Grain Size and Electrochemical Surface Modification Effects on Corrosion, Biological, and Technological Properties of CP Titanium Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Its Processing Parameters
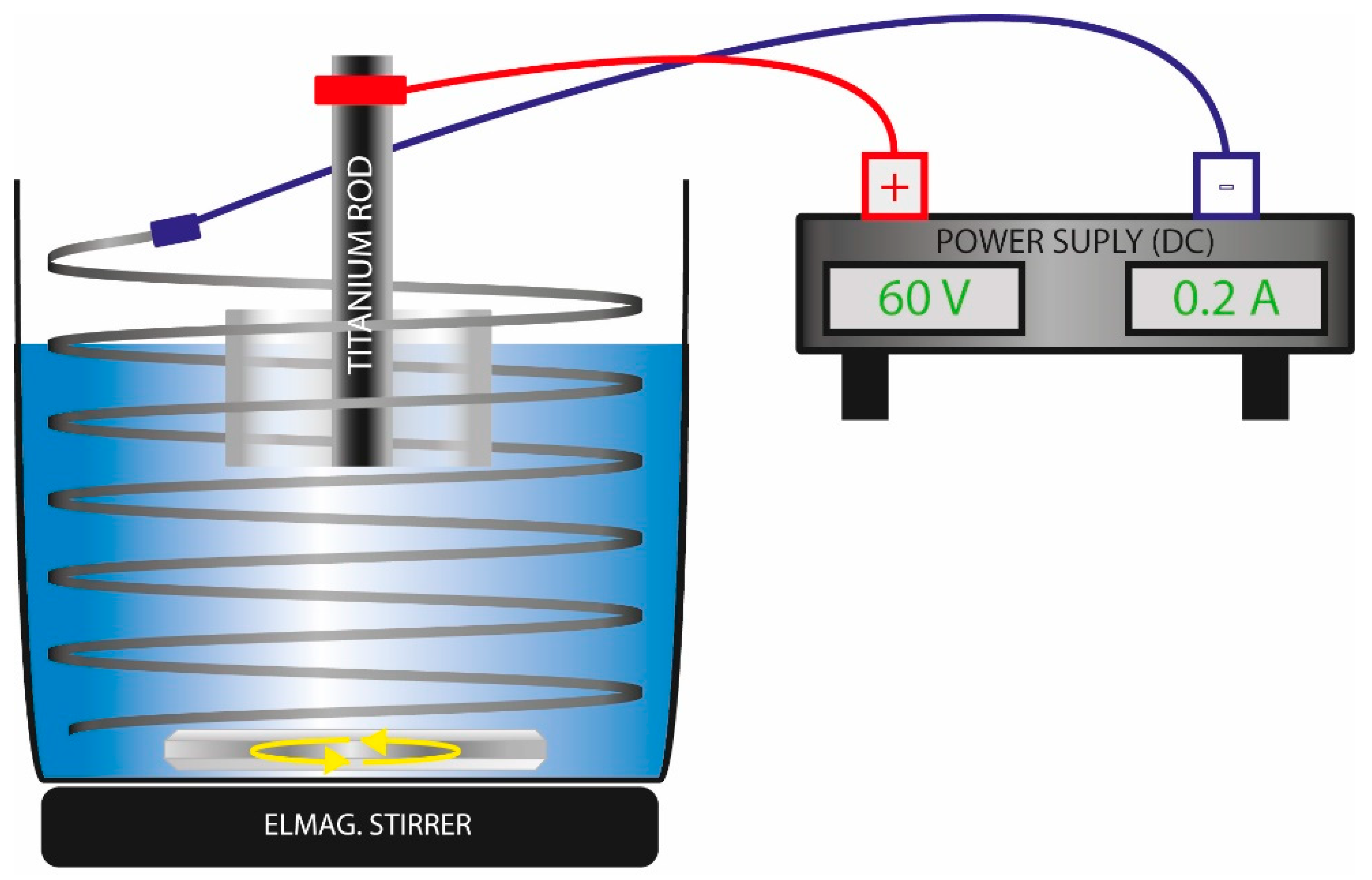
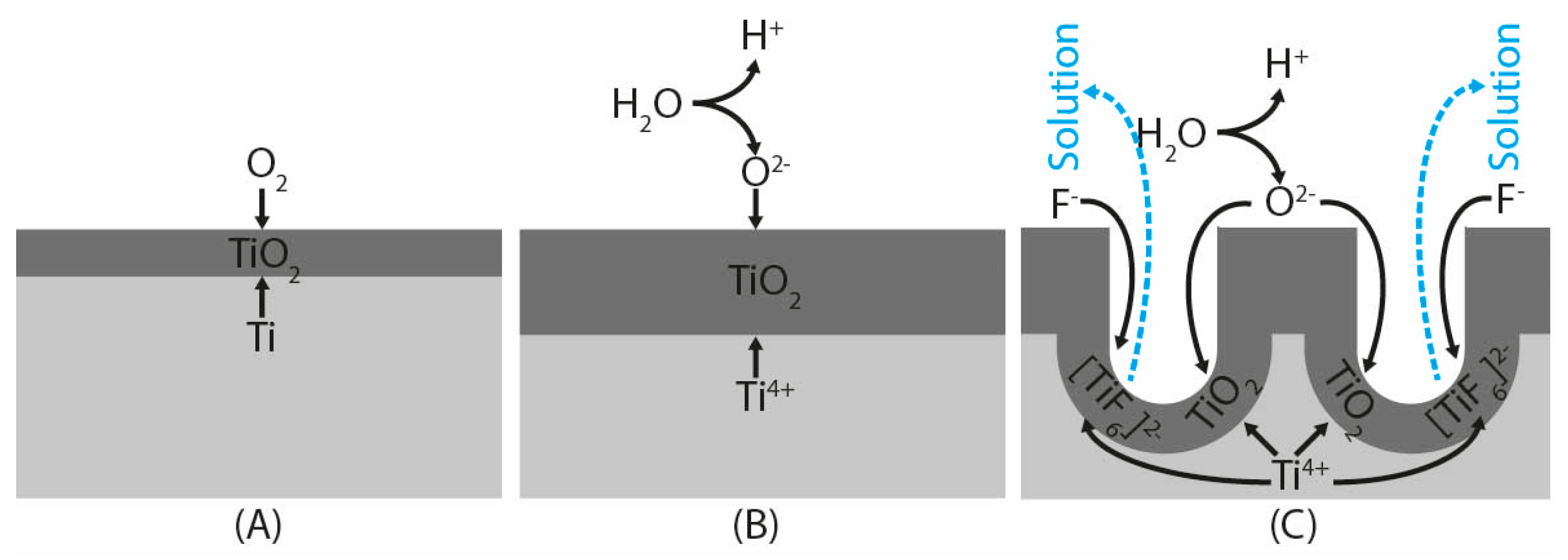
2.2. Electrochemical Surface Oxidation
2.3. Corrosion Tests
2.4. Surface Wettability Test
2.5. Drug Intercalation and Fourier-Transform Infrared Spectroscopy (FTIR) Characterization
2.6. Cell Adhesion and Proliferation
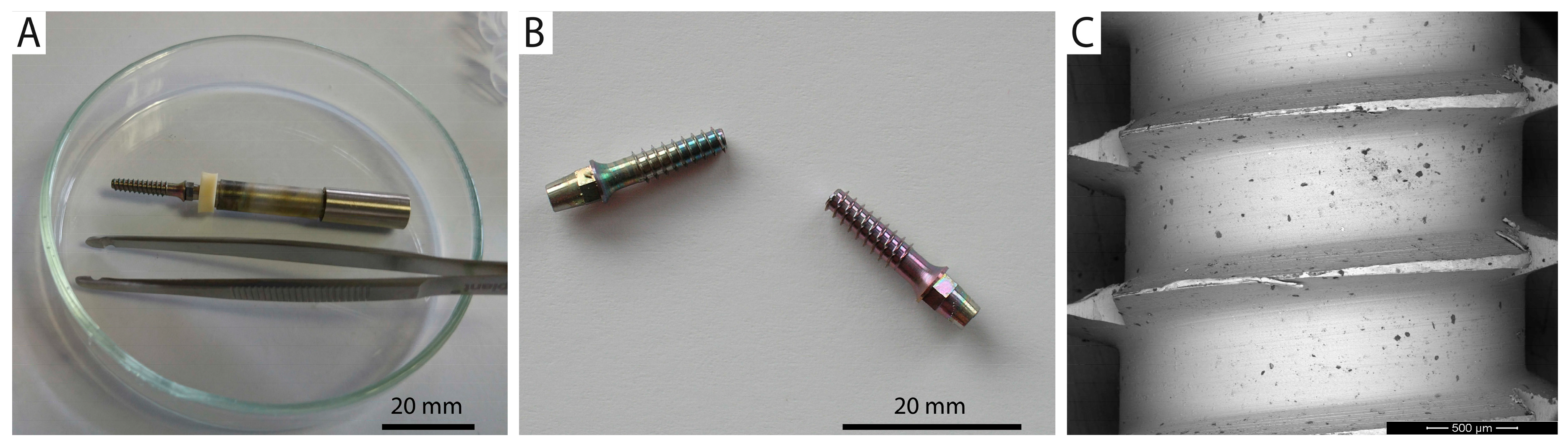
2.7. Practical Adhesion and Tribological Test
3. Results
3.1. Surface Morphology
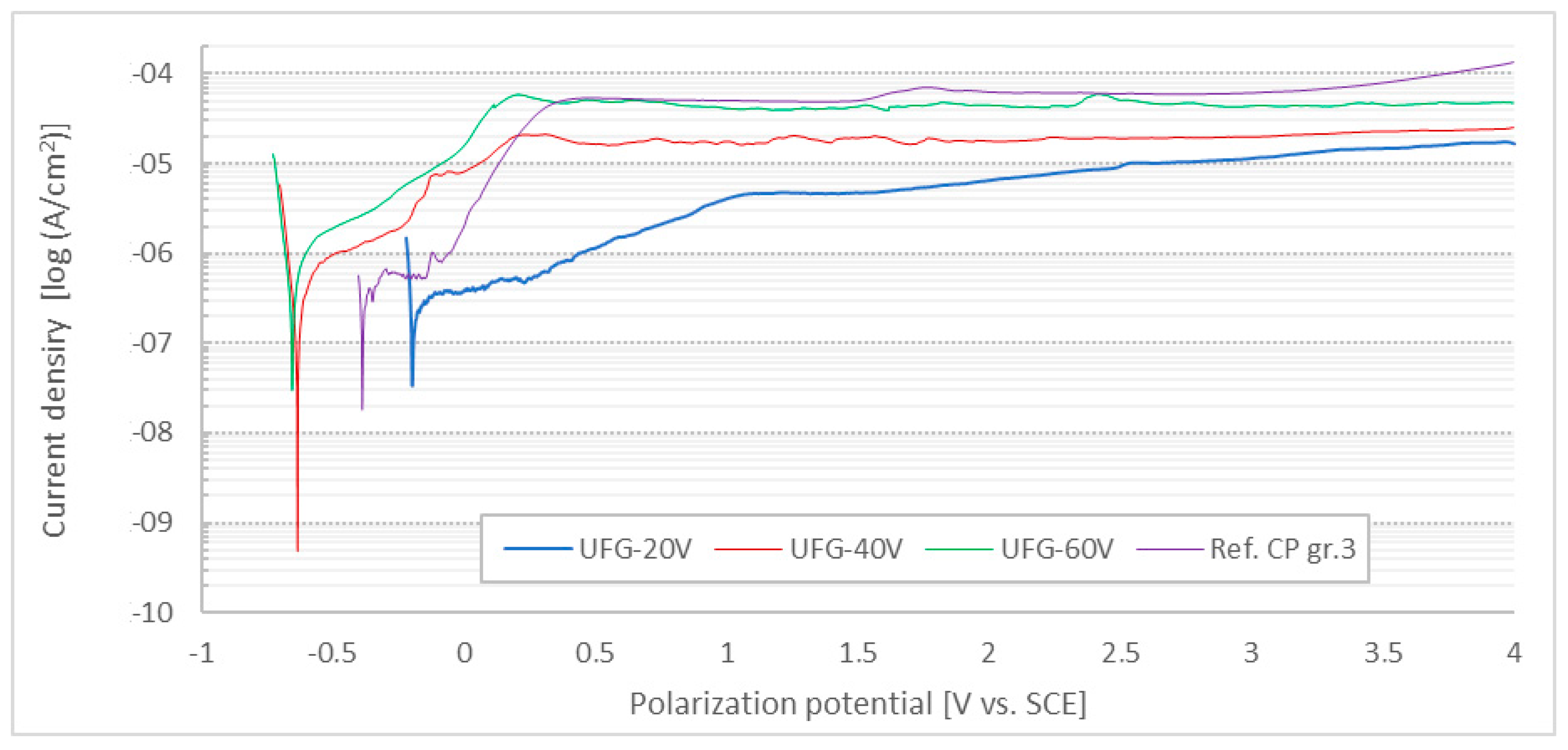
3.2. Potentiodynamic Corrosion Test
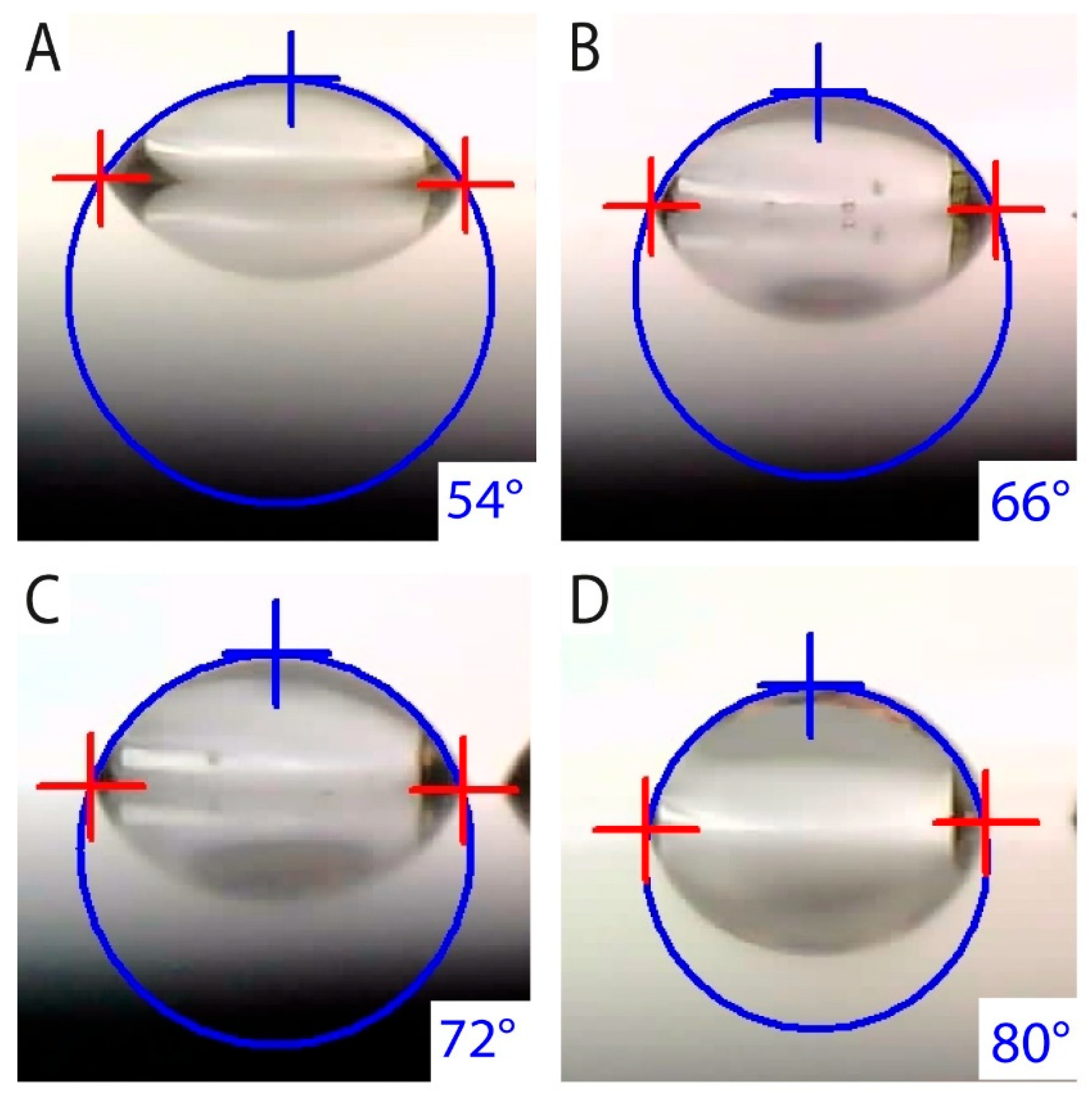
3.3. Surface Wettability
3.4. Practical Adhesion and Tribological Test
3.5. Layer Intercalation and FTIR
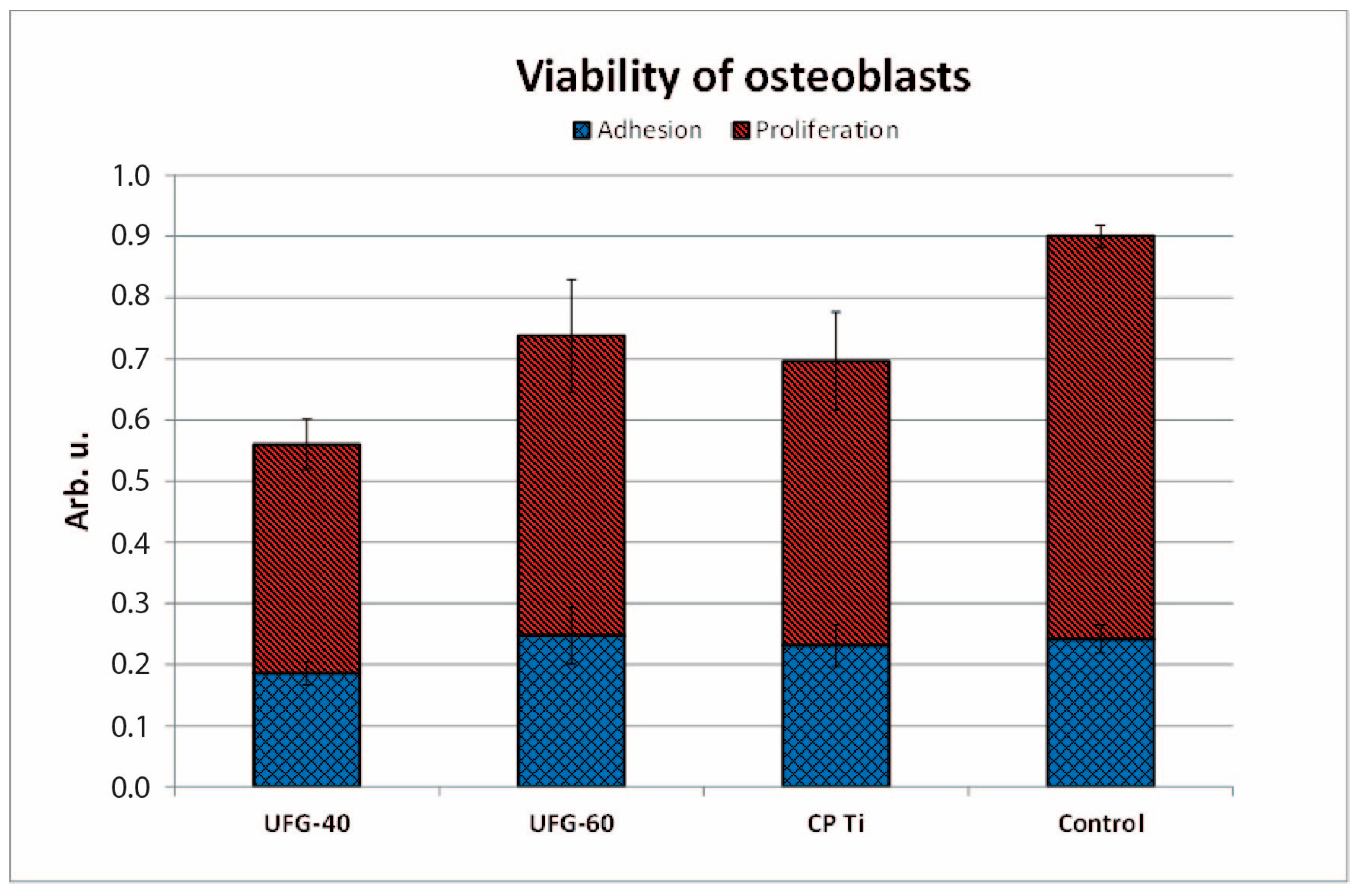
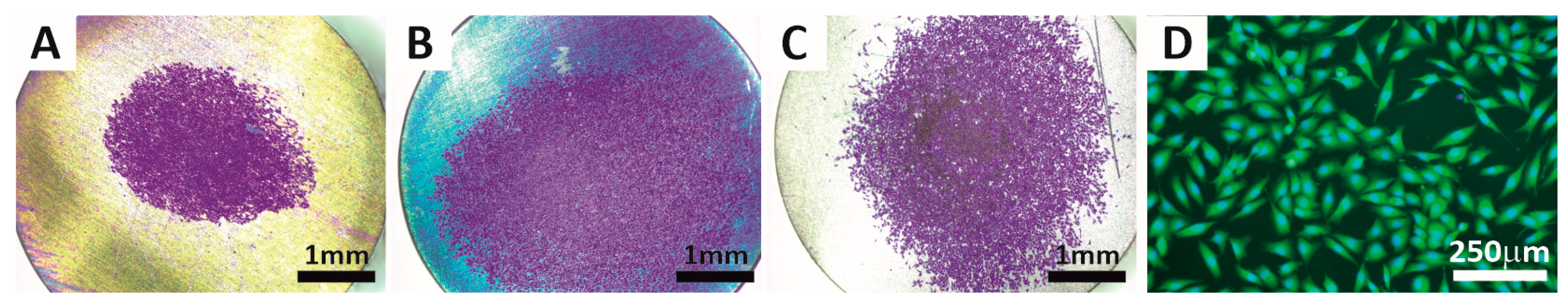
3.6. Cell Adhesion and Proliferation
4. Discussion
5. Conclusions
- Anodization at 20 V produced an oxide layer formed by clusters with typical nanotubular morphology. Anodization at 40 V and 60 V produced homogeneous TiO2 layers with nanoporous morphology. The pore size increased with the anodization voltage.
- The morphology of oxide layers on UFG CP Ti anodized at 40 V and 60 V matched the morphology of conventional grain size CP Ti anodized in a solution with a significantly lower water content.
- The corrosion rate of the anodized surfaces was slightly higher than that of the untreated CP Ti surface, but still deep under the limit recommended for implants.
- Corrosion potential of anodized surfaces was slightly increased compared to the untreated CP Ti surface, which increases the risk of their damage in the case of coupled corrosion initiation.
- Anodized layers were not prone to pitting corrosion even if polarized to 4000 mV vs. SCE in a solution containing a medium concentration of chlorine ions.
- Anodization of UFG CP Ti decreased the surface contact angle and, at the same time, increased the surface energy, which promotes easier colonization by hard tissue-forming cells.
- The anodized layer formed at 60 V on UFG CP Ti implants exhibited sufficient resistance to mechanical stress arising during simulated implantation procedures.
- The highly porous TiO2 surface produced at 60 V enabled the successful intercalation of DMSO and ibuprofen.
- All tested titanium surfaces were non-cytotoxic to osteoblasts. Cell adhesion and proliferation were slightly reduced on UFG-40V, but slightly enhanced on UFG-60V, when compared to the reference CP Ti. Overall, UFG-60V showed higher cell adhesion than UFG-40V and the reference titanium surface. Most parameters studied in this paper (corrosion, wettability, intercalation, biological) on UFG CP Ti and conventional grain size CP Ti anodized at similar conditions were comparable, and no significant differences were found between them.
- The results presented in this paper prove that specific electrochemical oxidation of miniaturized biomedical implants manufactured from UFG CP Ti had a positive effect on their biological performance and could also serve as a targeted drug delivery system while maintaining their excellent corrosion resistance.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, M.J. Etruscan Gold Dental Appliances: Three Newly “Discovered” Examples. Am. J. Archaeol. 1999, 103, 103–111. [Google Scholar] [CrossRef]
- Branemark, P.-I.; Zarb, G.A.; Albrektsson, T.; Rosen, H.M. Tissue-Integrated Prostheses. Osseointegration in Clinical Dentistry. Plast. Reconstr. Surg. 1986, 35, 259–265. [Google Scholar] [CrossRef]
- Nicholson, J. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Schroeder, V. Evolution of the Passive Film on Mechanically Damaged Nitinol. J. Biomed. Mater. Res. Part A 2009, 90, 1–17. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially Pure Titanium (Cp-Ti) versus Titanium Alloy (Ti6Al4V) Materials as Bone Anchored Implants—Is One Truly Better than the Other? Mater. Sci. Eng. C 2016, 62, 960–966. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Eduardo, E.R.; Zhao, X.; Yang, X.; Liu, X.; Cetlin, P.R.; Langdon, T.G. Improving the Fatigue Behavior of Dental Implants through Processing Commercial Purity Titanium by Equal-Channel Angular Pressing. Mater. Sci. Eng. A 2014, 619, 312–318. [Google Scholar] [CrossRef]
- Rotaru, H.; Armencea, G.; Spîrchez, D.; Berce, C.; Marcu, T.; Leordean, D.; Kim, S.G.; Lee, S.W.; Dinu, C.; Bǎciuţ, G.; et al. In Vivo Behavior of Surface Modified Ti6Al7Nb Alloys Used in Selective Laser Melting for Custom-Made Implants. A Preliminary Study. Rom. J. Morphol. Embryol. 2013, 54, 791–796. [Google Scholar]
- Cvijović-Alagić, I.; Cvijović, Z.; Mitrović, S.; Panić, V.; Rakin, M. Wear and Corrosion Behaviour of Ti-13Nb-13Zr and Ti-6Al-4V Alloys in Simulated Physiological Solution. Corros. Sci. 2011, 53, 796–808. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue Performance and Cyto-Toxicity of Low Rigidity Titanium Alloy, Ti-29Nb-13Ta-4.6Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M.P. Corrosion of Titanium: Part 1: Aggressive Environments and Main Forms of Degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, S.; Segal, R.J. Allergic Reaction to Vanadium Causes a Diffuse Eczematous Eruption and Titanium Alloy Orthopedic Implant Failure. Cutis 2017, 99, 245–249. [Google Scholar]
- Valiev, R.Z.; Semenova, I.P.; Latysh, V.V.; Rack, H.; Lowe, T.C.; Petruzelka, J.; Dluhos, L.; Hrusak, D.; Sochova, J. Nanostructured Titanium for Biomedical Applications. Adv. Eng. Mater. 2008, 10, B15–B17. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Sabirov, I.; Zemtsova, E.G.; Parfenov, E.V.; Dluhoš, L.; Lowe, T.C. Nanostructured Commercially Pure Titanium for Development of Miniaturized Biomedical Implants. In Titanium in Medical and Dental Applications; Woodhead Publishing: Cambridge, UK, 2018; ISBN 9780128124567. [Google Scholar]
- Hlinka, J.; Dluhos, L.; Dedkova, K. Corrosion Properties of ECAP Titanium with Bioactive Oxide Coating in Physiological Solution. Key Eng. Mater. 2019, 810, 52–57. [Google Scholar] [CrossRef]
- Lowe, T.C.; Reiss, R.A.; Illescas, P.E.; Davis, C.F.; Connick, M.C.; Sena, J.A. Effect of Surface Grain Boundary Density on Preosteoblast Proliferation on Titanium. Mater. Res. Lett. 2020, 8, 239–246. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Enhanced in Vitro Biocompatibility of Ultrafine-Grained Titanium with Hierarchical Porous Surface. Appl. Surf. Sci. 2011, 257, 5634–5640. [Google Scholar] [CrossRef]
- Abraham, C.M. A Brief Historical Perspective on Dental Implants, Their Surface Coatings and Treatments. Open Dent. J. 2014, 8, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zigterman, B.G.R.; Van den Borre, C.; Braem, A.; Mommaerts, M.Y. Titanium Surface Modifications and Their Soft-Tissue Interface on Nonkeratinized Soft Tissues—A Systematic Review (Review). Biointerphases 2019, 14, 040802. [Google Scholar] [CrossRef] [PubMed]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, Present and for the Future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- El-Gammal, M.Y.; El-Gammal, N.Y.; Fadhil, O.N.; Maria, O.M.; Ola Maria, C.M. Biological Reactions to Different Dental Implant Surface Treatments. Int. J. Contemp. Dent. Med. Rev. 2016, 2015, 1–9. [Google Scholar] [CrossRef]
- Hoseini, M.; Hamid Pourian, M.; Bridier, F.; Vali, H.; Szpunar, J.A.; Bocher, P. Thermal Stability and Annealing Behaviour of Ultrafine Grained Commercially Pure Titanium. Mater. Sci. Eng. A 2012, 532, 58–63. [Google Scholar] [CrossRef]
- Bagno, A.; Di Bello, C. Surface Treatments and Roughness Properties of Ti-Based Biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 935–949. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Leśniak, K.; Orzechowska, B.U.; Drab, M.; Wiśniewska, A.; Simka, W. Alkali Treatment of Anodized Titanium Alloys Affects Cytocompatibility. Metals 2018, 8, 29. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio-Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Lu, J.; Wei, G.; Yu, Y.; Zhao, X.; Dai, Y. Enhanced Corrosion Resistance of TA2 Titanium via Anodic Oxidation in Mixed Acid System. Int. J. Electrochem. Sci. 2017, 12, 2763–2776. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Chen, Z.; Zhao, A.Z.J.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Al-Deyab, S.S.; Lai, Y.K. TiO2 Nanotube Platforms for Smart Drug Delivery: A Review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Choi, D.S.; Lee, J.K.; Kim, W.T.; Cha, B.K.; Choi, W.Y. Effect of Drug-Loaded TiO2 Nanotube Arrays on Osseointegration in an Orthodontic Miniscrew: An in-Vivo Pilot Study. Biomed. Microdevices 2017, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Doadrio, A.L.; Conde, A.; Arenas, M.A.; Hernández-López, J.M.; De Damborenea, J.J.; Pérez-Jorge, C.; Esteban, J.; Vallet-Regí, M. Use of Anodized Titanium Alloy as Drug Carrier: Ibuprofen as Model of Drug Releasing. Int. J. Pharm. 2015, 492, 207–212. [Google Scholar] [CrossRef]
- ASTM B265-15; Standard Specification for Titanium and Titanium Alloy Strip, Sheet, and Plate. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2015.
- Cai, B.P.; Liu, Y.H.; Tian, X.J.; Li, H.; Ji, R.J.; Wang, F.; Zhang, Y.Z. Susceptibility of 316L Stainless Steel to Crevice Corrosion in Submersible Solenoid Valve. Mater. Corros. 2011, 62, 753–759. [Google Scholar] [CrossRef]
- Rohani, S.; El-Ruby, A. Synthesis of Titania Nanotube Arrays by Anodization. Chem. Eng. Trans. 2009, 17, 963–968. [Google Scholar]
- Fishtik, I. Thermodynamic Stability Relations in Redox Systems. Environ. Sci. Technol. 2006, 40, 1902–1910. [Google Scholar] [CrossRef]
- De Tacconi, N.R.; Chenthamarakshan, C.R.; Yogeeswaran, G.; Watcharenwong, A.; De Zoysa, R.S.; Basit, N.A.; Rajeshwar, K. Nanoporous TiO2 and WO3 Films by Anodization of Titanium and Tungsten Substrates: Influence of Process Variables on Morphology and Photoelectrochemical Response. J. Phys. Chem. B 2006, 110, 25347–25355. [Google Scholar] [CrossRef]
- Hlinka, J.; Lasek, S.; Faisal, N. Corrosion Properties of Anodized Titanium. Acta Met. Slovaca 2017, 23, 270–275. [Google Scholar] [CrossRef]
- Cheggou, R.; Ferhah, K.; Fraoucene, H.; Mougari, A.; Sam, S.; Rafai, S.; Khomeri, E.H. Experimental Investigation of Long-Term Ageing Effect on the Structural and Electrochemical Behaviour of Self-Organized TiO2 Array Nanotubes on Ti-6Al-4V Alloy. Nanosci. Nanotechnol.-Asia 2023, 13, e270423216293. [Google Scholar] [CrossRef]
- Fraoucene, H.; Hatem, D.; Vacandio, F.; Pasquinelli, M. Morphology and Electronic Properties of TiO2 Nanotubes Arrays Synthesized by Electrochemical Method. Nanosci. Nanotechnol.-Asia 2018, 9, 121–127. [Google Scholar] [CrossRef]
- Rosenbloom, S.N.; Corbett, R.A. An Assessment of ASTM F 2129 Test Results Comparing Nitinol to Other Implant Alloys. In SMST-2006-Proceedings of the International Conference on Shape Memory and Superelastic Technologies; Asilomar Conference Center: Pacific Grove, CA, USA, 2008. [Google Scholar]
- ASTM F746-04; Standard Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials. ASTM: West Conshohocken, PA, USA, 2014. [CrossRef]
- Olson, M.P.; Lacourse, W.R. Voltammetry. In Analytical Instrumentation Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780849390395. [Google Scholar]
- Pacheco, A.R.; Schokker, A.J.; Volz, J.S.; Hamilton, H.R. Linear Polarization Resistance Tests on Corrosion Protection Degree of Post-Tensioning Grouts. ACI Mater. J. 2011, 108, 365–370. [Google Scholar] [CrossRef]
- Kam, D.H.; Bhattacharya, S.; Mazumder, J. Control of the Wetting Properties of an AISI 316L Stainless Steel Surface by Femtosecond Laser-Induced Surface Modification. J. Micromech. Microeng. 2012, 22, 105019. [Google Scholar] [CrossRef]
- Calvimontes, A. The Measurement of the Surface Energy of Solids by Sessile Drop Accelerometry. Microgravity Sci. Technol. 2018, 30, 277–293. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, Z.; Haq, E.U.; Akhtar, W.; Arshad, M. Synthesis and Characterization of Titania Nanotubes by Anodizing of Titanium in Fluoride Containing Electrolytes. Appl. Nanosci. 2017, 7, 701–710. [Google Scholar] [CrossRef]
- Babuska, V.; Dobra, J.; Kulda, V.; Kripnerova, M.; Moztarzadeh, A.; Bolek, L.; Lahoda, J.; Hrusak, D. Comparison of Fibroblast and Osteoblast Response to Cultivation on Titanium Implants with Different Grain Sizes. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Babuska, V.; Dobra, J.K.; Dluhos, L.; Dvorakova, J.; Moztarzadeh, J.; Hrusak, D.; Kulda, V. Repeated Exposure of Nanostructured Titanium to Osteoblasts with Respect to Peri-Implantitis. Materials 2020, 13, 697. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical Applications of Titanium and Its Alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Elias, C.N.; Meyers, M.A.; Valiev, R.Z.; Monteiro, S.N. Ultrafine Grained Titanium for Biomedical Applications: An Overview of Performance. J. Mater. Res. Technol. 2013, 2, 340–350. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Semenova, I.P.; Jakushina, E.; Latysh, V.V.; Rack, H.; Lowe, T.C.; Petruželka, J.; Dluhoš, L.; Hrušák, D.; Sochová, J. Nanostructured SPD Processed Titanium for Medical Implants. Mater. Sci. Forum 2008, 584–586, 49–54. [Google Scholar] [CrossRef]
- Hsu, J.T.; Shen, Y.W.; Kuo, C.W.; Wang, R.T.; Fuh, L.J.; Huang, H.L. Impacts of 3D Bone-to- Implant Contact and Implant Diameter on Primary Stability of Dental Implant. J. Formos. Med. Assoc. 2017, 116, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Ezerietis, E. Juniper Wood as a Possible Implant Material. J. Biomed. Mater. Res. Part A 2003, 64A, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Kristen, H.; Bösch, P.; Bednar, H.; Plenk, H. The Effects of Dynamic Loading on Intracalcaneal Wood Implants and on the Tissues Surrounding Them. Arch. Orthop. Trauma. Surg. 1979, 93, 287–292. [Google Scholar] [CrossRef]
- Bilhan, H.; Geckili, O.; Mumcu, E.; Bozdag, E.; Sünbüloǧlu, E.; Kutay, O. Influence of Surgical Technique, Implant Shape and Diameter on the Primary Stability in Cancellous Bone. J. Oral Rehabil. 2010, 37, 900–907. [Google Scholar] [CrossRef]
- Falco, A.; Berardini, M.; Trisi, P. Correlation Between Implant Geometry, Implant Surface, Insertion Torque, and Primary Stability: In Vitro Biomechanical Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 824–830. [Google Scholar] [CrossRef]
- Handelsman, M. Surgical Guidelines for Dental Implant Placement. Br. Dent. J. 2006, 201, 139–152. [Google Scholar] [CrossRef]
- Becker, C.M.; Kaiser, D.A. Surgical Guide for Dental Implant Placement. J. Prosthet. Dent. 2000, 83, 248–251. [Google Scholar] [CrossRef]
- Jimenez, Y.S.; Gil, M.T.; Guerra, M.T.; Baltes, L.S.; Rosca, J.C.M. Interpretation of Open Circuit Potential of Two Titanium Alloys for a Long Time Immersion in Physiological Fluid. Transilv. Univ. Brasov 2009, 2, 197–204. [Google Scholar]
- McCafferty, E. Validation of Corrosion Rates Measured by the Tafel Extrapolation Method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Lee, B.C.; Kim, T.N.; Panigrahi, B.B. Corrosion Behaviour of Ultra Fine Grained Titanium in Simulated Body Fluid for Implant Application. Trends Biomater. Artif. Organs 2008, 22, 58–64. [Google Scholar]
- Zhong, X.; Xu, M.; Yang, L.; Qu, X.; Yang, L.; Zhang, M.; Liu, H.; Ma, Y. Predicting the Structure and Stability of Titanium Oxide Electrides. npj Comput. Mater. 2018, 4, 70. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium Alloys for Biomedical Applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Peters, M.; Hemptenmacher, J.; Kumpfert, J.; Leyens, C. Structure and Properties of Titanium and Titanium Alloys. In Titanium and Titanium Alloys; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Lorenzetti, M.; Pellicer, E.; Sort, J.; Baró, M.D.; Kovač, J.; Novak, S.; Kobe, S. Improvement to the Corrosion Resistance of Ti-Based Implants Using Hydrothermally Synthesized Nanostructured Anatase Coatings. Materials 2014, 7, 180–194. [Google Scholar] [CrossRef]
- Badea, G.E.; Caraban, A.; Sebesan, M.; Dzitac, S.; Cret, P.; Setel, A. Polarisation Measurements Used for Corrosion Rates Determination. J. Sustenable Energy 2010, 1, 1–4. [Google Scholar]
- De Assis, S.L.; Wolynec, S.; Costa, I. Corrosion Characterization of Titanium Alloys by Electrochemical Techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Wolf, F.G.; Dos Santos, L.O.E.; Philippi, P.C. Modeling and Simulation of the Fluid-Solid Interaction in Wetting. J. Stat. Mech. Theory Exp. 2009, 2009, P0600. [Google Scholar] [CrossRef]
- Viswanathan, B.; Raj, K.J.A. Effect of Surface Area, Pore Volume and Particle Size of P25 Titania on the Phase Transformation of Anatase to Rutile. Indian J. Chem. -Sect. A Inorg. Phys. Theor. Anal. Chem. 2009, 48, 1378–1382. [Google Scholar]
- Humphreys, F.J. Quantitative Metallography by Electron Backscattered Diffraction. J. Microsc. 1999, 195, 170–185. [Google Scholar] [CrossRef]
- Zeitler, V.A.; Brown, C.A. The Infrared Spectra of Some Ti-O-Si, Ti-O-Ti and Si-O-Si Compounds. J. Phys. Chem. 1957, 61, 1174–1177. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Danon, A.; Vijayan, B.K.; Gray, K.A.; Stair, P.C.; Weitz, E. Role of the Surface Lewis Acid and Base Sites in the Adsorption of CO2 on Titania Nanotubes and Platinized Titania Nanotubes: An in Situ FT-IR Study. J. Phys. Chem. C 2013, 117, 12661–12678. [Google Scholar] [CrossRef]
- Ramukutty, S.; Ramachandran, E. Growth, Spectral and Thermal Studies of Ibuprofen Crystals. Cryst. Res. Technol. 2012, 47, 31–38. [Google Scholar] [CrossRef]
- Kim, K.-H.; Ramaswamy, N. Electrochemical Surface Modification of Titanium in Dentistry. Dent. Mater. J. 2009, 28, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Thompson, G.E.; Habazaki, H.; Paez, M.A.; Shimizu, K.; Skeldon, P.; Wood, G.C. Morphological Development of Oxygen Bubbles in Anodic Alumina. J. Electrochem. Soc. 2000, 147, 1747–1750. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-Ordering Electrochemistry: A Review on Growth and Functionality of TiO2 Nanotubes and Other Self-Aligned MOx Structures. Chem. Commun. 2009, 20, 2791–2808. [Google Scholar] [CrossRef]
- Duvvuru, M.K.; Han, W.; Chowdhury, P.R.; Vahabzadeh, S.; Sciammarella, F.; Elsawa, S.F. Bone Marrow Stromal Cells Interaction with Titanium; Effects of Composition and Surface Modification. PLoS ONE 2019, 14, e0216087. [Google Scholar] [CrossRef]
- Indira, K.; Ningshen, S.; Mudali, U.K.; Rajendran, N. Effect of Anodization Parameters on the Structural Morphology of Titanium in Fluoride Containing Electrolytes. Mater. Charact. 2012, 71, 58–65. [Google Scholar] [CrossRef]
- Kaneco, S.; Chen, Y.; Westerhoff, P.; Crittenden, J.C. Fabrication of Uniform Size Titanium Oxide Nanotubes: Impact of Current Density and Solution Conditions. Scr. Mater. 2007, 56, 373–376. [Google Scholar] [CrossRef]
- Lee, A.R.; Kim, J.Y. Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells. Energies 2020, 13, 6100. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; Lai, P.; Hu, M.Z. A Comparative Study of Anodized Titania Nanotube Architectures in Aqueous and Nonaqueous Solutions. J. Mater. Res. 2011, 26, 2612–2623. [Google Scholar] [CrossRef]
- Li, Z.; Fu, L.; Fu, B.; Shan, A. Effects of Annealing on Microstructure and Mechanical Properties of Nano-Grained Titanium Produced by Combination of Asymmetric and Symmetric Rolling. Mater. Sci. Eng. A 2012, 558, 309–318. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and Its Prevention—A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Asumpinwong, W.; Saengkiettiyut, K.; Srimaneepong, V. Different Constant Voltages of Anodization on the Corrosion Behavior of Ti-6Al-4V Alloy. Chiang Mai J. Sci. 2015, 42, 239. [Google Scholar]
- Prando, D.; Brenna, A.; Bolzoni, F.M.; Diamanti, M.V.; Pedeferri, M.; Ormellese, M. Electrochemical Anodizing Treatment to Enhance Localized Corrosion Resistance of Pure Titanium. J. Appl. Biomater. Funct. Mater. 2017, 15, 19–24. [Google Scholar] [CrossRef]
- Tacchini, I.; Terrado, E.; Ansón, A.; Martínez, M.T. Anatase Nanotubes Synthesized by a Template Method and Their Application as a Green Photocatalyst. J. Mater. Sci. 2011, 46, 2097–2104. [Google Scholar] [CrossRef]
- Sopha, H.; Hromadko, L.; Nechvilova, K.; Macak, J.M. Effect of Electrolyte Age and Potential Changes on the Morphology of TiO2 Nanotubes. J. Electroanal. Chem. 2015, 759, 122–128. [Google Scholar] [CrossRef]
- Kadowaki, N.T.; Martinez, G.A.S.; Robin, A. Electrochemical Behavior of Three CP Titanium Dental Implants in Artificial Saliva. Mater. Res. 2009, 12, 363–366. [Google Scholar] [CrossRef]
- Bhola, R.; Bhola, S.; Mishra, B.; Ayers, R.; Olson, D.; Ohno, T. Surface Characterization of Anodically Treated Β Titanium Alloy for Biomedical Applications. Электронная Обработка Материалов 2011, 47, 75–82. [Google Scholar]
- Mohsen, Q.; Fadl-Allah, S.A. Improvement in Corrosion Resistance of Commercial Pure Titanium for the Enhancement of Its Biocompatibility. Mater. Corros. 2011, 62, 310–319. [Google Scholar] [CrossRef]
- Minhas, B.; Dino, S.; Zuo, Y.; Qian, H.; Zhao, X. Improvement of Corrosion Resistance of TiO2 Layers in Strong Acidic Solutions by Anodizing and Thermal Oxidation Treatment. Materials 2021, 14, 1188. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Burattini, M.; Miglio, R. Study of the Corrosion Resistance of Austenitic Stainless Steels during Conversion of Waste to Biofuel. Materials 2017, 10, 325. [Google Scholar] [CrossRef]
- Hlinka, J.; Lasek, S.; Siostrzonek, R.; Faisal, N. Characterization of Hydroxyapatite Layer on AISI 316L Stainless Steel. In Proceedings of the METAL 2017-26th International Conference on Metallurgy and Materials, Conference Proceedings, Brno, Czech Republic, 24–26 May 2017. [Google Scholar]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, M.V.; Ormellese, M.; Pedeferri, M.P. Application-Wise Nanostructuring of Anodic Films on Titanium: A Review. J. Exp. Nanosci. 2015, 10, 1285–1308. [Google Scholar] [CrossRef]
- Macak, J.M.; Jarosova, M.; Jäger, A.; Sopha, H.; Klementová, M. Influence of the Ti Microstructure on Anodic Self-Organized TiO2 nanotube Layers Produced in Ethyene Glycol Electrolytes. Appl. Surf. Sci. 2016, 371, 607–612. [Google Scholar] [CrossRef]
- ISO/ES10993-15; Biological Evaluation of Medical Devices—Part 15: Identification and Quantification of Degradation Products from Metals and Alloys. International Organization for Standardization: Geneva, Switzerland, 2001.
- Sato, N. The Basic Process of Metallic Corrosion in Aqueous Solution Consists of the Anodic Dissolution of Metals and the Cathodic Reduction of Oxidants Present in the Solution. In Basics of Corrosion Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual Effects and Mechanism of TiO2 Nanotube Arrays in Reducing Bacterial Colonization and Enhancing C3H10T1/2 Cell Adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of Surface Wettability on Competitive Protein Adsorption and Initial Attachment of Osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Anand, S.C.; Bhardwaj, J.R.; Sachdeva, V.P.; Sapru, B.L. BONE RESPONSE TO STAINLESS STEEL AND TITANIUM BONE PLATES: An Experimental Study on Animals. Med. J. Armed Forces India 1994, 50, 10–14. [Google Scholar] [CrossRef]
- Anil, S.; Anand, P.S.; Alghamdi, H.; Janse, J.A. Dental Implant Surface Enhancement and Osseointegration. In Implant Dentistry—A Rapidly Evolving Practice; Books on Demand: Hamburg, Germany, 2011. [Google Scholar]
- Kim, K.; Lee, B.A.; Piao, X.H.; Chung, H.J.; Kim, Y.J. Surface Characteristics and Bioactivity of an Anodized Titanium Surface. J. Periodontal Implant Sci. 2013, 43, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Osękowska, M.; Karuga-Kuźniewska, E.; Wojcieszak, D.; Mazur, M.; Poniedziałek, A.; Kaczmarek, D.; Szymonowicz, M.; Rybak, Z. Influence of Nanocrystalline Structure and Surface Properties of TiO2 Thin Films on the Viability of L929 Cells. Pol. J. Chem. Technol. 2015, 17, 33–39. [Google Scholar] [CrossRef]
- Goodarzi, S.; Moztarzadeh, F.; Nezafati, N.; Omidvar, H. Titanium Dioxide Nanotube Arrays: A Novel Approach into Periodontal Tissue Regeneration on the Surface of Titanium Implants. Adv. Mater. Lett. 2016, 7, 209–215. [Google Scholar] [CrossRef]
- Hashemi, A.; Ezati, M.; Mohammadnejad, J.; Houshmand, B.; Faghihi, S. Chitosan Coating of TiO2 Nanotube Arrays for Improved Metformin Release and Osteoblast Differentiation. Int. J. Nanomed. 2020, ume 15, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Chakrabarty, D.; Mukherjee, S.; Bhowmik, S. Adhesion Characteristics on Anodized Titanium and Its Durability under Aggressive Environments. Surf. Rev. Lett. 2016, 23, 1650033. [Google Scholar] [CrossRef]
- Tho, N.H.; Thy, T.T.M.; Dat, P.T.; Minh, V.C.; Sang, N.X. Physical Adsorption and Photocatalytic Activity of Titanium Dioxide Nanotube and Graphene Oxide Composite. VNU J. Sci. Nat. Sci. Technol. 2018, 34, 54–63. [Google Scholar] [CrossRef]
- NCT02096978 Comparing Implant Stability Using Two Accepted Techniques for Implant Sites. 2018. Available online: https://clinicaltrials.gov/show/NCT02096978 (accessed on 15 September 2025).
- García-Vives, N.; Andrés-García, R.; Rios-Santos, V.; Fernández-Palacín, A.; Bullón-Fernández, P.; Herrero-Climent, M.; Herrero-Climent, F. In Vitro Evaluation of the Type of Implant Bed Preparation with Osteotomes in Bone Type IV and Its Influence on the Stability of Two Implant Systems. Med. Oral Patol. Oral Cir. Bucal 2009, 14, e455–e460. [Google Scholar]
- Zabala, A.; Blunt, L.; Tejero, R.; Llavori, I.; Aginagalde, A.; Tato, W. Quantification of Dental Implant Surface Wear and Topographical Modification Generated during Insertion. Surf. Topogr. Metrol. Prop. 2020, 8, 015002. [Google Scholar] [CrossRef]
- Wang, Z.; Di-Franco, F.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Passivation-Induced Physicochemical Alterations of the Native Surface Oxide Film on 316L Austenitic Stainless Steel. J. Electrochem. Soc. 2019, 166, C3376–C3388. [Google Scholar] [CrossRef]
- Ion, R.; Necula, M.G.; Mazare, A.; Mitran, V.; Neacsu, P.; Schmuki, P.; Cimpean, A. Drug Delivery Systems Based on Titania Nanotubes and Active Agents for Enhanced Osseointegration of Bone Implants. Curr. Med. Chem. 2019, 27, 854–902. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Penha, N.; Ng, J.C. Anodization as a Promising Surface Treatment for Drug Delivery Implants and a Non-Cytotoxic Process for Surface Alteration: A Pilot Study. J. Osseointegration 2020, 12, 45–49. [Google Scholar] [CrossRef]
- Meng, F.; Yin, Z.; Ren, X.; Geng, Z.; Su, J. Construction of Local Drug Delivery System on Titanium-Based Implants to Improve Osseointegration. Pharmaceutics 2022, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
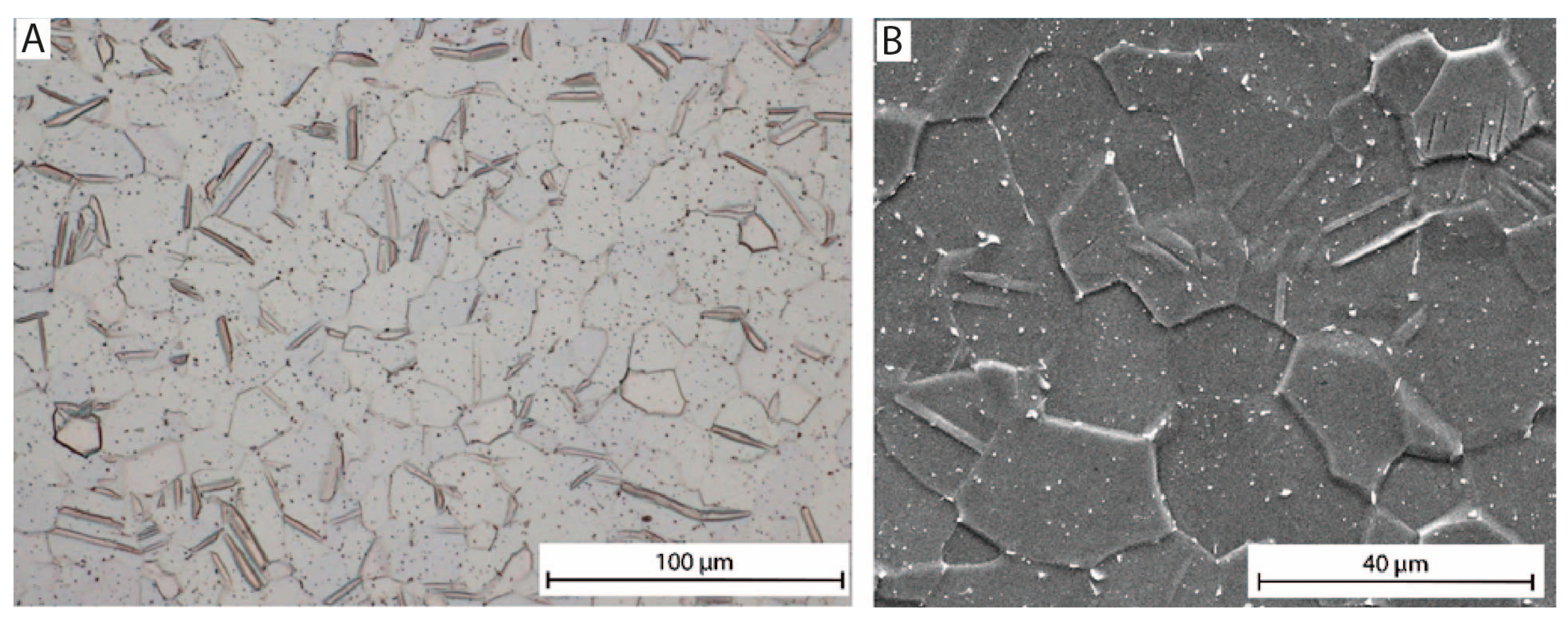
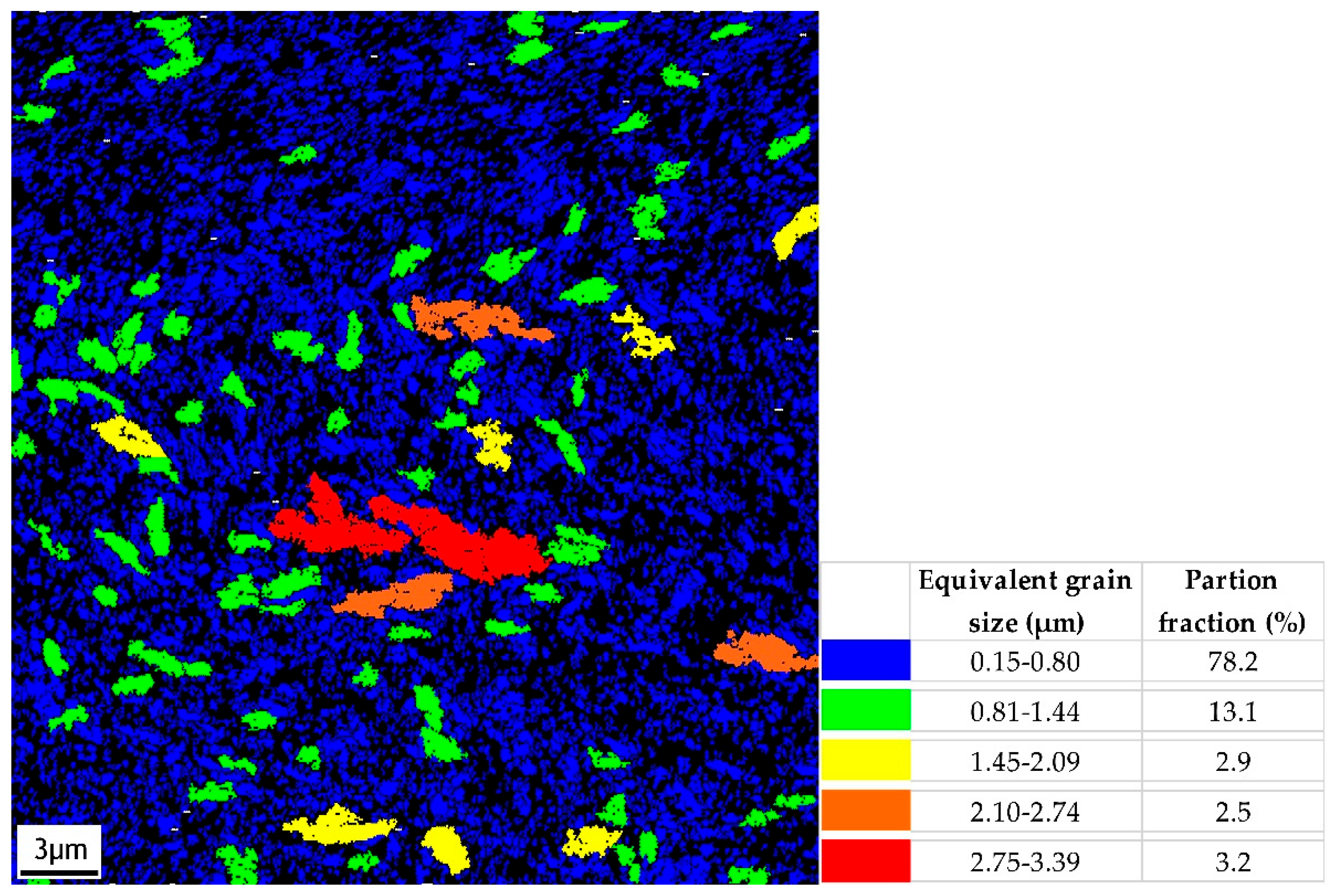
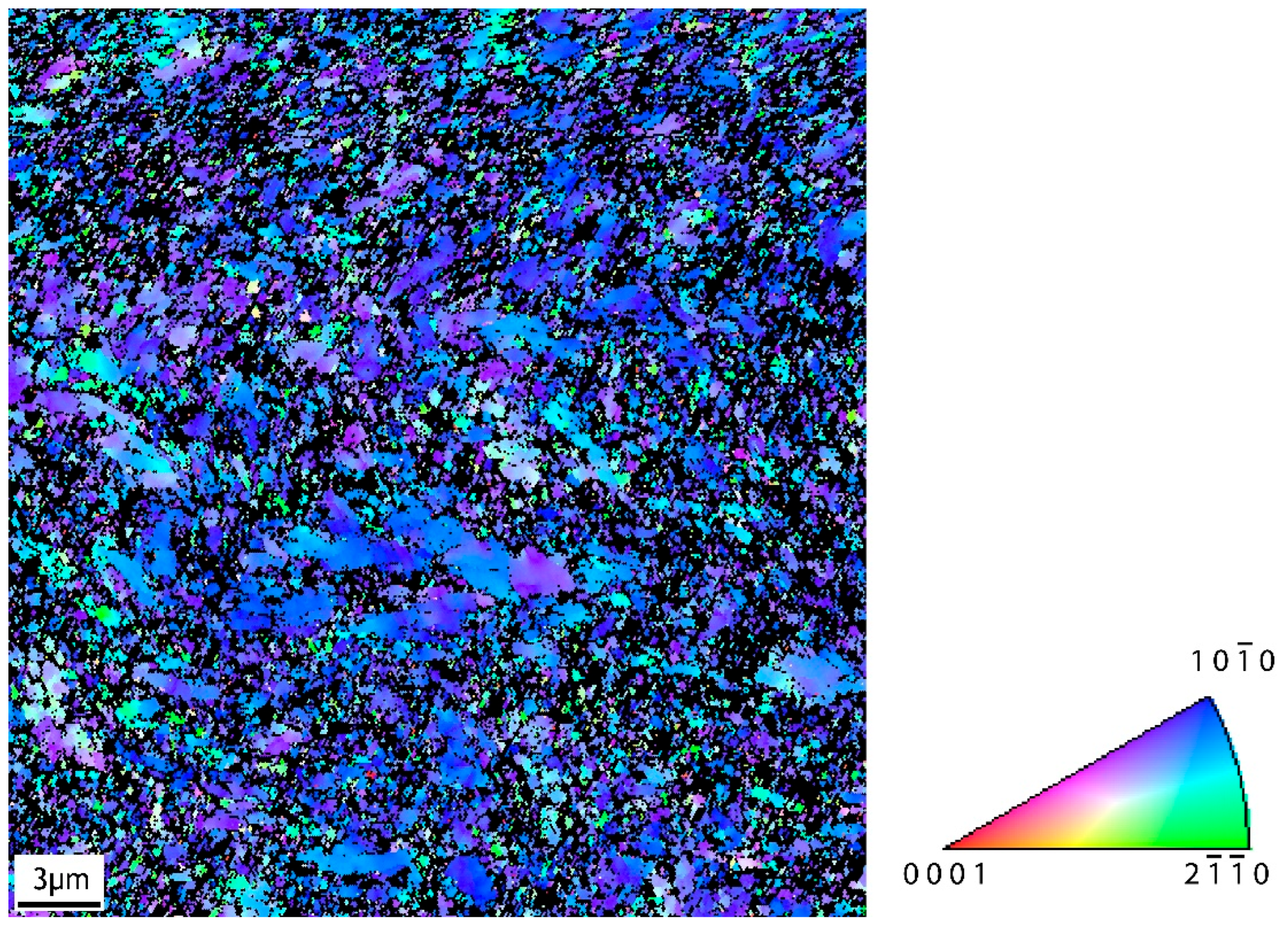



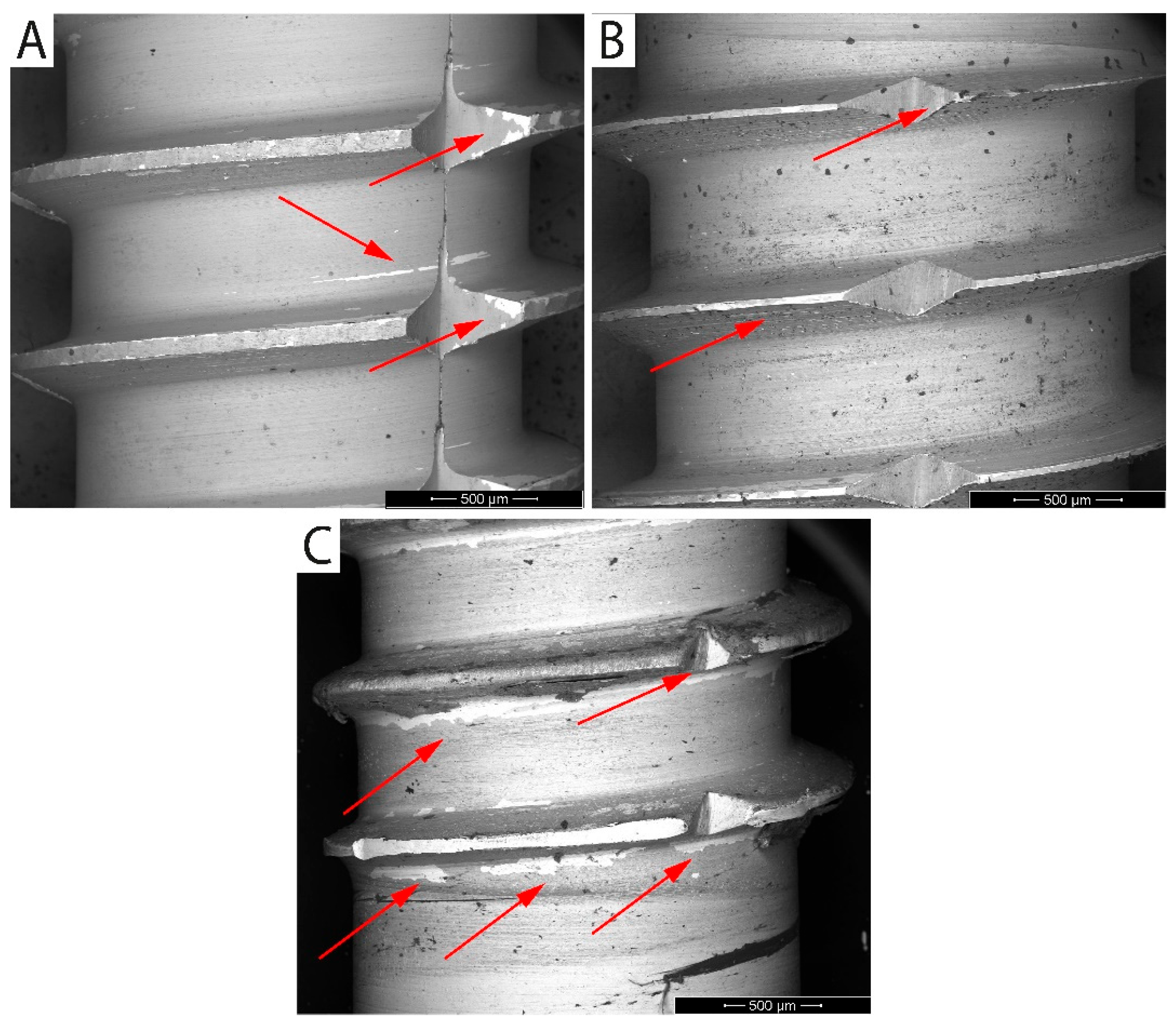
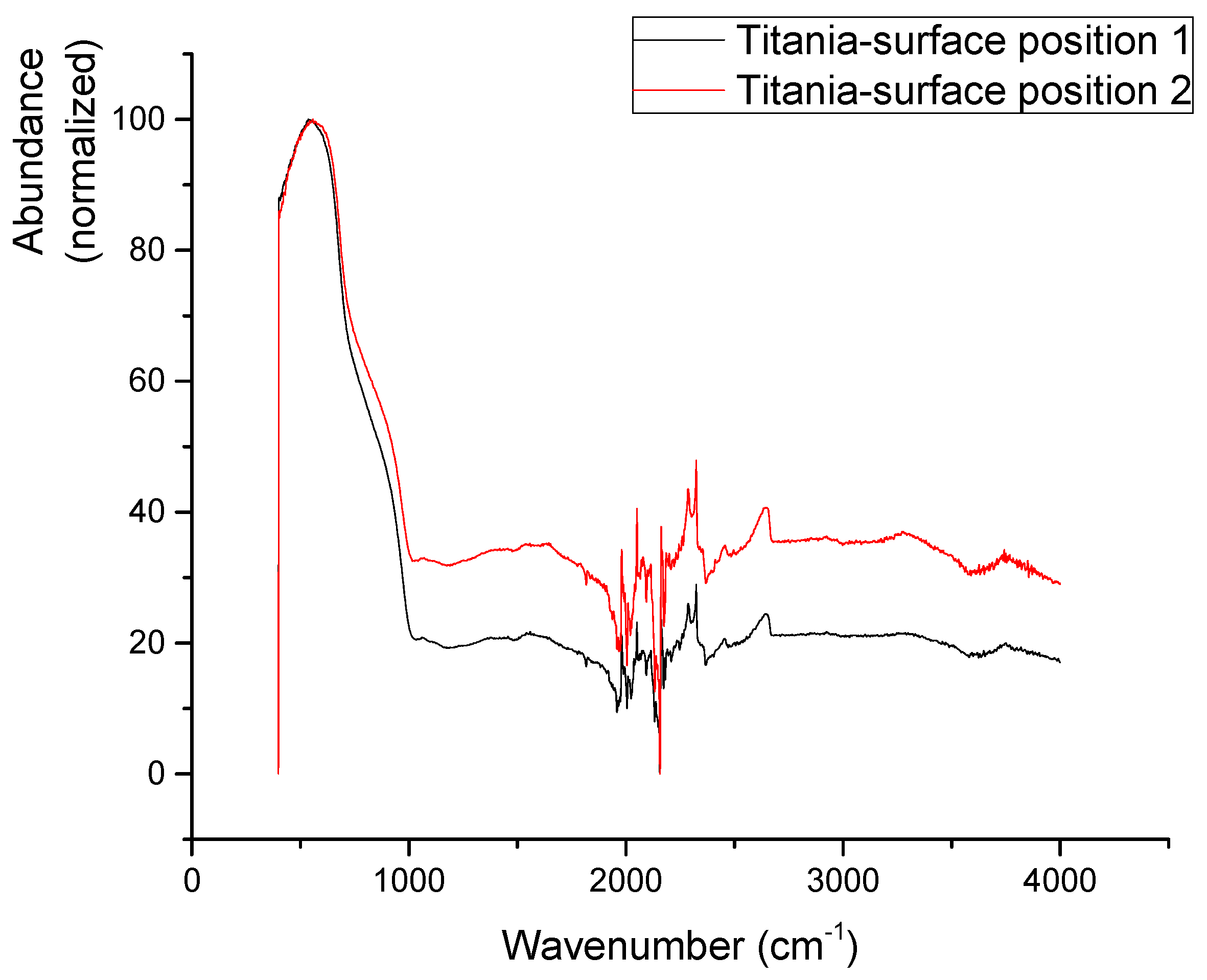
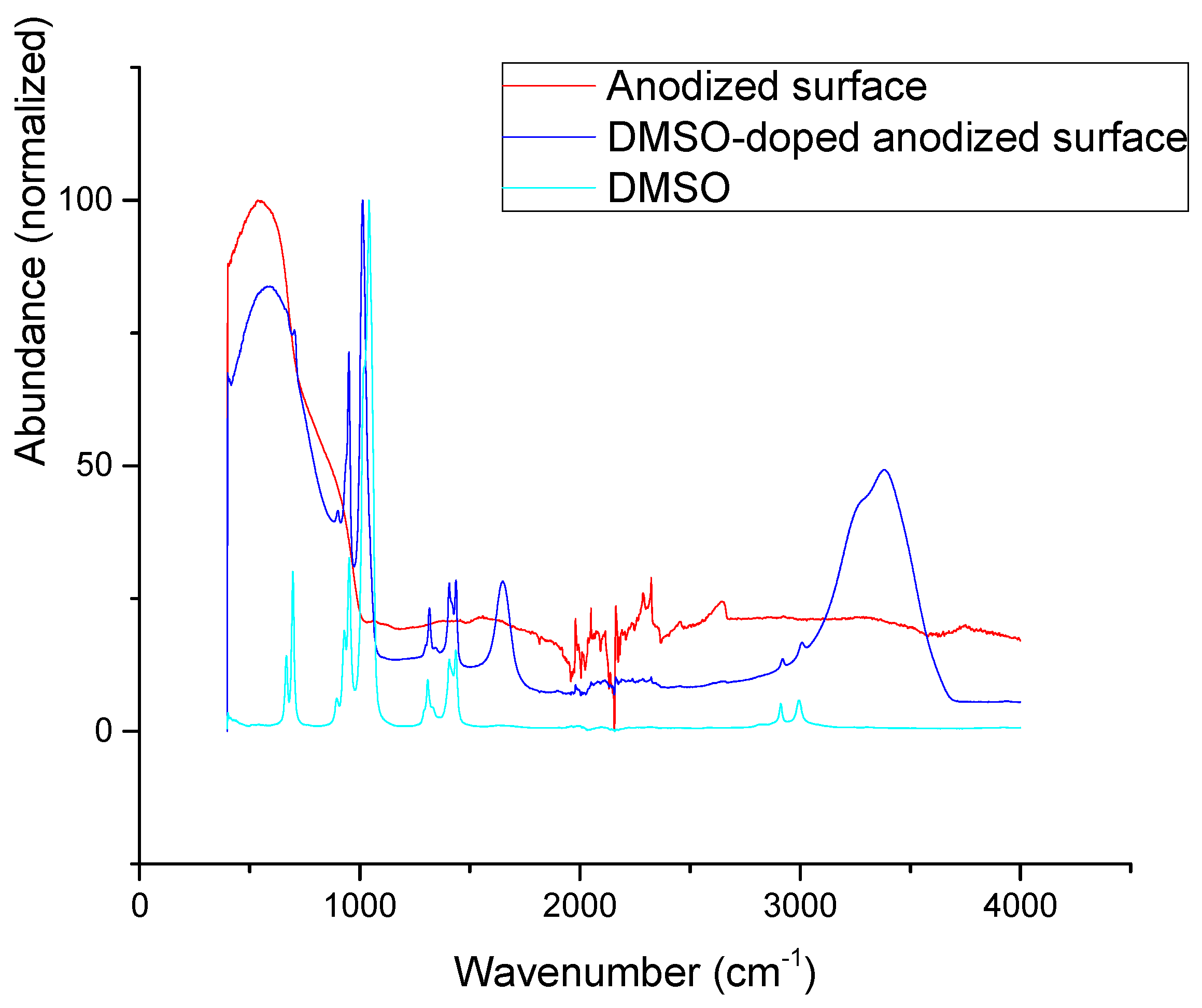
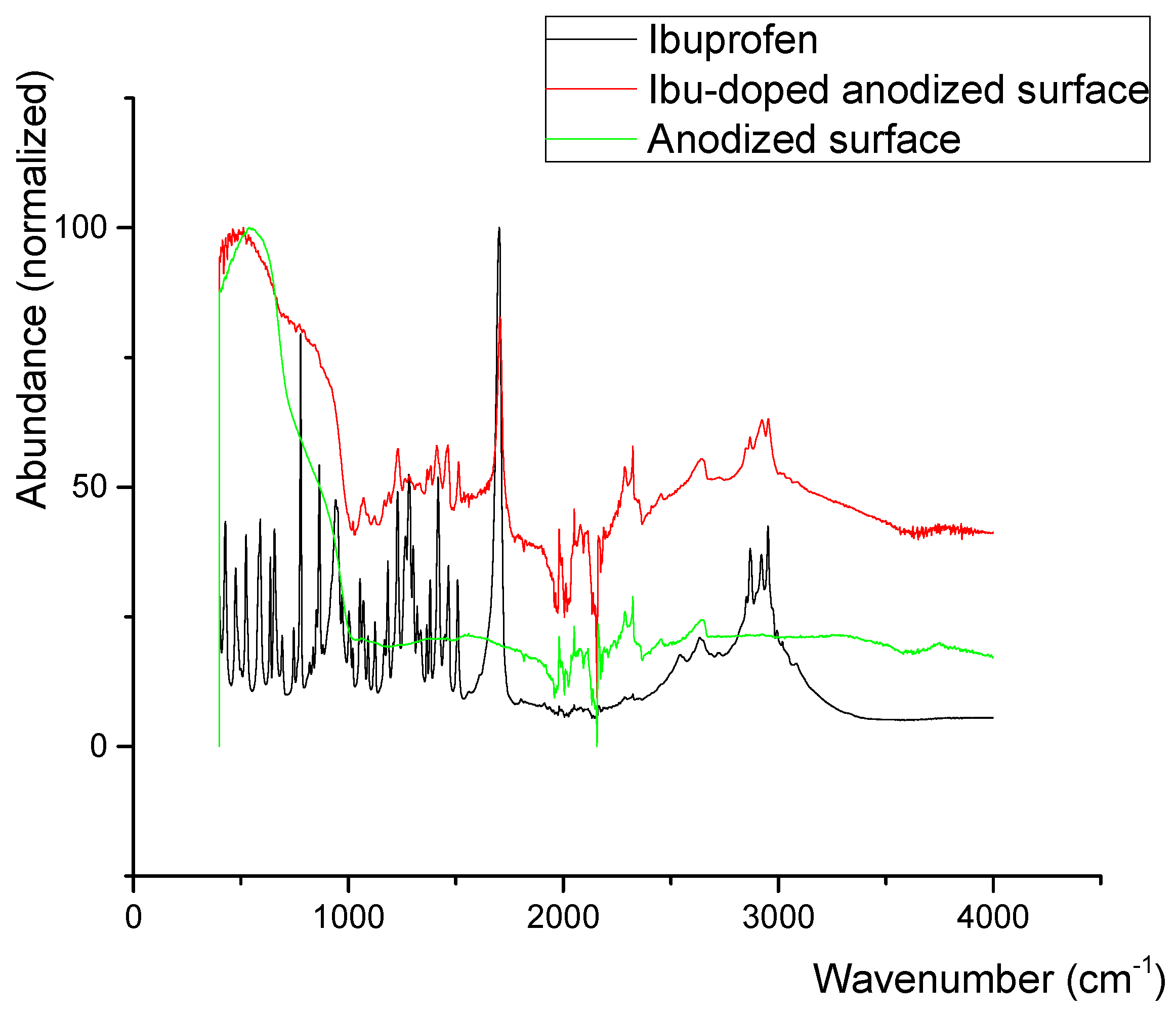
| Mechanical Properties | Chemical Composition (max. wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | N | C | H | Fe | O |
| CP Ti grade 3 | min. 450 | min. 380 | min. 18 | 0.3 | 0.8 | 0.015 | 0.3 | 0.35 |
| ECAP Ti grade 4 | 1143 | 864 | 15.4 | 0.3 | 0.8 | 0.015 | 0.5 | 0.4 |
| Sample | Material | Anodization Voltage (V) | Time of Anodization (s) |
|---|---|---|---|
| UFG-20V | UFG CP Ti grade 4 | 20 | 300 |
| UFG-40V | UFG CP Ti grade 4 | 40 | 300 |
| UFG-60V | UFG CP Ti grade 4 | 60 | 300 |
| Reference | CP Ti grade 3 | - | - |
| Taffel Method | Stearn–Geary Method | |||||
|---|---|---|---|---|---|---|
| Sample | Corrosion potential Ecor (mV vs. SCE) | Polarization resistance Rp (kΩ·cm2) | Cor. current density JC (nA·cm−2) | Corrosion rate CR (μm·year−1) | Corrosion potential Ecor (mV vs. SCE) | Polarization resistance Rp (kΩ·cm−2) |
| UFG-20V | −204 ± 18 | 125 ± 9 | 143 ± 14 | 1.3 ± 0.15 | −211 ± 21 | 128 ± 10 |
| UFG-40V | −632 ± 28 | 76 ± 8 | 184 ± 19 | 1.7 ± 0.22 | −634 ± 31 | 82 ± 9 |
| UFG-60V | −652 ± 33 | 41 ± 6 | 410 ± 48 | 3.7 ± 0.45 | −653 ± 35 | 48 ± 7 |
| Ref. CP Ti gr.3 | −376 ± 16 | 101 ± 8 | 137 ± 13 | 1.2 ± 0.14 | −375 ± 17 | 97 ± 7 |
| Sample | Contact Angle (°) | Calculated Free Surface Energy (mJ·m−2) |
|---|---|---|
| UFG-20V | 52.3 ± 2.1 | 53.6 ± 1.3 |
| UFG-40V | 67.9 ± 1.4 | 45.3 ± 0.8 |
| UFG-60V | 71.0 ± 1.5 | 39.5 ± 1.0 |
| Ref. CP gr.3 | 81.4 ± 2.9 | 32.6 ± 1.9 |
| Comparison | p (Adhesion) | Significance | p (Proliferation) | Significance |
|---|---|---|---|---|
| V4 vs. Control | 0.0049 | ** | 0.0016 | ** |
| V6 vs. Control | 0.8411 | ns | 0.0459 | * |
| CP Ti vs. Control | 0.6100 | ns | 0.0070 | ** |
| V4 vs. CP Ti | 0.0444 | * | 0.1585 | ns |
| V6 vs. CP Ti | 0.5899 | ns | 0.6392 | ns |
| V6 vs. V4 | 0.0393 | * | 0.1017 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlinka, J.; Cvejn, D.; Dluhos, L.; Babuska, V.; Cabanova, K.; Dvorakova, J.; Volodarskaja, A.; Valiev, R.Z.; Faisal, N.H.; Peterek Dedkova, K.; et al. Grain Size and Electrochemical Surface Modification Effects on Corrosion, Biological, and Technological Properties of CP Titanium Implants. J. Funct. Biomater. 2025, 16, 439. https://doi.org/10.3390/jfb16120439
Hlinka J, Cvejn D, Dluhos L, Babuska V, Cabanova K, Dvorakova J, Volodarskaja A, Valiev RZ, Faisal NH, Peterek Dedkova K, et al. Grain Size and Electrochemical Surface Modification Effects on Corrosion, Biological, and Technological Properties of CP Titanium Implants. Journal of Functional Biomaterials. 2025; 16(12):439. https://doi.org/10.3390/jfb16120439
Chicago/Turabian StyleHlinka, Josef, Daniel Cvejn, Ludek Dluhos, Vaclav Babuska, Kristina Cabanova, Jana Dvorakova, Anastasia Volodarskaja, Ruslan Z. Valiev, Nadimul H. Faisal, Katerina Peterek Dedkova, and et al. 2025. "Grain Size and Electrochemical Surface Modification Effects on Corrosion, Biological, and Technological Properties of CP Titanium Implants" Journal of Functional Biomaterials 16, no. 12: 439. https://doi.org/10.3390/jfb16120439
APA StyleHlinka, J., Cvejn, D., Dluhos, L., Babuska, V., Cabanova, K., Dvorakova, J., Volodarskaja, A., Valiev, R. Z., Faisal, N. H., Peterek Dedkova, K., Palupcikova, R., & Vodarek, V. (2025). Grain Size and Electrochemical Surface Modification Effects on Corrosion, Biological, and Technological Properties of CP Titanium Implants. Journal of Functional Biomaterials, 16(12), 439. https://doi.org/10.3390/jfb16120439










