In Vitro Bacterial Growth on Titanium Surfaces Treated with Nanosized Hydroxyapatite
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Preparation
2.2. Surface Characterization
2.3. X-Ray Diffraction Analysis
2.4. Calcium Assay
2.5. Bacterial Strains and Culture
2.6. Biofilm Assay
2.7. Confocal Microscopy
2.8. Statistical Analysis
3. Results
3.1. Surface Characterization
3.2. Confocal Laser Scanning Microscopy
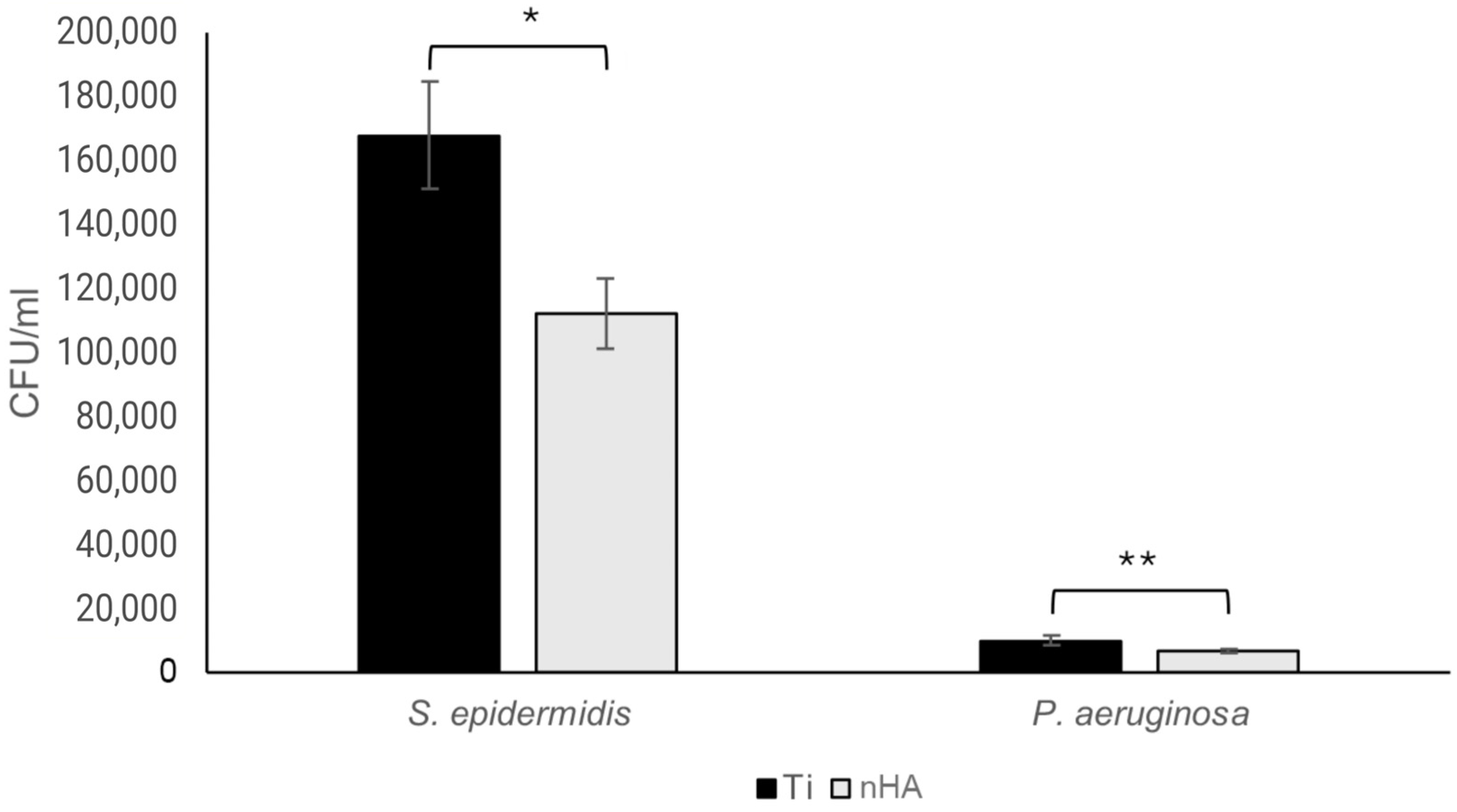
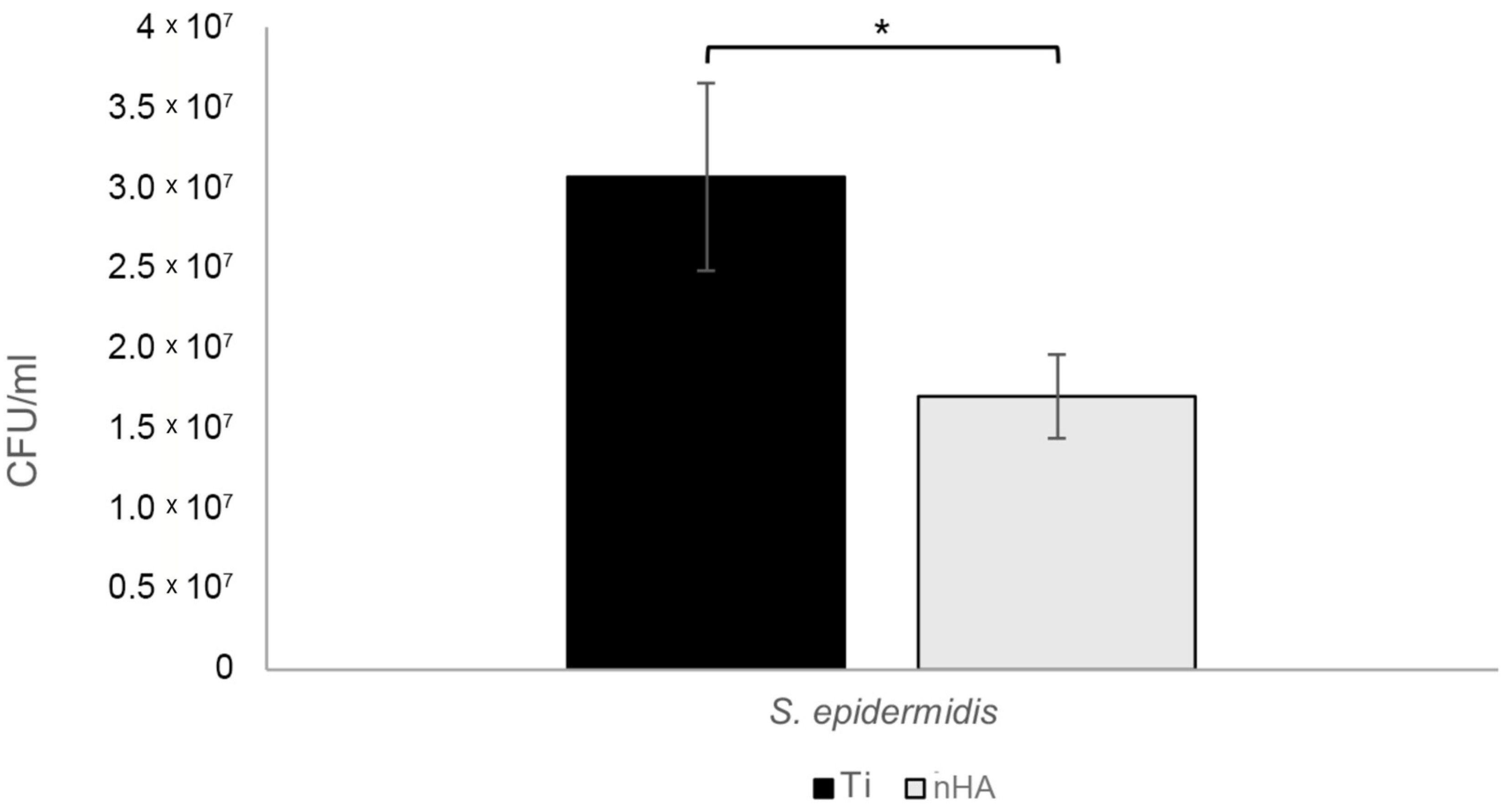
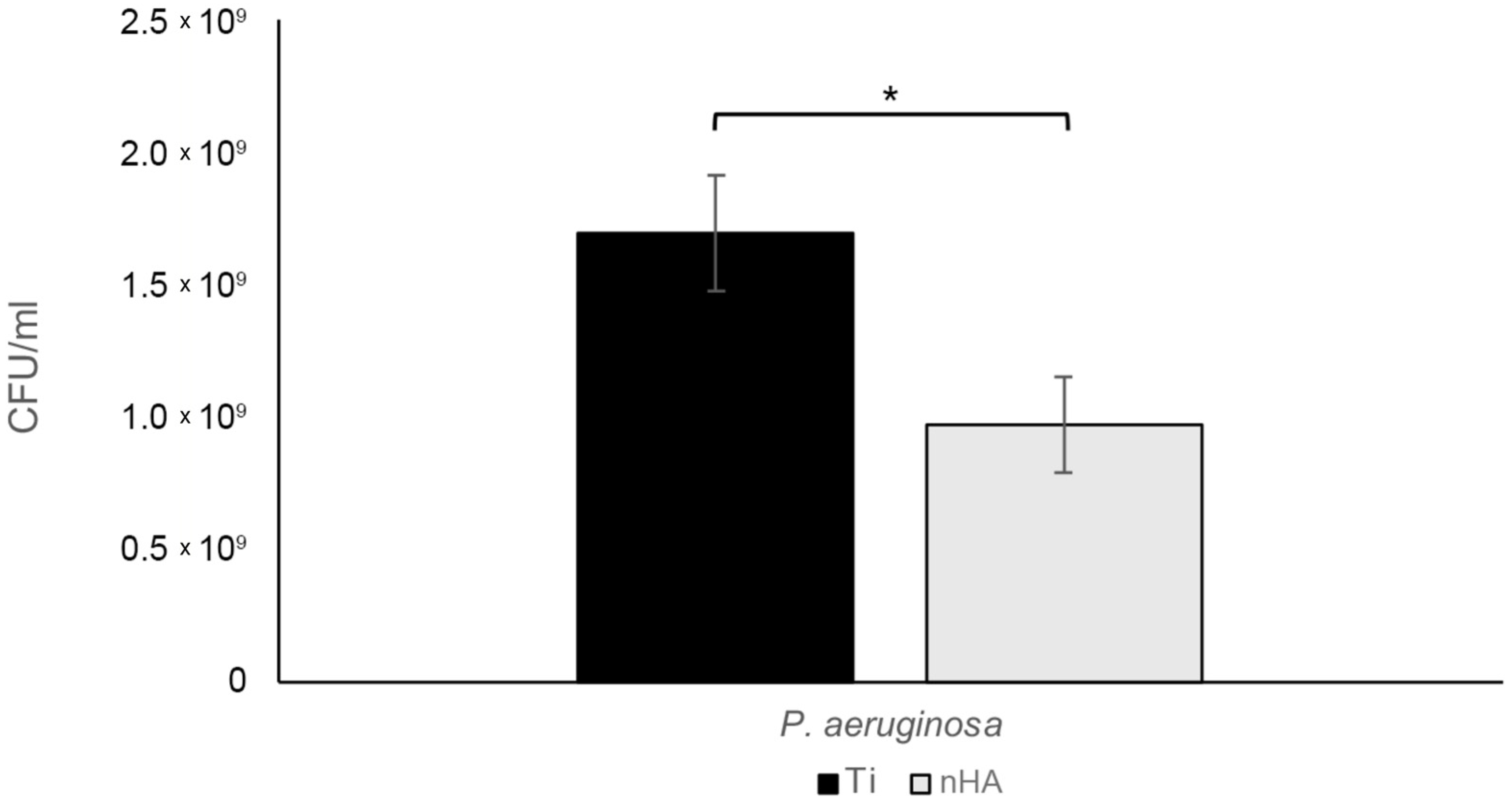
3.3. Biofilm Assay
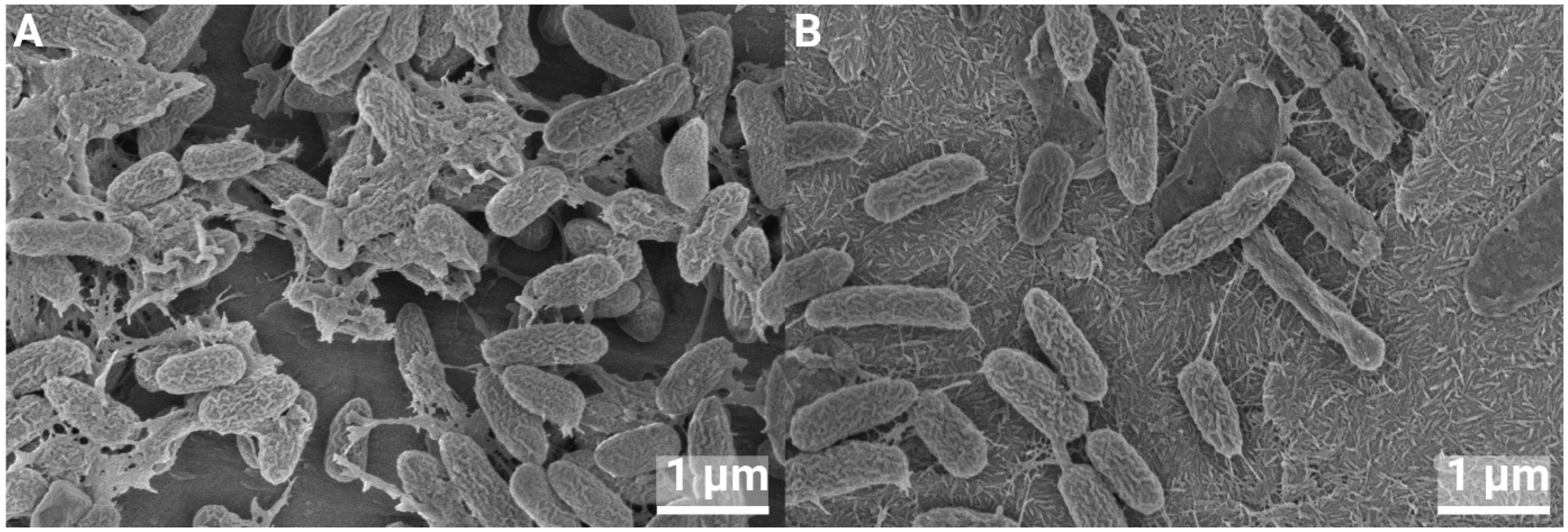
3.4. SEM of Biofilms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, V.K.; Rasouli, M.R.; Parvizi, J. Periprosthetic joint infection: Current concept. Indian J. Orthop. 2013, 47, 10–17. [Google Scholar] [CrossRef]
- Choi, S.W.; Lee, J.C.; Lee, W.S.; Hwang, J.Y.; Baek, M.J.; Choi, Y.S.; Jang, H.D.; Shin, B.J. Clinical differences between delayed and acute onset postoperative spinal infection. Medicine 2022, 101, e29366. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.E.; Shufflebarger, H.L. Late-developing infection in instrumented idiopathic scoliosis. Spine 1999, 24, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Parchi, P.D.; Evangelisti, G.; Andreani, L.; Girardi, F.; Darren, L.; Sama, A.; Lisanti, M. Postoperative Spine Infections. Orthop. Rev. 2015, 7, 5900. [Google Scholar] [CrossRef]
- Köder, K.; Hardt, S.; Gellert, M.S.; Haupenthal, J.; Renz, N.; Putzier, M.; Perka, C.; Trampuz, A. Outcome of spinal implant-associated infections treated with or without biofilm-active antibiotics: Results from a 10-year cohort study. Infection 2020, 48, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Dapunt, U.; Bürkle, C.; Günther, F.; Pepke, W.; Hemmer, S.; Akbar, M. Surgical site infections following instrumented stabilization of the spine. Ther. Clin. Risk Manag. 2017, 13, 1239–1245. [Google Scholar] [CrossRef][Green Version]
- Guo, G.; Wang, J.; You, Y.; Tan, J.; Shen, H. Distribution characteristics of Staphylococcus spp. in different phases of periprosthetic joint infection: A review. Exp. Ther. Med. 2017, 13, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, M.F.; Huddleston, P.M.; Piper, K.E.; Karau, M.J.; Dekutoski, M.B.; Yaszemski, M.J.; Currier, B.L.; Mandrekar, J.N.; Osmon, D.R.; McDowell, A.; et al. A biofilm approach to detect bacteria on removed spinal implants. Spine 2010, 35, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Elek, S.D.; Conen, P.E. The virulence of Staphylococcus pyogenes for man; a study of the problems of wound infection. Br. J. Exp. Pathol. 1957, 38, 573–586. [Google Scholar]
- Zimmerli, W.; Waldvogel, F.A.; Vaudaux, P.; Nydegger, U.E. Pathogenesis of foreign body infection: Description and characteristics of an animal model. J. Infect. Dis. 1982, 146, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Poelstra, K.A.; Barekzi, N.A.; Grainger, D.W.; Gristina, A.G.; Schuler, T.C. A novel spinal implant infection model in rabbits. Spine 2000, 25, 406–410. [Google Scholar] [CrossRef]
- Laratta, J.L.; Shillingford, J.N.; Hardy, N.; Lehman, R.A.; Lenke, L.G.; Riew, K.D. A Dose-Response Curve for a Gram-Negative Spinal Implant Infection Model in Rabbits. Spine 2017, 42, E1225–E1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Zheng, Z.; Liu, J.; Ji, X.; Sun, Y.; Xin, J.; Gong, W.; Na, S.; Jin, Y.; et al. Animal Models for Postoperative Implant-Related Spinal Infection. Orthop. Surg. 2022, 14, 1049–1058. [Google Scholar] [CrossRef]
- Alomar, A.Z.; Alfayez, S.M.; Binnasser, A.; Aljassir, F.F. Intraoperative evaluation and level of contamination during total knee arthroplasty. Acta Ortop. Bras. 2022, 30, e243232. [Google Scholar] [CrossRef]
- Khajanchi, B.K.; Kirtley, M.L.; Brackman, S.M.; Chopra, A.K. Immunomodulatory and protective roles of quorum-sensing signaling molecules N-acyl homoserine lactones during infection of mice with Aeromonas hydrophila. Infect. Immun. 2011, 79, 2646–2657. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.L.; Angle, A.; Kielian, T. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS ONE 2012, 7, e42476. [Google Scholar] [CrossRef] [PubMed]
- Kristian, S.A.; Birkenstock, T.A.; Sauder, U.; Mack, D.; Götz, F.; Landmann, R. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. J. Infect. Dis. 2008, 197, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Belgiovine, C.; Pellegrino, L.; Bulgarelli, A.; Lauta, F.C.; Di Claudio, A.; Ciceri, R.; Cancellara, A.; Calcaterra, F.; Mavilio, D.; Grappiolo, G.; et al. Interaction of Bacteria, Immune Cells, and Surface Topography in Periprosthetic Joint Infections. Int. J. Mol. Sci. 2023, 24, 9028. [Google Scholar] [CrossRef] [PubMed]
- Tucci, G.; Romanini, E.; Zanoli, G.; Pavan, L.; Fantoni, M.; Venditti, M. Prevention of surgical site infections in orthopaedic surgery: A synthesis of current recommendations. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 224–239. [Google Scholar] [CrossRef]
- Milleret, V.; Lienemann, P.S.; Gasser, A.; Bauer, S.; Ehrbar, M.; Wennerberg, A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. S1), 15–24. [Google Scholar] [CrossRef]
- Jeong, W.S.; Kwon, J.S.; Lee, J.H.; Uhm, S.H.; Ha Choi, E.; Kim, K.M. Bacterial attachment on titanium surfaces is dependent on topography and chemical changes induced by nonthermal atmospheric pressure plasma. Biomed. Mater. 2017, 12, 045015. [Google Scholar] [CrossRef]
- Skovdal, S.M.; Jørgensen, N.P.; Petersen, E.; Jensen-Fangel, S.; Ogaki, R.; Zeng, G.; Johansen, M.I.; Wang, M.; Rohde, H.; Meyer, R.L. Ultra-dense polymer brush coating reduces Staphylococcus epidermidis biofilms on medical implants and improves antibiotic treatment outcome. Acta Biomater. 2018, 76, 46–55. [Google Scholar] [CrossRef]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Savarino, L.; Fini, M.; Ciapetti, G.; Cenni, E.; Granchi, D.; Baldini, N.; Greco, M.; Rizzi, G.; Giardino, R.; Giunti, A. Biologic effects of surface roughness and fluorhydroxyapatite coating on osteointegration in external fixation systems: An in vivo experimental study. J. Biomed. Mater. Res. A 2003, 66A, 652–661. [Google Scholar] [CrossRef]
- Scheeren Brum, R.; Apaza-Bedoya, K.; Labes, L.G.; Volpato, C.Â.M.; Pimenta, A.L.; Benfatti, C.A.M. Early Biofilm Formation on Rough and Smooth Titanium Specimens: A Systematic Review of Clinical Studies. J. Oral Maxillofac. Res. 2021, 12, e1. [Google Scholar] [CrossRef] [PubMed]
- Saulacic, N.; Schaller, B. Prevalence of Peri-Implantitis in Implants with Turned and Rough Surfaces: A Systematic Review. J. Oral Maxillofac. Res. 2019, 10, e1. [Google Scholar] [CrossRef] [PubMed]
- Zeller, B.; Stöckli, S.; Zaugg, L.K.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Waltimo, T.; Zitzmann, N.U. Biofilm formation on metal alloys, zirconia and polyetherketoneketone as implant materials in vivo. Clin. Oral Implant. Res. 2020, 31, 1078–1086. [Google Scholar] [CrossRef]
- Meier, D.; Astasov-Frauenhoffer, M.; Waltimo, T.; Zaugg, L.K.; Rohr, N.; Zitzmann, N.U. Biofilm formation on metal alloys and coatings, zirconia, and hydroxyapatite as implant materials in vivo. Clin. Oral Implant. Res. 2023, 34, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Park, H.Y.; Dworsky, E.; Zoller, S.D.; Xi, W.; Johansen, D.O.; Loftin, A.H.; Hamad, C.D.; Segura, T.; Bernthal, N.M. The Use of a Novel Antimicrobial Implant Coating In Vivo to Prevent Spinal Implant Infection. Spine 2020, 45, E305–E311. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef]
- Asri, L.A.T.W.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; van der Mei, H.C.; Loontjens, T.J.A.; Busscher, H.J. A Shape-Adaptive, Antibacterial-Coating of Immobilized Quaternary-Ammonium Compounds Tethered on Hyperbranched Polyurea and its Mechanism of Action. Adv. Funct. Mater. 2014, 24, 346–355. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Chinnaraj, S.; Jana, S.; Bowen, L.; Charlton, S.; Duan, P.; Jakubovics, N.S.; Chen, J. Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci. Rep. 2018, 8, 1071. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. S1), 4–7. [Google Scholar] [CrossRef]
- Voigt, J.D.; Mosier, M. Hydroxyapatite (HA) coating appears to be of benefit for implant durability of tibial components in primary total knee arthroplasty. Acta Orthop. 2011, 82, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Vidalain, J.P. Twenty-year results of the cementless Corail stem. Int. Orthop. 2011, 35, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Røkkum, M.; Reigstad, A.; Johansson, C.B. HA particles can be released from well-fixed HA-coated stems: Histopathology of biopsies from 20 hips 2–8 years after implantation. Acta Orthop. 2002, 73, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, S.; Lind, M.; Rahbek, O.; Bünger, C.; Søballe, K. Improved fixation of porous-coated versus grit-blasted surface texture of hydroxyapatite-coated implants in dogs. Acta Orthop. 1997, 68, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.; Oliveira, P.; Bergamo, E.; Kjellin, P.; Novaes, A., Jr.; Ghiraldini, B.; Bezerra, F.; Scombatti de Souza, S. Effect of Smoke Exposure on Gene Expression in Bone Healing around Implants Coated with Nanohydroxyapatite. Nanomaterials 2022, 12, 3737. [Google Scholar] [CrossRef]
- Adam, M.; Ganz, C.; Xu, W.; Sarajian, H.R.; Götz, W.; Gerber, T. In vivo and in vitro investigations of a nanostructured coating material—A preclinical study. Int. J. Nanomed. 2014, 9, 975–984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johansson, P.; Jimbo, R.; Kozai, Y.; Sakurai, T.; Kjellin, P.; Currie, F.; Wennerberg, A. Nanosized Hydroxyapatite Coating on PEEK Implants Enhances Early Bone Formation: A Histological and Three-Dimensional Investigation in Rabbit Bone. Materials 2015, 8, 3815–3830. [Google Scholar] [CrossRef]
- Almeida, D.; Sartoretto, S.C.; Calasans-Maia, J.A.; Ghiraldini, B.; Bezerra, F.J.B.; Granjeiro, J.M.; Calasans-Maia, M.D. In vivo osseointegration evaluation of implants coated with nanostructured hydroxyapatite in low density bone. PLoS ONE 2023, 18, e0282067. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, E.T.P.; de Oliveira, P.; Campos, T.M.B.; Bonfante, E.A.; Tovar, N.; Boczar, D.; Nayak, V.V.; Coelho, P.G.; Witek, L. Osseointegration of implant surfaces in metabolic syndrome and type-2 diabetes mellitus. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35382. [Google Scholar] [CrossRef]
- ISO 25178-604:2025; Geometrical Product Specifications (GPS)—Surface Texture: Areal. Part 604: Nominal Characteristics of Non-Contact (Coherence Scanning Interferometry) Instruments. ISO: Geneva, Switzerland, 2025.
- ASTM D1193-99e1; Standard Specification for Reagent Water. ASTM: West Conshohocken, PA, USA, 2017.
- Johansson, P.; Jimbo, R.; Naito, Y.; Kjellin, P.; Currie, F.; Wennerberg, A. Polyether ether ketone implants achieve increased bone fusion when coated with nano-sized hydroxyapatite: A histomorphometric study in rabbit bone. Int. J. Nanomed. 2016, 11, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 172–184. [Google Scholar] [CrossRef] [PubMed]
- Moris, V.; Lam, M.; Amoureux, L.; Magallon, A.; Guilloteau, A.; Maldiney, T.; Zwetyenga, N.; Falentin-Daudre, C.; Neuwirth, C. What is the best technic to dislodge Staphylococcus epidermidis biofilm on medical implants? BMC Microbiol. 2022, 22, 192. [Google Scholar] [CrossRef]
- Karbysheva, S.; Di Luca, M.; Butini, M.E.; Winkler, T.; Schütz, M.; Trampuz, A. Comparison of sonication with chemical biofilm dislodgement methods using chelating and reducing agents: Implications for the microbiological diagnosis of implant associated infection. PLoS ONE 2020, 15, e0231389. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Alves, A.T.; Resende, R.F.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early osseointegration driven by the surface chemistry and wettability of dental implants. J. Appl. Oral Sci. 2015, 23, 279–287. [Google Scholar] [CrossRef]
- Guerreiro-Tanomaru, J.M.; Vázquez-García, F.A.; Bosso-Martelo, R.; Bernardi, M.I.; Faria, G.; Tanomaru, M.F. Effect of addition of nano-hydroxyapatite on physico-chemical and antibiofilm properties of calcium silicate cements. J. Appl. Oral Sci. 2016, 24, 204–210. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef]
- Ragab, H.; Ibrahim, F.; Abdallah, F.; Al-Ghamdi, A.; El-Tantawy, P.F.; Yakuphanoglu, F. Synthesis and In Vitro Antibacterial Properties of Hydroxyapatite Nanoparticles. IOSR J. Pharm. Biol. Sci. 2014, 9, 77–85. [Google Scholar] [CrossRef]
- Park, M.; Sutherland, J.B.; Rafii, F. Effects of nano-hydroxyapatite on the formation of biofilms by Streptococcus mutans in two different media. Arch. Oral Biol. 2019, 107, 104484. [Google Scholar] [CrossRef]
- Grenho, L.; Manso, M.C.; Monteiro, F.J.; Ferraz, M.P. Adhesion of Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa onto nanohydroxyapatite as a bone regeneration material. J. Biomed. Mater. Res. A 2012, 100, 1823–1830. [Google Scholar] [CrossRef]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.P.; Huang, J.; Pratten, J.; Spratt, D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Rybak, Z.; Pajączkowska, M.; Nowicka, J.; Szymonowicz, M.; Rusak, A.; Wiglusz, R.J.; Szyszka, K.; Chmielowiec, J.; Chodaczek, G.; et al. Antimicrobial Properties and Cytotoxic Effect Evaluation of Nanosized Hydroxyapatite and Fluorapatite Dedicated for Alveolar Bone Regeneration. Appl. Sci. 2024, 14, 7845. [Google Scholar] [CrossRef]
- Whyte, W.; Lidwell, O.M.; Lowbury, E.J.L.; Blowers, R. Suggested bacteriological standards for air in ultraclean operating rooms. J. Hosp. Infect. 1983, 4, 133–139. [Google Scholar] [CrossRef]
- Lidwell, O.M. Airborne bacteria and surgical infection. Am. J. Med. 1981, 70, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Stocks, G.W.; Self, S.D.; Thompson, B.; Adame, X.A.; O’Connor, D.P. Predicting bacterial populations based on airborne particulates: A study performed in nonlaminar flow operating rooms during joint arthroplasty surgery. Am. J. Infect. Control 2010, 38, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Guarch-Pérez, C.; Riool, M.; de Boer, L.; Kloen, P.; Zaat, S.A.J. Bacterial reservoir in deeper skin is a potential source for surgical site and biomaterial-associated infections. J. Hosp. Infect. 2023, 140, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Granato, R.; Bergamo, E.T.P.; Witek, L.; Bonfante, E.A.; Marin, C.; Greenberg, M.; Kurgansky, G.; Coelho, P.G. Clinical, histological, and nanomechanical parameters of implants placed in healthy and metabolically compromised patients. J. Dent. 2020, 100, 103436. [Google Scholar] [CrossRef]
- Baskar, K.; Anusuya, T.; Devanand Venkatasubbu, G. Mechanistic investigation on microbial toxicity of nano hydroxyapatite on implant associated pathogens. Mat. Sci. Eng. C 2017, 73, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Knabel, S.J.; Walker, H.W.; Hartman, P.A. Inhibition of Aspergillus flavus and Selected Gram-positive Bacteria by Chelation of Essential Metal Cations by Polyphosphates. J. Food Prot. 1991, 54, 360–365. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L. Calcium and Magnesium Ions Are Membrane-Active against Stationary-Phase Staphylococcus aureus with High Specificity. Sci. Rep. 2016, 6, 20628. [Google Scholar] [CrossRef]
- Shao, H.; Ma, M.; Wang, Q.; Yan, T.; Zhao, B.; Guo, S.; Tong, S. Advances in the superhydrophilicity-modified titanium surfaces with antibacterial and pro-osteogenesis properties: A review. Front. Bioeng. Biotechnol. 2022, 10, 1000401. [Google Scholar] [CrossRef] [PubMed]
- Kinnari, T.J.; Esteban, J.; Martin-de-Hijas, N.Z.; Sánchez-Muñoz, O.; Sánchez-Salcedo, S.; Colilla, M.; Vallet-Regí, M.; Gomez-Barrena, E. Influence of surface porosity and pH on bacterial adherence to hydroxyapatite and biphasic calcium phosphate bioceramics. J. Med. Microbiol. 2009, 58 Pt 1, 132–137. [Google Scholar] [CrossRef]



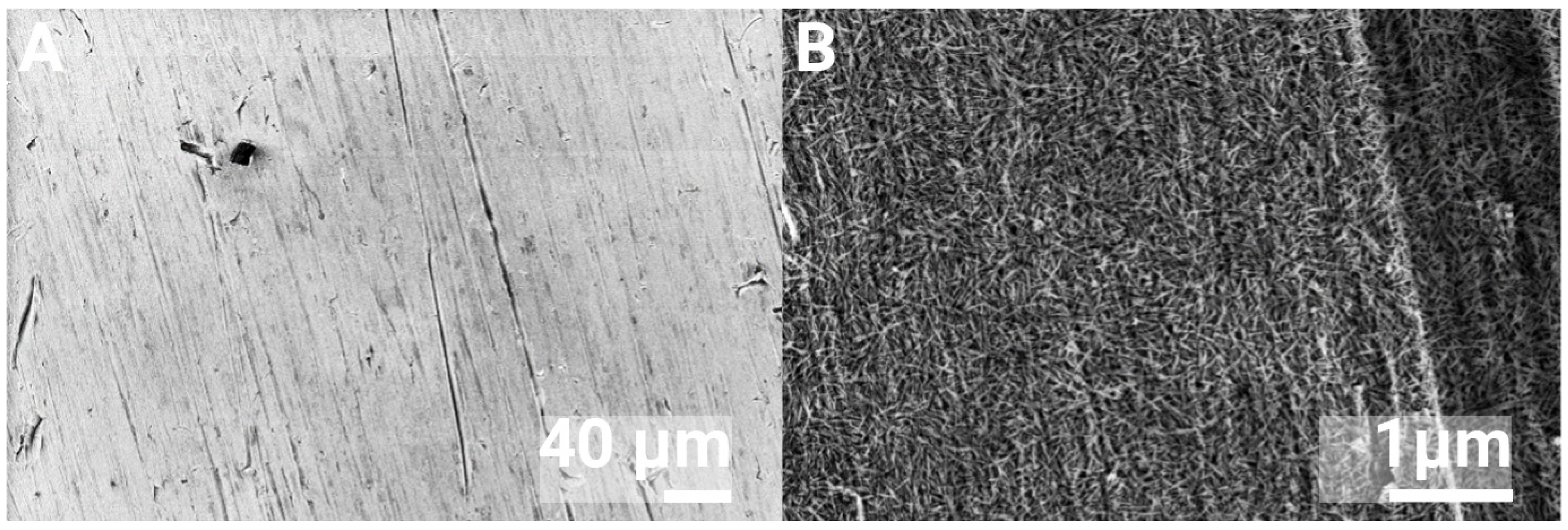
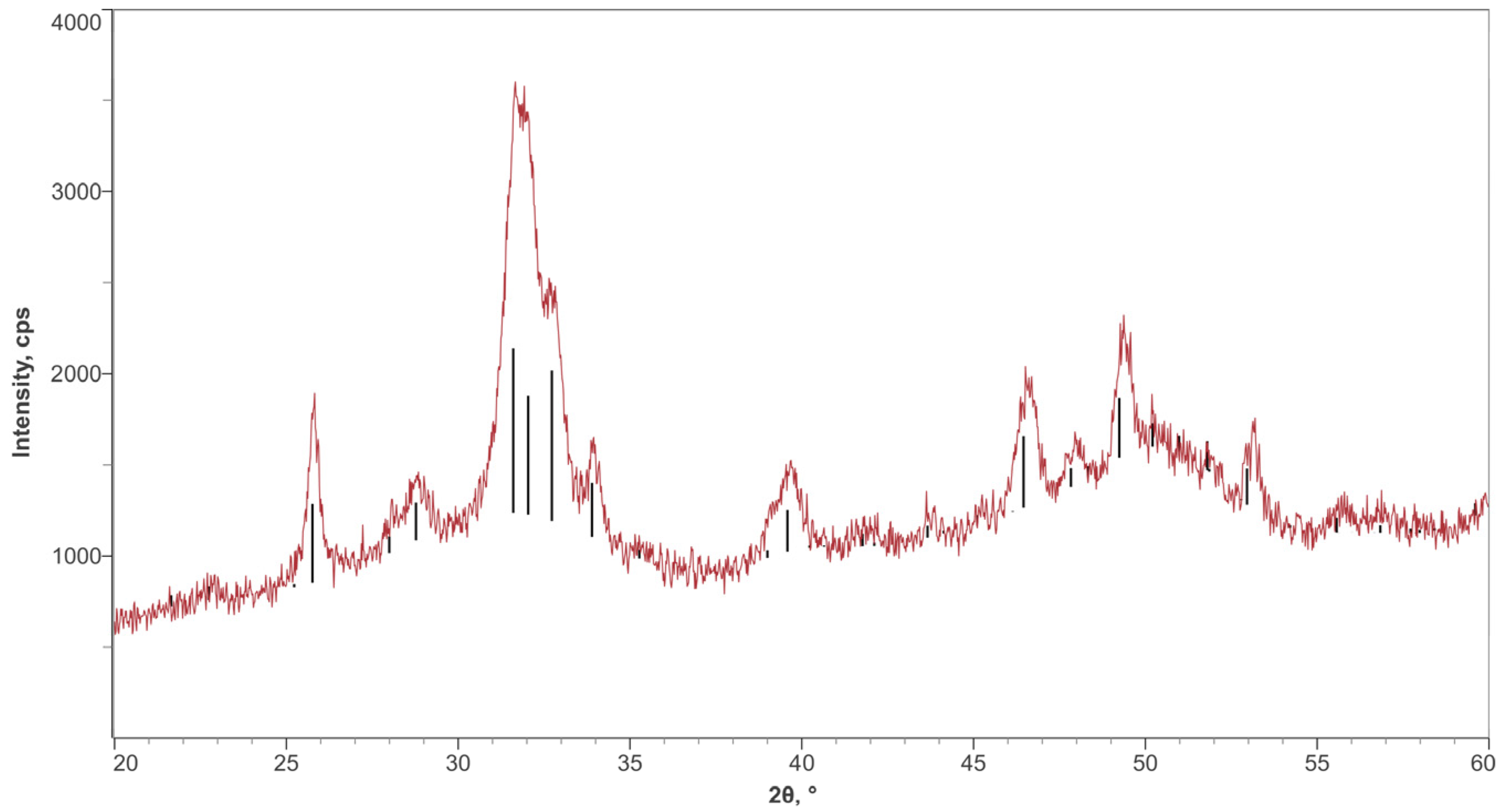
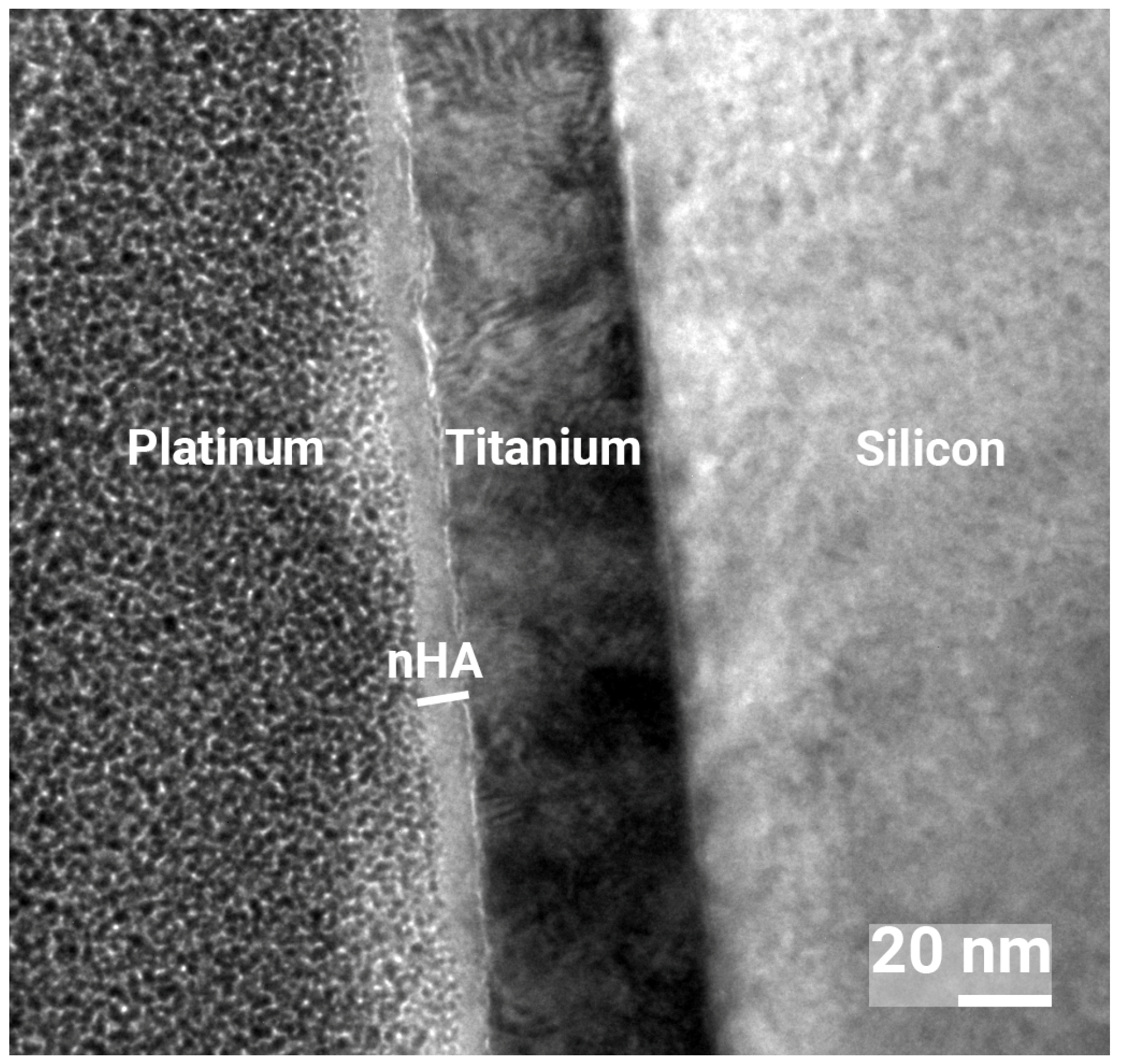
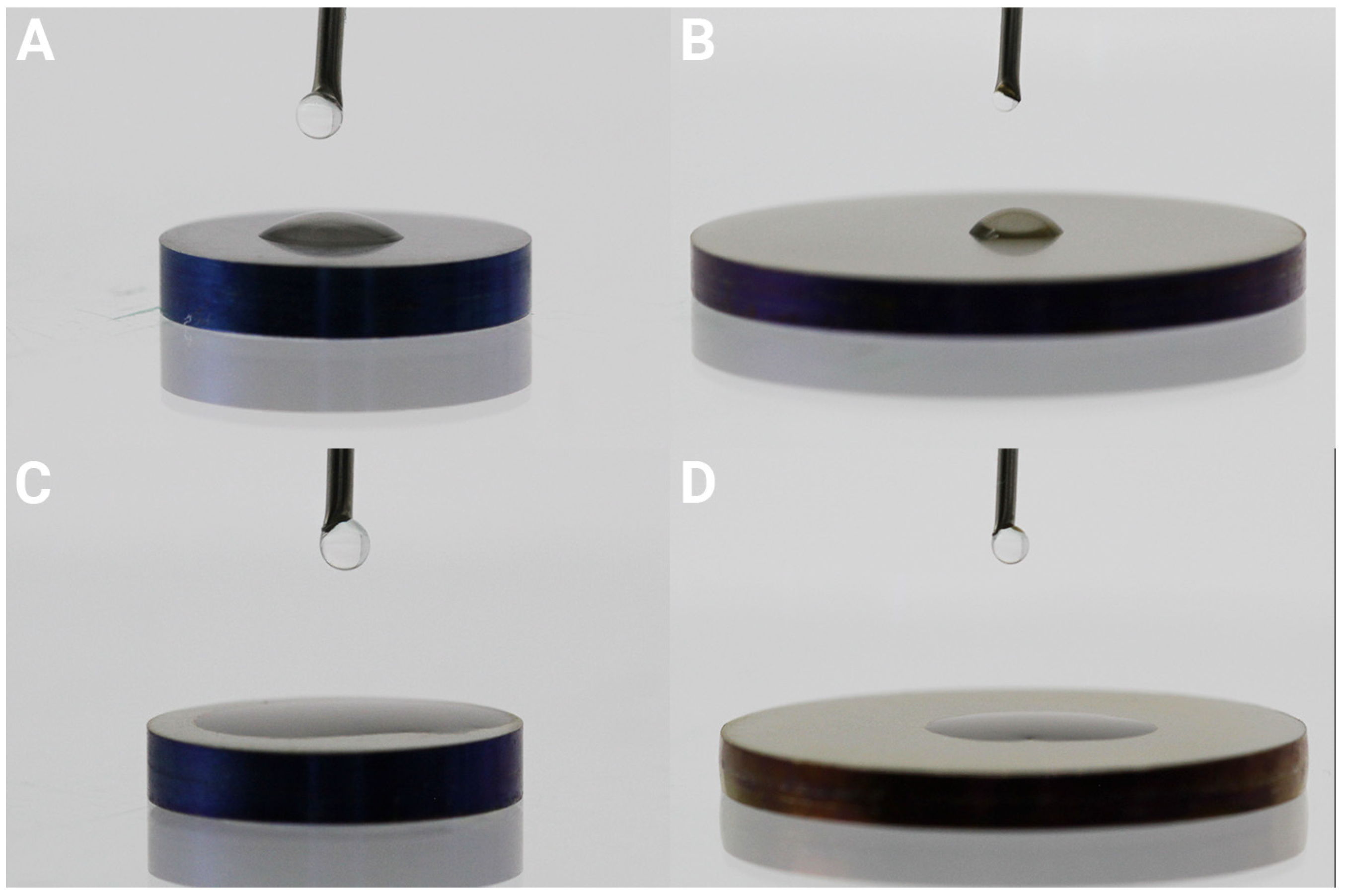
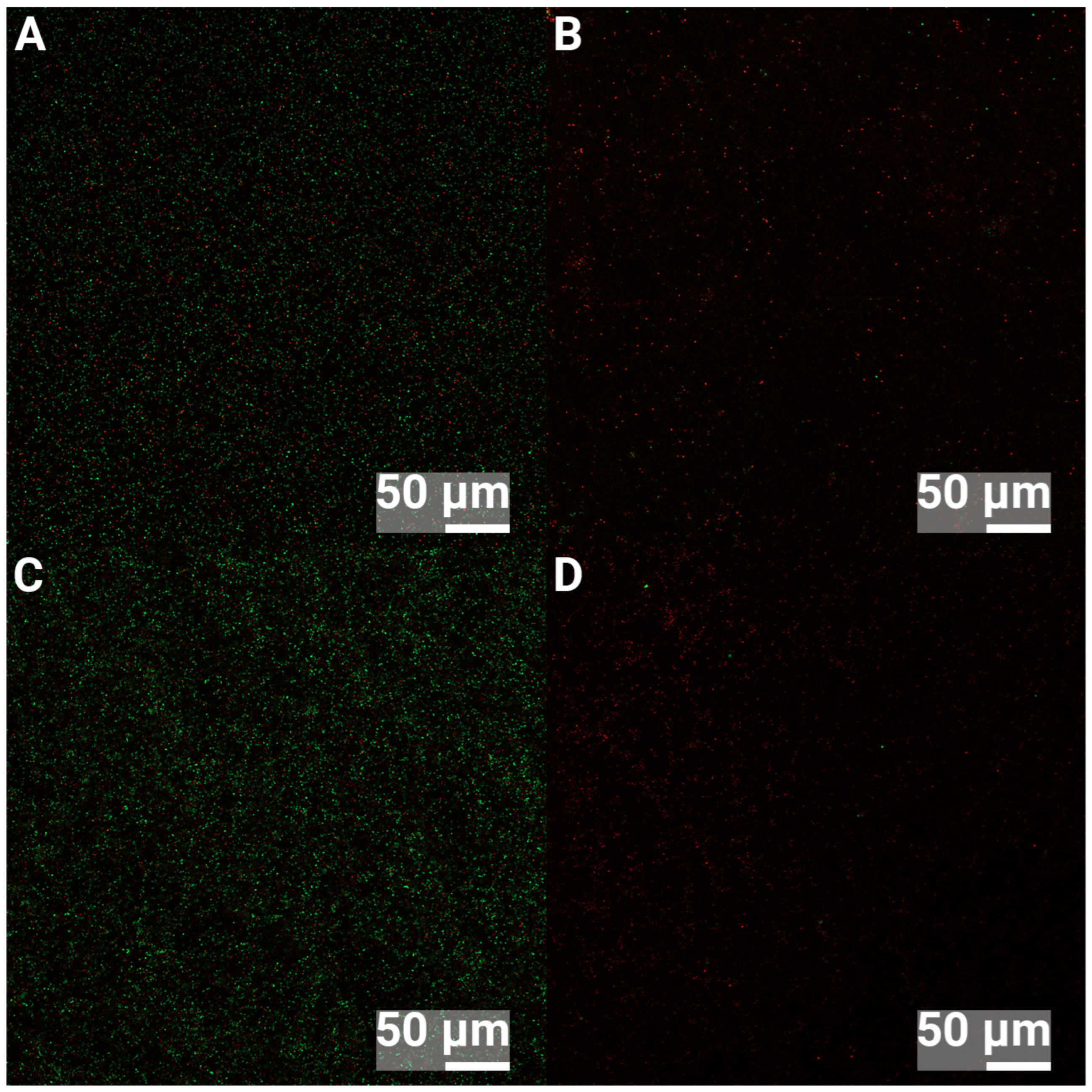
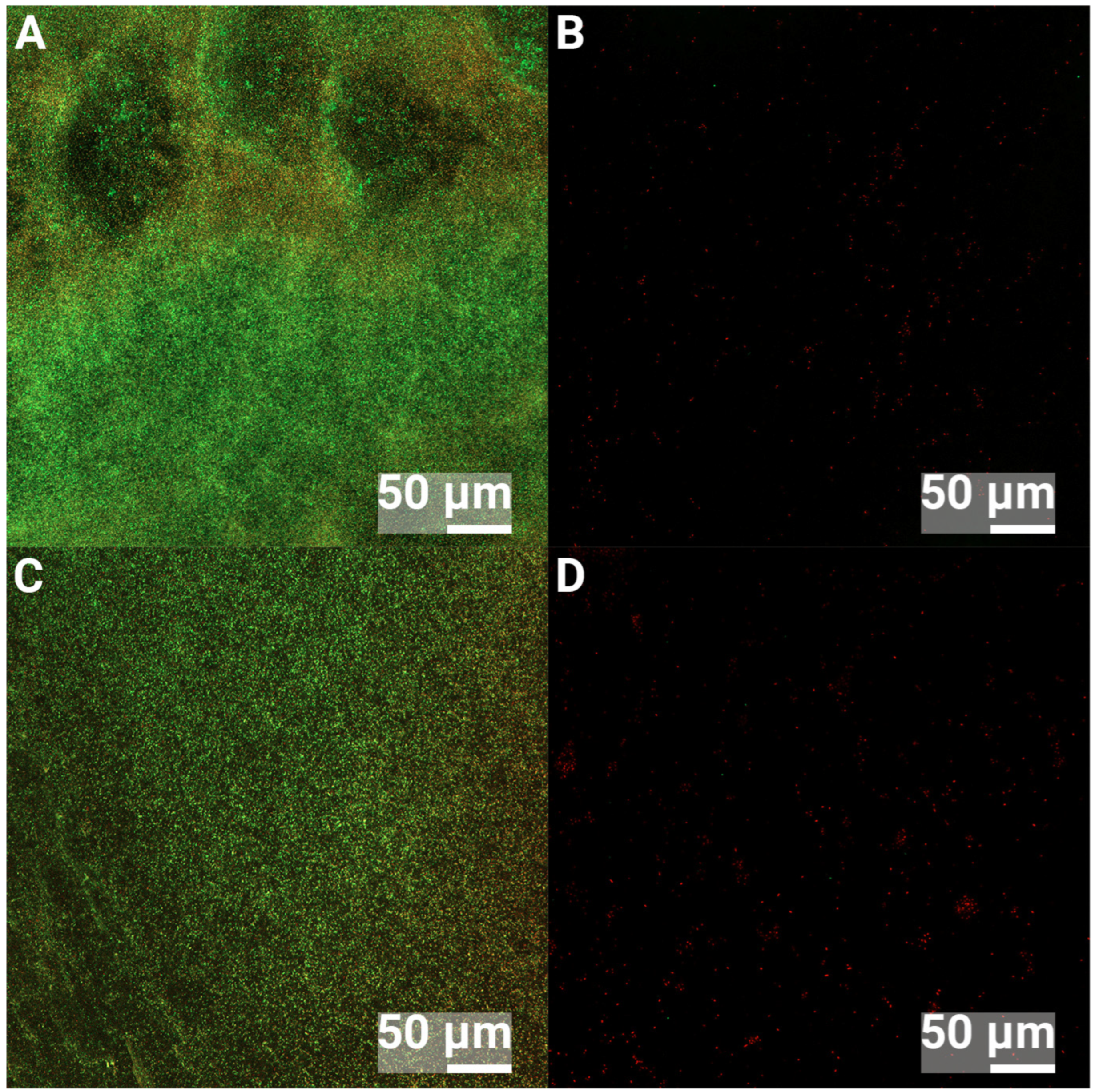

| Element | C | O | Ti | Ca | P |
|---|---|---|---|---|---|
| Ti grade 4 | 3.13 ± 0.23 | 12.90 ± 0.45 | 83.97 ± 0.61 | - | - |
| nHA, Ti grade 4 | 2.06 ± 0.25 | 28.09 ± 1.09 | 69.11 ± 1.58 | 0.42 ± 0.15 | 0.32 ± 0.13 |
| Ti grade 2 | 2.65 ± 0.13 | 25.61 ± 1.41 | 71.74 ± 1.46 | - | - |
| nHA, Ti grade 2 | 1.75 ± 0.19 | 30.09 ± 1.25 | 67.72 ± 1.28 | 0.25 ± 0.04 | 0.19 ± 0.05 |
| Substrate | Sa (µm) | Sdr (%) | Sds (1/µm2) |
|---|---|---|---|
| Ti grade 4 | 0.23 ± 0.04 | 6.20 ± 1.96 | 0.20 ± 0.02 |
| nHA, Ti grade 4 | 0.21 ± 0.05 | 5.29 ± 1.06 | 0.26 ± 0.03 |
| Ti grade 2 | 0.25 ± 0.04 | 7.51 ± 1.14 | 0.22 ± 0.01 |
| nHA, Ti grade 2 | 0.27 ± 0.05 | 7.10 ± 0.38 | 0.24 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmström, M.; Esko, S.; Danielsson, K.; Kjellin, P. In Vitro Bacterial Growth on Titanium Surfaces Treated with Nanosized Hydroxyapatite. J. Funct. Biomater. 2025, 16, 66. https://doi.org/10.3390/jfb16020066
Holmström M, Esko S, Danielsson K, Kjellin P. In Vitro Bacterial Growth on Titanium Surfaces Treated with Nanosized Hydroxyapatite. Journal of Functional Biomaterials. 2025; 16(2):66. https://doi.org/10.3390/jfb16020066
Chicago/Turabian StyleHolmström, Maria, Sonia Esko, Karin Danielsson, and Per Kjellin. 2025. "In Vitro Bacterial Growth on Titanium Surfaces Treated with Nanosized Hydroxyapatite" Journal of Functional Biomaterials 16, no. 2: 66. https://doi.org/10.3390/jfb16020066
APA StyleHolmström, M., Esko, S., Danielsson, K., & Kjellin, P. (2025). In Vitro Bacterial Growth on Titanium Surfaces Treated with Nanosized Hydroxyapatite. Journal of Functional Biomaterials, 16(2), 66. https://doi.org/10.3390/jfb16020066







