepared by 3D printing as hierarchically porous scaffolds. However, there are still existing challenges regarding their applicability due to insufficient mechanical strength, especially for load-bearing bones [31,65].
The mechanical characteristics of bioglass and CaP-based scaffolds have been compared to natural bone and presented in Table 2 [5,6,7,19,40,57,66,67].

Table 2.
Mechanical properties of the bioglass scaffolds, calcium phosphate-based scaffolds, and natural bones.
While dense bioceramics (bioglasses and CaPs) excel in compressive strength and hardness, their brittleness and low fracture toughness make them unsuitable for dynamic load-bearing applications. On the other hand, natural bone’s composite structure provides a unique balance of strength, toughness, and self-repair. Scaffold materials prioritize bioactivity and controlled degradation over mechanical performance, aligning with their role in temporary bone regeneration.
Another key challenge in the field of bone tissue engineering is the imitation of the extracellular matrix’s composition. Scaffolds derived from bioglass particles and hydroxyapatite feature a naturally porous architecture that enhances cell attachment and development. In addition, it can enable vascularity, migration of nutrients, and metabolic by-products. The unique structures of these materials ensure better adherence of osteoblast cells, promoting their faster proliferation, differentiation, and mineralization into bone [68]. This represented a significant advancement in the development and optimization of different bone filler materials [69].
4.1.2. Bioactive Ceramic–Polymer Composites
In tissue engineering, another interesting area is the development of composite materials that combine bioglasses and calcium phosphates with different biopolymers to enhance their suitability and adjustability.
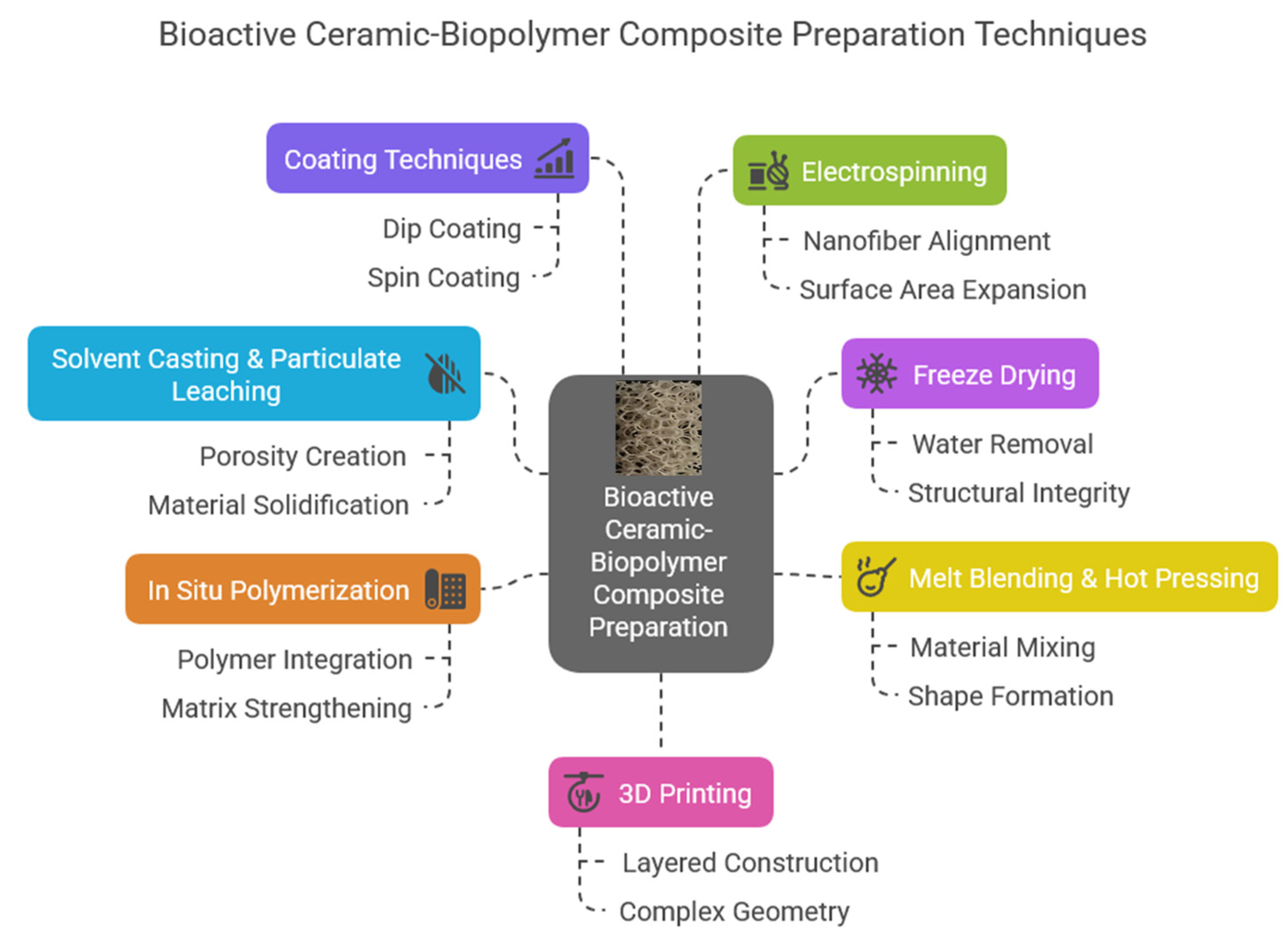
As Figure 7 shows, composite scaffolds can be prepared in many ways. Each has its advantages and disadvantages. These unique composite materials (both bioglasses and calcium phosphate derivatives) can be prepared as solid scaffolds, injectable composite hydrogels, fiber mats, thin films, or even matrices [70]. Among the various types of these composites, the injectable form is exceptionally attractive and useful [71,72,73]. The most commonly used biopolymers to form these composites can be classified into two main categories. One is the natural biopolymers, such as cellulose, cellulose acetate, alginates, chitosan, collagen, gelatin, and the other is the synthetic polymers like polylactic acid (PLA), polyvinyl pyrrolidone (PVP), polycaprolactone (PCL), and polyethylene glycol (PEG). A wide array of materials has been explored for the fabrication of these polymer-based scaffolds, each offering unique properties and functionalities tailored to specific applications. Chiu et al. [74] prepared injectable implants in the form of calcium sulfate and self-setting calcium phosphate composite. The developed injectable pastes were easy to handle, had excellent biocompatibility, and sufficient mechanical properties. These pastes also had great potential in minimally invasive surgery, and they can be utilized to treat maxillofacial defects or even in reconstructive rhinoplasty. In other interesting work, Cai et al. [75] prepared injectable chitosan oligosaccharide (COS) and bovine-derived hydroxyapatite (BHAp) composites. The intended use of this material was as a dental pulp cap. The developed biocomposite was slightly soluble in physiological solutions; however, the solubility rate can be changed by applying certain cross-linking agents. Regarding bone regeneration, a promising composite can also be a dextran-based injectable hydrogel [76] which can be utilized as scaffolds and drug-release systems simultaneously.
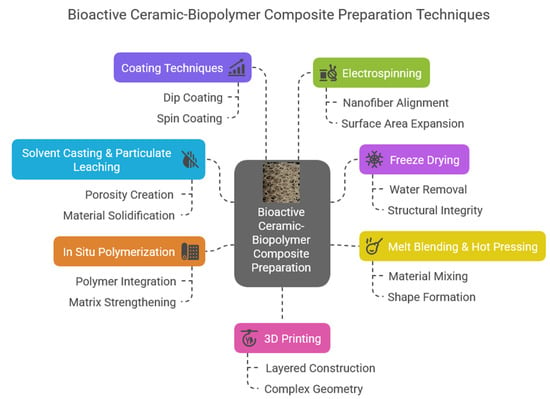
Figure 7.
Bioactive ceramic and biopolymer composite preparation possibilities and their forms.
The alginate-, chitosan-, collagen-, and gelatine-based composites are similarly ideal choices to prepare injectable materials. For example, Xu et al. [77] prepared bioactive glass containing sodium alginate hydrogel that had immunomodulatory and angiogenic properties to heal tendon tissues. According to their results of the biomechanical tests, the BG/SA hydrogel noticeably enhanced the load, failure stress, and tensile modulus of the repaired tendon. Therefore, applying injectable BG/SA hydrogel can be a novel and promising therapeutic approach to heal Achilles tendons along with surgical intervention. On the other hand, in a recent study, Estevez et al. [78] developed calcium phosphate containing sodium alginate and gelatin hydrogels as bone replacement injectable composites. They proved that the developed hydrogel composites had mineralization ability with slight antimicrobial properties and a slow-to-moderate degradation rate. Wu et al. [79] recently reported their work on the preparation of porous composites microspheres based on hydroxyapatite (HAp), di-calcium phosphate dihydrate (DCPD), and chitosan. They prepared the composites via the hydrothermal method, in which chitosan had an important role as a chelating agent to promote the calcium phosphate particles’ growth. They found that the formed microspheres comprising Ca2P2O7, β-TCP, and HAp can be utilized as injectable bone graft materials. Chitosan is also an important matrix for injectable hydrogel preparation. Ciotek et al. [80] examined chitosan and sodium hyaluronate hydrogels with bioglass particle addition under different conditions and found that these bioglass and polysaccharide hydrogels were microstructurally stable and bioactive. Besides the above-mentioned works, numerous other researchers are exploring these kinds of important injectable composite materials [81,82,83,84].
In the form of solid and stable scaffold materials, these novel composites can be utilized in cases of larger bone deficiency as filler materials. In this case, the main preparation methods are molding and additive manufacturing, such as 3D or 4D printing [85,86,87].
For instance, PLA polymer with embedded calcium phosphate particles has been evaluated for its bioactivity [88]. In vitro studies have shown that human mesenchymal stromal cells (hMSCs) proliferate on these composites, indicating potential for tissue engineering applications. In other research, Zhang et al. [89] investigated the effect of different amounts (5%, 10%, and 20% in wt%) of 63s bioglass on the properties of bioglass-PCL composites as scaffolds. They shaped the scaffold with 3D printing. The results revealed that the performance of the composites improved by increasing the 63s BG content. However, despite their advancements, they did not successfully reproduce the complex mechanical characteristics of cortical bone, which limits their application in load-bearing orthopedic settings, but they are appropriate for addressing minor bone or cartilage repairs. Rajzer et al. [90] prepared bioglass and zinc-doped bioglass containing PCL composite filaments by injection molding. They studied the mechanical and biological properties of the composites. In vitro mineralization studies revealed apatite formation on the surface of bioglass-added scaffolds after 7 days of immersion in SBF; however, in the case of Zn-doped bioglass, slower apatite formation was observed. An interesting work [91] reported a 3D preparation of biphasic calcium phosphate-PCL scaffolds with high and interconnected porosity as well as chemical degradability. They found that the PCL content improved the strength and toughness of composites, that is important to withstand mechanical loading in bone tissue engineering.
Hajiali et al. [92] compiled a thorough review paper on the PCL-bioglass and PCL-CP composites and studied their mechanical and biological performances as well as their biodegradability. In their paper, they focused on the preparation methods of scaffolds with ideal mechanical and structural properties; however, they did not discuss or draw any conclusion about the differences between the bioglass and CP containing PCL matrices. They concluded that the conventional preparation methods cannot produce scaffolds with controllable pore sizes, interconnectivity and reliable internal as well as external structures. Thus, their mechanical characteristics do not meet the requirements. However, the most ideal preparation method, according to their paper, is the FDM 3D printing process, which deposits melted composite filaments over a build platform layer by layer. This provides excellent mechanical and biological properties for the entire scaffold.
A recent study [93] reported that the PCL polymer has no inherent antibacterial characteristic; therefore, it can be subject to bacterial attachment and biofilm generation. They tried to make the composite bactericidal by CaP nanoparticle incorporation into the polymer matrix. They stated that the flake-like morphology of the prepared CaP particles could disrupt bacteria’s membranes, thus impeding bacterial growth.
Another paper [94] described the preparation of novel, three-layer calcium phosphate/polycaprolactone scaffolds. These scaffolds had a graded increased porosity fraction from the inner to the outer layer. In addition, they were bioresorbable at a gradual rate. The scaffolds are composed of a dense hydroxyapatite (HAp)/β-tricalcium phosphate inner layer, a macroporous HAp/β-TCP intermediate layer, and lastly a macroporous PCL/(HA/β-TCP) outer layer. The preparation methods were gel-casting for the inner layers, while the outer composite layer was formed by a solvent casting and particle leaching technique. They carried out in vitro dissolution tests in TRIS solution, and the results showed that the dissolution happened in a differentiated mechanism within the different layers. They concluded that a targeted multi-functionality can be reached with this layered structure.
In earlier research, Ródenas-Rochina et al. [95] compared the properties of hydroxyapatite and bioglass (BG) nanoparticles in a polycaprolactone composite scaffold. They found that pre-osteoblast cells proliferated well on all scaffolds. However, in their study, the addition of bioactive particles did not cause noticeably more positive effects on cell differentiation than that of a pure PCL scaffold.
Besides the widely investigated HAp and TCP calcium phosphate phases, another promising filler material can be the octacalcium phosphate (OCP), which has been combined with various polymers to create composites for tissue regeneration. For example, gelatin–OCP composites have been studied for their ability to support osteointegration due to their inherent porosity. In vivo preclinical studies have demonstrated that these composites can regenerate substantial amounts of bone within months of implantation, suggesting potential applications in both bone and soft tissue engineering [96].
4.2. Bioglasses and Calcium Phosphate Derivatives in Soft Tissue Engineering (STE)
While primarily associated with hard tissue repair, bioglasses and calcium phosphate derivatives can also find applications in soft tissue engineering. Unfortunately, their use in soft tissue regeneration is less common and requires further research. However, they can be prepared in ideally porous structures that can modulate cell behavior. This makes them suitable for cartilage, tendon, and ligament repair [97,98,99]. Techniques like freeze-drying, hydrogel incorporation, and electrospinning are employed to tailor these materials for soft tissue applications. Hence, nanostructured calcium phosphate is an essential biomaterial for hard tissue repair, but also has potential in some soft tissues, as reported by a recent review [10].
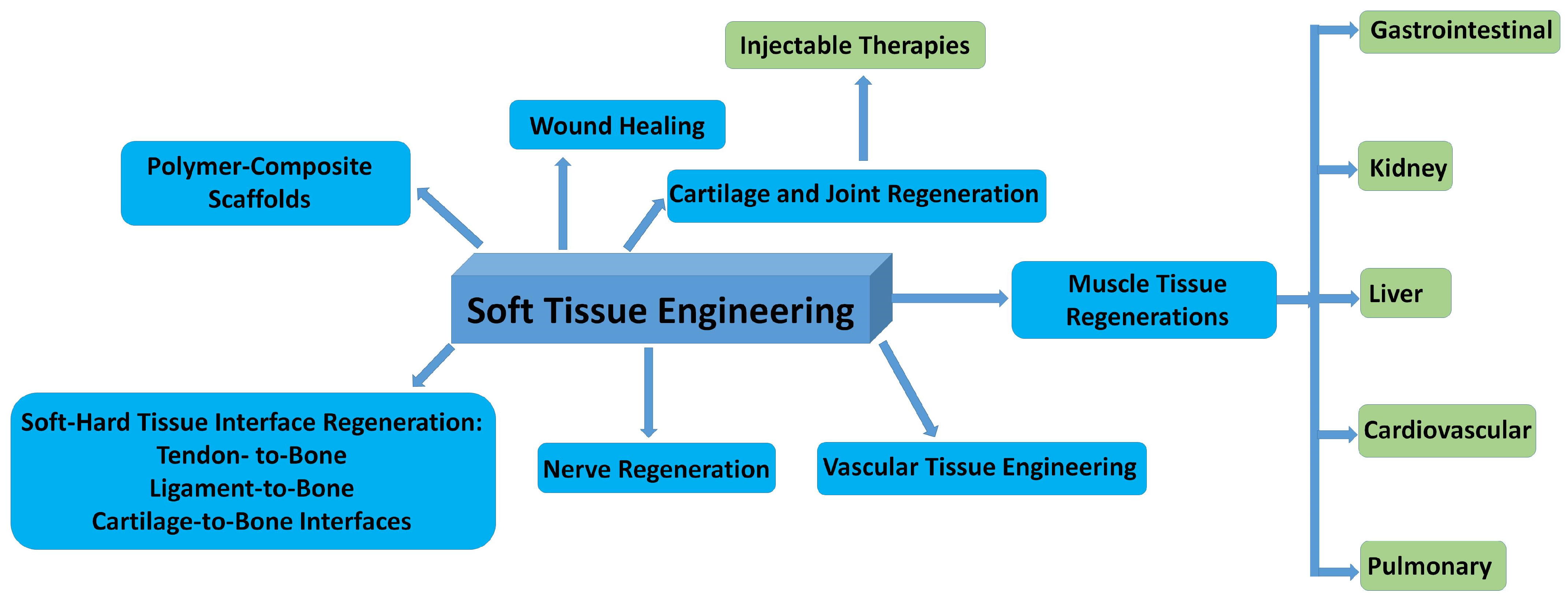
In Figure 8, the possible fields of applications in soft tissue repair are summarized.
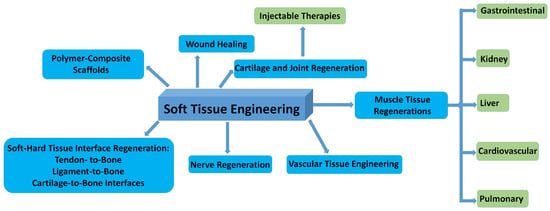
Figure 8.
Schematic illustration of the most important fields of bioceramic–biopolymer composites use in STE.
The main goal in soft tissue engineering is to repair and restore damaged or diseased tissues and remove the disadvantageous properties of both allografts and autografts. These materials can be prepared with optimized flexibility, biocompatibility, and biodegradability. These novel materials can initiate neovascularization, which is important for host tissue integration with the implanted structure [100]. Some reports describe their ability to aid the healing process of acute and chronic wounds [101,102,103,104]. In addition, bioglasses and calcium phosphate derivatives can be used to regenerate heart and lung tissues that are commonly known for their poor renewal capacity, as well as to repair peripheral nerve and musculoskeletal tissues [10,100,105,106,107,108]
Extensive research revealed that the incorporation of ceramic nanoparticles significantly improves mechanical strength and biological characteristics and alters the degradation kinetics of polymers. In addition, it can noticeably improve flexibility, which is crucial for soft tissue applications [109]. Furthermore, bioglasses have also proven their capacity to promote skin healing by improving blood vessel formation and collagen production during the proliferation phase, along with beneficial impacts on all other critical stages of the wound healing process [38].
It is noteworthy that since bioactive calcium phosphate-based ceramics are primarily used in association with bones, the number of reported data utilizing them in the field of wound healing is rather limited. For example, Eliaz et al. [110] discussed in a review paper that calcium phosphates have also shown potential as wound dressings since the cellular reaction is different for hydrophilic and hydrophobic implants, particularly in the initial stages of wound healing. CaPs can provide sufficient hydrophilicity to the surfaces with higher surface energy, resulting in faster cell activation and differentiation than those with lower surface energy.
Wang et al. [111] also mentioned in their mini review that traditional phosphate-based (Ca3(PO4)2, Ca5(PO4)3(OH)) and silicate-based (CaSiO3 and MgSiO3) powders can be changed into black bioceramics by the addition of magnesium and thermal reduction causing structural defects and oxygen vacancies. These black bioceramics could efficiently and controllably release Ca, Si, and Mg ions that were reportedly beneficial in the cases of skin-tumor-bearing mice [112]. Furthermore, black bioceramic-based materials have the potential to substantially boost the healing process of skin injuries in mice through improved tissue regeneration. In another review [103], calcium phosphate ceramics were also mentioned regarding their applications in wound treatment.
4.3. Bioglasses and Calcium Phosphate Derivatives as Drug Delivery Matrices
Nanostructured CaPs and mesoporous bioglasses with large surface areas are suitable for drug/gene delivery systems. These matrices are suitable architectures for incorporating and releasing drugs, growth factors, therapeutic agents, and even genes in a controlled way. Common preparation methods can be co-precipitation [113] and layer-by-layer assembly [114,115], which can control release kinetics and enhance efficacy. In addition, they have a moderate and gradual degradation rate in different biological conditions, as well as a large surface area. The CaP derivatives dissolve faster at a slightly acidic pH, which enables a controlled drug release into cells [116]. It is also discussed that the drug delivery ability of bioceramics (CaPs) is dependent on their crystalline rate, relative surface area, microstructure, micro- and nano-morphology, as well as particle sizes and forms [117]. Structures with low crystallinity or amorphous phases with high surface areas can incorporate more drugs and have lower release rates compared to crystalline structures, such as HAp [118]. The higher the solubility of the CaP phases, the faster the drug release rate [119]. For example, Uskoković et al. [120] reported that spheroid CaP particles had more effective drug loading and release capacity than flaky, brick-like, or elongated orthogonal-shaped particles. A large number of therapeutic agents can be delivered by bioactive ceramic nanoparticles, including antibiotics, anti-inflammatory drugs, and growth factors for bone healing [117].
Bioglasses can also serve as versatile platforms for drug delivery, capable of releasing therapeutic agents in a controlled manner. Their composition can be adjusted to achieve desired release profiles, utilizing techniques such as ion exchange and nanoparticle embedding. Their porous structure is an ideal matrix for drug inclusion, delivery, and controlled release [121]. The other unique structure of bioglasses is the mesoporous (MBGs) [122]. The mesoporous channels in bioglasses improve textural properties and bioactivity compared to sol–gel bioglasses, while their large pore volume allows simultaneous loading of drugs, growth factors, or other bioactive molecules [123]. Additionally, the silanol groups at the surface of MBGs enable further modification strategies with different functional groups for the controlled release of therapeutic compounds [124,125].
Yao et al. [126] developed a mesoporous bioactive glass (MBG) modified with alginate. They reported that by mixing MBGs with the polymer, the otherwise immediate and fast release of incorporated drug molecules can be better controlled. They have used simvastatin (SIM) as a model drug. Generally, any antibiotic incorporation is useful to prevent or cure infections at the implanted sites. For example, Huang et al. [127] prepared boron-doped bioactive glass (BG) scaffolds using the sol–gel method. Boron content aimed to enhance the bioactivity. The microstructural analyses showed mesoporous structures. They further revealed that the boron-doped BG had nano-sized pores and rougher microstructures than those of pure bioglasses, which is beneficial for drug delivery. In their case, the incorporated drug was teicoplanin. Teicoplanin and vancomycin are the last generation of antibiotics against infections caused mainly by Gram-positive bacteria. Teicoplanin is a better choice for use since it has a lower toxicity and longer half-life. According to the drug-release tests, the release rate gradually slowed down during immersion for 7 days.
The other important agents are the different growth factors, such as bone morphogenetic proteins (BMPs), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGF-β) are all critical factors in bone healing [128]. Hence, the addition of growth factors can further enhance the osteogenic ability of bioactive ceramics. For instance, BMP-2 connects with CaPs through the functional groups, such as -OH, -NH2, and -COO [129]. However, it is important to denaturalize the protein during its incorporation into the ceramic matrix, otherwise, it would lose its main functionality. The addition of different genes into the system also promotes and facilitates bone regeneration [130].
Bioactive element doping also alters the drug-encapsulation capacity of bioglasses. In a recent work [131], therapeutic element-added mesoporous bioactive glass nanoparticles (MBGNs) were evaluated as unique multifunctional systems. These ions were strontium and magnesium (SrMg-MBGNs). According to their results, the Sr and Mg-doping increased pore volume, thus higher solubility rate. In addition, the original mesoporous structure changed from worm-like to radial–dendritic, which caused a little faster drug release compared to undoped MBGNs. They used ibuprofen for the drug-release experiments. The experiments showed that the drug loading capacity slightly decreased while the release rate increased due to the open dendritic pores at the external surface, which resulted in accelerated ibuprofen emission. Additionally, several studies have been actively carried out to improve the efficacy of bioactive ceramics in combination with various healing agents [132,133,134,135,136,137,138]. The most important preparation techniques are described briefly in Table 3.

Table 3.
Preparation methods of drug-loaded bioactive ceramics.
4.4. Coatings on Metallic Implants to Improve Osseointegration
So far, an enormous amount of work has been undertaken to create coatings that improve the biocompatibility of commercially used metallic implant materials, such as titanium, Ti6Al4V, CoCrMo, and stainless steel, while also aiding in the prevention of implant-assisted infections. Coating materials such as bioglasses and phosphate-based ceramics are favored for bone implants due to their ability to promote integration with host tissues and enhance biological functionality, as it is widely discussed in many review papers [139,140,141,142]. At the junction of the bone implant and surrounding bone tissue, an apatite-like layer develops that simulates natural bone, due to the excellent bioactivity of these coatings. These layers form a robust and direct bond, ensuring the long-lasting stability of the implant within the human body [143]. In the research work of Nezami et al. [144], 58S tape bioglass powders were prepared via the sol–gel technique with subsequent calcination. The coatings were deposited electrophoretically. They studied the dissolution characteristics of the coatings by immersion tests. The results revealed excellent bioactivity and corrosion resistance of the coatings. While Zanca et al. [145] prepared CaP-bioglass composite coatings onto stainless steel substrates by the galvanic co-deposition of calcium phosphate and bioglass particles. According to their results, these coatings also had excellent biocompatibility and a low corrosion rate. In another recent paper, Farjam et al. [146] deposited CaP coatings onto polycarbonate–urethane (PCU) foils. In this case, the PCU samples were immersed in supersaturated SBFs, which resulted in a semi-crystalline CaP coating formation on the flexible foil over time. Such prepared CaP coatings were stable and intact when the foil was bent. The in vitro cell viability tests revealed that these coatings did not affect the cell viability and proliferation compared to the uncoated PCU substrate. Moreover, the CaP coatings promoted cell-mediated calcium deposition.
In another work [147], the preparation of double-layered porous CaP coatings onto Ti6Al4V was reported. The inner coating was deposited by plasma electrolytic oxidation (PEO), while the outer one was by radio frequency magnetron sputtering (RFMS). They used different CaP targets, such as β-tricalcium phosphate, hydroxyapatite, Mg-added β-tricalcium phosphate, Mg-added hydroxyapatite, Sr-added β-tricalcium phosphate, and Sr-substituted hydroxyapatite. They revealed that the RFMS treatment of PEO inner coating caused multileveled roughness, increased the Ca/P ratio and Young’s modulus, and helped to alter the crystallinity of the coating. Duta et al. [148], on the other hand, investigated the differences between the synthetic and naturally derived hydroxyapatite coatings. They reported that natural HAps contain a wide variety of trace elements (such as Na, Mg, Sr, and K) compared to synthetic ones. Therefore, they can have a more dynamic connection with the surrounding biological area.
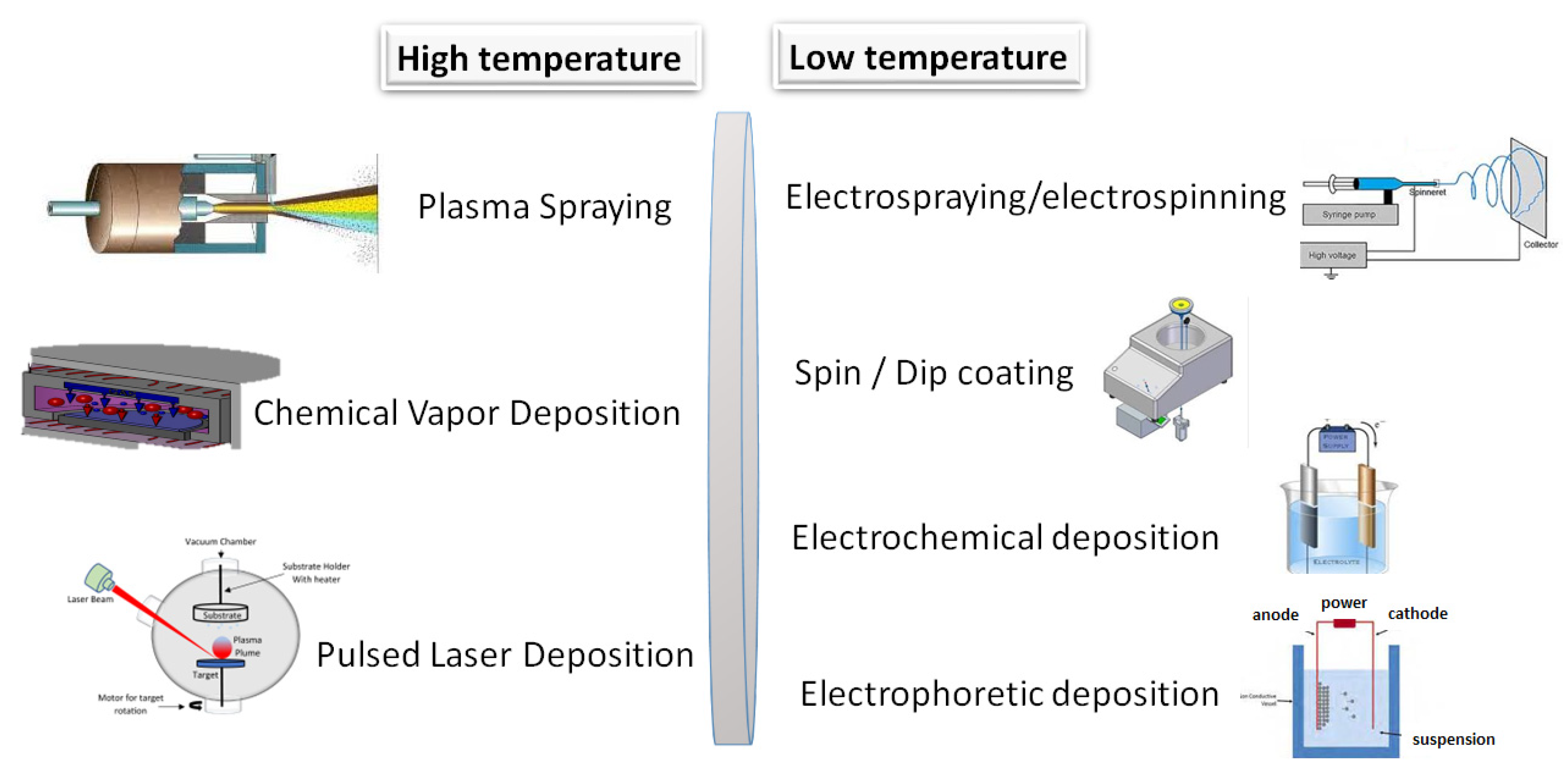
Gao et al. [149] applied CaP coatings onto magnesium alloy to enhance its biocompatibility and to decelerate the degradation rate of bare magnesium implant material. The most common methods are depicted in Figure 9 and categorized as high and low temperature methods.
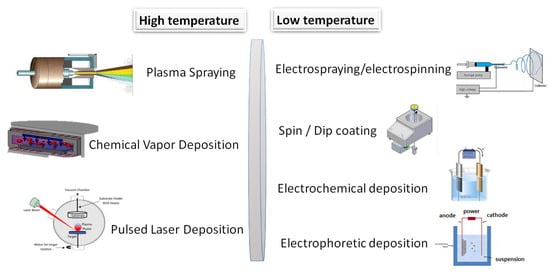
Figure 9.
Schematic illustration of the most utilized coating techniques.
These deposition techniques and their main characteristics are discussed in detail in many review papers [150].
Table 4 demonstrates the main characteristics (strengths and deficiencies) of coatings prepared by the described methods [150]. Even though these bioactive coatings have many benefits, their performance in load-bearing applications is constrained by several critical limitations, such as mechanical (for example, brittleness, insufficient fracture toughness, and cracking) as well as poor adhesion to substrates. In addition, there are degradation challenges, namely, uncontrollable dissolution rates. Bone regeneration requires coatings to degrade at a rate matching new bone formation (~3–6 months). However, HAp coatings often last for years, interfering with bone remodeling. On the other hand, bioglass coatings may degrade too quickly (<3 months), leaving the implant surface exposed before osseointegration is complete [151].

Table 4.
Preparation methods of ceramic coatings onto implant materials and coating properties [139,140,141,142,143,144,145,146,147,148,149,150].
4.5. Effects of Bioactive Element Doping of Bioglasses and CaP Derivatives
It has been extensively demonstrated that the mechanical, chemical, and morphological properties of both bioglasses and calcium phosphate-based bioceramics can be modified and optimized through the incorporation of various bioactive minerals or metallic ions. Commonly used dopants include elements such as silicon (Si), strontium (Sr), magnesium (Mg), zinc (Zn), iron (Fe), copper (Cu), cobalt (Co), fluoride (F), and silver (Ag), introduced in the form of either organic or inorganic salts [152,153,154]. These dopants have been shown to influence the crystal structure, nano- and micro-scale morphology, and surface topography of bioceramic substrates, thereby impacting their biological performance. Their effects have been extensively discussed and evaluated in the scientific literature [155,156,157,158,159,160].
In this context, the most commonly used and investigated substitute elements and their biological functions are further examined. Theoretically, these doping elements become incorporated within the interconnected pores of scaffolds and coatings. Upon implantation, surrounding body fluids infiltrate the porous structure, gradually dissolving the incorporated ions depending on their individual solubility characteristics. However, in the dynamic and fluctuating physiological environment, these dissolution rates may vary and, in some cases, accelerate.
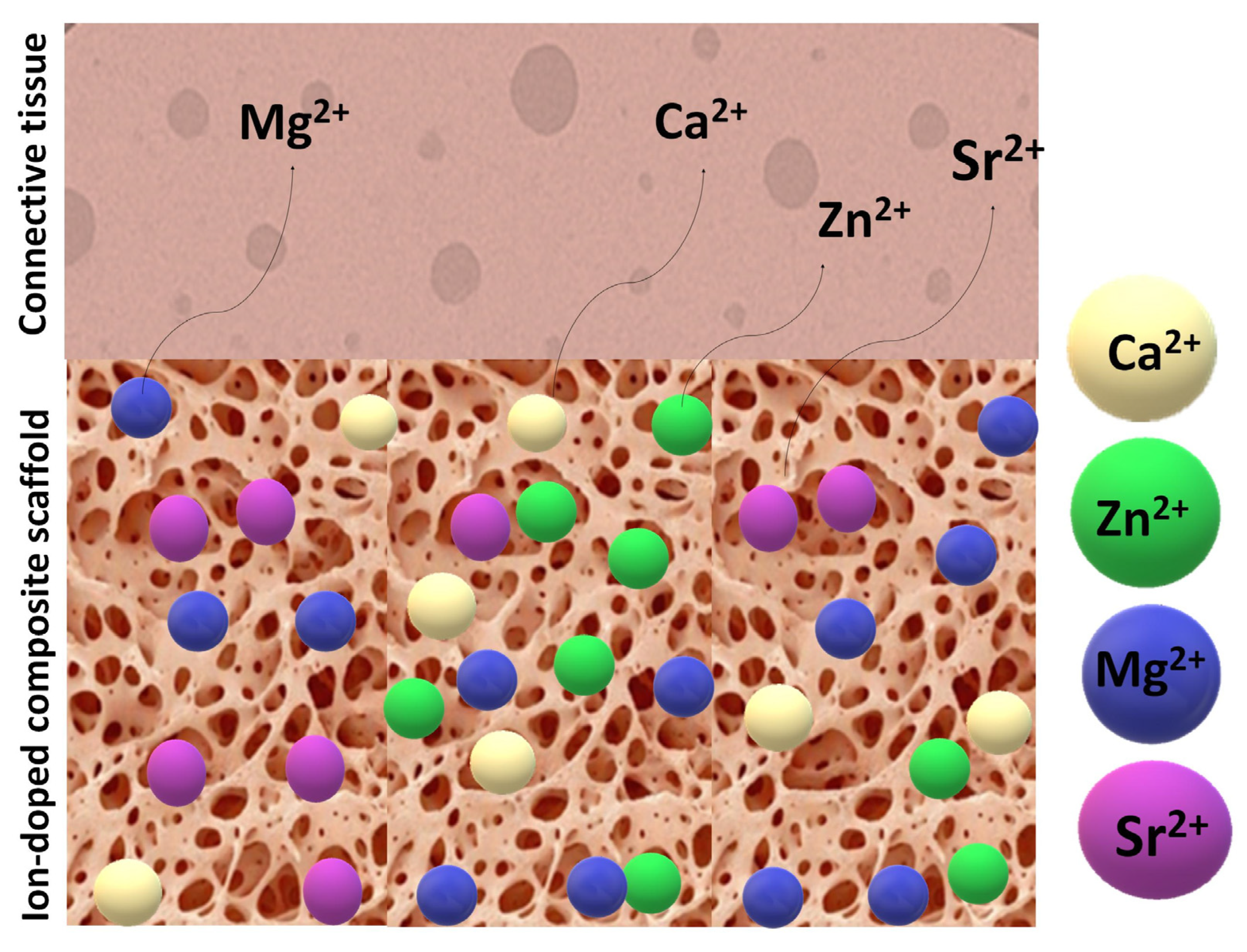
Figure 10 illustrates a porous scaffold material infused with bioactive ions interacting with adjacent tissue, alongside a proposed mechanism for ion release and leaching.
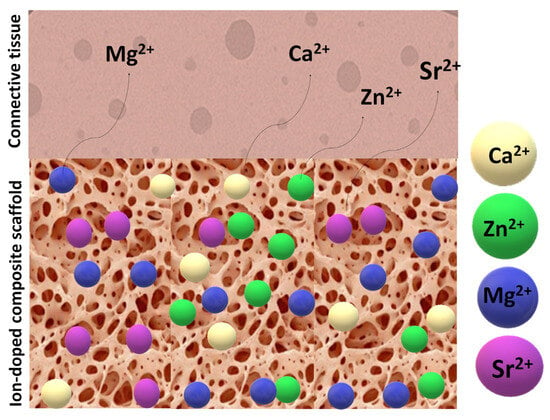
Figure 10.
Illustration of an ion-doped porous scaffold in contact with tissues and the doping ions’ dissolution pathway.
In Table 5, the biologically most active doping elements are listed (along with their roles) that can enhance the effectiveness and bioactivity of the base bioglasses and CaP ceramics or composites [156,157,158,159,160].

Table 5.
Different bioactive ion dopants and their effects on the base materials.
We further analyzed the doping components’ influence on a molecular scale and investigated their interactions with tissue or bone cells that can enhance and accelerate the healing process (Table 6) [156,157,158,159,160].

Table 6.
The biological effect of bioactive ion dopants on cell metabolisms.
5. Biodegradability and Clinical Evaluations of the Different Bioactive Ceramics and Bioglasses, as Well as Their Composites
Generally, both bioglasses and calcium phosphate (CaP) derivatives represent a unique class of bioactive materials capable of interacting with body fluids to form a biologically active hydroxycarbonate apatite (CHAp) layer on their surfaces. This reaction not only facilitates bonding with bone but also promotes new bone formation. The biodegradability of these materials is strongly influenced by their ionic dissolution mechanisms and rates [161,162,163,164,165,166,167], their chemical composition [168,169], and the surrounding environmental pH [170]. For example, Gharbi et al. [171] reported that high boron concentrations in bioglasses significantly increase their degradation rate. The resulting release of bioactive ions can enhance osteogenesis and angiogenesis and, in some cases, confer antibacterial properties.
Surface topography and micromorphology are also critical factors in the biological performance of both bioactive ceramics and bioglasses. Studies have shown that a rough and porous surface greatly enhances cell adhesion [172,173]. Such surface structures also facilitate controlled drug release and improved cellular interactions, rendering these materials highly suitable for a variety of medical applications.
Understanding the influence of the network structure of bioactive ceramics on the solubility of bioglasses is crucial for designing materials with tailored biological functions. A well-established correlation exists between the structural and morphological properties of bioactive ceramics and bioglasses and their biological performance [174,175].
Theoretically, the degradation of bioglass scaffolds begins with an ion exchange upon contact with body fluids [176]. In silicate-based bioglasses, for instance, sodium and calcium ions are leached out and replaced by hydrogen ions, increasing the local pH and leading to the formation of a silica-rich layer. This is followed by the precipitation of calcium and phosphate ions, forming an amorphous calcium phosphate layer, which subsequently crystallizes into CHAp [177]. Borate-based bioglasses exhibit a less dense network structure, resulting in faster degradation and more complete conversion to CHAp [178]. Phosphate-based bioglasses, on the other hand, are known for their even higher solubility, often undergoing complete dissolution under physiological conditions. An appropriate degradation rate is vital to ensure synchronization with new tissue formation, thus promoting optimal healing outcomes [179].
Conversely, the biodegradation of CaP ceramics predominantly occurs through a combination of dissolution and cell-mediated resorption [180]. As with bioglasses, the degradation rate of CaPs is a critical parameter in tissue engineering, ideally matching the rate of tissue regeneration. In aqueous or physiological environments, CaP materials dissolve gradually, releasing calcium and phosphate ions. Hydroxyapatite (HAp), for instance, is highly stable and exhibits slow degradation, making it ideal for load-bearing applications where longevity is essential. β-Tricalcium phosphate (β-TCP) possesses relatively higher solubility than HAp, releasing bioactive ions more readily and thus supporting osteogenesis. Another commonly used CaP phase is biphasic calcium phosphate (DCP or monetite), which offers a more adjustable degradation rate, allowing synchronization with bone regeneration. The degradation rate is further influenced by factors such as crystallinity, particle size, porosity, and the presence of ionic dopants. As degradation proceeds, the released ions can stimulate osteoblast proliferation and differentiation and enhance angiogenesis at the defect site [181].
The composition and microstructure of bioactive ceramics also significantly affect their degradability. Studies indicate that degradation rates can be fine-tuned to align with tissue regeneration needs [28]. Over the past decades, extensive preclinical and clinical research has demonstrated the effectiveness of bioglasses, such as the silicate-based 45S5 Bioglass®, the S53P4 formulation, borate-based variants, as well as calcium phosphate derivatives in various clinical applications [182,183,184].
Several studies have shown that combining bioglasses with local autografts leads to improved clinical outcomes without notable side effects, infections, or immune reactions [185]. For instance, Cottrill et al. [186] conducted a systematic review and meta-analysis on the use of bioglasses in spinal fusion, concluding that these materials offer promising potential as autograft extenders. Similarly, Van Vugt et al. [187] performed a mid-term clinical evaluation of S53P4 bioactive glass in the treatment of chronic long bone osteomyelitis. In this study involving 78 patients, infection was eliminated in 85% of cases, and 89% remained infection-free after one year. These outcomes support the use of S53P4 in one-stage osteomyelitis treatments.
Further clinical investigations have confirmed the effectiveness of S53P4 bioglass in osteomyelitis treatment [188]. In one such study involving 50 patients, 70.3% recovered within six months, and 83.3% showed no signs of infection after twelve months without the use of antibiotics—an effect attributed to the inherent antibacterial properties of the material. S53P4 has also been successfully applied in craniofacial reconstruction, with its sustained release of alkali ions increasing pH and eradicating infection, while concurrently degrading and supporting bone formation. Long-term follow-up studies report high fusion rates and favorable functional outcomes [189,190,191].
Additionally, composites integrating bioglass and fiber reinforcements have shown promise in maxillofacial and cranial surgery applications, demonstrating success in repairing various bone defects [182,192]. Animal model studies have also confirmed that certain bioglass derivatives enhance bone regeneration and improve implant osseointegration [193]. For example, in vivo studies using small animals (e.g., rats and rabbits) have demonstrated that 45S5 Bioglass® gradually degrades and is replaced by newly formed bone within a few months [194,195,196]. Borate-based bioglasses have been associated with accelerated bone healing due to their higher degradation rates, although potential toxicity is managed by regulating ion release in dynamic physiological conditions [12,197]. Phosphate-based bioglasses, due to their compositional similarity to bone minerals, also show enhanced osteogenic potential [198].
Several animal studies have reported complete degradation of bioglass scaffolds within 3–6 months, accompanied by the formation of well-vascularized mature bone tissue and minimal inflammatory response [199]. Clinical trials utilizing 45S5 Bioglass® (marketed as PerioGlas® or BioGran®) have shown success in periodontal therapy [200], with improvements in probing depth and bone defect filling. Long-term data indicate that bioglass facilitates not only bone formation but also regeneration of the periodontal ligament and cementum, resulting in predictable clinical outcomes [201].
Moreover, emerging clinical evidence supports the application of borate-based bioglasses in wound healing and soft tissue regeneration. Products such as MIRRAGEN® have been approved for treating chronic wounds, highlighting the material’s versatility [202]. Clinical studies further confirm the efficacy of bioglass-based treatments in enhancing bone regeneration with minimal long-term adverse effects [203].
Calcium phosphate derivatives have also been thoroughly investigated as bone substitutes. Their tunable degradation rate, governed by phase composition, crystallinity, porosity, and doping, allows for gradual replacement by newly formed bone [204,205,206,207]. Extensive in vitro and in vivo studies, along with clinical trials, have demonstrated the positive biological responses elicited by CaP materials in bone repair. In vitro evaluations focused on cell viability, adhesion, and cytocompatibility, highlighting the influence of surface morphology and crystallinity on osteoclast-mediated resorption and osteoblast proliferation [208,209,210,211]. Osteoblasts cultured on HAp and β-TCP surfaces exhibit high levels of viability, adhesion, and expression of osteogenic markers such as alkaline phosphatase, osteocalcin, and Runx2, which support tissue mineralization [212]. Similar ion-mediated mechanisms operate in bioglasses, where ionic dissolution promotes early tissue mineralization [213].
Simulated body fluid (SBF) immersion tests are frequently used to evaluate CaP bioactivity. These studies reveal that HAp and β-TCP can facilitate apatite layer deposition on their surfaces within 7–14 days, suggesting their strong bone-bonding potential [214,215,216]. In vivo assessments using various animal models (e.g., rabbits, dogs, and rodents) further confirm the performance of CaP materials in healing critical-sized bone defects [183,217,218,219,220]. HAp’s slow degradation rate makes it suitable for long-term scaffolding, with histological analyses demonstrating continuous bone remodeling beyond six months [184,221,222]. Conversely, β-TCP undergoes complete resorption and bone replacement within 3–6 months in non-load-bearing sites [223,224,225,226]. Monetite (DCP), with its balanced resorption rate, has shown favorable outcomes in animal models, particularly when used in biphasic forms (e.g., 60/40 HAp/β-TCP), where scaffolds were largely resorbed and replaced by vascularized bone within 12 weeks [227,228,229,230].
In dentistry, HAp and DCP have been employed as graft materials and coatings for implants, with studies reporting enhanced alveolar bone regeneration, improved implant stability, and superior long-term outcomes compared to autografts or allografts [231]. In orthopedic applications, CaP ceramics are widely used for fracture repair and reconstruction. Clinical data demonstrate that β-TCP and DCP granules support rapid bone healing and reduce implant loosening risk. Trials show high success rates in periodontal and long-bone defect treatments, with β-TCP yielding comparable results to autografts over 1–2 years [232,233].
In summary, clinical assessments have confirmed the efficacy of various calcium phosphate-based scaffolds in promoting bone regeneration in critical-sized defects [234,235]. Recent in vivo and clinical studies further support that the incorporation of bioactive elements into CaP scaffolds enhances biological performance, accelerates healing, and reduces infection rates. For instance, strontium-doped CaPs have demonstrated improved mechanical strength and bone formation both in vitro and in vivo [236,237,238,239].
Table 7 and Table 8 provide a succinct summary of the bioactive glass, calcium phosphate ceramics, and their composites that are currently on the market and in use.

Table 7.
Commercially available bioglass products and their main application fields.
The specific characteristics and possible clinical applications have been extensively reviewed and described in other reviews [194,252].
Similarly, there are numerous commercial CaP-based ceramic compounds, some of which are demonstrated in Table 8.

Table 8.
Commercially available calcium phosphate-based ceramic products and their main applications.
Table 8.
Commercially available calcium phosphate-based ceramic products and their main applications.
| Brand name | Description | Application | Supplier |
|---|---|---|---|
| Eurobone® 2 | Synthetic bone substitute, paste granules with a composition of 75% HA/25% β-TCP. | Orthopedic (Non-load-bearing applications) | [245] |
| Neobone® | Synthetic bone substitute, putty, is an injectable synthetic bone substitute used to fill defects without mechanical strength. | Orthopedics Dentistry Bone grafts Bone substitutes | |
| Ostibone® | |||
| NANOGEL® | Synthetic bone substitute. Absorbable bone void filler that provides support for bone ingrowth. Hydroxyapatite particles between 100 nm and 200 nm. | Orthopedic, Dental | |
| SKUHEAL™ SM-C | Biomimetic mineralized collagen synthetic bone graft material: It contains type I collagen and nano-hydroxyapatite. | Orthopedics Skull surgery Maxillofacial surgery | |
| HydroSet XT | Injectable, self-setting bone substitute composed of tetra-calcium phosphate that is formulated to convert to hydroxyapatite, the principal mineral component of bone. | Orthopedics Bone filler | [243] |
| DirectInject | The first and only on-demand, self-setting HAp cement. It is used to repair neurosurgical burr holes, contiguous craniotomy cuts and cranial defects. | Orthopedics Neurosurgery Bone filler | |
| Vitoss® | Beta-tricalcium phosphate and bioactive glass. Available in many forms such as moldable packs, malleable strips, and morsels. | Orthopedic Bone graft | |
| Osteoset® | Resorbable CaP ceramic that consists of synthetic calcium sulfate beads for bone grafting, calcium sulfate. | Bone graft | |
| Calcigen® | Synthetic calcium sulfate particles for bone grafting, calcium sulfate. | Bone Void Filler | [253] |
| ProOsteon® | Porous hydroxyapatite particles that are osteoconductive and have a structure and chemistry similar to human bone. | Orthopedics Bone grafts | |
| BonePlast® | Medical bone void fillers. Calcium sulfate powder, resorbable, extrudable, and moldable bone void fillers. | Bone graft Bone filler | |
| Norian ®SRS, Norian ®CRS | An injectable, moldable, and biocompatible bone void filler. It contains calcium phosphate powder and sodium phosphate. | [254] | |
| ChronOSTM Inject | Synthetic calcium phosphate bone substitute, injectable, osteoconductive, and resorbable. Irregular bone defects can be completely filled. It consists of a brushite matrix and tricalcium phosphate granules. | ||
| Neocement® | Calcium phosphate cement is intended for filling bone defects of the skeletal system. | Orthopedics Traumatology | [255] |
| Biopex®-R | Calcium phosphate cement which consists of powder and liquid components. | Bone tissue replacement. | [256] |
| Apaceram | Synthetic hydroxyapatite that has macro pores and micro pores. Macro pores are effective for new bone formation, while micro pores provide interconnectivity of the pores. | ||
| Superpore | It has a unique “triple pore structure”. Contains APACERAM Type-AX to absorbable tricalcium phosphate ceramics. | ||
| JectOS® TCH TCP Dental HP | Partially biodegradable cement with a composition of 45% TCP and 55% DCPD. Used to fill cancellous bone defects. | [257] | |
| CERAFORM® | Biocompatible synthetic biphasic ceramic made of hydroxyapatite and beta-tricalcium phosphate. | [258] | |
| Cerasorb® | Resorbable, pure-phase β-tricalcium phosphate with an interconnecting, open multi-porosity. | Implantology, General grafting | [259] |
| Bonetree® | Octacalcium phosphate (OCP)-based synthetic bone substitute material. | Orthopedics Dentistry Bone graft | [260] |
| MBCP® | Bioactive mixture of highly crystalline HAp and β-TCP (Tri Calcium Phosphate)-. | Ortopedics Dentistry Bone graft | [261] |
These commercial CaP composites are also reviewed and mentioned in other works [262,263,264].
6. Challenges
One of the primary challenges in utilizing bioactive ceramics for tissue engineering lies in optimizing their dissolution rates. Excessively slow degradation can disrupt the dynamic interaction between the material and surrounding bone, whereas overly rapid dissolution may hinder new bone formation. Stiller et al. [265] highlighted the necessity of fine-tuning dissolution behavior to promote bone regeneration without inducing adverse effects.
Equally critical is ensuring sufficient biocompatibility. Minimizing cytotoxic risks and maintaining appropriate biological activity are essential for patients’ safety post-implantation [266]. The development of safe implant materials, whether in the form of bone grafts, substitutes, surface coatings, or injectable hydrogels, requires the use of high-purity base materials and precisely controlled concentrations of doping elements.
Bone tissue engineering is inherently complex due to the variability in bone defects, differences in shape, size, location, and etiology across patients. Consequently, scaffolds, grafts, and fillers must be personalized to accommodate unique anatomical and functional demands, including complex geometries. This level of customization can be achieved through advancements in additive manufacturing (AM), which allow for the fabrication of highly porous, interconnected, and mechanically robust structures with uniform pore size distribution. AM techniques also facilitate the replication of bone’s natural structural and chemical features, thereby enhancing cellular integration and tissue regeneration.
In coatings, surface topography plays a key role—studies indicate that increased surface roughness improves osteoblast attachment. However, the optimal surface architecture and crystallinity of calcium phosphate-based (CaP) materials, which are detected by osteoclasts and influence their metabolic activity and resorption capacity, require further in vivo investigation. Moreover, CaP particles may enter cells, modulating cellular homeostasis and differentiation.
Similarly, the performance of bioglasses is highly composition-dependent. Elements such as lithium, silicon, boron, phosphorus pentoxide, and various dopants (e.g., Mg, Sr, and Zn) significantly influence bioactivity, degradation kinetics, and mechanical integrity. By tailoring these compositions, bioceramic materials can be customized for diverse medical applications. Further research should focus on elucidating the synergistic interactions among multiple dopants—especially in varying concentrations and ratios—within bioglass and CaP matrices, as these combinations hold potential to enhance tissue regeneration and wound healing outcomes.
Expanding clinical trials to assess the long-term performance of bioactive ceramic and bioglass-based implants is essential. Standardized, reproducible evaluation protocols are particularly critical for complex injuries involving both hard and soft tissues, where self-repair mechanisms are limited, and pathogenic factors complicate treatment.
Despite existing challenges, bioactive ceramics continue to demonstrate considerable potential for applications in the regeneration of bone, skin, tendons, cartilage, blood vessels, and myocardium. However, the development of bioceramic-based wound healing materials remains limited, with ongoing challenges that include the following:
- Variability in mechanical, chemical, and biological properties, driven by the type of bioceramic, biopolymer matrix, and fabrication methods.
- Difficulty in achieving precise control over scaffold vascularization, degradation kinetics, and standardized manufacturing processes.
- Limited reproducibility in the distribution of ceramic particles within polymer matrices.
To overcome these barriers, advanced and standardized protocols for material synthesis and processing are needed to improve implant performance and quality. In summary, key obstacles remain in achieving optimal mechanical strength, controlled biodegradability, and cost-effective scalability for widespread clinical use.
7. Future Perspectives
To date, the precise methods for regulating the types, dimensions, and forms of bioactive ceramics, along with their specific interactions with biological responses, remain poorly understood. Thus, future work must continue to refine these materials to optimize their mechanical properties, control degradation kinetics, and enhance biological performance for improved clinical outcomes. The enhancement of mechanical properties is especially critical for load-bearing applications, while optimized composition, interconnected and highly porous morphology are essential for delivering therapeutic ions or drugs incorporated into their network. Standardization of in vivo protocols and extended clinical trials will help clarify the long-term safety and efficacy of these versatile biomaterials.
Calcium phosphate-based ceramics (CaPs) are widely used in bone tissue engineering due to their bone-like composition, which confers excellent biocompatibility and osteoconductivity. With optimized chemical compositions, they have demonstrated tunable degradation rates beneficial for bone regeneration. In vitro studies consistently confirm their ability to support cell adhesion, proliferation, and osteogenic differentiation, while in vivo studies show gradual resorption and replacement by new bone tissue. However, further research is needed to optimize phase composition (e.g., tailoring Ca/P ratios and doping element concentrations), improve mechanical properties through composite formulations, and enhance biological performance via organic/inorganic additives or therapeutic agents.
In contrast, bioglasses, with their complex oxide-based compositions, are versatile for both soft and hard tissue engineering. Their chemical compositions, component ratios, and bioactive dopants can be flexibly adjusted to optimize biological characteristics and degradation rates. However, the molecular dynamics between cells and bioactive particles/bioglasses remain underexplored, yet this knowledge is crucial for maximizing their biological potential.
Regarding scaffold fabrication, increased adoption of advanced techniques like 3D/4D printing could enable patient-specific, highly porous scaffolds with controlled architectures. Future efforts should prioritize novel fabrication methods and explore synergistic combinations of bioactive ceramics and bioglasses to advance regenerative medicine.
Hybrid scaffolds combining CaPs, bioglasses, and polymers offer a straightforward and efficient strategy. Incorporating bioactive ions in carefully balanced amounts can enhance biological functionality, though further refinement is required. Notably, metallic ions (e.g., Mn2+, Cu2+, Fe3+, and Ag+) may trigger unexpected biological responses, necessitating deeper investigation. Understanding the long-term effects of these materials is vital for clinical translation, requiring more in vivo studies and clinical trials.
Given existing insights into CaP-based biomaterials, future innovations should focus on customizable designs, including tailored shapes and biodegradation rates [211]. Additionally, studies suggest that scaffold/implant surfaces mimicking native tissue microstructure, texture, and topography can enhance cell adhesion and organization. Future research should investigate cellular responses to nanoscale surface features and the underlying biological mechanisms.
8. Conclusions
This review discussed that bioglasses and calcium phosphate-based ceramics (CaPs) are not only highly biocompatible and osteoconductive but also possess tunable degradation profiles that can be matched to the rate of bone regeneration. Both in vitro and in vivo studies confirm their potential for diverse clinical applications in hard and soft tissue engineering, with primary uses including scaffolds, grafts, composites, hydrogels, and thin matrices. These materials have been shown to promote osteoconduction, osteoinduction, and antibacterial activity via controlled ion release, while clinical trials validate their efficacy in dentistry, craniofacial, orthopedic surgery (e.g., bone grafts, fillers, and injectable composites), wound healing, and drug delivery systems.
This review emphasized that in vivo and clinical studies consistently support bioactive ceramics as controllably degradable materials that stimulate bone regeneration and may exhibit antibacterial properties. Advances in fabrication technologies have significantly expanded the diversity of bioactive ceramics, enabling customization of composition, particle size, and morphology to suit specific tissue regeneration needs.
Key findings revealed that bioactive CaPs and bioglasses are promising for tissue engineering due to their unique bioactivity, biocompatibility, and mechanical adaptability. Their capacity to enhance bone regeneration, angiogenesis, and soft tissue repair positions them as versatile tools for clinical use. Notably, CaP-based materials are particularly suited to bone tissue engineering, as their calcium phosphate composition mimics the mineral phase of natural bone. In contrast, bioglasses, owing to their oxide-based structure and adaptable bioactivity, are effective in both soft and hard tissue repair, despite their inherent reactivity in biological systems.
In conclusion, the tunable degradation and biological responses of these materials make them ideal candidates for advancing regenerative medicine. Future efforts should focus on optimizing their design for targeted applications while addressing the remaining challenges in scalability and clinical translation.
Funding
This research was funded by the National Research, Development and Innovation Office—NKFIH OTKA-FK 146141.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Bedir, T.; Altan, E.; Aranci-Ciftci, K.; Gunduz, O. Bioceramics. In Biomaterials and Tissue Engineering. Stem Cell Biology and Regenerative Medicine, 74; Gunduz, O., Egles, C., Pérez, R.A., Ficai, D., Ustundag, C.B., Eds.; Springer: Cham, Switzerland, 2023; pp. 175–203. [Google Scholar]
- Raucci, M.G.; Giugliano, D.; Ambrosio, L. Fundamental Properties of Bioceramics and Biocomposites. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–19. [Google Scholar]
- Li, Y.; Coughlan, A.; Laffir, F.R.; Pradhan, D.; Mellott, N.P.; Wren, A.W. Investigating the mechanical durability of bioactive glasses as a function of structure, solubility and incubation time. J. Non-Cryst. Sol. 2013, 380, 25–34. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E. Elastic Mechanical Properties of 45S5-Based Bioactive Glass–Ceramic Scaffolds. Materials 2019, 12, 3244. [Google Scholar] [CrossRef]
- Morales-Hernandez, D.G.; Genetos, D.C.; Working, D.M.; Murphy, K.C.; Leach, J.K. Ceramic Identity Contributes to Mechanical Properties and Osteoblast Behavior on Macroporous Composite Scaffolds. J. Funct. Biomater. 2012, 3, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Souza, M.T.; Li, W.; Schubert, D.W.; Boccaccini, A.R.; Roether, J.A. Bioactive Glass-Biopolymer Composites. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–26. [Google Scholar]
- Niemelä, T.; Kellomäki, M. 11-Bioactive glass and biodegradable polymer composites. In Bioactive Glasses; Ylänen, H.O., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2011; pp. 227–245. [Google Scholar]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 9613787. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomat. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
- Ege, D.; Zheng, K.; Boccaccini, A.R. Borate Bioactive Glasses (BBG): Bone Regeneration, Wound Healing Applications, and Future Directions. ACS Appl. Bio Mater. 2022, 5, 3608–3622. [Google Scholar] [CrossRef]
- Shearer, A.; Montazerian, M.; Mauro, J.C. Modern definition of bioactive glasses and glass-ceramics. J. Non-Cryst. Sol. 2023, 608, 122228. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Bioceramics for drug delivery. Acta Mater. 2013, 61, 890–911. [Google Scholar] [CrossRef]
- Szewczyk, A.; Skwira, A.; Konopacka, A.; Sądej, R.; Prokopowicz, M. Mesoporous Silica-Bioglass Composite Pellets as Bone Drug Delivery System with Mineralization Potential. Int. J. Mol. Sci. 2021, 22, 4708. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Indurkar, A.; Locs, J.; Haugen, H.J.; Loca, D. Calcium Phosphates: A Key to Next-Generation In Vitro Bone Modeling. Adv. Healthc. Mater. 2024, 13, e2401307. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Briglia, M.; Zammuto, V.; Morganti, D.; Faggio, C.; Impellitteri, F.; Multisanti, C.R.; Graziano, A.C.E. Innovation in Osteogenesis Activation: Role of Marine-Derived Materials in Bone Regeneration. Curr. Issues Mol. Biol. 2025, 47, 175. [Google Scholar] [CrossRef]
- Klenke, F.M.; Siebenrock, K.A. Osteology in Orthopedics–Bone Repair, Bone Grafts, and Bone Graft Substitutes. In Encyclopedia of Bone Biology; Zaidi, M., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 778–792. [Google Scholar]
- Alexandre, N.; Simões, G.; Martinho Lopes, A.; Guerra Guimarães, T.; Lopes, B.; Sousa, P.; Sousa, A.C.; Alvites, R.D.; Maurício, A.C. Biomechanical Basis of Bone Fracture and Fracture Osteosynthesis in Small Animals. In Biomechanical Insights into Osteoporosis; IntechOpen: Vienna, Austria, 2023. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Mihály, J.; Balázsi, K.; Balázsi, C. Comparison of the Morphological and Structural Characteristic of Bioresorbable and Biocompatible Hydroxyapatite-Loaded Biopolymer Composites. Nanomaterials 2021, 11, 3194. [Google Scholar] [CrossRef] [PubMed]
- Rheima, A.M.; Abdul-Rasool, A.A.; Al-Sharify, Z.T.; Zaidan, H.K.; Athair, D.M.; Mohammed, S.H.; kianfar, E. Nano bioceramics: Properties, applications, hydroxyapatite, nanohydroxyapatite and drug delivery. Case Stud. Chem. Environ. Eng. 2024, 10, 100869. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2020, 6, e10206. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Ma, Y.; Jiang, Y. Calcium Phosphate-Based Nanomaterials: Preparation, Multifunction, and Application for Bone Tissue Engineering. Molecules 2023, 28, 4790. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Birgani, Z.T.; Habibovic, P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef]
- Pandayil, J.T.; Boetti, N.G.; Janner, D. Advancements in Biomedical Applications of Calcium Phosphate Glass and Glass-Based Devices—A Review. J. Funct. Biomater. 2024, 15, 79. [Google Scholar] [CrossRef]
- Kawsar, M.; Hossain, S.; Alam, K.; Bahadur, N.M.; Shaikh, A.A.; Ahmed, S. Synthesis of pure and doped nano-calcium phosphates using different conventional methods for biomedical applications: A review. J. Mater. Chem. B 2024, 12, 3376–3391. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Seles, M.A.; Rajan, M. Role of bioglass derivatives in tissue regeneration and repair: A review. Rev. Adv. Mater. Sci. 2023, 62, 20220318. [Google Scholar] [CrossRef]
- Vizureanu, P.; Baltatu, M.; Sandu, A.; Achitei, D.; Negris, D.D.B.; Perju, M. New Trends in Bioactive Glasses for Bone Tissue: A Review. In Current Concepts in Dental Implantology—From Science to Clinical Research; IntechOpen: Vienna, Austria, 2021; Corpus ID: 244581791. [Google Scholar]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.; Shinde, V. Bioglass and hybrid bioactive material: A review on the fabrication, therapeutic potential and applications in wound healing. Hybrid Adv. 2024, 6, 100196. [Google Scholar] [CrossRef]
- Maximov, M.; Maximov, O.-C.; Craciun, L.; Ficai, D.; Ficai, A.; Andronescu, E. Bioactive Glass—An Extensive Study of the Preparation and Coating Methods. Coatings 2021, 11, 1386. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Holz, L.I.V.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glasses Containing Strontium or Magnesium Ions to Enhance the Biological Response in Bone Regeneration. Nanomaterials 2023, 13, 2717. [Google Scholar] [CrossRef]
- Dang, T.H.; Bui, T.H.; Guseva, E.V.; Ta, A.T.; Nguyen, A.T.; Hoang, T.T.H.; Bui, X.V. Characterization of Bioactive Glass Synthesized by Sol-Gel Process in Hot Water. Crystals 2020, 10, 529. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Pullar, R.C. A Comparison of Bioactive Glass Scaffolds Fabricated by Robocasting from Powders Made by Sol–Gel and Melt-Quenching Methods. Processes 2020, 8, 615. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Fiume, E.; Akbarov, A.; Ziyadullaeva, N.; Murtazaev, S.; Rahdar, A.; Massera, J.; Verné, E.; Baino, F. In Vivo Evaluation of 3D-Printed Silica-Based Bioactive Glass Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 74. [Google Scholar] [CrossRef]
- Kermani, F.; Sadidi, H.; Ahmadabadi, A.; Hoseini, S.J.; Tavousi, S.H.; Rezapanah, A.; Nazarnezhad, S.; Hosseini, S.A.; Mollazadeh, S.; Kargozar, S. Modified Sol–Gel Synthesis of Mesoporous Borate Bioactive Glasses for Potential Use in Wound Healing. Bioengineering 2022, 9, 442. [Google Scholar] [CrossRef]
- Negut, I.; Ristoscu, C. Bioactive Glasses for Soft and Hard Tissue Healing Applications—A Short Review. Appl. Sci. 2023, 13, 6151. [Google Scholar] [CrossRef]
- Molinar-Díaz, J.; Arjuna, A.; Abrehart, N.; McLellan, A.; Harris, R.; Islam, M.T.; Alzaidi, A.; Bradley, C.R.; Gidman, C.; Prior, M.J.W.; et al. Development of Resorbable Phosphate-Based Glass Microspheres as MRI Contrast Media Agents. Molecules 2024, 29, 4296. [Google Scholar] [CrossRef] [PubMed]
- Mohan Babu, M.; Syam Prasad, P.; Hima Bindu, S.; Prasad, A.; Venkateswara Rao, P.; Putenpurayil Govindan, N.; Veeraiah, N.; Özcan, M. Investigations on Physico-Mechanical and Spectral Studies of Zn2+ Doped P2O5-Based Bioglass System. J. Compos. Sci. 2020, 4, 129. [Google Scholar] [CrossRef]
- Rajendran, A.K.; Anthraper, M.S.J.; Hwang, N.S.; Rangasamy, J. Osteogenesis and angiogenesis promoting bioactive ceramics. Mater. Sci. Eng. R Rep. 2024, 159, 100801. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Brauer, D.S.; Wilson, R.M.; Law, R.V.; Hill, R.G.; Karpukhina, N. Sodium Is Not Essential for High Bioactivity of Glasses. Int. J. Appl. Glass Sci. 2017, 8, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Arango-Ospina, M.; Boccaccini, A.R. Chapter 4—Bioactive glasses and ceramics for tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 3rd ed.; Woodhead Publishing Series in Biomaterials; Boccaccini, A.R., Ma, P.X., Liverani, L., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 111–178. [Google Scholar]
- Kowalska, K.J.; Czechowska, J.P.; Yousef, E.S.; Zima, A. Novel phosphate bioglasses and bioglass-ceramics for bone regeneration. Ceram. Int. 2024, 50, 45976–45985. [Google Scholar] [CrossRef]
- Wallace, K.E.; Hill, R.G.; Pembroke, J.T.; Brown, C.J.; Hatton, P.V. Influence of Sodium Oxide Content on Bioactive Glass Composite. J. Mater. Sci. Mater. Med. 2000, 10, 697–701. [Google Scholar] [CrossRef]
- Kaimonov, M.; Safronova, T.; Shatalova, T.; Filippov, Y.; Tikhomirova, I.; Sergeev, N. Composite Ceramics in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes including Hydroxyapatite and an Aqueous Solution of Sodium Silicate. Ceramics 2022, 5, 550–561. [Google Scholar] [CrossRef]
- Kaimonov, M.R.; Safronova, T.V. Materials in the Na2O–CaO–SiO2–P2O5 System for Medical Applications. Materials 2023, 16, 5981. [Google Scholar] [CrossRef]
- Zhang, N.-Z.; Zhang, M.; Tang, H.-Y.; Qin, L.; Cheng, C.-K. P2O5 enhances the bioactivity of lithium silicate glass ceramics via promoting phase transformation and forming Li3PO4. Ceram. Int. 2024, 50, 13308–13317. [Google Scholar] [CrossRef]
- Siqueira, R.L.; Zanotto, E.D. The influence of phosphorus precursors on the synthesis and bioactivity of SiO2-CaO-P2O5 sol-gel glasses and glass-ceramics. J. Mater. Sci. Mater. Med. 2012, 24, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.A.; Mirsky, N.A.; Silva, B.L.G.; Shinde, A.R.; Arakelians, A.R.L.; Nayak, V.V.; Marcantonio, R.A.C.; Gupta, N.; Witek, L.; Coelho, P.G. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J. Funct. Biomater. 2024, 15, 280. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Calcium Phosphates and Bioactive Glasses for Bone Implant Applications. Coatings 2023, 13, 1217. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Agathopoulos, S.; Dimitriadis, K.; Fernandes, H.R.; Gabrieli, R.; Baino, F. The Story, Properties and Applications of Bioactive Glass “1d”: From Concept to Early Clinical Trials. Inorganics 2024, 12, 224. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Tong, S.; Wang, Y.; Wang, Z.; Sui, R.; Yang, K.; Witte, F.; Yang, S. Porous metal materials for applications in orthopedic field: A review on mechanisms in bone healing. J. Orthop. Translat. 2024, 49, 135–155. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Babu, P.J. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Parfenov, V.A.; Mironov, V.A.; Koudan, E.V.; Nezhurina, E.K.; Karalkin, P.A.; Pereira, F.D.A.S.; Petrov, S.V.; Krokhmal, A.A.; Aydemir, T.; Vakhrushev, I.V.; et al. Fabrication of calcium phosphate 3D scaffolds for bone repair using magnetic levitational assembly. Sci. Rep. 2020, 10, 4013. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, J.-H.; Young, D.; Kim, S.; Nishimoto, S.K.; Yang, Y. Novel template-casting technique for fabricating β-tricalcium phosphate scaffolds with high interconnectivity and mechanical strength and in vitro cell responses. J. Biomed. Mater. Res. 2010, 92A, 997–1006. [Google Scholar] [CrossRef]
- Sanzana, E.S.; Navarro, M.; Ginebra, M.-P.; Planell, J.A.; Ojeda, A.C.; Montecinos, H.A. Role of porosity and pore architecture in the in vivo bone regeneration capacity of biodegradable glass scaffolds. J. Biomed. Mater. Res. 2014, 102, 1767–1773. [Google Scholar] [CrossRef]
- Velayudhan, S.; Ramesh, P.; Sunny, M.C.; Varma, H.K. Extrusion of hydroxyapatite to clinically significant shapes. Mater. Lett. 2000, 46, 142–146. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Lu, T.; He, F.; Ye, J. Preparation and properties of porous calcium phosphate ceramic microspheres modified with magnesium phosphate surface coating for bone defect repair. Ceram. Int. 2024, 50, 7514–7527. [Google Scholar] [CrossRef]
- Shoushtari, M.S.; Hoey, D.; Biak, D.R.A.; Abdullah, N.; Kamarudin, S.; Zainuddin, H.S. Sol–gel-templated bioactive glass scaffold: A review. Res. Biomed. Eng. 2024, 40, 281–296. [Google Scholar] [CrossRef]
- Chan, S.S.L.; Black, J.R.; Franks, G.V.; Heath, D.E. Hierarchically porous 3D-printed ceramic scaffolds for bone tissue engineering. Biomater. Adv. 2025, 169, 214149. [Google Scholar] [CrossRef] [PubMed]
- Mirzavandi, Z.; Poursamar, S.A.; Amiri, F.; Bigham, A.; Rafienia, M. 3D printed polycaprolactone/gelatin/ordered mesoporous calcium magnesium silicate nanocomposite scaffold for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2024, 35, 58. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent advances and future perspectives of sol–gel derived porous bioactive glasses: A review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef]
- Meng, Y.; Li, X.; Yun, B. Preparation and Characterization of Mechanical Properties of HAP/45S5 Bioglass Laminated Ceramic Composites via Spark Plasma Sintering. Materials 2024, 17, 5413. [Google Scholar] [CrossRef]
- Waletzko-Hellwig, J.; Saemann, M.; Schulze, M.; Frerich, B.; Bader, R.; Dau, M. Mechanical Characterization of Human Trabecular and Formed Granulate Bone Cylinders Processed by High Hydrostatic Pressure. Materials 2021, 14, 1069. [Google Scholar] [CrossRef]
- Venugopal, J.; Vadgma, P.; Sampath Kumar, T.; Ramakrishna, S. Biocomposite nanofibres and osteoblasts for bone tissue engineering. Nanotechnology 2007, 18, 055101. [Google Scholar] [CrossRef]
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef] [PubMed]
- El-Okaily, M.S.; Mostafa, A.A.; Dulnik, J.; Denis, P.; Sajkiewicz, P.; Mahmoud, A.A.; Dawood, R.; Maged, A. Nanofibrous Polycaprolactone Membrane with Bioactive Glass and Atorvastatin for Wound Healing: Preparation and Characterization. Pharmaceutics 2023, 15, 1990. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Chowdhury, S.D. Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels 2023, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Mîrț, A.-L.; Ficai, D.; Oprea, O.-C.; Vasilievici, G.; Ficai, A. Current and Future Perspectives of Bioactive Glasses as Injectable Material. Nanomaterials 2024, 14, 1196. [Google Scholar] [CrossRef]
- Barreto, M.E.V.; Medeiros, R.P.; Shearer, A.; Fook, M.V.L.; Montazerian, M.; Mauro, J.C. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. J. Funct. Biomater. 2023, 14, 23. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chen, I.-C.; Su, C.-Y.; Tsai, H.-H.; Young, T.-H.; Fang, H.-W. Development of Injectable Calcium Sulfate and Self-Setting Calcium Phosphate Composite Bone Graft Materials for Minimally Invasive Surgery. Int. J. Mol. Sci. 2022, 23, 7590. [Google Scholar] [CrossRef]
- Cai, M.; Ratnayake, J.; Cathro, P.; Gould, M.; Ali, A. Investigation of a Novel Injectable Chitosan Oligosaccharide—Bovine Hydroxyapatite Hybrid Dental Biocomposite for the Purposes of Conservative Pulp Therapy. Nanomaterials 2022, 12, 3925. [Google Scholar] [CrossRef]
- Alves, P.; Simão, A.F.; Graça, M.F.P.; Mariz, M.J.; Correia, I.J.; Ferreira, P. Dextran-Based Injectable Hydrogel Composites for Bone Regeneration. Polymers 2023, 15, 4501. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Xu, J.; Tong, W.; Hu, S.; Chen, Y.F.; Deng, S.; Yao, H.; Li, J.; Lee, C.W.; et al. Injectable bioactive glass/sodium alginate hydrogel with immunomodulatory and angiogenic properties for enhanced tendon healing. Bioeng. Transl. Med. 2022, 8, e10345. [Google Scholar] [CrossRef]
- Estevez, A.T.; Abdallah, Y.K. Biomimetic Approach for Enhanced Mechanical Properties and Stability of Self-Mineralized Calcium Phosphate Dibasic–Sodium Alginate–Gelatine Hydrogel as Bone Replacement and Structural Building Material. Processes 2024, 12, 944. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Huang, S.-W.; Kao, I.-F.; Yen, S.-K. The Preparation and Characterization of Chitosan/Calcium Phosphate Composite Microspheres for Biomedical Applications. Polymers 2024, 16, 167. [Google Scholar] [CrossRef]
- Ciołek, L.; Zaczyńska, E.; Krok-Borkowicz, M.; Biernat, M.; Pamuła, E. Chitosan and Sodium Hyaluronate Hydrogels Supplemented with Bioglass for Bone Tissue Engineering. Gels 2024, 101, 128. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Thormann, U.; Kramer, I.; Sommer, U.; Budak, M.; Schumacher, M.; Bernhardt, A.; Lode, A.; Kern, C.; Rohnke, M.; et al. Mesoporous Bioactive Glass-Incorporated Injectable Strontium-Containing Calcium Phosphate Cement Enhanced Osteoconductivity in a Critical-Sized Metaphyseal Defect in Osteoporotic Rats. Bioengineering 2023, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Tan, R.; Zhang, Y.; Wang, C.; Wan, Y.; Li, J. Multi-Crosslinked Strong and Elastic Bioglass/Chitosan-Cysteine Hydrogels with Controlled Quercetin Delivery for Bone Tissue Engineering. Pharmaceutics 2022, 14, 2048. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Eftekhari Yekta, B.; Rezaie, H.; Naimi-Jamal, M.R.; Kumar, A.; Cochis, A.; Miola, M.; Rimondini, L. Enhancing Mechanical Properties and Biological Performances of Injectable Bioactive Glass by Gelatin and Chitosan for Bone Small Defect Repair. Biomedicines 2020, 8, 616. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Salvatori, R.; Cannillo, V. Chitosan-Based Bioactive Glass Gauze: Microstructural Properties, In Vitro Bioactivity, and Biological Tests. Materials 2020, 13, 2819. [Google Scholar] [CrossRef]
- Brachet, A.; Bełżek, A.; Furtak, D.; Geworgjan, Z.; Tulej, D.; Kulczycka, K.; Karpiński, R.; Maciejewski, M.; Baj, J. Application of 3D Printing in Bone Grafts. Cells 2023, 12, 859. [Google Scholar] [CrossRef]
- Anwajler, B.; Witek-Krowiak, A. Three-Dimensional Printing of Multifunctional Composites: Fabrication, Applications, and Biodegradability Assessment. Materials 2023, 16, 7531. [Google Scholar] [CrossRef]
- Pereira, A.C.; Nayak, V.V.; Coelho, P.G.; Witek, L. Integrative Modeling and Experimental Insights into 3D and 4D Printing Technologies. Polymers 2024, 16, 2686. [Google Scholar] [CrossRef]
- Danoux, C.B.; Barbieri, D.; Yuan, H.; de Bruijn, J.D.; van Blitterswijk, C.A.; Habibovic, P. In vitro and in vivo bioactivity assessment of a polylactic acid/hydroxyapatite composite for bone regeneration. Biomatter 2014, 4, e27664. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Vigneshwaran, M.; Qi, Y.; Zhou, Y.; Fu, G.; Li, Z.; Wang, J. Effect of Different Contents of 63s Bioglass on the Performance of Bioglass-PCL Composite Bone Scaffolds. Inventions 2023, 8, 138. [Google Scholar] [CrossRef]
- Rajzer, I.; Kurowska, A.; Frankova, J.; Sklenářová, R.; Nikodem, A.; Dziadek, M.; Jabłoński, A.; Janusz, J.; Szczygieł, P.; Ziąbka, M. 3D-Printed Polycaprolactone Implants Modified with Bioglass and Zn-Doped Bioglass. Materials 2023, 16, 1061. [Google Scholar] [CrossRef] [PubMed]
- Vella, J.B.; Trombetta, R.P.; Hoffman, M.D.; Inzana, J.; Awad, H.; Benoit, D.S.W. Three dimensional printed calcium phosphate and poly(caprolactone) composites with improved mechanical properties and preserved microstructure. J. Biomed. Mater. Res. A 2018, 106, 663–672. [Google Scholar] [CrossRef]
- Hajiali, F.; Tajbakhsh, S.; Shojaei, A. Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polym. Rev. 2017, 58, 164–207. [Google Scholar] [CrossRef]
- Ganbaatar, S.E.; Kim, H.-K.; Kang, N.-U.; Kim, E.C.; U, H.J.; Cho, Y.-S.; Park, H.-H. Calcium Phosphate (CaP) Composite Nanostructures on Polycaprolactone (PCL): Synergistic Effects on Antibacterial Activity and Osteoblast Behavior. Polymers 2025, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Tulliani, J.-M.; Tadier, S.; Meille, S.; Chevalier, J.; Palmero, P. Novel calcium phosphate/PCL graded samples: Design and development in view of biomedical applications. Mat. Sci. Eng. C 2019, 97, 336–346. [Google Scholar] [CrossRef]
- Ródenas-Rochina, J.; Ribelles, J.L.; Lebourg, M. Comparative study of PCL-HAp and PCL-bioglass composite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1293–1308. [Google Scholar] [CrossRef]
- Elshazly, N.; Nasr, F.E.; Hamdy, A.; Saied, S.; Elshazly, M. Advances in clinical applications of bioceramics in the new regenerative medicine era. World J. Clin. Cases 2024, 12, 1863–1869. [Google Scholar] [CrossRef]
- D’Amora, U.; Gloria, A.; Ambrosio, L. 10-Composite materials for ligaments and tendons replacement. In Biomedical Composites, 2nd ed.; Woodhead Publishing Series in Biomaterials; Ambrosio, L., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 215–235. ISBN 9780081007525. [Google Scholar]
- Huang, L.; Chen, L.; Chen, H.; Wang, M.; Jin, L.; Zhou, S.; Gao, L.; Li, R.; Li, Q.; Wang, H.; et al. Biomimetic Scaffolds for Tendon Tissue Regeneration. Biomimetics 2023, 8, 246. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021, 6, 2491–2510. [Google Scholar] [CrossRef]
- Majumdar, S.; Gupra, S.; Krishnamurthy, S. Multifarious applications of bioactive glasses in soft tissue engineering. Biomater. Sci. 2021, 9, 8111–8147. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.D.; de Sousa, V.R.; de Sousa, B.V.; de Araújo Neves, G.; de Lima Santana, L.N.; Menezes, R.R. Ceramic Nanofiber Materials for Wound Healing and Bone Regeneration: A Brief Review. Materials 2022, 15, 3909. [Google Scholar] [CrossRef]
- Asefnejad, A.; Shadman-Manesh, V. Exploring the Impact of Ceramic Reinforcements on the Mechanical Properties of Wound Dressings and their Influence on Wound Healing and Drug Release. Sci. Hypotheses 2024, 1, 1. [Google Scholar] [CrossRef]
- Al-Naymi, H.A.S.; Al-Musawi, M.H.; Mirhaj, M.; Valizadeh, H.; Momeni, A.; Pajooh, A.M.D.; Shahriari-Khalaji, M.; Sharifianjazi, F.; Tavamaishvili, K.; Kazemi, N.; et al. Exploring nanobioceramics in wound healing as effective and economical alternatives. Heliyon 2024, 10, e38497. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.K.; Martinez-Rodriguez, S.; Md Fadilah, N.I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280. [Google Scholar] [CrossRef] [PubMed]
- Moosvi, S.R.; Day, R.M. Bioactive glass modulation of intestinal epithelial cell restitution. Acta Biomater. 2009, 5, 76–83. [Google Scholar] [CrossRef]
- Yao, X.; Xue, T.; Chen, B.; Zhou, X.; Ji, Y.; Gao, Z.; Liu, B.; Yang, J.; Shen, Y.; Sun, H.; et al. Advances in biomaterial-based tissue engineering for peripheral nerve injury repair. Bioactive Mater. 2025, 46, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 16, 554257. [Google Scholar] [CrossRef]
- Ren, Z.; Tang, S.; Wang, J.; Lv, S.; Zheng, K.; Xu, Y.; Li, K. Bioactive Glasses: Advancing Skin Tissue Repair through Multifunctional Mechanisms and Innovations. Biomater. Res. 2025, 29, 0134. [Google Scholar] [CrossRef]
- Palmero, P. 15-Ceramic–polymer nanocomposites for bone-tissue regeneration. In Nanocomposites for Musculoskeletal Tissue Regeneration; Liu, H., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 331–367. [Google Scholar]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar]
- Wang, X.; Tang, M. Bioceramic materials with ion-mediated multifunctionality for wound healing. Smart Med. 2022, 1, e20220032. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Ma, B.; Wu, J.; Chang, J.; Gelinsky, M.; Wu, C. Black Bioceramics: Combining Regeneration with Therapy. Adv. Mater. 2020, 32, 2005140. [Google Scholar] [CrossRef] [PubMed]
- Popova, E.; Tikhomirova, V.; Akhmetova, A.; Ilina, I.; Kalinina, N.; Taliansky, M.; Kost, O. Calcium Phosphate Nanoparticles as Carriers of Low and High Molecular Weight Compounds. Int. J. Mol. Sci. 2024, 25, 12887. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Encapsulation of Calcium Phosphates on Electrospun Nanofibers for Tissue Engineering Applications. Crystals 2021, 11, 199. [Google Scholar] [CrossRef]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.-C.; Tseng, Y.-C.; Mozumdar, S.; Huang, L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control. Release 2010, 142, 416–421. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Palazzo, B.; Marchetti, M.; Margiotta, N.; Ostuni, R.; Natile, G.; Morpurgo, M.; Gandin, V.; Marzano, C.; Roveri, N. Smart delivery of antitumoral platinum complexes from biomimetic hydroxyapatite nanocrystals. J. Mater. Chem. 2009, 19, 8385–8392. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef]
- Uskoković, V.; Batarni, S.S.; Schweicher, J.; King, A.; Desai, T.A. Effect of calcium phosphate particle shape and size on their antibacterial and osteogenic activity in the delivery of antibiotics in vitro. ACS Appl. Mater. Interfaces 2013, 5, 2422–2431. [Google Scholar] [CrossRef]
- Soundrapandian, C.; Datta, S.; Kundu, B.; Basu, D.; Sa, B. Porous bioactive glass scaffolds for local drug delivery in osteomyelitis: Development and in vitro characterization. AAPS PharmSciTech 2010, 11, 1675–1683. [Google Scholar] [CrossRef]
- Cui, Y.; Hong, S.; Jiang, W.; Li, X.; Zhou, X.; He, X.; Liu, J.; Lin, K.; Mao, L. Engineering mesoporous bioactive glasses for emerging stimuli-responsive drug delivery and theranostic applications. Bioact. Mater. 2024, 34, 436–462. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Vitale-Brovarone, C.; Fiorilli, S. Achievements in Mesoporous Bioactive Glasses for Biomedical Applications. Pharmaceutics 2022, 14, 2636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-Functional Silica-Based Mesoporous Materials for Simultaneous Delivery of Biologically Active Ions and Therapeutic Biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Sharifi, E.; Bigham, A.; Yousefiasl, S.; Trovato, M.; Ghomi, M.; Esmaeili, Y.; Samadi, P.; Zarrabi, A.; Ashrafizadeh, M.; Sharifi, S.; et al. Mesoporous Bioactive Glasses in Cancer Diagnosis and Therapy: Stimuli-Responsive, Toxicity, Immunogenicity, and Clinical Translation. Adv. Sci. 2022, 9, 2102678. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Luo, J.; Deng, Y.; Li, Z.; Wei, J. Alginate-modified mesoporous bioactive glass and its drug delivery, bioactivity, and osteogenic properties. Front. Bioeng. Biotechnol. 2022, 10, 994925. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-L.; Fang, W.; Huang, B.-R.; Wang, Y.-H.; Dong, G.-C.; Lee, T.-M. Bioactive Glass as a Nanoporous Drug Delivery System for Teicoplanin. Appl. Sci. 2020, 10, 2595. [Google Scholar] [CrossRef]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Q.; Wu, T.; Pan, H. Understanding adsorption-desorption dynamics of BMP-2 on hydroxyapatite (001) surface. Biophys. J. 2007, 93, 750–759. [Google Scholar] [CrossRef]
- Qadir, A.; Gao, Y.; Suryaji, P.; Tian, Y.; Lin, X.; Dang, K.; Jiang, S.; Li, Y.; Miao, Z.; Qian, A. Non-Viral Delivery System and Targeted Bone Disease Therapy. Int. J. Mol. Sci. 2019, 20, 565. [Google Scholar] [CrossRef]
- Matic, T.; Daou, F.; Cochis, A.; Barac, N.; Ugrinovic, V.; Rimondini, L.; Veljovic, D. Multifunctional Sr,Mg-Doped Mesoporous Bioactive Glass Nanoparticles for Simultaneous Bone Regeneration and Drug Delivery. Int. J. Mol. Sci. 2024, 25, 8066. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Sánchez, M.; Landin, M. Drug-Loaded Biomimetic Ceramics for Tissue Engineering. Pharmaceutics 2018, 10, 272. [Google Scholar] [CrossRef]
- Gupta, S.; Majumdar, S.; Krishnamurthy, S. Bioactive glass: A multifunctional delivery system. J. Control. Release 2021, 335, 481–497. [Google Scholar] [CrossRef]
- Tangri, S.; Hasan, N.; Kaur, J.; Mohammad, F.; Maan, S.; Kesharwani, P.; Ahmad, F.J. Drug loaded bioglass nanoparticles and their coating for efficient tissue and bone regeneration. J. Non-Cryst. Solids 2023, 616, 122469. [Google Scholar] [CrossRef]
- Miola, M.; Vitale-Brovarone, C.; Mattu, C.; Verné, E. Antibiotic loading on bioactive glasses and glass-ceramics: An approach to surface modification. J. Biomater. Appl. 2012, 28, 308–319. [Google Scholar] [CrossRef]
- Piatti, E.; Miola, M.; Verné, E. Tailoring of bioactive glass and glass-ceramics properties for in vitro and in vivo response optimization: A review. Biomater. Sci. 2024, 12, 4546–4589. [Google Scholar] [CrossRef] [PubMed]
- Auniq, R.B.Z.; Hirun, N.; Boonyang, U. Three-Dimensionally Ordered Macroporous-Mesoporous Bioactive Glass Ceramics for Drug Delivery Capacity and Evaluation of Drug Release. In Advanced Ceramic Materials; Mhadhbi, M., Ed.; IntechOpen: Vienna, Austria, 2021; p. 95290. [Google Scholar]
- Zhao, C.; Liu, W.; Zhu, M.; Wu, C.; Zhu, Y. Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: A review. Bioact. Mater. 2022, 18, 383–398. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Comprehensive Review of Bioactive Glass Coatings: State of the Art, Challenges and Future Perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.; Bellucci, D.; Cannillo, V.; Cattini, A. Bioactive glass coatings: A review. Surf. Eng. 2011, 27, 560–572. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Electrophoretic Deposition of Bioactive Glass Coatings for Bone Implant Applications: A Review. Coatings 2024, 14, 1084. [Google Scholar] [CrossRef]
- Helsen, J.A.; Proost, J.; Schrooten, J.; Timmermans, G.; Brauns, E.; Vanderstraeten, J. Glasses and bioglasses: Synthesis and coatings. J. Eur. Cer. Soc. 1997, 17, 147–152. [Google Scholar]
- Mohammad Nezami, M.; Abbasi Khazaei, B. Applying 58S Bioglass Coating on Titanium Substrate: Effect of Multiscale Roughness on Bioactivity, Corrosion Resistance and Coating Adhesion. J. Bio Tribo Corros. 2024, 10, 37. [Google Scholar] [CrossRef]
- Zanca, C.; Milazzo, A.; Campora, S.; Capuana, E.; Pavia, F.C.; Patella, B.; Lopresti, F.; Brucato, V.; La Carrubba, V.; Inguanta, R. Galvanic Deposition of Calcium Phosphate/Bioglass Composite Coating on AISI 316L. Coatings 2023, 13, 1006. [Google Scholar] [CrossRef]
- Farjam, P.; Luckabauer, M.; de Vries, E.G.; Rangel, V.R.; Hekman, E.E.G.; Verkerke, G.J.; Rouwkema, J. Bioactive calcium phosphate coatings applied to flexible poly(carbonate urethane) foils. Surf. Coat. Technol. 2023, 470, 129838. [Google Scholar] [CrossRef]
- Kozelskaya, A.; Dubinenko, G.; Vorobyev, A.; Fedotkin, A.; Korotchenko, N.; Gigilev, A.; Shesterikov, E.; Zhukov, Y.; Tverdokhlebov, S. Porous CaP Coatings Formed by Combination of Plasma Electrolytic Oxidation and RF-Magnetron Sputtering. Coatings 2020, 10, 1113. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.N. Synthetic and Biological-Derived Hydroxyapatite Implant Coatings. Coatings 2024, 14, 39. [Google Scholar] [CrossRef]
- Gao, J.; Su, Y.; Qin, Y.-X. Calcium phosphate coatings enhance biocompatibility and degradation resistance of magnesium alloy: Correlating in vitro and in vivo studies. Bioact. Mater. 2021, 6, 1223–1229. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Bioactive Calcium Phosphate Coatings for Bone Implant Applications: A Review. Coatings 2023, 13, 1091. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Lebedev, V.N.; Kharovskaya, M.I.; Lazoryak, B.I.; Solovieva, A.O.; Fadeeva, I.V.; Amirov, A.A.; Koliushenkov, M.A.; Orudzhev, F.F.; Baryshnikova, O.V.; Yankova, V.G.; et al. Strontium and Copper Co-Doped Multifunctional Calcium Phosphates: Biomimetic and Antibacterial Materials for Bone Implants. Biomimetics 2024, 9, 252. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Tolnai, I.; Balázsi, K.; Balázsi, C. Investigation of Calcium Phosphate-Based Biopolymer Composite Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2024, 25, 13716. [Google Scholar] [CrossRef]
- Kubiak-Mihkelsoo, Z.; Kostrzębska, A.; Błaszczyszyn, A.; Pitułaj, A.; Dominiak, M.; Gedrange, T.; Nawrot-Hadzik, I.; Matys, J.; Hadzik, J. Ionic Doping of Hydroxyapatite for Bone Regeneration: Advances in Structure and Properties over Two Decades—A Narrative Review. Appl. Sci. 2025, 15, 1108. [Google Scholar] [CrossRef]
- Md Dali, S.S.; Wong, S.K.; Chin, K.-Y.; Ahmad, F. The Osteogenic Properties of Calcium Phosphate Cement Doped with Synthetic Materials: A Structured Narrative Review of Preclinical Evidence. Int. J. Mol. Sci. 2023, 24, 7161. [Google Scholar] [CrossRef]
- Niziołek, K.; Słota, D.; Ronowska, A.; Sobczak-Kupiec, A. Calcium Phosphate Biomaterials Modified with Mg2+ or Mn2+ Ions: Structural, Chemical, and Biological Characterization. Ceram. Int. 2025, in press. [CrossRef]
- Wang, X.; Huang, S.; Peng, Q. Metal Ion-Doped Hydroxyapatite-Based Materials for Bone Defect Restoration. Bioengineering 2023, 10, 1367. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Czömpöly, O.; Balázsi, K.; Balázsi, C. Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites. Int. J. Mol. Sci. 2022, 23, 15737. [Google Scholar] [CrossRef]
- Kostka, K.; Hosseini, S.; Epple, M. In-Vitro Cell Response to Strontium/Magnesium-Doped Calcium Phosphate Nanoparticles. Micro 2023, 3, 156–171. [Google Scholar] [CrossRef]
- Alves Côrtes, J.; Dornelas, J.; Duarte, F.; Messora, M.R.; Mourão, C.F.; Alves, G. The Effects of the Addition of Strontium on the Biological Response to Calcium Phosphate Biomaterials: A Systematic Review. Appl. Sci. 2024, 14, 7566. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Lacan, I.; Moldovan, M.; Sarosi, C.; Cuc, S.; Pastrav, M.; Petean, I.; Ene, R. Mechanical Properties and Liquid Absorption of Calcium Phosphate Composite Cements. Materials 2023, 16, 5653. [Google Scholar] [CrossRef]
- Kowalewicz, K.; Vorndran, E.; Feichtner, F.; Waselau, A.-C.; Brueckner, M.; Meyer-Lindenberg, A. In-Vivo Degradation Behavior and Osseointegration of 3D Powder-Printed Calcium Magnesium Phosphate Cement Scaffolds. Materials 2021, 14, 946. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.-N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Lyyra, I.; Leino, K.; Hukka, T.; Hannula, M.; Kellomäki, M.; Massera, J. Impact of Glass Composition on Hydrolytic Degradation of Polylactide/Bioactive Glass Composites. Materials 2021, 14, 667. [Google Scholar] [CrossRef]
- Backes, E.H.; Pires, L.d.N.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Analysis of the Degradation During Melt Processing of PLA/Biosilicate® Composites. J. Compos. Sci. 2019, 3, 52. [Google Scholar] [CrossRef]
- Ewald, A.; Fuchs, A.; Boegelein, L.; Grunz, J.-P.; Kneist, K.; Gbureck, U.; Hoelscher-Doht, S. Degradation and Bone-Contact Biocompatibility of Two Drillable Magnesium Phosphate Bone Cements in an In Vivo Rabbit Bone Defect Model. Materials 2023, 16, 4650. [Google Scholar] [CrossRef]
- He, L.; Yin, J.; Gao, X. Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review. Bioengineering 2023, 10, 672. [Google Scholar] [CrossRef]
- Martelli, A.; Bellucci, D.; Cannillo, V. An Enhanced Bioactive Glass Composition with Improved Thermal Stability and Sinterability. Materials 2024, 17, 6175. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Lin, J. Effect of pH on the In Vitro Degradation of Borosilicate Bioactive Glass and Its Modulation by Direct Current Electric Field. Materials 2022, 15, 7015. [Google Scholar] [CrossRef]
- Gharbi, A.; Oudadesse, H.; el Feki, H.; Cheikhrouhou-Koubaa, W.; Chatzistavrou, X.; Rau, J.V.; Heinämäki, J.; Antoniac, I.; Ashammakhi, N.; Derbel, N. High Boron Content Enhances Bioactive Glass Biodegradation. J. Funct. Biomater. 2023, 14, 364. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, W.; Yu, L.; Camacho, L.C.; Nie, C.; Zhang, M.; Haag, R.; Wei, Q. Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells. Nano Micro Small 2020, 16, 1905422. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Z.; Gu, L.; Ma, Z.; Zheng, H.; Wang, Q.; Yang, Y. Unraveling the relationship between the structural features and solubility properties in Sr-containing bioactive glasses. Ceram. Inter. Part A 2024, 50, 4245–4255. [Google Scholar] [CrossRef]
- Tilocca, A. Structural models of bioactive glasses from molecular dynamics simulations. Proc. R. Soc. A 2009, 465, 1003–1027. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Huang, Y.W.; Semon, J.A.; Leu, M.C. 3D-printed Biomimetic Bioactive Glass Scaffolds for Bone Regeneration in Rat Calvarial Defects. Int. J. Bioprint. 2020, 6, 274, Erratum in Int. J. Bioprint. 2020, 6, 309. [Google Scholar]
- Galusková, D.; Kaňková, H.; Švančárková, A.; Galusek, D. Early-Stage Dissolution Kinetics of Silicate-Based Bioactive Glass under Dynamic Conditions: Critical Evaluation. Materials 2021, 14, 3384. [Google Scholar] [CrossRef]
- Yao, A.; Wang, D.; Huang, W.; Fu, Q.; Rahaman, M.N.; Day, D.E. In Vitro Bioactive Characteristics of Borate-Based Glasses with Controllable Degradation Behavior. J. Am. Cer. Soc. 2007, 90, 303–306. [Google Scholar] [CrossRef]
- Christie, J.K.; Ainsworth, R.I.; Di Tommaso, D.; de Leeuw, N.H. Nanoscale chains control the solubility of phosphate glasses for biomedical applications. J. Phys. Chem. B. 2013, 117, 10652–10657. [Google Scholar] [CrossRef]
- Lu, J.; Descamps, M.; Dejou, J.; Hardouin, P.; Lemaitre, J.; Proust, J.-P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef]
- Andrade, A.L.; Valério, P.; Goes, A.M.; de Fátima Leite, M.; Domingues, R.Z. Influence of morphology on in vitro compatibility of bioactive glasses. J. Non. Cryst. Solids 2006, 352, 3508–3511. [Google Scholar] [CrossRef]
- De Pace, R.; Molinari, S.; Mazzoni, E.; Perale, G. Bone Regeneration: A Review of Current Treatment Strategies. J. Clin. Med. 2025, 14, 1838. [Google Scholar] [CrossRef]
- Alonso-Fernández, I.; Haugen, H.J.; Nogueira, L.P.; López-Álvarez, M.; González, P.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz-Guzón, F. Enhanced Bone Healing in Critical-Sized Rabbit Femoral Defects: Impact of Helical and Alternate Scaffold Architectures. Polymers 2024, 16, 1243. [Google Scholar] [CrossRef]
- Brochu, B.M.; Sturm, S.R.; Kawase De Queiroz Goncalves, J.A.; Mirsky, N.A.; Sandino, A.I.; Panthaki, K.Z.; Panthaki, K.Z.; Nayak, V.V.; Daunert, S.; Witek, L.; et al. Advances in Bioceramics for Bone Regeneration: A Narrative Review. Biomimetics 2024, 9, 690. [Google Scholar] [CrossRef]
- Kojima, K.E.; de Andrade E Silva, F.B.; Leonhardt, M.C.; de Carvalho, V.C.; de Oliveira, P.R.D.; Lima, A.L.L.M.; Roberto Dos Reis, P.; Silva, J.D.S. Bioactive glass S53P4 to fill-up large cavitary bone defect after acute and chronic osteomyelitis treated with antibiotic-loaded cement beads: A prospective case series with a minimum 2-year follow-up. Injury 2021, 52 (Suppl. S3), S23–S28. [Google Scholar] [CrossRef]
- Cottrill, E.; Pennington, Z.; Lankipalle, N.; Ehresman, J.; Valencia, C.; Schilling, A.; Feghali, J.; Perdomo-Pantoja, A.; Theodore, N.; Sciubba, D.M.; et al. The Effect of Bioactive Glasses on Spinal Fusion: A Cross-Disciplinary Systematic Review and Meta-Analysis of the Preclinical and Clinical Data. J. Clin. Neurosci. 2020, 78, 34–46. [Google Scholar] [CrossRef]
- Van Vugt, T.A.G.; Heidotting, J.; Arts, J.J.; Ploegmakers, J.J.W.; Jutte, P.C.; Geurts, J.A.P. Mid-term clinical results of chronic cavitary long bone osteomyelitis treatment using S53P4 bioactive glass: A multi-center study. J. Bone Jt. Infection. 2021, 6, 413–421. [Google Scholar] [CrossRef]
- Malat, T.A.; Glombitza, M.; Dahmen, J.; Hax, P.-M.; Steinhausen, E. The Use of Bioactive Glass S53P4 as Bone Graft Substitute in the Treatment of Chronic Osteomyelitis and Infected Non-Unions—A Retrospective Study of 50 Patients. Z. Orthop. Unfall. 2018, 156, 152–159. [Google Scholar] [CrossRef]
- Paiva, J.C.C.; Oliveira, L.; Vaz, M.F.; Costa-de-Oliveira, S. Biodegradable Bone Implants as a New Hope to Reduce Device-Associated Infections-A Systematic Review. Bioengineering 2022, 9, 409. [Google Scholar] [CrossRef]
- Lindfors, N.C.; Hyvönen, P.; Nyyssönen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef]
- van Gestel, N.A.; Geurts, J.; Hulsen, D.J.; van Rietbergen, B.; Hofmann, S.; Arts, J.J. Clinical Applications of S53P4 Bioactive Glass in Bone Healing and Osteomyelitic Treatment: A Literature Review. Biomed. Res. Int. 2015, 2015, 684826. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Vallittu, P.K.; Özcan, M.; Lassila, L.V.J.; Gandini, P.; Sfondrini, M.F. Travel beyond Clinical Uses of Fiber Reinforced Composites (FRCs) in Dentistry: A Review of Past Employments, Present Applications, and Future Perspectives. BioMed Res. Int. 2018, 2018, 1498901. [Google Scholar] [CrossRef] [PubMed]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Hamdi, M.; Basirun, W.J. Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J. Biomed. Mater. Res. 2017, 105, 3197–3223. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.M.B.; Rosso, M.P.O.; Buchaim, D.V.; Zangrando, M.S.R.; Buchaim, R.L. Update on the use of 45S5 bioactive glass in the treatment of bone defects in regenerative medicine. World J. Orthop. 2024, 15, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair. Mater. Sci. Eng. C 2017, 79, 286–295. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In Vitro, In Vivo and clinical trials. J. Non-Cryst. Solids 2016, 432 Pt A, 90–110. [Google Scholar]
- Fakher, S.; Westenberg, D. Properties and antibacterial effectiveness of metal-ion doped borate-based bioactive glasses. Future Microbiol. 2025, 20, 315–331. [Google Scholar] [CrossRef]
- Madival, H.; Rajiv, A.; Muniraju, C.; Reddy, M.S. Advancements in Bioactive Glasses: A Comparison of Silicate, Borate, and Phosphate Network Based Materials. Biomed. Mater. Devices 2025. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Cannillo, V.; Salvatori, R.; Bergamini, S.; Bellucci, D.; Bertoldi, C. Bioactive Glasses in Periodontal Regeneration: Existing Strategies and Future Prospects—A Literature Review. Materials 2022, 15, 2194. [Google Scholar] [CrossRef]
- Nicholson, J.W. Periodontal Therapy Using Bioactive Glasses: A Review. Prosthesis 2022, 4, 648–663. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Orgill, D.P.; Galiano, R.D.; Glat, P.M.; DiDomenico, L.A.; Carter, M.J.; Zelen, C.M. A multi-centre, single-blinded randomised controlled clinical trial evaluating the effect of resorbable glass fibre matrix in the treatment of diabetic foot ulcers. Int. Wound J. 2022, 9, 791–801. [Google Scholar] [CrossRef]
- Schaefer, S.; Detsch, R.; Uhl, F.; Deisinger, U.; Ziegler, G. How Degradation of Calcium Phosphate Bone Substitute Materials is influenced by Phase Composition and Porosity. Adv. Eng. Mat. 2011, 13, 342–350. [Google Scholar] [CrossRef]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielov, M. Synthetic Calcium–Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef] [PubMed]
- Skibiński, S.; Czechowska, J.P.; Guzik, M.; Vivcharenko, V.; Przekora, A.; Szymczak, P.; Zima, A. Scaffolds based on β tricalcium phosphate and polyhydroxyalkanoates as biodegradable and bioactive bone substitutes with enhanced physicochemical properties. Sustain. Mater. Technol. 2023, 38, e00722. [Google Scholar] [CrossRef]
- Oliveira, C.S.F.; Negut, I.; Bita, B. The Use of Calcium Phosphate Bioceramics for the Treatment of Osteomyelitis. Ceramics 2024, 7, 1779–1809. [Google Scholar] [CrossRef]
- Xiao, L.; Shiwaku, Y.; Hamai, R.; Tsuchiya, K.; Sasaki, K.; Suzuki, O. Macrophage Polarization Related to Crystal Phases of Calcium Phosphate Biomaterials. Int. J. Mol. Sci. 2021, 22, 11252. [Google Scholar] [CrossRef]
- Leitão, M.; Mavropoulos, E.; Sader, M.S.; Costa, A.; Lopez, E.; Fontes, G.N.; Granjeiro, J.M.; Romasco, T.; Di Pietro, N.; Piattelli, A.; et al. Effects of Physically Adsorbed and Chemically Immobilized RGD on Cell Adhesion to a Hydroxyapatite Surface. Appl. Sci. 2024, 14, 7479. [Google Scholar] [CrossRef]
- Humbert, P.; Kampleitner, C.; De Lima, J.; Brennan, M.Á.; Lodoso-Torrecilla, I.; Sadowska, J.M.; Blanchard, F.; Canal, C.; Ginebra, M.-P.; Hoffmann, O.; et al. Phase composition of calcium phosphate materials affects bone formation by modulating osteoclastogenesis. Acta Biomater. 2024, 176, 417–431. [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, T.; Wang, S.; Liu, Z.; Cheng, Y.; Li, A.; Pei, D. The Osteoinductivity of Calcium Phosphate-Based Biomaterials: A Tight Interaction With Bone Healing. Front. Bioeng. Biotechnol. 2022, 10, 911180. [Google Scholar] [CrossRef]
- An, S.; Ling, J.; Gao, Y.; Xiao, Y. Effects of varied ionic calcium and phosphate on the proliferation, osteogenic differentiation and mineralization of human periodontal ligament cells in vitro. J. Periodont. Res. 2012, 47, 374–382. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Owyoung, J.B.; Saiz, E.; Marshall, S.J.; Marshall, G.W.; Loomer, P.M. The ionic products of bioactive glass particle dissolution enhance periodontal ligament fibroblast osteocalcin expression and enhance early mineralized tissue development. J. Biomed. Mater. Res. A 2011, 98, 177–184. [Google Scholar] [CrossRef]
- Ftiti, S.; Cifuentes, S.C.; Guidara, A.; Rams, J.; Tounsi, H.; Fernández-Blázquez, J.P. The Structural, Thermal and Morphological Characterization of Polylactic Acid/Β-Tricalcium Phosphate (PLA/Β-TCP) Composites upon Immersion in SBF: A Comprehensive Analysis. Polymers 2024, 16, 719. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yagi, R.; Waki, T.; Wada, T.; Ohkubo, C.; Hayakawa, T. Study of Apatite Deposition in a Simulated Body Fluid Immersion Experiment. J. Oral Tissue Eng. 2016, 14, 9–14. [Google Scholar]
- Ebrahimian-Hosseinabadi, M.; Etemadifar, M.; Ashrafizadeh, F. Effects of Nano-biphasic Calcium Phosphate Composite on Bioactivity and Osteoblast Cell Behavior in Tissue Engineering Applications. J. Med. Signals Sens. 2016, 6, 237–242. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Translat. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, Y.; Shang, Z.; Zhao, B.; Jiao, M.; Liu, W.; Cheng, M.; Zhai, B.; Guo, Y.; Liu, B.; et al. Biodegradable metals for bone defect repair: A systematic review and meta-analysis based on animal studies. Bioact. Mater. 2021, 6, 4027–4052. [Google Scholar] [CrossRef]
- Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Ann. Anat. Anat. Anz. 2017, 213, 83–90. [Google Scholar] [CrossRef]
- Ono, H.; Sase, T.; Tanaka, Y.; Takasuna, H. Histological assessment of porous custom-made hydroxyapatite implants 6 months and 2.5 years after cranioplasty. Surg. Neurol. Int. 2017, 8, 8. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Shen, C.; Wang, M.M.; Witek, L.; Tovar, N.; Cronstein, B.N.; Torroni, A.; Flores, R.L.; Coelho, P.G. Transforming the Degradation Rate of β-tricalcium Phosphate Bone Replacement Using 3-Dimensional Printing. Ann. Plast. Surg. 2021, 87, e153–e162. [Google Scholar] [CrossRef]
- Liu, B.; Lun, D. Current Application of β-tricalcium Phosphate Composites in Orthopaedics. Orthopaedic Surgery 2012, 4, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Komaki, H.; Chazono, M.; Kitasato, S.; Kakuta, A.; Akiyama, S.; Marumo, K. Basic research and clinical application of beta-tricalcium phosphate (β-TCP). Morphologie 2017, 101, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.C.; Mingrone, L.E.; Sá, M.J.C. Evaluation of Osseointegration and Bone Healing Using Pure-Phase β-TCP Ceramic Implant in Bone Critical Defects. A Systematic Review. Front. Vet. Sci. 2022, 9, 859920. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Jolic, M.; Micheletti, C.; Omar, O.; Norlindh, B.; Emanuelsson, L.; Engqvist, H.; Engstrand, T.; Palmquist, A.; Thomsen, P. Bone without borders—Monetite-based calcium phosphate guides bone formation beyond the skeletal envelope. Bioact. Mater. 2023, 19, 103–114. [Google Scholar] [CrossRef]
- Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. Int. J. Mol. Sci. 2025, 26, 3085. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Pyo, S.-W.; Paik, J.-W.; Lee, D.-N.; Seo, Y.-W.; Park, J.-Y.; Kim, S.; Choi, S.-H. Comparative Analysis of Bone Regeneration According to Particle Type and Barrier Membrane for Octacalcium Phosphate Grafted into Rabbit Calvarial Defects. Bioengineering 2024, 11, 215. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.G. Advancements in alveolar bone grafting and ridge preservation: A narrative review on materials, techniques, and clinical outcomes. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 14. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Jasser, R.A.; AlSubaie, A.; AlShehri, F. Effectiveness of beta-tricalcium phosphate in comparison with other materials in treating periodontal infra-bony defects around natural teeth: A systematic review and meta-analysis. BMC Oral Health 2021, 21, 219. [Google Scholar] [CrossRef]
- Witek, L.; Alifarag, A.M.; Tovar, N.; Lopez, C.D.; Cronstein, B.N.; Rodriguez, E.D.; Coelho, P.G. Repair of Critical-Sized Long Bone Defects Using Dipyridamole-Augmented 3D-Printed Bioactive Ceramic Scaffolds. J. Orthopeadic Res. 2019, 37, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Ishack, S.; Mediero, A.; Wilder, T.; Ricci, J.L.; Cronstein, B.N. Bone regeneration in critical bone defects using three-dimensionally printed β-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 366–375. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, Y.; Zhou, Y. Strontium Functionalized in Biomaterials for Bone Tissue Engineering: A Prominent Role in Osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 928799. [Google Scholar] [CrossRef]
- Sheng, X.; Li, C.; Wang, Z.; Xu, Y.; Sun, Y.; Zhang, W.; Liu, H.; Wang, J. Advanced applications of strontium-containing biomaterials in bone tissue engineering. Mater. Today Bio 2023, 20, 100636. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, S.; Zhou, J.; Gholipourmalekabadi, M.; Cao, Y.; Lin, K.; Zhuang, Y.; Yuan, C. From hard tissues to beyond: Progress and challenges of strontium-containing biomaterials in regenerative medicine applications. Bioact. Mater. 2025, 49, 85–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, H.; Zhang, J.; Sun, T.; Zhang, W.; Li, Z. Recent Advance of Strontium Functionalized in Biomaterials for Bone Regeneration. Bioengineering 2023, 10, 414. [Google Scholar] [CrossRef]
- Available online: http://www.porexsurgical.com (accessed on 14 July 2002).
- Available online: https://novabone.com/ (accessed on 9 March 2001).
- Available online: https://www.bonalive.com (accessed on 8 February 2011).
- Available online: https://www.stryker.com/hk/en/interventional-spine/products/cortoss-bone-augmentation-material.html (accessed on 2 December 2022).
- Available online: https://www.novetech-surgery.com/en/product/glassbone/ (accessed on 13 June 2018).
- Available online: https://www.biospace.com/repregen-ltd-formerly-known-as-bioceramic-therapeutics-raises-1-03m-1-6m-in-additional-capital-from-existing-investors-b-imperial-innovations (accessed on 16 November 2010).
- Available online: https://regenity.com/ (accessed on 15 June 2013).
- Available online: https://skulleimplants.com/ (accessed on 30 September 2011).
- Available online: https://www.bioventussurgical.com/product/signafuse (accessed on 18 July 2019).
- Available online: https://www.gsk.com/en-gb/ (accessed on 1 April 2024).
- Available online: https://avalonmed.com/products/ (accessed on 20 October 2020).
- Available online: https://theraglass.co.uk/ (accessed on 1 April 2024).
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Available online: https://www.zimmerbiomet.com/en (accessed on 13 August 2021).
- Available online: https://www.jnjmedtech.com/en-US/companies/depuy-synthes (accessed on 29 March 2022).
- Available online: https://www.bioceramed.com/ (accessed on 18 August 2018).
- Available online: https://www.hoyatechnosurgical.co.jp/en/ (accessed on 31 March 2022).
- Available online: https://pro-healthint.com/kasios/ (accessed on 1 April 2023).
- Available online: https://www.teknimed.com/ (accessed on 25 January 1999).
- Available online: https://www.curasan.com/cerasorb-m/ (accessed on 23 March 2023).
- Available online: https://bonegraft.com.tr/ (accessed on 18 January 2018).
- Available online: https://biomatlante.com/en/products/mbcp-synthetic-bone-graft-substitute (accessed on 21 April 2021).
- Yousefi, A.-M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Func. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef]
- Hettich, G.; Schierjott, R.A.; Epple, M.; Gbureck, U.; Heinemann, S.; Mozaffari-Jovein, H.; Grupp, T.M. Calcium Phosphate Bone Graft Substitutes with High Mechanical Load Capacity and High Degree of Interconnecting Porosity. Materials 2019, 12, 3471. [Google Scholar] [CrossRef]
- Ribeiro, N.; Reis, M.; Figueiredo, L.; Pimenta, A.; Santos, L.F.; Branco, A.C.; Alves de Matos, A.P.; Salema-Oom, M.; Almeida, A.; Pereira, M.F.C.; et al. Improvement of a commercial calcium phosphate bone cement by means of drug delivery and increased injectability. Ceram. Int. 2022, 48, 33361–33372. [Google Scholar] [CrossRef]
- Stiller, A.; Engblom, M.; Karlström, O.; Lindén, M.; Hupa, L. Impact of Fluid Flow Rate on the Dissolution Behavior of Bioactive Glass S53P4. J. Non-Cryst. Solids 2023, 607, 122219. [Google Scholar] [CrossRef]
- Meskher, H.; Sharifianjazi, F.; Tavamaishvili, K.; Irandoost, M.; Nejadkoorki, D.; Makvandi, P. Limitations, Challenges and Prospective Solutions for Bioactive Glasses-Based Nanocomposites for Dental Applications: A Critical Review. J. Dent. 2024, 150, 105331. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).