Comparison of the Properties of Acellular Matrix from the Skins of Cod (Gadus morhua) and Tilapia (Oreochromis mossambicus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AFSM
2.3. Acellular Process
2.3.1. Determination of DNA Content
2.3.2. Histological Staining Analysis
2.4. Determination of Endotoxin Content
2.5. Characterization of the Physical Properties of AFSM
2.5.1. Microstructure Analysis
2.5.2. Determination of Porosity
2.5.3. Determination of Tensile Stress
2.6. Biocompatibility Evaluation
2.6.1. Skin Irritation Test
2.6.2. Cytotoxicity Assay
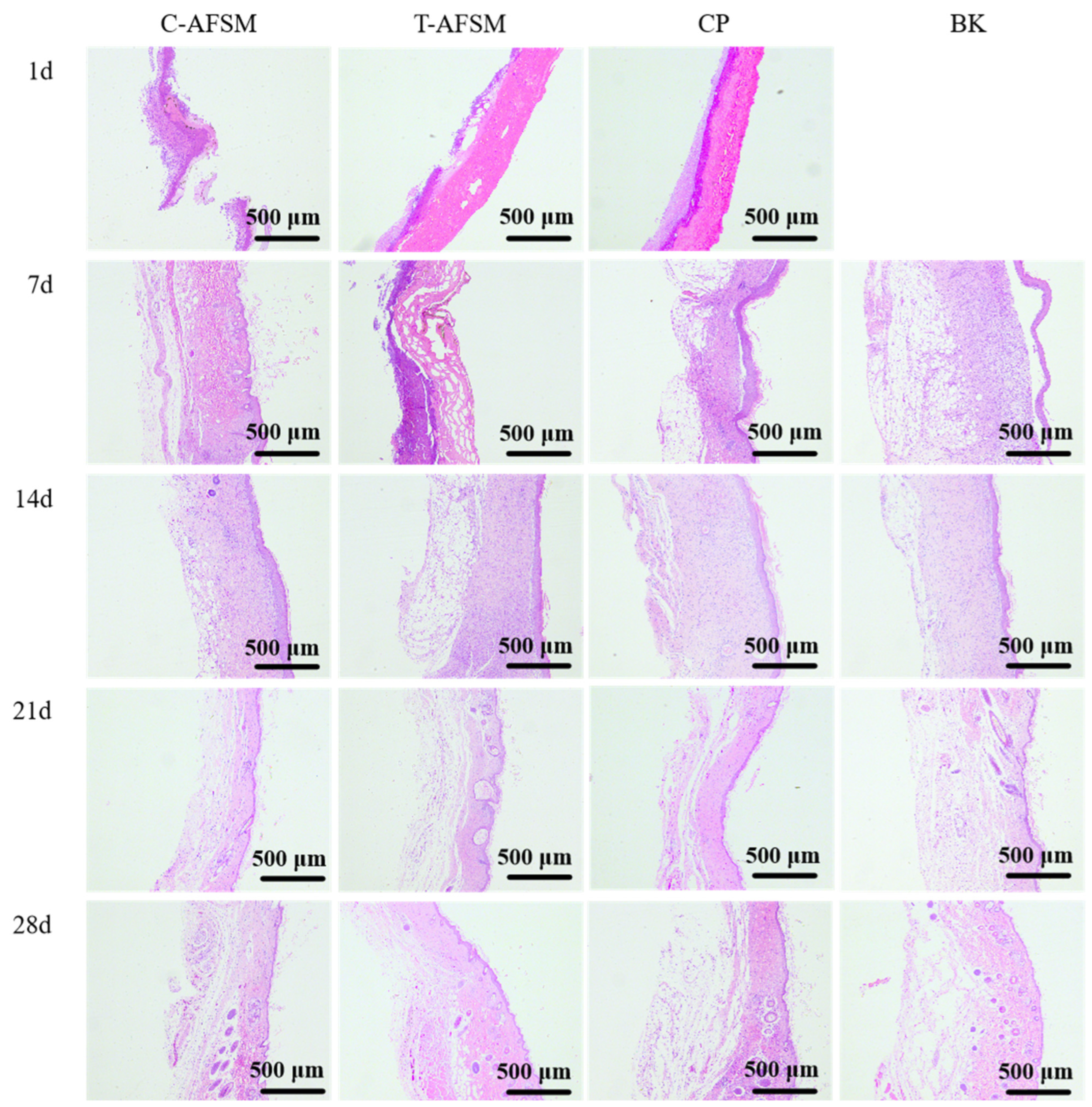
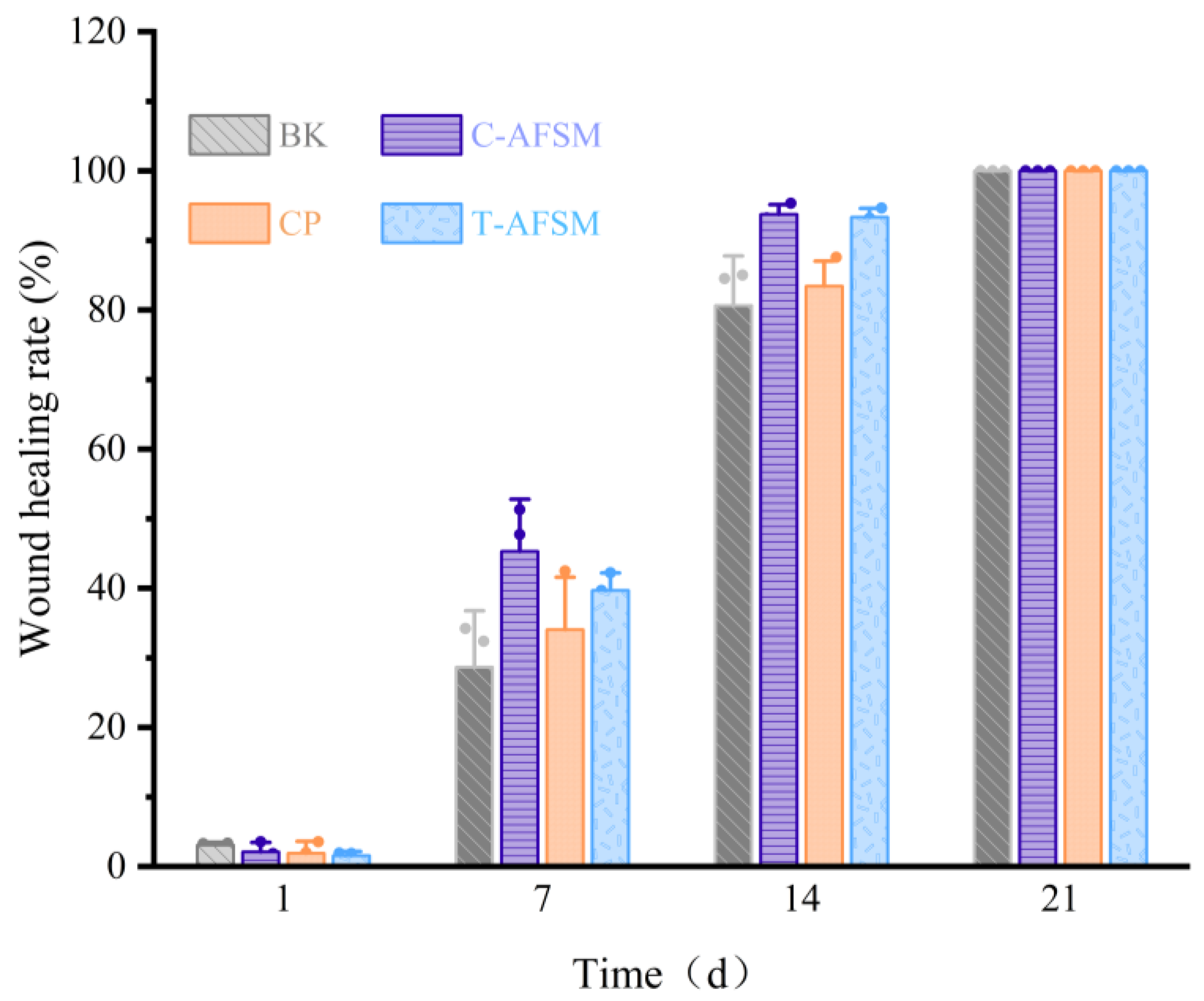
2.6.3. Wound Healing Test
2.7. Statistical Analysis of Data
3. Results and Discussion
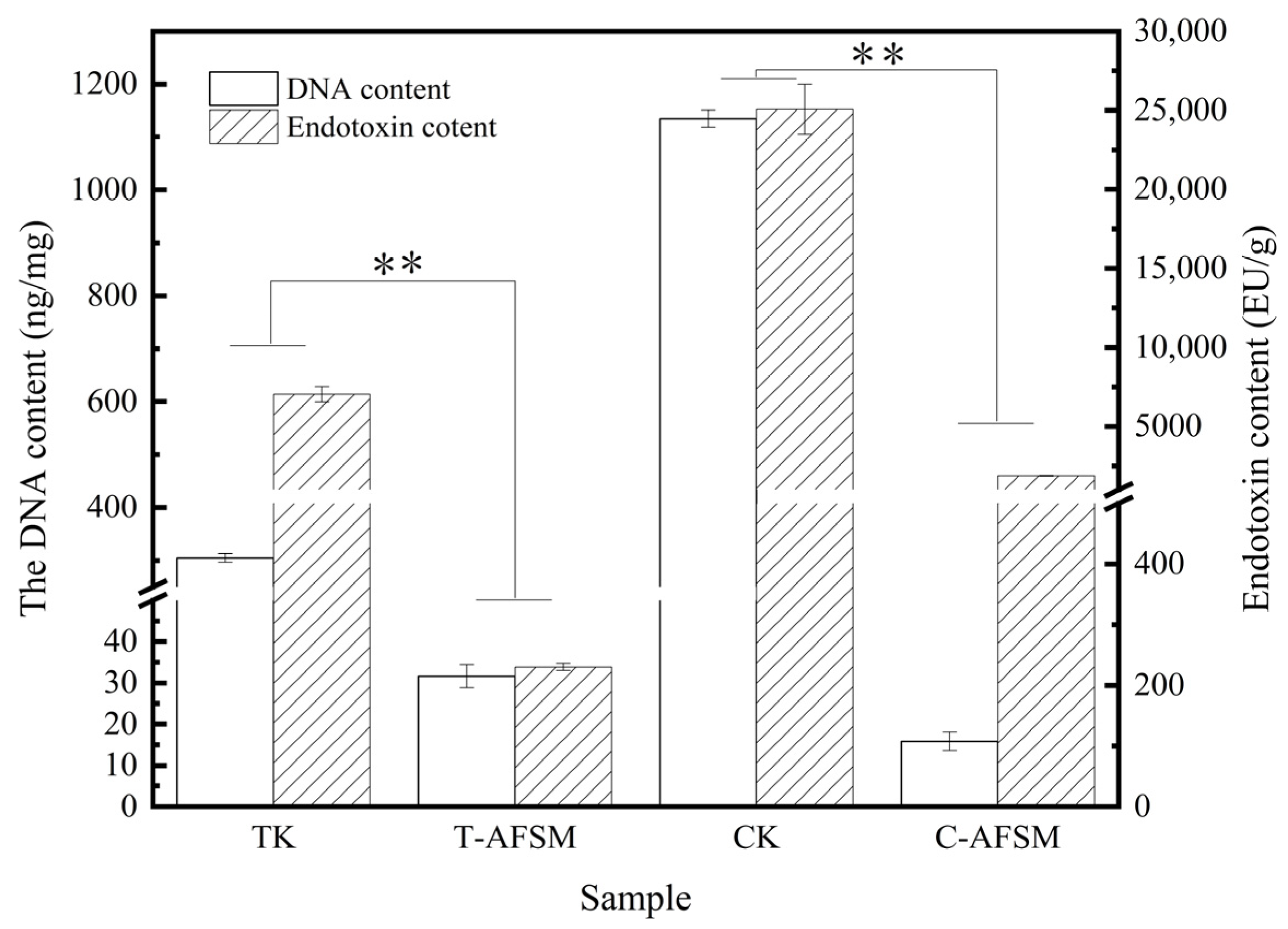
3.1. DNA Residue and Endotoxin Content
3.2. Physical and Chemical Characterization
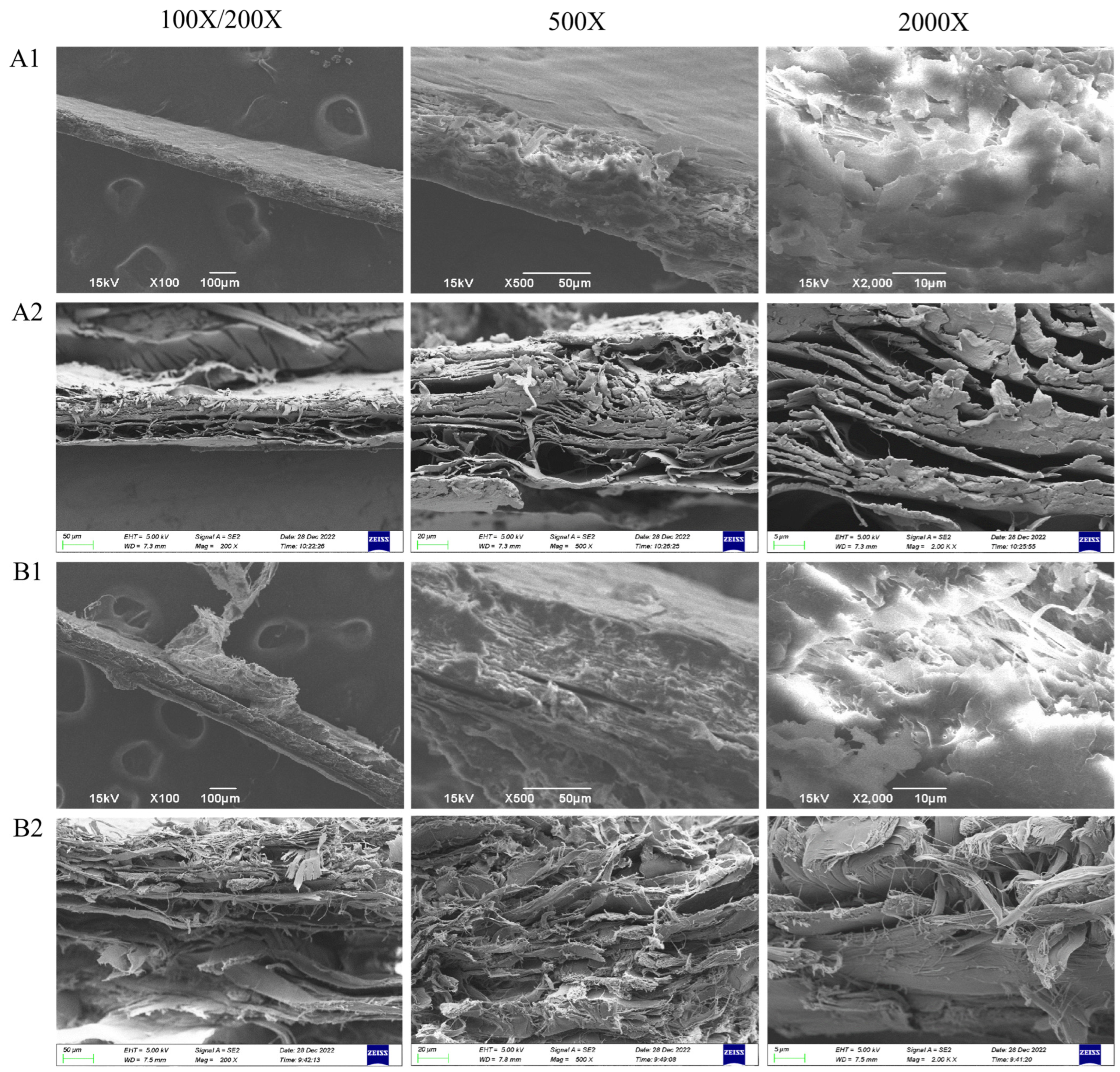
3.2.1. Microstructure Analysis
3.2.2. Porosity
3.2.3. Tensile Strength and Elongation at Break
3.3. Biocompatibility Evaluation of AFSM
3.3.1. Skin Irritation Test
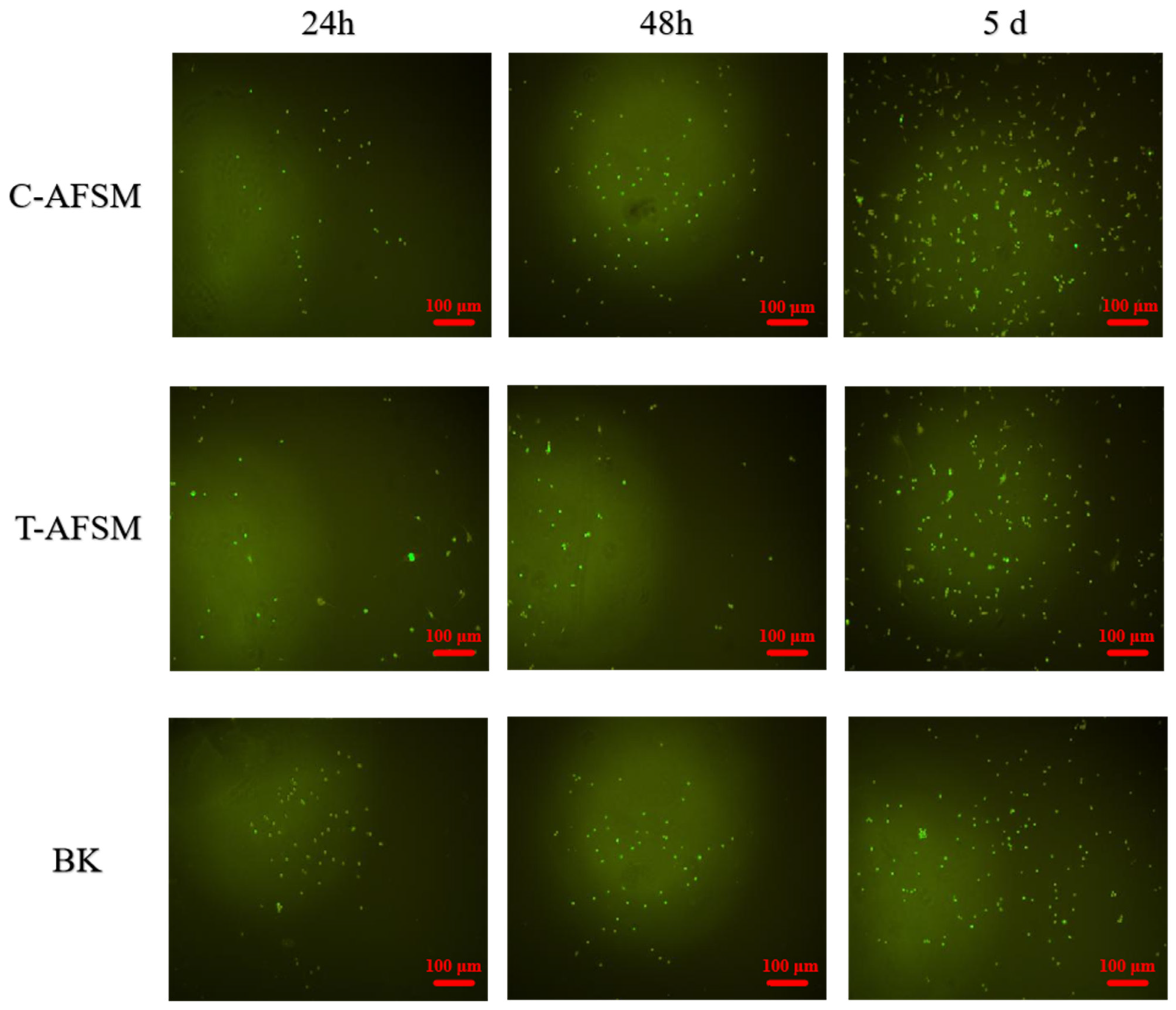
3.3.2. Cytotoxicity Assay
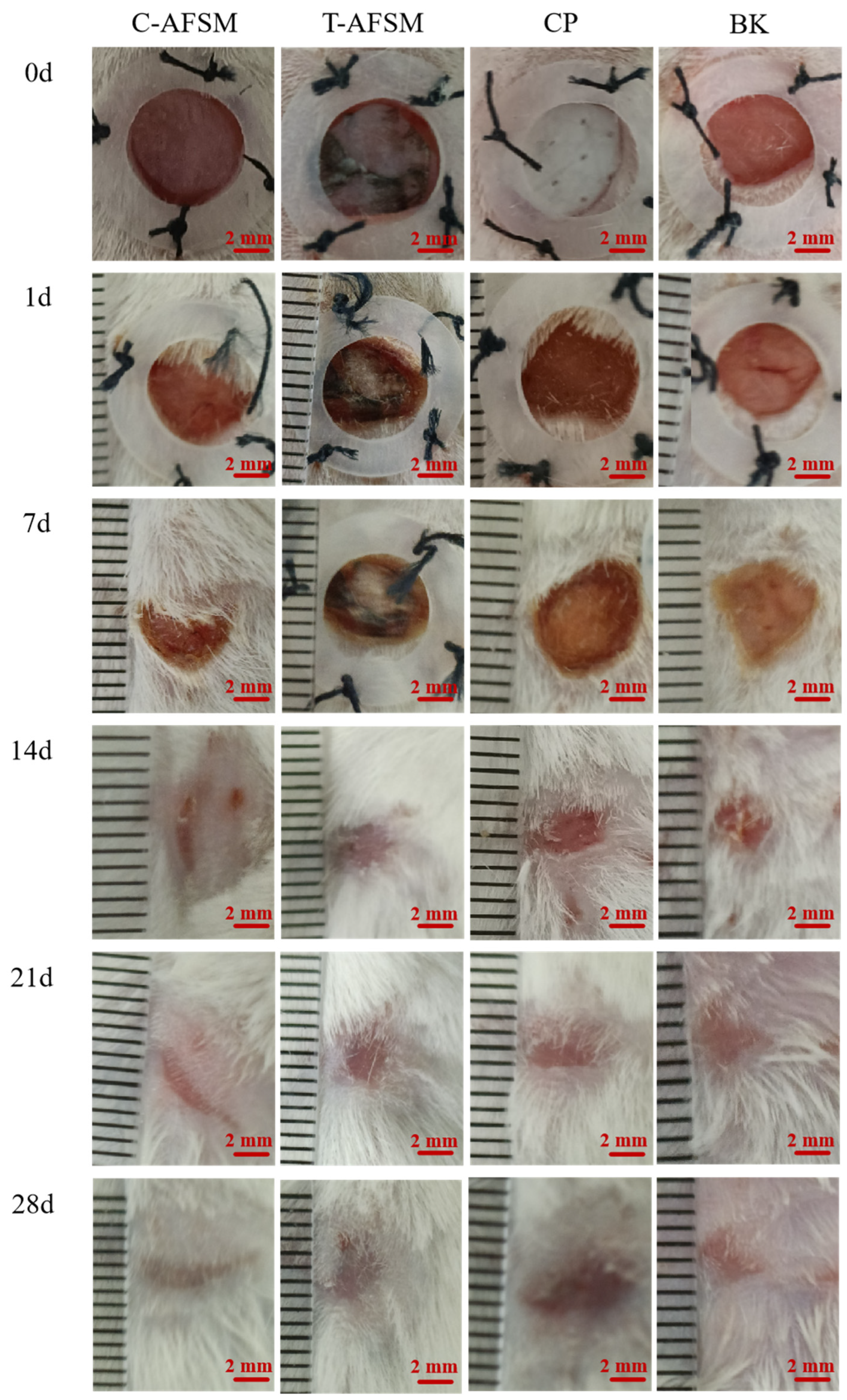
3.3.3. Wound Healing Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C-AFSM | Cod acellular fish skin matrix |
| T-AFSM | Tilapia acellular fish skin matrix |
| SC-AFSM | Silver carp acellular fish skin matrix |
| CP | Commercially available product |
| BK | Blank control |
| ACTM | Acellular tissue matrix |
| ECM | Extracellular matrix |
| CK | Cod skin |
| TK | Tilapia skin |
| DMEM | Dulbecco’s modification of Eagle’s medium |
| WHR | Wound healing rate |
| 3T3 | Mouse fibroblasts |
| TS | Tensile strength |
| EAB | Elongation at break |
References
- Wiggenhauser, P.S.; Schwarz, S.; Koerber, L.; Hoffmann, T.K.; Rotter, N. Addition of decellularized extracellular matrix of porcine nasal cartilage improves cartilage regenerative capacities of PCL-based scaffolds in vitro. J. Mater.Sci. Mater. Med. 2019, 30, 121. [Google Scholar] [CrossRef]
- Esmaeili, A.; Biazar, E.; Ebrahimi, M.; Heidari Keshel, S.; Kheilnezhad, B.; Saeedi Landi, F. Acellular fish skin for wound healing. Int. Wound J. 2023, 20, 2924–2941. [Google Scholar] [CrossRef]
- Dadlani, S. Porcine acellular dermal matrix: An alternative to connective tissue graft—A narrative review. Int. J. Dent. 2021, 2021, 1652032. [Google Scholar] [CrossRef]
- Zajicek, R.; Mandys, V.; Mestak, O.; Sevcik, J.; Königova, R.; Matouskova, E. Human keratinocyte growth and differentiation on acellular porcine dermal matrix in relation to wound healing potential. Sci. World J. 2012, 2012, 727352. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Sun, J.; Liu, G.; Li, L.; Wu, S.; Xiang, Z. Graphene oxide–modified 3D acellular cartilage extracellular matrix scaffold for cartilage regeneration. Mater. Sci. Eng. 2021, 119, 111603. [Google Scholar] [CrossRef]
- Bondioli, E.; Purpura, V.; Orlandi, C.; Carboni, A.; Minghetti, P.; Cenacchi, G.; De Luca, G.; Capirossi, D.; Nigrisoli, E.; Melandri, D. The use of an acellular matrix derived from human dermis for the treatment of full-thickness skin wounds. Cell Tissue Bank. 2019, 20, 183–192. [Google Scholar] [CrossRef]
- Lau, C.S.; Hassanbhai, A.; Wen, F.; Wang, D.; Chanchareonsook, N.; Goh, B.T.; Yu, N.; Teoh, S.H. Evaluation of decellularized tilapia skin as a tissue engineering scaffold. J. Tissue Eng. Regen. Med. 2019, 13, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, X.; Liu, X.; Wen, G.; Yu, Y. Recent advances in decellularized biomaterials for wound healing. Mater. Today Bio. 2023, 19, 100589. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, J.; Guo, Z.; Wu, Z.; Sun, Y.; Wang, K.; Duan, R. Study on the preparation and properties of acellular matrix from the skin of silver carp (Hypophthalmichthys molitrix). J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1328–1335. [Google Scholar] [CrossRef]
- Luo, Y.; Tao, F.; Wang, J.; Chai, Y.; Ren, C.; Wang, Y.; Wu, T.; Chen, Z. Development and evaluation of tilapia skin-derived gelatin, collagen, and acellular dermal matrix for potential use as hemostatic sponges. Int. J. Biol. Macromol. 2023, 253, 127014. [Google Scholar] [CrossRef]
- Li, D.; Sun, W.Q.; Wang, T.; Gao, Y.; Wu, J.; Xie, Z.; Zhao, J.; He, C.; Zhu, M.; Zhang, S. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater. Sci. Eng. 2021, 127, 112202. [Google Scholar] [CrossRef]
- Badois, N.; Bauër, P.; Cheron, M.; Hoffmann, C.; Nicodeme, M.; Choussy, O.; Lesnik, M.; Poitrine, F.C.; Fromantin, I. Acellular fish skin matrix on thin-skin graft donor sites: A preliminary study. J. Wound Care 2019, 28, 624–628. [Google Scholar] [CrossRef]
- Volova, T.G.; Vinnik, Y.S.; Shishatskaya, E.I.; Markelova, N.M.; Zaikov, G.E. Natural-Based Polymers for Biomedical Applications; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2017. [Google Scholar]
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J. Funct. Foods 2017, 35, 418–427. [Google Scholar] [CrossRef]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K. Omega-3 polyunsaturated fatty acids suppress the inflammatory responses of lipopolysaccharide-stimulated mouse microglia by activating SIRT1 pathways. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized scaffolds for skin repair and regeneration. Appl. Sci. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Fitzsimmons, K.; Martinez-Garcia, R.; Gonzalez-Alanis, P. Why tilapia is becoming the most important food fish on the planet. In Proceedings of the Better Science, Better Fish, Better Life Proceedings of the Ninth International Symposium on Tilapia in Aquaculture (ISTA 9), Shanghai, China, 22–24 April 2011. [Google Scholar]
- Li, X.; Deng, Y.; Qiu, W.; Feng, Y.; Jin, Y.; Deng, S.; Tao, N.; Jin, Y. Alteration of collagen thermal denaturation, structural and the abrogation of allergenicity in eel skin induced by ohmic heating. Food Chem. 2022, 391, 133272. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.; Li, J.; Zhong, X.; Konno, K.; Wen, H. The effect of the subunit composition on the thermostability of collagens from the scales of freshwater fish. Food Chem. 2012, 135, 127–132. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid-and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Kimura, S.; Ohno, Y.; Miyauchi, Y.; Uchida, N. Fish skin type I collagen: Wide distribution of an alpha 3 subunit in teleosts. Comp. Biochem. Physiology. B Comp. Biochem. 1987, 88, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gazi, S.K.N.; Hosseini, S.E.; Yakhchali, S.H.; Borajee, M. The Application of the statistical methods to measure the effect of adding a bamboo fiber and combine it with animal collagen on the physical, chemical and sensory characteristics of nags tilapia fish. In Proceedings of the International Conference on Literature, History, Humanities and Social Sciences, Klaipeda, Lithuania, 16–17 May 2017. [Google Scholar]
- Ablikim, Z. Preparation of five kinds of acellular fish skin matrices and in vitro toxicity analysis. Acad. J. Second. Mil. Med. Univ. 2019, 40, 162–168. [Google Scholar] [CrossRef]
- Yang, C.; Guo, G.; Wang, J.; Fan, J.; Xing, Y.; Zhang, L.; Ang, M. Effects of two common acellular methods on the physicochemical properties of dermal acellular matrix. J. Biomed. Eng. 2021, 38, 911–918. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Kamalvand, M.; Biazar, E.; Daliri-Joupari, M.; Montazer, F.; Rezaei-Tavirani, M.; Heidari-Keshel, S. Design of a decellularized fish skin as a biological scaffold for skin tissue regeneration. Tissue Cell. 2021, 71, 101509. [Google Scholar] [CrossRef]
- Greco, K.; Francis, L.; Somasundaram, M.; Greco, G.; English, N.R.; Roether, J.A.; Boccaccini, A.R.; Sibbons, P.; Ansari, T. Characterisation of porcine dermis scaffolds decellularised using a novel non-enzymatic method for biomedical applications. J. Biomater. Appl. 2015, 30, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, M.-Z.; Peng, H.; Liu, R.-T.; Lim, T.; Zhang, C.-Q.; Zhu, Z.-Z.; Wei, X.-J. Decellularized tilapia fish skin: A novel candidate for tendon tissue engineering. Mater. Today Bio. 2022, 17, 100488. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dan, N.; Dan, W. Preparation and characterization of an advanced collagen aggregate from porcine acellular dermal matrix. Int. J. Biol. Macromol. 2016, 88, 179–188. [Google Scholar] [CrossRef]
- Ma, P.; Wang, Y.; Li, B.; Hou, H. Cross-linking effects of carbodiimide, oxidized chitosan oligosaccharide and glutaraldehyde on acellular dermal matrix of basa fish (Pangasius bocourti). Int. J. Biol. Macromol. 2020, 164, 677–686. [Google Scholar] [CrossRef]
- Jiang, S.; Zhuang, Y.; Cai, M.; Wang, X.; Lin, K. Decellularized extracellular matrix: A promising strategy for skin repair and regeneration. Eng. Regen. 2023, 4, 357–374. [Google Scholar] [CrossRef]
- Acharya, P.P.; Kupendra, M.H.; Fasim, A.; More, S.S.; Murthy, V.K. A comparative assessment of collagen type 1 from silver carp (fresh water) and milk shark (marine) fish waste. 3 Biotech. 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Li, B.; Zhao, X.; Zhuang, Y.; Ren, G.; Yan, M.; Cai, Y.; Zhang, X.; Chen, L. The effect of pacific cod (Gadus macrocephalus) skin gelatin polypeptides on UV radiation-induced skin photoaging in ICR mice. Food Chem. 2009, 115, 945–950. [Google Scholar] [CrossRef]
- Sung, H.-J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, J. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly (D, L-lactide-co-glycolide) scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2005, 75, 767–777. [Google Scholar] [CrossRef] [PubMed]



| Sample | CK | C-AFSM | TK | T-AFSM |
|---|---|---|---|---|
| Thicknesses (mm) | 0.24 ± 0.02 | 0.17 ± 0.03 | 0.48 ± 0.02 | 0.49 ± 0.05 |
| TS (MPa) | 13.14 ± 1.39 | - | 17.08 ± 0.62 | 5.79 ± 0.11 |
| EAB (%) | 39.23 ± 9.67 | - | 88.37 ± 8.57 | 64.79 ± 5.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wei, Z.; Duan, R.; Wang, K.; Xu, T.; Mao, B.; Zhang, J. Comparison of the Properties of Acellular Matrix from the Skins of Cod (Gadus morhua) and Tilapia (Oreochromis mossambicus). J. Funct. Biomater. 2025, 16, 81. https://doi.org/10.3390/jfb16030081
Liu Y, Wei Z, Duan R, Wang K, Xu T, Mao B, Zhang J. Comparison of the Properties of Acellular Matrix from the Skins of Cod (Gadus morhua) and Tilapia (Oreochromis mossambicus). Journal of Functional Biomaterials. 2025; 16(3):81. https://doi.org/10.3390/jfb16030081
Chicago/Turabian StyleLiu, Yu, Zeyu Wei, Rui Duan, Ke Wang, Tianyue Xu, Binxian Mao, and Junjie Zhang. 2025. "Comparison of the Properties of Acellular Matrix from the Skins of Cod (Gadus morhua) and Tilapia (Oreochromis mossambicus)" Journal of Functional Biomaterials 16, no. 3: 81. https://doi.org/10.3390/jfb16030081
APA StyleLiu, Y., Wei, Z., Duan, R., Wang, K., Xu, T., Mao, B., & Zhang, J. (2025). Comparison of the Properties of Acellular Matrix from the Skins of Cod (Gadus morhua) and Tilapia (Oreochromis mossambicus). Journal of Functional Biomaterials, 16(3), 81. https://doi.org/10.3390/jfb16030081









