Next-Generation Biomaterials for Wound Healing: Development and Evaluation of Collagen Scaffolds Functionalized with a Heparan Sulfate Mimic and Fibroblast Growth Factor 2
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Scaffolds
2.2. Scaffold Characterization
2.2.1. Quantification of OTR4120 and Heparin Content
2.2.2. Effect of γ-Irradiation on Collagen Scaffolds
2.2.3. Localization of Heparin, OTR4120, and FGF-2
2.2.4. Quantification of FGF-2 Captured by Scaffolds
2.2.5. Quantification of FGF-2 Release by Scaffolds
2.3. Anti-Fibrotic Response In Vitro
2.3.1. Cell Culture
2.3.2. Quantitative Gene Expression Analysis (qPCR) and α-SMA Protein Expression
2.3.3. Data Analysis
3. Results
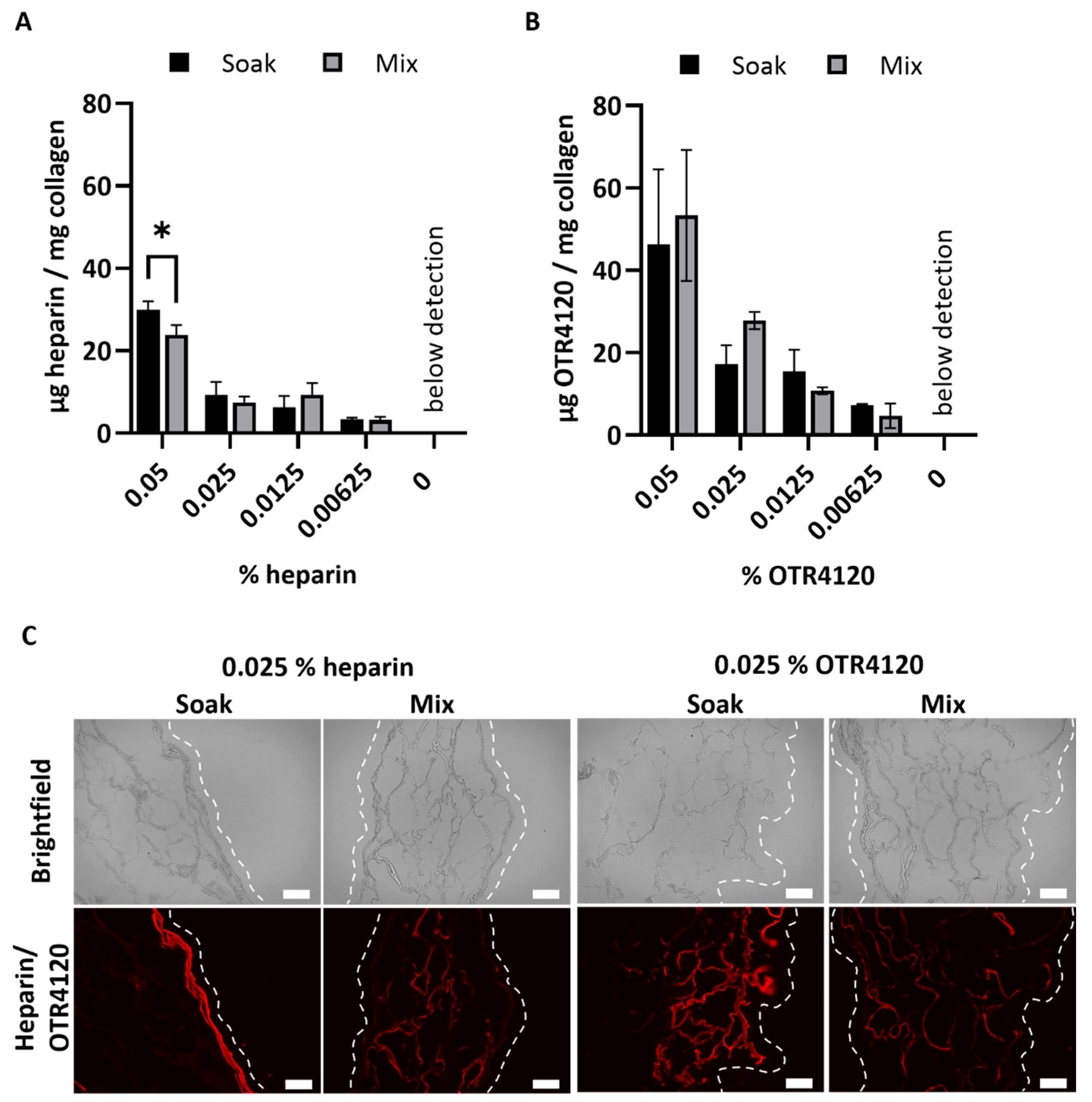
3.1. Production and Characterization of Scaffolds
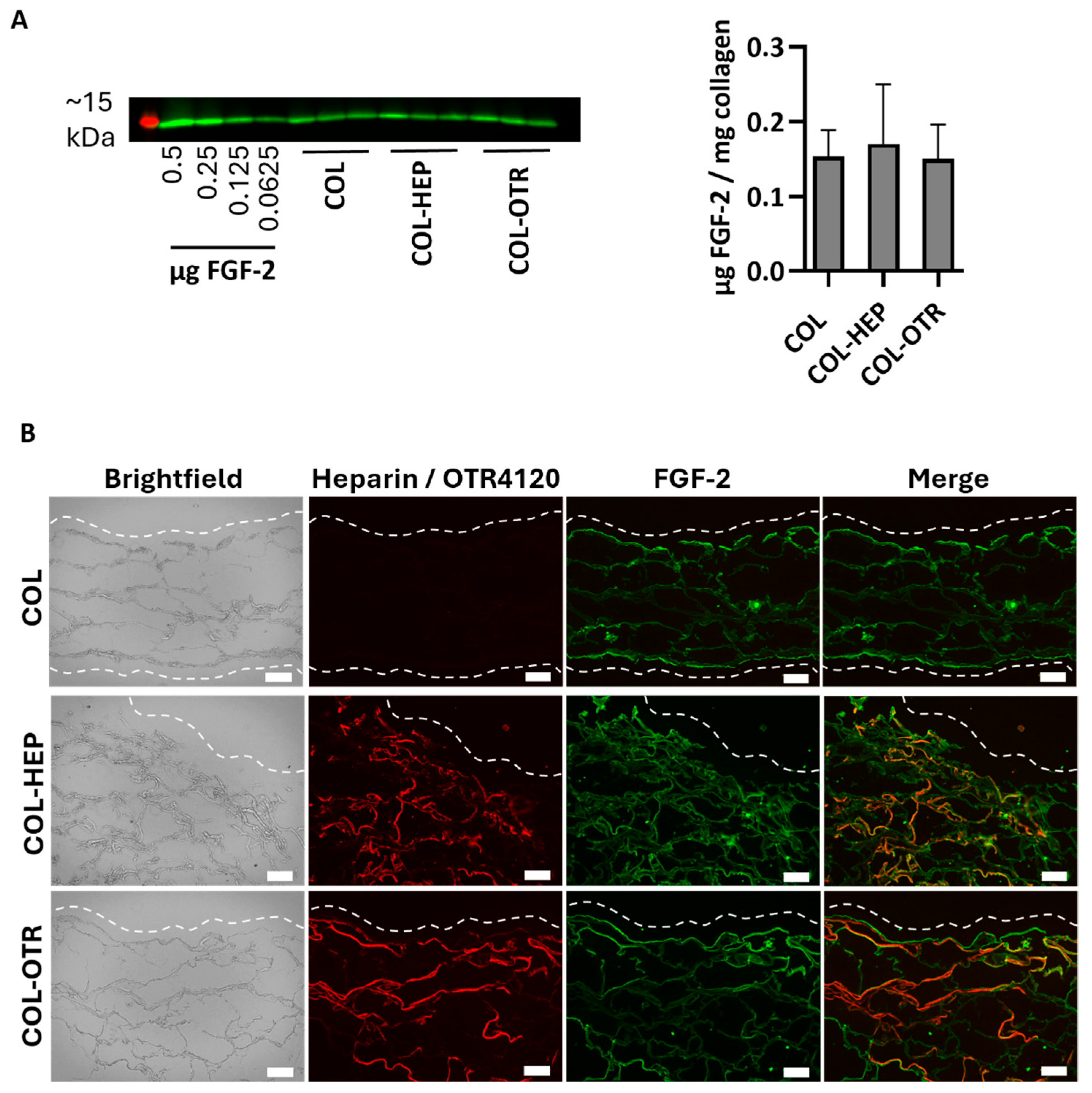
3.2. Collagen–OTR4120 Scaffolds Capture FGF-2 Comparably to Collagen–Heparin
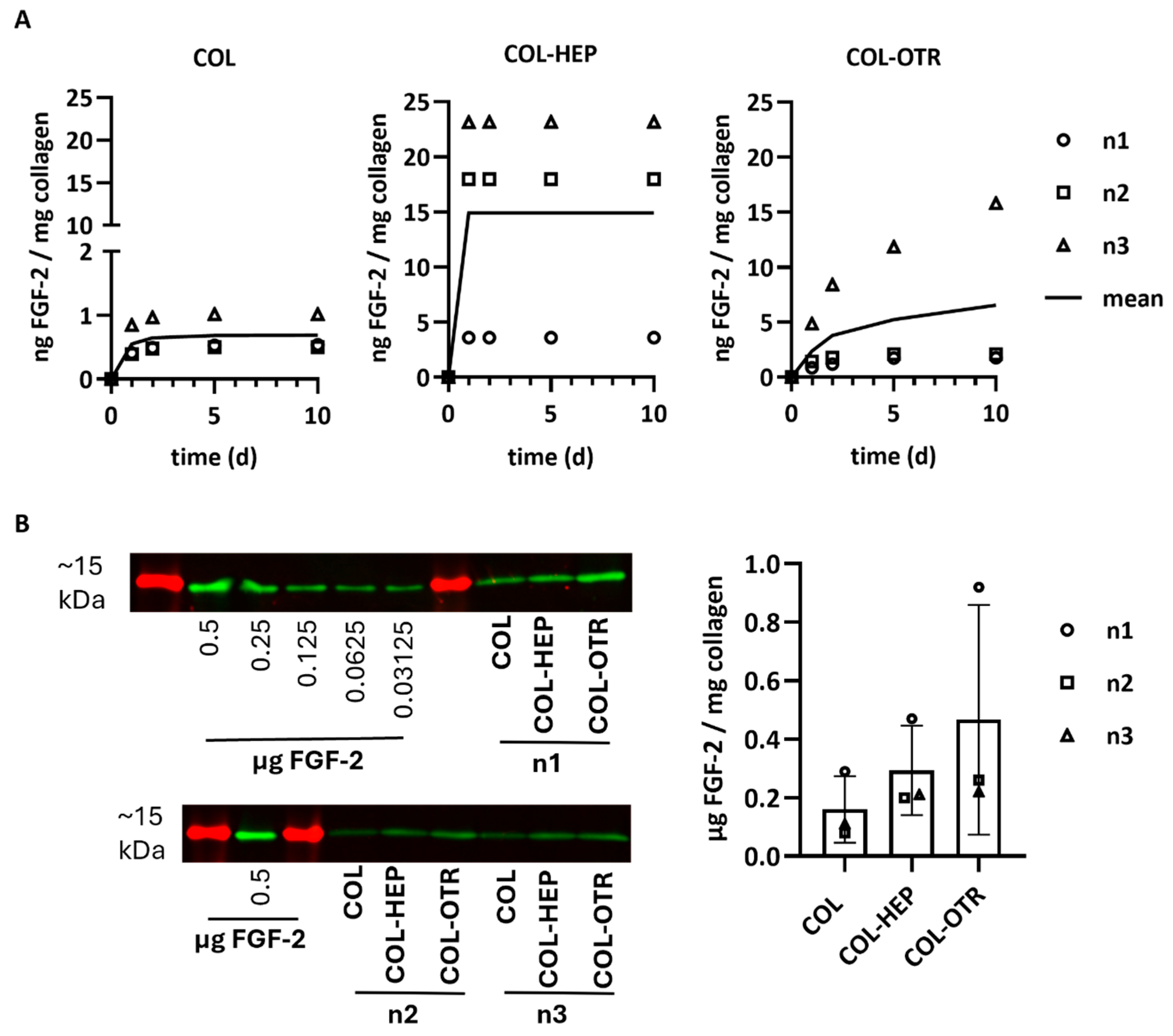
3.3. Collagen–OTR4120 Scaffolds Gradually Release FGF-2
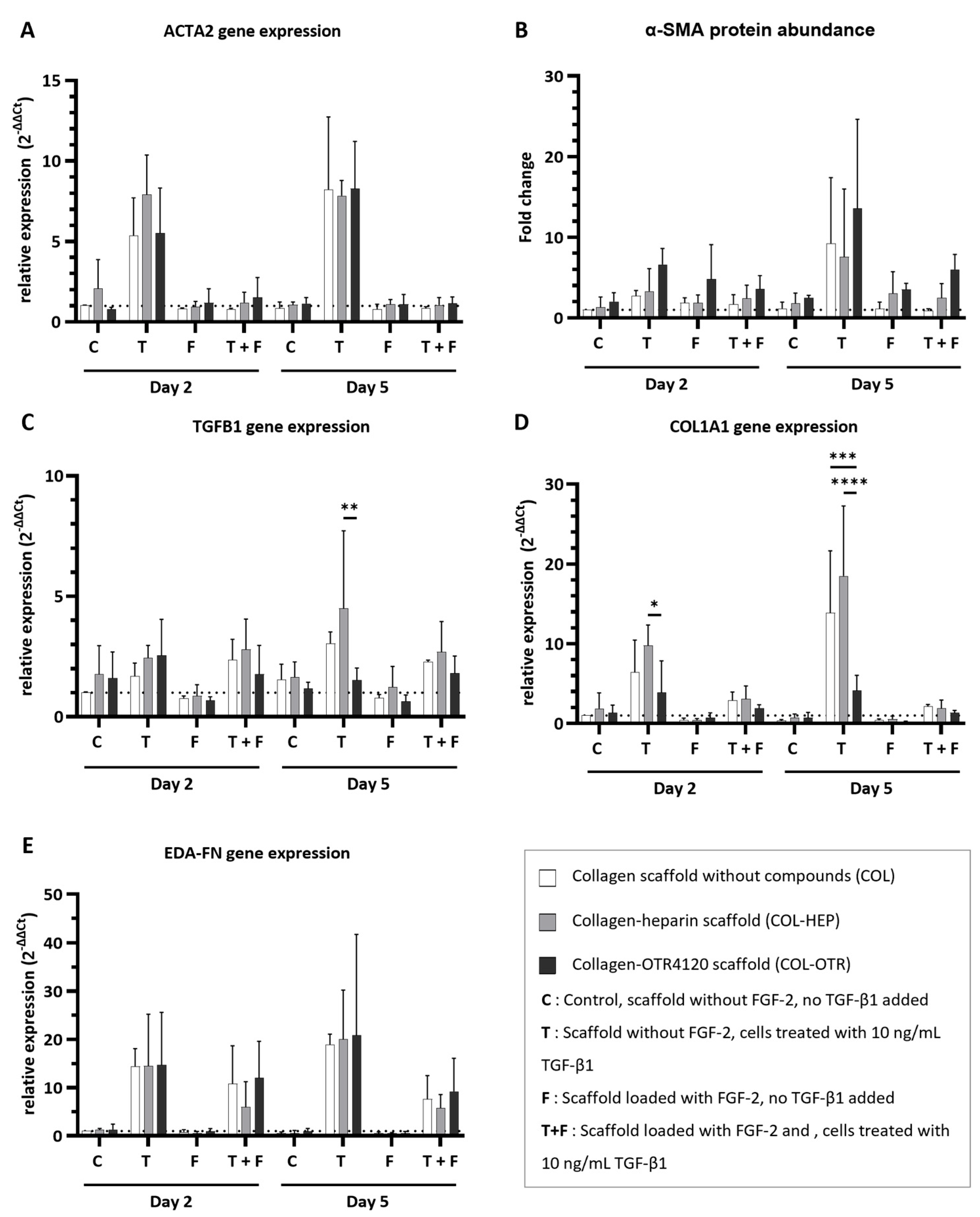
3.4. Collagen–OTR4120(-FGF-2) Scaffolds Possess Inherent Anti-Fibrotic Properties In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Bucan, V.; Vogt, P.M.; Krezdorn, N. A Short History of Skin Grafting in Burns: From the Gold Standard of Autologous Skin Grafting to the Possibilities of Allogeneic Skin Grafting with Immunomodulatory Approaches. Medicina 2021, 57, 225. [Google Scholar] [CrossRef]
- Ayaz, M.; Najafi, A.; Karami, M.Y. Thin Split Thickness Skin Grafting on Human Acellular Dermal Matrix Scaffold for the Treatment of Deep Burn Wounds. Int. J. Organ Transpl. Med. 2021, 12, 44–51. [Google Scholar]
- Biazar, E.; Heidari Keshel, S.; Rezaei Tavirani, M.; Kamalvand, M. Healing effect of acellular fish skin with plasma rich in growth factor on full-thickness skin defects. Int. Wound J. 2022, 19, 2154–2162. [Google Scholar] [CrossRef]
- Press, I.; Moiemen, N.; Ahmed, Z. Efficacy and Complications Associated with Acellular Dermal Substitute Use in the Treatment of Acute Burns: A Systematic Review and Meta-Analysis. Eur. Burn J. 2023, 4, 548–562. [Google Scholar] [CrossRef]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Godin, M.; Pelling, A.E. Mechanical stretch sustains myofibroblast phenotype and function in microtissues through latent TGF-beta1 activation. Integr. Biol. 2020, 12, 199–210. [Google Scholar] [CrossRef]
- Hall, C.L.; Wells, A.R.; Leung, K.P. Pirfenidone reduces profibrotic responses in human dermal myofibroblasts, in vitro. Lab. Investig. 2018, 98, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Samandari, M.; Endo, Y.; Zhang, Y.; Quint, J.; Schmidt, T.A.; Tamayol, A.; Sinha, I. printing of growth factor-eluting adhesive scaffolds improves wound healing. Bioact. Mater. 2022, 8, 296–308. [Google Scholar] [CrossRef]
- Niu, C.; Wang, L.; Ji, D.; Ren, M.; Ke, D.; Fu, Q.; Zhang, K.; Yang, X. Fabrication of SA/Gel/C scaffold with 3D bioprinting to generate micro-nano porosity structure for skin wound healing: A detailed animal in vivo study. Cell Regen. 2022, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, T.H.; Kang, M.S.; Kim, H.W. Angiogenic Effects of Collagen/Mesoporous Nanoparticle Composite Scaffold Delivering VEGF. Bio. Res. Int. 2016, 2016, 9676934. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type, I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- De Francesco, F.; Busato, A.; Mannucci, S.; Zingaretti, N.; Cottone, G.; Amendola, F.; De Francesco, M.; Merigo, F.; Riccio, V.; Vaienti, L.; et al. Artificial dermal substitutes for tissue regeneration: Comparison of the clinical outcomes and histological findings of two templates. J. Int. Med. Res. 2020, 48, 300060520945508. [Google Scholar] [CrossRef]
- Sarmin, A.M.; El Moussaid, N.; Suntornnond, R.; Tyler, E.J.; Kim, Y.H.; Di Cio, S.; Megone, W.V.; Pearce, O.; Gautrot, J.E.; Dawson, J.; et al. Multi-Scale Analysis of the Composition, Structure, and Function of Decellularized Extracellular Matrix for Human Skin and Wound Healing Models. Biomolecules 2022, 12, 837. [Google Scholar] [CrossRef]
- Dolivo, D.M.; Larson, S.A.; Dominko, T. FGF2-mediated attenuation of myofibroblast activation is modulated by distinct MAPK signaling pathways in human dermal fibroblasts. J. Dermatol. Sci. 2017, 88, 339–348. [Google Scholar] [CrossRef]
- Guimond, S.; Maccarana, M.; Olwin, B.B.; Lindahl, U.; Rapraeger, A.C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J. Biol. Chem. 1993, 268, 23906–23914. [Google Scholar] [CrossRef]
- Forsten-Williams, K.; Chua, C.C.; Nugent, M.A. The kinetics of FGF-2 binding to heparan sulfate proteoglycans and MAP kinase signaling. J. Theor. Biol. 2005, 233, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, Y.; Mizumachi, H.; Yoshida, K.; Ijima, H. Heparin-conjugated collagen as a potent growth factor-localizing and stabilizing scaffold for regenerative medicine. Regen. Ther. 2020, 15, 236–242. [Google Scholar] [CrossRef]
- Pieper, J.S.; Hafmans, T.; van Wachem, P.B.; van Luyn, M.J.; Brouwer, L.A.; Veerkamp, J.H.; van Kuppevelt, T.H. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J. Biomed. Mater. Res. 2002, 62, 185–194. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Mencner, L.; Komosinska-Vassev, K. Diverse Roles of Heparan Sulfate and Heparin in Wound Repair. Bio. Res. Int. 2015, 2015, 549417. [Google Scholar] [CrossRef]
- Barritault, D.; Gilbert-Sirieix, M.; Rice, K.L.; Sineriz, F.; Papy-Garcia, D.; Baudouin, C.; Desgranges, P.; Zakine, G.; Saffar, J.L.; van Neck, J. RGTA((R)) or ReGeneraTing Agents mimic heparan sulfate in regenerative medicine: From concept to curing patients. Glycoconj. J. 2017, 34, 325–338. [Google Scholar] [CrossRef]
- Samakidou, G.; Eleftheriadou, I.; Anastasiou, I.A.; Kosta, O.; Tentolouris, A.; Evangelou, K.; Tentolouris, N. A Single Center, Randomized Controlled Trial on the Efficacy of Topical Application of ReGenerating Tissue Agents (RGTA) Technology in Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2024, 15347346241259893. [Google Scholar] [CrossRef] [PubMed]
- ISO 11137-1:2006; Sterilization of Health Care Products—Radiation. Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices. ISO: Geneva, Switzerland, 2006.
- ISO 13485:2016; Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes. ISO: Geneva, Switzerland, 2016.
- Clausen, T.M.; Kumar, G.; Ibsen, E.K.; Orum-Madsen, M.S.; Hurtado-Coll, A.; Gustavsson, T.; Agerbaek, M.O.; Gatto, F.; Todenhofer, T.; Basso, U.; et al. A simple method for detecting oncofetal chondroitin sulfate glycosaminoglycans in bladder cancer urine. Cell Death Discov. 2020, 6, 65. [Google Scholar] [CrossRef]
- Ten Dam, G.B.; Kurup, S.; van de Westerlo, E.M.; Versteeg, E.M.; Lindahl, U.; Spillmann, D.; van Kuppevelt, T.H. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J. Biol. Chem. 2006, 281, 4654–4662. [Google Scholar] [CrossRef]
- Benjamini, Y.; Krieger, A.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Zhou, H.; Wu, X.; Yao, K.D. pH-responsive swelling behavior of collagen complex materials. Artif. Cells Blood Substit. Immobil. Biotechnol. 2000, 28, 255–262. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Wang, T.; Goff, H.D.; Zhong, F. Fabrication of films with tailored properties by regulating the swelling of collagen fiber through pH adjustment. Food Hydrocoll. 2020, 108, 106016. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef]
- Ikeda, Y.; Charef, S.; Ouidja, M.O.; Barbier-Chassefière, V.; Sineriz, F.; Duchesnay, A.; Narasimprakash, H.; Martelly, I.; Kern, P.; Barritault, D.; et al. Synthesis and biological activities of a library of glycosaminoglycans mimetic oligosaccharides. Biomaterials 2011, 32, 769–776. [Google Scholar] [CrossRef] [PubMed]
- DePhillips, P.; Lenhoff, A.M. Relative retention of the fibroblast growth factors FGF-1 and FGF-2 on strong cation-exchange sorbents. J. Chromatogr. A. 2004, 1036, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gresham, R.C.H.; Bahney, C.S.; Leach, J.K. Growth factor delivery using extracellular matrix-mimicking substrates for musculoskeletal tissue engineering and repair. Bioact. Mater. 2021, 6, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Rushton, G.; Gallagher, J.T. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J. Biol. Chem. 1997, 272, 18000–18006. [Google Scholar] [CrossRef]
- Rider, C.C.; Mulloy, B. Heparin, Heparan Sulphate and the TGF-beta Cytokine Superfamily. Molecules 2017, 22, 713. [Google Scholar] [CrossRef]
- Hulme, E.C.; Trevethick, M.A. Ligand binding assays at equilibrium: Validation and interpretation. Br. J. Pharmacol. 2010, 161, 1219–1237. [Google Scholar] [CrossRef]
- Chogan, F.; Mirmajidi, T.; Rezayan, A.H.; Sharifi, A.M.; Ghahary, A.; Nourmohammadi, J.; Kamali, A.; Rahaie, M. Design, fabrication, and optimization of a dual function three-layer scaffold for controlled release of metformin hydrochloride to alleviate fibrosis and accelerate wound healing. Acta. Biomater. 2020, 113, 144–163. [Google Scholar] [CrossRef] [PubMed]
- Mestries, P.; Alexakis, C.; Papy-Garcia, D.; Duchesnay, A.; Barritault, D.; Caruelle, J.P.; Kern, P. Specific RGTA increases collagen V expression by cultured aortic smooth muscle cells via activation and protection of transforming growth factor-beta1. Matrix Biol. 2001, 20, 171–181. [Google Scholar] [CrossRef]
- Urciuolo, F.; Passariello, R.; Imparato, G.; Casale, C.; Netti, P.A. Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro. Bioengineering 2022, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ding, J.; Agrawal, B.; Kwan, P.O.; Tredget, E.E. Stimulation of toll-like receptor pathways by burn eschar tissue as a possible mechanism for hypertrophic scarring. Wound Repair. Regen. 2021, 29, 810–819. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Hofmann, E.; Schwarz, A.; Fink, J.; Kamolz, L.P.; Kotzbeck, P. Modelling the Complexity of Human Skin In Vitro. Biomedicines 2023, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, X.; Chen, J.; Zhen, Z.; Cao, B.; Wan, W.; Dou, Y.; Pan, H.; Xu, F.; Zhang, Z.; et al. Key considerations on the development of biodegradable biomaterials for clinical translation of medical devices: With cartilage repair products as an example. Bioact. Mater. 2022, 9, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.T.; Han, Y.P.; Yan, C.; Shaw, M.C.; Garner, W.L. TNF-alpha suppresses alpha-smooth muscle actin expression in human dermal fibroblasts: An implication for abnormal wound healing. J. Investig. Dermatol. 2007, 127, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gansevoort, M.; Wentholt, S.; Li Vecchi, G.; de Vries, M.; Versteeg, E.M.M.; Boekema, B.K.H.L.; Choppin, A.; Barritault, D.; Chiappini, F.; van Kuppevelt, T.H.; et al. Next-Generation Biomaterials for Wound Healing: Development and Evaluation of Collagen Scaffolds Functionalized with a Heparan Sulfate Mimic and Fibroblast Growth Factor 2. J. Funct. Biomater. 2025, 16, 51. https://doi.org/10.3390/jfb16020051
Gansevoort M, Wentholt S, Li Vecchi G, de Vries M, Versteeg EMM, Boekema BKHL, Choppin A, Barritault D, Chiappini F, van Kuppevelt TH, et al. Next-Generation Biomaterials for Wound Healing: Development and Evaluation of Collagen Scaffolds Functionalized with a Heparan Sulfate Mimic and Fibroblast Growth Factor 2. Journal of Functional Biomaterials. 2025; 16(2):51. https://doi.org/10.3390/jfb16020051
Chicago/Turabian StyleGansevoort, Merel, Sabine Wentholt, Gaia Li Vecchi, Marjolein de Vries, Elly M. M. Versteeg, Bouke K. H. L. Boekema, Agnes Choppin, Denis Barritault, Franck Chiappini, Toin H. van Kuppevelt, and et al. 2025. "Next-Generation Biomaterials for Wound Healing: Development and Evaluation of Collagen Scaffolds Functionalized with a Heparan Sulfate Mimic and Fibroblast Growth Factor 2" Journal of Functional Biomaterials 16, no. 2: 51. https://doi.org/10.3390/jfb16020051
APA StyleGansevoort, M., Wentholt, S., Li Vecchi, G., de Vries, M., Versteeg, E. M. M., Boekema, B. K. H. L., Choppin, A., Barritault, D., Chiappini, F., van Kuppevelt, T. H., & Daamen, W. F. (2025). Next-Generation Biomaterials for Wound Healing: Development and Evaluation of Collagen Scaffolds Functionalized with a Heparan Sulfate Mimic and Fibroblast Growth Factor 2. Journal of Functional Biomaterials, 16(2), 51. https://doi.org/10.3390/jfb16020051







