An Overview of 3D Bioprinting Impact on Cell Viability: From Damage Assessment to Protection Solutions
Abstract
1. Introduction
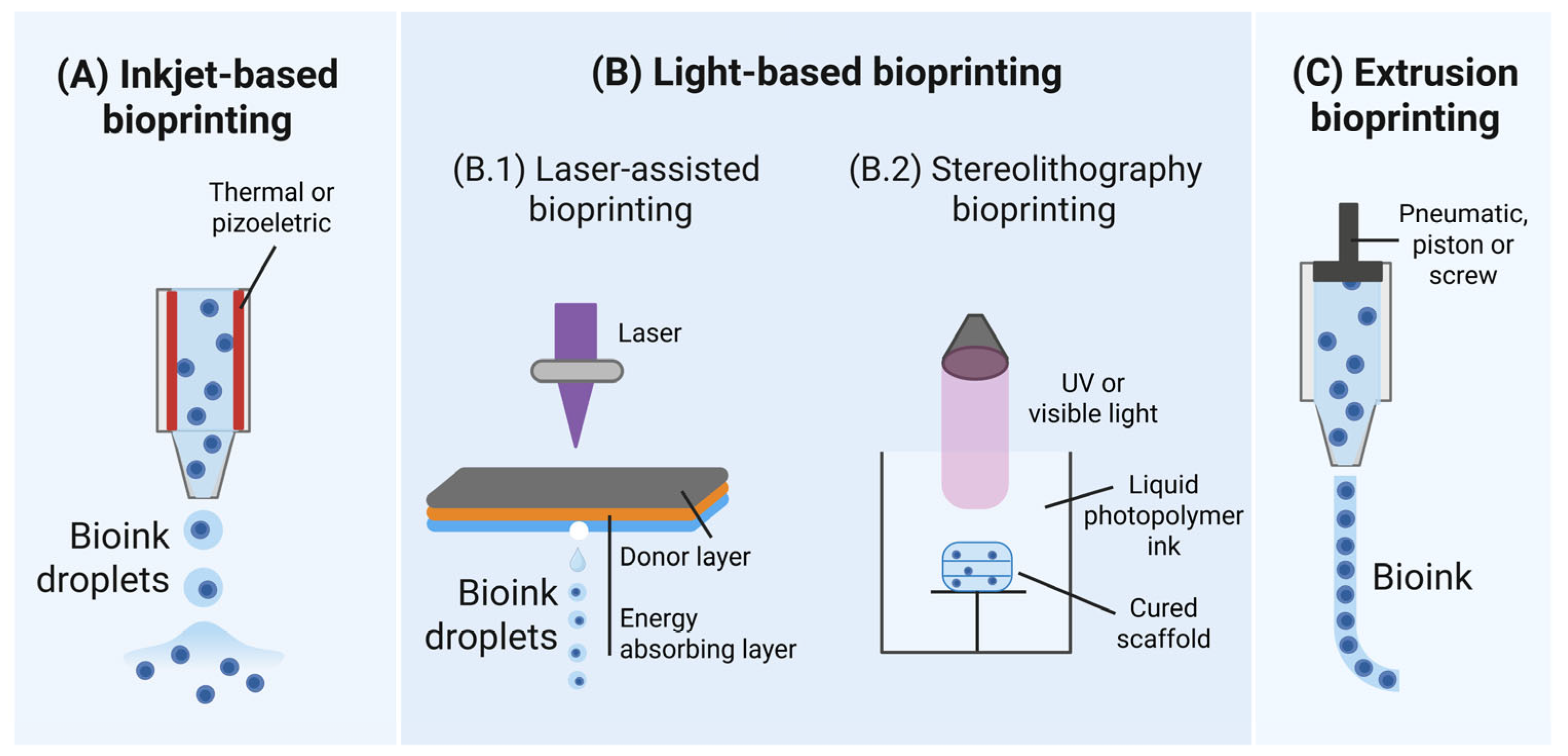
2. Bioprinting Technologies and Associated Cell Injuries
2.1. Inkjet Bioprinting
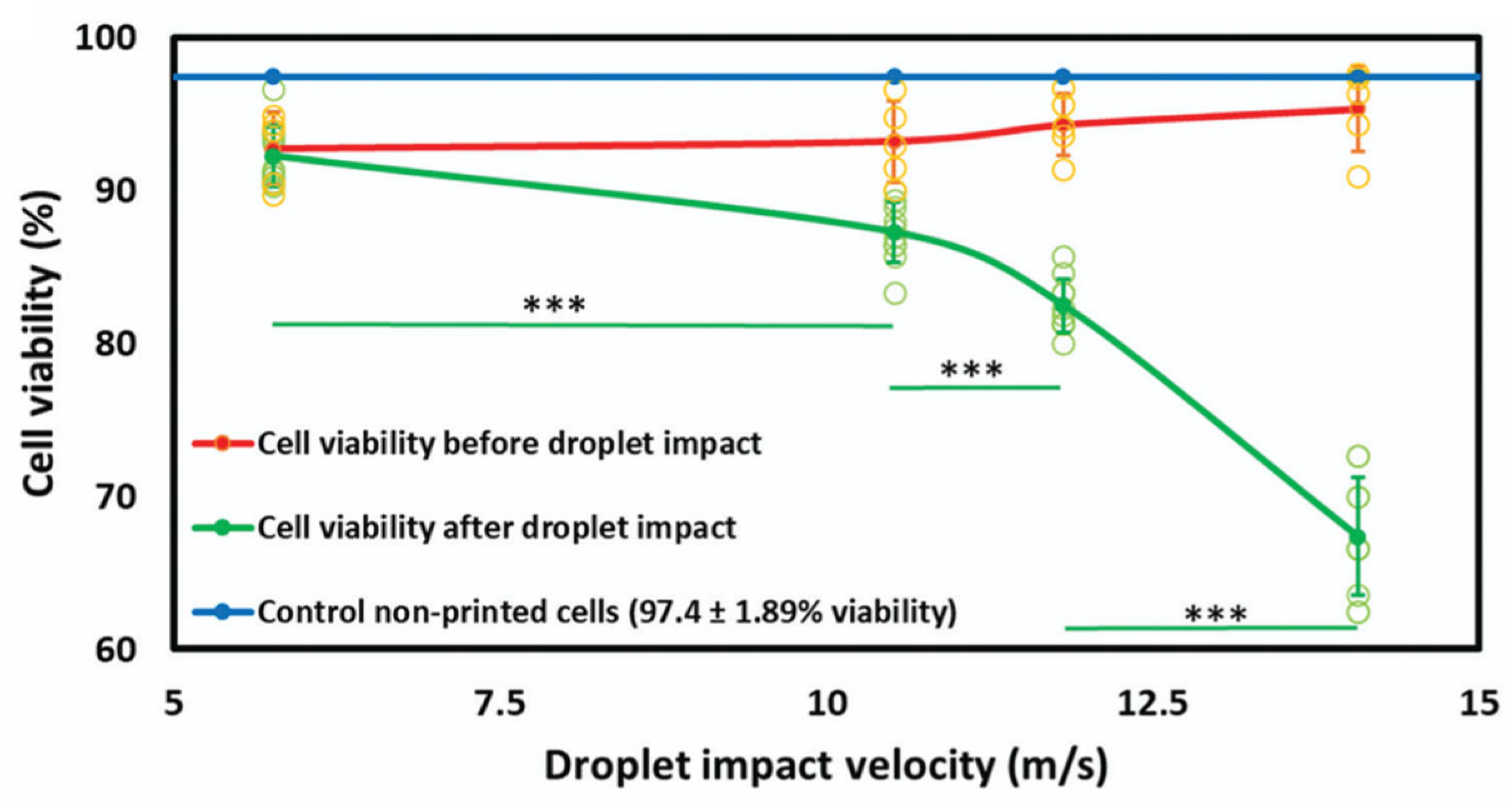
Cell Damage in Inkjet Bioprinting
2.2. Light-Based Bioprinting
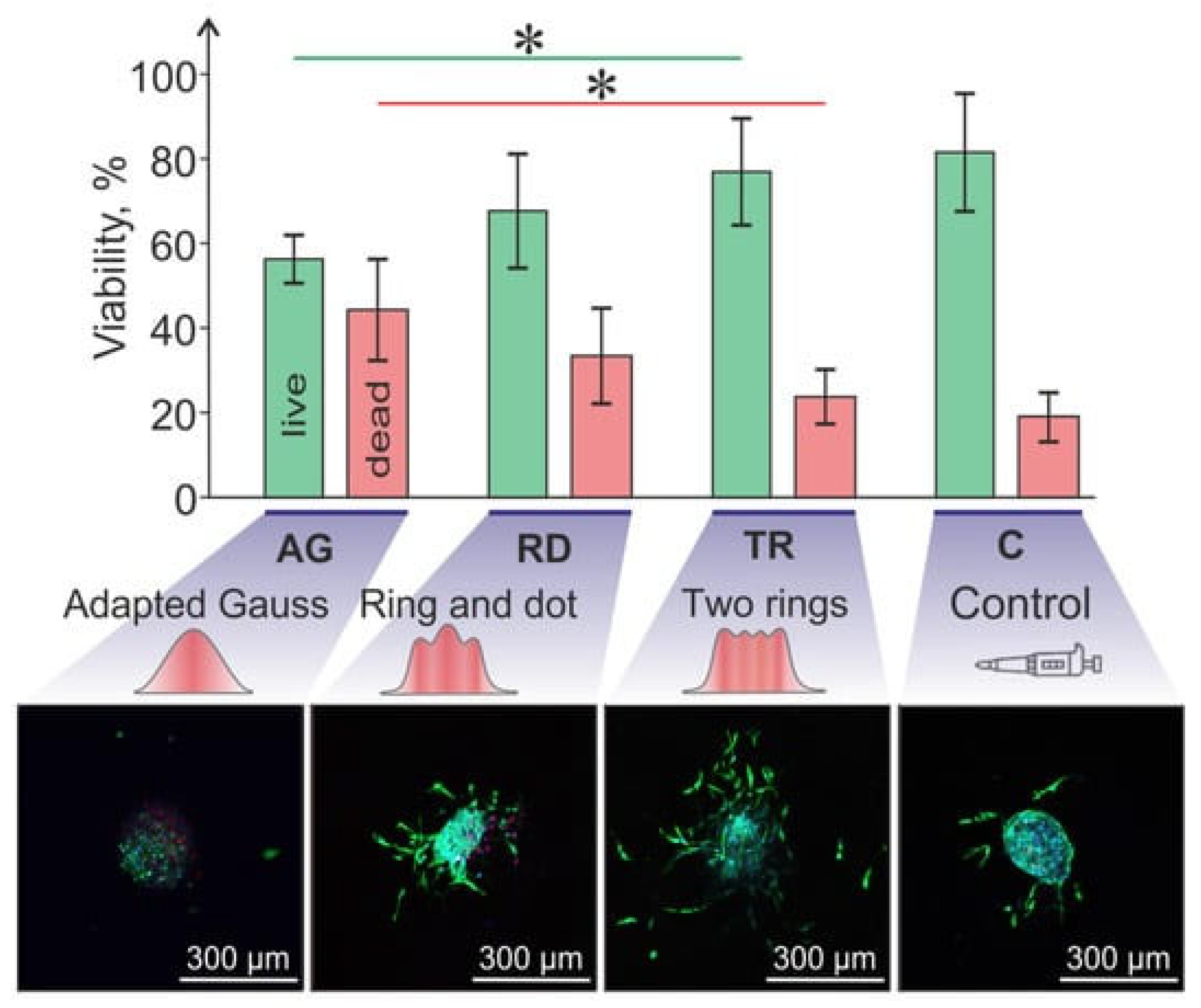
Cell Damage in Laser-Assisted Bioprinting
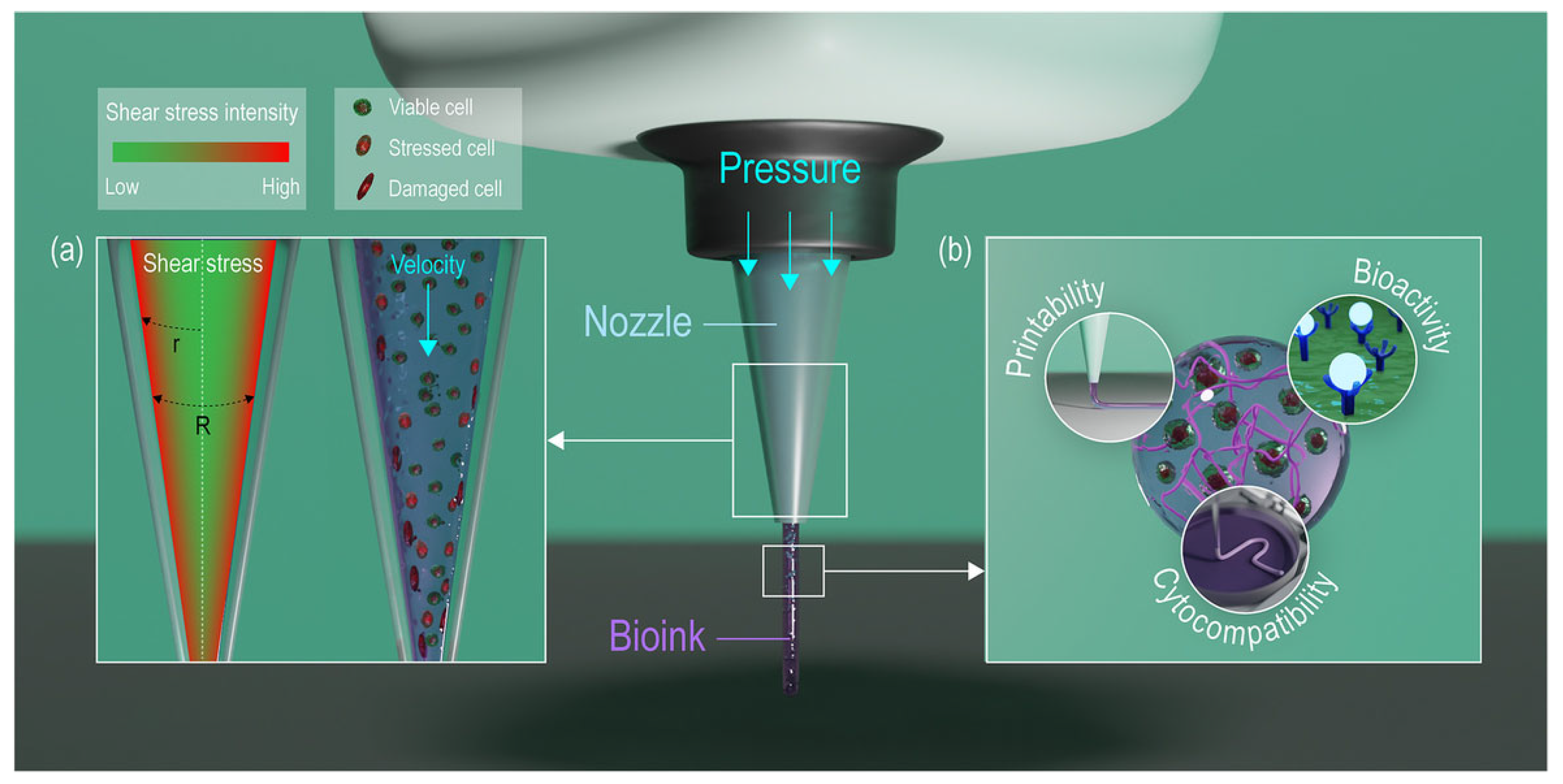
2.3. Extrusion-Based Bioprinting
2.3.1. Cell Damage in Extrusion-Based Bioprinting
| Bioink | Pressure (kPa) | Nozzle Diameter (μm) | Cell Viability (%) | Reference |
|---|---|---|---|---|
| 0.5–1.5% w/v alginate, mouse fibroblasts | 50 ÷ 150 | 150 ÷ 300 | 76 ÷ 96 | [34] |
| 0.2% w/v alginate, Schwann cells and myoblasts | 100 ÷ 330 | 100 ÷ 330 | 75 ÷ 100 | [68] |
| 5–20% w/v gelatin methacrylamide, HepG2 cells | 0 ÷ 500 | 150 ÷ 200 | 50 ÷ 100 | [66] |
| 2% w/v alginate and 8% w/v gelatine, aortic valve interstitial cells | 10 ÷ 200 | 500 ÷ 700 | >50 | [69] |
| (5% w/v) Alginate—(0.6% w/v) Collagen and 5% w/v gelatine, human umbilical vein endothelial cells and human dermal fibroblasts | 100 ÷ 900 | 900 ÷ 1900 | >70 | [70] |
| 3% w/v gelatine methacryloyl (GelMA) and 3–4% w/v alginate, mouse fibroblasts | 80.5 and 129.1 | 610 | >66 | [71] |
| CELLINK ink (composition/concentration not available), murine myoblasts | 35 and 80 | 200 ÷ 330 | 77 ÷ 82.1 | [72] |
| 2–4–6% w/v alginate, bovine cartilage progenitor cells | 35 ÷ 138 | 230 ÷ 330 (inner) 1190 (outer) | >60 | [73] |
2.3.2. Hand-Held Deposition
2.3.3. Cell Damage in Hand-Held Deposition
| Bioprinting Method | Bioink | Cell Viability | Reference |
|---|---|---|---|
| Inkjet-based | 1% w/v sodium alginate, mouse fibroblasts | >90% after 24 h | [81] |
| 1–2% w/v sodium alginate, mouse fibroblasts | >82% after 72 h | [82] | |
| 10–20 w/v poly(ethylene glycol) dimethacrylate, human chondrocytes | >85% after 24 h | [83] | |
| Laser-based | (Concentration not determined) biopaper, human endothelial cells | >90% after 30 min | [84] |
| 4% w/v alginate and murine fibroblasts | >90% | [85] | |
| 15% hyaluronic acid solution (1% w/v), 85% E8 medium, human induced pluripotent stem cells | >90% | [86] | |
| Extrusion-based | 1% w/v alginate sulfate—1.36% nanocellulose, bovine chondrocytes | >81% after 24 h | [87] |
| Methacrylamide-modified gelatin (Gel-MA), methacrylated κ-carrageenan (Car-MA) (final concentrations not determined), adipose tissue-derived stem cells | >90% after 14 days | [88] | |
| 2–4% w/v sodium alginate, mouse fibroblasts and smooth muscle cells | >90% after 7 days | [89] |
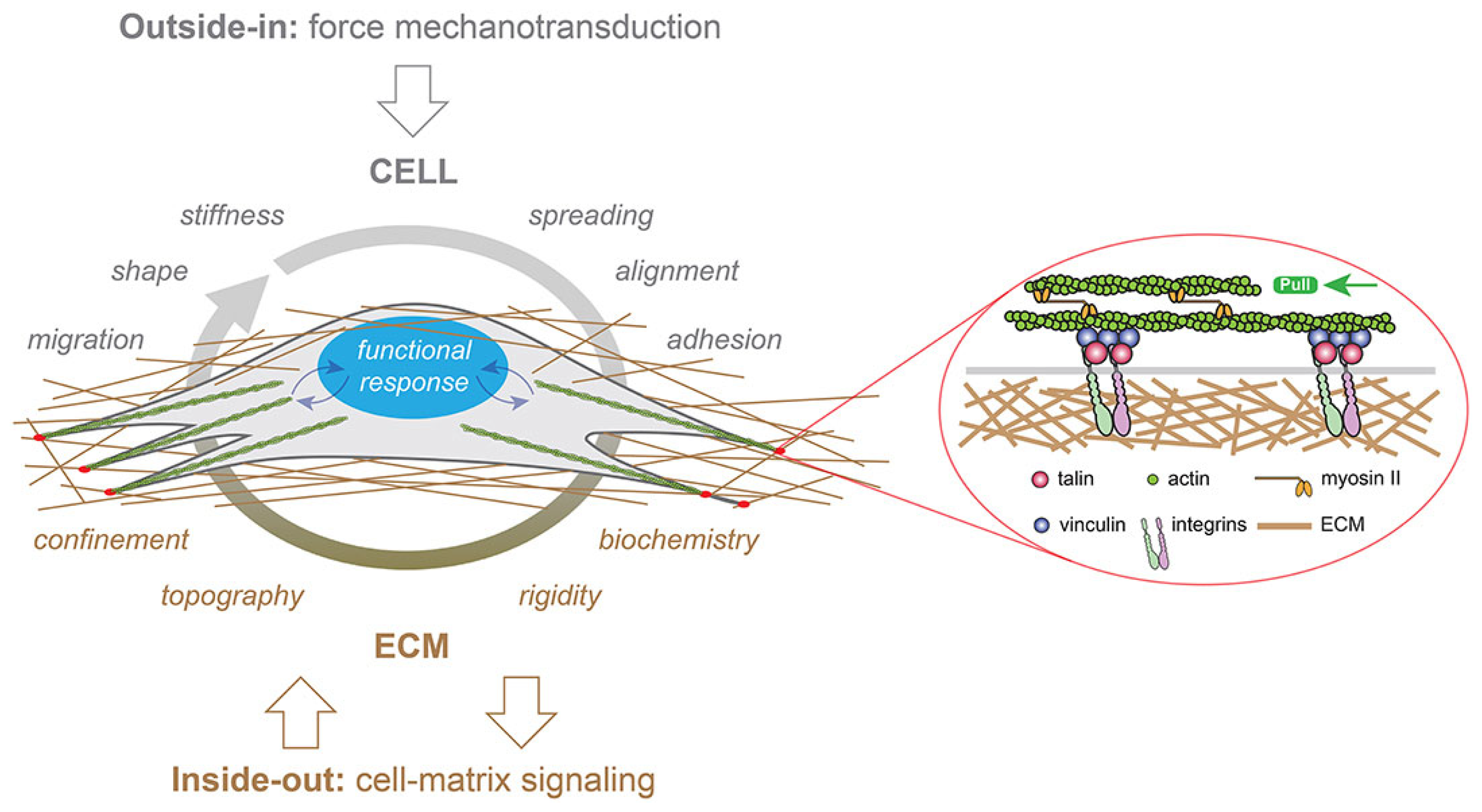
3. Cell Mechanotransduction Mechanism: Cell Response to Mechanical Stress
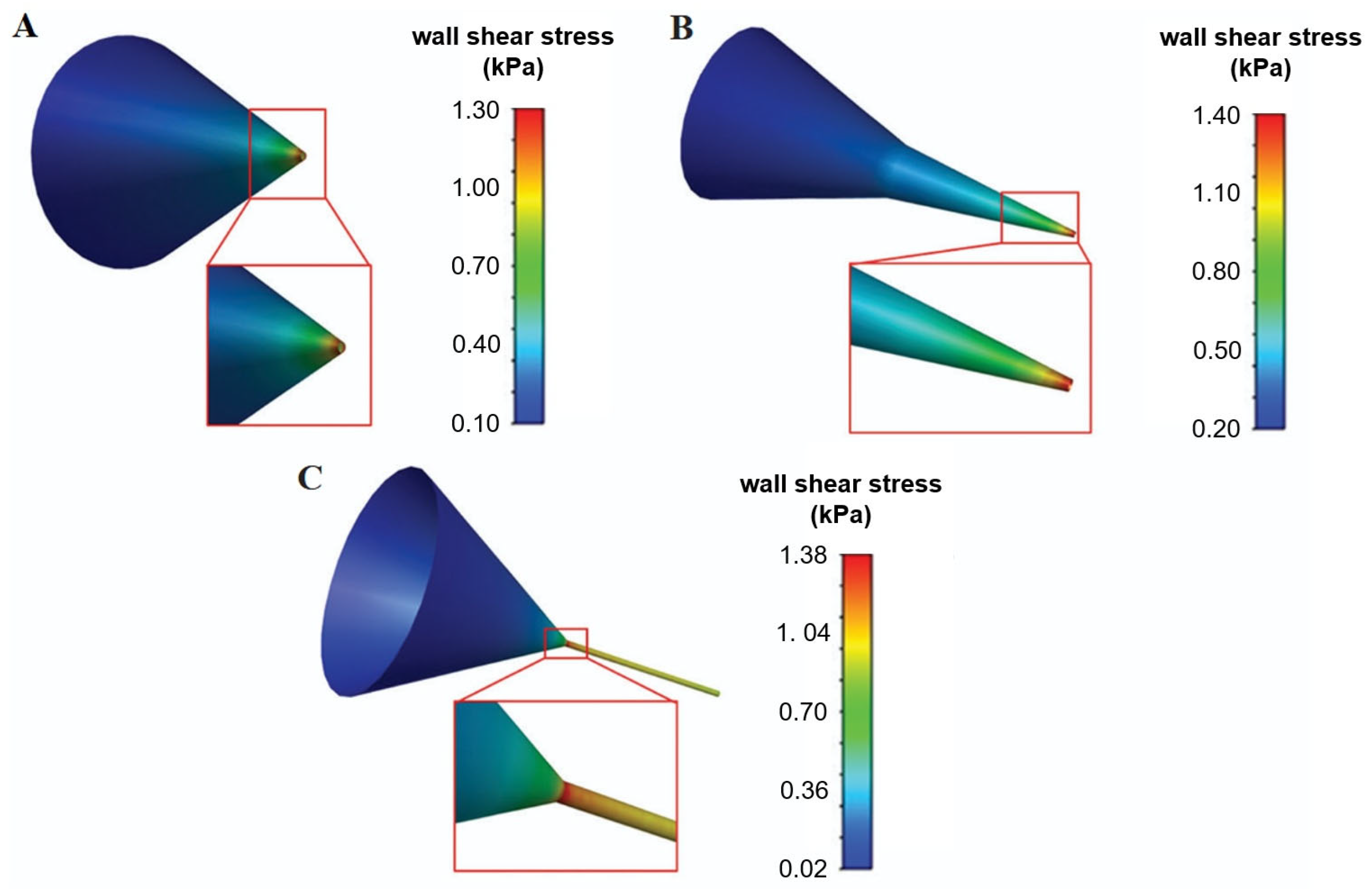
4. Simulation of the Printing Process Through Computational Fluid Dynamics
Predicting Cell-Associated Stress During Extrusion Bioprinting
5. Methods to Enhance Printing and Post-Printing Cell Viability
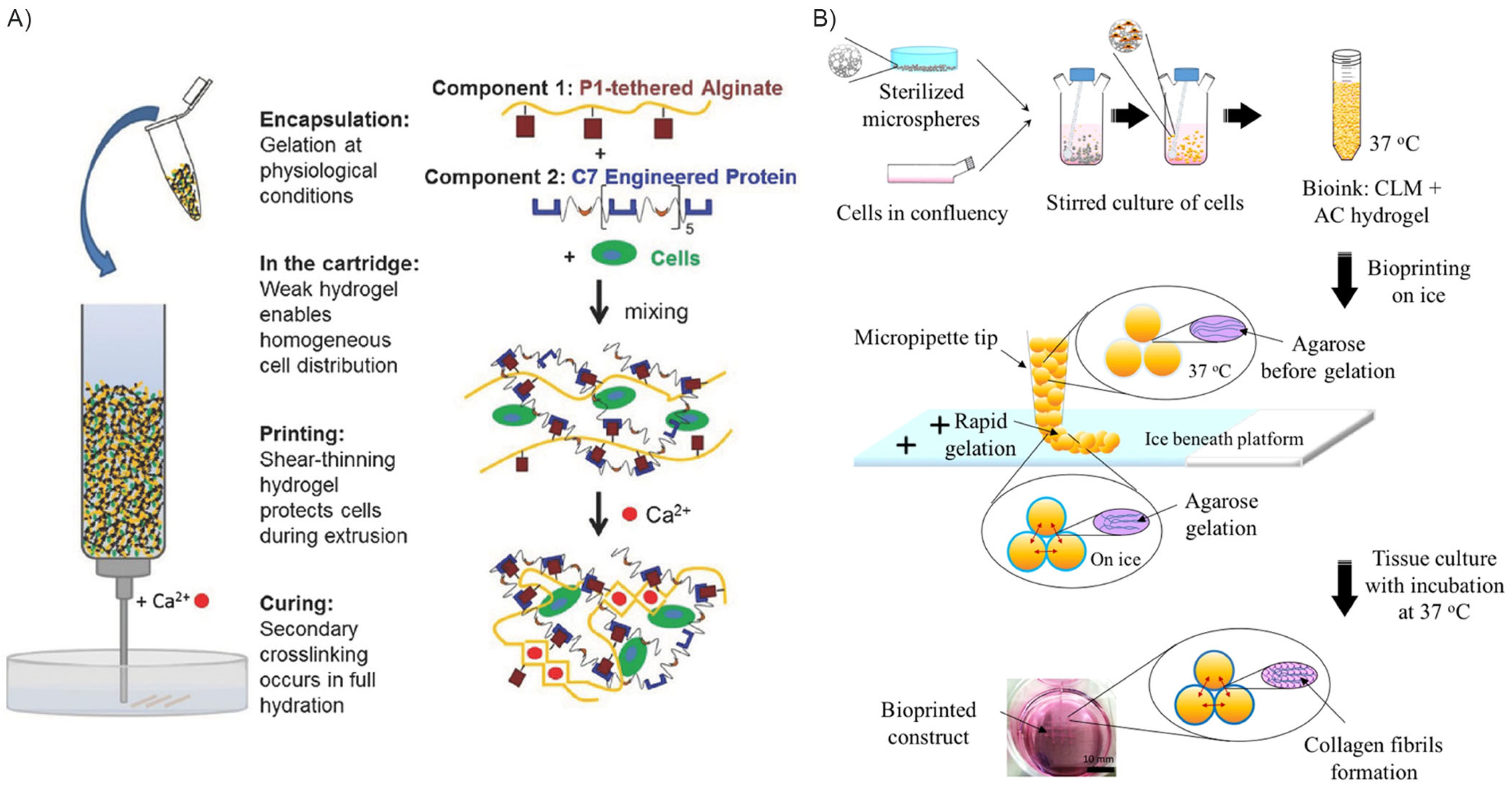
5.1. Interpenetrating, Thixotropic, and Reversibly Crosslinked Hydrogels
5.2. Three-Dimensionally Printed Hydrogel Curing Though Calcium Ion Deposition
5.3. Hybrid Scaffold Production Inkjet Bioprinting
5.4. Cell Encapsulation into Microparticles and Microbeads Inkjet Bioprinting
5.5. Shear Stress Preconditioning
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| UV | Ultraviolet |
| CFD | Computational fluid dynamics |
| LIFT | Laser-induced forward transfer |
| hMSCs | Human mesenchymal stem cells |
| HUVEC | Human umbilical-vein endothelial cells |
| HUVSMC | Human umbilical-vein smooth muscle cells |
| YAP | Yes-associated protein |
| EDTA | Ethylene Diamine Tetra-acetic Acid |
| PCL | Polycaprolactone |
| PLGA | Poly(lactic-co-glycolic acid) |
| MITCH | Mixing-induced two component hydrogels |
| TCP | Tricalcium phosphate |
| ECM | Extracellular matrix |
References
- Gudapati, H.; Dey, M.; Ozbolat, I. A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef]
- Balters, L.; Reichl, S. 3D Bioprinting of Corneal Models: A Review of the Current State and Future Outlook. J. Tissue Eng. 2023, 14, 20417314231197793. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Ricci, G.; Gibelli, F.; Sirignano, A. Three-Dimensional Bioprinting of Human Organs and Tissues: Bioethical and Medico-Legal Implications Examined through a Scoping Review. Bioengineering 2023, 10, 1052. [Google Scholar] [CrossRef]
- Veeravalli, R.S.; Vejandla, B.; Savani, S.; Nelluri, A.; Peddi, N.C. Three-Dimensional Bioprinting in Medicine: A Comprehensive Overview of Current Progress and Challenges Faced. Cureus 2023, 15, e41624. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; Gao, G.; Yonezawa, T.; Cui, X. 3D Bioprinting and the Current Applications in Tissue Engineering. Biotechnol. J. 2017, 12, 1600734. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, M.; Yang, X. Organ Bioprinting: Progress, Challenges and Outlook. J. Mater. Chem. B 2023, 11, 10263–10287. [Google Scholar] [CrossRef]
- da Silva, R.G.L.; Au, L.; Blasimme, A. Organizational Aspects of Tissue Engineering Clinical Translation: Insights from a Qualitative Case Study. Transl. Med. Commun. 2024, 9, 17. [Google Scholar] [CrossRef]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A Perspective on the Clinical Translation of Scaffolds for Tissue Engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef]
- Ahadian, S.; Khademhosseini, A. A Perspective on 3D Bioprinting in Tissue Regeneration. Bio-Des. Manuf. 2018, 1, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Ramos, T.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication—A Year in Review. Tissue Eng. Part C Methods 2020, 26, 91–106. [Google Scholar] [CrossRef]
- Xu, H.Q.; Liu, J.C.; Zhang, Z.Y.; Xu, C.X. A Review on Cell Damage, Viability, and Functionality during 3D Bioprinting. Mil. Med. Res. 2022, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Restan Perez, M.; da Silva, V.A.; Thomsen, J.; Bhardwaj, L.; Andrade, T.A.M.; Alhussan, A.; Willerth, S.M. 3D Bioprinting Complex Models of Cancer. Biomater. Sci. 2023, 11, 3414–3430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xie, Q.; Gimple, R.C.; Zhong, Z.; Tam, T.; Tian, J.; Kidwell, R.L.; Wu, Q.; Prager, B.C.; Qiu, Z.; et al. Three-Dimensional Bioprinted Glioblastoma Microenvironments Model Cellular Dependencies and Immune Interactions. Cell Res. 2020, 30, 833–853. [Google Scholar] [CrossRef]
- Quigley, C.; Tuladhar, S.; Habib, A. A Roadmap to Fabricate Geometrically Accurate Three-Dimensional Scaffolds CO-Printed by Natural and Synthetic Polymers. J. Micro-Nano-Manuf. 2022, 10, 021001. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.; Zhang, Z.; Xu, C. Cell Sedimentation during 3D Bioprinting: A Mini Review. Bio-Des. Manuf. 2022, 5, 617–626. [Google Scholar] [CrossRef]
- Barbee, K.A. Mechanical Cell Injury. Ann. N. Y. Acad. Sci. 2006, 1066, 67–84. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Park, J.A.; Lee, Y.; Jung, S. Inkjet-Based Bioprinting for Tissue Engineering. Organoid 2023, 3, e12. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef]
- Liu, J.; Shahriar, M.; Xu, H.; Xu, C. Cell-Laden Bioink Circulation-Assisted Inkjet-Based Bioprinting to Mitigate Cell Sedimentation and Aggregation. Biofabrication 2022, 14, 045020. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The Cell in the Ink: Improving Biofabrication by Printing Stem Cells for Skeletal Regenerative Medicine. Biomaterials 2019, 209, 10–24. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet Printing Biomaterials for Tissue Engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; D.D’Lima, D.; Lotz, M.K. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent. Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kobayashi, A.; Takagi, F.; Watanabe, A.; Hiruma, Y.; Ohuchi, K.; Iwasaki, Y.; Horie, M.; Morita, I.; Takatani, S. Biocompatible Inkjet Printing Technique for Designed Seeding of Individual Living Cells. Tissue Eng. 2005, 11, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, J.; Roy, A.; Das, A.; Ghosh, M.; Thomas, S.; Sinha, A.; Kim, J.; Saha, P. Effects of Processing Parameters of 3D Bioprinting on the Cellular Activity of Bioinks. Macromol. Biosci. 2021, 21, e2000179. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, J.; Park, S.A.; Kim, W.D. Three-Dimensional Bio-Printing Equipment Technologies for Tissue Engineering and Regenerative Medicine. Tissue Eng. Regen. Med. 2016, 13, 663–676. [Google Scholar] [CrossRef]
- Koçak, E.; Yıldız, A.; Acartürk, F. Three Dimensional Bioprinting Technology: Applications in Pharmaceutical and Biomedical Area. Colloids Surf. B Biointerfaces 2021, 197, 111396. [Google Scholar] [CrossRef]
- Derby, B. Bioprinting: Inkjet Printing Proteins and Hybrid Cell-Containing Materials and Structures. J. Mater. Chem. 2008, 18, 5717. [Google Scholar] [CrossRef]
- Maîtrejean, G.; Cousin, M.; Truong, F.; Verdoot, V.; Hugenell, F.; Roux, D.C.D. Comprehensive Experimental Dataset on Large-Amplitude Rayleigh-Plateau Instability in Continuous InkJet Printing Regime. Data Brief. 2024, 52, 109941. [Google Scholar] [CrossRef] [PubMed]
- Workman, V.L.; Tezera, L.B.; Elkington, P.T.; Jayasinghe, S.N. Controlled Generation of Microspheres Incorporating Extracellular Matrix Fibrils for Three-Dimensional Cell Culture. Adv. Funct. Mater. 2014, 24, 2648–2657. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting Is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, J.; Li, X.; Xu, F.; Li, D. Micro/Nanoscale Electrohydrodynamic Printing: From 2D to 3D. Nanoscale 2016, 8, 15376–15388. [Google Scholar] [CrossRef]
- Ng, W.L.; Xi, H.; Shkolnikov, V.; Goh, G.L.; Suntornnond, R.; Yeong, W.Y. Controlling Droplet Impact Velocity and Droplet Volume: Key Factors to Achieving High Cell Viability in Sub-Nanoliter Droplet-Based Bioprinting. Int. J. Bioprint. 2021, 8, 424. [Google Scholar] [CrossRef]
- Tasoglu, S.; Kaynak, G.; Szeri, A.J.; Demirci, U.; Muradoglu, M. Impact of a Compound Droplet on a Flat Surface: A Model for Single Cell Epitaxy. Phys. Fluids 2010, 22, 082103. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-Thinning Hydrogels for Biomedical Applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Kim, W.; Lee, Y.; Kang, D.; Kwak, T.; Lee, H.-R.; Jung, S. 3D Inkjet-Bioprinted Lung-on-a-Chip. ACS Biomater. Sci. Eng. 2023, 9, 2806–2815. [Google Scholar] [CrossRef]
- Oropeza, B.P.; Serna, C.; Furth, M.E.; Solis, L.H.; Gonzalez, C.E.; Altamirano, V.; Alvarado, D.C.; Castor, J.A.; Cedeno, J.A.; Chaparro Vega, D.; et al. Assessment of Angiogenesis and Cell Survivability of an Inkjet Bioprinted Biological Implant in an Animal Model. Materials 2022, 15, 4468. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and Its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Nahmias, Y.; Schwartz, R.E.; Verfaillie, C.M.; Odde, D.J. Laser-guided Direct Writing for Three-dimensional Tissue Engineering. Biotechnol. Bioeng. 2005, 92, 129–136. [Google Scholar] [CrossRef]
- Odde, D.J.; Renn, M.J. Laser-Guided Direct Writing of Living Cells. Biotechnol. Bioeng. 2000, 67, 312–318. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.; Huang, Y. Modeling of Bubble Expansion-Induced Cell Mechanical Profile in Laser-Assisted Cell Direct Writing. J. Manuf. Sci. Eng. 2009, 131, 051013. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Barna, N.; Vass, C.; Antal, Z.; Kredics, L.; Chrisey, D. Time-Resolved Study of Absorbing Film Assisted Laser Induced Forward Transfer of Trichoderma longibrachiatum Conidia. J. Phys. D Appl. Phys. 2005, 38, 833–837. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, Z.; Chai, W.; Chrisey, D.B.; Huang, Y. Study of Gelatin as an Effective Energy Absorbing Layer for Laser Bioprinting. Biofabrication 2017, 9, 024103. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, X.; Tian, Z.; Menard, F.; Holzman, J.F.; Kim, K. A Novel, Well-Resolved Direct Laser Bioprinting System for Rapid Cell Encapsulation and Microwell Fabrication. Adv. Healthc. Mater. 2018, 7, e1701249. [Google Scholar] [CrossRef]
- Minaeva, E.D.; Antoshin, A.A.; Kosheleva, N.V.; Koteneva, P.I.; Gonchukov, S.A.; Tsypina, S.I.; Yusupov, V.I.; Timashev, P.S.; Minaev, N.V. Laser Bioprinting with Cell Spheroids: Accurate and Gentle. Micromachines 2023, 14, 1152. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a Clay Based Bioink for 3D Cell Printing for Skeletal Application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Mao, S.; Sun, W.; Yao, R. The Influence of Printing Parameters on Cell Survival Rate and Printability in Microextrusion-Based 3D Cell Printing Technology. Biofabrication 2015, 7, 045002. [Google Scholar] [CrossRef]
- Rossi, A.; Pescara, T.; Gambelli, A.M.; Gaggia, F.; Asthana, A.; Perrier, Q.; Basta, G.; Moretti, M.; Senin, N.; Rossi, F.; et al. Biomaterials for Extrusion-Based Bioprinting and Biomedical Applications. Front. Bioeng. Biotechnol. 2024, 12, 1393641. [Google Scholar] [CrossRef]
- Müller, S.J.; Mirzahossein, E.; Iftekhar, E.N.; Bächer, C.; Schrüfer, S.; Schubert, D.W.; Fabry, B.; Gekle, S. Flow and Hydrodynamic Shear Stress inside a Printing Needle during Biofabrication. PLoS ONE 2020, 15, e0236371. [Google Scholar] [CrossRef]
- Blanco, J.C.G.; Macías-García, A.; Rodríguez-Rego, J.M.; Mendoza-Cerezo, L.; Sánchez-Margallo, F.M.; Marcos-Romero, A.C.; Pagador-Carrasco, J.B. Optimising Bioprinting Nozzles through Computational Modelling and Design of Experiments. Biomimetics 2024, 9, 460. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An Overview of Extrusion-Based Bioprinting with a Focus on Induced Shear Stress and Its Effect on Cell Viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- White, C.R.; Frangos, J.A. The Shear Stress of It All: The Cell Membrane and Mechanochemical Transduction. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chella, R.; Ma, T. Effects of Shear Stress on 3-D Human Mesenchymal Stem Cell Construct Development in a Perfusion Bioreactor System: Experiments and Hydrodynamic Modeling. Biotechnol. Bioeng. 2007, 96, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.K.; Chan, J.M.; Kamm, R.D.; Tien, J. Microfluidic Models of Vascular Functions. Annu. Rev. Biomed. Eng. 2012, 14, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Yao, B.; Wang, Y.; Wang, R.; Yang, S.; Li, Z.; Zhang, Y.; Huang, S.; Fu, X. The Stiffness of Hydrogel-Based Bioink Impacts Mesenchymal Stem Cells Differentiation toward Sweat Glands in 3D-Bioprinted Matrix. Mater. Sci. Eng. C 2021, 118, 111387. [Google Scholar] [CrossRef]
- Heo, G.; Noh, H.; Yoon, D.; Chae, S.; Hwangbo, H.; Park, J.H.; Lim, W.H.; Kim, W.; Kim, G. Mechanotransduction-Enhanced Bioconstructs Fabricated Using a Bioink Comprising Collagen and Omega-3 Fatty Acids for Gingival Tissue Regeneration. Theranostics 2025, 15, 6476–6496. [Google Scholar] [CrossRef]
- Egorova, V.V.; Lavrenteva, M.P.; Makhaeva, L.N.; Petrova, E.A.; Ushakova, A.A.; Bozhokin, M.S.; Krivoshapkina, E.F. Fibrillar Hydrogel Inducing Cell Mechanotransduction for Tissue Engineering. Biomacromolecules 2024, 25, 7674–7684. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Nam, J.; Sun, W. Effects of Dispensing Pressure and Nozzle Diameter on Cell Survival from Solid Freeform Fabrication–Based Direct Cell Writing. Tissue Eng. Part A 2008, 14, 41–48. [Google Scholar] [CrossRef]
- Yang, G.H.; Kang, D.; An, S.; Ryu, J.Y.; Lee, K.; Kim, J.S.; Song, M.-Y.; Kim, Y.-S.; Kwon, S.-M.; Jung, W.-K.; et al. Advances in the Development of Tubular Structures Using Extrusion-Based 3D Cell-Printing Technology for Vascular Tissue Regenerative Applications. Biomater. Res. 2022, 26, 73. [Google Scholar] [CrossRef]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of Cell Viability during Bioprinting Processes. Biotechnol. J. 2009, 4, 1168–1177. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D Printing of Gelatin Methacrylamide Cell-Laden Tissue-Engineered Constructs with High Cell Viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Kozinski, J.A.; Chen, X.; Hwang, D.K. Modeling mechanical cell damage in the bioprinting process employing a conical needle. J. Mech. Med. Biol. 2015, 15, 1550073. [Google Scholar] [CrossRef]
- Ning, L.; Betancourt, N.; Schreyer, D.J.; Chen, X. Characterization of Cell Damage and Proliferative Ability during and after Bioprinting. ACS Biomater. Sci. Eng. 2018, 4, 3906–3918. [Google Scholar] [CrossRef]
- Immohr, M.B.; Dos Santos Adrego, F.; Teichert, H.L.; Schmidt, V.; Sugimura, Y.; Bauer, S.; Barth, M.; Lichtenberg, A.; Akhyari, P. 3D-Bioprinting of Aortic Valve Interstitial Cells: Impact of Hydrogel and Printing Parameters on Cell Viability. Biomed. Mater. 2023, 18, 015004. [Google Scholar] [CrossRef]
- Sun, J.; Gong, Y.; He, Y.; Fan, C.; Chen, H.; Shao, H.; Zhou, R. Process Optimization for Coaxial Extrusion-Based Bioprinting: A Comprehensive Analysis of Material Behavior, Structural Precision, and Cell Viability. Addit. Manuf. 2025, 100, 104682. [Google Scholar] [CrossRef]
- Strauß, S.; Grijalva Garces, D.; Hubbuch, J. Analytics in Extrusion-Based Bioprinting: Standardized Methods Improving Quantification and Comparability of the Performance of Bioinks. Polymers 2023, 15, 1829. [Google Scholar] [CrossRef]
- Boularaoui, S.; Shanti, A.; Khan, K.A.; Iacoponi, S.; Christoforou, N.; Stefanini, C. Harnessing Shear Stress Preconditioning to Improve Cell Viability in 3D Post-Printed Biostructures Using Extrusion Bioprinting. Bioprinting 2022, 25, e00184. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of Cell Viability and Functionality in Vessel-like Bioprintable Cell-Laden Tubular Channels. J. Biomech. Eng. 2013, 135, 091011. [Google Scholar] [CrossRef]
- Demir, E.; Metli, S.N.; Tutum, B.E.; Gokyer, S.; Oto, C.; Yilgor, P. Hand-Held Bioprinters Assisting in Situ Bioprinting. Biomed. Mater. 2025, 20, 022012. [Google Scholar] [CrossRef]
- Pazhouhnia, Z.; Beheshtizadeh, N.; Namini, M.S.; Lotfibakhshaiesh, N. Portable Hand-held Bioprinters Promote in Situ Tissue Regeneration. Bioeng. Transl. Med. 2022, 7, e10307. [Google Scholar] [CrossRef]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld Skin Printer: In Situ Formation of Planar Biomaterials and Tissues. Lab Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Di Bella, C.; Thompson, F.; Augustine, C.; Beirne, S.; Cornock, R.; Richards, C.J.; Chung, J.; Gambhir, S.; Yue, Z.; et al. Development of the Biopen: A Handheld Device for Surgical Printing of Adipose Stem Cells at a Chondral Wound Site. Biofabrication 2016, 8, 015019. [Google Scholar] [CrossRef] [PubMed]
- MacAdam, A.; Chaudry, E.; McTiernan, C.D.; Cortes, D.; Suuronen, E.J.; Alarcon, E.I. Development of in Situ Bioprinting: A Mini Review. Front. Bioeng. Biotechnol. 2022, 10, 940896. [Google Scholar] [CrossRef]
- Gironi, P.; Petraro, L.; Santoni, S.; Dedé, L.; Colosimo, B.M. A Computational Model of Cell Viability and Proliferation of Extrusion-Based 3D-Bioprinted Constructs during Tissue Maturation Process. Int. J. Bioprint. 2023, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Guarnera, D.; Restaino, F.; Vannozzi, L.; Trucco, D.; Mazzocchi, T.; Worwąg, M.; Gapinski, T.; Lisignoli, G.; Zaffagnini, S.; Russo, A.; et al. Arthroscopic Device with Bendable Tip for the Controlled Extrusion of Hydrogels on Cartilage Defects. Sci. Rep. 2024, 14, 19904. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform Inkjet Printing of Cellular Structures with Bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Xu, C.; Chai, W.; Huang, Y.; Markwald, R.R. Scaffold-free Inkjet Printing of Three-dimensional Zigzag Cellular Tubes. Biotechnol. Bioeng. 2012, 109, 3152–3160. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef]
- Kawecki, F.; Clafshenkel, W.P.; Auger, F.A.; Bourget, J.-M.; Fradette, J.; Devillard, R. Self-Assembled Human Osseous Cell Sheets as Living Biopapers for the Laser-Assisted Bioprinting of Human Endothelial Cells. Biofabrication 2018, 10, 035006. [Google Scholar] [CrossRef]
- Koch, L.; Brandt, O.; Deiwick, A.; Chichkov, B. Laser-Assisted Bioprinting at Different Wavelengths and Pulse Durations with a Metal Dynamic Release Layer: A Parametric Study. Int. J. Bioprint. 2017, 3, 42–53. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Franke, A.; Schwanke, K.; Haverich, A.; Zweigerdt, R.; Chichkov, B. Laser Bioprinting of Human Induced Pluripotent Stem Cells—The Effect of Printing and Biomaterials on Cell Survival, Pluripotency, and Differentiation. Biofabrication 2018, 10, 035005. [Google Scholar] [CrossRef]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Ortega Arevalo, M.d.P.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-Based 3D Printing of Photo-Crosslinkable Gelatin and κ-Carrageenan Hydrogel Blends for Adipose Tissue Regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, Z.; Lin, Z.; Qiu, J.; Liu, Y.; Liu, A.; Wang, Y.; Xiang, M.; Chen, B.; Fu, J.; et al. 3D Bioprinting of Vessel-like Structures with Multilevel Fluidic Channels. ACS Biomater. Sci. Eng. 2017, 3, 399–408. [Google Scholar] [CrossRef] [PubMed]
- DuFort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing Forces: Architectural Control of Mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef]
- Mohammed, D.; Versaevel, M.; Bruyère, C.; Alaimo, L.; Luciano, M.; Vercruysse, E.; Procès, A.; Gabriele, S. Innovative Tools for Mechanobiology: Unraveling Outside-In and Inside-Out Mechanotransduction. Front. Bioeng. Biotechnol. 2019, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Parzel, C.A.; Pepper, M.E.; Burg, T.; Groff, R.E.; Burg, K.J.L. EDTA Enhances High-Throughput Two-Dimensional Bioprinting by Inhibiting Salt Scaling and Cell Aggregation at the Nozzle Surface. J. Tissue Eng. Regen. Med. 2009, 3, 260–268. [Google Scholar] [CrossRef]
- Ponce-Torres, A.; Ortega, E.; Rubio, M.; Rubio, A.; Vega, E.J.; Montanero, J.M. Gaseous Flow Focusing for Spinning Micro and Nanofibers. Polymer 2019, 178, 121623. [Google Scholar] [CrossRef]
- Ponce-Torres, A.; Vega, E.J.; Castrejón-Pita, A.A.; Montanero, J.M. Smooth Printing of Viscoelastic Microfilms with a Flow Focusing Ejector. J. Nonnewton Fluid. Mech. 2017, 249, 1–7. [Google Scholar] [CrossRef]
- Shi, J.; Wu, B.; Li, S.; Song, J.; Song, B.; Lu, W.F. Shear Stress Analysis and Its Effects on Cell Viability and Cell Proliferation in Drop-on-Demand Bioprinting. Biomed. Phys. Eng. Express 2018, 4, 045028. [Google Scholar] [CrossRef]
- Reina-Romo, E.; Mandal, S.; Amorim, P.; Bloemen, V.; Ferraris, E.; Geris, L. Towards the Experimentally-Informed In Silico Nozzle Design Optimization for Extrusion-Based Bioprinting of Shear-Thinning Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 701778. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; An, Y.; Li, M.; Zhao, X. Research on the Flow Behavior of Bio-Ink inside the Extrusion Nozzle during Printing. J. Appl. Phys. 2024, 136. [Google Scholar] [CrossRef]
- Smith, C.M.; Stone, A.L.; Parkhill, R.L.; Stewart, R.L.; Simpkins, M.W.; Kachurin, A.M.; Warren, W.L.; Williams, S.K. Three-Dimensional BioAssembly Tool for Generating Viable Tissue-Engineered Constructs. Tissue Eng. 2004, 10, 1566–1576. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of Three-Dimensional Hepatocyte/Gelatin Structures with Rapid Prototyping System. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef]
- Cohen, D.L.; Malone, E.; Lipson, H.; Bonassar, L.J. Direct Freeform Fabrication of Seeded Hydrogels in Arbitrary Geometries. Tissue Eng. 2006, 12, 1325–1335. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Zhu, N.; Schreyer, D.J.; Chen, X. Modeling Process-Induced Cell Damage in the Biodispensing Process. Tissue Eng. Part C Methods 2010, 16, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.J.; Fabry, B.; Gekle, S. Predicting Cell Stress and Strain during Extrusion Bioprinting. Phys. Rev. Appl. 2023, 19, 064061. [Google Scholar] [CrossRef]
- Chand, R.; Muhire, B.S.; Vijayavenkataraman, S. Computational Fluid Dynamics Assessment of the Effect of Bioprinting Parameters in Extrusion Bioprinting. Int. J. Bioprint. 2022, 8, 545. [Google Scholar] [CrossRef]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering Considerations on Extrusion-Based Bioprinting: Interactions of Material Behavior, Mechanical Forces and Cells in the Printing Needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef]
- Gómez-Blanco, J.C.; Pagador, J.B.; Galván-Chacón, V.P.; F. Sánchez-Peralta, L.; Matamoros, M.; Marcos, A.; M. Sánchez-Margallo, F. Computational Simulation-Based Comparative Analysis of Standard 3D Printing and Conical Nozzles for Pneumatic and Piston-Driven Bioprinting. Int. J. Bioprint. 2024, 9, 730. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Haweet, A.; Jayash, S.N.; Morgan, G.; Moore, J.E.; Candeo, A. Real-Time Imaging and Analysis of Cell-Hydrogel Interplay within an Extrusion-Bioprinting Capillary. Bioprinting 2021, 23, e00144. [Google Scholar] [CrossRef]
- Ouyang, L.; Salmeron-Sanchez, M. Editorial 3D Bioprinting and Advanced Biofabrication of Biomaterials. Biomater. Adv. 2024, 156, 213725. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Chen, X.B.; Fazel Anvari-Yazdi, A.; Duan, X.; Zimmerling, A.; Gharraei, R.; Sharma, N.K.; Sweilem, S.; Ning, L. Biomaterials/Bioinks and Extrusion Bioprinting. Bioact. Mater. 2023, 28, 511–536. [Google Scholar] [CrossRef] [PubMed]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and Its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Fang, W.; Yang, M.; Wang, L.; Li, W.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; et al. Hydrogels for 3D Bioprinting in Tissue Engineering and Regenerative Medicine: Current Progress and Challenges. Int. J. Bioprint. 2023, 9, 759. [Google Scholar] [CrossRef]
- Loukelis, K.; Koutsomarkos, N.; Mikos, A.G.; Chatzinikolaidou, M. Advances in 3D Bioprinting for Regenerative Medicine Applications. Regen. Biomater. 2024, 11, rbae033. [Google Scholar] [CrossRef]
- Dubbin, K.; Hori, Y.; Lewis, K.K.; Heilshorn, S.C. Dual-Stage Crosslinking of a Gel-Phase Bioink Improves Cell Viability and Homogeneity for 3D Bioprinting. Adv. Healthc. Mater. 2016, 5, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Wong Po Foo, C.T.S.; Lee, J.S.; Mulyasasmita, W.; Parisi-Amon, A.; Heilshorn, S.C. Two-Component Protein-Engineered Physical Hydrogels for Cell Encapsulation. Proc. Natl. Acad. Sci. USA 2009, 106, 22067–22072. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.E.; Rakoski, A.; Jivan, F.; Pérez, L.M.; Alge, D.L. Hydrogel Synthesis and Stabilization via Tetrazine Click-Induced Secondary Interactions. Macromol. Rapid Commun. 2020, 41, e2000287. [Google Scholar] [CrossRef] [PubMed]
- Mulyasasmita, W.; Lee, J.S.; Heilshorn, S.C. Molecular-Level Engineering of Protein Physical Hydrogels for Predictive Sol–Gel Phase Behavior. Biomacromolecules 2011, 12, 3406–3411. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Cui, R.; Li, S.; Li, T.; Gou, X.; Jing, T.; Zhang, G.; Wei, G.; Jin, Z.; Xiong, X.; Qu, S. Natural Polymer Derived Hydrogel Bioink with Enhanced Thixotropy Improves Printability and Cellular Preservation in 3D Bioprinting. J. Mater. Chem. B 2023, 11, 3907–3918. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Gorkin III, R.; in het Panhuis, M. An Overview of the Suitability of Hydrogel-Forming Polymers for Extrusion-Based 3D-Printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, e1901648. [Google Scholar] [CrossRef]
- Jiao, T.; Lian, Q.; Lian, W.; Wang, Y.; Li, D.; Reis, R.L.; Oliveira, J.M. Properties of Collagen/Sodium Alginate Hydrogels for Bioprinting of Skin Models. J. Bionic Eng. 2023, 20, 105–118. [Google Scholar] [CrossRef]
- Wang, J.K.; Cheam, N.M.J.; Irvine, S.A.; Tan, N.S.; Venkatraman, S.; Tay, C.Y. Interpenetrating Network of Alginate–Human Adipose Extracellular Matrix Hydrogel for Islet Cells Encapsulation. Macromol. Rapid Commun. 2020, 41, e2000275. [Google Scholar] [CrossRef]
- Fischer, L.; Nosratlo, M.; Hast, K.; Karakaya, E.; Ströhlein, N.; Esser, T.U.; Gerum, R.; Richter, S.; Engel, F.B.; Detsch, R.; et al. Calcium Supplementation of Bioinks Reduces Shear Stress-Induced Cell Damage during Bioprinting. Biofabrication 2022, 14, 045005. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, X.; Yu, L.; Xu, H. Calcium Signaling in Membrane Repair. Semin. Cell Dev. Biol. 2015, 45, 24–31. [Google Scholar] [CrossRef]
- Klenow, M.B.; Heitmann, A.S.B.; Nylandsted, J.; Simonsen, A.C. Timescale of Hole Closure during Plasma Membrane Repair Estimated by Calcium Imaging and Numerical Modeling. Sci. Rep. 2021, 11, 4226. [Google Scholar] [CrossRef] [PubMed]
- Wales, P.; Schuberth, C.E.; Aufschnaiter, R.; Fels, J.; García-Aguilar, I.; Janning, A.; Dlugos, C.P.; Schäfer-Herte, M.; Klingner, C.; Wälte, M.; et al. Calcium-Mediated Actin Reset (CaAR) Mediates Acute Cell Adaptations. eLife 2016, 5, e19850. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The Versatility and Universality of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Lin, R.C.; Scheller, R.H. Mechanisms of Synaptic Vesicle Exocytosis. Annu. Rev. Cell Dev. Biol. 2000, 16, 19–49. [Google Scholar] [CrossRef]
- Shao, X.; Li, Q.; Mogilner, A.; Bershadsky, A.D.; Shivashankar, G.V. Mechanical Stimulation Induces Formin-Dependent Assembly of a Perinuclear Actin Rim. Proc. Natl. Acad. Sci. USA 2015, 112, E2595–E2601. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Jang, J.; Chae, S.; Gao, G.; Kong, J.-S.; Ahn, M.; Cho, D.-W. Three-Dimensional Bioprinting of Cell-Laden Constructs with Polycaprolactone Protective Layers for Using Various Thermoplastic Polymers. Biofabrication 2016, 8, 035013. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Tan, X.; Yeong, W.Y.; Tor, S.B. Hybrid Microscaffold-Based 3D Bioprinting of Multi-Cellular Constructs with High Compressive Strength: A New Biofabrication Strategy. Sci. Rep. 2016, 6, 39140. [Google Scholar] [CrossRef]
- Rousselle, A.; Ferrandon, A.; Mathieu, E.; Godet, J.; Ball, V.; Comperat, L.; Oliveira, H.; Lavalle, P.; Vautier, D.; Arntz, Y. Enhancing Cell Survival in 3D Printing of Organoids Using Innovative Bioinks Loaded with Pre-Cellularized Porous Microscaffolds. Bioprinting 2022, 28, e00247. [Google Scholar] [CrossRef]
- Sattari, S.; Mariano, C.A.; Eskandari, M. Biaxial Mechanical Properties of the Bronchial Tree: Characterization of Elasticity, Extensibility, and Energetics, Including the Effect of Strain Rate and Preconditioning. Acta Biomater. 2023, 155, 410–422. [Google Scholar] [CrossRef]
- Park, J.; Shin, A.; Jafari, S.; Demer, J.L. Material Properties and Effect of Preconditioning of Human Sclera, Optic Nerve, and Optic Nerve Sheath. Biomech. Model. Mechanobiol. 2021, 20, 1353–1363. [Google Scholar] [CrossRef]
- Sasajima, S.; Kubo, K. Influence of Preconditioning on Morphological and Mechanical Properties of Human Achilles Tendon in Vivo. J. Biomech. 2024, 170, 112168. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Zagonel, F.; Hammerman, M.; Bernhardsson, M.; Eliasson, P. Effect of Storage and Preconditioning of Healing Rat Achilles Tendon on Structural and Mechanical Properties. Sci. Rep. 2021, 11, 958. [Google Scholar] [CrossRef]
- Moeinabadi-Bidgoli, K.; Babajani, A.; Yazdanpanah, G.; Farhadihosseinabadi, B.; Jamshidi, E.; Bahrami, S.; Niknejad, H. Translational Insights into Stem Cell Preconditioning: From Molecular Mechanisms to Preclinical Applications. Biomed. Pharmacother. 2021, 142, 112026. [Google Scholar] [CrossRef]
- Orive, G.; Santos, E.; Poncelet, D.; Hernández, R.M.; Pedraz, J.L.; Wahlberg, L.U.; De Vos, P.; Emerich, D. Cell Encapsulation: Technical and Clinical Advances. Trends Pharmacol. Sci. 2015, 36, 537–546. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoli, S.; Merotto, E.; Piccoli, M.; Gobbo, P.; Todros, S.; Pavan, P.G. An Overview of 3D Bioprinting Impact on Cell Viability: From Damage Assessment to Protection Solutions. J. Funct. Biomater. 2025, 16, 436. https://doi.org/10.3390/jfb16120436
Manzoli S, Merotto E, Piccoli M, Gobbo P, Todros S, Pavan PG. An Overview of 3D Bioprinting Impact on Cell Viability: From Damage Assessment to Protection Solutions. Journal of Functional Biomaterials. 2025; 16(12):436. https://doi.org/10.3390/jfb16120436
Chicago/Turabian StyleManzoli, Sara, Elena Merotto, Martina Piccoli, Pierangelo Gobbo, Silvia Todros, and Piero G. Pavan. 2025. "An Overview of 3D Bioprinting Impact on Cell Viability: From Damage Assessment to Protection Solutions" Journal of Functional Biomaterials 16, no. 12: 436. https://doi.org/10.3390/jfb16120436
APA StyleManzoli, S., Merotto, E., Piccoli, M., Gobbo, P., Todros, S., & Pavan, P. G. (2025). An Overview of 3D Bioprinting Impact on Cell Viability: From Damage Assessment to Protection Solutions. Journal of Functional Biomaterials, 16(12), 436. https://doi.org/10.3390/jfb16120436









