Three-Dimensional Human Neurovascular Unit Modeling Reveals Cell-Specific Mechanisms of Traumatic Brain Injury
Abstract
1. Introduction
2. Methods
3. Results and Discussion
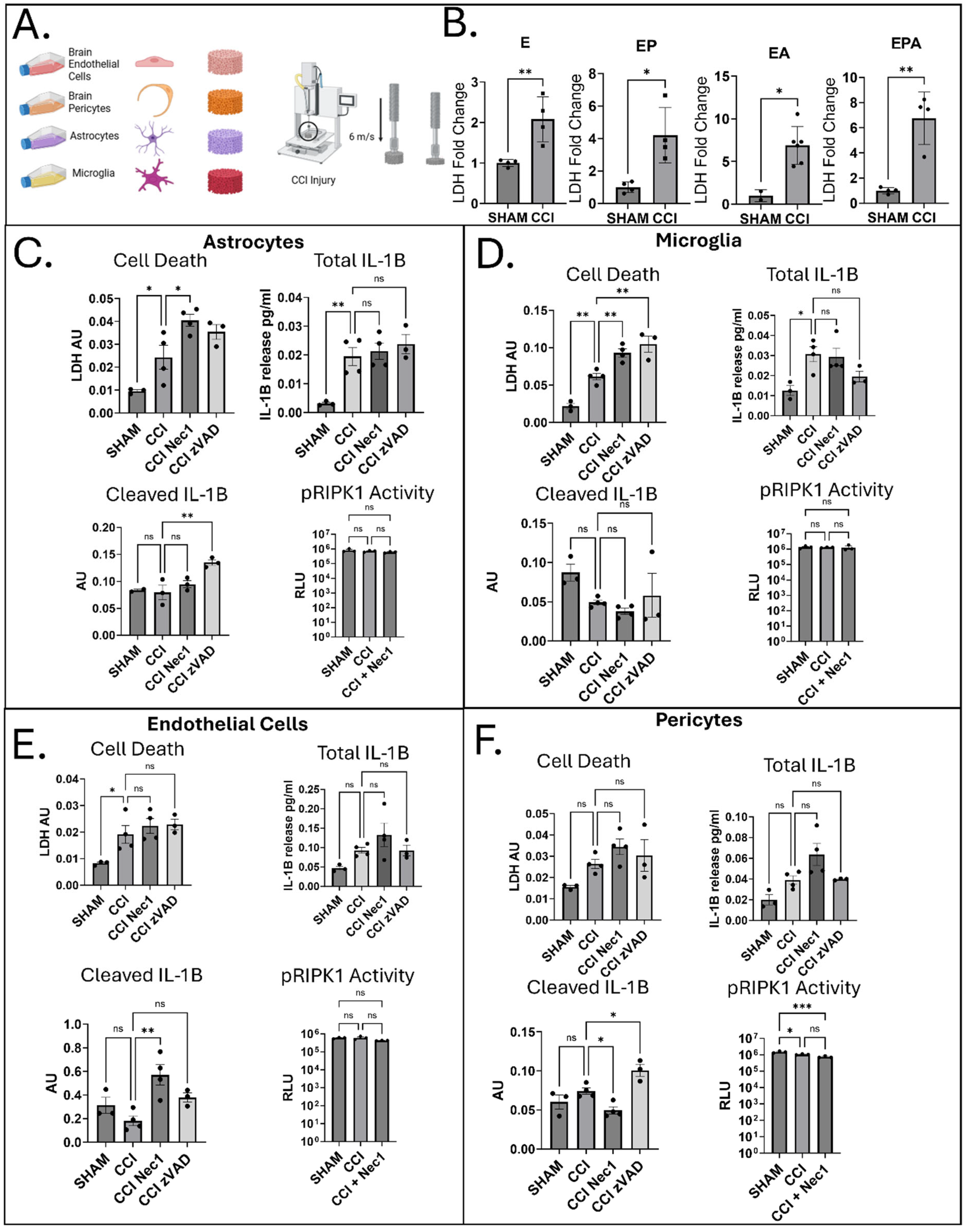
3.1. Acute Cell-Type-Specific Cell Death and IL-1β Responses
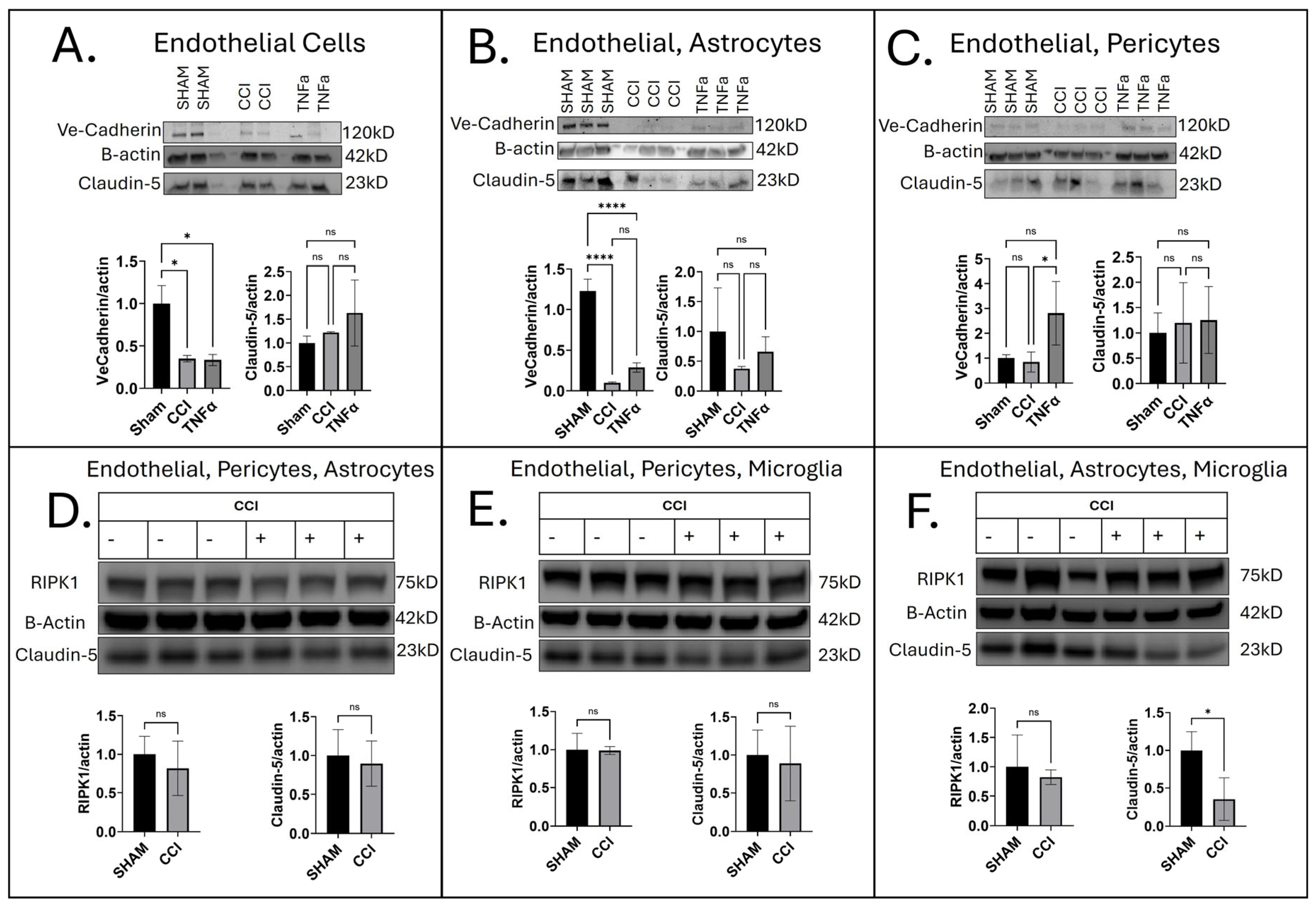
3.2. Cell-Specific Influence on Barrier Integrity Following CCI
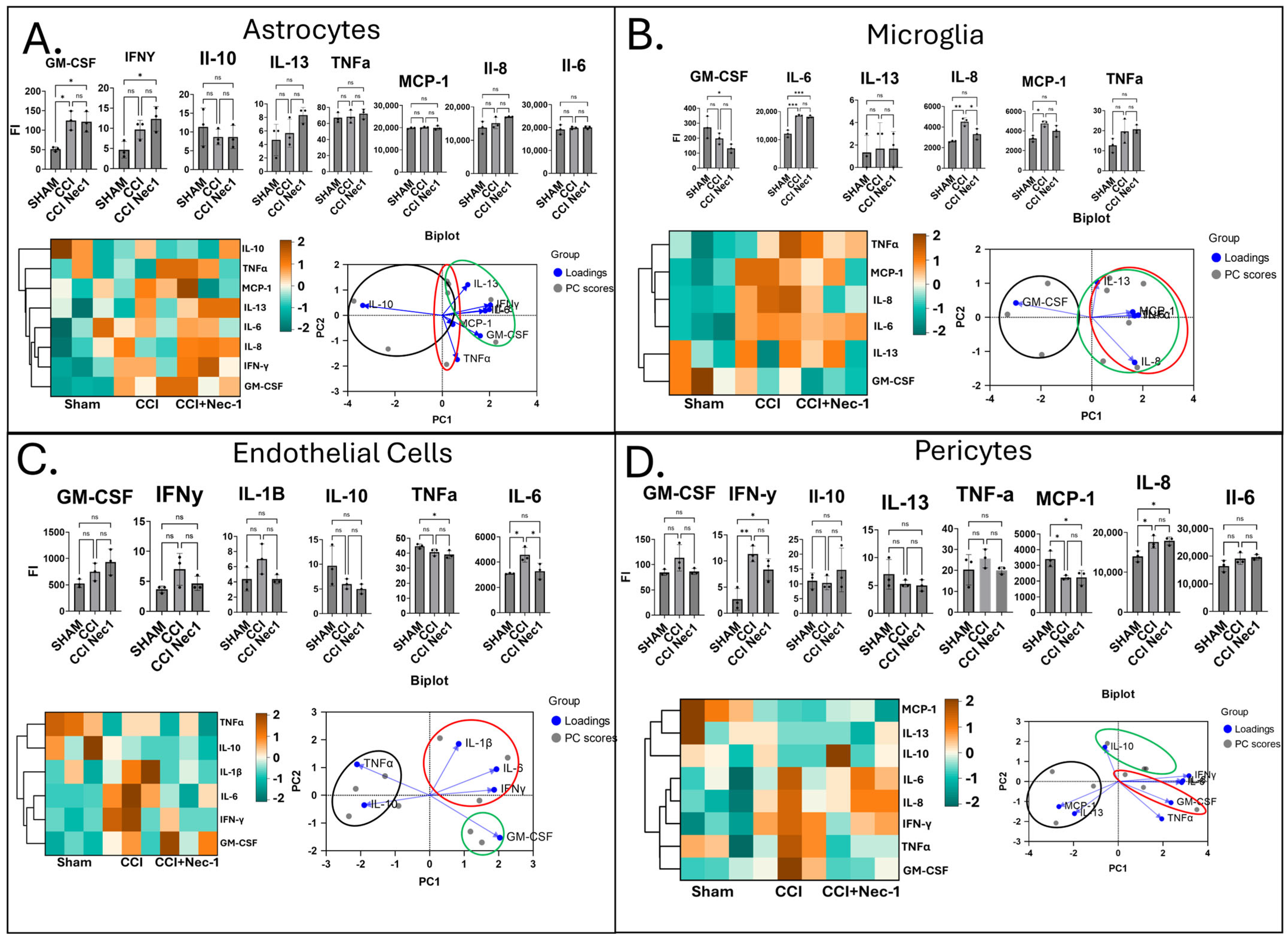
3.3. Paracrine Media Analysis from Injured Monocultures
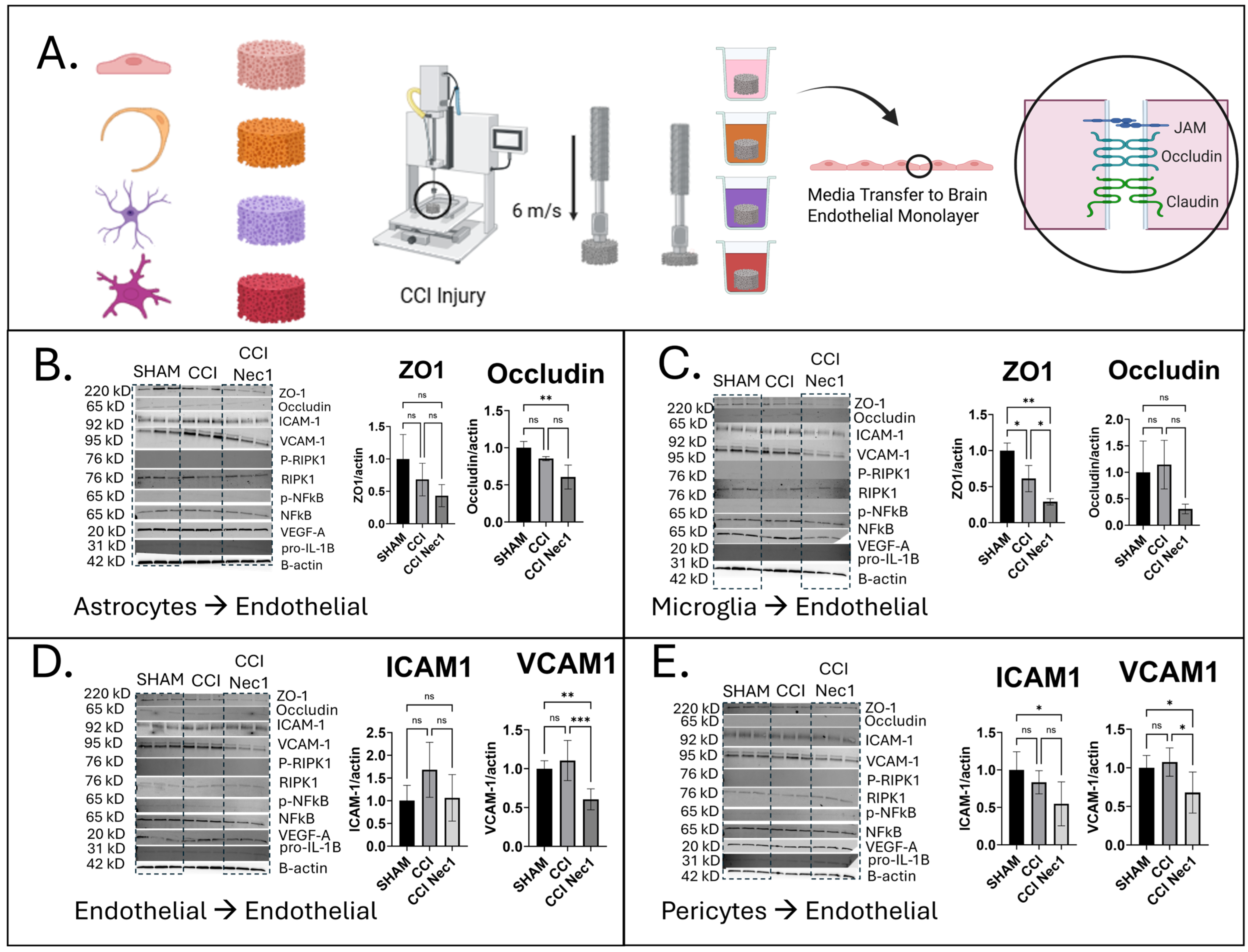
3.4. Effects of Paracrine Media from Injured Monocultures on Brain Endothelial Monolayers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andriessen, T.M.J.C.; Horn, J.; Franschman, G.; van der Naalt, J.; Haitsma, I.; Jacobs, B.; Steyerberg, E.W.; Vos, P.E. Epidemiology, Severity Classification, and Outcome of Moderate and Severe Traumatic Brain Injury: A Prospective Multicenter Study. J. Neurotrauma 2011, 28, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Esterov, D.; Bellamkonda, E.; Mandrekar, J.; Ransom, J.E.; Brown, A.W. Cause of Death after Traumatic Brain Injury: A Population-Based Health Record Review Analysis Referenced for Nonhead Trauma. Neuroepidemiology 2021, 55, 180–187. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The Epidemiology and Impact of Traumatic Brain Injury: A Brief Overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef]
- Tomkins, O.; Feintuch, A.; Benifla, M.; Cohen, A.; Friedman, A.; Shelef, I. Blood-Brain Barrier Breakdown Following Traumatic Brain Injury: A Possible Role in Posttraumatic Epilepsy. Cardiovasc. Psychiatry Neurol. 2011, 2011, 765923. [Google Scholar] [CrossRef]
- Glushakova, O.Y.; Johnson, D.; Hayes, R.L. Delayed Increases in Microvascular Pathology after Experimental Traumatic Brain Injury Are Associated with Prolonged Inflammation, Blood–Brain Barrier Disruption, and Progressive White Matter Damage. J. Neurotrauma 2014, 31, 1180–1193. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Ndode-Ekane, X.E.; Lehto, L.J.; Gorter, J.A.; Andrade, P.; Aronica, E.; Gröhn, O.; Pitkänen, A. Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury. Neurobiol. Dis. 2020, 145, 105080. [Google Scholar] [CrossRef]
- Vázquez-Rosa, E.; Shin, M.-K.; Dhar, M.; Chaubey, K.; Cintrón-Pérez, C.J.; Tang, X.; Liao, X.; Miller, E.; Koh, Y.; Barker, S.; et al. P7C3-A20 treatment one year after TBI in mice repairs the blood–brain barrier, arrests chronic neurodegeneration, and restores cognition. Proc. Natl. Acad. Sci. USA 2020, 117, 27667–27675. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. Modeling the blood–brain barrier: Beyond the endothelial cells. Curr. Opin. Biomed. Eng. 2018, 5, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, J.J.; Gerecht, S. Chipping Away at Blood-Brain-Barrier Modeling. Cell Stem Cell 2019, 24, 831–832. [Google Scholar] [CrossRef]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Moreira, M.; Sarropoulos, I.; Velten, B.; Mort, M.; Cooper, D.N.; Huber, W.; Kaessmann, H. Developmental Gene Expression Differences between Humans and Mammalian Models. Cell Rep. 2020, 33, 108308. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, S.M.; Leclercq, P.D.; Moyes, L.; Graham, D.I.; Smith, C.; Griffin, W.S.T.; Nicoll, J.A.R. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci. Int. 2004, 146, 97–104. [Google Scholar] [CrossRef]
- Liaudanskaya, V.; Chung, J.Y.; Mizzoni, C.; Rouleau, N.; Berk, A.N.; Wu, L.; Turner, J.A.; Georgakoudi, I.; Whalen, M.J.; Nieland, T.J.F.; et al. Modeling Controlled Cortical Impact Injury in 3D Brain-Like Tissue Cultures. Adv. Healthc. Mater. 2020, 9, 2000122. [Google Scholar] [CrossRef] [PubMed]
- Power, L.; Shuhmaher, R.; Houtz, P.; Chen, J.; Rudolph, S.; Yuen, J.; Machour, M.; Levy, E.; Wu, L.; Levenberg, S.; et al. 3D Neurovascular Unit Tissue Model to Assess Responses to Traumatic Brain Injury. J. Biomed. Mater. Res. A 2025, 113, e37816. [Google Scholar] [CrossRef]
- Omelchenko, A.; Singh, N.K.; Firestein, B.L. Current advances in in vitro models of central nervous system trauma. Curr. Opin. Biomed. Eng. 2020, 14, 34–41. [Google Scholar] [CrossRef]
- Schlotterose, L.; Beldjilali-Labro, M.; Schneider, G.; Vardi, O.; Hattermann, K.; Even, U.; Shohami, E.; Haustein, H.D.; Leichtmann-Bardoogo, Y.; Maoz, B.M. Traumatic Brain Injury in a Well: A Modular Three-Dimensional Printed Tool for Inducing Traumatic Brain Injury In vitro. Neurotrauma Rep. 2023, 4, 255–266. [Google Scholar] [CrossRef]
- Dwyer, M.K.R.; Morrison, B. Recent advancements in in vitro models of traumatic brain injury. Curr. Opin. Biomed. Eng. 2022, 23, 100396. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Jiang, Y.; Shi, S.; Niamnud, A.; Vodovoz, S.J.; Katakam, P.V.G.; Vidoudez, C.; Dumont, A.S.; Wang, X. Establishment and Application of a Novel In Vitro Model of Microglial Activation in Traumatic Brain Injury. J. Neurosci. 2023, 43, 319–332. [Google Scholar] [CrossRef]
- Seo, S.; Choi, C.-H.; Yi, K.S.; Kim, S.U.; Lee, K.; Choi, N.; Lee, H.J.; Cha, S.-H.; Kim, H.N. An engineered neurovascular unit for modeling neuroinflammation. Biofabrication 2021, 13, 035039. [Google Scholar] [CrossRef]
- Liaudanskaya, V.; Fiore, N.J.; Zhang, Y.; Milton, Y.; Kelly, M.F.; Coe, M.; Barreiro, A.; Rose, V.K.; Shapiro, M.R.; Mullis, A.S.; et al. Mitochondria dysregulation contributes to secondary neurodegeneration progression post-contusion injury in human 3D in vitro triculture brain tissue model. Cell Death Dis. 2023, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chung, J.Y.; Cao, T.; Jin, G.; Edmiston, W.J.; Hickman, S.; Levy, E.S.; Whalen, J.A.; Abrams, E.S.L.; Degterev, A.; et al. Genetic inhibition of RIPK3 ameliorates functional outcome in controlled cortical impact independent of necroptosis. Cell Death Dis. 2021, 12, 1064. [Google Scholar] [CrossRef]
- Hu, T.; Li, L.; Cao, Q.; Tu, W.; Huang, X.; Yuan, T. Positive association between serum lactate dehydrogenase levels and blood pressure: Evidence from NHANES 2015–2016. Front. Cardiovasc. Med. 2025, 12, 1554702. [Google Scholar] [CrossRef]
- Cao, L.; Mu, W. Necrostatin-1 and necroptosis inhibition: Pathophysiology and therapeutic implications. Pharmacol. Res. 2021, 163, 105297. [Google Scholar] [CrossRef] [PubMed]
- Lentini, G.; Famà, A.; De Gaetano, G.V.; Coppolino, F.; Mahjoub, A.K.; Ryan, L.; Lien, E.; Espevik, T.; Beninati, C.; Teti, G. Caspase-8 inhibition improves the outcome of bacterial infections in mice by promoting neutrophil activation. Cell Rep. Med. 2023, 4, 101098. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Li, J.; Zhang, Y.; Gao, D.; Zhao, S. Caspase-independent cell death mediated by apoptosis-inducing factor (AIF) nuclear translocation is involved in ionizing radiation induced HepG2 cell death. Biochem. Biophys. Res. Commun. 2016, 472, 137–143. [Google Scholar] [CrossRef]
- Mihaly, S.R.; Sakamachi, Y.; Ninomiya-Tsuji, J.; Morioka, S. Noncanonical cell death program independent of caspase activation cascade and necroptotic modules is elicited by loss of TGFβ-activated kinase 1. Sci. Rep. 2017, 7, 2918. [Google Scholar] [CrossRef]
- Bodnar, C.N.; Watson, J.B.; Higgins, E.K.; Quan, N.; Bachstetter, A.D. Inflammatory Regulation of CNS Barriers After Traumatic Brain Injury: A Tale Directed by Interleukin-1. Front. Immunol. 2021, 12, 688254. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Gicquel, T.; Victoni, T.; Valença, S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 2016, 36, e00360. [Google Scholar] [CrossRef]
- Oelschlaegel, D.; Weiss Sadan, T.; Salpeter, S.; Krug, S.; Blum, G.; Schmitz, W.; Schulze, A.; Michl, P. Cathepsin Inhibition Modulates Metabolism and Polarization of Tumor-Associated Macrophages. Cancers 2020, 12, 2579. [Google Scholar] [CrossRef]
- Alfaidi, M.; Wilson, H.; Daigneault, M.; Burnett, A.; Ridger, V.; Chamberlain, J.; Francis, S. Neutrophil Elastase Promotes Interleukin-1β Secretion from Human Coronary Endothelium. J. Biol. Chem. 2015, 290, 24067–24078. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Tummers, B.; Green, D.R. Crashing the computer: Apoptosis vs. necroptosis in neuroinflammation. Cell Death Differ. 2019, 26, 41–52. [Google Scholar] [CrossRef]
- England, H.; Summersgill, H.R.; Edye, M.E.; Rothwell, N.J.; Brough, D. Release of Interleukin-1α or Interleukin-1β Depends on Mechanism of Cell Death. J. Biol. Chem. 2014, 289, 15942–15950. [Google Scholar] [CrossRef]
- Yao, K.; Shi, Z.; Zhao, F.; Tan, C.; Zhang, Y.; Fan, H.; Wang, Y.; Li, X.; Kong, J.; Wang, Q.; et al. RIPK1 in necroptosis and recent progress in related pharmaceutics. Front. Immunol. 2025, 16, 1480027. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Y.; Sun, J.; Dai, K.; Wang, Z.; Zhang, J. Inhibiting RIPK1-driven neuroinflammation and neuronal apoptosis mitigates brain injury following experimental subarachnoid hemorrhage. Exp. Neurol. 2024, 374, 114705. [Google Scholar] [CrossRef] [PubMed]
- Wehn, A.C.; Khalin, I.; Duering, M.; Hellal, F.; Culmsee, C.; Vandenabeele, P.; Plesnila, N.; Terpolilli, N.A. RIPK1 or RIPK3 deletion prevents progressive neuronal cell death and improves memory function after traumatic brain injury. Acta Neuropathol. Commun. 2021, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Costigan, A.; Hollville, E.; Martin, S.J. Discriminating Between Apoptosis, Necrosis, Necroptosis, and Ferroptosis by Microscopy and Flow Cytometry. Curr. Protoc. 2023, 3, e951. [Google Scholar] [CrossRef]
- Nie, Z.; Tan, L.; Niu, J.; Wang, B. The role of regulatory necrosis in traumatic brain injury. Front. Mol. Neurosci. 2022, 15, 1005422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, Q. Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling. Front. Immunol. 2023, 14, 1128358. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Yang, J.; Chen, Y.; Zhou, B.; Abbott, D.W.; Xiao, T.S. Caspase-1 Engages Full-Length Gasdermin D through Two Distinct Interfaces That Mediate Caspase Recruitment and Substrate Cleavage. Immunity 2020, 53, 106–114.e5. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef]
- Shapira, Y.; Setton, D.; Artru, A.A.; Shohami, E. Blood-Brain Barrier Permeability, Cerebral Edema, and Neurologic Function After Closed Head Injury in Rats. Anesth. Analg. 1993, 77, 141–148. [Google Scholar] [CrossRef]
- Başkaya, M.K.; Muralikrishna Rao, A.; Doğan, A.; Donaldson, D.; Dempsey, R.J. The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci. Lett. 1997, 226, 33–36. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G. Immune regulation in neurovascular units after traumatic brain injury. Neurobiol. Dis. 2023, 179, 106060. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Saviuk, M.; Vedunova, M.V. Necroptosis in CNS diseases: Focus on astrocytes. Front. Aging Neurosci. 2023, 14, 1016053. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Li, Y.; Zou, C.; Chen, C.; Li, S.; Zhu, Z.; Fan, Q.; Pang, R.; Li, F.; Chen, Z.; Wang, Z.; et al. Myeloid-derived MIF drives RIPK1-mediated cerebromicrovascular endothelial cell death to exacerbate ischemic brain injury. Proc. Natl. Acad. Sci. USA 2023, 120, e2219091120. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Singh, V.; Kumari, P.; Singh, R.; Chopra, H.; Emran, T.B. Novel insights on the role of VCAM-1 and ICAM-1: Potential biomarkers for cardiovascular diseases. Ann. Med. Surg. 2022, 84. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Z. Regulation of RIPK1 Phosphorylation: Implications for Inflammation, Cell Death, and Therapeutic Interventions. Biomedicines 2024, 12, 1525. [Google Scholar] [CrossRef]
- Madangarli, N.; Bonsack, F.; Dasari, R.; Sukumari–Ramesh, S. Intracerebral Hemorrhage: Blood Components and Neurotoxicity. Brain Sci. 2019, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.N.; Robinson, S.R.; Dringen, R.; Bishop, G.M. Uptake, metabolism and toxicity of hemin in cultured neurons. Neurochem. Int. 2011, 58, 804–811. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, J.G.; Jamieson, J.J.; Linville, R.M.; Searson, P.C. Benchmarking in vitro tissue-engineered blood–brain barrier models. Fluids Barriers CNS 2018, 15, 32. [Google Scholar] [CrossRef]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Power, L.H.; Marcet, E.C.; Chen, Z.; Chen, J.; Arkhangelskiy, A.; Whalen, M.J.; Chen, Y.; Kaplan, D.L. Three-Dimensional Human Neurovascular Unit Modeling Reveals Cell-Specific Mechanisms of Traumatic Brain Injury. J. Funct. Biomater. 2025, 16, 454. https://doi.org/10.3390/jfb16120454
Power LH, Marcet EC, Chen Z, Chen J, Arkhangelskiy A, Whalen MJ, Chen Y, Kaplan DL. Three-Dimensional Human Neurovascular Unit Modeling Reveals Cell-Specific Mechanisms of Traumatic Brain Injury. Journal of Functional Biomaterials. 2025; 16(12):454. https://doi.org/10.3390/jfb16120454
Chicago/Turabian StylePower, Liam H., Evan C. Marcet, Zihong Chen, Jinpeng Chen, Artem Arkhangelskiy, Michael J. Whalen, Ying Chen, and David L. Kaplan. 2025. "Three-Dimensional Human Neurovascular Unit Modeling Reveals Cell-Specific Mechanisms of Traumatic Brain Injury" Journal of Functional Biomaterials 16, no. 12: 454. https://doi.org/10.3390/jfb16120454
APA StylePower, L. H., Marcet, E. C., Chen, Z., Chen, J., Arkhangelskiy, A., Whalen, M. J., Chen, Y., & Kaplan, D. L. (2025). Three-Dimensional Human Neurovascular Unit Modeling Reveals Cell-Specific Mechanisms of Traumatic Brain Injury. Journal of Functional Biomaterials, 16(12), 454. https://doi.org/10.3390/jfb16120454








