Endothelial Cells Differentiated from Human Induced Pluripotent Stem Cells Form Aligned Network Structures in Engineered Neural Tissue
Abstract
1. Introduction
2. Materials and Methods
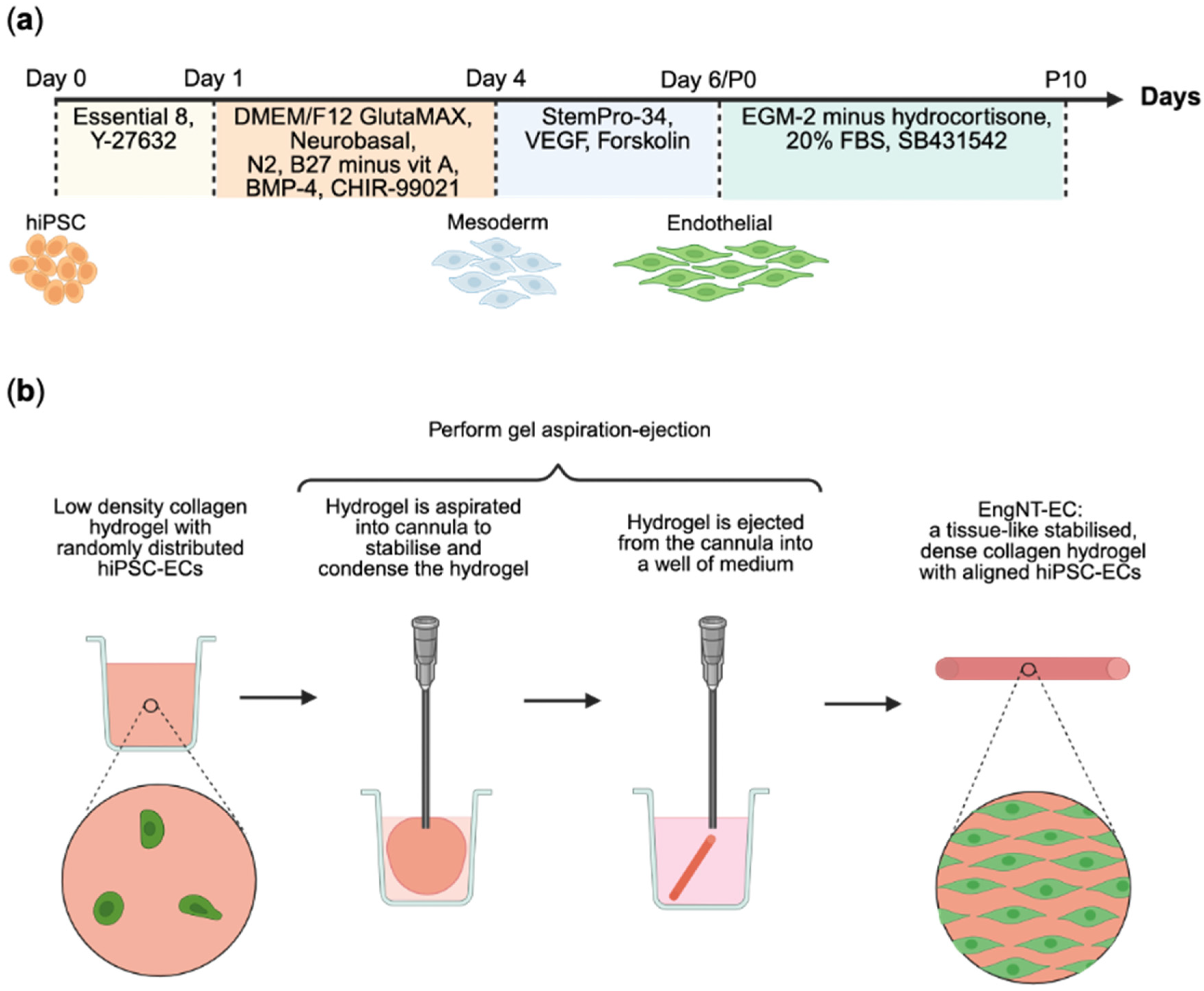
2.1. Cell Culture and Differentiation
2.2. Tubulogenesis Assay
2.3. Tissue Engineering
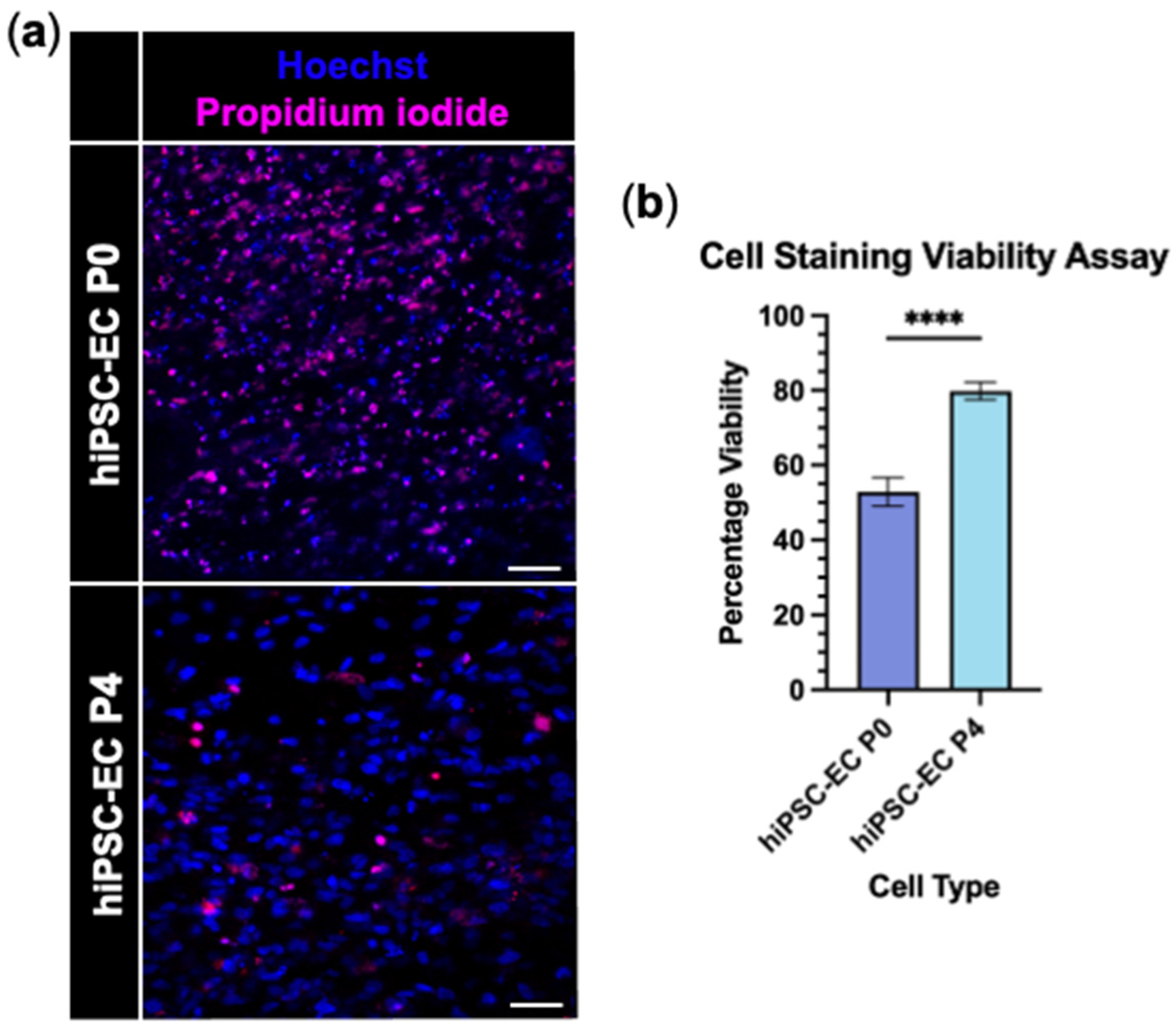
2.4. Viability Assays
2.5. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Neurite Extension Assay
2.7. Immunofluorescence Staining
2.8. Microscopy and Image Analysis
2.9. Statistical Analysis
3. Results
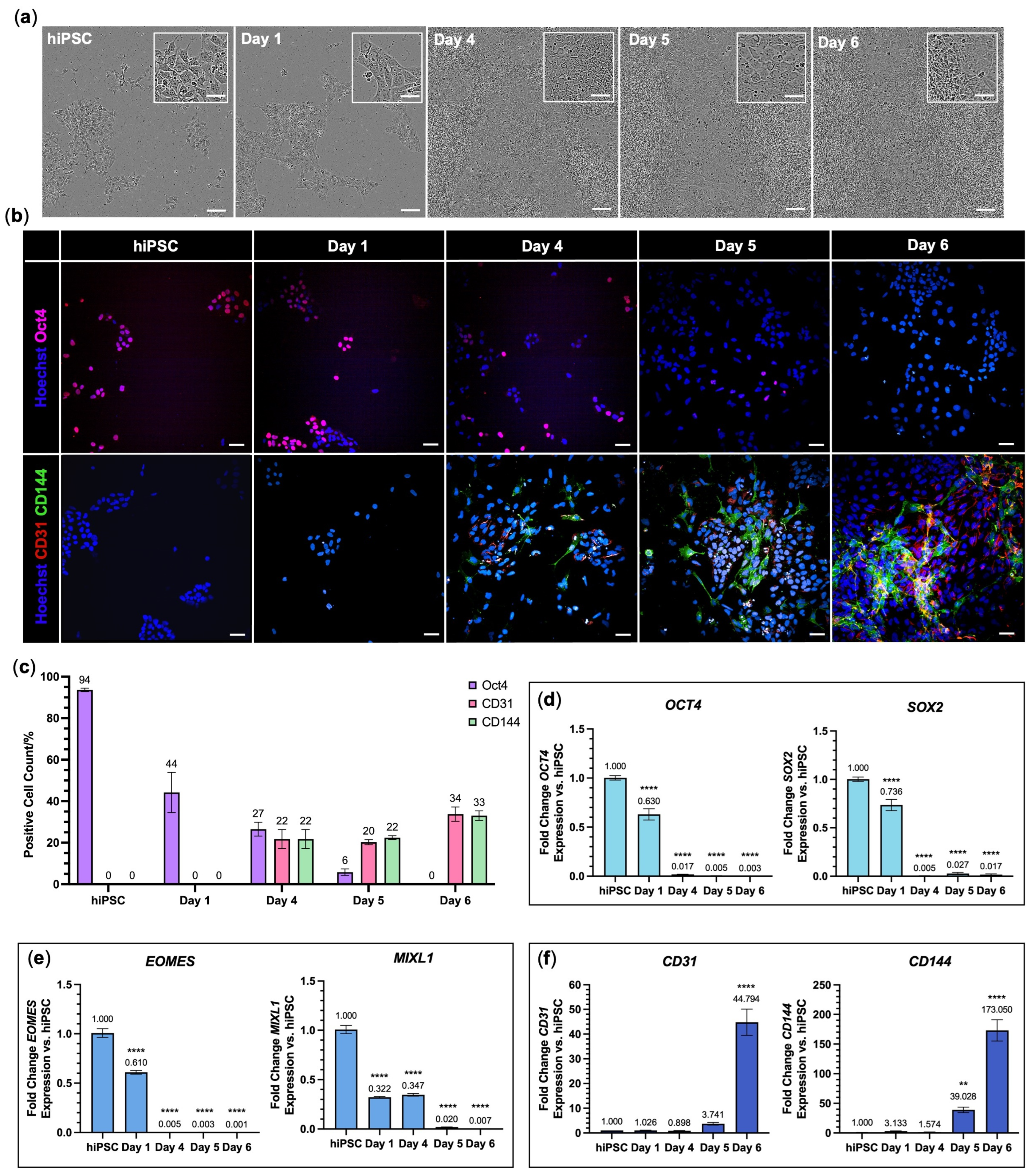
3.1. Endothelial Cells Were Successfully Differentiated from hiPSCs
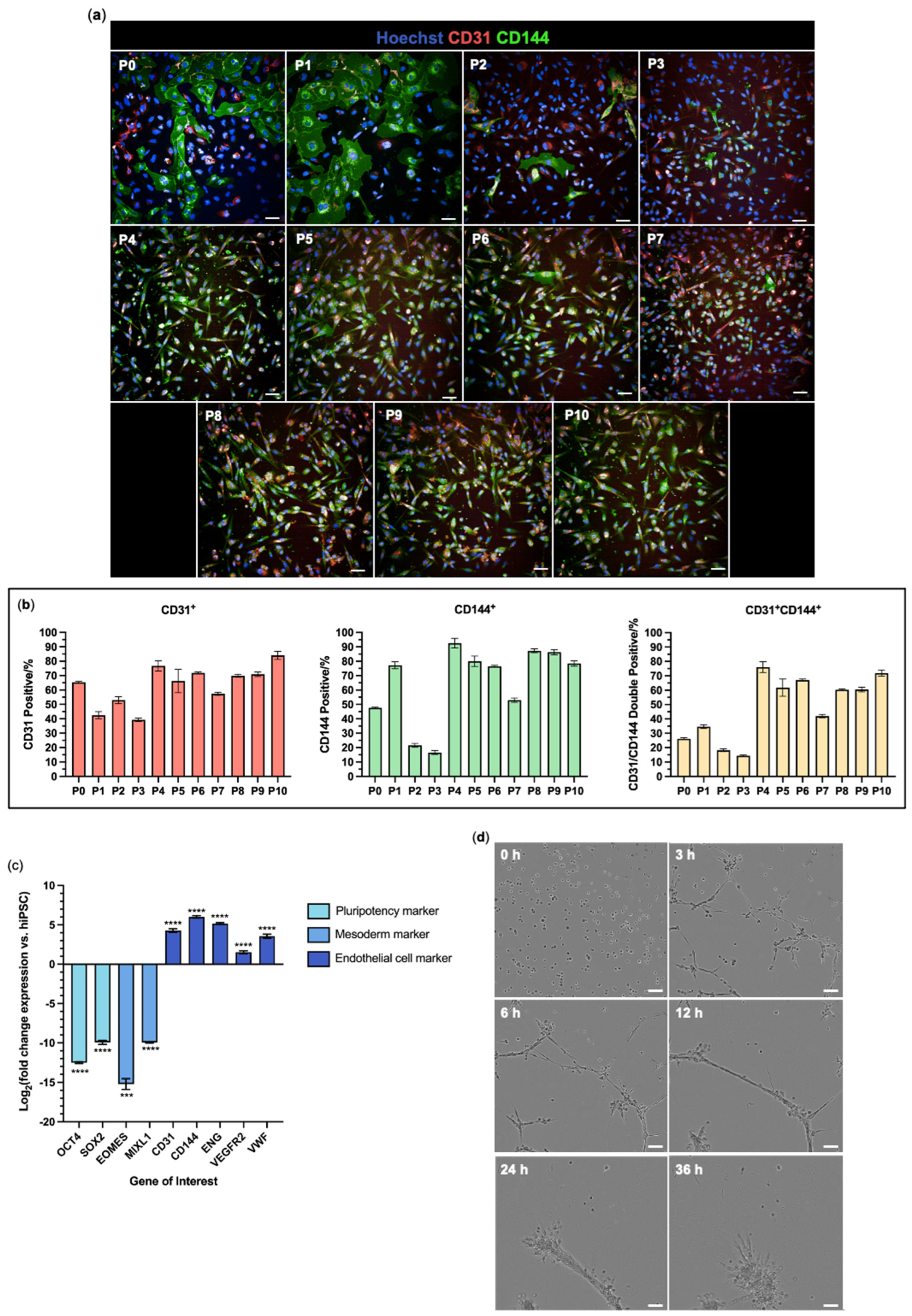
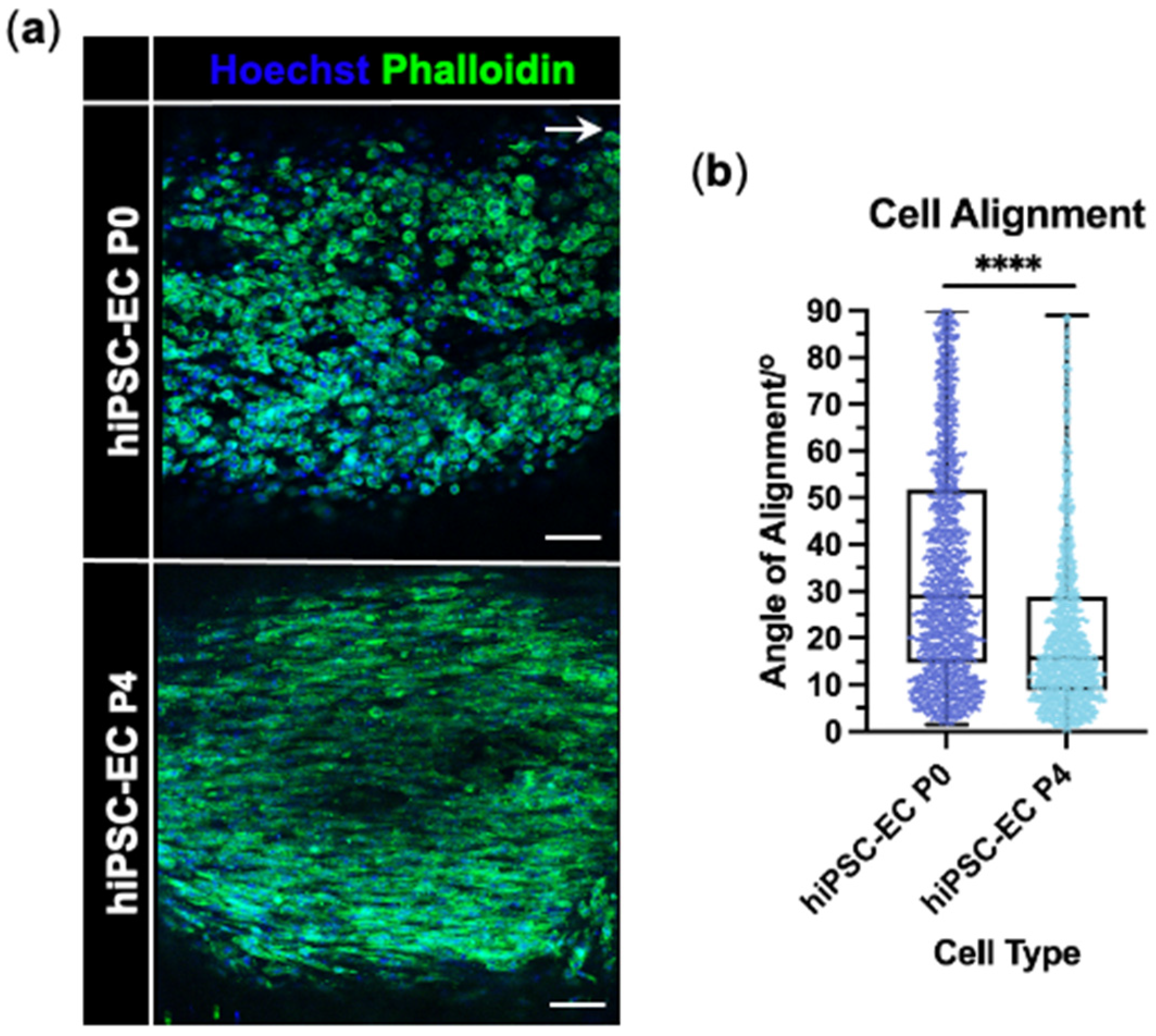
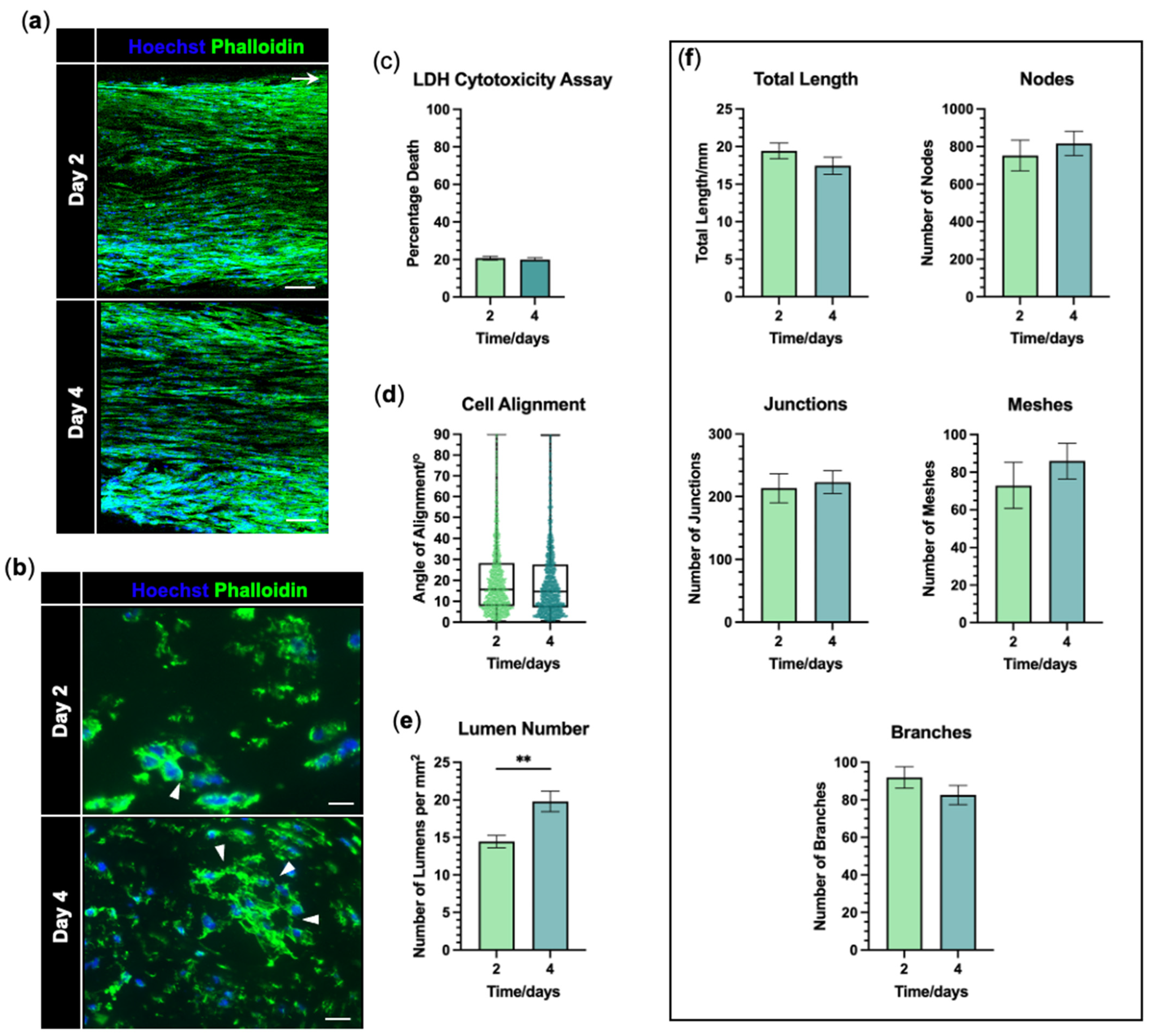
3.2. hiPSC-Derived Endothelial Cells Form Viable and Aligned EngNT
3.3. hiPSC-EC Form Aligned Tube-like Structures Within EngNT
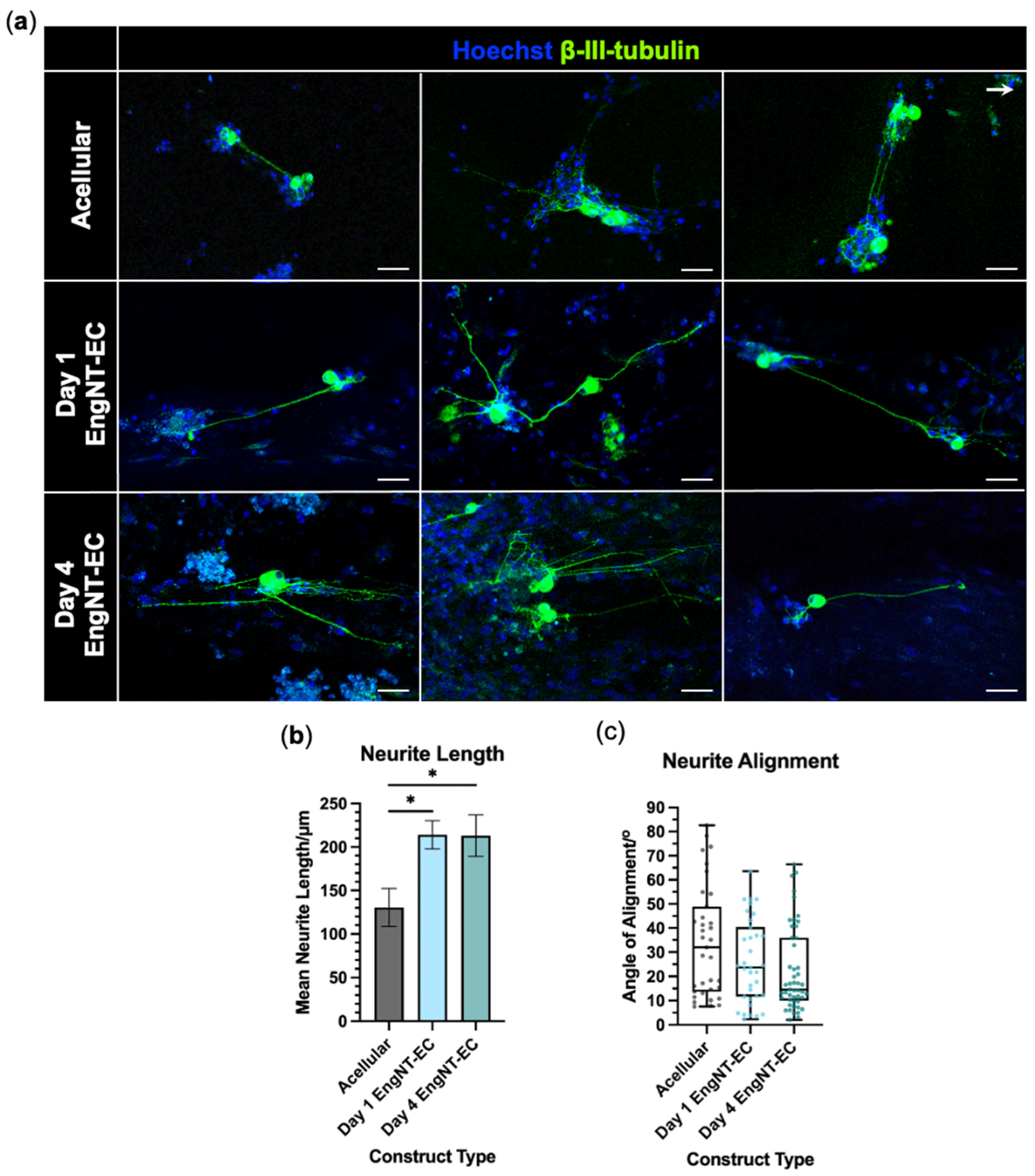
3.4. EngNT-EC Constructs Support Neuronal Outgrowth In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 16G | 16 gauge |
| Ara-C | Cytosine arabinoside |
| ATMP | Advanced therapy medicinal product |
| BDNF | Brain-derived neurotrophic factor |
| BMP4 | Bone morphogenetic protein 4 |
| cDNA | Complementary deoxyribose nucleic acid |
| D-PBS | Dulbecco’s phosphate-buffered saline |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| DRG | Dorsal root ganglion |
| EDTA | Ethylenediaminetetraacetic acid |
| EGM-2 | Endothelial Growth Medium-2 |
| EngNT | Engineered neural tissue |
| EngNT-EC | Engineered neural tissue containing endothelial cells |
| FBS | Foetal bovine serum |
| GAE | Gel aspiration-ejection |
| GDNF | Glial cell line-derived neurotrophic factor |
| GSK-3 | Glycogen synthase kinase-3 |
| hiPSCs | Human induced pluripotent stem cells |
| hiPSC-ECs | Human induced pluripotent stem cell-derived endothelial cells |
| HUVECs | Human umbilical vein endothelial cell |
| LDH | Lactate dehydrogenase |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PDL | Poly-D-lysine |
| Pen-Strep | Penicillin-streptomycin |
| PFA | Paraformaldehyde |
| ROCK | Rho-associated protein kinase |
| TGF-β | Transforming growth factor-β |
| VEGF(A) | Vascular endothelial growth factor (A) |
References
- Murphy, R.N.; de Schoulepnikoff, C.; Chen, J.H.; Columb, M.O.; Bedford, J.; Wong, J.K.; Reid, A.J. The incidence and management of peripheral nerve injury in England (2005–2020). J. Plast. Reconstr. Aesthetic Surg. 2023, 80, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. The history of nerve repair. In Peripheral Nerve Tissue Engineering and Regeneration; Springer: Cham, Switzerland, 2022; pp. 1–32. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Blood vessels and endothelial cells. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Gao, H.; You, Y.; Zhang, G.; Zhao, F.; Sha, Z.; Shen, Y. The use of fiber-reinforced scaffolds cocultured with schwann cells and vascular endothelial cells to repair rabbit sciatic nerve defect with vascularization. BioMed Res. Int. 2013, 2013, 362918. [Google Scholar] [CrossRef]
- Muangsanit, P.; Roberton, V.; Costa, E.; Phillips, J.B. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021, 126, 224–237. [Google Scholar] [CrossRef]
- Thibodeau, A.; Galbraith, T.; Fauvel, C.M.; Khuong, H.T.; Berthod, F. Repair of peripheral nerve injuries using a prevascularized cell-based tissue-engineered nerve conduit. Biomaterials 2022, 280, 121269. [Google Scholar] [CrossRef]
- Muangsanit, P.; Smith, P. Harnessing endothelial cells and vascularization strategies for nerve regeneration. Neural Regen. Res. 2024, 19, 2337–2338. [Google Scholar] [CrossRef]
- Grasman, J.; Kaplan, D. Human endothelial cells secrete neurotropic factors to direct axonal growth of peripheral nerves. Sci. Rep. 2017, 7, 4092. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, G.; Li, S.; Li, J.; Wang, W.; Xue, J.; Wang, Y.; Fang, M.; Zhou, N. Correction: Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J. Nanobiotechnology 2024, 22, 81. [Google Scholar] [CrossRef]
- Cattin, A.-L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Calavia, N.G.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.P.; Maurizio, A.; Motta, A.; Podini, P.; Diprima, S.; Malpighi, C.; Brambilla, I.; Martins, L.; Badaloni, A.; Boselli, D.; et al. Structured wound angiogenesis instructs mesenchymal barrier compartments in the regenerating nerve. Neuron 2024, 112, 209–229.e11. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi, A.O. A novel way of harvesting a large population of endothelial cells for clinical and experimental use. Biomed. Res. Rev. 2018, 2, 120. [Google Scholar] [CrossRef]
- Gulati, R.; Lerman, A.; Simari, R.D. Therapeutic uses of autologous endothelial cells for vascular disease. Clin. Sci. 2005, 109, 27–37. [Google Scholar] [CrossRef]
- Keighron, C.; Lyons, C.J.; Creane, M.; O’Brien, T.; Liew, A. Recent advances in endothelial progenitor cells toward their use in clinical translation. Front. Med. 2018, 5, 354. [Google Scholar] [CrossRef]
- Alexander, M.D.; Sun, Z.; Conrad, M.B.; Cooke, D.L. Assessment of cell yield among different devices for endovascular biopsy to harvest vascular endothelial cells. BioTechniques 2019, 66, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Von Recum, H. Endothelial stem cells and precursors for tissue engineering: Cell source, differentiation, selection, and application. Tissue Eng. Part B Rev. 2008, 14, 133–147. [Google Scholar] [CrossRef]
- Gordon, T.; Eva, P.; Borschel, G.H. Delayed peripheral nerve repair: Methods, including surgical ‘cross-bridging’to promote nerve regeneration. Neural Regen. Res. 2015, 10, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Nakanishi, E.; Yamakado, H.; Sawamoto, N.; Takahashi, J. Allogenic transplantation therapy of iPS cell-derived dopamine progenitors for Parkinson’s disease—Current status of the Kyoto Trial and future perspectives. Park. Relat. Disord. 2025, 135, 107833. [Google Scholar] [CrossRef]
- Phillips, J.B. ‘EngNT’—Engineering live neural tissue for nerve replacement. Emerg. Top. Life Sci. 2021, 5, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced pluripotent stem cells (iPSCs): Molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.-J.; Yoon, Y.-s. Vascular Regeneration with New Sources of Endothelial Cells: Pluripotent Stem Cells and Directly Reprogrammed Cells. Circ. Res. 2019, 124, 29–31. [Google Scholar] [CrossRef]
- Jang, S.; de l’Hortet, A.C.; Soto-Gutierrez, A. Induced pluripotent stem cell–derived endothelial cells: Overview, current advances, applications, and future directions. Am. J. Pathol. 2019, 189, 502–512. [Google Scholar] [CrossRef]
- Navarro, S.A.; Carrillo, E.; Grinan-Lison, C.; Martin, A.; Peran, M.; Marchal, J.A.; Boulaiz, H. Cancer suicide gene therapy: A patent review. Expert. Opin. Ther. Pat. 2016, 26, 1095–1104. [Google Scholar] [CrossRef]
- Hildebrandt, S.; Kampfrath, B.; Fischer, K.; Hildebrand, L.; Haupt, J.; Stachelscheid, H.; Knaus, P. ActivinA induced SMAD1/5 signaling in an iPSC derived EC model of fibrodysplasia ossificans progressiva (FOP) can be rescued by the drug candidate saracatinib. Stem Cell Rev. Rep. 2021, 17, 1039–1052. [Google Scholar] [CrossRef]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial cell tube formation assay for the in vitro study of angiogenesis. JoVE (J. Vis. Exp.) 2014, 91, e51312. [Google Scholar]
- Powell, R.; Phillips, J.B. Engineered Tissues Made from Human iPSC-Derived Schwann Cells for Investigating Peripheral Nerve Regeneration In Vitro. Methods and Protocols. 2021, 2269, 245–254. [Google Scholar]
- Smith, P.O.; Huang, G.; Devries, K.; Nazhat, S.N.; Phillips, J.B. Automated production of nerve repair constructs containing endothelial cell tube-like structures. Biofabrication 2024, 17, 015024. [Google Scholar] [CrossRef]
- Panina, Y.; Germond, A.; Masui, S.; Watanabe, T. Validation of common housekeeping genes as reference for qPCR gene expression analysis during ips reprogramming process. Sci. Rep. 2018, 8, 8716. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Diao, H.; Zhao, L.; Xing, Y.; Zhang, J.; Liu, N.; Yan, Y.; Tian, X.; Sun, W.; Liu, B. Identification of suitable reference genes for real-time quantitative PCR analysis of hydrogen peroxide-treated human umbilical vein endothelial cells. BMC Mol. Biol. 2017, 18, 10. [Google Scholar] [CrossRef]
- Bakhashab, S.; Lary, S.; Ahmed, F.; Schulten, H.-J.; Bashir, A.; Ahmed, F.W.; Al-Malki, A.L.; Jamal, H.S.; Gari, M.A.; Weaver, J.U. Reference genes for expression studies in hypoxia and hyperglycemia models in human umbilical vein endothelial cells. G3 Genes Genomes Genet. 2014, 4, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Martinelli, M.; Courty, J.; Cascone, I. Angiogenesis analyzer for ImageJ. In Proceedings of the 4th ImageJ User and Developer Conference Proceedings, Luxembourg, 24–26 October 2012; pp. 198–201. [Google Scholar]
- Patsch, C.; Challet-Meylan, L.; Thoma, E.C.; Urich, E.; Heckel, T.; O’Sullivan, J.F.; Grainger, S.J.; Kapp, F.G.; Sun, L.; Christensen, K.; et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015, 17, 994–1003. [Google Scholar] [CrossRef]
- Lu, T.M.; Houghton, S.; Magdeldin, T.; Durán, J.G.B.; Minotti, A.P.; Snead, A.; Sproul, A.; Nguyen, D.-H.T.; Xiang, J.; Fine, H.A.; et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc. Natl. Acad. Sci. USA 2021, 118, e2016950118. [Google Scholar] [CrossRef]
- van Duinen, V.; Stam, W.; Mulder, E.; Famili, F.; Reijerkerk, A.; Vulto, P.; Hankemeier, T.; van Zonneveld, A.J. Robust and scalable angiogenesis assay of perfused 3D human iPSC-derived endothelium for anti-angiogenic drug screening. Int. J. Mol. Sci. 2020, 21, 4804. [Google Scholar] [CrossRef]
- Abutaleb, N.O.; Truskey, G.A. Differentiation and characterization of human iPSC-derived vascular endothelial cells under physiological shear stress. STAR Protoc. 2021, 2, 100394. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Narayanan, K.; Leong, M.F.; Wan, A.C. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials 2014, 35, 6006–6014. [Google Scholar] [CrossRef]
- Gu, M. Efficient differentiation of human pluripotent stem cells to endothelial cells. Curr. Protoc. Hum. Genet. 2018, 98, e64. [Google Scholar] [CrossRef]
- Harding, A.; Cortez-Toledo, E.; Magner, N.L.; Beegle, J.R.; Coleal-Bergum, D.P.; Hao, D.; Wang, A.; Nolta, J.A.; Zhou, P. Highly efficient differentiation of endothelial cells from pluripotent stem cells requires the MAPK and the PI3K pathways. Stem Cells 2017, 35, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, T.; Masumoto, H.; Yamamizu, K.; Yoshioka, M.; Minakata, K.; Ikeda, T.; Sakata, R.; Yamashita, J.K. Efficient and robust differentiation of endothelial cells from human induced pluripotent stem cells via lineage control with VEGF and cyclic AMP. PLoS ONE 2017, 12, e0173271. [Google Scholar]
- Lian, X.; Bao, X.; Al-Ahmad, A.; Liu, J.; Wu, Y.; Dong, W.; Dunn, K.K.; Shusta, E.V.; Palecek, S.P. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 2014, 3, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, J.; Xu, X.; Zeisberg, M.; Guan, K.; Zeisberg, E.M. Differentiation of functional endothelial cells from human induced pluripotent stem cells: A novel, highly efficient and cost effective method. Differentiation 2016, 92, 225–236. [Google Scholar] [CrossRef]
- Palpant, N.J.; Pabon, L.; Friedman, C.E.; Roberts, M.; Hadland, B.; Zaunbrecher, R.J.; Bernstein, I.; Zheng, Y.; Murry, C.E. Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat. Protoc. 2017, 12, 15–31. [Google Scholar] [CrossRef]
- Wang, L.; Xiang, M.; Liu, Y.; Sun, N.; Lu, M.; Shi, Y.; Wang, X.; Meng, D.; Chen, S.; Qin, J. Human induced pluripotent stem cells derived endothelial cells mimicking vascular inflammatory response under flow. Biomicrofluidics 2016, 10, 014106. [Google Scholar] [CrossRef]
- Zhang, S.; Dutton, J.R.; Su, L.; Zhang, J.; Ye, L. The influence of a spatiotemporal 3D environment on endothelial cell differentiation of human induced pluripotent stem cells. Biomaterials 2014, 35, 3786–3793. [Google Scholar] [CrossRef]
- Boonkaew, B.; Suwanpitak, S.; Pattanapanyasat, K.; Sermsathanasawadi, N.; Wattanapanitch, M. Efficient generation of endothelial cells from induced pluripotent stem cells derived from a patient with peripheral arterial disease. Cell Tissue Res. 2022, 388, 89–104. [Google Scholar] [CrossRef]
- Pavlova, M.; McGarvey, S.S.; Bilousova, G.; Kogut, I. A high-efficiency method for the production of endothelial cells from human induced pluripotent stem cells. In Induced Pluripotent Stem Cells and Human Disease: Methods and Protocols; Springer: New York, NY, USA, 2021; pp. 169–186. [Google Scholar]
- Song, C.; Wang, L.; Li, Q.; Liao, B.; Qiao, W.; Li, Q.; Dong, N.; Li, L. Generation of individualized immunocompatible endothelial cells from HLA-I-matched human pluripotent stem cells. Stem Cell Res. Ther. 2022, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Muangsanit, P.; Day, A.; Dimiou, S.; Ataç, A.F.; Kayal, C.; Park, H.; Nazhat, S.N.; Phillips, J.B. Rapidly formed stable and aligned dense collagen gels seeded with Schwann cells support peripheral nerve regeneration. J. Neural Eng. 2020, 17, 046036. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.; Fierstein, S.; Purkey, L.; DeCicco-Skinner, K. Endothelial cell tube formation assay: An in vitro model for angiogenesis. In VEGF Signaling: Methods and Protocols; Springer: New York, NY, USA, 2022; pp. 187–196. [Google Scholar]
- Conway, E.M.; Collen, D.; Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef]
- Jakubowska, W.; Chabaud, S.; Saba, I.; Galbraith, T.; Berthod, F.; Bolduc, S. Prevascularized tissue-engineered human vaginal mucosa: In vitro optimization and in vivo validation. Tissue Eng. Part A 2020, 26, 811–822. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, L.; Sun, S.; Xu, J.; Han, Q.; Liu, Y.; Wu, R.; Li, G. Co-culture of Schwann cells and endothelial cells for synergistically regulating dorsal root ganglion behavior on chitosan-based anisotropic topology for peripheral nerve regeneration. Burn. Trauma. 2022, 10, tkac030. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef]
| Antibody | Species | Manufacturer | Dilution | Catalogue |
|---|---|---|---|---|
| CD31 Polyclonal Antibody | Rabbit | Invitrogen, Paisley, UK | 1:200 | PA5-32321 |
| CD144 (VE-cadherin) Monoclonal Antibody (16B1), eBioscience™ | Mouse | Invitrogen, Paisley, UK | 1:200 | 14-1449-82 |
| Oct3/4 Antibody (C-20) | Goat | Santa Cruz, Welwyn Garden City, UK | 1:200 | SC-8629 |
| Alexa Fluor® 488 Anti-beta III tubulin antibody | Mouse | Abcam, Cambridge, UK | 1:500 | ab195879 |
| Horse anti-goat IgG Antibody 594 DyLight® | Horse | VectorLabs, London, UK | 1:200 | DI-3094-1.5 |
| Goat anti-mouse IgG Alexa Fluor™ Plus 488 | Goat | Invitrogen, Paisley, UK | 1:500 | A21121 |
| Goat anti-rabbit IgG Alexa Fluor™ Plus 488 | Goat | Invitrogen, Paisley, UK | 1:500 | A32731 |
| Goat anti-rabbit IgG Alexa Fluor™ Plus 647 | Goat | Invitrogen, Paisley, UK | 1:500 | A32733 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, P.O.; Jat, P.; Phillips, J.B. Endothelial Cells Differentiated from Human Induced Pluripotent Stem Cells Form Aligned Network Structures in Engineered Neural Tissue. J. Funct. Biomater. 2025, 16, 425. https://doi.org/10.3390/jfb16110425
Smith PO, Jat P, Phillips JB. Endothelial Cells Differentiated from Human Induced Pluripotent Stem Cells Form Aligned Network Structures in Engineered Neural Tissue. Journal of Functional Biomaterials. 2025; 16(11):425. https://doi.org/10.3390/jfb16110425
Chicago/Turabian StyleSmith, Poppy O., Parmjit Jat, and James B. Phillips. 2025. "Endothelial Cells Differentiated from Human Induced Pluripotent Stem Cells Form Aligned Network Structures in Engineered Neural Tissue" Journal of Functional Biomaterials 16, no. 11: 425. https://doi.org/10.3390/jfb16110425
APA StyleSmith, P. O., Jat, P., & Phillips, J. B. (2025). Endothelial Cells Differentiated from Human Induced Pluripotent Stem Cells Form Aligned Network Structures in Engineered Neural Tissue. Journal of Functional Biomaterials, 16(11), 425. https://doi.org/10.3390/jfb16110425







