Laser-Induced Photothermal Hydrogels Promote the Proliferation of MC3T3-E1 Preosteoblasts for Enhanced Bone Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Synthesis of Alginate-CaCl2-AuNPs Hydrogel
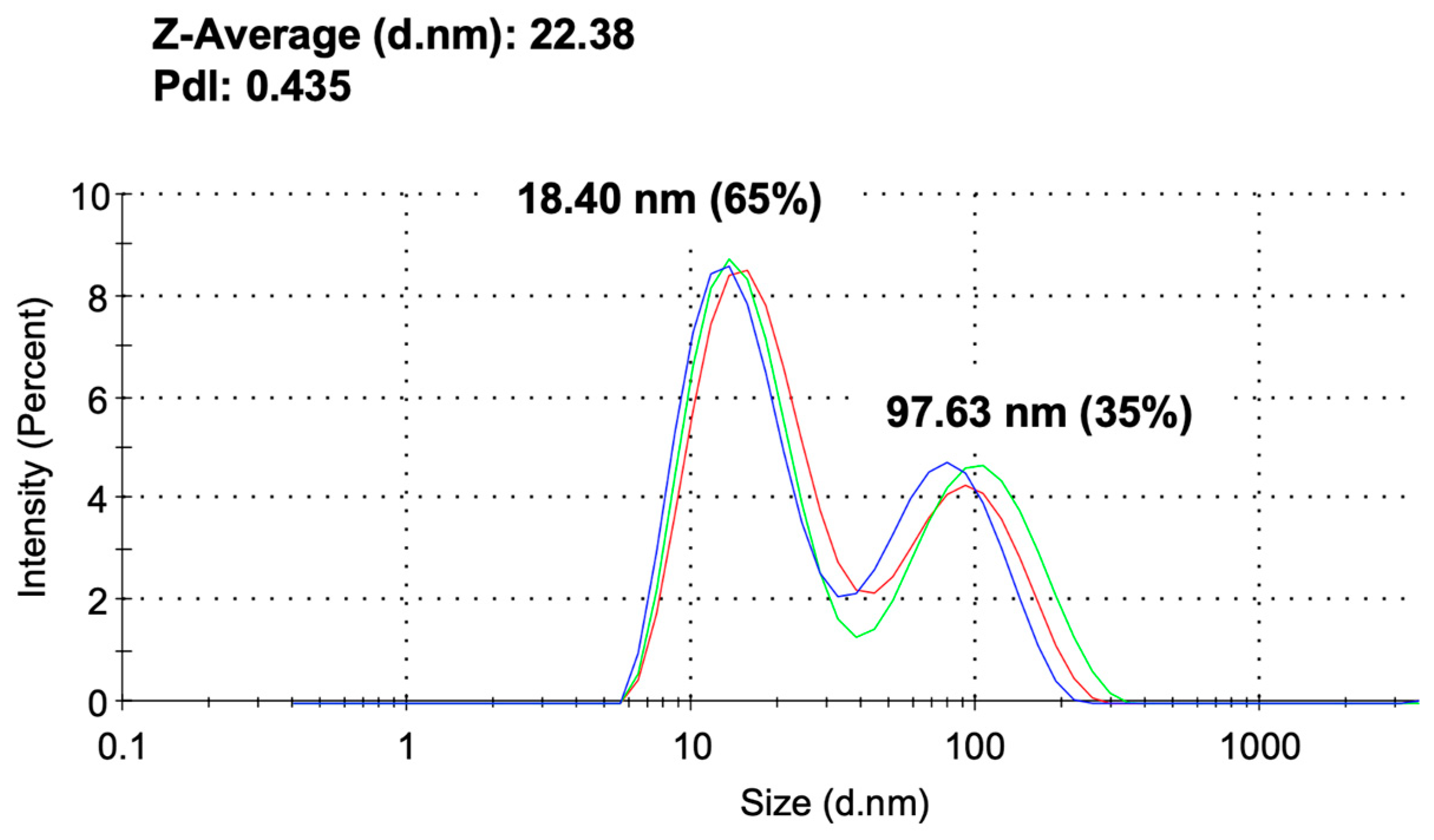
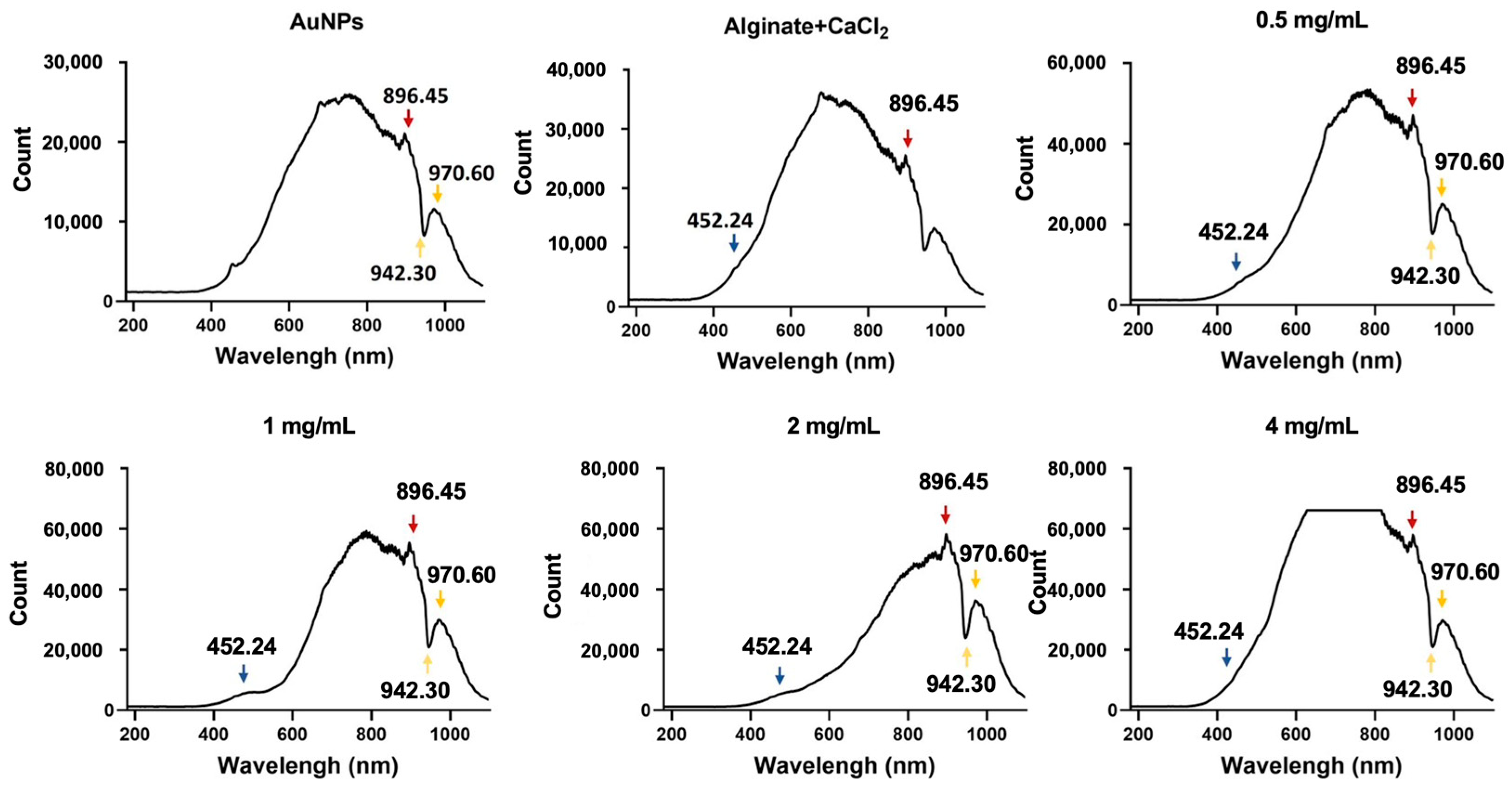
2.3. Analysis of Alginate-CaCl₂-AuNPs Hydrogel via DLS, FTIR, and UV-Vis-NIR
2.4. Laser-Induced Temperature Change in Alginate-CaCl2-AuNPs Hydrogel
2.5. Viscoelastic Behavior of Alginate-CaCl2-AuNPs Hydrogel
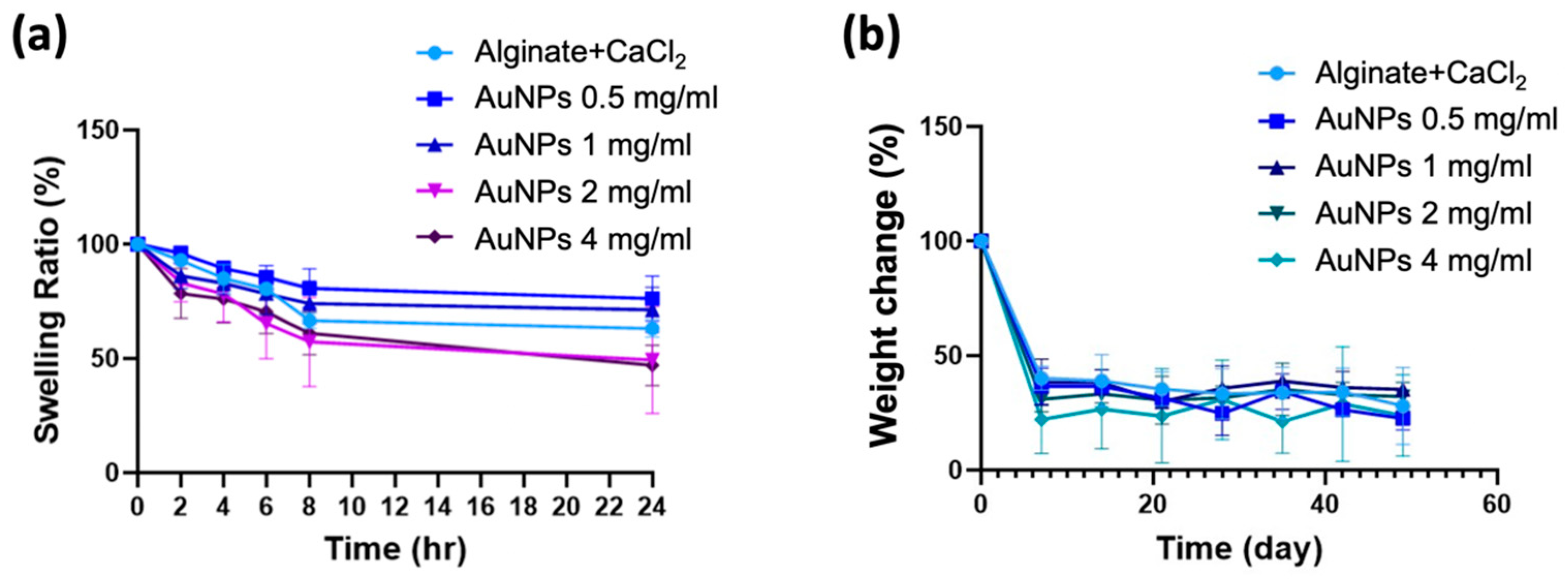
2.6. Swelling Ratio of Alginate-AuNPs Hydrogel
2.7. Degradation Behavior of Alginate-CaCl2-AuNPs Hydrogel
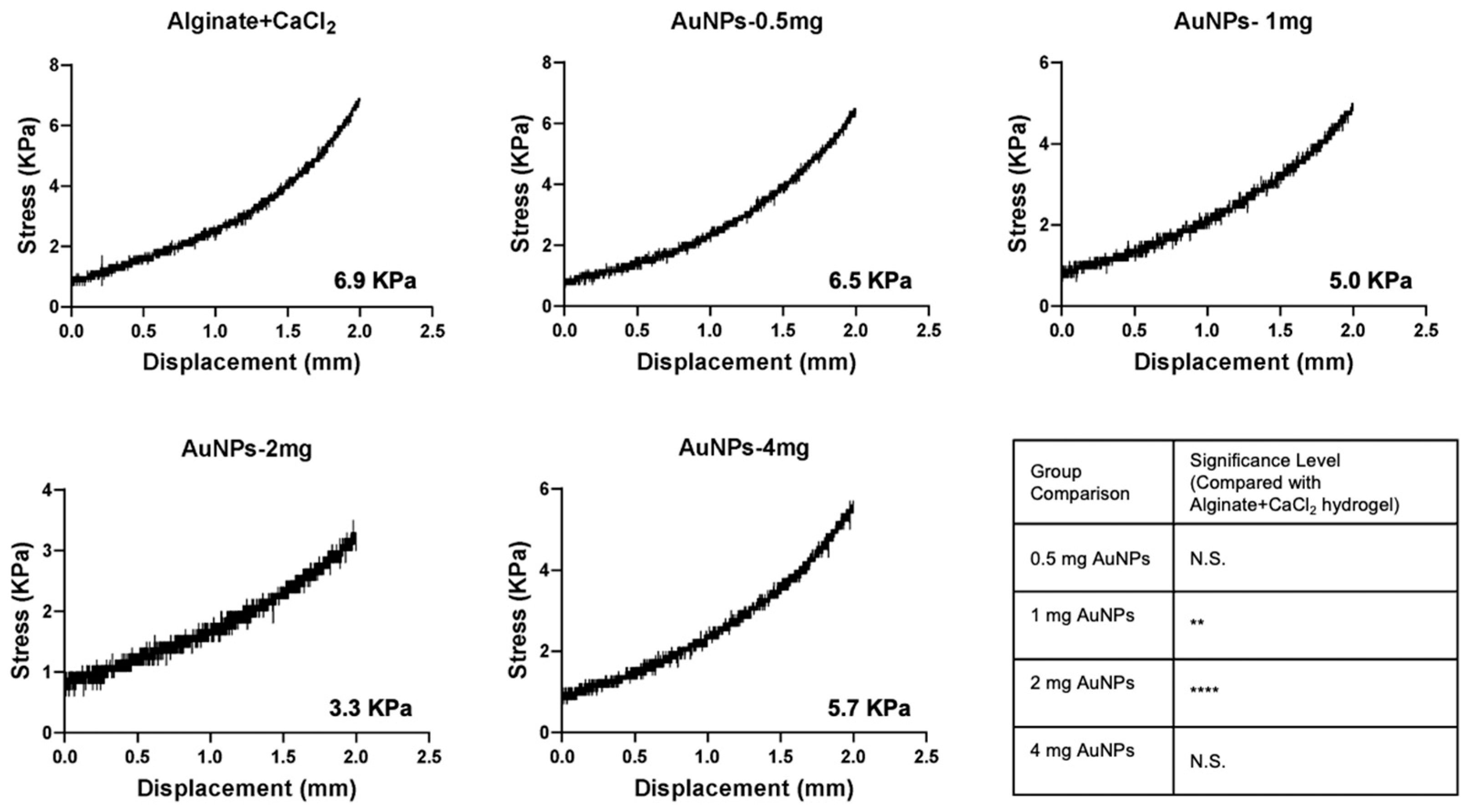
2.8. Evaluation of the Mechanical Properties of Alginate-CaCl₂-AuNPs Hydrogel
2.9. Cell Viability of MC3T3-E1 Preosteoblasts
2.9.1. Indirect Contact Method
2.9.2. Direct Contact Method with Laser Irradiation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Alginate-CaCl2-AuNPs Hydrogel
3.2. Temperature Change in Alginate-CaCl2-AuNPs Hydrogels During Laser Induction
3.3. Viscoelastic Behavior of Alginate-CaCl2-AuNPs Hydrogels
3.4. Swelling Ratio and Degradation Behavior of Alginate-CaCl2-AuNPs Hydrogels
3.5. Mechanical Properties of Alginate-CaCl2-AuNPs Hydrogel
3.6. Cell Viability of MC3T3-E1 Preosteobasts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| CaCl2 | Calcium chloride |
| NIR | Near-infrared |
| LSPR | Localized surface plasmon resonance |
| PTT | Photothermal therapy |
References
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. Interfaces 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Liao, J. A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers 2021, 13, 2100. [Google Scholar] [CrossRef]
- Short, A.R.; Koralla, D.; Deshmukh, A.; Wissel, B.; Stocker, B.; Calhoun, M.; Dean, D.; Winter, J.O. Hydrogels That Allow and Facilitate Bone Repair, Remodeling, and Regeneration. J. Mater. Chem. B 2015, 3, 7818–7830. [Google Scholar] [CrossRef] [PubMed]
- Shui, C.; Scutt, A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J. Bone Miner. Res. 2001, 16, 731–741. [Google Scholar] [CrossRef]
- Ota, T.; Nishida, Y.; Ikuta, K.; Kato, R.; Kozawa, E.; Hamada, S.; Sakai, T.; Ishiguro, N. Heat-stimuli-enhanced osteogenesis using clinically available biomaterials. PLoS ONE 2017, 12, e0181404. [Google Scholar] [CrossRef]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Amini, M.; Jalilzadeh, N.; Baradaran, B.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Oroojalian, F. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J. Control. Release 2022, 349, 269–303. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Thakur, D.; Alexander, A.; Ajazuddin; Badwaik, H.; Tripathi, D.K. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: Present status and applications. Curr. Drug Deliv. 2012, 9, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sun, Z.; Sun, C.; Li, F.; Chen, K.; Zhang, Z.; Hou, G.; Cheng, H.M.; Li, F. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature. Adv. Funct. Mater. 2020, 30, 2007172. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate based scaffolds for cartilage tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Aguero, L.; Alpdagtas, S.; Ilhan, E.; Zaldivar-Silva, D.; Gunduz, O. Functional role of crosslinking in alginate scaffold for drug delivery and tissue engineering: A review. Eur. Polym. J. 2021, 160, 110807. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D. Hydrogel-based flexible electronics. Adv. Mater. 2023, 35, 2205326. [Google Scholar] [CrossRef]
- Teng, K.; An, Q.; Chen, Y.; Zhang, Y.; Zhao, Y. Recent development of alginate-based materials and their versatile functions in biomedicine, flexible electronics, and environmental uses. ACS Biomater. Sci. Eng. 2021, 7, 1302–1337. [Google Scholar] [CrossRef]
- Gundewadi, G.; Rudra, S.G.; Sarkar, D.J.; Singh, D. Nanoemulsion based alginate organic coating for shelf life extension of okra. Food Packag. Shelf Life 2018, 18, 1–12. [Google Scholar]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Li, D.; Wei, Z.; Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369. [Google Scholar] [CrossRef] [PubMed]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous polysaccharide ionic gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Pricker, S.P. Medical uses of gold compounds: Past, present and future. Gold Bull. 1996, 29, 53–60. [Google Scholar] [CrossRef]
- Everts, M.; Saini, V.; Leddon, J.L.; Kok, R.J.; Stoff-Khalili, M.; Preuss, M.A.; Millican, C.L.; Perkins, G.; Brown, J.M.; Bagaria, H.; et al. Covalently linked Au nanoparticles to a viral vector: Potential for combined photothermal and gene cancer therapy. Nano Lett. 2006, 6, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Lim, D.K. Photothermal Effect of Gold Nanoparticles as a Nanomedicine for Diagnosis and Therapeutics. Pharmaceutics 2023, 15, 2349. [Google Scholar] [CrossRef]
- International, A. Standard Test Method for Compressive Properties of Rigid Plastics; ASTM International: West Conshohocken, PA, USA, 2023. [Google Scholar]
- Jiang, K.; Smith, D.A.; Pinchuk, A. Size-dependent photothermal conversion efficiencies of plasmonically heated gold nanoparticles. J. Phys. Chem. C 2013, 117, 27073–27080. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, Y.; Randrianalisoa, J.; Raeesi, V.; Chan, W.C.W.; Lipiński, W.; Bischof, J.C. Quantitative comparison of photothermal heat generation between gold nanospheres and nanorods. Sci. Rep. 2016, 6, 29826. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yue, Z.; Shi, M.; Jiang, L.; Chen, S.; Yao, M.; Yu, Q.; Wu, X.; Zhang, H.; Yao, F.; et al. An intrapericardial injectable hydrogel patch for mechanical-electrical coupling with infarcted myocardium. ACS Nano 2022, 16, 16234–16248. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, Y. Weak Bond-Based Injectable and Stimuli Responsive Hydrogels for Biomedical Applications. J. Mater. Chem. B 2017, 5, 887–906. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, A.L.; Wu, A.F.; Chen, C.-Y.; Moreno, R.L.H.; Wu, J.-L.; Wong, P.-C. Laser-Induced Photothermal Hydrogels Promote the Proliferation of MC3T3-E1 Preosteoblasts for Enhanced Bone Healing. J. Funct. Biomater. 2025, 16, 63. https://doi.org/10.3390/jfb16020063
Wu AL, Wu AF, Chen C-Y, Moreno RLH, Wu J-L, Wong P-C. Laser-Induced Photothermal Hydrogels Promote the Proliferation of MC3T3-E1 Preosteoblasts for Enhanced Bone Healing. Journal of Functional Biomaterials. 2025; 16(2):63. https://doi.org/10.3390/jfb16020063
Chicago/Turabian StyleWu, Audrey L., Abigail F. Wu, Chieh-Ying Chen, Ruaina Lily Hope Moreno, Jia-Lin Wu, and Pei-Chun Wong. 2025. "Laser-Induced Photothermal Hydrogels Promote the Proliferation of MC3T3-E1 Preosteoblasts for Enhanced Bone Healing" Journal of Functional Biomaterials 16, no. 2: 63. https://doi.org/10.3390/jfb16020063
APA StyleWu, A. L., Wu, A. F., Chen, C.-Y., Moreno, R. L. H., Wu, J.-L., & Wong, P.-C. (2025). Laser-Induced Photothermal Hydrogels Promote the Proliferation of MC3T3-E1 Preosteoblasts for Enhanced Bone Healing. Journal of Functional Biomaterials, 16(2), 63. https://doi.org/10.3390/jfb16020063










