Evaluation of the Characteristics of Digital Light Processing 3D-Printed Magnesium Calcium Phosphate for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Preparation
2.2. Fabrication of MCP Photopolymer Suspensions
2.3. Rheological Measurement
2.4. Measurement of Cure Depth
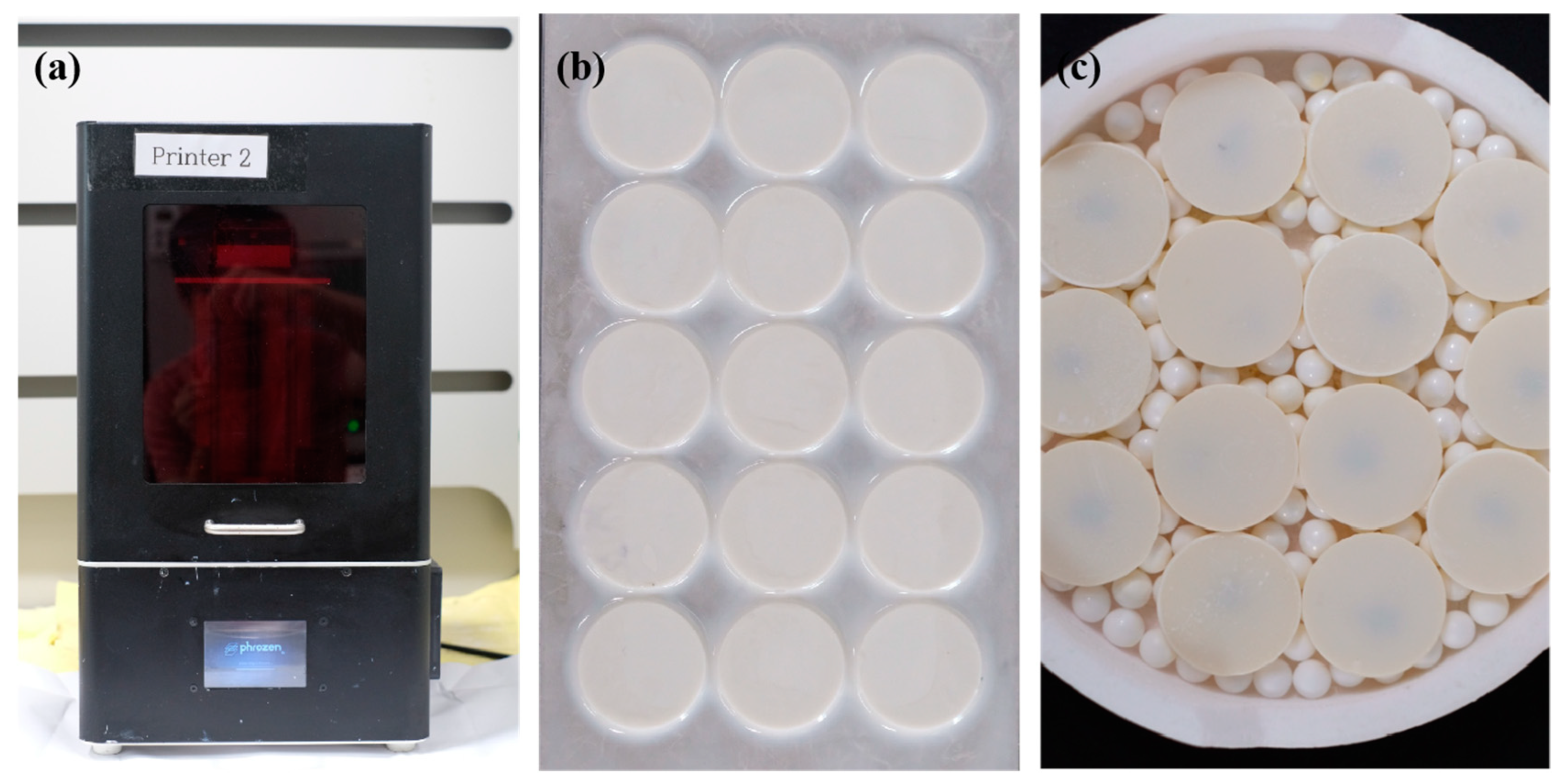
2.5. Additive Manufacturing
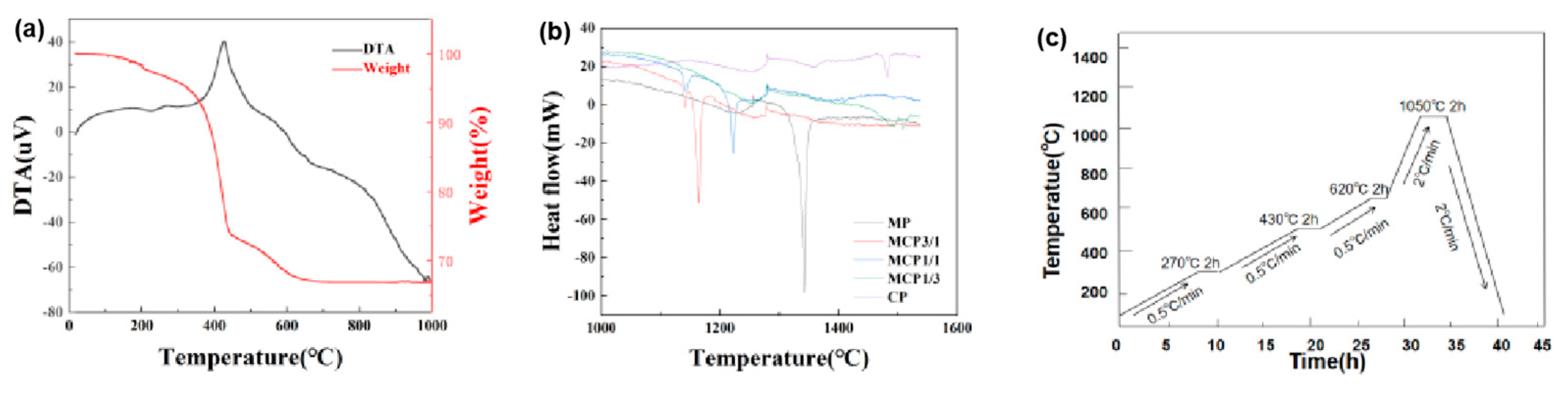
2.6. Thermal Analysis Method
2.7. Characterization of Samples
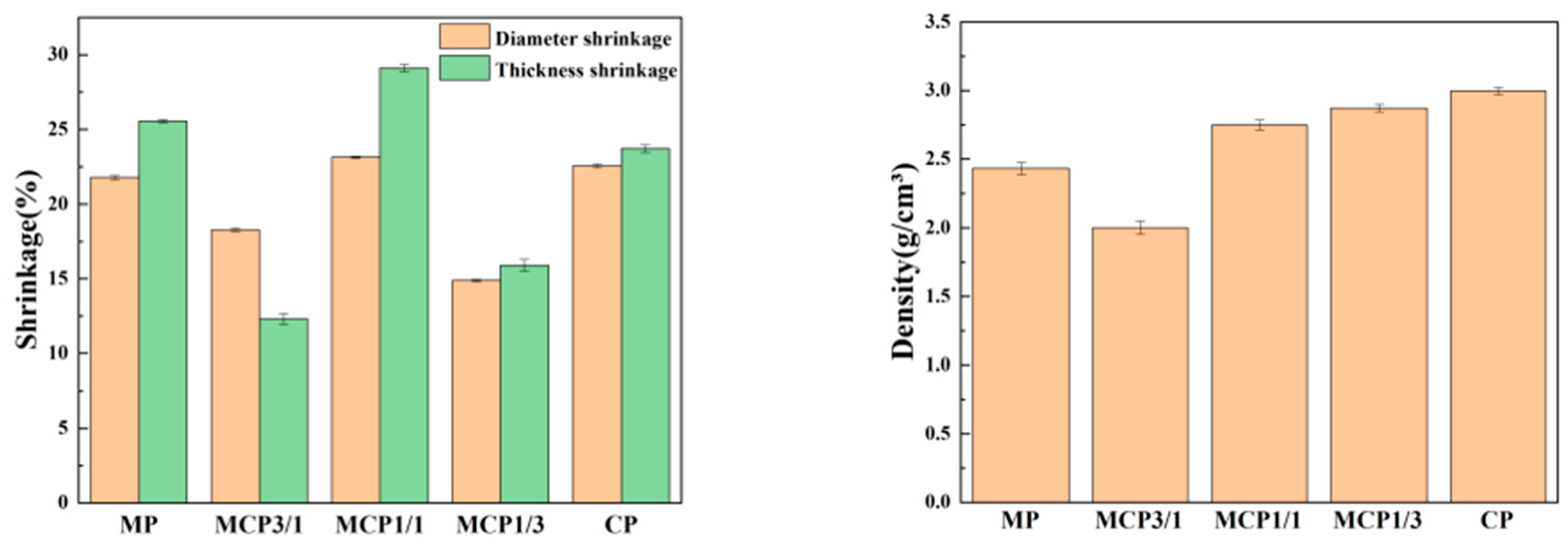
2.7.1. Density and Shrinkage
2.7.2. Microstructural and X-Ray Diffraction (XRD) Analyses
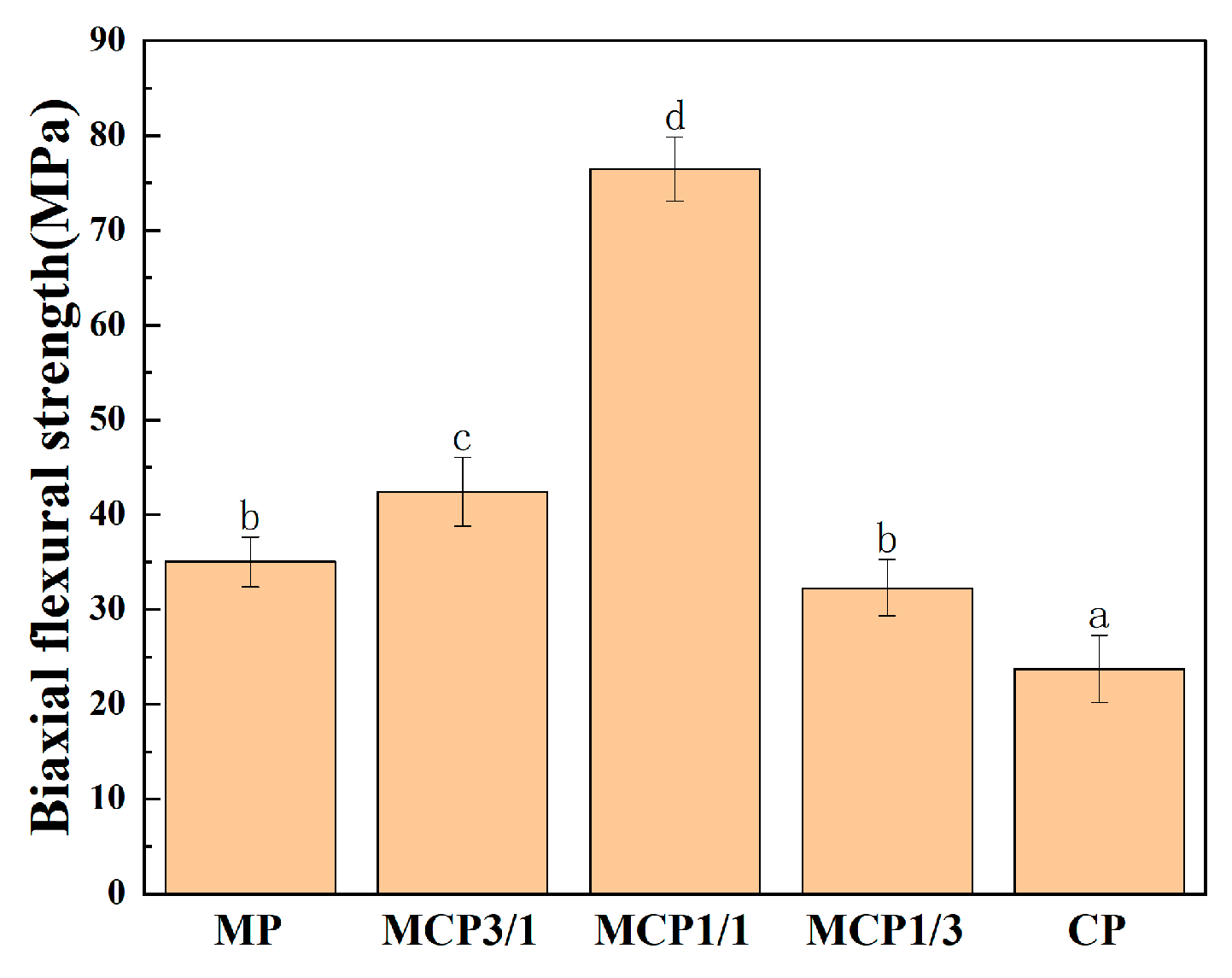
2.7.3. Biaxial Flexural Strength Measurement
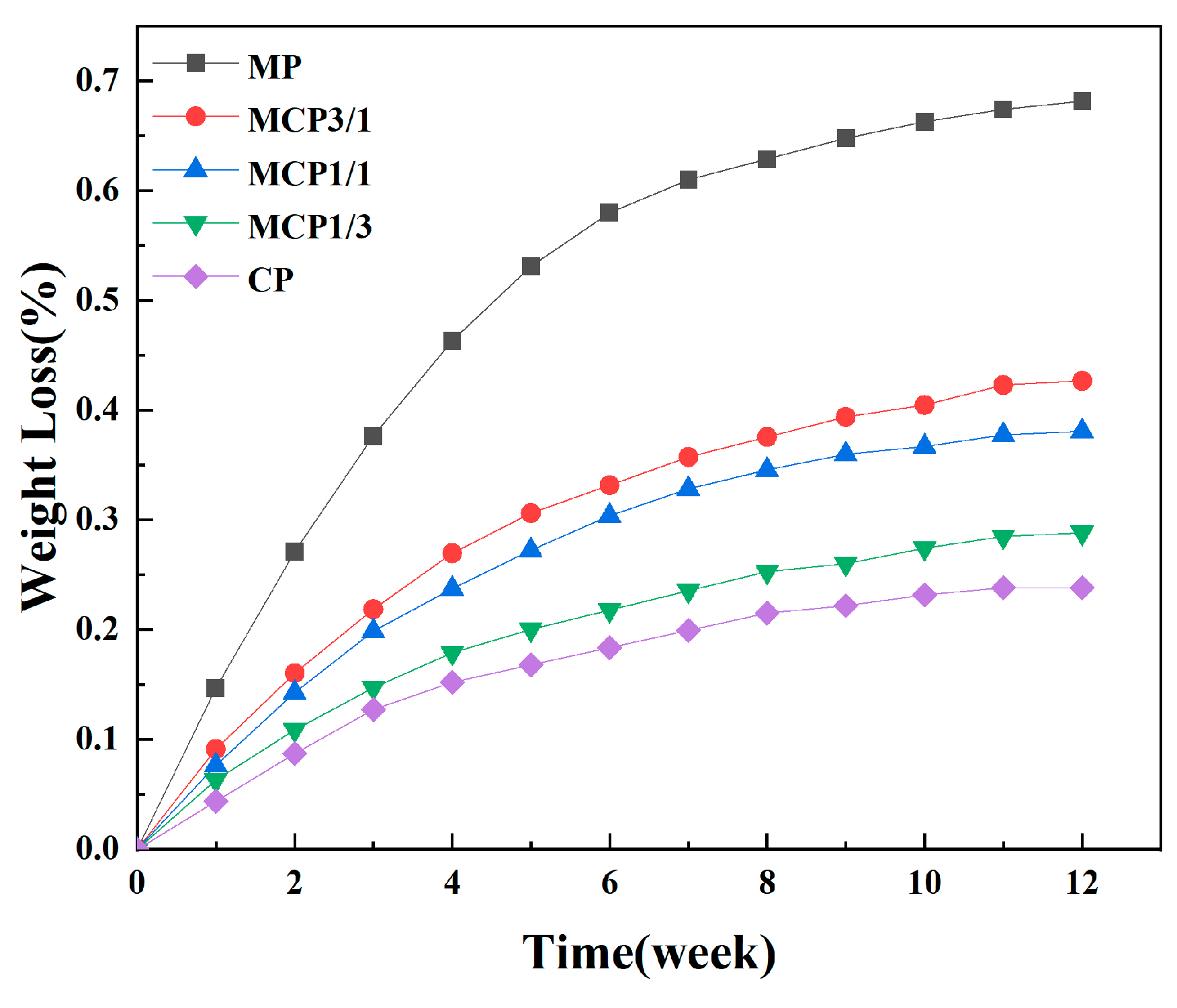
2.7.4. Degradation Analysis
2.8. In Vitro Cell Experiments
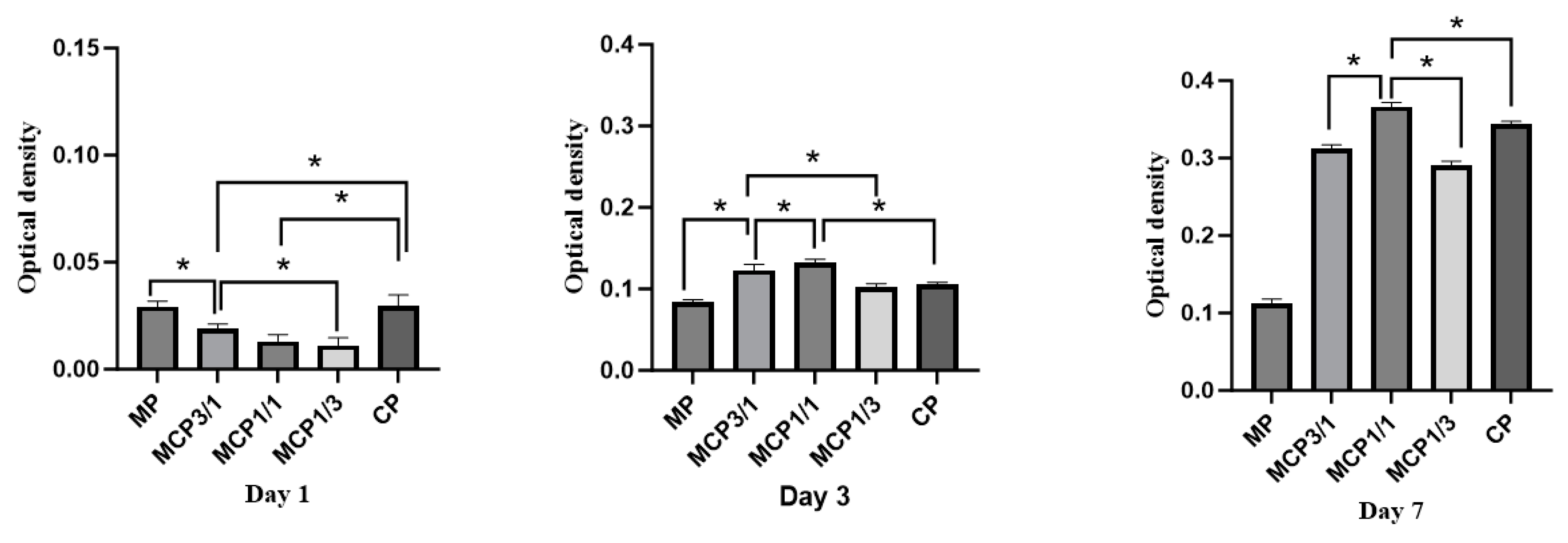
2.8.1. Cell Proliferation
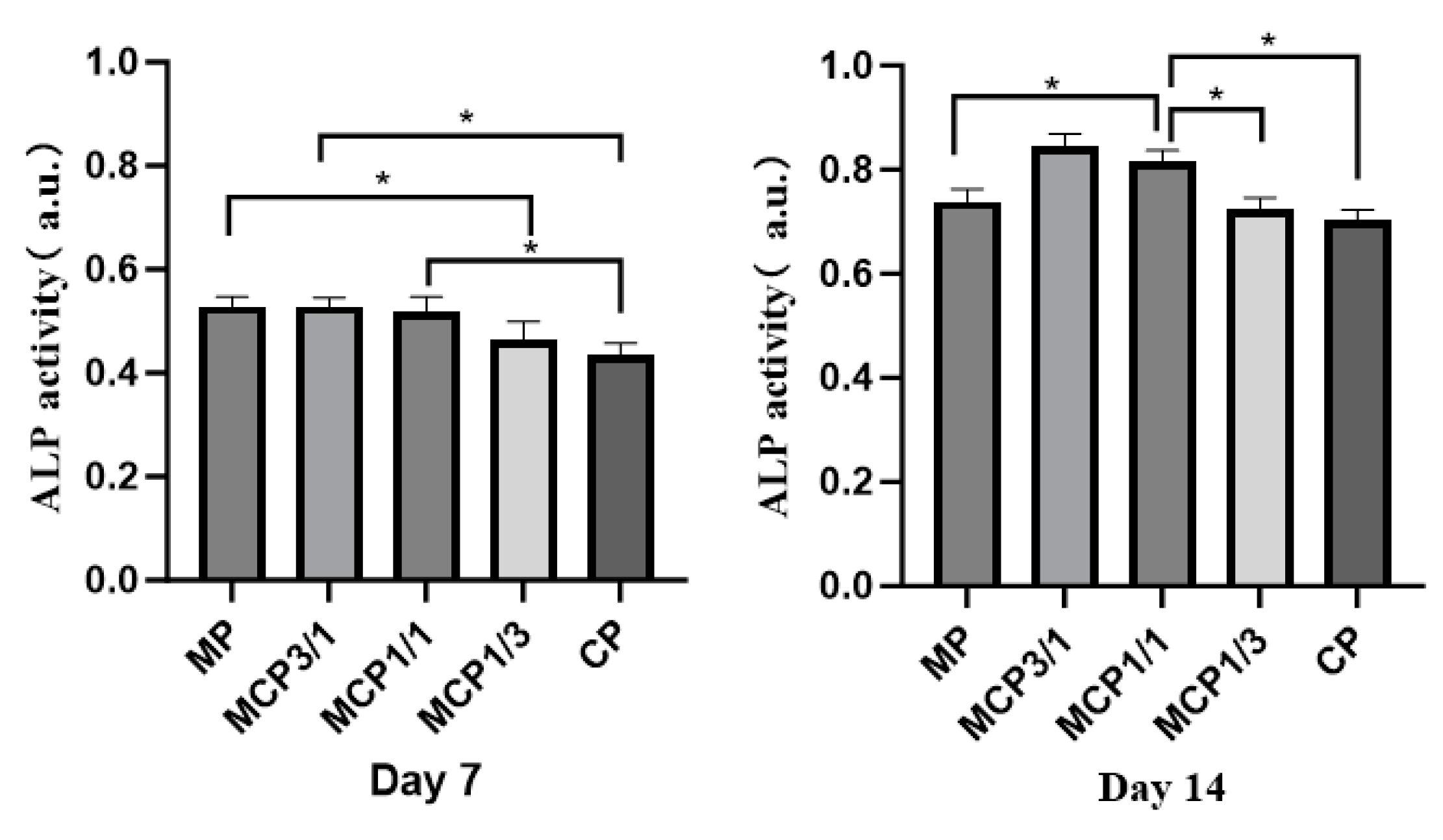
2.8.2. Cell Differentiation
2.9. Statistical Analysis
3. Results
3.1. Characterization of Powder
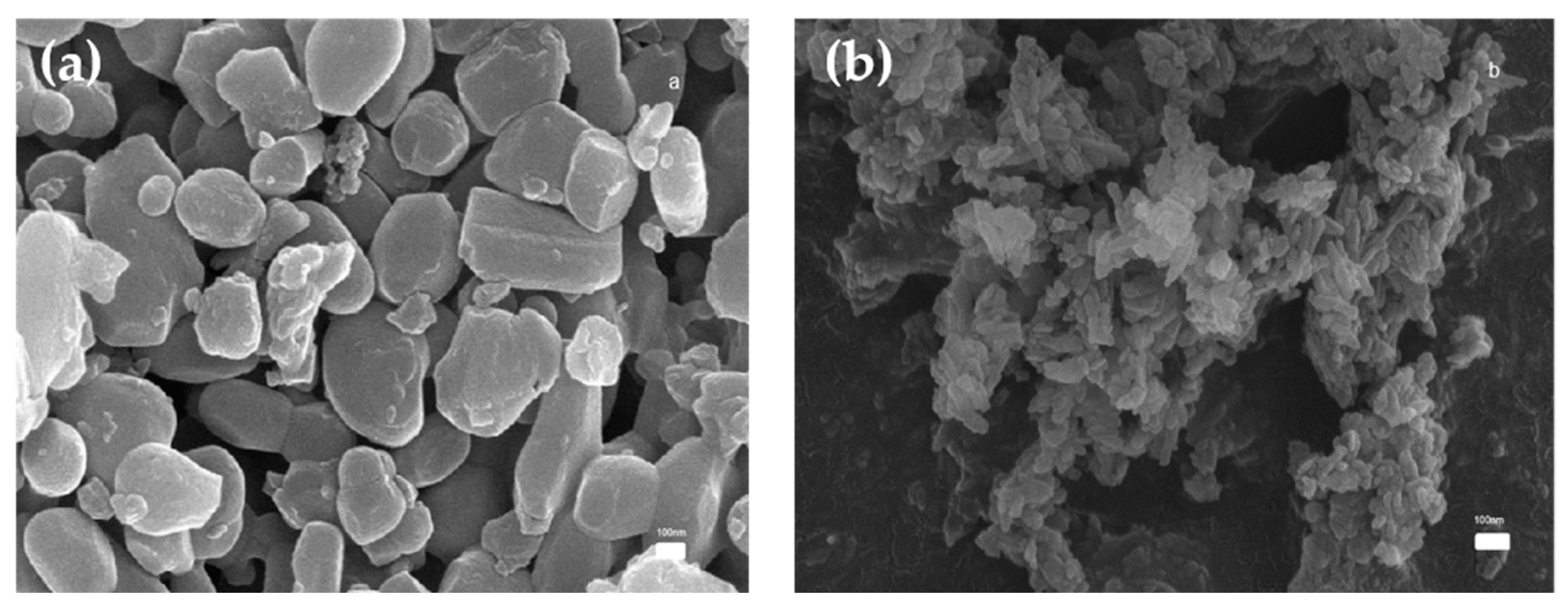
3.1.1. SEM Analysis
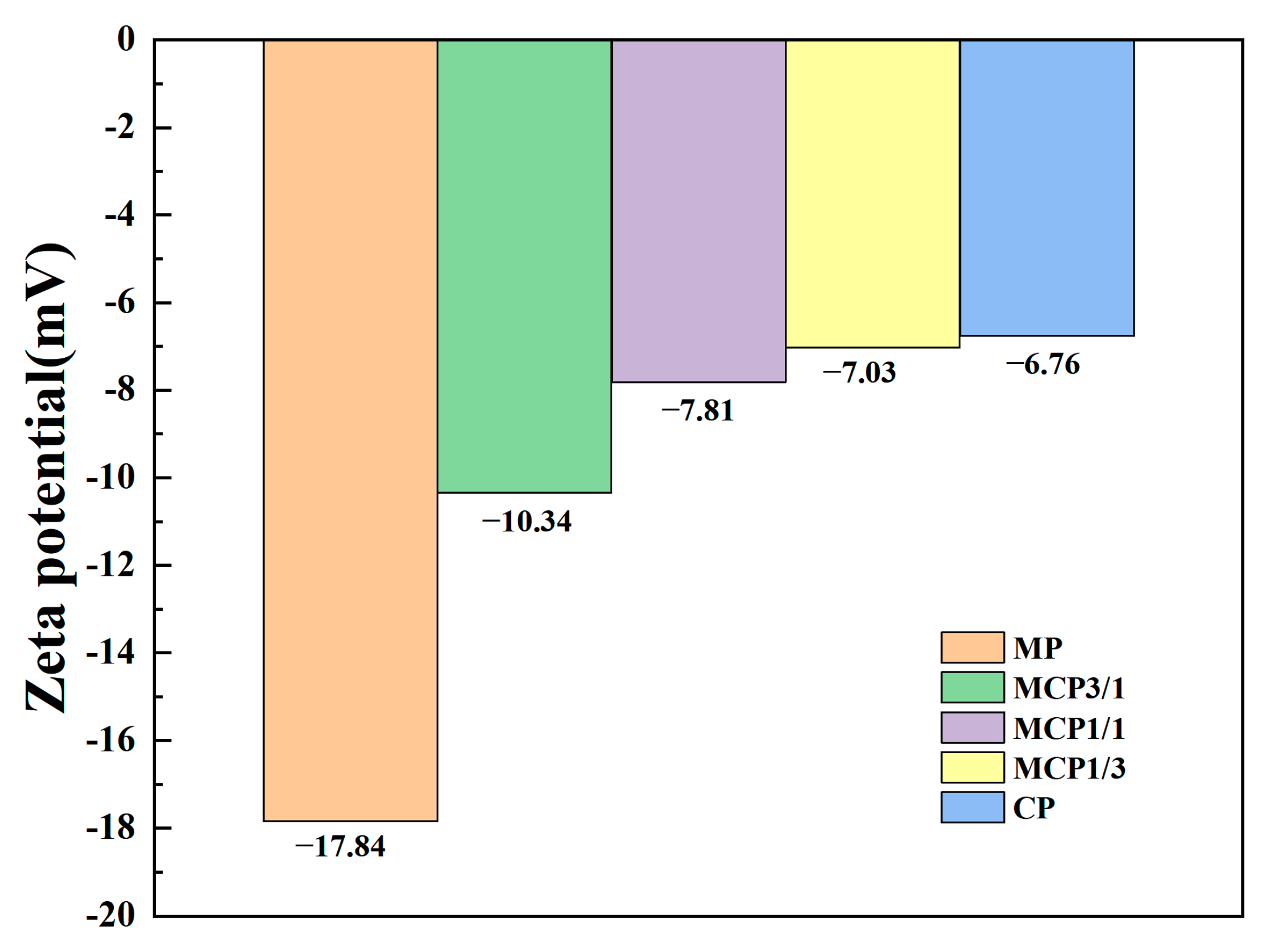
3.1.2. Zeta Potential
3.2. Characterization of Suspensions
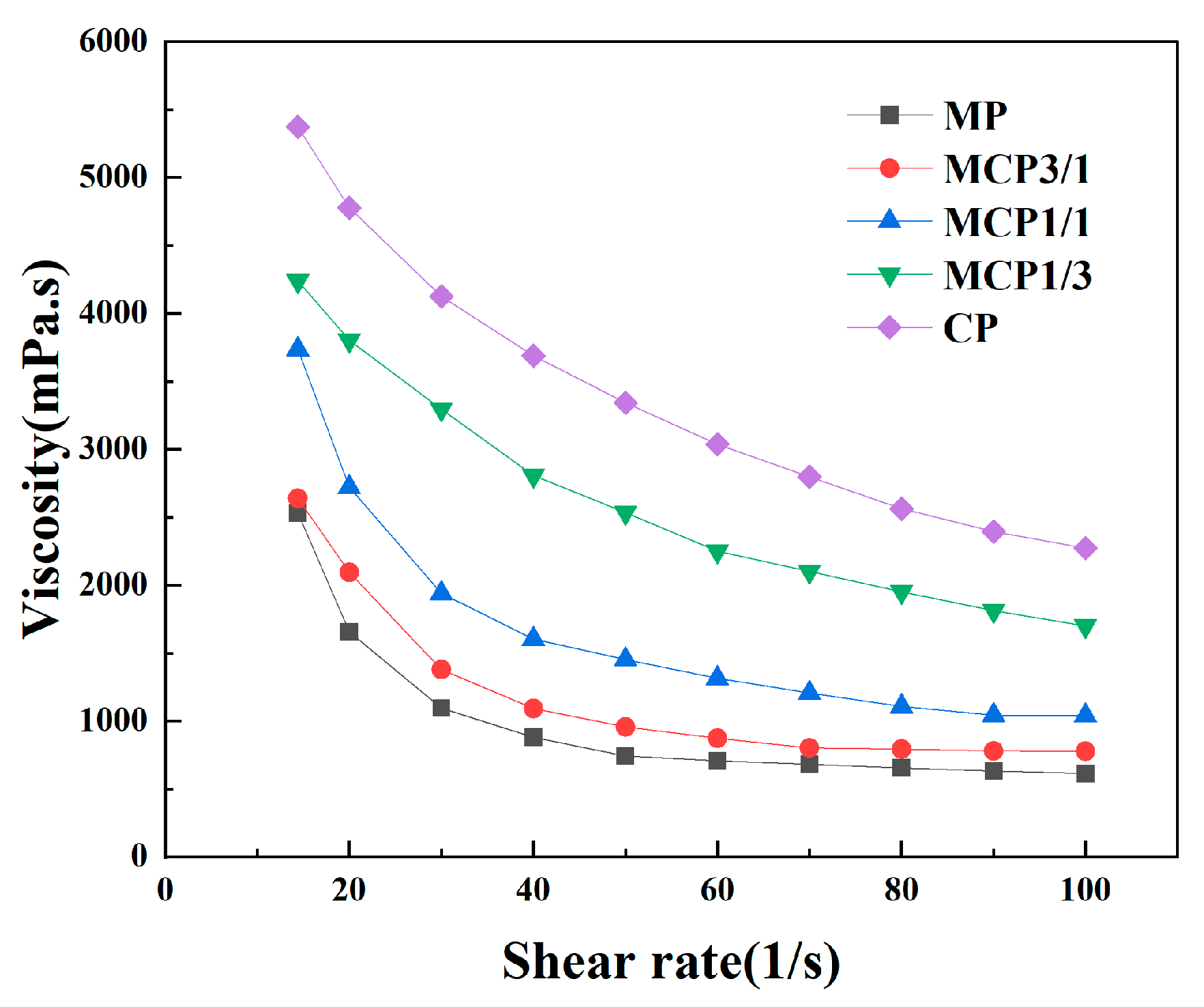
3.2.1. Viscosity
3.2.2. Cure Depth
3.3. Thermal Analysis
3.4. Microstructural Analysis
3.5. XRD
3.6. Linear Shrinkage Rate and Density
3.7. Biaxial Flexural Strength
3.8. Degradation
3.9. Cell Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| DLP | digital light processing |
| MCP | magnesium calcium phosphate |
| CP | calcium phosphate |
| MP | magnesium phosphate |
| AM | additive manufacturing |
| SLA | stereolithography |
| FDM | fused deposition modeling |
| TG-DTA | thermogravimetric–differential thermal analysis |
| HDDA | 1,6-hexanediol diacrylate |
| UV | ultraviolet |
| STL | standard triangle language |
| DMEM | Dulbecco’s modified Eagle medium |
| PBS | phosphate-buffered saline |
| WST-8 | water-soluble tetrazolium 8 |
| ALP | alkaline phosphatase |
| DSC | differential scanning calorimetry |
References
- Smith, B.T.; Shum, J.; Wong, M.; Mikos, A.G.; Young, S. Bone Tissue Engineering Challenges in Oral & Maxillofacial Surgery, Engineering Mineralized and Load Bearing Tissues; Springer: Berlin/Heidelberg, Germany, 2015; pp. 57–78. [Google Scholar]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, K.; Wu, T.; Chen, J.; Li, S.; Zhang, X. Research progress in degradable metal-based multifunctional scaffolds for bone tissue engineering. Med. Comm. Biomater. Appl. 2023, 2, e60. [Google Scholar] [CrossRef]
- Zare, Y.; Shabani, I. Polymer/metal nanocomposites for biomedical applications. Mater. Sci. Eng. C 2016, 60, 195–203. [Google Scholar] [CrossRef]
- Ribas, R.G.; Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Stegemann, C.; Gonçalves Leite, D.M.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-setting bioactive calcium–magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef]
- Eugen, G.; Claus, M.; Anna-Maria, S.; Niklas, D.; Philipp, S.; Andrea, E.; Andrea, M.-L.; Elke, V. Degradation of 3D-printed magnesium phosphate ceramics in vitro and a prognosis on their bone regeneration potential. Bioact. Mater. 2023, 19, 376–391. [Google Scholar] [CrossRef]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef]
- Gelli, R.; Ridi, F. An Overview of Magnesium-Phosphate-Based Cements as Bone Repair Materials. J. Funct. Biomater. 2023, 14, 424. [Google Scholar] [CrossRef]
- Zhao, Q.; Ni, Y.; Wei, H.; Duan, Y.; Chen, J.; Xiao, Q.; Gao, J.; Yu, Y.; Cui, Y.; Ouyang, S.; et al. Ion incorporation into bone grafting materials. Periodontology 2023, 94, 213–230. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, H.; Wu, J.-M.; Chen, S.; Cheng, L.-J.; Shi, Y.-S.; Mo, Y.-C.; Li, C.-H.; Xiao, J. Preparation and biological evaluation of ZrO2 all-ceramic teeth by DLP technology. Ceram. Int. 2020, 46, 11268–11274. [Google Scholar] [CrossRef]
- Lei, B.; Gao, X.; Zhang, R.; Yi, X.; Zhou, Q. In situ magnesium phosphate/polycaprolactone 3D-printed scaffold induce bone regeneration in rabbit maxillofacial bone defect model. Mater. Des. 2022, 215, 110477. [Google Scholar] [CrossRef]
- Schaufler, C.; Schmitt, A.-M.; Moseke, C.; Stahlhut, P.; Geroneit, I.; Brückner, M.; Meyer-Lindenberg, A.; Vorndran, E. Physicochemical degradation of calcium magnesium phosphate (stanfieldite) based bone replacement materials and the effect on their cytocompatibility. Biomed. Mater. 2022, 18, 015022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Liang, H.; Liu, Y.; Bai, J.; Wang, M. Digital light processing (DLP) of nano biphasic calcium phosphate bioceramic for making bone tissue engineering scaffolds. Ceram. Int. 2022, 48, 27681–27692. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital light processing based three-dimensional printing for medical applications. Int. J. Bioprinting 2019, 6, 242. [Google Scholar] [CrossRef]
- Coppola, B.; Montanaro, L.; Palmero, P. DLP Fabrication of Zirconia Scaffolds Coated with HA/β-TCP Layer: Role of Scaffold Architecture on Mechanical and Biological Properties. J. Funct. Biomater. 2022, 13, 148. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Zhang, H.; Liu, X.; Wei, Q.; Li, M.; Liu, Z.; Bao, C.; Zhang, K. Effects of dispersant concentration on the properties of hydroxyapatite slurry and scaffold fabricated by digital light processing. J. Manuf. Process. 2023, 109, 460–470. [Google Scholar] [CrossRef]
- ASTM C326-09(2018); Standard Test Method for Drying and Firing Shrinkages of Ceramic Whiteware Clays. ASTM International: West Conshohocken, PA, USA, 1997.
- Esteves, A.V.; Martins, M.I.; Soares, P.; Rodrigues, M.; Lopes, M.; Santos, J. Additive manufacturing of ceramic alumina/calcium phosphate structures by DLP 3D printing. Mater. Chem. Phys. 2022, 276, 125417. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Salehi, R.; Fathi, M.; Karbasi, S.; Pieles, U.; Daniels, A. Preparation, chemistry and physical properties of bone-derived hydroxyapatite particles having a negative zeta potential. Mater. Chem. Phys. 2012, 132, 446–452. [Google Scholar] [CrossRef]
- Jun, M.-J.; Kang, J.-H.; Sakthiabirami, K.; Toopghara, S.A.H.; Kim, Y.-S.; Yun, K.-D.; Park, S.-W. The Impact of Particle Size and Surface Treatment of Zirconia Suspension for Photocuring Additive Manufacturing. Materials 2023, 16, 1670. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, J.; Yu, F.; Zhang, W.; Meng, X.; Yang, N.; Liu, S. A novel fabrication of yttria-stabilized-zirconia dense electrolyte for solid oxide fuel cells by 3D printing technique. Int. J. Hydrogen Energy 2019, 44, 6182–6191. [Google Scholar] [CrossRef]
- Scalera, F.; Corcione, C.E.; Montagna, F.; Sannino, A.; Maffezzoli, A. Development and characterization of UV curable epoxy/hydroxyapatite suspensions for stereolithography applied to bone tissue engineering. Ceram. Int. 2014, 40, 15455–15462. [Google Scholar] [CrossRef]
- Muralithran, G.; Ramesh, S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Int. 2000, 26, 221–230. [Google Scholar] [CrossRef]
- Thomas-Vielma, P.; Cervera, A.; Levenfeld, B.; Várez, A. Production of alumina parts by powder injection molding with a binder system based on high density polyethylene. J. Eur. Ceram. Soc. 2008, 28, 763–771. [Google Scholar] [CrossRef]
- He, R.; Liu, W.; Wu, Z.; An, D.; Huang, M.; Wu, H.; Jiang, Q.; Ji, X.; Wu, S.; Xie, Z. Fabrication of complex-shaped zirconia ceramic parts via a DLP- stereolithography-based 3D printing method. Ceram. Int. 2018, 44, 3412–3416. [Google Scholar] [CrossRef]
- Zakeri, S.; Vippola, M.; Levänen, E. A comprehensive review of the photopolymerization of ceramic resins used in stereolithography. Addit. Manuf. 2020, 35, 101177. [Google Scholar] [CrossRef]
- Sim, J.-H.; Koo, B.-K.; Jung, M.; Kim, D.-S. Study on Debinding and Sintering Processes for Ceramics Fabricated Using Digital Light Processing (DLP) 3D Printing. Processes 2022, 10, 2467. [Google Scholar] [CrossRef]
- Santoliquido, O.; Colombo, P.; Ortona, A. Additive Manufacturing of ceramic components by Digital Light Processing: A comparison between the “bottom-up” and the “top-down” approaches. J. Eur. Ceram. Soc. 2019, 39, 2140–2148. [Google Scholar] [CrossRef]
- Sun, J.; Binner, J.; Bai, J. Effect of surface treatment on the dispersion of nano zirconia particles in non-aqueous suspensions for stereolithography. J. Eur. Ceram. Soc. 2019, 39, 1660–1667. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, C.; Wang, G.; He, R.; Ding, G.; Wang, M.; Dai, D.; Fang, D. High solid loading, low viscosity photosensitive Al2O3 slurry for stereolithography based additive manufacturing. Ceram. Int. 2019, 45, 203–208. [Google Scholar] [CrossRef]
- Zhang, K.Q.; Meng, Q.Y.; Zhang, X.Q.; Qu, Z.L.; Jing, S.K.; He, R.J. Roles of solid loading in stereolithography additive manufacturing of ZrO2 ceramic. Int. J. Refract. Met. Hard Mater. 2021, 99, 105604. [Google Scholar] [CrossRef]
- Zhang, S.; Sha, N.; Zhao, Z. Surface modification of α-Al2O3 with dicarboxylic acids for the preparation of UV-curable ceramic suspensions. J. Eur. Ceram. Soc. 2017, 37, 1607–1616. [Google Scholar] [CrossRef]
- Borlaf, M.; Serra-Capdevila, A.; Colominas, C.; Graule, T. Development of UV-curable ZrO2 slurries for additive manufacturing (LCM-DLP) technology. J. Eur. Ceram. Soc. 2019, 39, 3797–3803. [Google Scholar] [CrossRef]
- Halloran, J.W.; Tomeckova, V.; Gentry, S.; Das, S.; Cilino, P.; Yuan, D.; Guo, R.; Rudraraju, A.; Shao, P.; Wu, T. Photopolymerization of powder suspensions for shaping ceramics. J. Eur. Ceram. Soc. 2011, 31, 2613–2619. [Google Scholar] [CrossRef]
- Jacobs, P.F. Rapid Prototyping & Manufacturing: Fundamentals of Stereolithography; Society of Manufacturing Engineers: Southfield, MI, USA, 1992. [Google Scholar]
- Ge, M.; Xie, D.; Yang, Y.; Tian, Z. Sintering densification mechanism and mechanical properties of the 3D-printed high-melting-point-difference magnesium oxide/calcium phosphate composite bio-ceramic scaffold. J. Mech. Behav. Biomed. Mater. 2023, 144, 105978. [Google Scholar] [CrossRef]
- Sader, M.S.; LeGeros, R.Z.; Soares, G.A. Human osteoblasts adhesion and proliferation on magnesium-substituted tricalcium phosphate dense tablets. J. Mater. Sci. Mater. Med. 2008, 20, 521–527. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, E.; Pan, Y.-H.; Sun, Y.-S.; Hsueh, H.-W.; Dorj, O.; Yao, W.-L.; Lin, J.C.-Y.; Teng, N.-C.; Watanabe, I.; Abe, S.; et al. Magnesium Modified β-Tricalcium Phosphate Induces Cell Osteogenic Differentiation In Vitro and Bone Regeneration In Vivo. Int. J. Mol. Sci. 2022, 23, 1717. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, H.; Wei, J.; Jiang, X.; Hua, H.; Chen, F.; Wei, S.; Shin, J.W.; Liu, C. Development of magnesium calcium phosphate biocement for bone regeneration. J. R. Soc. Interface 2010, 7, 1171–1180. [Google Scholar] [CrossRef] [PubMed]



| Group | MCP (vol%) (Mg:CP) | Resin (vol%) | HDDA (vol%) | Photoinitiator (vol%) | Dispersant (vol%) | Total (vol%) |
|---|---|---|---|---|---|---|
| MP | 45% (4:0) | 8.52% | 37.30% | 0.04% | 9.14% | 100% |
| MCP3/1 | 45% (3:1) | 8.36% | 36.62% | 0.04% | 9.98% | 100% |
| MCP1/1 | 45% (1:1) | 8.19% | 35.86% | 0.04% | 10.91% | 100% |
| MCP1/3 | 45% (1:3) | 8.00% | 35.02% | 0.04% | 11.94% | 100% |
| CP | 45% (0:4) | 7.78% | 34.09% | 0.04% | 13.09% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhang, M.; Jung, Y.-N.; Choi, S.-W.; Lee, Y.-S.; Hwang, G.; Yun, K.-D. Evaluation of the Characteristics of Digital Light Processing 3D-Printed Magnesium Calcium Phosphate for Bone Regeneration. J. Funct. Biomater. 2025, 16, 139. https://doi.org/10.3390/jfb16040139
Zhang P, Zhang M, Jung Y-N, Choi S-W, Lee Y-S, Hwang G, Yun K-D. Evaluation of the Characteristics of Digital Light Processing 3D-Printed Magnesium Calcium Phosphate for Bone Regeneration. Journal of Functional Biomaterials. 2025; 16(4):139. https://doi.org/10.3390/jfb16040139
Chicago/Turabian StyleZhang, Peng, Meiling Zhang, Yoo-Na Jung, Seong-Won Choi, Yong-Seok Lee, Geelsu Hwang, and Kwi-Dug Yun. 2025. "Evaluation of the Characteristics of Digital Light Processing 3D-Printed Magnesium Calcium Phosphate for Bone Regeneration" Journal of Functional Biomaterials 16, no. 4: 139. https://doi.org/10.3390/jfb16040139
APA StyleZhang, P., Zhang, M., Jung, Y.-N., Choi, S.-W., Lee, Y.-S., Hwang, G., & Yun, K.-D. (2025). Evaluation of the Characteristics of Digital Light Processing 3D-Printed Magnesium Calcium Phosphate for Bone Regeneration. Journal of Functional Biomaterials, 16(4), 139. https://doi.org/10.3390/jfb16040139







