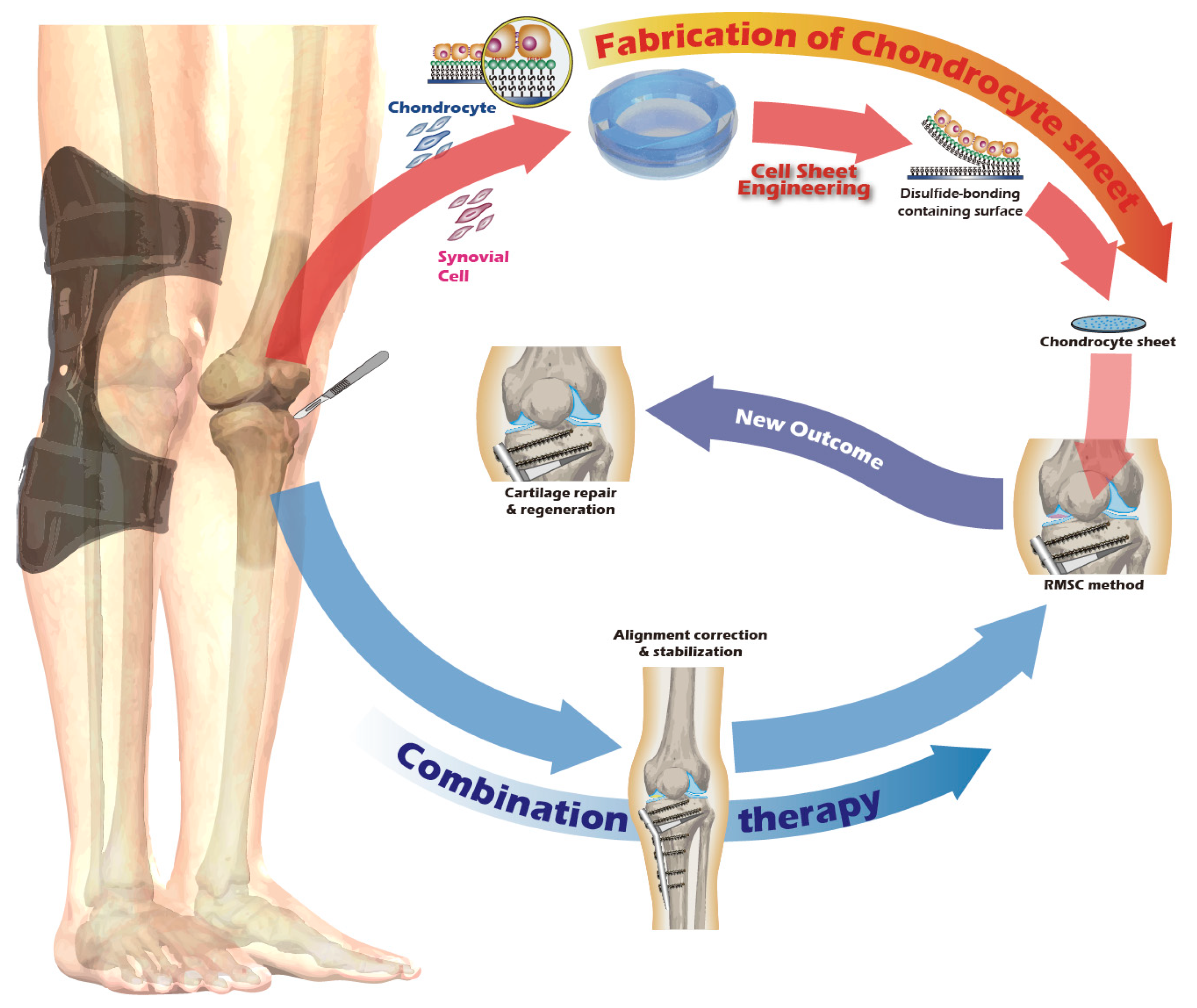
A New Way to Engineer Cell Sheets for Articular Cartilage Regeneration
Abstract
1. Introduction
2. Materials and Methods
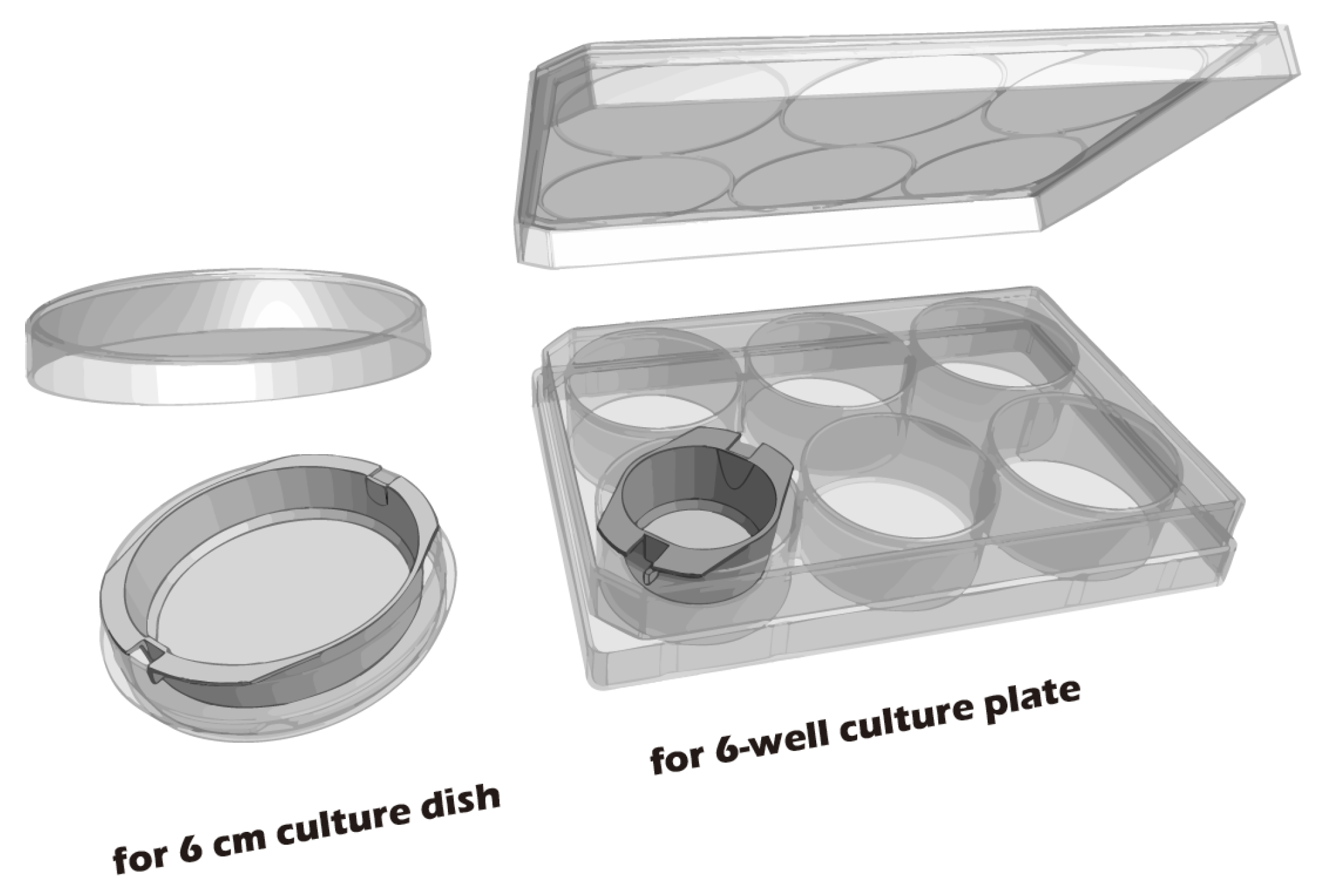
2.1. Preparation of Cell Culture Inserts
2.2. Characterization of Surface-Modified PET Membrane
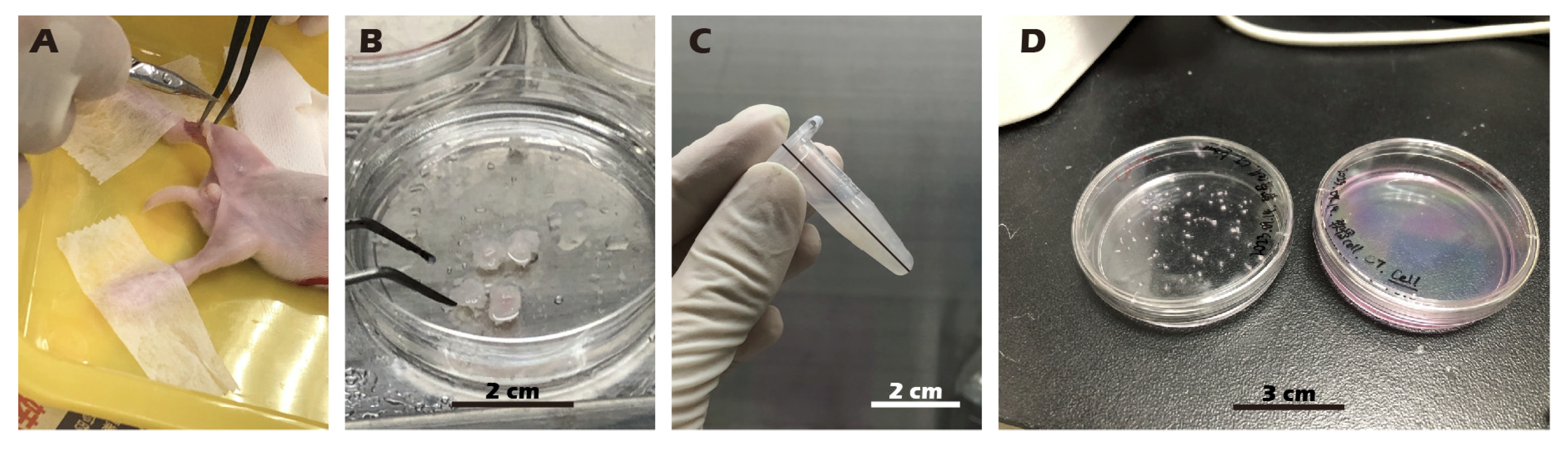
2.3. Juvenile Rabbit Articular Chondrocyte Isolation and Cultivation
2.4. Rabbit Articular Chondrocyte Isolation and Cultivation
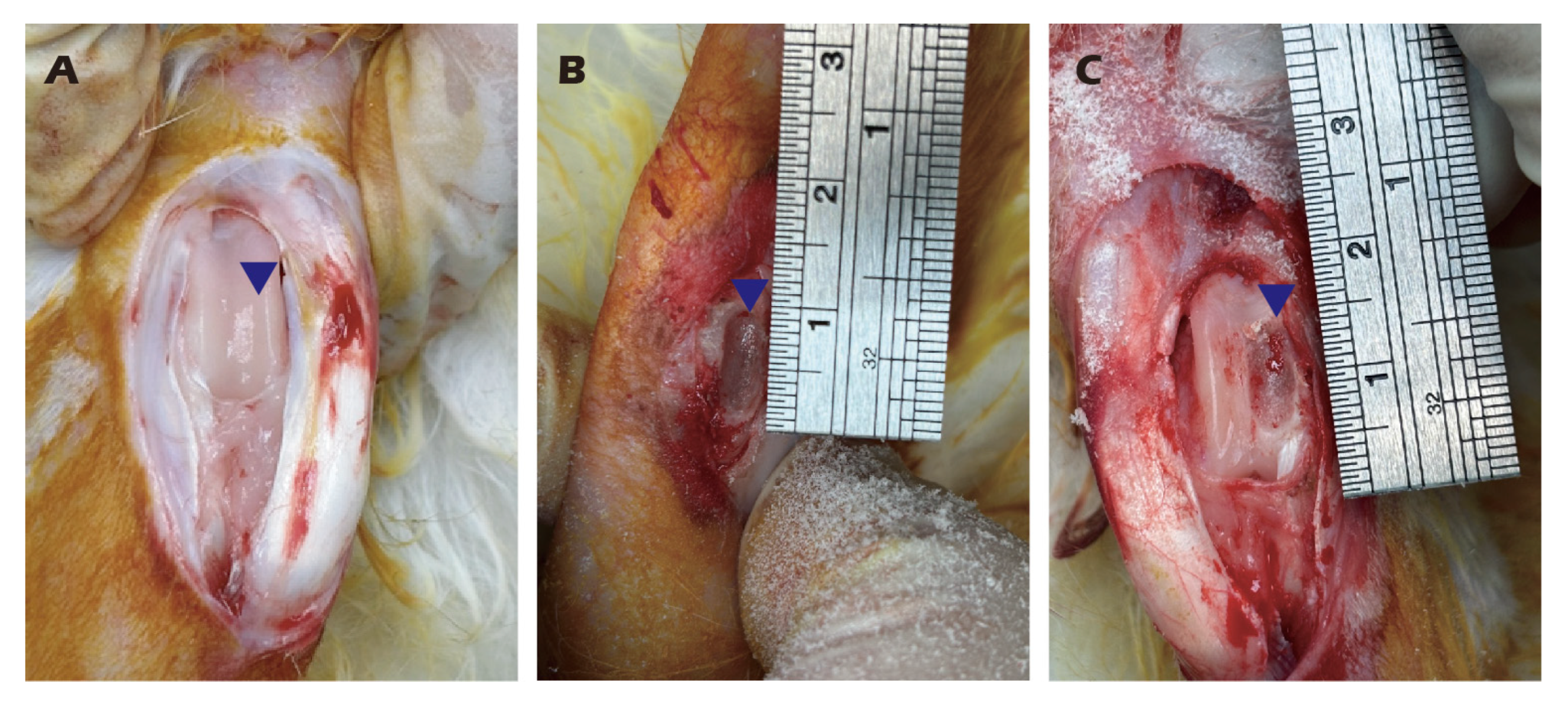
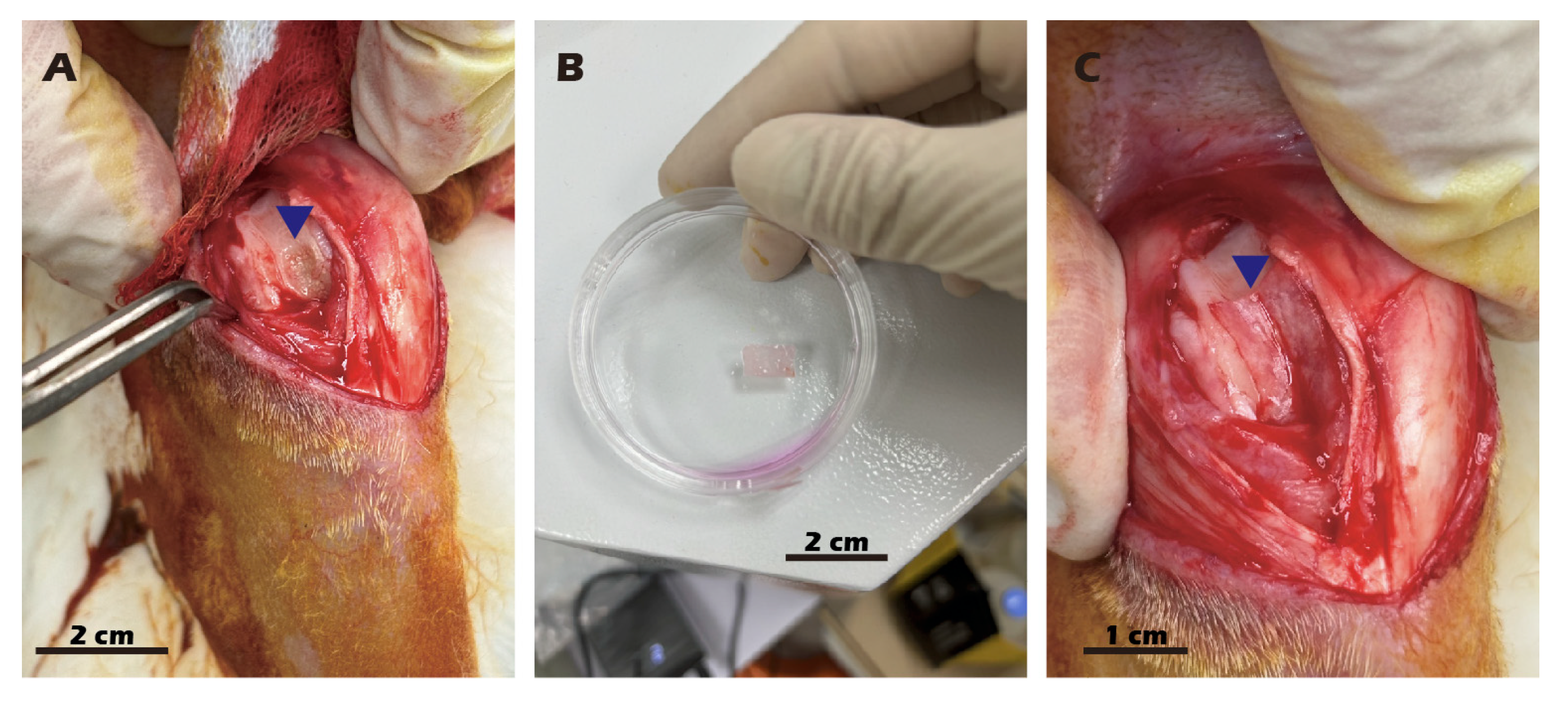
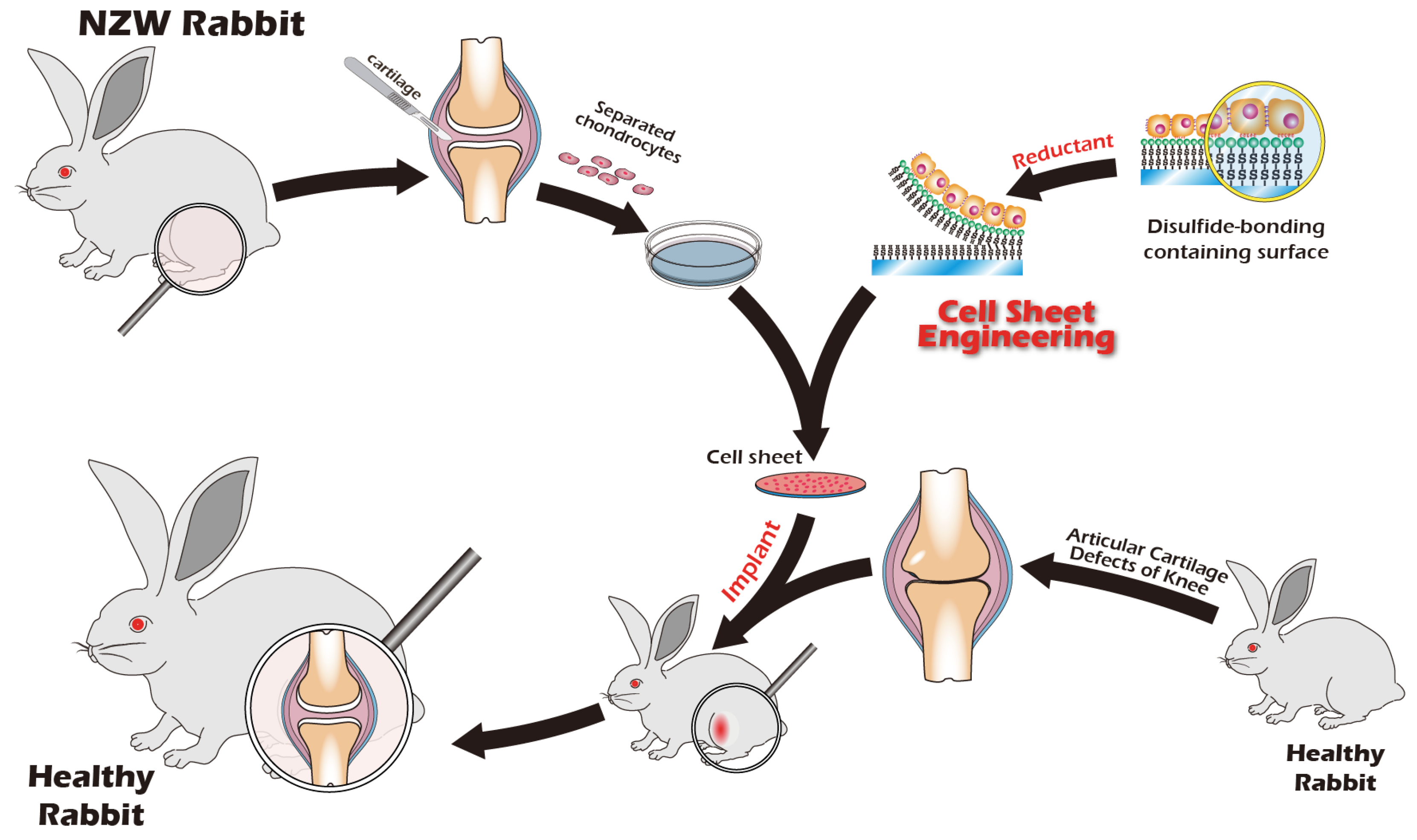
2.5. Preparation and Transplantation of Chondrocyte Sheets
2.6. Histological and Immunocytochemistry (ICC) Staining
2.7. Chondrocyte Sheet Histological and Immunohistochemical Staining
2.8. Magnetic Resonance Imaging (MRI) and Micro-CT Protocol for Ex Vivo Rabbit Knee Specimens
2.8.1. Ethical Approval and Specimen Preparation
2.8.2. Micro-Computed Tomography (Micro-CT) Imaging
2.8.3. Magnetic Resonance Imaging (MRI)
3. Results
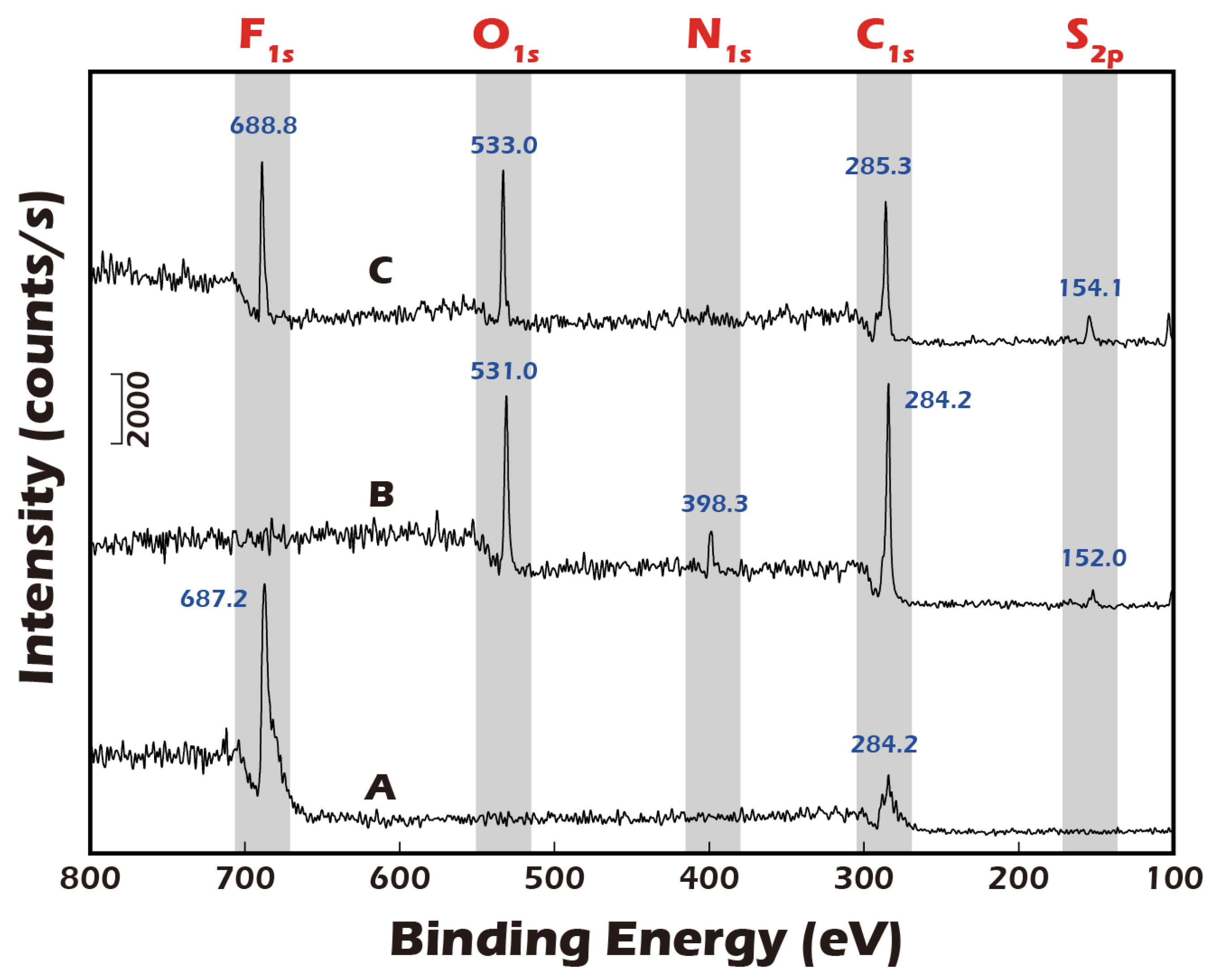
3.1. ESCA (XPS) Analysis Confirms Successful Surface Modification and Reversible Detachment Chemistry
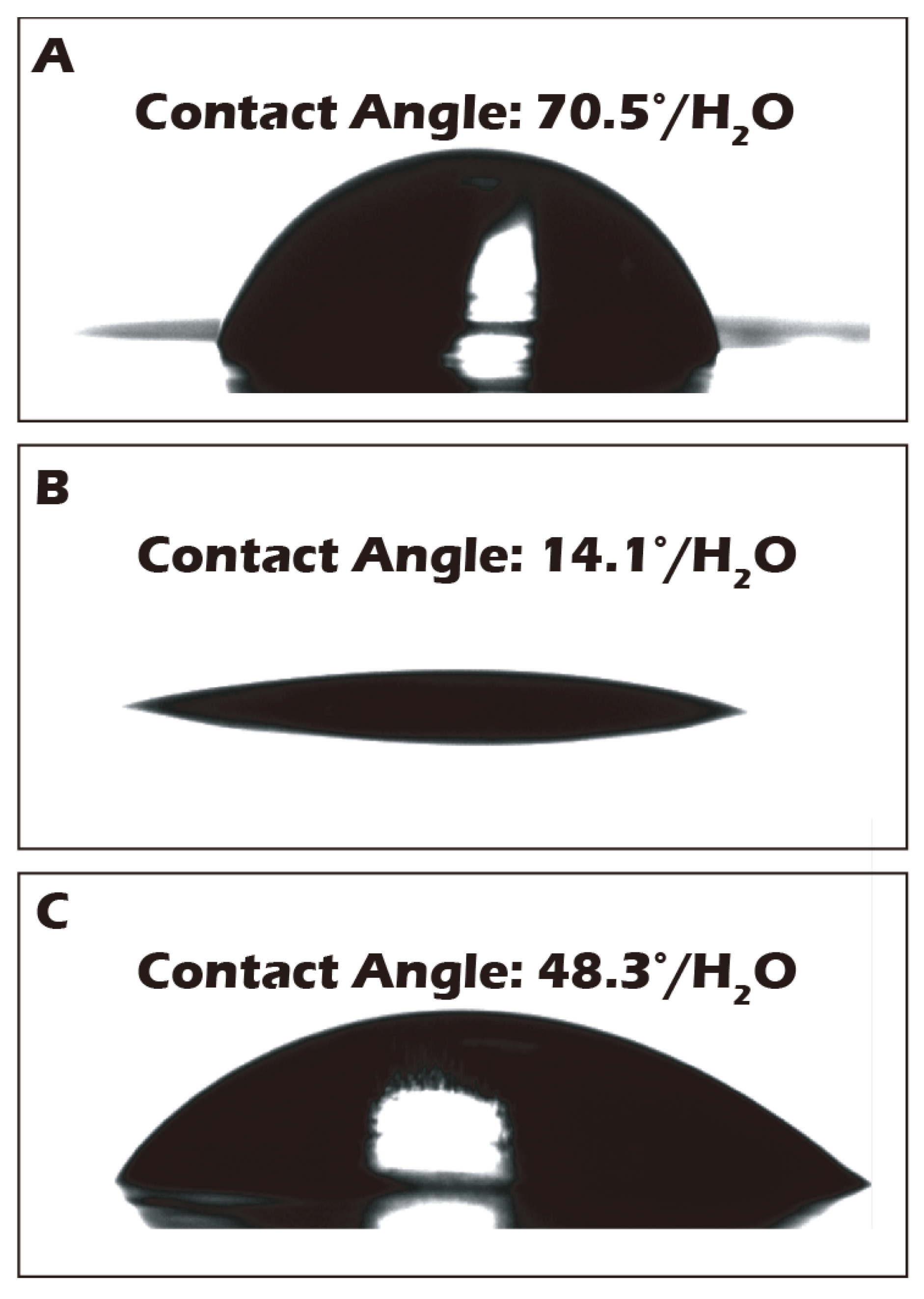
3.2. Water Contact Angle Measurements Corroborate Macroscopic Surface Property Changes
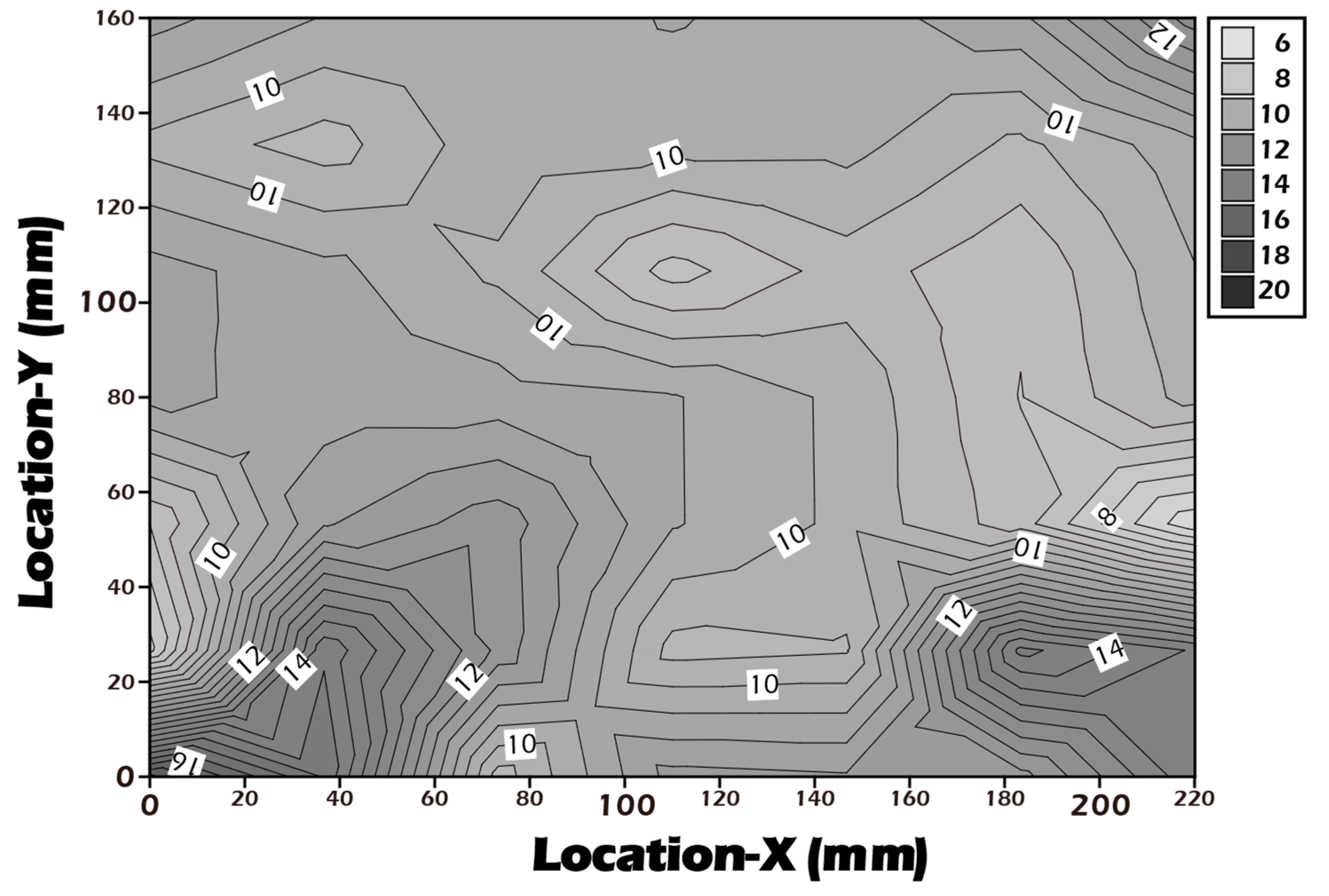
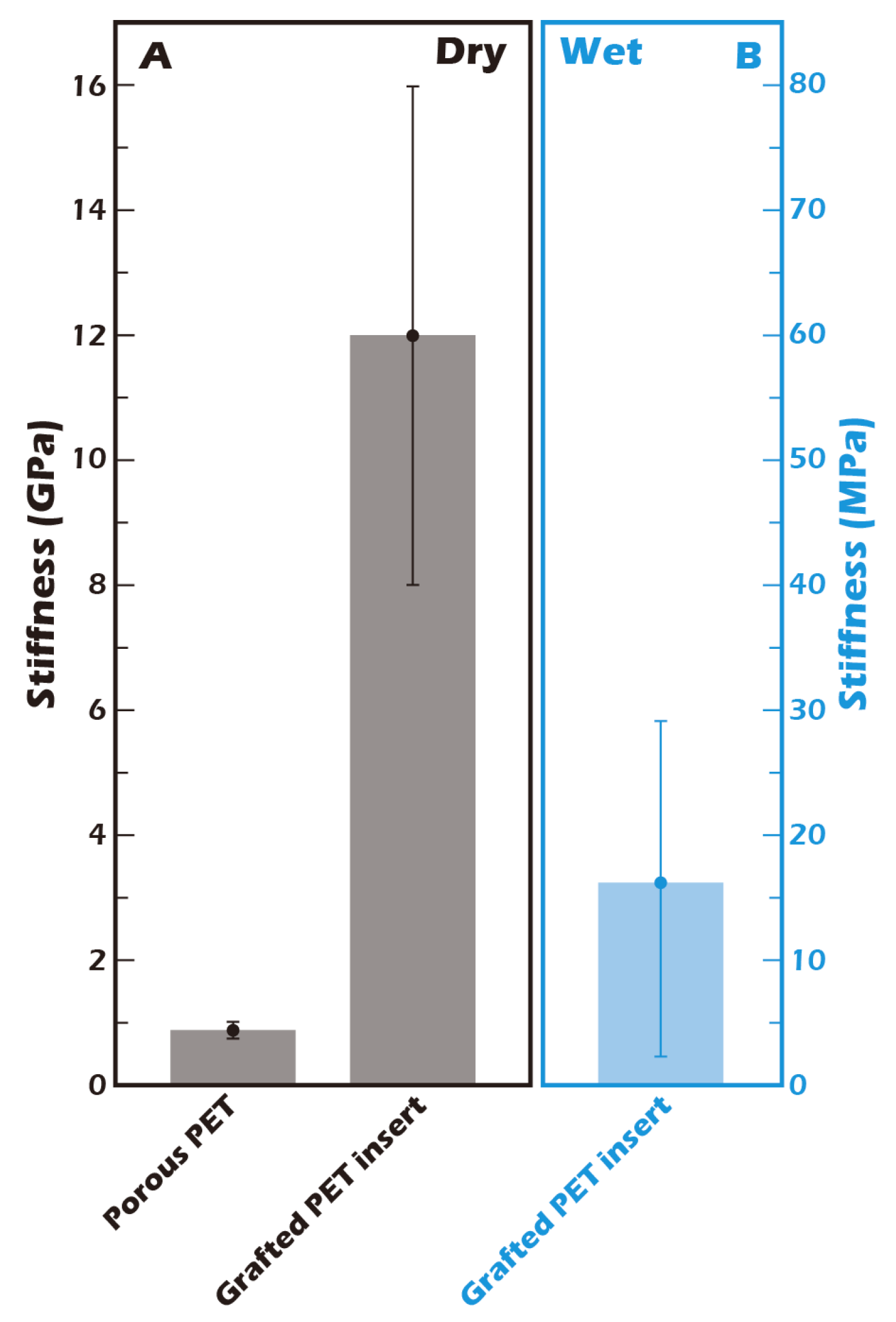
3.3. AFM Reveals Biomimetic Stiffness of the Hydrated Culture Substrate
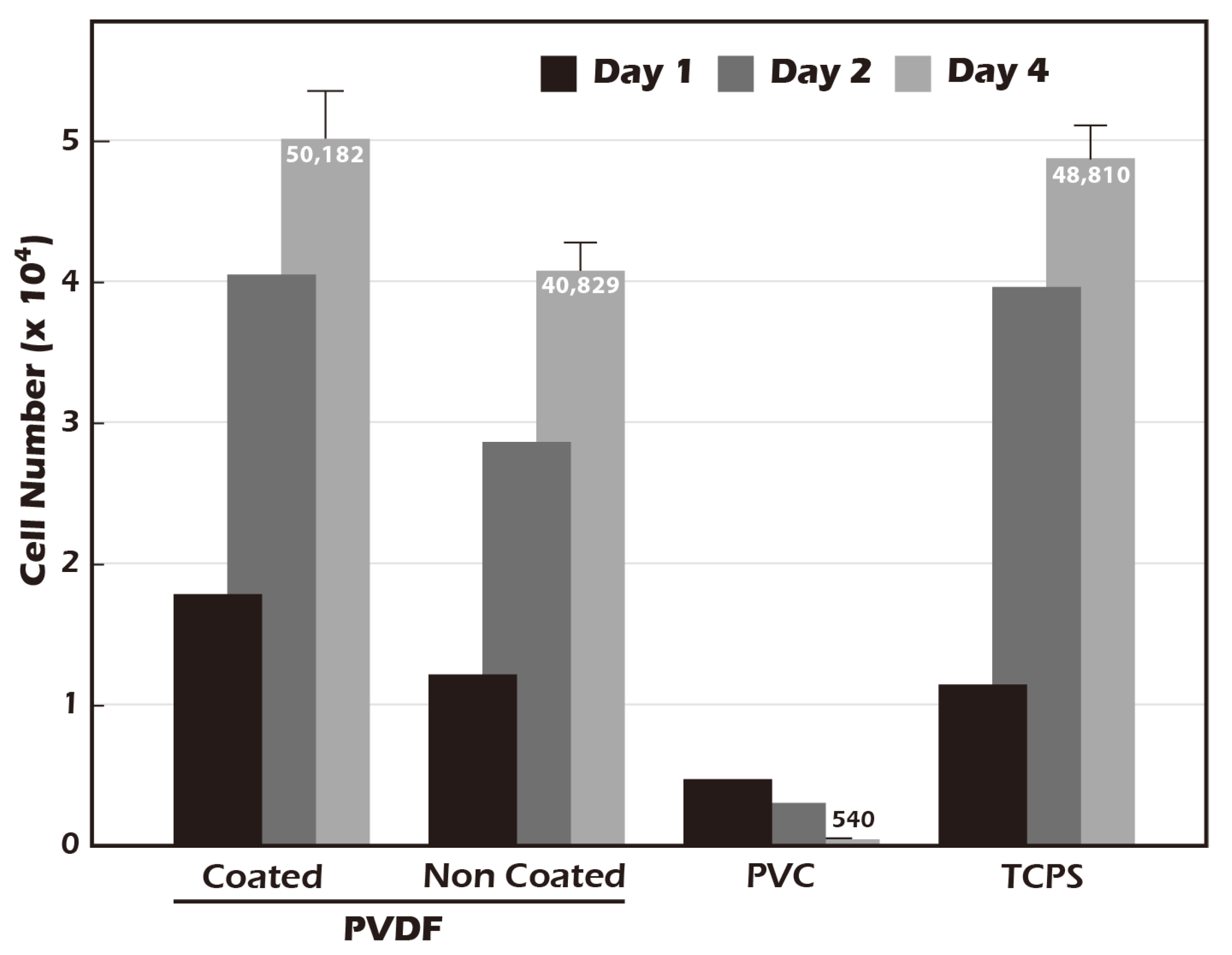
3.4. Grafted Membrane Exhibits Excellent Biocompatibility and Promotes Cell Proliferation
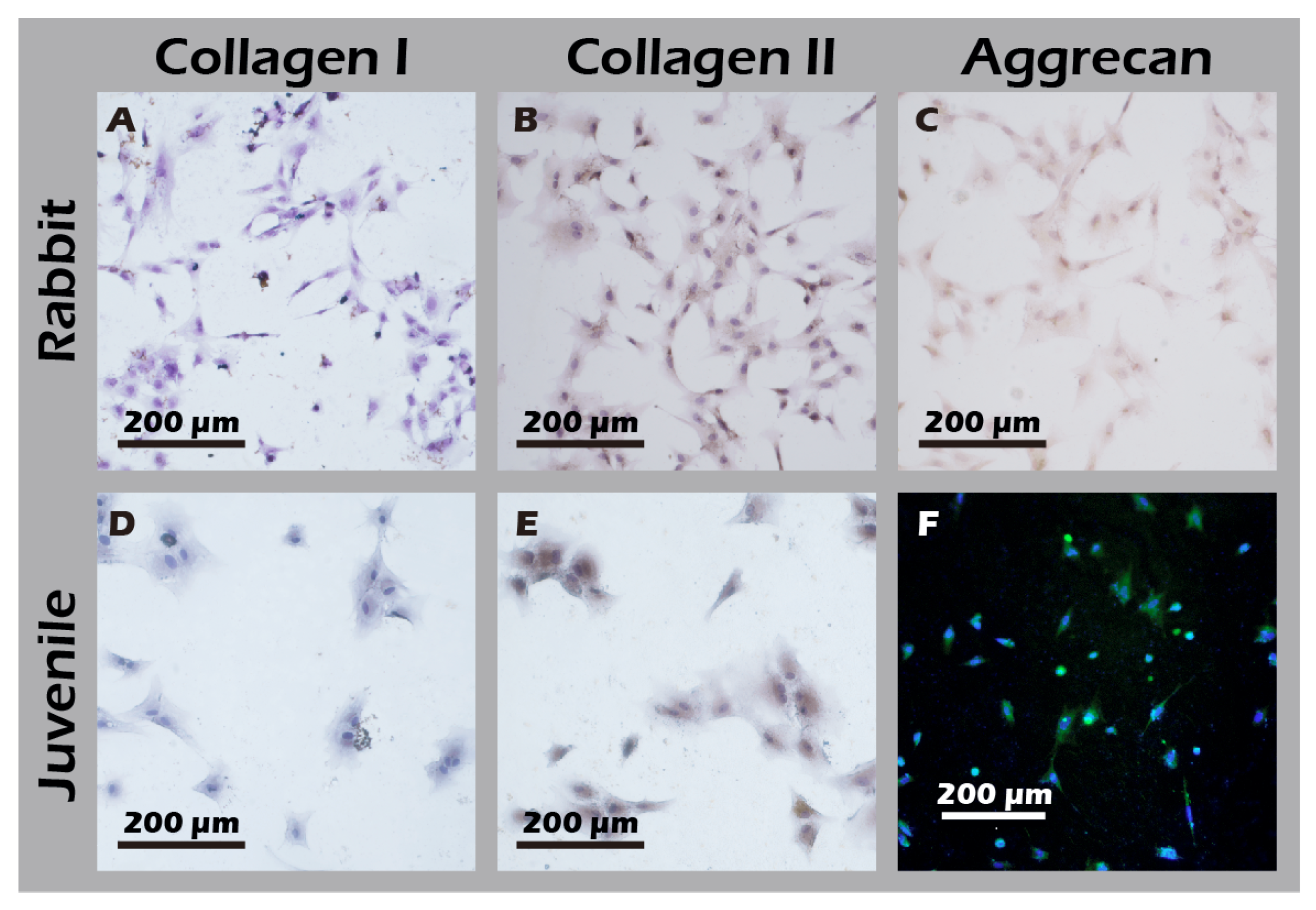
3.5. Cultured Rabbit Chondrocytes Maintain a Stable, Healthy Phenotype
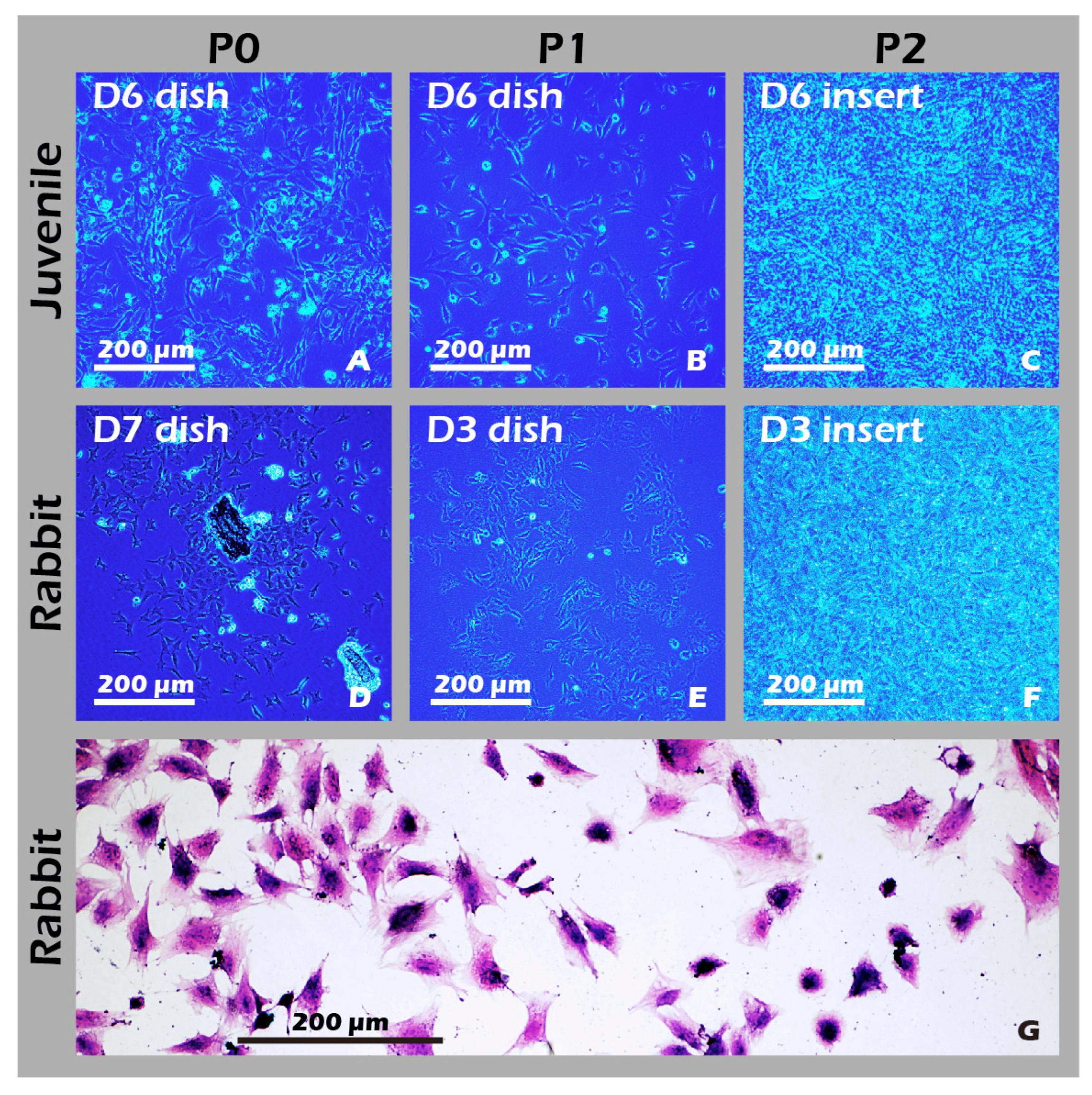
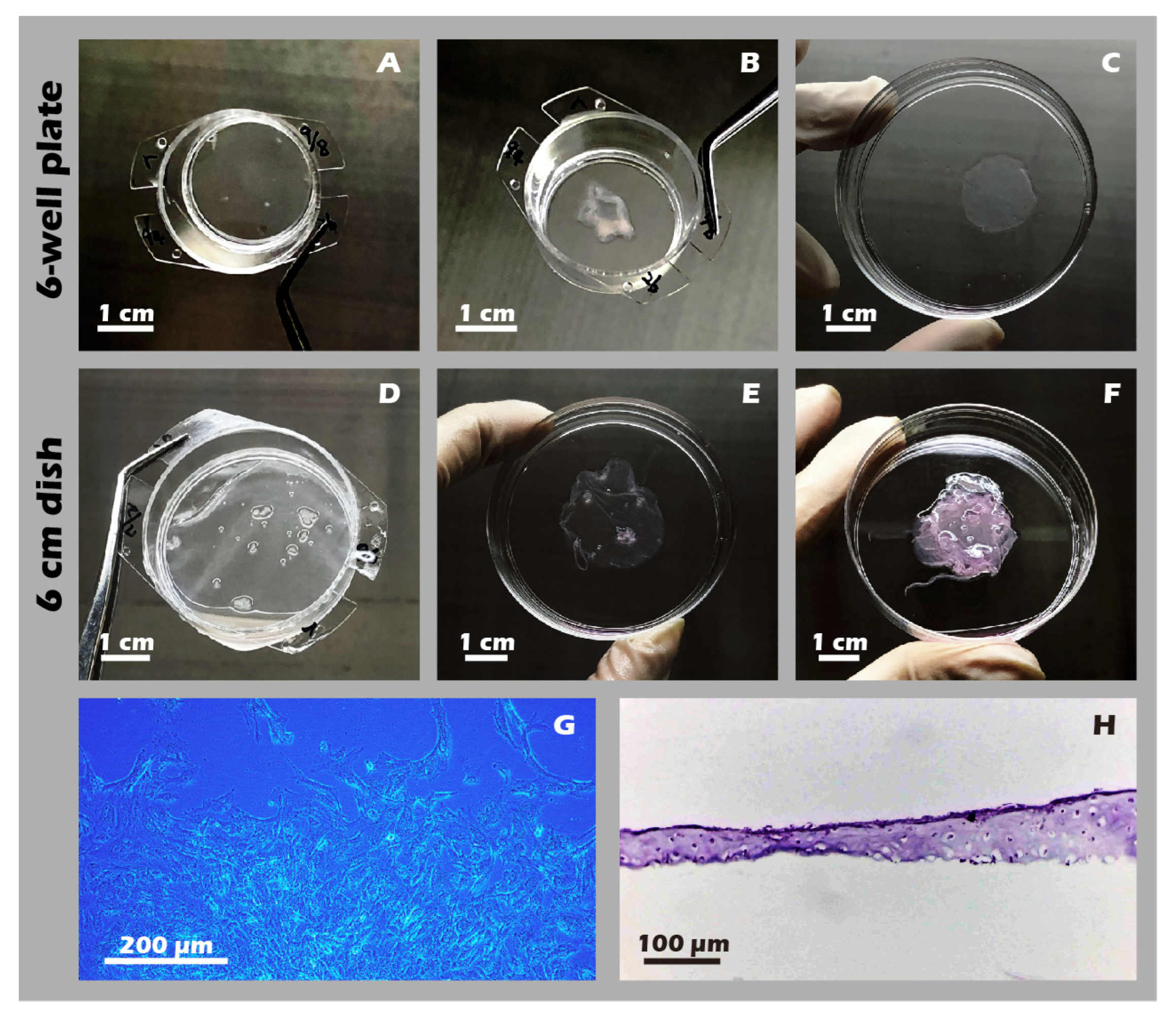
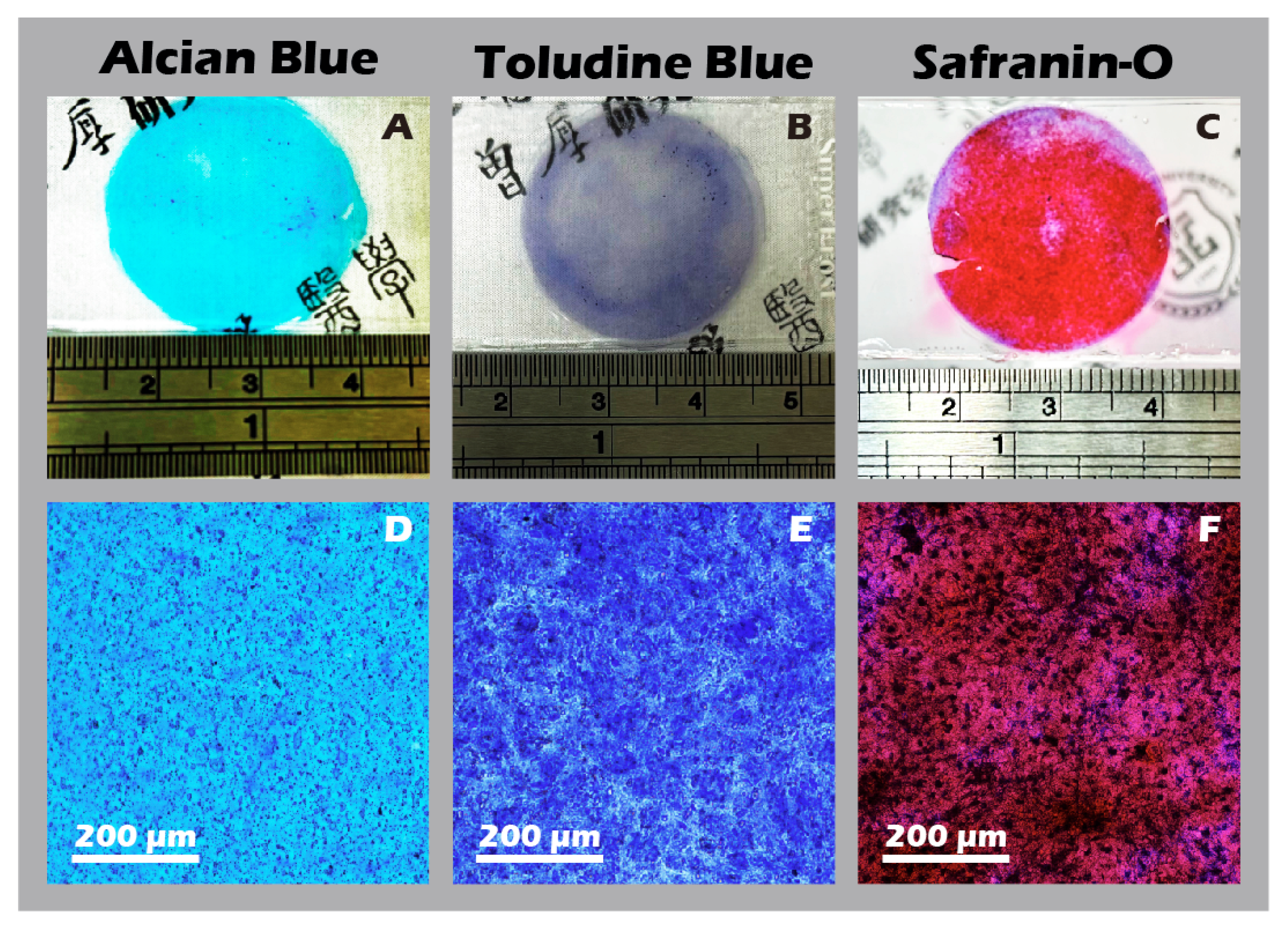
3.6. Robust Fabrication and Characterization of Multilayered Chondrocyte Sheets with Developed ECM
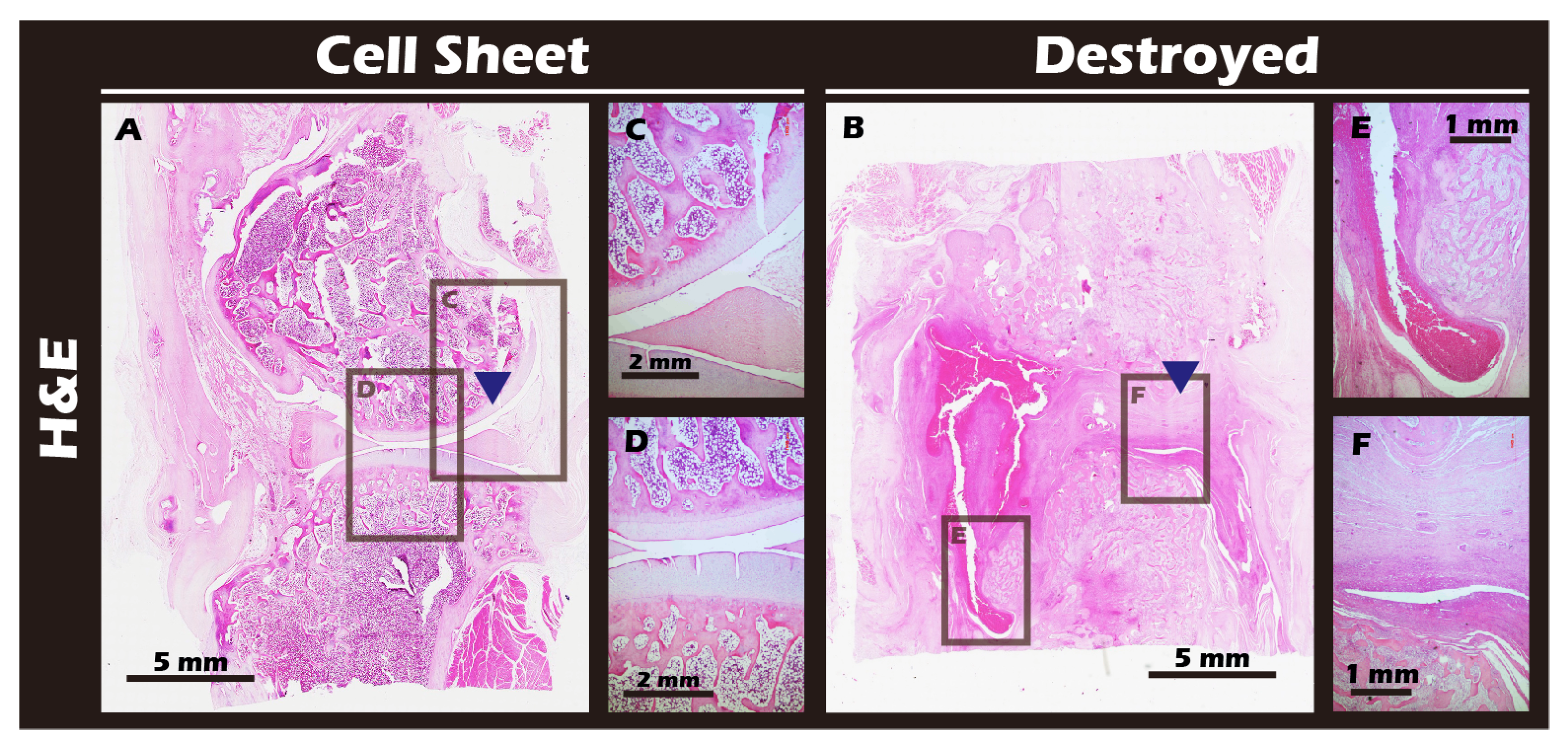
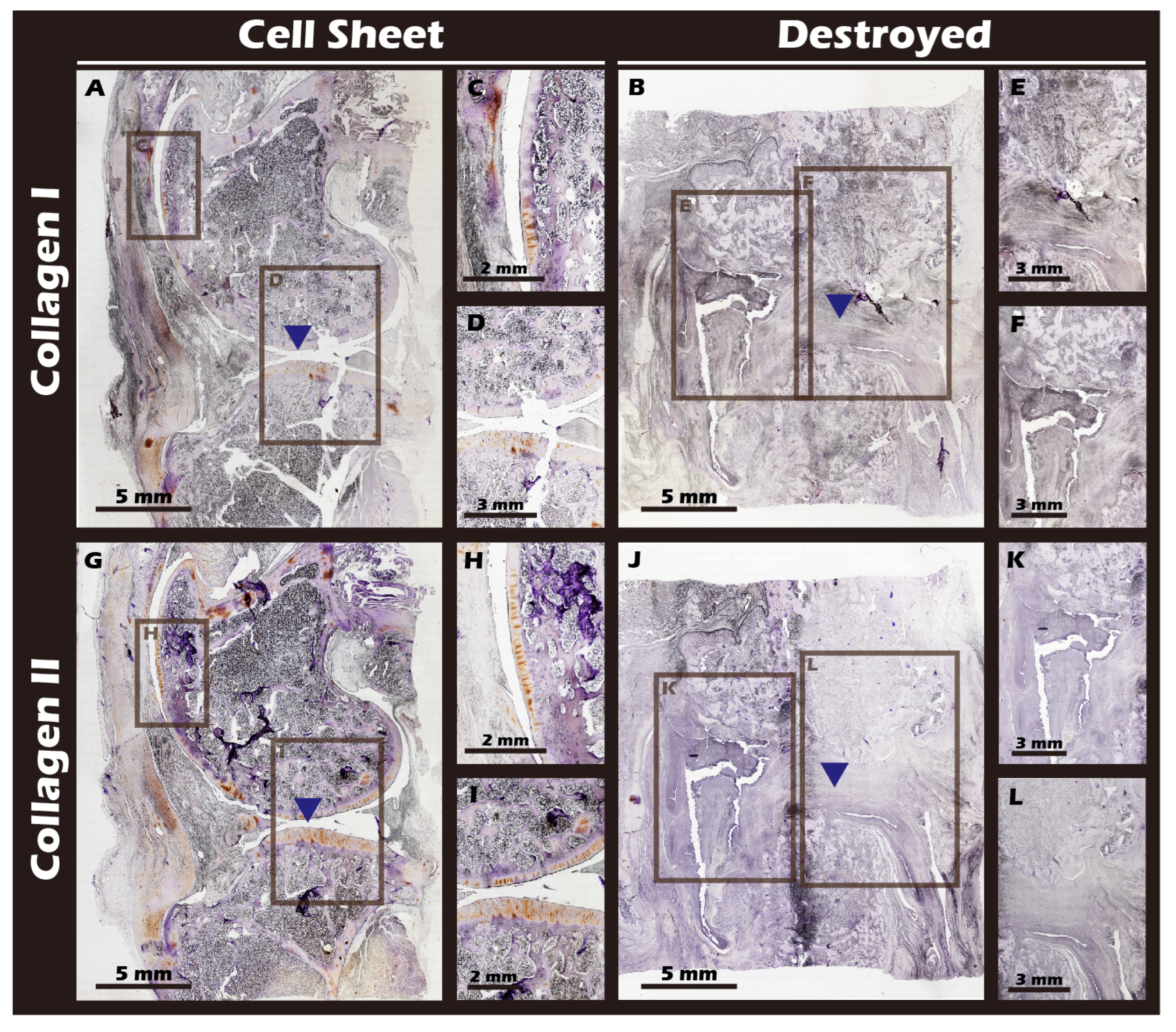
3.7. In Vivo Transplantation Leads to Successful Regeneration of Hyaline-like Cartilage
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSE | Cell Sheet Engineering |
| OA | Osteoarthritis |
| ACI | Autologous Chondrocyte Implantation |
| MACI | matrix-induced ACI |
| ECM | extracellular matrix |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| LKS | Lysholm Knee Score |
| JCCs | juvenile chondrocytes |
| ATMPs | advanced therapy medicinal products |
| pNIPAAm | poly(N-isopropylacrylamide) |
| γ-PGA | gamma-polyglutamic acid |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| NHS | N-hydroxysuccinimide |
| PVDF | Poly vinyl difluoride |
| PET | polyethylene terephthalate |
| CCD | Charge-coupled Device |
| ESCA | Electron Spectroscopy for Chemical Analysis |
| XPS | X-ray photoelectron spectroscopy |
| H&E | hematoxylin and eosin |
| ICC | Immunocytochemistry |
| IHC | Immunohistochemistry |
| IF | Immuno-fluorescence |
| IACUC | Institutional Animal Care and Use Committee |
| Micro-CT | Micro-Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| PVC | Poly vinyl chloride |
| TCPS | Tissue Culture Polystyrene |
| AFM | Atomic Force Microscope |
| GMP | Good Manufacturing Practice |
References
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef]
- Chen, X.; Tian, B.; Wang, Y.; Zheng, J.; Kang, X. Potential and challenges of utilizing exosomes in osteoarthritis therapy (Review). Int. J. Mol. Med. 2025, 55, 43. [Google Scholar] [CrossRef]
- Aabedi, A.; Fraix, M.; Agrawal, D. Surgical interventions in Severe Osteoarthritis: Pros and Cons. J. Orthop. Sports Med. 2025, 7, 169–178. [Google Scholar] [CrossRef]
- Kim, E.H.; Jeon, S.; Park, J.; Ryu, J.H.; Mobasheri, A.; Matta, C.; Jin, E.-J. Progressing future osteoarthritis treatment toward precision medicine: Integrating regenerative medicine, gene therapy and circadian biology. Exp. Mol. Med. 2025, 57, 1133–1142. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, S.; Kim, S.; Ju, G.-i. Past, present, and future of cartilage restoration: From localized defect to arthritis. Knee Surg. Relat. Res. 2022, 34, 1. [Google Scholar] [CrossRef] [PubMed]
- Gopinatth, V.; Jackson, G.R.; Touhey, D.C.; Chahla, J.; Smith, M.V.; Matava, M.J.; Brophy, R.H.; Knapik, D.M. Microfracture for medium size to large knee chondral defects has limited long-term efficacy: A systematic review. J. Exp. Orthop. 2024, 11, e70060. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Eschweiler, J.; Goetze, C.; Tingart, M.; Maffulli, N. Membrane scaffolds for matrix-induced autologous chondrocyte implantation in the knee: A systematic review. Br. Med. Bull. 2021, 140, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-N.; Wang, X.; Yang, M.; Chen, Y.-R.; Zhang, J.-Y.; Deng, R.-H.; Zhang, Z.-N.; Yu, J.-K.; Yuan, F.-Z. Scaffold-Based Tissue Engineering Strategies for Osteochondral Repair. Front. Bioeng. Biotechnol. 2022, 9, 812383. [Google Scholar] [CrossRef]
- Trengove, A.; Di Bella, C.; O’Connor, A.J. The Challenge of Cartilage Integration: Understanding a Major Barrier to Chondral Repair. Tissue Eng. Part B Rev. 2020, 28, 114–128. [Google Scholar] [CrossRef]
- Hu, D.; Li, X.; Li, J.; Tong, P.; Li, Z.; Lin, G.; Sun, Y.; Wang, J. The preclinical and clinical progress of cell sheet engineering in regenerative medicine. Stem Cell Res. Ther. 2023, 14, 112. [Google Scholar] [CrossRef]
- Thummarati, P.; Laiwattanapaisal, W.; Nitta, R.; Fukuda, M.; Hassametto, A.; Kino-Oka, M. Recent Advances in Cell Sheet Engineering: From Fabrication to Clinical Translation. Bioengineering 2023, 10, 211. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Gao, C.; Li, J.; Tong, P.; Sun, Y. The preparation methods and types of cell sheets engineering. Stem Cell Res. Ther. 2024, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. npj Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Bornes, T.D.; Adesida, A.B.; Jomha, N.M. Articular Cartilage Repair with Mesenchymal Stem Cells After Chondrogenic Priming: A Pilot Study. Tissue Eng. Part A 2018, 24, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Yamato, M.; Mitani, G.; Takagaki, T.; Hamahashi, K.; Nakamura, Y.; Ishihara, M.; Matoba, R.; Kobayashi, H.; Okano, T.; et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. npj Regen. Med. 2019, 4, 4. [Google Scholar] [CrossRef]
- Sato, M.; Toyoda, E.; Hamahashi, K. Regenerative Medicine for Osteoarthritis of the Knee Using Cell Sheets. In Regenerative Medicine in Sports and Orthopaedics: A New Approach; Gobbi, A., Nakamura, N., Lane, J.G., Dallo, I., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 363–373. [Google Scholar]
- Yu, J.; Tu, Y.-K.; Tang, Y.-B.; Cheng, N.-C. Stemness and transdifferentiation of adipose-derived stem cells using l-ascorbic acid 2-phosphate-induced cell sheet formation. Biomaterials 2014, 35, 3516–3526. [Google Scholar] [CrossRef]
- Corporation, J.T.E. Business Plan and Growth Potential. Available online: https://www.jpte.co.jp/en/investors/library/financial/index.html (accessed on 11 October 2025).
- Metzler, N.F.; Kondo, M.; Matsukura, K.; Ford, A.J.; Grainger, D.W.; Okano, T. Differentiated and Untreated Juvenile Chondrocyte Sheets Regenerate Cartilage Similarly In Vivo. Tissue Eng. Part A 2025, 31, 184–194. [Google Scholar] [CrossRef]
- Matsukura, K.; Kondo, M.; Metzler, N.F.; Ford, A.J.; Maak, T.G.; Hutchinson, D.T.; Wang, A.A.; Sato, M.; Grainger, D.W.; Okano, T. Cell source-derived variabilities in human juvenile chondrocyte-derived cell sheet cartilage regenerative effects in a nude rat chondral defect implantation model. bioRxiv 2023. bioRxiv:16.540535. [Google Scholar] [CrossRef]
- Zheng, K.; Ma, Y.; Chiu, C.; Xue, M.; Zhang, C.; Du, D. Enhanced articular cartilage regeneration using costal chondrocyte-derived scaffold-free tissue engineered constructs with ascorbic acid treatment. J. Orthop. Translat. 2024, 45, 140–154. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Zhang, Q.; Xu, Z.; Xu, F.; Li, D.; Li, Y. Chondrocyte sheet in vivo cartilage regeneration technique using miR-193b-3p to target MMP16. Aging 2019, 11, 7070–7082. [Google Scholar] [CrossRef]
- Abolarinwa, B.A.; Shaw, M.K.; Lee, C.-H. Perspectives on Challenges to Cell Therapy Development in Taiwan: Strengthening Evidential Standards and Ways Forward. Front. Bioeng. Biotechnol. 2021, 9, 789043. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, J. From the Editors—Cell Therapy Regulation in 2025: A Turning Point for Global Innovation and Access. Available online: https://www.isctglobal.org/telegrafthub/blogs/isct-head-office1/2025/10/07/from-the-editors-cell-therapy-regulation-in-2025-a (accessed on 20 October 2025).
- Owaki, T.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnol. J. 2014, 9, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.H.; Hung, S.-H.; Tseng, Y.; Quang, L.X.; Le, N.T.N.; Fang, C.-L.; Tseng, H. Partial Decellularized Scaffold Combined with Autologous Nasal Epithelial Cell Sheet for Tracheal Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 10322. [Google Scholar] [CrossRef]
- Viet-Nhi, N.-K.; Chen, Y.-C.; Dang, L.H.; Tseng, H.; Hung, S.-H. Degassing a Decellularized Scaffold Enhances Wound Healing and Reduces Fibrosis during Tracheal Defect Reconstruction: A Preliminary Animal Study. J. Funct. Biomater. 2023, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.J.; Ramesh, A.; Brama, P.A.J.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 2016, 3, 1. [Google Scholar] [CrossRef]
- Takahashi, T.; Sato, M.; Toyoda, E.; Maehara, M.; Takizawa, D.; Maruki, H.; Tominaga, A.; Okada, E.; Okazaki, K.; Watanabe, M. Rabbit xenogeneic transplantation model for evaluating human chondrocyte sheets used in articular cartilage repair. J. Tissue Eng. Regen. Med. 2018, 12, 2067–2076. [Google Scholar] [CrossRef]
- Kim, H.; Witt, H.; Oswald, T.A.; Tarantola, M. Adhesion of Epithelial Cells to PNIPAm Treated Surfaces for Temperature-Controlled Cell-Sheet Harvesting. ACS Appl. Mater. Interfaces 2020, 12, 33516–33529. [Google Scholar] [CrossRef]
- ANSI/AAMI/ISO 10993-5:2009/(R)2022; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. American National Standards Institute: Washington, DC, USA, 2009.
- Yoshida, H.; Matsusaki, M.; Akashi, M. Development of Thick and Highly Cell-Incorporated Engineered Tissues by Hydrogel Template Approach with Basic Fibroblast Growth Factor or Ascorbic Acid. J. Biomater. Sci. Polym. Ed. 2010, 21, 415–428. [Google Scholar] [CrossRef]
- Khalil, I.; Irorere, V.; Radecka, I.; Burns, A.; Kowalczuk, M.; Mason, J.; Khechara, M. Poly-γ-Glutamic Acid: Biodegradable Polymer for Potential Protection of Beneficial Viruses. Materials 2016, 9, 28. [Google Scholar] [CrossRef]
- Dadsetan, M.; Pumberger, M.; Casper, M.E.; Shogren, K.; Giuliani, M.; Ruesink, T.; Hefferan, T.E.; Currier, B.L.; Yaszemski, M.J. The effects of fixed electrical charge on chondrocyte behavior. Acta Biomater. 2011, 7, 2080–2090. [Google Scholar] [CrossRef]
- Tsimbouri, P. Adult Stem Cell Responses to Nanostimuli. J. Funct. Biomater. 2015, 6, 598–622. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Models Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Chen, C.-H.; Shih, T.-C.; Liu, Y.-L.; Peng, Y.-J.; Huang, Y.-L.; Chen, B.S.; Tseng, H. Engineered Immature Testicular Tissue by Electrospun Mats for Prepubertal Fertility Preservation in a Bioluminescence Imaging Transgenic Mouse Model. Int. J. Mol. Sci. 2022, 23, 12145. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, L.; Liu, Z.; Geng, B.; Teng, Y.; Liu, X.; Yi, Q.; Yu, D.; Chen, X.; Zhao, D.; et al. Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis. Channels 2021, 15, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhu, M.; Wu, Z.; Liu, W.; Cao, C.; Shi, J. The role of YAP/TAZ on joint and arthritis. FASEB J. 2024, 38, e23636. [Google Scholar] [CrossRef]
- Liu, S.; Li, P.; Liu, X.; Wang, P.; Xue, W.; Ren, Y.; Yang, R.; Chi, B.; Ye, Z. Bioinspired mineral-polymeric hybrid hyaluronic acid/poly (γ-glutamic acid) hydrogels as tunable scaffolds for stem cells differentiation. Carbohydr. Polym. 2021, 264, 118048. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Schmittlein, B.; Sadhu, S.; Nayak, S.; Lares, A.; Uboldi, M.; Zema, L.; di Robilant, B.N.; Feldman, S.A.; Esensten, J.H. Automated manufacturing of cell therapies. J. Control. Release 2025, 381, 113561. [Google Scholar] [CrossRef]
- Guilak, F.; Estes, B.T.; Moutos, F.T. Functional tissue engineering of articular cartilage for biological joint resurfacing—The 2021 Elizabeth Winston Lanier Kappa Delta Award. J. Orthop. Res. 2022, 40, 1721–1734. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Xiang, Y.; Bao, X.; Sun, T. A Paradigm Shift in Tissue Engineering: From a Top–Down to a Bottom–Up Strategy. Processes 2021, 9, 935. [Google Scholar] [CrossRef]


| Target Joint/Defect | Cell Type Used | Model | Key Findings and Advancements | Study/Product [Citation] |
|---|---|---|---|---|
| Knee Joint | Autologous Cultured Cartilage | Clinical (Human) | Approved in Japan for relief of OA symptoms (defect > 2 cm2) and for traumatic cartilage defects (defect > 4 cm2) in patients refractory to other treatments. | Clinical Application: JACC [20] |
| Articular Cartilage (Focal Defect) | Juvenile Chondrocyte (JCC) sheets (Allogeneic source) | In vivo (Nude Rat) | In vitro pre-differentiation of JCC sheets did not speed healing. Conventional sheets produced similar hyaline cartilage in vivo, suggesting the environment dominates and a simpler process is possible. | Metzler et al. (2025) [21] |
| Articular Cartilage (Chondral Defect) | Human Juvenile Chondrocyte (JCC) sheets (P2 vs. P9) | In vivo (Nude Rat) | Confirmed donor-to-donor variability and cell passage dependency affect JCC sheet efficacy, correlating with cartilage regeneration and subchondral bone remodeling. | Matsukura et al. (2023) [22] |
| Articular Cartilage (Defect Site) | Costal Chondrocytes (CCs) (Scaffold-free TEC) | In vivo (Animal) | Proposes CCs as a cost-effective, direct cell source. Ascorbic acid boosts matrix production, leading to better hyaline-like cartilage regeneration in vivo. | Zheng et al. (2024) [23] |
| Ectopic Model (Subcutaneous) | Chondrocyte sheet + miR-193b-3p mimics | In vivo (Nude Mice) | In vitro data (not full text) indicate miR-193b-3p is involved in ECM regulation. This in vivo model was used to assess ECM component synthesis post-implantation. | Chen et al. (2019) [24] |
| Articular Cartilage | Mesenchymal Stem Cells (MSCs) | In vitro | It has been demonstrated that 3D MSC sheets, using a scaffold-free approach, can effectively induce chondrogenic differentiation to produce transplantable hyaline-like cartilage tissue. | Liu et al. (2020) [2] |
| Articular Cartilage | Autologous Cultured Cartilage | In vivo (NZW rabbit) | An autologous multilayer knee chondrocyte sheet was developed using a scaffolded disulfide bond and gamma-PGA-based culture and detachment system. MRI and CT scans confirmed that this method successfully restored knee cartilage tissue. | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, T.-L.; Tseng, Y.; Li, J.-W.; Yang, C.-T.; Chen, H.-Y.; Lee, H.-I.; Liu, J.-J.; Yang, Y.-Y.; Tseng, H. A New Way to Engineer Cell Sheets for Articular Cartilage Regeneration. J. Funct. Biomater. 2025, 16, 437. https://doi.org/10.3390/jfb16120437
Tan T-L, Tseng Y, Li J-W, Yang C-T, Chen H-Y, Lee H-I, Liu J-J, Yang Y-Y, Tseng H. A New Way to Engineer Cell Sheets for Articular Cartilage Regeneration. Journal of Functional Biomaterials. 2025; 16(12):437. https://doi.org/10.3390/jfb16120437
Chicago/Turabian StyleTan, Ta-Lun, Yuan Tseng, Jia-Wei Li, Cheng-Tse Yang, Hsuan-Yu Chen, Her-I Lee, Jun-Jen Liu, Yi-Yuan Yang, and How Tseng. 2025. "A New Way to Engineer Cell Sheets for Articular Cartilage Regeneration" Journal of Functional Biomaterials 16, no. 12: 437. https://doi.org/10.3390/jfb16120437
APA StyleTan, T.-L., Tseng, Y., Li, J.-W., Yang, C.-T., Chen, H.-Y., Lee, H.-I., Liu, J.-J., Yang, Y.-Y., & Tseng, H. (2025). A New Way to Engineer Cell Sheets for Articular Cartilage Regeneration. Journal of Functional Biomaterials, 16(12), 437. https://doi.org/10.3390/jfb16120437







