Phase-Pure Hydroxyapatite/β-Tricalcium Phosphate Scaffolds from Ultra-Pure Precursors: Composition Governs Porosity, Strength, and SBF Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HA and β-TCP Powders
2.3. Preparation of BCP Scaffolds
2.4. Characterization
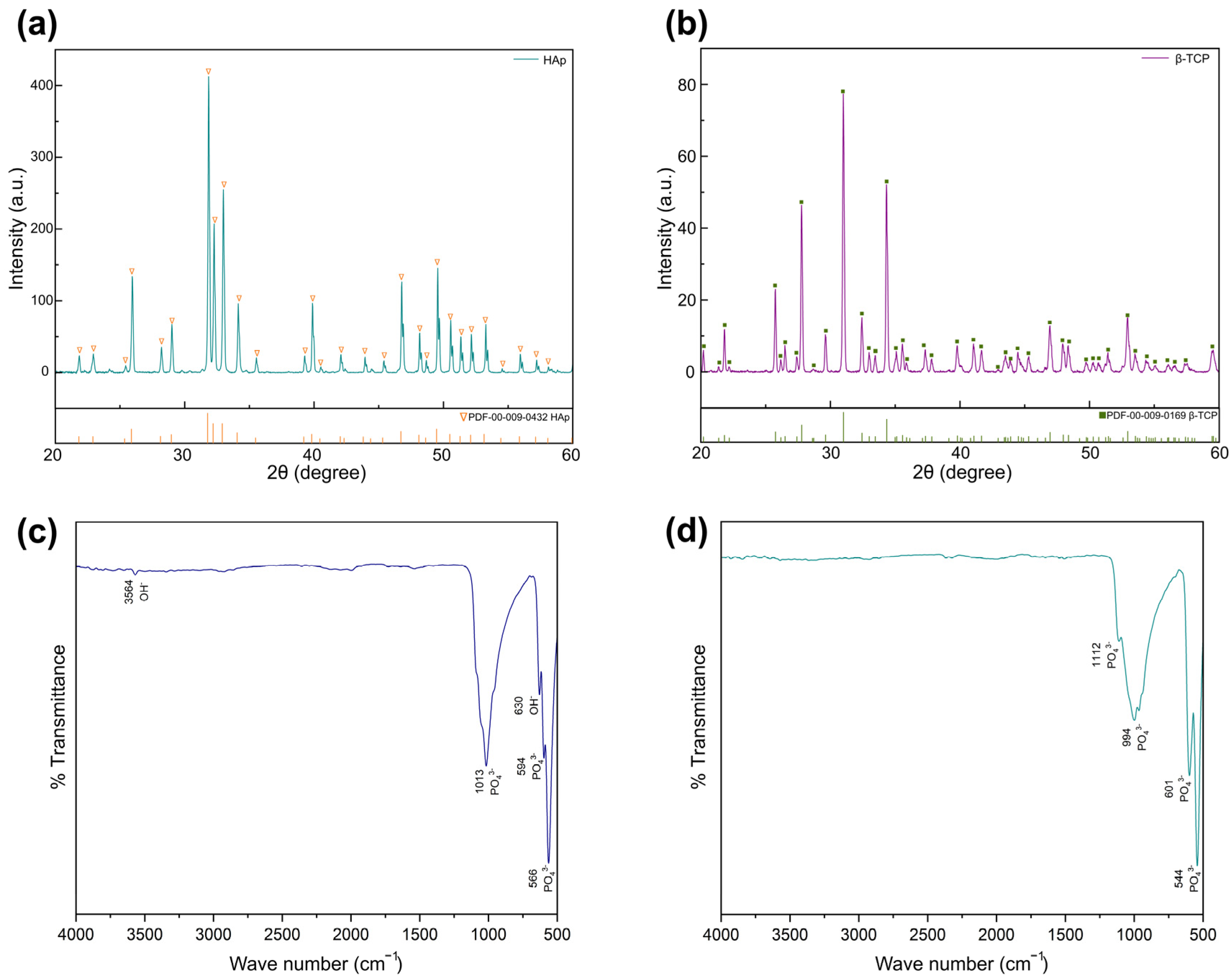
2.4.1. X-Ray Diffractometer (XRD)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
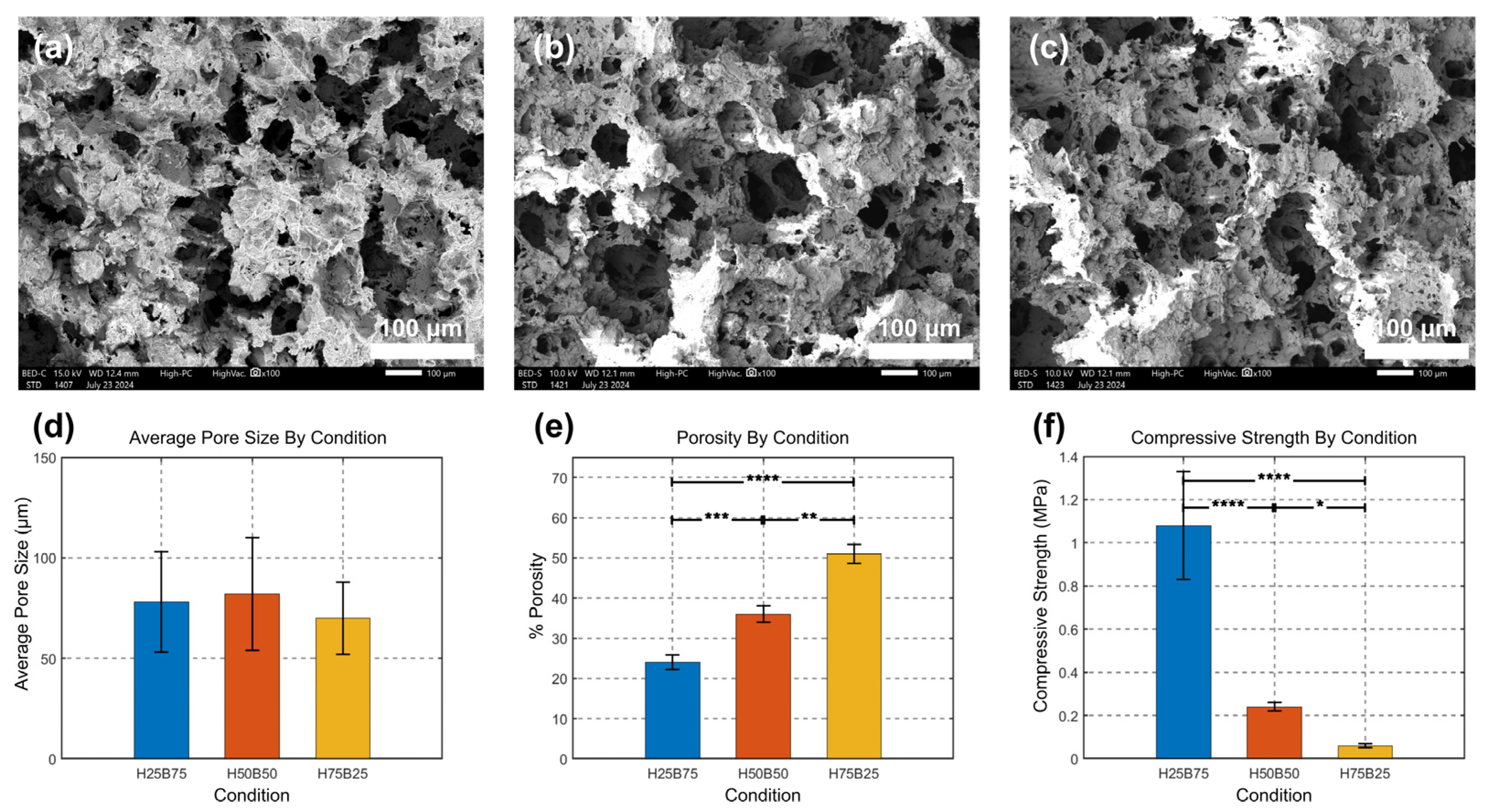
2.4.3. Scanning Electron Microscopy and Image-Based Pore Metrics
2.4.4. Porosity Measurement
2.4.5. Compression Test
2.5. In Vitro Degradation Test in Simulated Body Fluid (SBF)
2.6. Ion Release Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterizations of HA and β-TCP Powders
3.2. Characterization and Morphology of BCP Scaffolds
3.3. Mechanical Properties of BCP Scaffolds
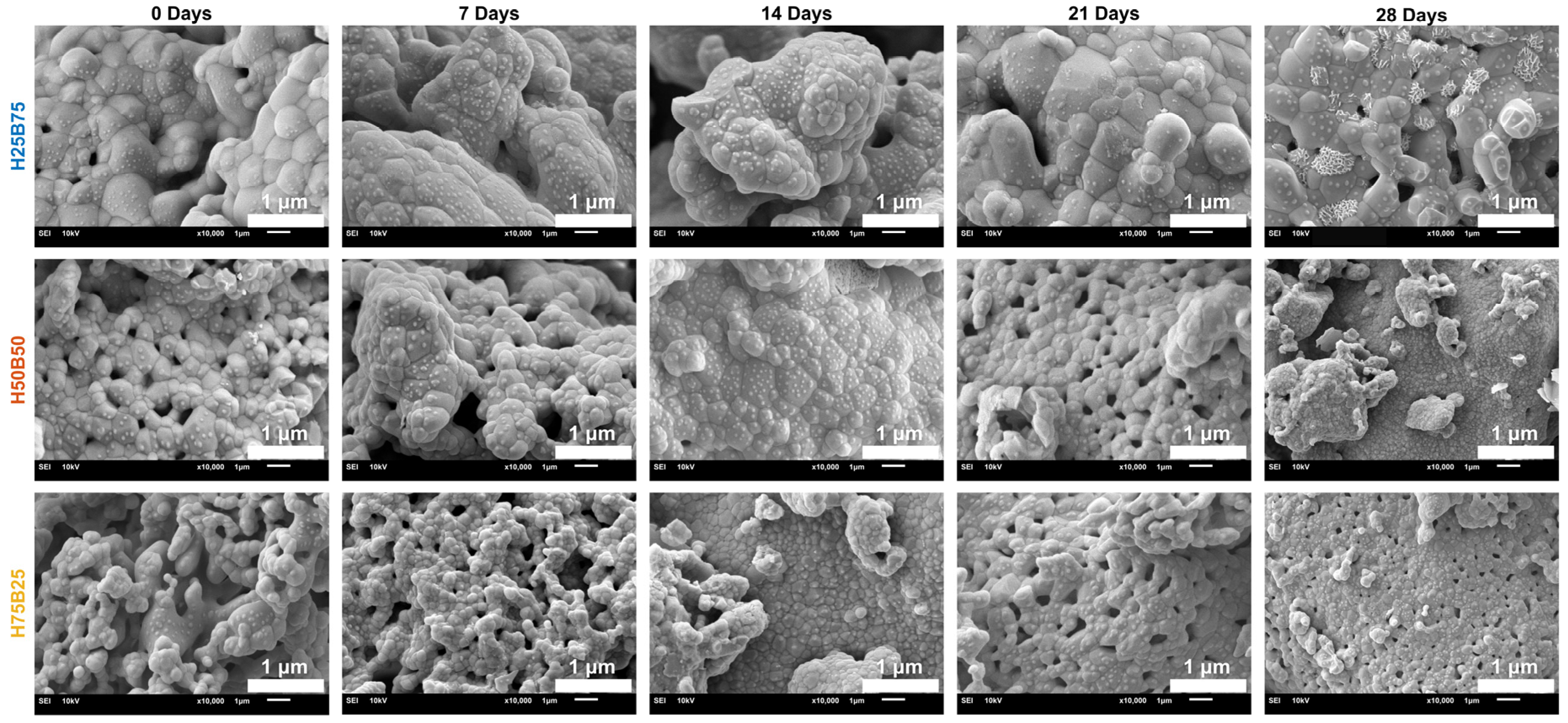
3.4. Degradation Studies
3.4.1. Medium Stability & Gross Morphology in SBF
3.4.2. Degradation Kinetics in SBF
3.4.3. SEM Analysis of BCP Scaffold Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-TCP | Alpha-tricalcium phosphate |
| β-TCP | Beta-tricalcium phosphate |
| APC | Article processing charge |
| ATR | Attenuated total reflectance |
| BCP | Biphasic calcium phosphate |
| BGMN | Rietveld refinement engine/software “BGMN” |
| BSE | Backscattered electron |
| CaP | Calcium phosphate |
| DOI | Digital object identifier |
| FE-SEM | Field-emission scanning electron microscopy |
| FTIR | Fourier transform infrared |
| GBR | Guided bone regeneration |
| HA | Hydroxyapatite |
| HEC | Hydroxyethyl cellulose |
| ICDD | International Centre for Diffraction Data |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| ISO | International Organization for Standardization |
| LoD | limit of detection |
| MIL | Mean intercept length |
| PDF (ICDD) | Powder Diffraction File (ICDD) |
| Rwp | Weighted-profile R-factor |
| ROI | Region of interest |
| SBF | Simulated body fluid |
| SD | Standard deviation |
| SE | Secondary electron |
| SEM | Scanning electron microscopy |
| TTCP | Tetracalcium phosphate |
| XRD | X-ray diffraction |
References
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Sohn, H.-S.; Oh, J.-K. Review of Bone Graft and Bone Substitutes with an Emphasis on Fracture Surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, S81–S98. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Property-Relevant Design Parameters. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Dorcioman, G.; Grumezescu, V. A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards. Nanomaterials 2021, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kuh, S.-U.; Park, J.-Y.; Park, H.-S.; Kim, K.-S.; Chin, D.-K.; Cho, Y.-E. The Influences of Different Ratios of Biphasic Calcium Phosphate and Collagen Augmentation on Posterior Lumbar Spinal Fusion in Rat Model. Yonsei Med. J. 2017, 58, 407–414. [Google Scholar] [CrossRef]
- Al-Harbi, F.; ArRejaie, A. Hydroxyapatite-Based Bone Substitutes in Maxillofacial Reconstruction: A Systematic Review. J. Cranio-Maxillofac. Surg. 2022, 50, 726–736. [Google Scholar]
- Ni, P.; Wang, Y.; Tang, Y.; Cheng, G. Enhancing Bone Repair with β-Tricalcium Phosphate–Based Composite Scaffolds: A Review of Material Design and Biological Performance. Orthop. Res. Rev. 2024, 16, 55–73. [Google Scholar]
- Park, J.Y.; Kim, Y.; Kim, J.H.; Wang, H. Clinical and Histologic Evaluation of 3D-Printed PCL/β-TCP Scaffolds for Alveolar Ridge Preservation in Humans. J. Clin. Med. 2024, 13, 4561. [Google Scholar] [CrossRef]
- Indurkar, A.; Choudhary, R.; Rubenis, K.; Locs, J. Advances in Sintering Techniques for Calcium Phosphates Ceramics. Materials 2021, 14, 6133. [Google Scholar] [CrossRef]
- Trzaskowska, M.; Vivcharenko, V.; Przekora, A. The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties. Int. J. Mol. Sci. 2023, 24, 5083. [Google Scholar] [CrossRef]
- Santoni, B.L.G.; Panova, A.; Chamary, S.; Scholze, M.; Bohner, M. Effects of Minor β-Calcium Pyrophosphate and Hydroxyapatite Impurities on the Properties of β-Tricalcium Phosphate. Bioact. Mater. 2022, 10, 222–235. [Google Scholar] [CrossRef]
- Bohner, M.; Wustmann, N.; Wasilewski, M.; Santoni, B.L.G.; Dey, A.; Cattani-Lorente, M.; Doebelin, N. Reactivity of α-Tricalcium Phosphate Powders Is Affected by Minute Amounts of β-Calcium Pyrophosphate and by the Synthesis Temperature. Open Ceram. 2024, 15, 100647. [Google Scholar] [CrossRef]
- Baino, F.; Yamaguchi, S. Advances in 3D-Printed Scaffolds for Bone Regeneration: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef]
- Wen, J.; Zhai, W.; Lu, H.; Fu, G. Biphasic Calcium Phosphate Scaffolds for Craniofacial Bone Repair: Composition-Dependent Mechanical and Biological Outcomes. Tissue Eng. Part B Rev. 2023, 29, 412–428. [Google Scholar]
- Pandit, A.; Indurkar, A.; Locs, J.; Haugen, H.J.; Loca, D. Calcium Phosphates: A Key to Next-Generation In Vitro Bone Modeling. Adv. Healthc. Mater. 2024, 13, e2401307. [Google Scholar] [CrossRef]
- Döbelin, N. Interlaboratory Study on the Quantification of Calcium Phosphate Phases by Rietveld Refinement. Powder Diffr. 2015, 30, 231–241. [Google Scholar] [CrossRef]
- Döbelin, N. Profex User Manual—Part 2: Using Profex, Version 4.3, 25 January 2021. Available online: https://www.profex-xrd.org/wp-content/uploads/2022/04/Profex-BGMN-Part-2-Application-EN.pdf (accessed on 16 September 2025).
- ISO 23317:2014; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. ISO: Geneva, Switzerland, 2014; (Note: Superseded by ISO 23317:2025).
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Stoch, A.; Jastrzębski, W.; Brożek, A.; Trybalska, B.; Cichocińska, M.; Szarawara, E. FTIR monitoring of the growth of the carbonate containing apatite layers from simulated and natural body fluids. J. Mol. Struct. 1999, 511–512, 287–294. [Google Scholar] [CrossRef]
- Carvalho, G.K.; Farias, J.R.S.; Lima, I.S.; Nascimento, A.M.; Neves, G.A.; Menezes, R.; Osajima, J.A.; Braga, A.; Silva-Filho, E.C. HAp/β-TCP Biphasic Ceramics Obtained by the Pechini Method: An Antibacterial Approach. Minerals 2022, 12, 1482. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, A.H. α-Tricalcium Phosphate: Synthesis, Properties and Biomedical Applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Tronco, M.C.; Cassel, J.B.; dos Santos, L.A. α-TCP-Based Calcium Phosphate Cements: A Critical Review. Acta Biomater. 2022, 151, 70–87. [Google Scholar] [CrossRef]
- Böhme, N.; Hauke, K.; Dohrn, M.; Neuroth, M.; Geisler, T. High-temperature phase transformations of hydroxylap-atite and the formation of silicocarnotite in the hydroxylapatite–quartz–lime system studied in situ and in operan-do by Raman spectroscopy. J. Mater. Sci. 2022, 57, 15239–15266. [Google Scholar] [CrossRef]
- Brown, O.; McAfee, M.; Clarke, S.; Buchanan, F. Sintering of Biphasic Calcium Phosphates. J. Mater. Sci. Mater. Med. 2010, 21, 2271–2279. [Google Scholar] [CrossRef]
- Kondo, Y.; Takahashi, H.; Takahashi, M. Effect of silicon dioxide nanoparticles on the sintering behavior and mechanical properties of β-tricalcium phosphate. Materials 2023, 17, 797. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Liou, S.-P.; Ho, W.-F. Phase Transformation and Mechanical Optimization of Hydroxyapatite Ceramics at Different Sintering Temperatures. Materials 2024, 17, 4062. [Google Scholar] [CrossRef]
- ISO 23317:2025; Implants for Surgery—Materials—Simulated Body Fluid (SBF) Preparation Procedure and Test Method to Detect Apatite Formation in SBF for Initial Screening of Bone-Contacting Implant Materials. 4th ed. ISO: Geneva, Switzerland, 2025.
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and Mechanobiology of Trabecular Bone. J. Biomech. Eng. 2015, 137, 010802–01080215. [Google Scholar] [CrossRef]
- Öhman-Mägi, C.; Holub, O.; Wu, D.; Hall, R.M.; Persson, C. Density and mechanical properties of vertebral trabecular bone—A review. JOR Spine 2021, 4, e1176. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.; Kampleitner, C.; De Lima, J.; Brennan, M.Á.; Lodoso-Torrecilla, I.; Sadowska, J.M.; Blanchard, F.; Canal, C.; Ginebra, M.-P.; Hoffmann, O.; et al. Phase composition of calcium phosphate materials affects bone formation by modulating osteoclastogenesis. Acta Biomater. 2024, 176, 417–431. [Google Scholar] [CrossRef] [PubMed]
- León-Reina, L.; García-Matías, P.R.; Cuberos, A.J.M.; Santacruz, I.; Vallcorba, O.; De la Torre, A.G.; Aranda, M.A.G. Accuracy in Rietveld quantitative phase analysis: A comparative study of strictly monochromatic Mo and Cu radiations. J. Appl. Crystallogr. 2016, 49, 722–735. [Google Scholar] [CrossRef] [PubMed]



| Ion | Concentration (mM) | Compound Source |
|---|---|---|
| Na+ | 142.0 | NaCl |
| K+ | 5.0 | KCl |
| Mg2+ | 1.5 | MgSO4·7H2O |
| Ca2+ | 2.5 | CaCl2·2H2O |
| Cl− | 147.8 | NaCl, KCl, CaCl2·2H2O |
| HCO3− | 4.2 | NaHCO3 |
| HPO42− | 1.0 | K2HPO4 |
| SO42− | 0.5 | MgSO4·7H2O |
| BCP Scaffolds | HA (wt%) | β-TCP (wt%) | α-TCP (wt%) | Rwp (%) | χ2 |
|---|---|---|---|---|---|
| H25B75 | 27.57 | 72.43 | <LoD | 22.29 | 2.10 |
| H50B50 | 68.51 | 31.49 | <LoD | 24.37 | 3.98 |
| H75B25 | 99.26 | 0.74 | <LoD | 20.30 | 4.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monviset, P.; Srirussamee, K.; Khantachawana, A.; Naruphontjirakul, P. Phase-Pure Hydroxyapatite/β-Tricalcium Phosphate Scaffolds from Ultra-Pure Precursors: Composition Governs Porosity, Strength, and SBF Kinetics. J. Funct. Biomater. 2025, 16, 407. https://doi.org/10.3390/jfb16110407
Monviset P, Srirussamee K, Khantachawana A, Naruphontjirakul P. Phase-Pure Hydroxyapatite/β-Tricalcium Phosphate Scaffolds from Ultra-Pure Precursors: Composition Governs Porosity, Strength, and SBF Kinetics. Journal of Functional Biomaterials. 2025; 16(11):407. https://doi.org/10.3390/jfb16110407
Chicago/Turabian StyleMonviset, Panuwat, Kasama Srirussamee, Anak Khantachawana, and Parichart Naruphontjirakul. 2025. "Phase-Pure Hydroxyapatite/β-Tricalcium Phosphate Scaffolds from Ultra-Pure Precursors: Composition Governs Porosity, Strength, and SBF Kinetics" Journal of Functional Biomaterials 16, no. 11: 407. https://doi.org/10.3390/jfb16110407
APA StyleMonviset, P., Srirussamee, K., Khantachawana, A., & Naruphontjirakul, P. (2025). Phase-Pure Hydroxyapatite/β-Tricalcium Phosphate Scaffolds from Ultra-Pure Precursors: Composition Governs Porosity, Strength, and SBF Kinetics. Journal of Functional Biomaterials, 16(11), 407. https://doi.org/10.3390/jfb16110407






