Properties of 3-Dimensional Cell Cultivation Matrices and Scaffolds in Magnetic Resonance Imaging at 3 Tesla
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Spheroid Generation
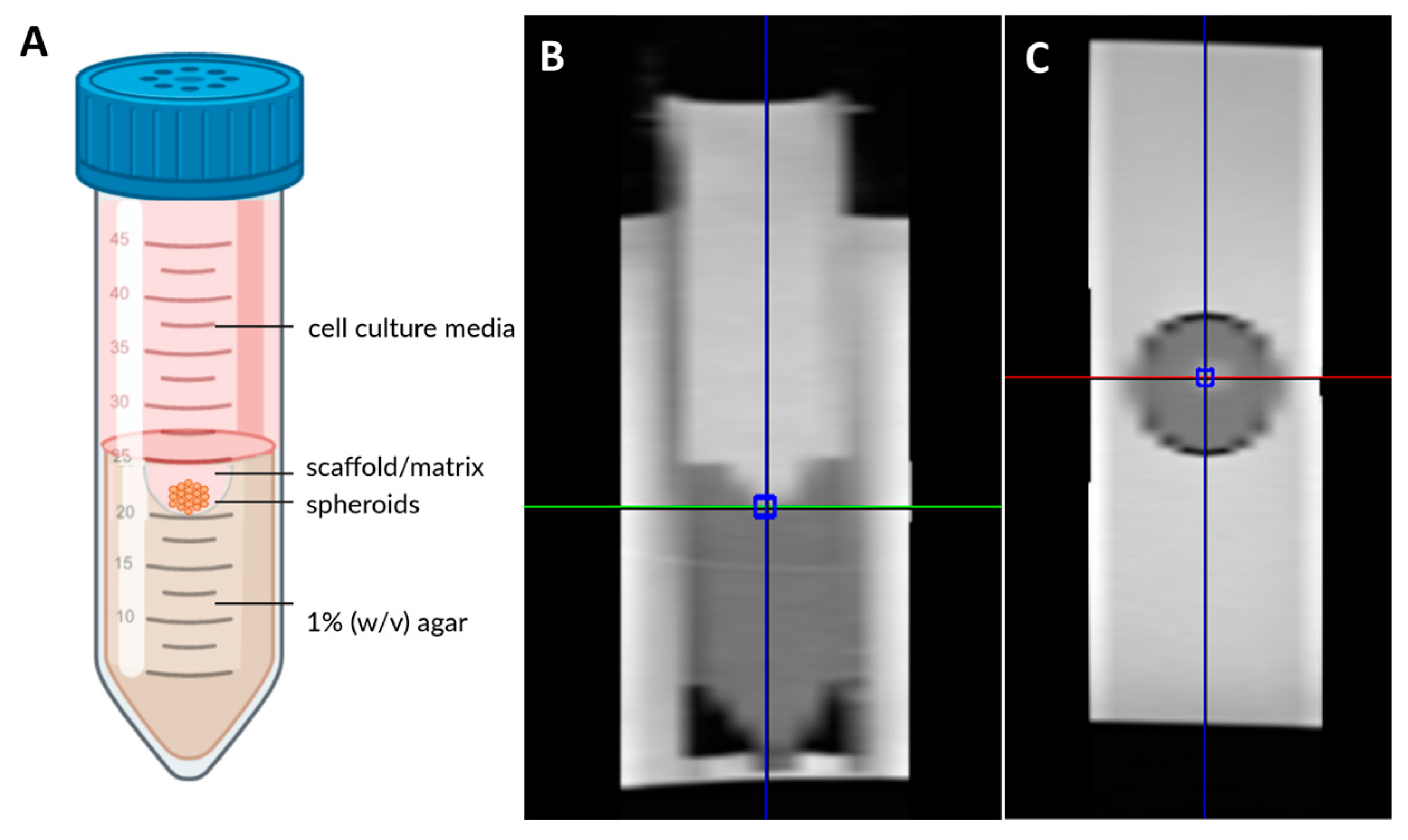
2.2. Scaffolds Preparation
Matrigel®
2.3. Hydrogels
2.4. Fibrin Glue
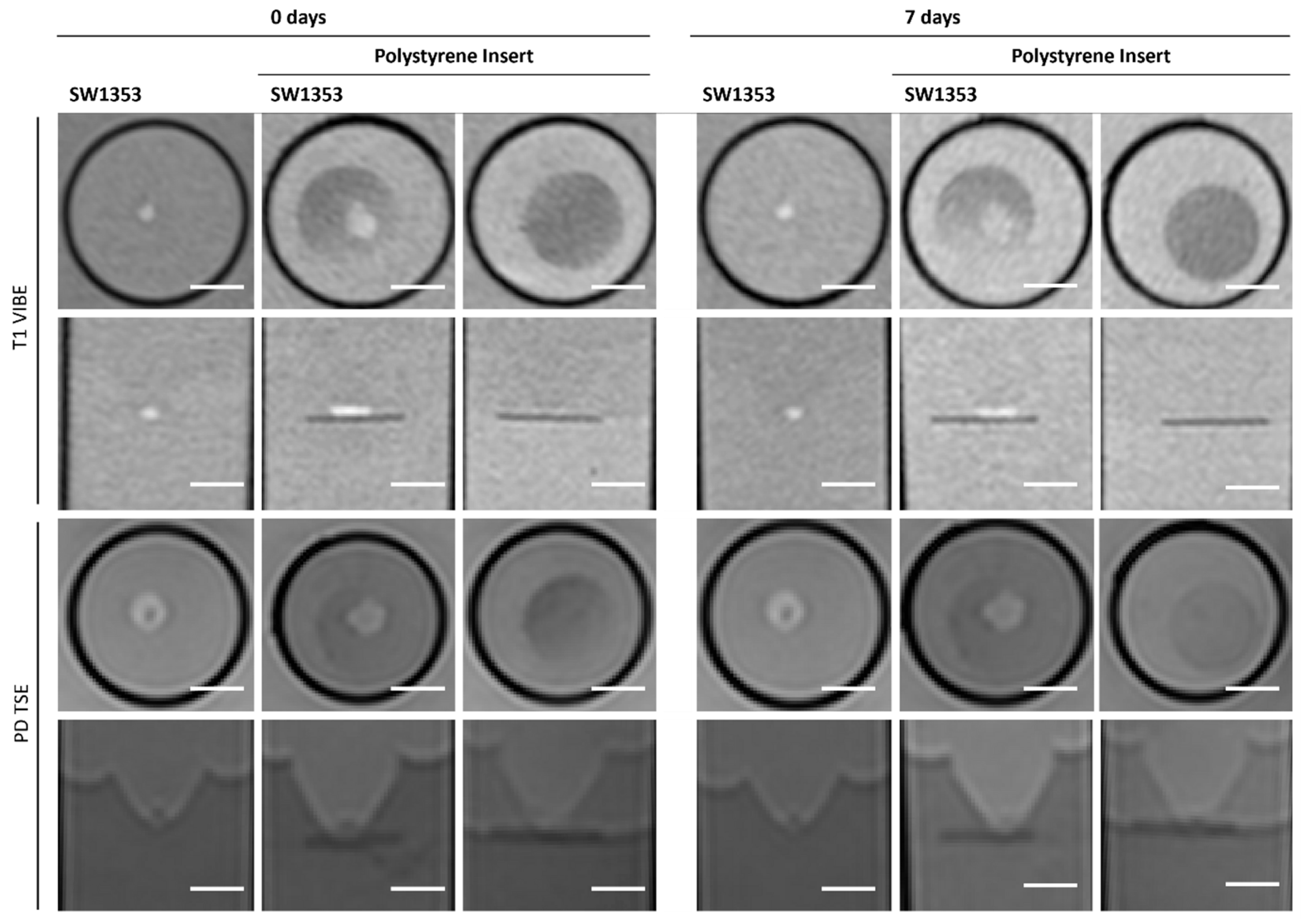
2.5. Polystyrene Insert
2.6. Magnetic Resonance Imaging and Volume Localized Spectroscopy Techniques
MRI
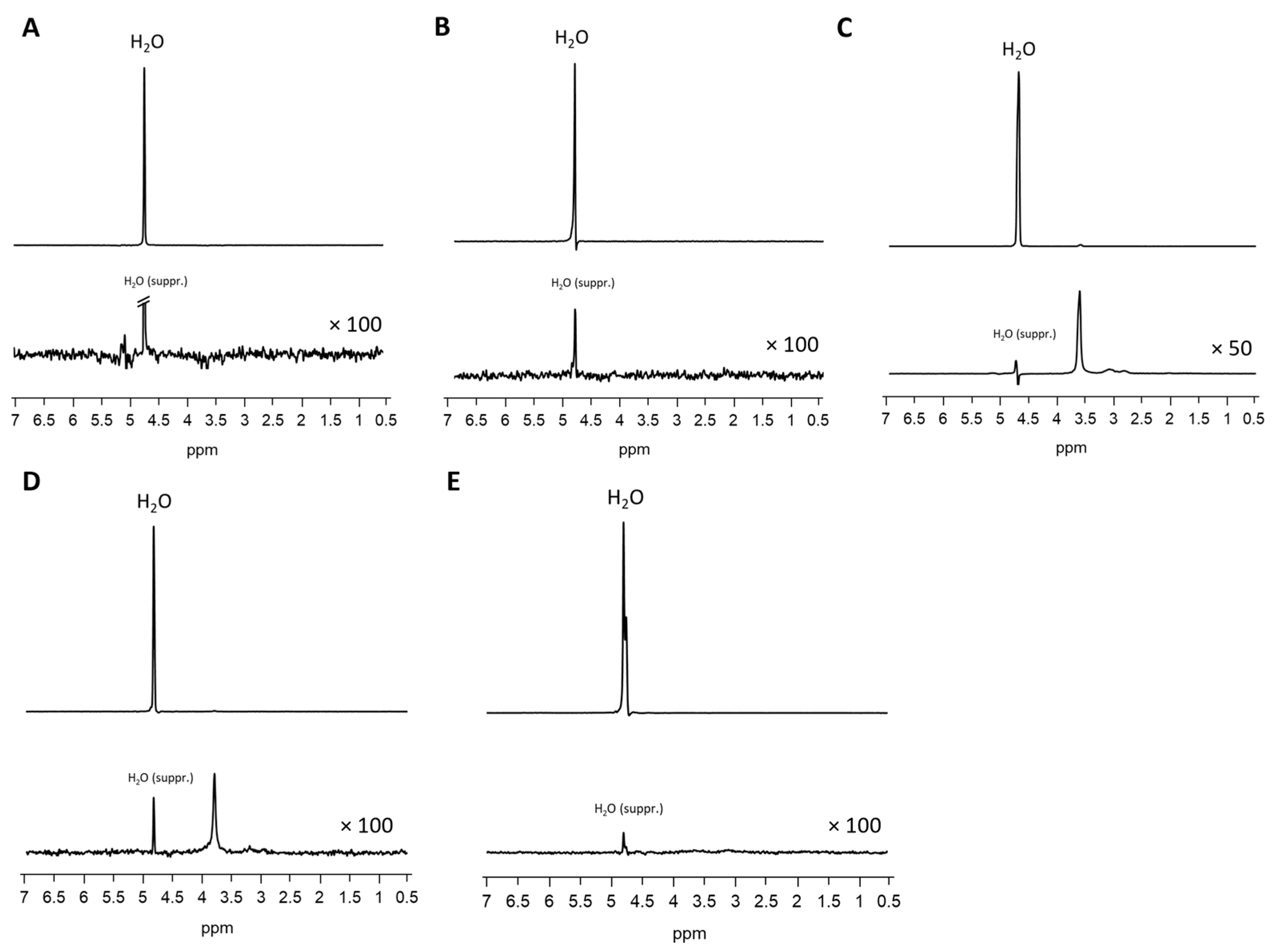
2.7. MR Spectroscopy
2.8. Quantification and Statistical Data Analysis
3. Results
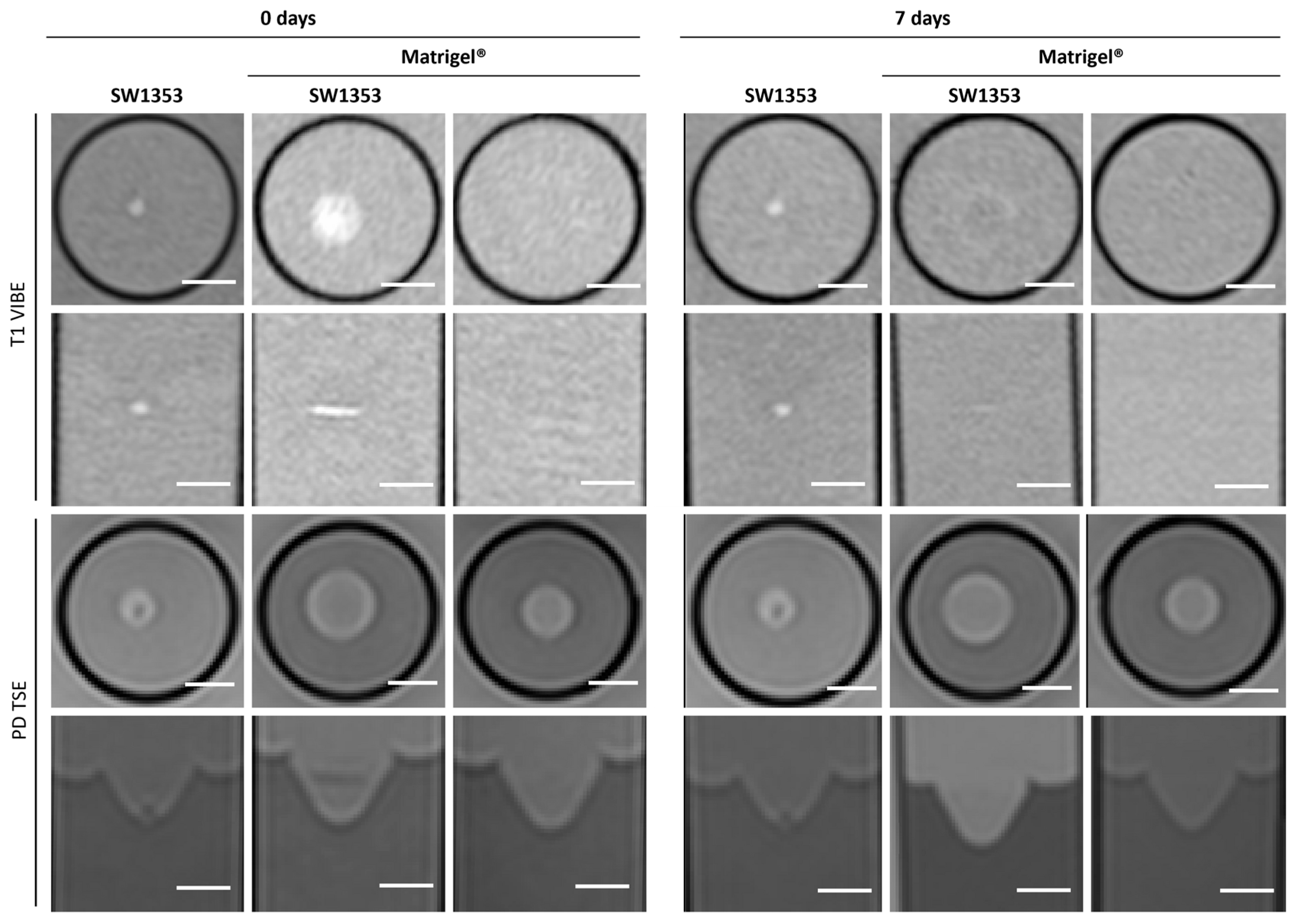
3.1. Matrigel®
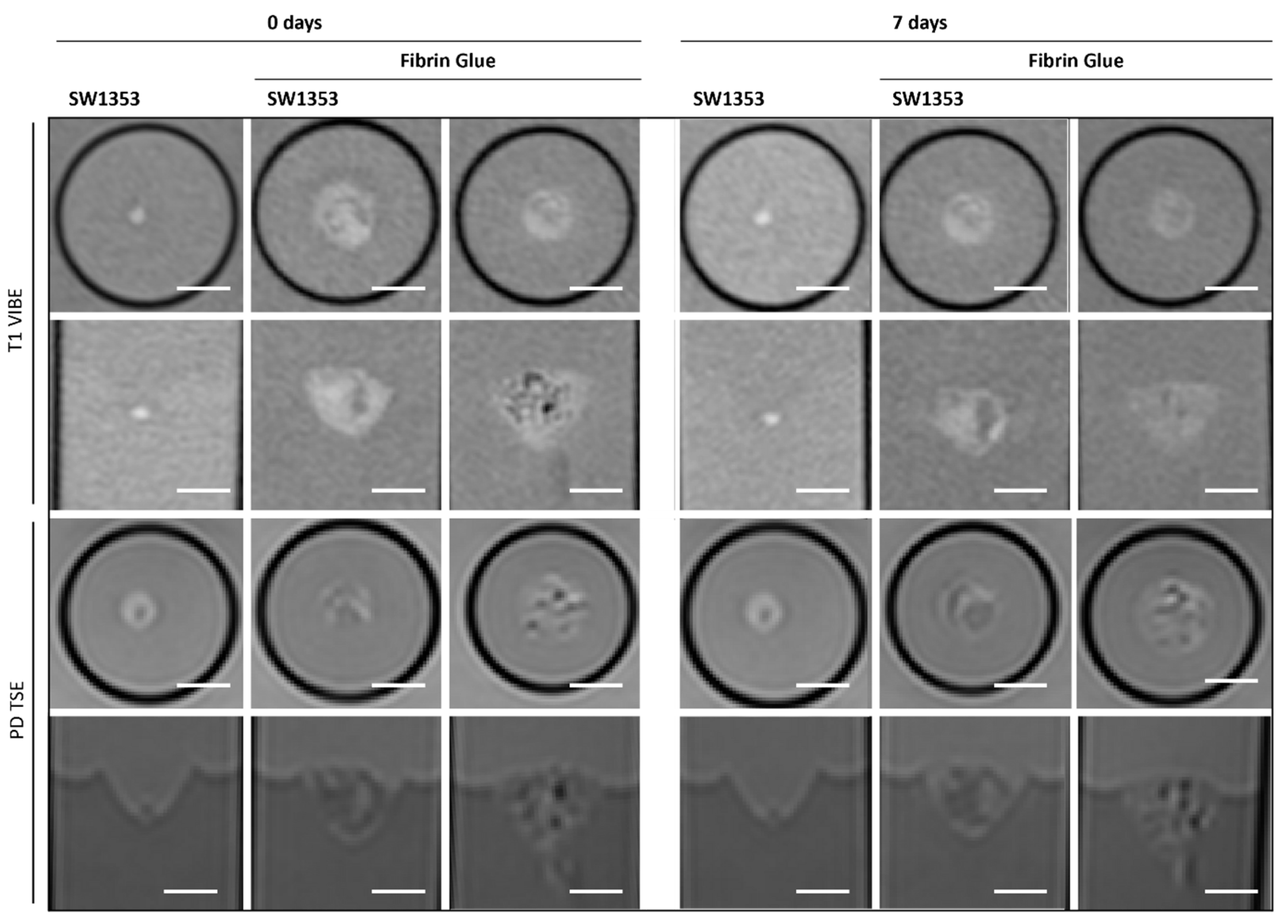
3.2. Fibrin Glue
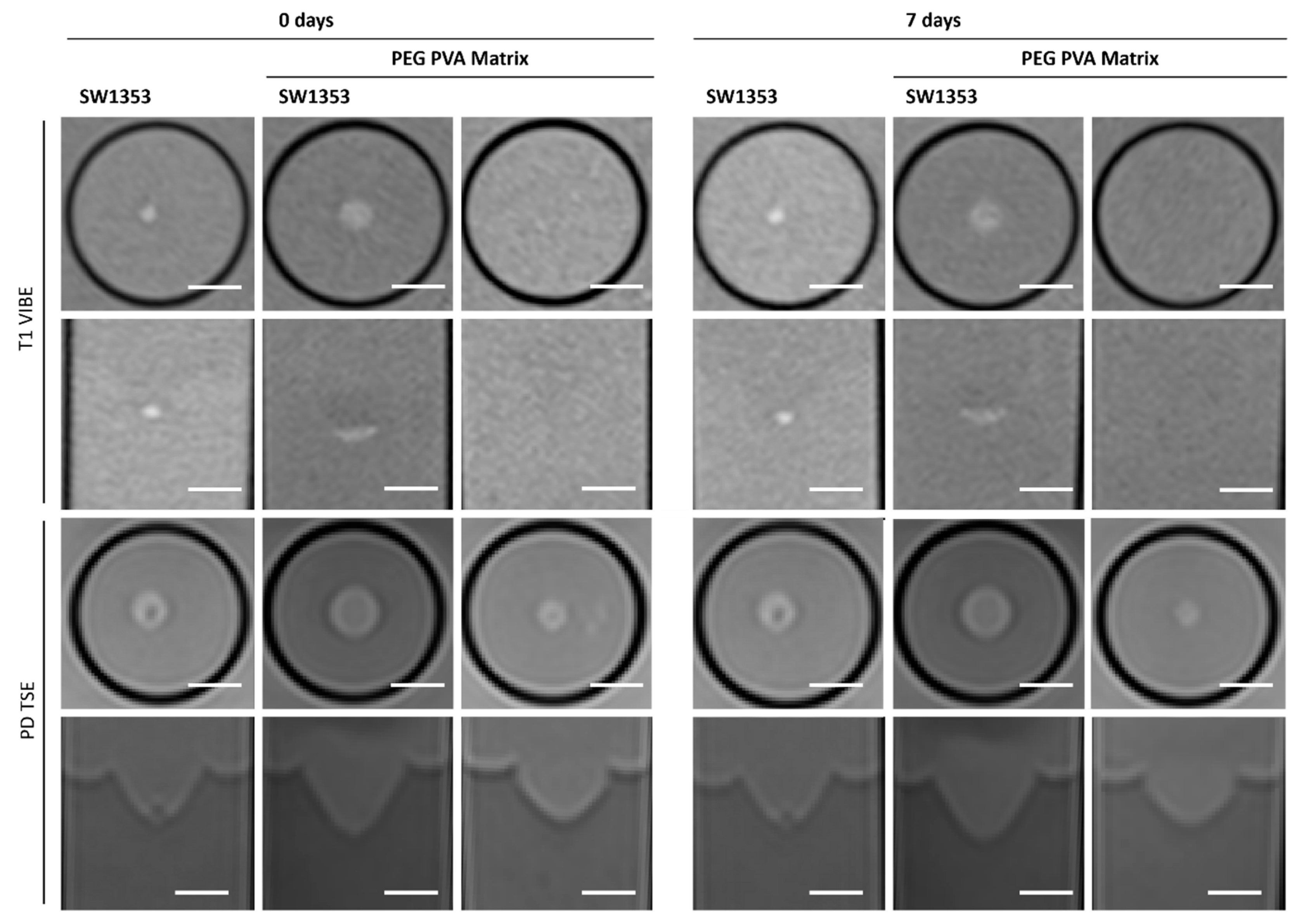
3.3. PEG PVA Hydrogel
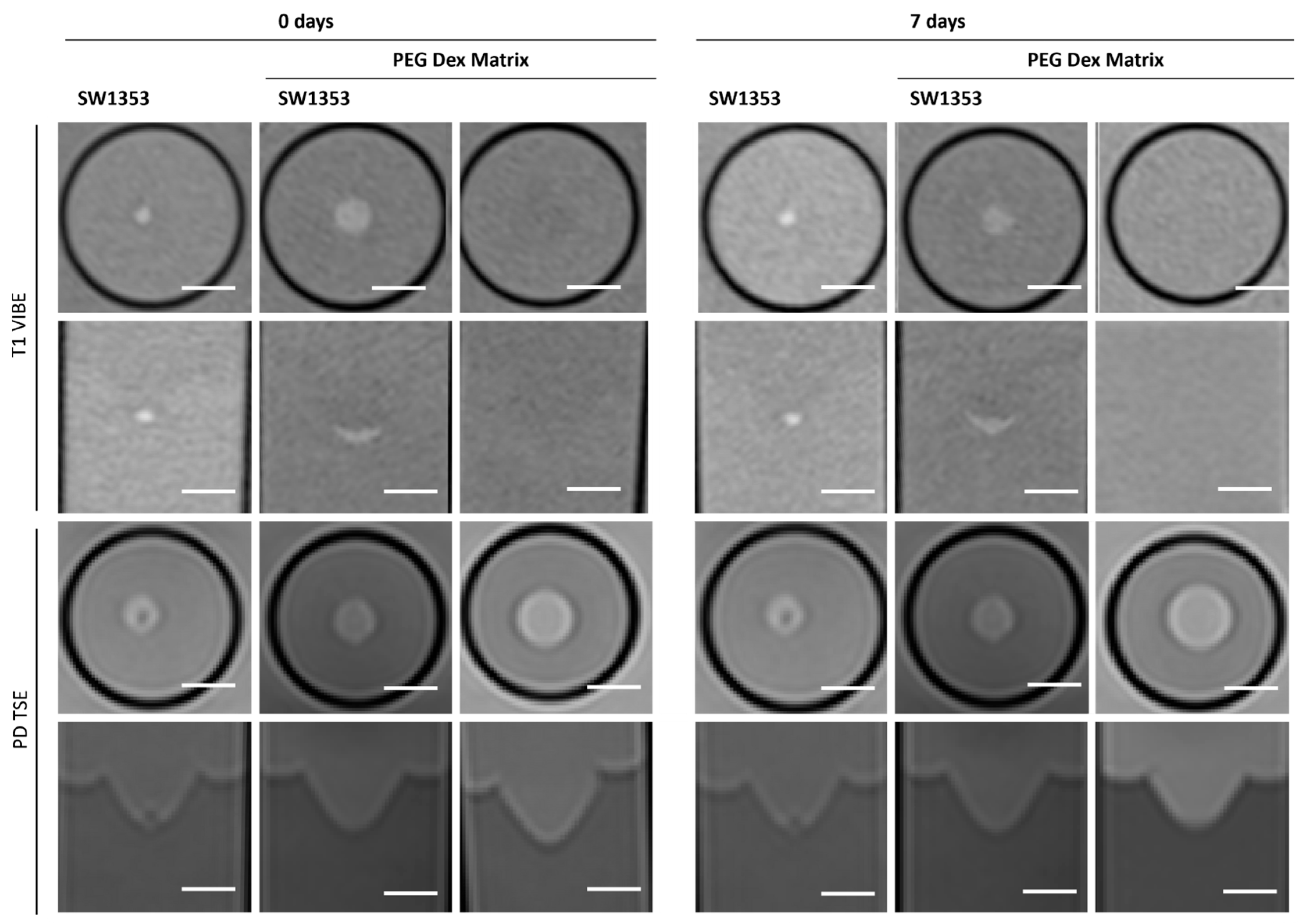
3.4. PEG Dex Hydrogel
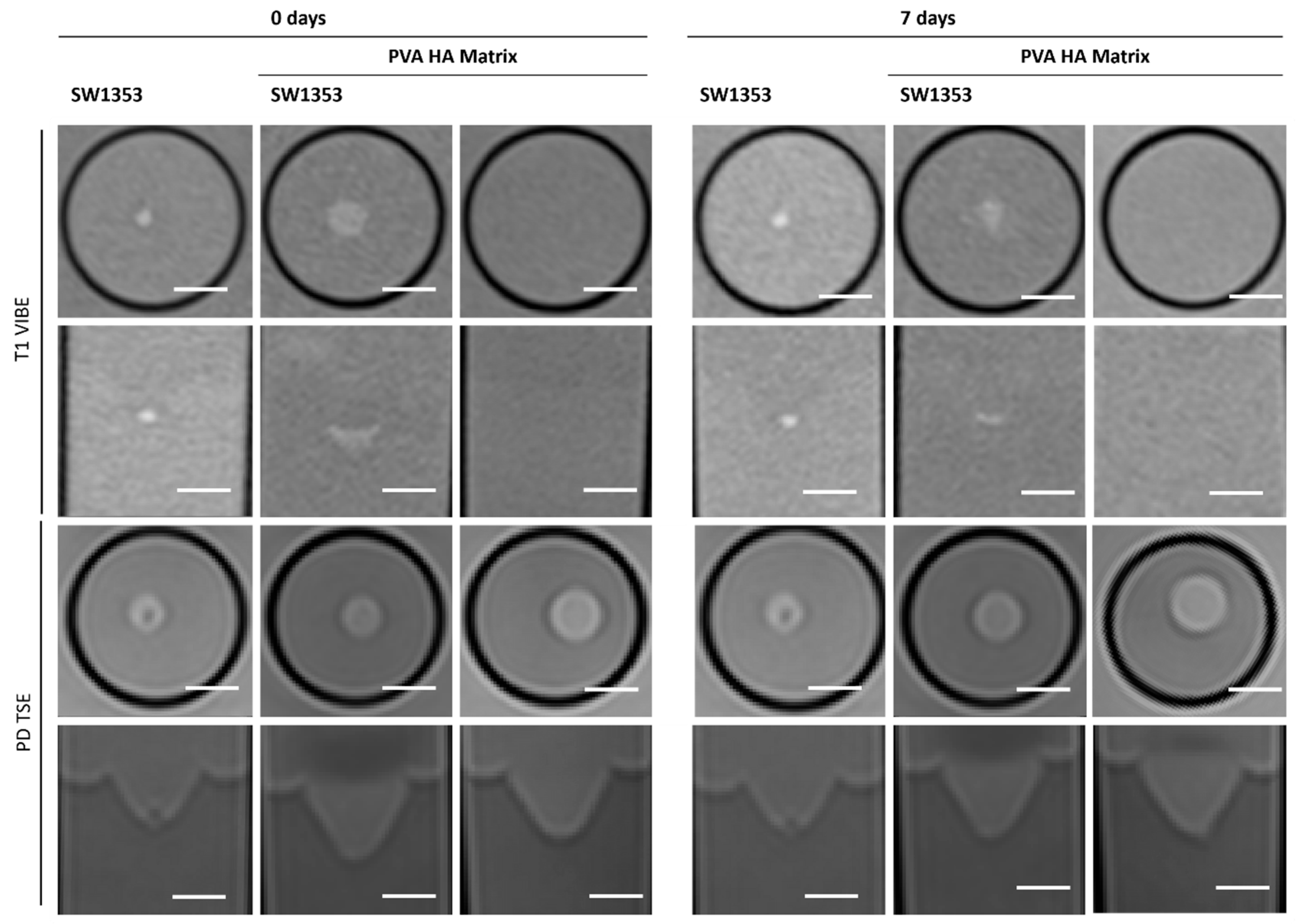
3.5. PEG HA Hydrogel
3.6. Polystyrene Insert
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | apparent diffusion coefficient |
| CPMG | Carr–Purcell–Meiboom–Gill |
| Dex | dextran |
| DWI-EPI | diffusion-weighted echo planar imaging |
| ECM | extracellular matrix |
| EHS | Elbrecht–Holm–Swarm |
| FG | fibrin glue |
| HA | hyaluronic acid |
| MRI | magnetic resonance imaging |
| MRS | magnetic resonance spectroscopy |
| MTR | magnetization transfer ratios |
| PD | proton density |
| PEG | polyethylene glycol |
| PS | polystyrene |
| PVA | polyvinyl alcohol |
| SNR | signal-to-noise ratio |
| STEAM | stimulated echo acquisition mode |
| T | Tesla |
| TSE | turbo spin echo |
| VFA | variable flip angle |
| VIBE | volumetric interpolated breath-hold examination |
References
- Lu, Y.; Zhang, G.; Shen, C.; Uygun, K.; Yarmush, M.L.; Meng, Q. A novel 3D liver organoid system for elucidation of hepatic glucose metabolism. Biotechnol. Bioeng. 2012, 109, 595–604. [Google Scholar] [CrossRef]
- Purpura, K.A.; Bratt-Leal, A.M.; Hammersmith, K.A.; McDevitt, T.C.; Zandstra, P.W. Systematic engineering of 3D pluripotent stem cell niches to guide blood development. Biomaterials 2012, 33, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, A.I.; Maya-Mendoza, A.; Streuli, C.H. Cellular microenvironment influences the ability of mammary epithelia to undergo cell cycle. PLoS ONE 2011, 6, e18144. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Kannan, D.; Elumalai, K.; Karunakar, K.; Jayaraj, S.; Devaraji, M.; Jayaprakash, N. Advanced 3D biomaterials and bioprinting strategies for in vitro modeling of neurodegenerative diseases. Biomed. Technol. 2025, 11, 100089. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To scaffold, or not to scaffold, that is the question. Int. J. Mol. Sci. 2021, 22, 12690. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Peña, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.; Mestroni, L.; Park, D. Injectable hydrogels for cardiac tissue engineering. Macromol. Biosci. 2018, 18, 1800079. [Google Scholar] [CrossRef]
- Kwee, B.J.; Mooney, D.J. Biomaterials for skeletal muscle tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Pugliese, R.; Marchini, A.; Saracino, G.A.; Gelain, F. Functionalization of self-assembling peptides for neural tissue engineering. In Self-Assembling Biomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 475–493. [Google Scholar]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Passaniti, A.; Kleinman, H.K.; Martin, G.R. Matrigel: History/background, uses, and future applications. J. Cell Commun. Signal. 2022, 16, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Dubiak-Szepietowska, M.; Karczmarczyk, A.; Jönsson-Niedziółka, M.; Winckler, T.; Feller, K.-H. Development of complex-shaped liver multicellular spheroids as a human-based model for nanoparticle toxicity assessment in vitro. Toxicol. Appl. Pharmacol. 2016, 294, 78–85. [Google Scholar] [CrossRef]
- Kramer, R.H.; Bensch, K.G.; Wong, J. Invasion of reconstituted basement membrane matrix by metastatic human tumor cells. Cancer Res. 1986, 46 Pt 2, 1980–1989. [Google Scholar] [PubMed]
- Philp, D.; Chen, S.S.; Fitzgerald, W.; Orenstein, J.; Margolis, L.; Kleinman, H.K. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells 2005, 23, 288–296. [Google Scholar] [CrossRef]
- Grefte, S.; Vullinghs, S.; Kuijpers-Jagtman, A.; Torensma, R.; Von den Hoff, J. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed. Mater. 2012, 7, 055004. [Google Scholar] [CrossRef]
- Uemura, M.; Refaat, M.M.; Shinoyama, M.; Hayashi, H.; Hashimoto, N.; Takahashi, J. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J. Neurosci. Res. 2010, 88, 542–551. [Google Scholar] [CrossRef]
- Nyga, A.; Cheema, U.; Loizidou, M. 3D tumour models: Novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 2011, 5, 239–248. [Google Scholar] [CrossRef]
- Asghar, W.; El Assal, R.; Shafiee, H.; Pitteri, S.; Paulmurugan, R.; Demirci, U. Engineering cancer microenvironments for in vitro 3-D tumor models. Mater. Today 2015, 18, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-D.; Wang, H.-J.; Tan, Y.-Z.; Wu, J.-H. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng. Part A 2011, 17, 45–58. [Google Scholar] [CrossRef]
- Ho, W.; Tawil, B.; Dunn, J.C.; Wu, B.M. The behavior of human mesenchymal stem cells in 3D fibrin clots: Dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006, 12, 1587–1595. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, L.; Ren, X.; Gao, G. Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur. Polym. J. 2019, 112, 660–669. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.R.; Adebowale, K.; Zhao, Z.; Mitragotri, S. Hydrogels in the clinic: An update. Bioeng. Transl. Med. 2024, 9, e10680. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Liu, Q.; Yu, L.; Ding, J. Enzymatically cross-linked hydrogels based on a linear poly (ethylene glycol) analogue for controlled protein release and 3D cell culture. J. Mater. Chem. B 2018, 6, 6067–6079. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Carvajal, C.E.; Liu, Q.; Remache, Y.; Goy, A.; Suh, K.S. Cancer tissue engineering: A novel 3D polystyrene scaffold for in vitro isolation and amplification of lymphoma cancer cells from heterogeneous cell mixtures. J. Tissue Eng. 2011, 2011, 362326. [Google Scholar] [CrossRef] [PubMed]
- Pilehrood, M.K.; Atashi, A.; Sadeghi-Aliabadi, H.; Nousiainen, P.; Harlin, A. 3D micro-nano structured hybrid scaffolds: An investigation into the role of nanofiber coating on viability, proliferation and differentiation of seeded mesenchymal stem cells. J. Nanosci. Nanotechnol. 2016, 16, 9000–9007. [Google Scholar] [CrossRef]
- Henkelman, R.; Stanisz, G.; Graham, S. Magnetization transfer in MRI: A review. NMR Biomed. 2001, 14, 57–64. [Google Scholar] [CrossRef]
- Tognarelli, J.M.; Dawood, M.; Shariff, M.I.; Grover, V.P.; Crossey, M.M.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J. Magnetic resonance spectroscopy: Principles and techniques: Lessons for clinicians. J. Clin. Exp. Hepatol. 2015, 5, 320–328. [Google Scholar] [CrossRef]
- Ladd, M.E.; Bachert, P.; Meyerspeer, M.; Moser, E.; Nagel, A.M.; Norris, D.G.; Schmitter, S.; Speck, O.; Straub, S.; Zaiss, M. Pros and cons of ultra-high-field MRI/MRS for human application. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 109, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Poser, B.A.; Deng, W.; Stenger, V.A. Spectral decomposition of susceptibility artifacts for spectral-spatial radiofrequency pulse design. Magn. Reson. Med. 2012, 68, 1905–1910. [Google Scholar] [CrossRef]
- Wißmann, R.; Martirosian, P.; Danalache, M.; Elser, S.; Schick, F. Protocol for the quantitative characterization of cell aggregates using an MRI setup maintaining optimal cultivation conditions. STAR Protoc. 2025, 6, 104002. [Google Scholar] [CrossRef] [PubMed]
- Wißmann, R.; Martirosian, P.; Danalache, M.; Grözinger, G.; Schick, F.; Elser, S. Imaging cell spheroid clusters: An MRI protocol for non-invasive standardized characterization. Heliyon 2025, 11, e41803. [Google Scholar] [CrossRef]
- Guz, W.; Podgórski, R.; Aebisher, D.; Truszkiewicz, A.; Machorowska-Pieniążek, A.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Utility of 1.5 Tesla MRI Scanner in the Management of Small Sample Sizes Driven from 3D Breast Cell Culture. Int. J. Mol. Sci. 2024, 25, 3009. [Google Scholar] [CrossRef]
- Löhr, M.; Linsenmann, T.; Jawork, A.; Kessler, A.F.; Timmermann, N.; Homola, G.A.; Ernestus, R.I.; Hagemann, C. Implanting Glioblastoma Spheroids into Rat Brains and Monitoring Tumor Growth by MRI Volumetry. In RNAi and Small Regulatory RNAs in Stem Cells; Humana Press: Totowa, NJ, USA, 2017; Volume 1622, pp. 149–159. [Google Scholar] [CrossRef]
- Yan, J.; Chen, H.; Pan, Y.; Yan, Y.; Tang, S.; Zhou, Q.; Hu, K.; Guo, Z.; Gu, N.; Zhang, F. Magnetic labeling of physically tunable hydrogel-induced mesenchymal stem cell spheroids with IONPs for MRI tracking and bone regeneration. Nano Today 2025, 61, 102620. [Google Scholar] [CrossRef]
- Yan, S.; Hu, K.; Zhang, M.; Sheng, J.; Xu, X.; Tang, S.; Li, Y.; Yang, S.; Si, G.; Mao, Y.; et al. Extracellular magnetic labeling of biomimetic hydrogel-induced human mesenchymal stem cell spheroids with ferumoxytol for MRI tracking. Bioact. Mater. 2023, 19, 418–428. [Google Scholar] [CrossRef]
- Kimberlee, P.; Adrian, C.T.; Laurence, H. Mapping of the spatial variation in alginate concentration in calcium alginate gels by magnetic resonance imaging (MRI). Carbohydr. Res. 1993, 246, 43–49. [Google Scholar] [CrossRef]
- Degrassi, A.; Toffanin, R.; Paoletti, S.; Hall, L.D. A better understanding of the properties of alginate solutions and gels by quantitative magnetic resonance imaging (MRI). Carbohydr. Res. 1998, 306, 19–26. [Google Scholar] [CrossRef]
- Gallo, E.; Rosa, E.; Diaferia, C.; Rossi, F.; Tesauro, D.; Accardo, A. Systematic overview of soft materials as a novel frontier for MRI contrast agents. RSC Adv. 2020, 10, 27064–27080. [Google Scholar] [CrossRef]
- Arends, F.; Nowald, C.; Pflieger, K.; Boettcher, K.; Zahler, S.; Lieleg, O. The biophysical properties of Basal lamina gels depend on the biochemical composition of the gel. PLoS ONE 2015, 10, e0118090. [Google Scholar] [CrossRef]
- Mun, S.J.; Hong, Y.H.; Ahn, H.S.; Ryu, J.S.; Chung, K.S.; Son, M.J. Long-Term Expansion of Functional Human Pluripotent Stem Cell-Derived Hepatic Organoids. Int. J. Stem Cells 2020, 13, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef]
- Gopal, S.; Kwon, S.-J.; Ku, B.; Lee, D.W.; Kim, J.; Dordick, J.S. 3D tumor spheroid microarray for high-throughput, high-content natural killer cell-mediated cytotoxicity. Commun. Biol. 2021, 4, 893. [Google Scholar] [CrossRef]
- Francis, M.E.; Uriel, S.; Brey, E.M. Endothelial cell–matrix interactions in neovascularization. Tissue Eng. Part B Rev. 2008, 14, 19–32. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sung, C.H.; Chung, S.H.; Kwak, S.J.; Koh, Y.G. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am. J. Sports Med. 2017, 45, 2010–2018. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, Y.J.; Lee, S.W.; Kwon, O.R.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: A prospective study. Osteoarthr. Cartil. 2016, 24, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013, 12, 458–465. [Google Scholar] [CrossRef]
- Zhong, J.; Paul, A.; Kellie, S.J.; O′ Neill, G.M. Mesenchymal migration as a therapeutic target in glioblastoma. J. Oncol. 2010, 2010, 430142. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef]
- Patterson, J.; Hubbell, J.A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 2010, 31, 7836–7845. [Google Scholar] [CrossRef]
- Pasek-Allen, J.L.; Wilharm, R.K.; Bischof, J.C.; Pierre, V.C. NMR characterization of polyethylene glycol conjugates for nanoparticle functionalization. ACS Omega 2023, 8, 4331–4336. [Google Scholar] [CrossRef]
- Clop, E.M.; Perillo, M.A.; Chattah, A.K. 1H and 2H NMR spin–lattice relaxation probing water: PEG molecular dynamics in solution. J. Phys. Chem. B 2012, 116, 11953–11958. [Google Scholar] [CrossRef] [PubMed]
| PD/T2-w | T1-w | T1-Map | T2-Map | DWI | MTR | |
|---|---|---|---|---|---|---|
| Sequence name | 2D TSE | 3D VIBE | 3D VFA VIBE | 2D CPMG | RESOLVE | 3D GRE |
| Echo train length | 18 | 1 | 1 | 32 | 1 | 1 |
| TR (ms) | 3000 | 6.8 | 9.2 | 3000 | 3000 | 25 |
| TE (ms) | 12/160 | 2.74 | 2.71 | 10–320 | 47 | 3.18 |
| Flip angle (deg) | 90–180n | 10 | 2/8/15 | 90–180n | 180 | 10 |
| BW (Hz/Px) | 191 | 190 | 190 | 235 | 744 | 190 |
| Matrix | 120 × 160, 15 slices | 173 × 256 × 128 | 120 × 160 × 30 | 120 × 160 single slice | 120 × 160 single slice | 120 × 160 × 30 |
| FOV (mm2/mm3) | 120 × 160 × 16 | 116 × 154 × 38 | 120 × 1600 × 15 | 120 × 160 × 2 | 120 × 160 × 3 | 120 × 160 × 15 |
| Voxel size (mm3) | 1.0 × 1.0 × 1.0 | 0.6 × 0.6 × 0.6 | 1.0 × 1.0 × 1.0 | 1.0 × 1.0 × 2.0 | 0.5 × 0.5 × 3.0 | 0.5 × 0.5 × 0.5 |
| Scan time (min:s) | 3:41 | 4:28 | 5:37 | 6:05 | 6:53 | 4:48 × 2 |
| MRS | |
|---|---|
| Sequence Name | STEAM |
| TR (ms) | 4000 |
| TE (ms) | 5.4 |
| Voxel size (mm3) | 3 × 3 × 3 |
| Bandwidth | 1400 Hz |
| nAcq | 8 (without water suppression), 128 (with water suppression), 2 preparation scans |
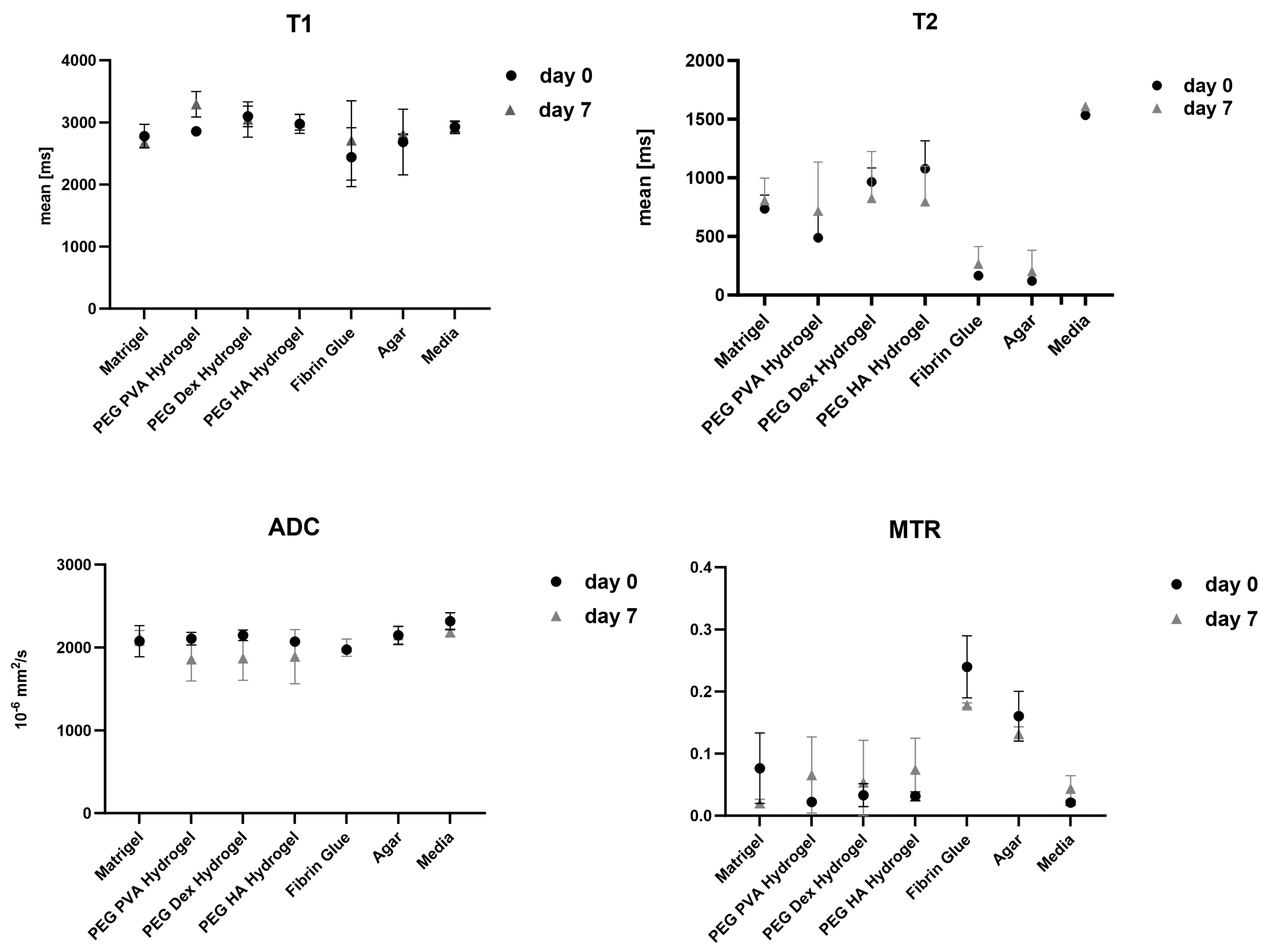
| Matrigel | PEG PVA Hydrogel | PEG Dex Hydrogel | PEG HA Hydrogel | Fibrin Glue | Agar | Media | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNR (PD) | 127 | 103 | 116 | 108 | 125 | 104 | 119 | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| day 0 | T1 [ms] | 2779 | 191 | 2856 | 44 | 3098 | 166 | 2977 | 156 | 2441 | 475 | 2684 | 528 | 2929 | 85 |
| Contrast in T1 | 30% | 24% | 23% | 20% | n.d. * | 23% | 24% | ||||||||
| T2 [ms] | 736 | 117 | 488 | 196 | 966 | 119 | 1078 | 238 | 166 | 25 | 121 | 6 | 1534 | 12 | |
| Contrast in T2 | −17% | −1% | −27% | −32% | n.d. * | −3% | −35% | ||||||||
| ADC [10−6 mm2/s] | 2077 | 188 | 2106 | 76 | 2147 | 64 | 2071 | 24 | 1975 | 14 | 2147 | 110 | 2317 | 103 | |
| Contrast in ADC | −7% | −19% | −61% | −70% | n.d. * | −8% | −15% | ||||||||
| MTR | 0.0765 | 0.0567 | 0.0222 | 0.0069 | 0.0332 | 0.0183 | 0.0316 | 0.0070 | 0.2398 | 0.0501 | 0.1603 | 0.0402 | 0.0210 | 0.0041 | |
| Contrast in MTR | 60% | 84% | 90% | 82% | n.d. * | −2% | 86% | ||||||||
| day 7 | T1 [ms] | 2675 | 83 | 3292 | 205 | 3048 | 284 | 3002 | 124 | 2710 | 638 | 2810 | 6 | 2923 | 101 |
| Contrast in T1 | 3% | 40% | 15% | 20% | n.d. * | 3% | 3% | ||||||||
| T2 [ms] | 807 | 190 | 718 | 416 | 826 | 399 | 797 | 303 | 267 | 147 | 203 | 180 | 1609 | 15 | |
| Contrast in T2 | −2% | −1% | −4% | −18% | n.d. * | 19% | −3% | ||||||||
| ADC [10−6 mm2/s] | 2116 | 89 | 1858 | 262 | 1870 | 265 | 1890 | 327 | 1997 | 104 | 2149 | 102 | 2183 | 44 | |
| Contrast in ADC | −1% | −20% | −10% | −40% | n.d. * | 0 | −6% | ||||||||
| MTR | 0.0200 | 0.0066 | 0.0654 | 0.0613 | 0.0537 | 0.0676 | 0.0742 | 0.0505 | 0.1779 | 0.0036 | 0.1316 | 0.0113 | 0.0437 | 0.0209 | |
| Contrast in MTR | 54% | 53% | 69% | 63% | n.d. * | −2% | −82% | ||||||||
| Matrigel® | PEG PVA Hydrogel | HA PVA Hydrogel | PEG Dex Hydrogel | Fibrin Glue | Poly- Styrene Insert | |
|---|---|---|---|---|---|---|
| MRI Susceptibility | Isointense with media | Isointense with media | Isointense with media | Isointense with media | Signal- enhancing | Signal depleting |
| Discernibility of cells | +/− | + | + | + | − | + |
| Stability in long-term culture | + | +/− | +/− | +/− | + | + |
| Artefact generation | − | − | − | − | Artefact generation through air inclusion | − |
| Approx. Cost | − EUR 48/mL | + EUR 21/mL | − EUR 50/mL | + EUR 23/mL | − EUR 52/mL | + EUR 1.19 per plate |
| MRS Compatibility | − | + | + | + | + | − |
| Usability | + | + | + | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wißmann, R.; Martirosian, P.; Danalache, M.; Elser, S.; Machann, J.; Schick, F. Properties of 3-Dimensional Cell Cultivation Matrices and Scaffolds in Magnetic Resonance Imaging at 3 Tesla. J. Funct. Biomater. 2025, 16, 440. https://doi.org/10.3390/jfb16120440
Wißmann R, Martirosian P, Danalache M, Elser S, Machann J, Schick F. Properties of 3-Dimensional Cell Cultivation Matrices and Scaffolds in Magnetic Resonance Imaging at 3 Tesla. Journal of Functional Biomaterials. 2025; 16(12):440. https://doi.org/10.3390/jfb16120440
Chicago/Turabian StyleWißmann, Rebecca, Petros Martirosian, Marina Danalache, Stefanie Elser, Jürgen Machann, and Fritz Schick. 2025. "Properties of 3-Dimensional Cell Cultivation Matrices and Scaffolds in Magnetic Resonance Imaging at 3 Tesla" Journal of Functional Biomaterials 16, no. 12: 440. https://doi.org/10.3390/jfb16120440
APA StyleWißmann, R., Martirosian, P., Danalache, M., Elser, S., Machann, J., & Schick, F. (2025). Properties of 3-Dimensional Cell Cultivation Matrices and Scaffolds in Magnetic Resonance Imaging at 3 Tesla. Journal of Functional Biomaterials, 16(12), 440. https://doi.org/10.3390/jfb16120440






