Cell Viability Assay of Chitosan-Modified Glass Ionomer Restorative Cements
Abstract
1. Introduction
- Null Hypothesis for Material/Modification: There are no significant differences in mean cell viability among the six CS-modified material groups.
- Null Hypothesis for Time: There are no significant differences in mean cell viability across the three time points.
- Null Hypothesis for Interaction: The effect of the material/modification on cell viability is consistent across all time points. That is, the changes in viability over time for one material are not significantly different from the changes observed for another.
2. Materials and Methods
2.1. Materials and Modification
2.2. 3T3 Balb/c Fibroblast Culture
2.3. Diluent Specimen Preparation
2.4. MTT Colorimetric and 3T3 Balb/c Fibroblast Cell Viability Assays
2.5. 3T3 Balb/c Fibroblast Disk Surface Contact Evaluation
2.6. Fixing Technique for Scanning Electron Microscopy (SEM) of 3T3 Cells
2.7. Statistical Analysis
- Factor A: Material/Modification with six levels: FN 5%CS, FN 10%CS, KU 5%CS, KU 10%CS, RSC 5%CS, and RSC 10%CS.
- Factor B: Time with three levels: Day 1, Day 7, and Day 21. Analysis was performed with a significance level (alpha) of 0.05.
3. Results
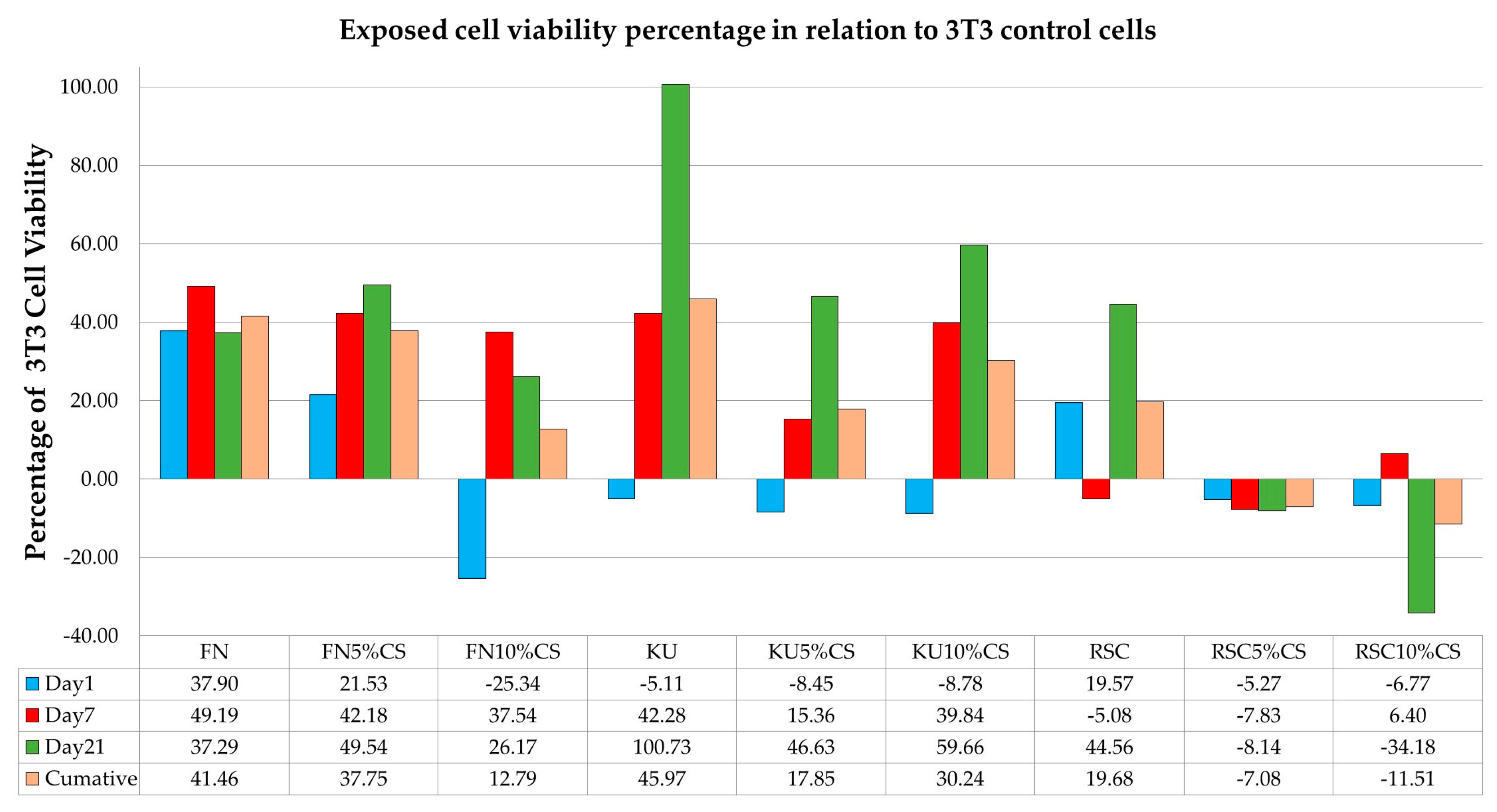
3.1. Effect of Different GICs on Cell Viability
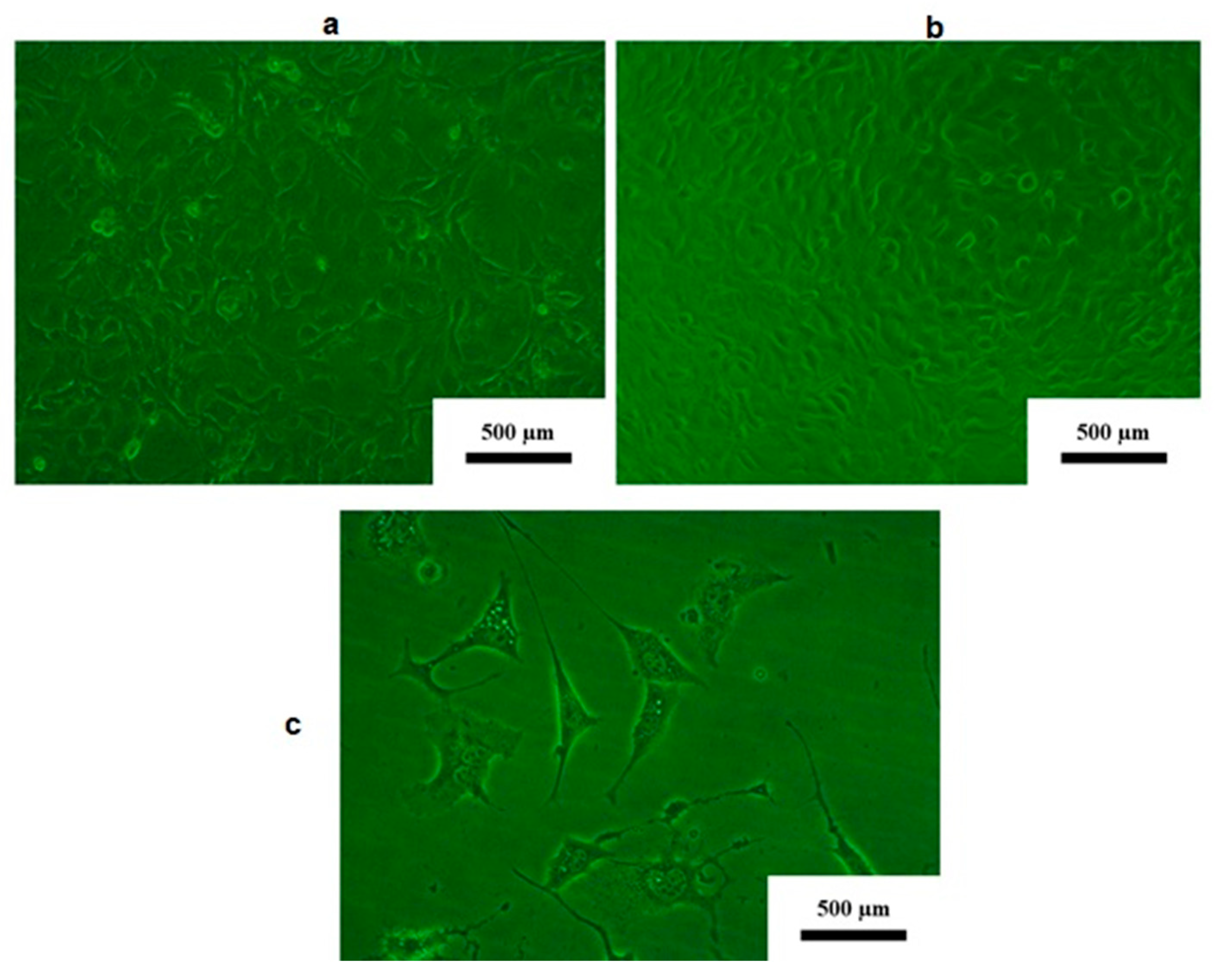
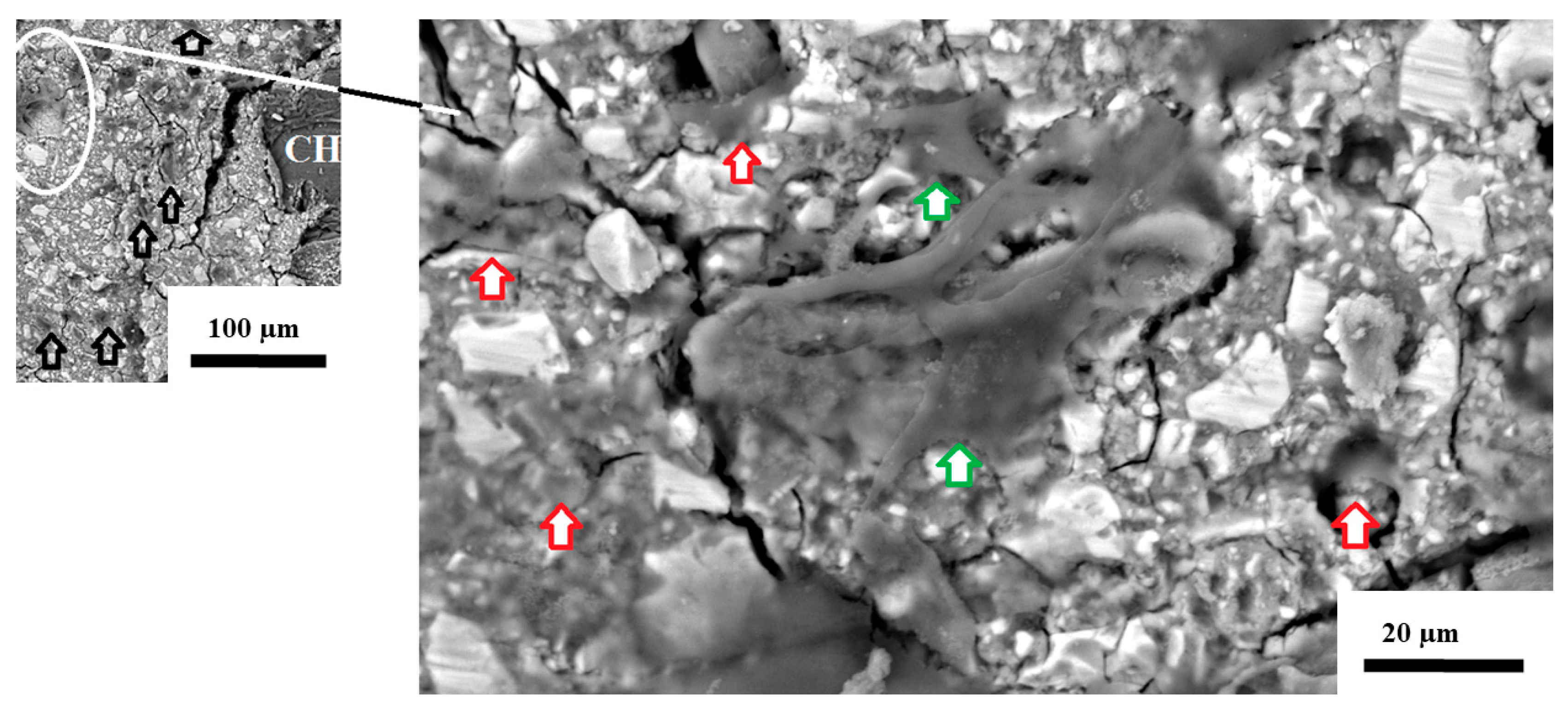
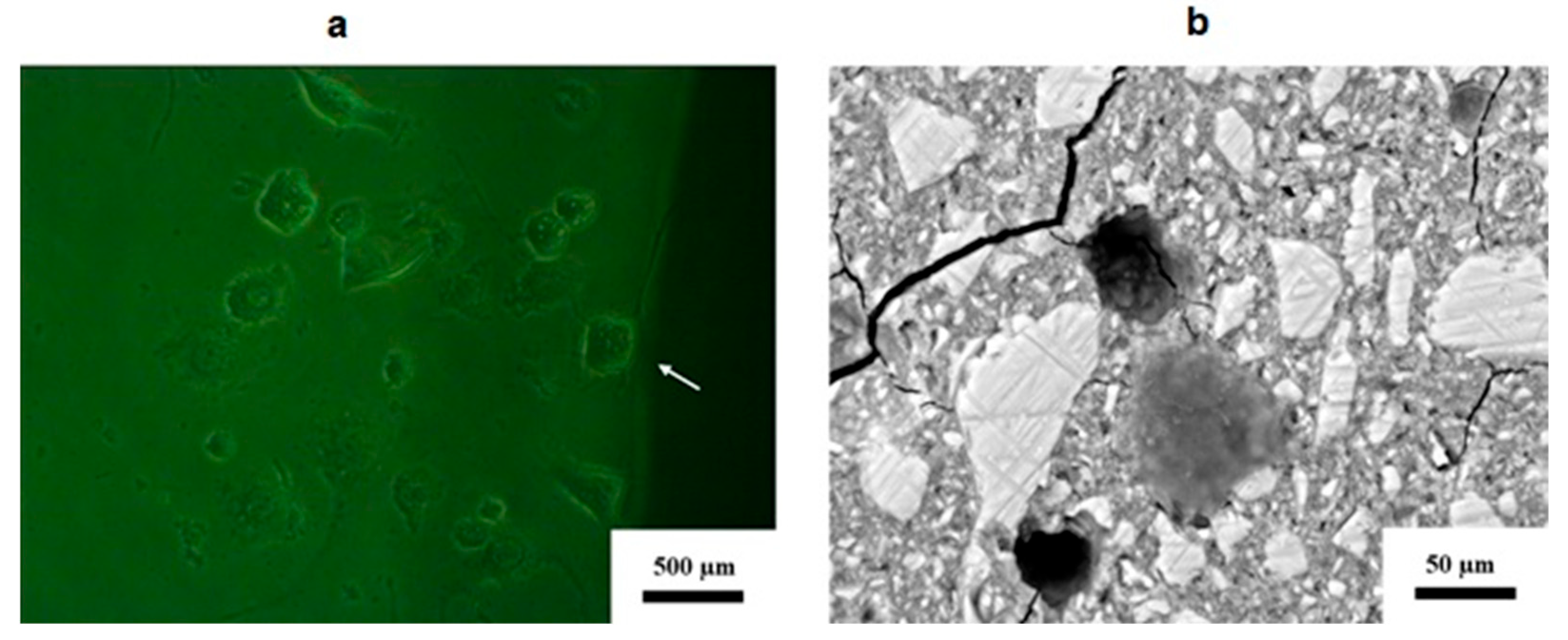
3.2. Cell Morphology in Response to Contact with the Disc’s Surface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GIC | glass ionomer cement |
| CS | Chitosan |
| CS-modified | chitosan-modified |
| FN | Fuji IX GP |
| KU | Ketac Universal |
| RSC | Riva Self Cure |
| S. mutans | Streptococcus Mutans Species |
References
- Glasspoole, E.A.; Erickson, R.L.; Davidson, C.L. Effect of surface treatments on the bond strength of glass ionomers to enamel. Dent. Mater. 2002, 18, 454–462. [Google Scholar] [CrossRef]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Snauwaert, J.; Hellemans, L.; Lambrechts, P.; Vanherle, G.; Wakasa, K. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J. Dent. Res. 2000, 79, 709–714. [Google Scholar] [CrossRef]
- Maeda, T.; Mukaeda, K.; Shimohira, T.; Katsuyama, S. Ion distribution in matrix parts of glass-polyalkenoate cement by SIMS. J. Dent. Res. 1999, 78, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Hatton, P.; Brook, I. Characterisation of the ultrastructure of glass-ionomer (poly-alkenoate) cement. Br. Dent. J. 1992, 173, 275–277. [Google Scholar] [CrossRef]
- Nicholson, J.W. Chemistry of glass-ionomer cements: A review. Biomaterials 1998, 19, 485–494. [Google Scholar] [CrossRef]
- Ten Bosch, J.J.; Fennis-le, Y.; Verdonschot, E.H. Time-dependent decrease and seasonal variation of the porosity of recently erupted sound dental enamel in vivo. J. Dent. Res. 2000, 79, 1556–1559. [Google Scholar] [CrossRef]
- Matsuya, S.; Maeda, T.; Ohta, M. IR and NMR analyses of hardening and maturation of glass-ionomer cement. J. Dent. Res. 1996, 75, 1920–1927. [Google Scholar] [CrossRef]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical properties and microstructures of glass-ionomer cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef]
- Chau, N.P.; Pandit, S.; Cai, J.N.; Lee, M.H.; Jeon, J.G. Relationship between fluoride release rate and anti-cariogenic biofilm activity of glass ionomer cements. Dent. Mater. 2015, 31, e100–e108. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.C.; Mount, G.; Mc Intyre, J.; Tuisuva, J.; Von Doussa, R.J. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: An in vivo study. J. Dent. 2006, 34, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.F.; Donegá, J.; Benassi, A.M.; Bocangel, J.A. Preliminary study on chitosan modified glass ionomer restoratives. Dent. Mater. 2007, 23, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Meera Priyadarshini, B.; Neo, J.; Fawzy, A.S. Characterization of Chitosan/TiO2 Nano-Powder Modified Glass-Ionomer Cement for Restorative Dental Applications. J. Esthet. Restor. Dent. 2017, 29, 146–156. [Google Scholar] [CrossRef]
- ISO 10993–5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 18 July 2025).
- ISO 7405:2025; Dentistry—Evaluation of Biocompatibility of Medical Devices Used in Dentistry. International Organization for Standardization: Geneva, Switzerland, 2025. Available online: https://www.iso.org/standard/7405 (accessed on 18 July 2025).
- Piszko, A.; Piszko, P.J.; Kulus, M.J.; Pajączkowska, M.; Nowicka, J.; Chwirot, A.; Rusak, A.; Chodaczek, G.; Szymonowicz, M.; Dobrzyński, M. Fluoride Release and Biological Properties of Resin-Modified Glass Ionomer Cement Doped with Copper. Appl. Sci. 2025, 15, 9506. [Google Scholar] [CrossRef]
- Geurtsen, W.; Lehmann, F.; Spahl, W.; Leyhausen, G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J. Biomed. Mater. Res. 1998, 41, 474–480. [Google Scholar] [CrossRef]
- Li, K.-Y.; Tsai, C.-C.; Lin, T.-C.; Wang, Y.-L.; Lin, F.-H.; Lin, C.-P. Fluorinated Montmo-rillonite and 3YSZ as the Inorganic Fillers in Fluoride-Releasing and Rechargeable Dental Composition Resin. Polymers 2020, 12, 223. [Google Scholar] [CrossRef]
- Moodley, D.; Grobler, S.R.; Olivier, A. Cytotoxicity of a dentine bonding agent on four different cell-lines. SADJ 2005, 60, 234–236. Available online: https://pubmed.ncbi.nlm.nih.gov/16119020/ (accessed on 1 July 2023).
- Grobler, S.R.; Oliver, A.; Moodley, D.; Van Wyk Kotze, T.J. Cytotoxicity of recent dentin bonding agents on mouse fibroblast cells. Quintessence Int. 2008, 39, 511–516. Available online: https://pubmed.ncbi.nlm.nih.gov/19057749/ (accessed on 1 July 2023). [PubMed]
- Olivier, A.; Grobler, S.R.; Osman, Y. Cytotoxicity of seven recent dentine bonding agents on mouse 3T3 fibroblast cells. OJST 2012, 2, 244–250. [Google Scholar] [CrossRef]
- Novotná, B.; Holík, P.; Morozova, Y.; Rosa, M.; Galandáková, A.; Langová, K. Evaluation of Cytotoxicity of the Dental Materials TheraCal LC, TheraCal PT, ApaCal ART and Biodentine Used in Vital Pulp Therapy: In Vitro Study. Dent. J. 2024, 12, 249. [Google Scholar] [CrossRef]
- Skośkiewicz-Malinowska, K.; Mysior, M.; Rusak, A.; Kuropka, P.; Kozakiewicz, M.; Jurczyszyn, K. Application of Texture and Fractal Dimension Analysis to Evaluate Subgingival Cement Surfaces in Terms of Biocompatibility. Materials 2021, 14, 5857. [Google Scholar] [CrossRef]
- ISO 9917-1:2025; Dentistry—Water-Based Cements Part 1: Acid-Base Cements. International Organization for Standardization: Geneva, Switzerland, 2025. Available online: https://www.iso.org/standard/76037.html (accessed on 18 July 2025).
- Mulder, R.; Anderson-Small, C. Ion release of chitosan and nanodiamond modified glass ionomer restorative cements. Clin. Cosmet. Investig. Dent. 2019, 11, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R. Variation in the Dispersions of Powder Liquid Ratios in Hand-Mix Glass Ionomers. Open Dent. J. 2018, 12, 647–654. [Google Scholar] [CrossRef]
- Basak, V.; Bahar, T.E.; Emine, K.; Yelda, K.; Mine, K.; Figen, S.; Rustem, N. Evaluation of cytotoxicity and gelatinases activity in 3T3 fibroblast cell by root repair materials. Biotechnol. Equip. 2016, 30, 984–990. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–56. [Google Scholar] [CrossRef]
- Persaud, S.J. Cell and tissue culture: Laboratory procedures in biotechnology. In Cell Biochemistry and Function; Doyle, A., Griffiths, J.B., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1999; pp. 289–332. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; Al-Sa’Eed, O.R.; Darmani, H. Quality of cellular attachment to various root-end filling materials. J. Appl. Oral. Sci. 2012, 20, 82–88. [Google Scholar] [CrossRef]
- Camilleri, J.; Montesin, F.E.; Papaioannou, S.; McDonald, F.; Pitt Ford, T.R. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int. Endod. J. 2004, 37, 699–704. [Google Scholar] [CrossRef]
- ProSciTech Pty Ltd. Usage of Cacodylate, the Most Favoured Buffering Fixative in Specimen Preparation for Electron Microscopy. Sodium Cacodylate Trihydrate. 2014. Available online: https://proscitech.com.au/products/sodium-cacodylate-trihydrate-dg (accessed on 18 July 2025).
- Selimović-Dragaš, M.; Hasić-Branković, L.; Korać, F.; Đapo, N.; Huseinbegović, A.; Kobašlija, S.; Lekić, M.; Hatibović-Kofman, Š. In vitro fluoride release from a different kind of conventional and resin modified glass-ionomer cements. Bosn. J. Basic Med. Sci. 2013, 13, 197–202. [Google Scholar] [CrossRef]
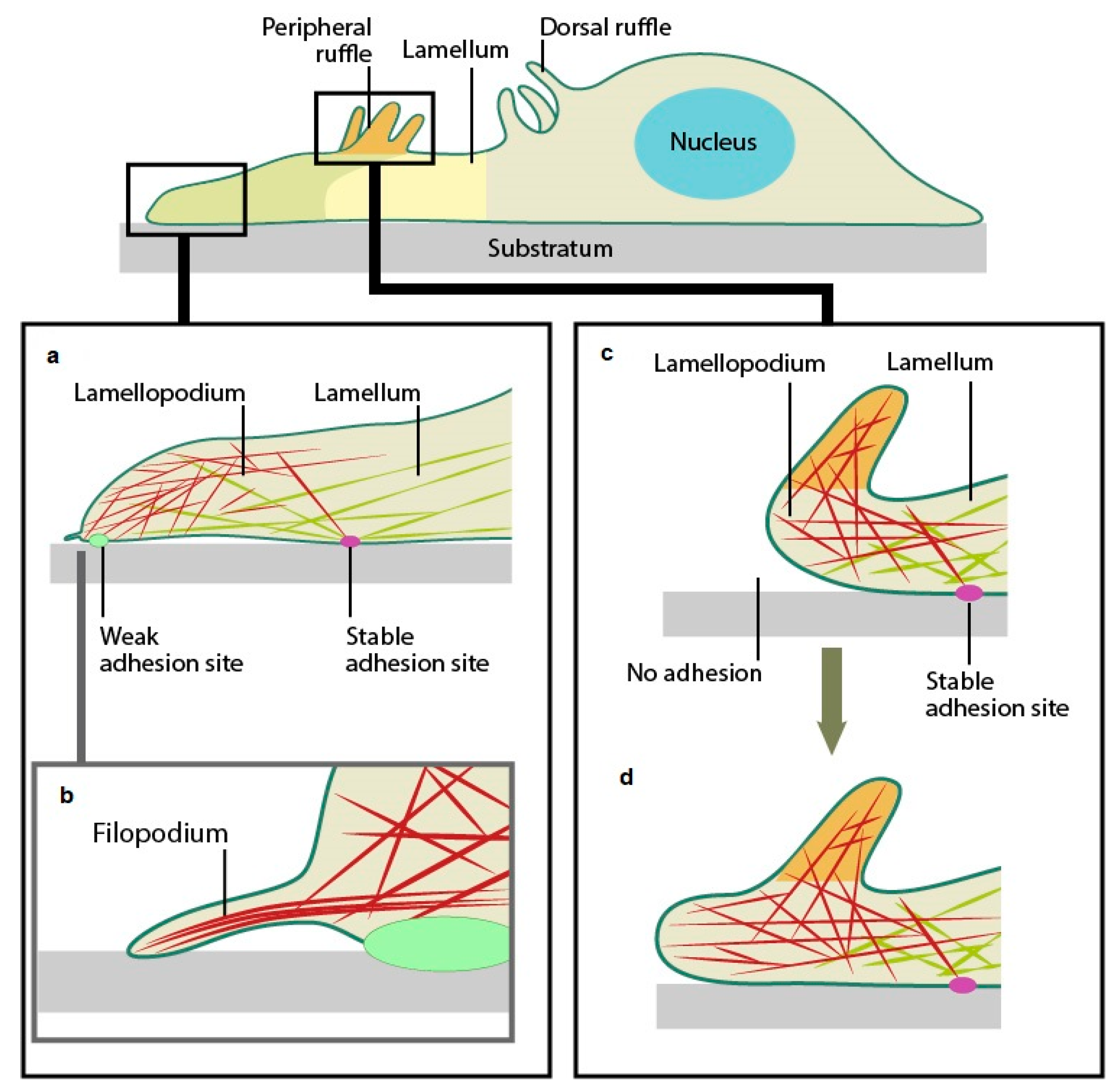
- Mechanobiology Institute, National University of Singapore (MBI). Cytoskeleton Dynamics. 2022. Available online: https://www.mbi.nus.edu.sg/mbinfo/what-is-the-role-of-the-lamellipodia-in-mechanosensing-and-cell-motility/ (accessed on 18 July 2025).
- Six, N.; Lasfargues, J.J.; Goldberg, M. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J. Dent. 2000, 28, 413–422. [Google Scholar] [CrossRef]
- Guida, A.; Hill, R.G.; Towler, M.R.; Eramo, S. Fluoride release from model glass ionomer cements. J. Mater. Sci. Mater. Med. 2002, 13, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Sasanaluckit, P.; Albustany, K.R.; Doherty, P.J.; Williams, D.F. Biocompatibility of glass ionomer cements. Biomaterials 1993, 14, 906–916. [Google Scholar] [CrossRef]
- Elshenawy, E.A.; El-Ebiary, M.A.; Kenawy, E.R.; El-Olimy, G.A. Modification of glass-ionomer cement properties by quaternized chitosan-coated nanoparticles. Odontology 2023, 111, 328–441. [Google Scholar] [CrossRef]
- Wilson, A.D.; Groffman, D.M.; Kuhn, A.T. The release of fluoride and other chemical species from a glass-ionomer cement. Biomaterials 1985, 6, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.; Hill, R. Glass ceramic approach to controlling the properties of a glass-ionomer bone cement. Biomaterials 1991, 12, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Baxter, L.C.; Frauchiger, M.; Textor, M.; Gwynn, I.; Richards, R.G. Fibroblast and osteoblast adhesion and morphology on calcium phosphate surfaces. Eur. Cells Mater. 2002, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hensten-Pettersen, A. Comparison of the methods available for assessing cytotoxicity. Int. Endod. J. 2007, 21, 89–99. [Google Scholar] [CrossRef]
- Abdullah, D.; Pitt Ford, T.R.; Papaioannou, S.; Nicholson, J.; McDonald, F. An evaluation of accelerated Portland cement as a restorative material. Biomaterials 2002, 23, 4001–4010. [Google Scholar] [CrossRef]
- De Souza Costa, C.A.; Hebling, J.; Garcia-Godoy, F.; Hanks, C.T. In vitro cytotoxicity of five glass-ionomer cements. Biomaterials 2003, 24, 3853–3858. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y.; Komoda, Y. 1H and 13C NMR Studies of the Interaction of Eugenol, Phenol, and Triethyleneglycol Dimethacrylate with Phospholipid Liposomes as a Model System for Odontoblast Membranes. J. Dent. Res. 1988, 67, 1438–1441. [Google Scholar] [CrossRef]
- Corral Nuñez, C.M.; Bosomworth, H.J.; Field, C.; Whitworth, J.M.; Valentine, R.A. Biodentine and Mineral Trioxide Aggregate Induce Similar Cellular Responses in a Fibroblast Cell Line. J. Endod. 2014, 40, 406–411. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ozeki, M.; Yagi, T.; Hosoi, T.; Takei, M. X-ray Diffraction Analysis of the Reaction Products of Hydroxyapatite and Lanthanum. J. Dent. Health 1980, 29, 354–360. [Google Scholar] [CrossRef]
- Kotyńska, J.; Figaszewski, Z.A. Binding of trivalent metal ions (Al3+, In3+, La3+) with phosphatidylcholine liposomal membranes investigated by microelectrophoresis. Eur. Phys. J. E Soft Matter 2018, 41, 70. [Google Scholar] [CrossRef]
- Smith, J.B.; Smith, L. Initiation of DNA synthesis in quiescent Swiss 3T3 and 3T6 cells by lanthanum. Biosci. Rep. 1984, 4, 777–782. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, L.; Yang, X.; Wang, K.; Ke, Y.; Qian, Z.M. La3+-promoted proliferation is interconnected with apoptosis in NIH 3T3 cells. J. Cell Biochem. 2005, 94, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.; Maboza, E.; Ahmed, R. Streptococcus Mutans Growth and Resultant Material Surface Roughness on Modified Glass Ionomers. Front. Oral Health 2020, 17, 613384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Q.; Fan, C.; Ren, H.; Xu, S.; Hu, F.; Wang, L.; Yang, K.; Ji, Q. Characteristics of chitosan-modified glass ionomer cement and their effects on the adhesion and proliferation of human gingival fibroblasts: An in vitro study. J. Mater. Sci. Mater. Med. 2019, 30, 39. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R. An in Vitro Study of the Properties of GICs with Bioactive Biomaterial Modification. Ph.D. Thesis, The University of the Western Cape, Cape Town, South Africa, 2019; pp. 230–275. Available online: https://uwcscholar.uwc.ac.za/items/ba74d13a-d4fd-4f3f-a9ca-b367ccc96703 (accessed on 18 July 2025).



| Comparison between Groups and Time | Mean Difference | Standard Error | 95% Confidence Interval | Adjusted p-Value |
|---|---|---|---|---|
| KU Day 21 vs. FN Day 21 | 0.164 | 0.044 | (0.076, 0.252) | <0.001 |
| KU Day 21 vs. RSC Day 21 | 0.154 | 0.044 | (0.066, 0.242) | <0.001 |
| FN Day 1 vs. FN Day 21 | 0.311 | 0.044 | (0.223, 0.399) | <0.001 |
| RSC Day 1 vs. RSC Day 21 | 0.204 | 0.044 | (0.116, 0.292) | <0.001 |
| KU Day 1 vs. KU Day 21 | −0.052 | 0.044 | (−0.140, 0.036) | 0.540 |
| Day(s) | Material | |||
| Comparison within groups | FN | KU | RSC | |
| Day 1 3T3 cells | 37.90 * | −5.11 | 19.57 ^ | |
| Day 7 3T3 cells | 49.19 * | 42.28 * | −5.08 | |
| Day 21 3T3 cells | 37.29 ^ | 100.73 * | 44.56 * | |
| Day | Material | ||
|---|---|---|---|
| FN | FN5%CS | FN10%CS | |
| Day 1 3T3 cells | 37.90 * | 21.53 ^ | −25.34 ^ |
| Day 7 3T3 cells | 49.19 * | 42.18 * | 37.54 * |
| Day 21 3T3 cells | 37.29 | 49.54 ^ | 26.17 |
| KU | KU5%CS | KU10%CS | |
| Day 1 3T3 cells | −5.11 | −8.45 | −8.78 * |
| Day 7 3T3 cells | 42.28 * | 15.36 | 39.84 * |
| Day 21 3T3 cells | 100.73 * | 46.63 * | 59.66 * |
| RSC | RSC5%CS | RSC10%CS | |
| Day 1 3T3 cells | 19.57 | −5.27 | −6.77 ^ |
| Day 7 3T3 cells | −5.08 | −7.83 ^ | 6.40 |
| Day 21 3T3 cells | 44.56 * | −8.14 | −34.18 ^ |
| Day | Material | ||
|---|---|---|---|
| FN | FN5%CS | FN10%CS | |
| Day 1 DMEM pH | 8.93 | 8.85 | 8.88 |
| Day 7 DMEM pH | 8.69 | 8.65 | 8.71 |
| Day 21 DMEM pH | 8.29 | 8.41 | 8.78 |
| KU | KU5%CS | KU10%CS | |
| Day 1 DMEM pH | 8.9 | 8.8 | 8.84 |
| Day 7 DMEM pH | 8.91 | 8.65 | 8.41 |
| Day 21 DMEM pH | 8.84 | 7.77 | 8.03 |
| RSC | RSC5%CS | RSC10%CS | |
| Day 1 DMEM pH | 8.71 | 8.89 | 9.02 |
| Day 7 DMEM pH | 8.76 | 8.88 | 8.77 |
| Day 21 DMEM pH | 8.93 | 8.87 | 8.93 |
| DMEM pH | 7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulder, R.; Mulder-van Staden, S.; Olivier, A. Cell Viability Assay of Chitosan-Modified Glass Ionomer Restorative Cements. J. Funct. Biomater. 2025, 16, 432. https://doi.org/10.3390/jfb16120432
Mulder R, Mulder-van Staden S, Olivier A. Cell Viability Assay of Chitosan-Modified Glass Ionomer Restorative Cements. Journal of Functional Biomaterials. 2025; 16(12):432. https://doi.org/10.3390/jfb16120432
Chicago/Turabian StyleMulder, Riaan, Suné Mulder-van Staden, and Annette Olivier. 2025. "Cell Viability Assay of Chitosan-Modified Glass Ionomer Restorative Cements" Journal of Functional Biomaterials 16, no. 12: 432. https://doi.org/10.3390/jfb16120432
APA StyleMulder, R., Mulder-van Staden, S., & Olivier, A. (2025). Cell Viability Assay of Chitosan-Modified Glass Ionomer Restorative Cements. Journal of Functional Biomaterials, 16(12), 432. https://doi.org/10.3390/jfb16120432









