Fabrication of PVA Coatings Applied to Electrospun PLGA Scaffolds to Prevent Postoperative Adhesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Electrospun PLGA Scaffolds
2.2. Preparation of PVA Solutions and Viscosity Measurements
2.3. PVA Coating Application by Dip-Coating
2.4. Mass and Thickness Analysis
2.5. Optical Microscopy of Sample Surfaces and Their Cross-Sections
2.6. Scanning Electron Microscopy
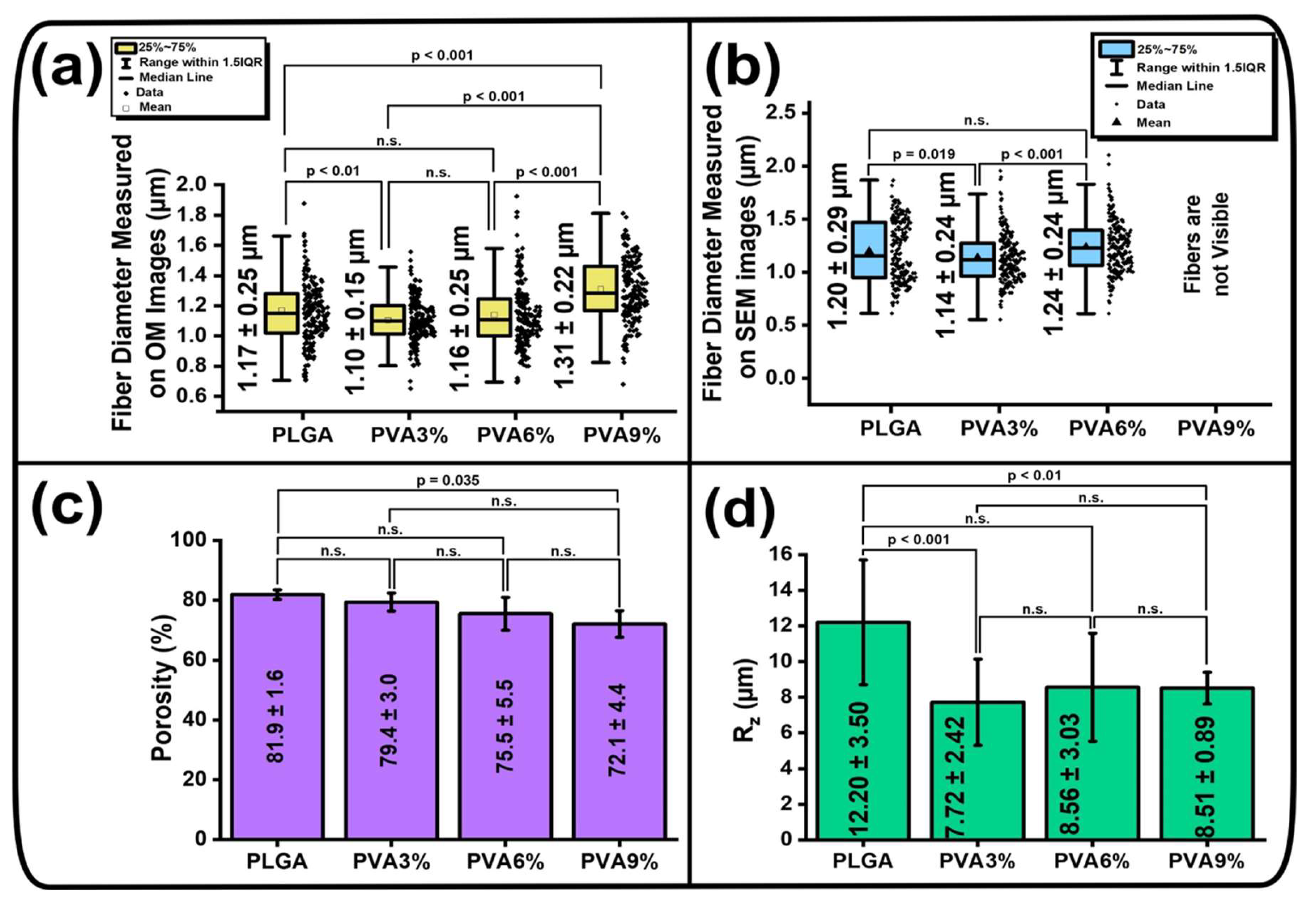
2.7. Optical Profilometry
2.8. Porosity of the Scaffold Samples
2.9. Elemental Composition by Energy-Dispersive X-Ray Spectroscopy Analysis
2.10. Fourier-Transform Infrared Spectroscopy
2.11. X-Ray Diffraction (XRD)
2.12. Thermogravimetric Analysis
2.13. Wettability of the Samples
2.14. Mechanical Properties
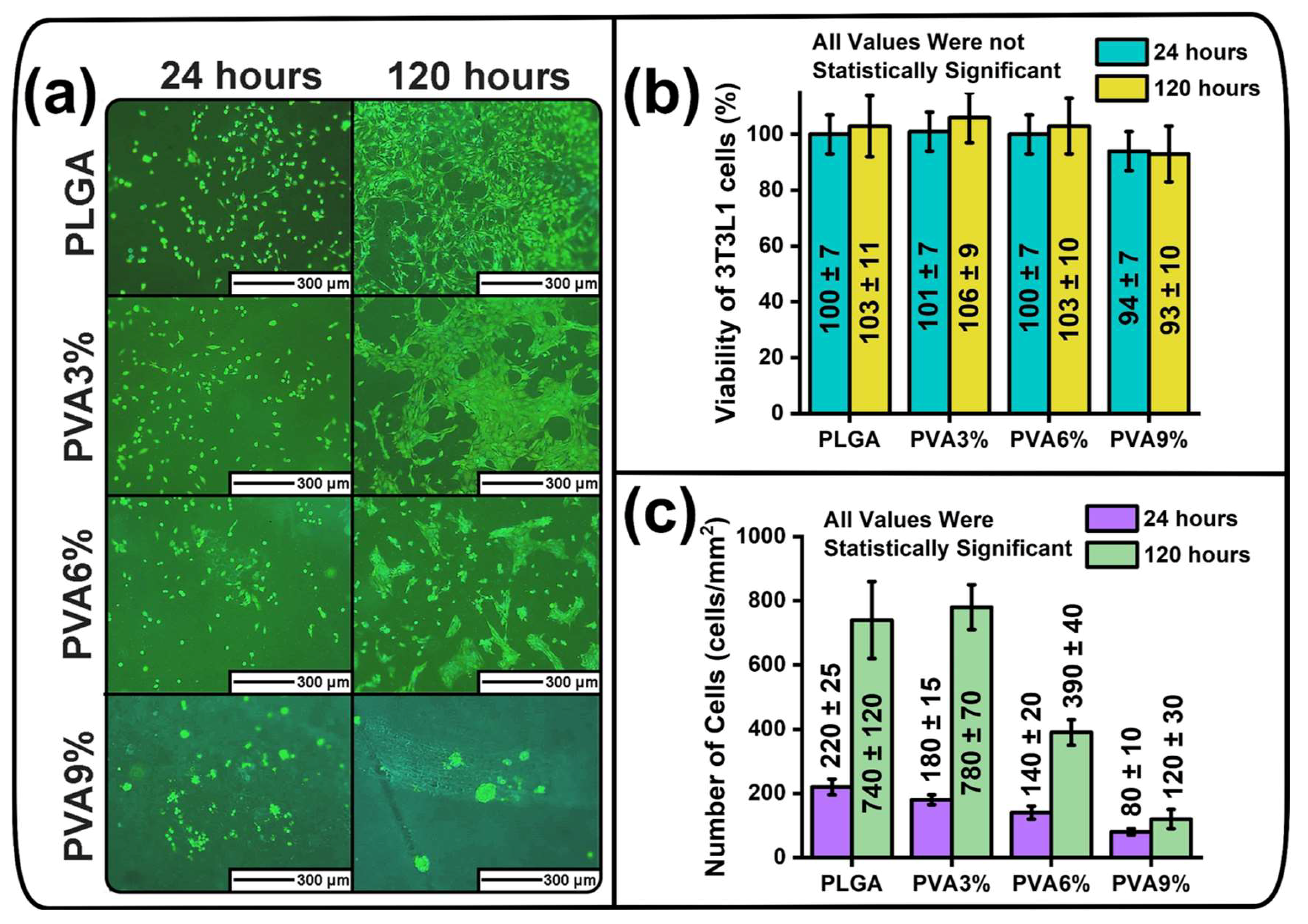
2.15. Cytotoxicity and Anti-Adhesive Properties
2.16. Statistics
3. Results and Discussion
3.1. Viscosity of the PVA Solutions and Changes in Mass and Thickness of PLGA Scaffolds After Dip-Coating
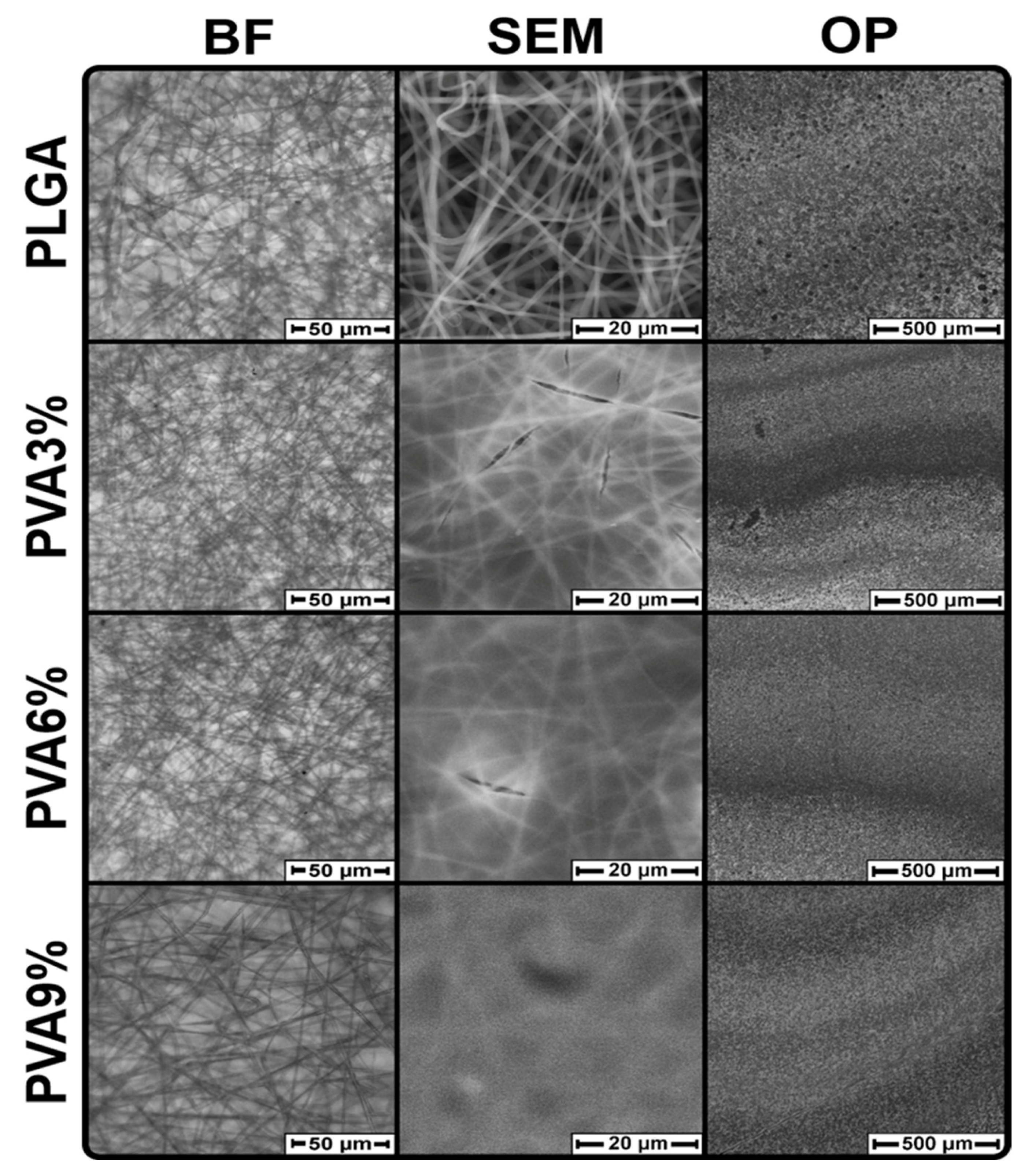
3.2. Surface Morphology of the PLGA Scaffolds
3.3. Morphology of the PLGA Scaffold Cross-Sections
3.4. Elemental and Chemical Composition, Thermal Properties and Crystalline Structure of the PLGA Scaffolds
3.5. Wettability of the PLGA Scaffold Samples
3.6. Mechanical Properties of the PLGA Scaffolds
3.7. Cytotoxicity and Anti-Adhesive Properties of the PLGA Scaffold Samples
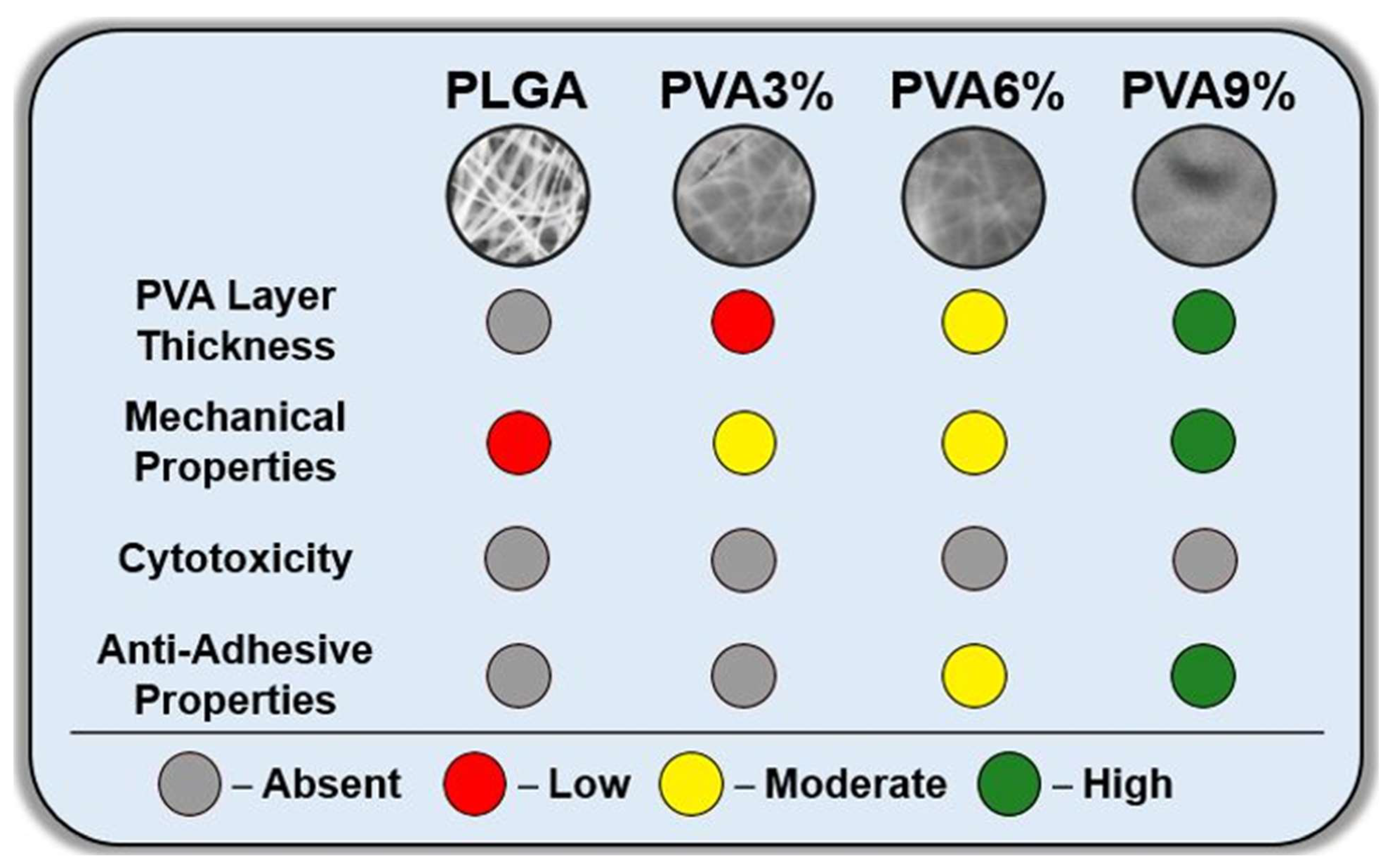
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fatehi Hassanabad, A.; Zarzycki, A.N.; Jeon, K.; Deniset, J.F.; Fedak, P.W.M. Post-Operative Adhesions: A Comprehensive Review of Mechanisms. Biomedicines 2021, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Brüggmann, D.; Tchartchian, G.; Wallwiener, M.; Münstedt, K.; Tinneberg, H.-R.; Hackethal, A. Intra-Abdominal Adhesions. Dtsch. Arztebl. Int. 2010, 107, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, K.; Ashrafian, H.; Zacharakis, E.; Hasegawa, H.; Kitagawa, Y.; Athanasiou, T.; Darzi, A. Adhesions after Abdominal Surgery: A Systematic Review of the Incidence, Distribution and Severity. Surg. Today 2014, 44, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiang, Z.; Bernards, M.T.; Chen, S. Peritoneal Adhesions: Occurrence, Prevention and Experimental Models. Acta Biomater. 2020, 116, 84–104. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, R.; das Neves, J.; Tang, J.; Xiao, J.; Ni, Q.; Liu, X.; Pan, G.; Li, D.; Cui, W.; et al. Advances in Biomaterials for Preventing Tissue Adhesion. J. Control. Release 2017, 261, 318–336. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, C.; Zhang, X.; Lin, Y.; Yang, H.; Zhang, Y.S.; Ding, J. An Oxidative Stress-Responsive Electrospun Polyester Membrane Capable of Releasing Anti-Bacterial and Anti-Inflammatory Agents for Postoperative Anti-Adhesion. J. Control. Release 2021, 335, 359–368. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, M.; Guidoin, R.; Li, Y.; Wang, F.; Brochu, G.; Zhang, Z.; Wang, L. Potential of a Facile Sandwiched Electrospun Scaffold Loaded with Ibuprofen as an Anti-Adhesion Barrier. Mater. Sci. Eng. C 2021, 118, 111451. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.Y.; Park, S.H.; Kim, M.J.; Song, B.R.; Yun, H.-W.; Kang, T.W.; Choi, H.S.; Kim, Y.J.; Min, B.H.; et al. Cross-Linked Electrospun Cartilage Acellular Matrix/Poly(Caprolactone-Co-Lactide-Co-Glycolide) Nanofiber as an Antiadhesive Barrier. Acta Biomater. 2018, 74, 192–206. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; He, T.; Li, W.; Zhao, Y.; Chen, Z.; Zhang, J.; Wan, H.; Li, R. Prevention of Intra-Abdominal Adhesion Using Electrospun PEG/PLGA Nanofibrous Membranes. Mater. Sci. Eng. C 2017, 78, 988–997. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Yang, W.J.; Park, J.-C.; Hyon, S.-H.; Han, D.-W. Epigallocatechin-3-O-Gallate-Loaded Poly(Lactic-Co-Glycolic Acid) Fibrous Sheets as Anti-Adhesion Barriers. J. Biomed. Nanotechnol. 2015, 11, 1461–1471. [Google Scholar] [CrossRef]
- MengQing, S.; RuiZhe, T.; Peng, L.; Peng, Z. Study on Prevention of Postoperative Abdominal Adhesions with PLGA Nanofiber Membrane. J. Phys. Conf. Ser. 2020, 1676, 012129. [Google Scholar] [CrossRef]
- Liu, K.-S.; Kao, C.-W.; Tseng, Y.-Y.; Chen, S.-K.; Lin, Y.-T.; Lu, C.-J.; Liu, S.-J. Assessment of Antimicrobial Agents, Analgesics, and Epidermal Growth Factors-Embedded Anti-Adhesive Poly(Lactic-Co-Glycolic Acid) Nanofibrous Membranes: In Vitro and in Vivo Studies. Int. J. Nanomed. 2021, 16, 4471–4480. [Google Scholar] [CrossRef]
- Bazgir, M.; Saeinasab, M.; Zhang, W.; Zhang, X.; Min Tsui, K.; Maasoumi Sarvestani, A.; Nawaz, S.; Coates, P.; Youseffi, M.; Elies, J.; et al. Investigation of Cell Adhesion and Cell Viability of the Endothelial and Fibroblast Cells on Electrospun PCL, PLGA and Coaxial Scaffolds for Production of Tissue Engineered Blood Vessel. J. Funct. Biomater. 2022, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- El Khatib, M.; Mauro, A.; Wyrwa, R.; Di Mattia, M.; Turriani, M.; Di Giacinto, O.; Kretzschmar, B.; Seemann, T.; Valbonetti, L.; Berardinelli, P.; et al. Fabrication and Plasma Surface Activation of Aligned Electrospun PLGA Fiber Fleeces with Improved Adhesion and Infiltration of Amniotic Epithelial Stem Cells Maintaining Their Teno-Inductive Potential. Molecules 2020, 25, 3176. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical Properties of Electrospun Fibers—A Critical Review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface Modification of Electrospun PLGA Scaffold with Collagen for Bioengineered Skin Substitutes. Mater. Sci. Eng. C 2016, 66, 130–137. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Fan, Y. Prevention Strategies of Postoperative Adhesion in Soft Tissues by Applying Biomaterials: Based on the Mechanisms of Occurrence and Development of Adhesions. Bioact. Mater. 2023, 26, 387–412. [Google Scholar] [CrossRef]
- Maadani, A.M.; Davoodian, F.; Salahinejad, E. Effects of PLGA Coating on Biological and Mechanical Behaviors of Tissue Engineering Scaffolds. Prog. Org. Coat. 2023, 176, 107406. [Google Scholar] [CrossRef]
- Abbasi Soureshjani, F.; Nilforoushan, M.R.; Sharifi, H.; Arefpour, A.; Doostmohammadi, A. Improvement in Mechanical and Biological Performance of Porous Baghdadite Scaffold by Applying Chitosan Coating. Appl. Phys. A 2021, 127, 335. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Loutfy, S.A.; Hussein, Y.; Kenawy, E.-R.S. Recent Advances in PVA-Polysaccharide Based Hydrogels and Electrospun Nanofibers in Biomedical Applications: A Review. Int. J. Biol. Macromol. 2021, 187, 755–768. [Google Scholar] [CrossRef]
- Lan, X.; Lei, Y.; He, Z.; Yin, A.; Li, L.; Tang, Z.; Li, M.; Wang, Y. A Transparent Hydrophilic Anti-Biofouling Coating for Intraocular Lens Materials Prepared by “Bridging” of the Intermediate Adhesive Layer. J. Mater. Chem. B 2021, 9, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- P Libel, G.; Facchi, S.P.; de Almeida, D.A.; Madruga, L.C.; Kipper, M.J.; Schrekker, H.S.; Martins, A.F.; Radovanovic, E. Cross-Linked Poly(Vinyl Alcohol)/Citric Acid Electrospun Fibers Containing Imidazolium Ionic Liquid with Enhanced Antiadhesive and Antimicrobial Properties. Mater. Chem. Phys. 2024, 316, 129087. [Google Scholar] [CrossRef]
- Jin, D.; Yang, S.; Wu, S.; Yin, M.; Kuang, H. A Functional PVA Aerogel-Based Membrane Obtaining Sutureability through Modified Electrospinning Technology and Achieving Promising Anti-Adhesion Effect after Cardiac Surgery. Bioact. Mater. 2022, 10, 355–366. [Google Scholar] [CrossRef]
- Liang, W.; He, W.; Huang, R.; Tang, Y.; Li, S.; Zheng, B.; Lin, Y.; Lu, Y.; Wang, H.; Wu, D. Peritoneum-Inspired Janus Porous Hydrogel with Anti-Deformation, Anti-Adhesion, and Pro-Healing Characteristics for Abdominal Wall Defect Treatment. Adv. Mater. 2022, 34, 2108992. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, J.; Zeng, G.; Xu, H.; Lv, Y.; Liang, X.; Jin, L.; Jiang, X. Chitosan-Crosslinked Polyvinyl Alcohol Anti-Swelling Hydrogel Designed to Prevent Abdominal Wall Adhesion. Mater. Today Bio 2024, 24, 100931. [Google Scholar] [CrossRef]
- Silvério, H.A.; Flauzino Neto, W.P.; Pasquini, D. Effect of Incorporating Cellulose Nanocrystals from Corncob on the Tensile, Thermal and Barrier Properties of Poly(Vinyl Alcohol) Nanocomposites. J. Nanomater. 2013, 2013, 289641. [Google Scholar] [CrossRef]
- Zamanian, M.; Sadrnia, H.; Khojastehpour, M.; Hosseini, F.; Thibault, J. Effect of TiO2 Nanoparticles on Barrier and Mechanical Properties of PVA Films. J. Membr. Sci. Res. 2021, 7, 67–73. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, N.S. Bio-Inspired Strategies for Designing Antifouling Biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. A Synergistic Antibacterial Effect between Terbium Ions and Reduced Graphene Oxide in a Poly(Vinyl Alcohol)–Alginate Hydrogel for Treating Infected Chronic Wounds. J. Mater. Chem. B 2019, 7, 538–547. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent Advance in Surface Modification for Regulating Cell Adhesion and Behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Ren, X.; Zou, B.; Guo, W.; Song, L.; Hu, Y. Construction of Hierarchical Functionalized Black Phosphorus with Polydopamine: A Novel Strategy for Enhancing Flame Retardancy and Mechanical Properties of Polyvinyl Alcohol. Chem. Eng. J. 2020, 402, 126212. [Google Scholar] [CrossRef]
- Gu, Y.-H.; Liu, H.-W.; Dong, X.-H.; Ma, Z.-Z.; Li, Y.-X.; Li, L.; Gan, D.-L.; Liu, P.-S.; Shen, J. Zwitterionic-Phosphonate Block Polymer as Anti-Fouling Coating for Biomedical Metals. Rare Met. 2022, 41, 700–712. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.; Xiao, B.; Zhou, C.; Jiang, Z.; Liang, Y.; Sun, Z.; Xiong, J.; Chen, G.; Zhu, H.; et al. Visible-Light Photocatalytic Degradation of Water-Soluble Polyvinyl Alcohol in Aqueous Solution by Cu2O@TiO2: Optimization of Conditions, Mechanisms and Toxicity Analysis. J. Environ. Manag. 2023, 341, 118054. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Vale, A.C.; Alves, N.M. Spin-Coated Freestanding Films for Biomedical Applications. J. Mater. Chem. B 2021, 9, 3778–3799. [Google Scholar] [CrossRef]
- Liao, T.-Y.; Biesiekierski, A.; Berndt, C.C.; King, P.C.; Ivanova, E.P.; Thissen, H.; Kingshott, P. Multifunctional Cold Spray Coatings for Biological and Biomedical Applications: A Review. Prog. Surf. Sci. 2022, 97, 100654. [Google Scholar] [CrossRef]
- Choudhury, M.; Mohanty, S.; Nayak, S. Effect of Different Solvents in Solvent Casting of Porous PLA Scaffolds—In Biomedical and Tissue Engineering Applications. J. Biomater. Tissue Eng. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Zhitomirsky, I. Deposition of Poly(Methyl Methacrylate) and Composites Containing Bioceramics and Bioglass by Dip Coating Using Isopropanol-Water Co-Solvent. Prog. Org. Coatings 2020, 148, 105883. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chen, F.-H.; Wu, G.-L.; Ching, P.C.O.; Yeh, M.-L. Influence of Spin Coating and Dip Coating with Gelatin/Hydroxyapatite for Bioresorbable Mg Alloy Orthopedic Implants: In Vitro and In Vivo Studies. ACS Biomater. Sci. Eng. 2023, 9, 705–718. [Google Scholar] [CrossRef]
- Sing, J.; Singh, S.; Gill, R. Applications of Biopolymer Coatings in Biomedical Engineering. J. Electrochem. Sci. Eng. 2022, 13, 63–81. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. Dip-Coating for Fibrous Materials: Mechanism, Methods and Applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Badaraev, A.D.; Tran, T.-H.; Drozd, A.G.; Plotnikov, E.V.; Dubinenko, G.E.; Kozelskaya, A.I.; Rutkowski, S.; Tverdokhlebov, S.I. Effect of PLGA Concentration in Electrospinning Solution on Biocompatibility, Morphology and Mechanical Properties of Nonwoven Scaffolds. Technologies 2023, 11, 137. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Papastavrou, G.; Borkovec, M.; Behrens, S.H. Imaging the Coil-to-Globule Conformational Transition of a Weak Polyelectrolyte by Tuning the Polyelectrolyte Charge Density. Nano Lett. 2004, 4, 149–152. [Google Scholar] [CrossRef]
- Kemme, M.; Heinzel-Wieland, R. Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol. J. Funct. Biomater. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.P.; Pawar, S.; Chougule, M.; Godse, P.; Sakhare, R.; Sen, S.; Joshi, P. Effect of Annealing on Structural, Morphological, Electrical and Optical Studies of Nickel Oxide Thin Films. J. Surf. Eng. Mater. Adv. Technol. 2011, 1, 35–41. [Google Scholar] [CrossRef]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A Comparative Study of the Crystallinity of Polyetheretherketone by Using Density, DSC, XRD, and Raman Spectroscopy Techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Ningrum, D.R.; Hanif, W.; Mardhian, D.F.; Asri, L.A.T.W. In Vitro Biocompatibility of Hydrogel Polyvinyl Alcohol/Moringa Oleifera Leaf Extract/Graphene Oxide for Wound Dressing. Polymers 2023, 15, 468. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Bin Sabri, A.H.; Moreno-Castellanos, N.; Utomo, E.; Cárcamo-Martínez, Á.; Domínguez-Robles, J.; Wardoyo, L.A.H.; Donnelly, R.F. Soluplus®-Based Dissolving Microarray Patches Loaded with Colchicine: Towards a Minimally Invasive Treatment and Management of Gout. Biomater. Sci. 2022, 10, 5838–5855. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Masse, L.; Kim, S.; Schlezinger, J.J.; Webster, T.F.; Stapleton, H.M. Characterization of Adipogenic Chemicals in Three Different Cell Culture Systems: Implications for Reproducibility Based on Cell Source and Handling. Sci. Rep. 2017, 7, 42104. [Google Scholar] [CrossRef]
- Bakibaev, A.A.; Tuguldurova, V.P.; Lyapunova, M.V.; Ivanov, V.V.; Kaidash, O.A.; Udut, E.V.; Bukterov, M.V.; Buyko, E.E.; Kasyanova, A.S.; Malkov, V.S. Anti-Adhesion Effect of Composite Film Materials Based on Glycoluril-Modified Sodium Carboxymethyl Cellulose. Sovrem. Tehnol. Med. 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Shams, T.; Parhizkar, M.; Illangakoon, U.E.; Orlu, M.; Edirisinghe, M. Core/Shell Microencapsulation of Indomethacin/Paracetamol by Co-Axial Electrohydrodynamic Atomization. Mater. Des. 2017, 136, 204–213. [Google Scholar] [CrossRef]
- Bastidas, J.G.; Maurmann, N.; da Silveira, M.R.; Ferreira, C.A.; Pranke, P. Development of Fibrous PLGA/Fibrin Scaffolds as a Potential Skin Substitute. Biomed. Mater. 2020, 15, 055014. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of Ultrasonication Duration of Polyvinyl Alcohol (PVA) Gel on Characterizations of PVA Film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Mat Hussin, R.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, Optical, Opto-Thermal and Thermal Properties of ZnS–PVA Nanofluids Synthesized through a Radiolytic Approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Mojet, B.L.; Ebbesen, S.D.; Lefferts, L. Light at the Interface: The Potential of Attenuated Total Reflection Infrared Spectroscopy for Understanding Heterogeneous Catalysis in Water. Chem. Soc. Rev. 2010, 39, 4643. [Google Scholar] [CrossRef]
- García-Pérez, C.A.; González-Dueñas, V.A.; Rodríguez-Macías, F.J.; Menchaca-Campos, C.; González-Noriega, O.A.; Vega-Cantú, Y.I. Functional and Structural Modification of Polyvinyl Alcohol/Carbon Nanotubes Composite Fibers. Results Mater. 2024, 23, 100603. [Google Scholar] [CrossRef]
- Freire, T.F.; Quinaz, T.; Fertuzinhos, A.; Quyền, N.T.; de Moura, M.F.S.M.; Martins, M.; Zille, A.; Dourado, N. Thermal, Mechanical and Chemical Analysis of Poly(Vinyl Alcohol) Multifilament and Braided Yarns. Polymers 2021, 13, 3644. [Google Scholar] [CrossRef]
- Li, K.; Li, P.; Jia, Z.; Qi, B.; Xu, J.; Kang, D.; Liu, M.; Fan, Y. Enhanced Fluorescent Intensity of Magnetic-Fluorescent Bifunctional PLGA Microspheres Based on Janus Electrospraying for Bioapplication. Sci. Rep. 2018, 8, 17117. [Google Scholar] [CrossRef]
- Song, B.; Cao, Y.; Wang, L.; Shen, Y.; Qian, X. Properties and Structure of Thermoplastic Polyvinyl Alcohol/Polyamide Sea-Island Fibers. Polymers 2023, 15, 2071. [Google Scholar] [CrossRef]
- Gupta, B.; Agarwal, R.; Sarwar Alam, M. Preparation and Characterization of Polyvinyl Alcohol-polyethylene Oxide-carboxymethyl Cellulose Blend Membranes. J. Appl. Polym. Sci. 2013, 127, 1301–1308. [Google Scholar] [CrossRef]
- Batra, R.; Bansal, P.; Yadav, R.; Purwar, R.; Kulanthaivel, S.; Mishra, P. Enhancement of Functional Properties by Blending Cocoon Extracted Antheraea Mylitta Silk Fibroin with Polyvinyl Alcohol for Applications in Biomedical Field. J. Appl. Polym. Sci. 2022, 139, 51913. [Google Scholar] [CrossRef]
- Paneru, R.; Lamichhane, P.; Chandra Adhikari, B.; Ki, S.H.; Choi, J.; Kwon, J.S.; Choi, E.H. Surface Modification of PVA Thin Film by Nonthermal Atmospheric Pressure Plasma for Antifogging Property. AIP Adv. 2019, 9, 075008. [Google Scholar] [CrossRef]
- Motealleh, A.; Eqtesadi, S.; Perera, F.H.; Pajares, A.; Guiberteau, F.; Miranda, P. Understanding the Role of Dip-Coating Process Parameters in the Mechanical Performance of Polymer-Coated Bioglass Robocast Scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 64, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Duan, K.; Zhang, J.W.; Lu, X.; Weng, J. The Influence of Polymer Concentrations on the Structure and Mechanical Properties of Porous Polycaprolactone-Coated Hydroxyapatite Scaffolds. Appl. Surf. Sci. 2010, 256, 4586–4590. [Google Scholar] [CrossRef]
- Hexig, B.; Nakaoka, R.; Tsuchiya, T. Safety Evaluation of Surgical Materials by Cytotoxicity Testing. J. Artif. Organs 2008, 11, 204–211. [Google Scholar] [CrossRef]
- Kang, S.; Park, S.; Baek, I.; Song, Y.; Kim, S.; Choi, D.; Kim, J.; Lee, Y. Development of Poly(D,L-Lactic-Co-Glycolic Acid) Films Coated with Biomembrane-Mimicking Polymers for Anti-Adhesion Activity. Mater. Sci. Eng. C 2021, 120, 111780. [Google Scholar] [CrossRef]
- Weis, C.; Odermatt, E.K.; Kressler, J.; Funke, Z.; Wehner, T.; Freytag, D. Poly(Vinyl Alcohol) Membranes for Adhesion Prevention. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70B, 191–202. [Google Scholar] [CrossRef]
- Ino, J.M.; Sju, E.; Ollivier, V.; Yim, E.K.F.; Letourneur, D.; Le Visage, C. Evaluation of Hemocompatibility and Endothelialization of Hybrid Poly(Vinyl Alcohol) (PVA)/Gelatin Polymer Films. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1549–1559. [Google Scholar] [CrossRef]
- Kraskouski, A.; Hileuskaya, K.; Kulikouskaya, V.; Kabanava, V.; Agabekov, V.; Pinchuk, S.; Vasilevich, I.; Volotovski, I.; Kuznetsova, T.; Lapitskaya, V. Polyvinyl Alcohol and Pectin Blended Films: Preparation, Characterization, and Mesenchymal Stem Cells Attachment. J. Biomed. Mater. Res. Part A 2021, 109, 1379–1392. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of Glutaraldehyde Crosslinked Collagen/Poly(Vinyl Alcohol) Films Is by the Mechanism of Apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Huang, Y.; Liang, M.; Ren, P.; Tao, Y.; Xu, L.; Zhang, T.; Ji, Z.; Zhang, Q. Polypropylene Composite Hernia Mesh with Anti-Adhesion Layer Composed of PVA Hydrogel and Liposomes Drug Delivery System. Colloids Surf. B Biointerfaces 2023, 223, 113159. [Google Scholar] [CrossRef] [PubMed]
- Kerch, G. Polymer Hydration and Stiffness at Biointerfaces and Related Cellular Processes. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface Hydration: Principles and Applications toward Low-Fouling/Nonfouling Biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Cabrera-Sanfelix, P.; Arnau, A.; Darling, G.R.; Sanchez-Portal, D. On the Structure of the First Hydration Layer on NaCl(100): Role of Hydrogen Bonding. J. Chem. Phys. 2007, 126, 214707. [Google Scholar] [CrossRef]
- Huang, Z.; Ghasemi, H. Hydrophilic Polymer-Based Anti-Biofouling Coatings: Preparation, Mechanism, and Durability. Adv. Colloid Interface Sci. 2020, 284, 102264. [Google Scholar] [CrossRef]
- Randriantsilefisoa, R.; Hou, Y.; Pan, Y.; Camacho, J.L.C.; Kulka, M.W.; Zhang, J.; Haag, R. Interaction of Human Mesenchymal Stem Cells with Soft Nanocomposite Hydrogels Based on Polyethylene Glycol and Dendritic Polyglycerol. Adv. Funct. Mater. 2020, 30, 1905200. [Google Scholar] [CrossRef]
- Zhou, M.-C.; Wu, W.-M.; Yang, S.-H.; Zhou, B.; Zhang, Z.; Liu, Z.-T.; Li, K.-N.; Hu, X.-L. Advances in Polyethylene Glycol-Based Materials in Peritoneal Adhesions. J. Biomater. Tissue Eng. 2024, 14, 97–114. [Google Scholar] [CrossRef]
- Chen, Z. Surface Hydration and Antifouling Activity of Zwitterionic Polymers. Langmuir 2022, 38, 4483–4489. [Google Scholar] [CrossRef]
- Daley, K.R.; Kubarych, K.J. An “Iceberg” Coating Preserves Bulk Hydration Dynamics in Aqueous PEG Solutions. J. Phys. Chem. B 2017, 121, 10574–10582. [Google Scholar] [CrossRef]
- Molino, P.J.; Yang, D.; Penna, M.; Miyazawa, K.; Knowles, B.R.; MacLaughlin, S.; Fukuma, T.; Yarovsky, I.; Higgins, M.J. Hydration Layer Structure of Biofouling-Resistant Nanoparticles. ACS Nano 2018, 12, 11610–11624. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.; Sun, S.; Zhang, K.; Jiang, S.; Chen, Z. Molecular Level Studies on Interfacial Hydration of Zwitterionic and Other Antifouling Polymers in Situ. Acta Biomater. 2016, 40, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Wang, B.B.; Li, W.X.; Fei, W.M.; Cui, Y.; Guo, X.D. In Vivo Safety Assessment, Biodistribution and Toxicology of Polyvinyl Alcohol Microneedles with 160-Day Uninterruptedly Applications in Mice. Eur. J. Pharm. Biopharm. 2021, 160, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Davey, A.K.; Maher, P.J. Surgical Adhesions: A Timely Update, a Great Challenge for the Future. J. Minim. Invasive Gynecol. 2007, 14, 15–22. [Google Scholar] [CrossRef]
- Rudra, R.; Kumar, V.; Kundu, P.P. Acid Catalysed Cross-Linking of Poly Vinyl Alcohol (PVA) by Glutaraldehyde: Effect of Crosslink Density on the Characteristics of PVA Membranes Used in Single Chambered Microbial Fuel Cells. RSC Adv. 2015, 5, 83436–83447. [Google Scholar] [CrossRef]
- Sau, S.; Pandit, S.; Kundu, S. Crosslinked Poly (Vinyl Alcohol): Structural, Optical and Mechanical Properties. Surf. Interfaces 2021, 25, 101198. [Google Scholar] [CrossRef]
- Atlan, M.; Simon-Yarza, T.; Ino, J.M.; Hunsinger, V.; Corté, L.; Ou, P.; Aid-Launais, R.; Chaouat, M.; Letourneur, D. Design, Characterization and in Vivo Performance of Synthetic 2 Mm-Diameter Vessel Grafts Made of PVA-Gelatin Blends. Sci. Rep. 2018, 8, 7417. [Google Scholar] [CrossRef]
- Yang, X.; Sha, D.; Sun, L.; Chen, L.; Xu, J.; Shi, K.; Yu, C.; Wang, B.; Ji, X. Charged Group-Modified Poly(Vinyl Alcohol) Hydrogels: Preparation and Antibacterial Property. React. Funct. Polym. 2020, 154, 104635. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badaraev, A.D.; Plotnikov, E.V.; Bukal, V.R.; Dubinenko, G.E.; Frueh, J.; Rutkowski, S.; Tverdokhlebov, S.I. Fabrication of PVA Coatings Applied to Electrospun PLGA Scaffolds to Prevent Postoperative Adhesions. J. Funct. Biomater. 2025, 16, 57. https://doi.org/10.3390/jfb16020057
Badaraev AD, Plotnikov EV, Bukal VR, Dubinenko GE, Frueh J, Rutkowski S, Tverdokhlebov SI. Fabrication of PVA Coatings Applied to Electrospun PLGA Scaffolds to Prevent Postoperative Adhesions. Journal of Functional Biomaterials. 2025; 16(2):57. https://doi.org/10.3390/jfb16020057
Chicago/Turabian StyleBadaraev, Arsalan D., Evgenii V. Plotnikov, Vladislav R. Bukal, Gleb E. Dubinenko, Johannes Frueh, Sven Rutkowski, and Sergei I. Tverdokhlebov. 2025. "Fabrication of PVA Coatings Applied to Electrospun PLGA Scaffolds to Prevent Postoperative Adhesions" Journal of Functional Biomaterials 16, no. 2: 57. https://doi.org/10.3390/jfb16020057
APA StyleBadaraev, A. D., Plotnikov, E. V., Bukal, V. R., Dubinenko, G. E., Frueh, J., Rutkowski, S., & Tverdokhlebov, S. I. (2025). Fabrication of PVA Coatings Applied to Electrospun PLGA Scaffolds to Prevent Postoperative Adhesions. Journal of Functional Biomaterials, 16(2), 57. https://doi.org/10.3390/jfb16020057










