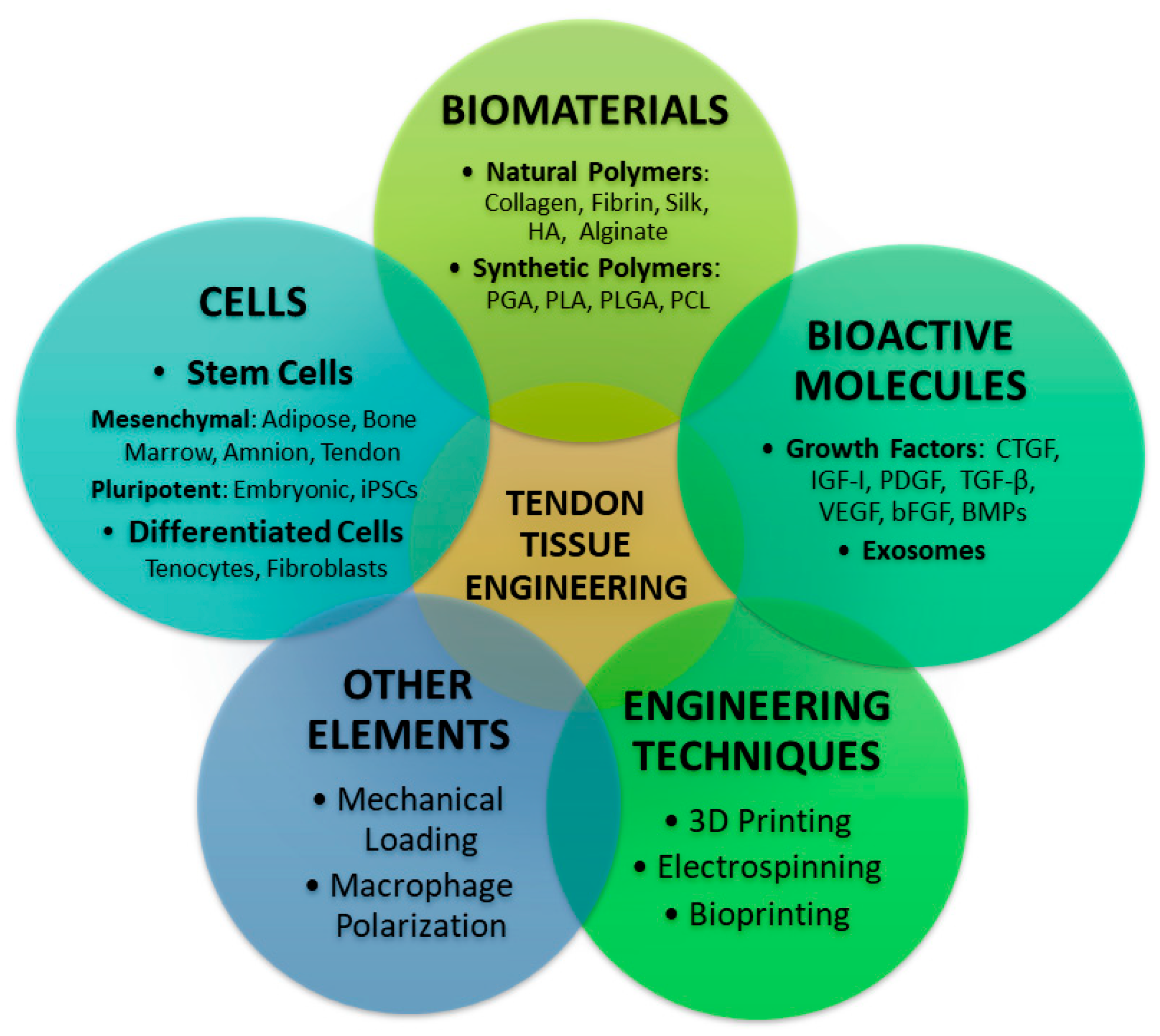
Implications of Tissue Engineering for Tendon Repair and Regeneration
Abstract
1. Introduction
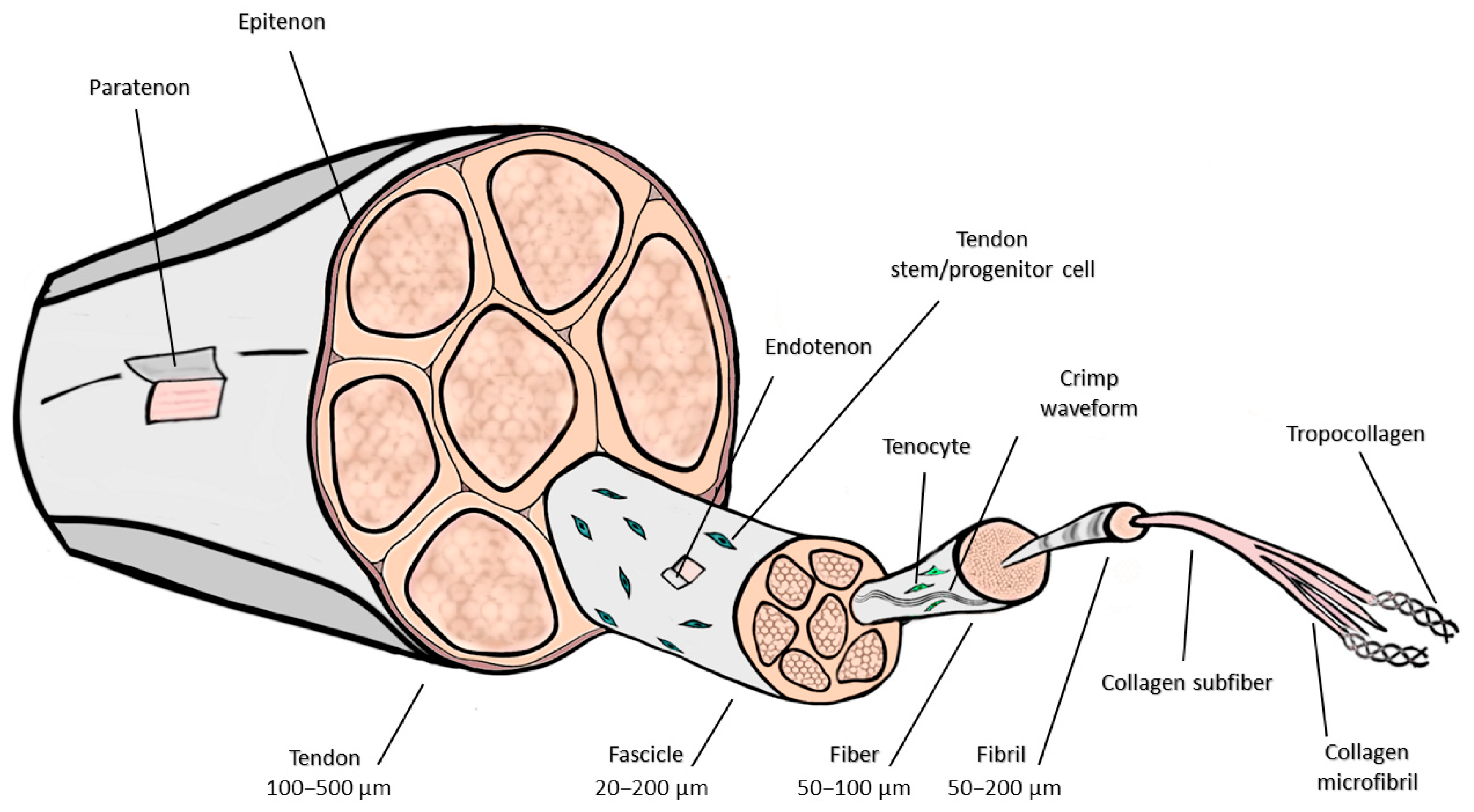
2. Anatomic and Histologic Considerations
3. Cells Involved in Tendon Tissue Engineering
4. Scaffolds Used for Tissue Engineering of the Tendon
4.1. Natural Scaffolds
4.1.1. Collagen
4.1.2. Silk
4.1.3. Fibrin
4.1.4. Chitin
4.1.5. Decellularized Tissues
4.2. Synthetic Scaffolds
4.2.1. Polylactic Acid
4.2.2. Polyglycolic Acid
4.2.3. Poly (Lactic-co-Glycolic) Acid
4.2.4. Polyurethanes
4.2.5. Poly (ε-Caprolactone)
4.3. Hybrid Composites
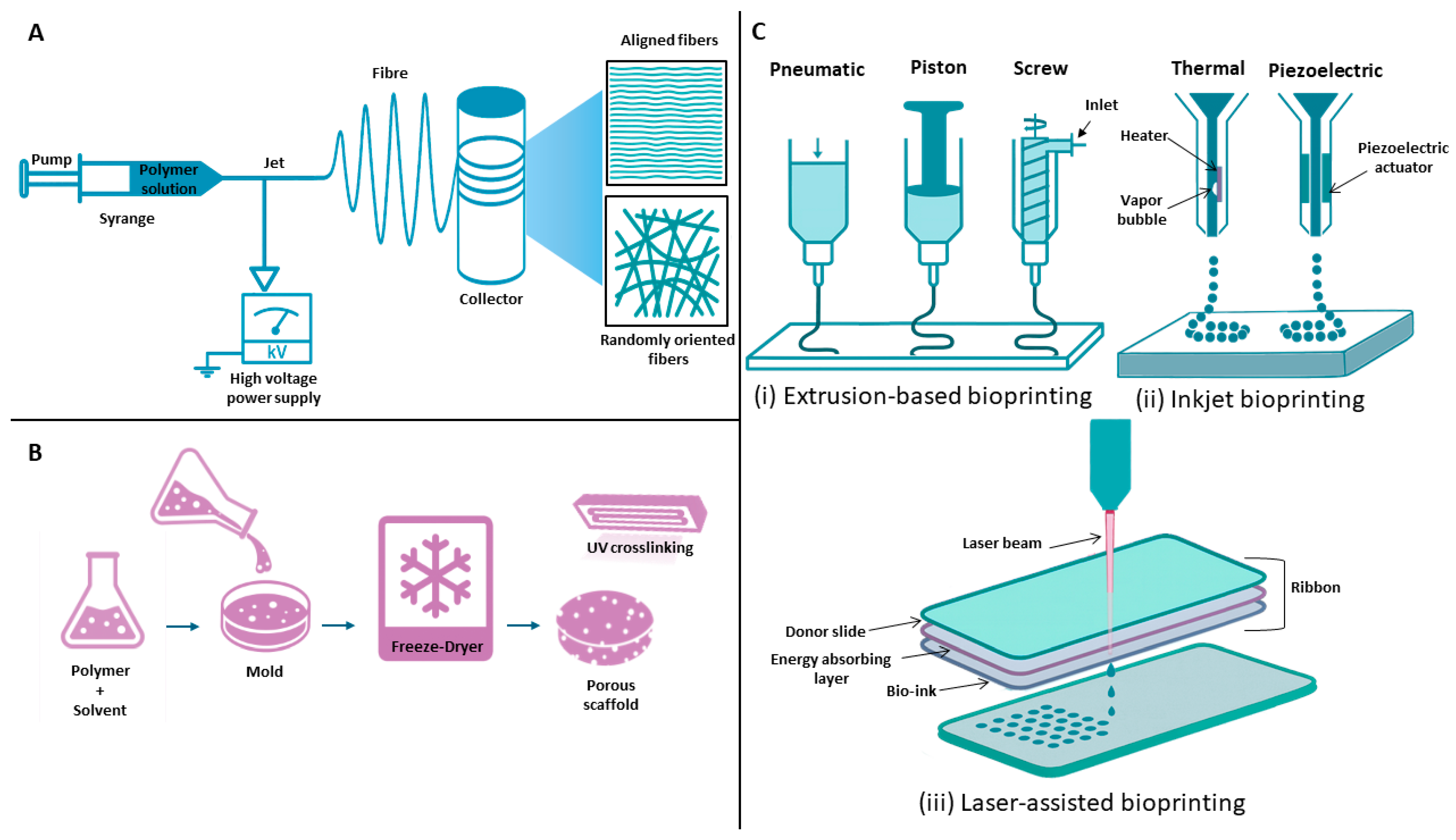
4.4. Additive Manufacturing
3D Bioprinting
4.5. Electrospinning
4.6. Freeze-Drying
5. Discussion
6. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snedeker, J.G.; Foolen, J. Tendon Injury and Repair–A Perspective on the Basic Mechanisms of Tendon Disease and Future Clinical Therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef]
- Federer, A.E.; Steele, J.R.; Dekker, T.J.; Liles, J.L.; Adams, S.B. Tendonitis and Tendinopathy: What Are They and How Do They Evolve? Foot Ankle Clin. 2017, 22, 665–676. [Google Scholar] [CrossRef]
- Bianchi, E.; Ruggeri, M.; Rossi, S.; Vigani, B.; Miele, D.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Innovative Strategies in Tendon Tissue Engineering. Pharmaceutics 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The Cellular Basis of Fibrotic Tendon Healing: Challenges and Opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.; Lafuente-Merchan, M.; Ciriza, J.; Saenz-del-Burgo, L.; Pedraz, J.L. Tendon Tissue Engineering: Cells, Growth Factors, Scaffolds and Production Techniques. J. Control. Release 2021, 333, 448–486. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Black, A.C.; Varacallo, M.A. Anatomy, Tendons. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kannus, P. Structure of the Tendon Connective Tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef]
- Doha, U.; Aydin, O.; Joy, M.S.H.; Emon, B.; Drennan, W.; Saif, M.T.A. Disorder to Order Transition in Cell-ECM Systems Mediated by Cell-Cell Collective Interactions. Acta Biomater. 2022, 154, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Minkwitz, S.; Schmock, A.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B.; Klatte-Schulz, F. Time-Dependent Alterations of MMPs, TIMPs and Tendon Structure in Human Achilles Tendons after Acute Rupture. Int. J. Mol. Sci. 2017, 18, 2199. [Google Scholar] [CrossRef]
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-Function Relationships in Tendons: A Review. J. Anat. 2008, 212, 211–228. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, B.; Li, Y.; Liu, X.; Guo, S.; Wang, C.; Li, S.; Wang, D. The Role of Vascular Endothelial Growth Factor in Tendon Healing. Front. Physiol. 2021, 12, 766080. [Google Scholar] [CrossRef]
- Lee, K.J.; Clegg, P.D.; Comerford, E.J.; Canty-Laird, E.G. A Comparison of the Stem Cell Characteristics of Murine Tenocytes and Tendon-Derived Stem Cells. BMC Musculoskelet. Disord. 2018, 19, 116. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.; Liu, S. Identification and Distinction of Tenocytes and Tendon-Derived Stem Cells. Front. Cell Dev. Biol. 2021, 9, 629515. [Google Scholar] [CrossRef] [PubMed]
- Sevivas, N.; Teixeira, F.G.; Portugal, R.; Direito-Santos, B.; Espregueira-Mendes, J.; Oliveira, F.J.; Silva, R.F.; Sousa, N.; Sow, W.T.; Nguyen, L.T.H.; et al. Mesenchymal Stem Cell Secretome Improves Tendon Cell Viability In Vitro and Tendon-Bone Healing In Vivo When a Tissue Engineering Strategy Is Used in a Rat Model of Chronic Massive Rotator Cuff Tear. Am. J. Sports Med. 2018, 46, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Lohan, A.; Kohl, B.; Meier, C.; Schulze-Tanzil, G. Tenogenesis of Decellularized Porcine Achilles Tendon Matrix Reseeded with Human Tenocytes in the Nude Mice Xenograft Model. Int. J. Mol. Sci. 2018, 19, 2059. [Google Scholar] [CrossRef]
- Karimi, H.; Seyed-Forootan, K.; Karimi, A.-M. Stem Cells and Tendon Regeneration. In Regenerative Medicine and Plastic Surgery: Skin and Soft Tissue, Bone, Cartilage, Muscle, Tendon and Nerves; Springer International Publishing: Cham, Switzerland, 2019; pp. 369–384. [Google Scholar] [CrossRef]
- Mao, X.-F.; Zhang, X.-Q.; Yao, Z.-Y.; Mao, H.-J. Advances in Mesenchymal Stem Cells Therapy for Tendinopathies. Chin. J. Traumatol. 2024, 27, 11–17. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, L.; Song, Y.; Fang, Y.; Liu, J.; Chen, P.; Wang, S.; Wang, C.; Xia, T.; Liu, W.; et al. MSC-Derived Immunomodulatory Extracellular Matrix Functionalized Electrospun Fibers for Mitigating Foreign-Body Reaction and Tendon Adhesion. Acta Biomater. 2021, 133, 280–296. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Zhang, G.; Yu, W.; He, Y. Adipose Stem Cell-Derived Exosomes Ameliorate Chronic Rotator Cuff Tendinopathy by Regulating Macrophage Polarization: From a Mouse Model to a Study in Human Tissue. Am. J. Sports Med. 2021, 49, 2321–2331. [Google Scholar] [CrossRef]
- Ragni, E.; Papait, A.; Perucca Orfei, C.; Silini, A.R.; Colombini, A.; Viganò, M.; Libonati, F.; Parolini, O.; de Girolamo, L. Amniotic Membrane-mesenchymal Stromal Cells Secreted Factors and Extracellular Vesicle-miRNAs: Anti-inflammatory and Regenerative Features for Musculoskeletal Tissues. Stem Cells Transl. Med. 2021, 10, 1044–1062. [Google Scholar] [CrossRef]
- McClellan, P.; Ina, J.G.; Knapik, D.M.; Isali, I.; Learn, G.; Valente, A.; Wen, Y.; Wen, R.; Anderson, J.M.; Gillespie, R.J.; et al. Mesenchymal Stem Cell Delivery via Topographically Tenoinductive Collagen Biotextile Enhances Regeneration of Segmental Tendon Defects. Am. J. Sports Med. 2022, 50, 2281–2291. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive Polymeric Scaffolds for Tissue Engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Jiang, Q.; He, S.; Wang, B.; Yi, Q.; Cheng, X.; Gao, X.; Bai, Y. Structure, Ingredient, and Function-Based Biomimetic Scaffolds for Accelerated Healing of Tendon-Bone Interface. J. Orthop. Transl. 2024, 48, 70–88. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, T.; Tang, C.; Chen, Y.; Liao, Y.; Ju, W.; Zhang, H.; Zhou, B.; Liang, R.; Zhang, T.; et al. 3D Printing of Chemical-Empowered Tendon Stem/Progenitor Cells for Functional Tissue Repair. Biomaterials 2021, 271, 120722. [Google Scholar] [CrossRef]
- Xuan, H.; Zhang, Z.; Jiang, W.; Li, N.; Sun, L.; Xue, Y.; Guan, H.; Yuan, H. Dual-Bioactive Molecules Loaded Aligned Core-Shell Microfibers for Tendon Tissue Engineering. Colloids Surf. B Biointerfaces 2023, 228, 113416. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, L.; Chen, H.; Wang, M.; Jin, L.; Zhou, S.; Gao, L.; Li, R.; Li, Q.; Wang, H.; et al. Biomimetic Scaffolds for Tendon Tissue Regeneration. Biomimetics 2023, 8, 246. [Google Scholar] [CrossRef]
- Später, T.; Del Rio, P.; Shelest, O.; Wechsler, J.T.; Kaneda, G.; Chavez, M.; Sheyn, J.; Yu, V.; Metzger, W.; Huang, D.; et al. Collagen Scaffold-Seeded ITenocytes Accelerate the Healing and Functional Recovery of Achilles Tendon Defects in a Rat Model. Front. Bioeng. Biotechnol. 2024, 12, 1407729. [Google Scholar] [CrossRef]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef] [PubMed]
- Loiacono, C.; Palermi, S.; Massa, B.; Belviso, I.; Romano, V.; Di Gregorio, A.; Sirico, F.; Sacco, A.M. Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina 2019, 55, 447. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.; Kawamura, R.; Suzuki, T.; Matsumoto, T. Rapid Fabrication of Tendon-like Collagen Gel via Simultaneous Fibre Alignment and Intermolecular Cross-Linking under Mechanical Loading. Biomed. Mater. 2022, 17, 045018. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Yang, S.; Shi, X.; Li, X.; Wang, J.; Wang, Y.; Luo, Y. Oriented Collagen Fiber Membranes Formed through Counter-Rotating Extrusion and Their Application in Tendon Regeneration. Biomaterials 2019, 207, 61–75. [Google Scholar] [CrossRef]
- Bianchi, E.; Vigani, B.; Ruggeri, M.; Del Favero, E.; Ricci, C.; Grisoli, P.; Ferraretto, A.; Rossi, S.; Viseras, C.; Sandri, G. Electrospun Scaffolds Based on Poly(Butyl Cyanoacrylate) for Tendon Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3172. [Google Scholar] [CrossRef]
- Caralt, M.; Uzarski, J.S.; Iacob, S.; Obergfell, K.P.; Berg, N.; Bijonowski, B.M.; Kiefer, K.M.; Ward, H.H.; Wandinger-Ness, A.; Miller, W.M.; et al. Optimization and Critical Evaluation of Decellularization Strategies to Develop Renal Extracellular Matrix Scaffolds as Biological Templates for Organ Engineering and Transplantation. Am. J. Transpl. 2015, 15, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Roßbach, B.P.; Gülecyüz, M.F.; Kempfert, L.; Pietschmann, M.F.; Ullamann, T.; Ficklscherer, A.; Niethammer, T.R.; Zhang, A.; Klar, R.M.; Müller, P.E. Rotator Cuff Repair With Autologous Tenocytes and Biodegradable Collagen Scaffold: A Histological and Biomechanical Study in Sheep. Am. J. Sports Med. 2020, 48, 450–459. [Google Scholar] [CrossRef]
- Sindhi, K.; Pingili, R.B.; Beldar, V.; Bhattacharya, S.; Rahaman, J.; Mukherjee, D. The Role of Biomaterials-Based Scaffolds in Advancing Skin Tissue Construct. J. Tissue Viability 2025, 34, 100858. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Harley, B.A.C. The Effect of Anisotropic Collagen-GAG Scaffolds and Growth Factor Supplementation on Tendon Cell Recruitment, Alignment, and Metabolic Activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, S.; Zhou, Q.; Mo, X.; Song, L.; Hou, T.; Wu, J.; Li, S.; Li, Y.; Li, P.; et al. The Effect of Mechanical Stimulation on the Maturation of TDSCs-Poly(L-Lactide-Co-e-Caprolactone)/Collagen Scaffold Constructs for Tendon Tissue Engineering. Biomaterials 2014, 35, 2760–2772. [Google Scholar] [CrossRef]
- Puetzer, J.L.; Ma, T.; Sallent, I.; Gelmi, A.; Stevens, M.M. Driving Hierarchical Collagen Fiber Formation for Functional Tendon, Ligament, and Meniscus Replacement. Biomaterials 2021, 269, 120527. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, B.; Wang, X.; Zhou, G.; Zhang, W.; Yi, B.; Wang, W.; Liu, W. Synergistic Effects of Mechanical Stimulation and Crimped Topography to Stimulate Natural Collagen Development for Tendon Engineering. Acta Biomater. 2022, 145, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Madappura, A.P.; Madduri, S. A Comprehensive Review of Silk-Fibroin Hydrogels for Cell and Drug Delivery Applications in Tissue Engineering and Regenerative Medicine. Comput. Struct. Biotechnol. J. 2023, 21, 4868–4886. [Google Scholar] [CrossRef]
- Yao, D.; Liu, H.; Fan, Y. Silk Scaffolds for Musculoskeletal Tissue Engineering. Exp. Biol. Med. 2016, 241, 238–245. [Google Scholar] [CrossRef]
- Kardestuncer, T.; McCarthy, M.B.; Karageorgiou, V.; Kaplan, D.; Gronowicz, G. RGD-Tethered Silk Substrate Stimulates the Differentiation of Human Tendon Cells. Clin. Orthop. Relat. Res. 2006, 448, 234–239. [Google Scholar] [CrossRef]
- Chen, J.; Mo, Q.; Sheng, R.; Zhu, A.; Ling, C.; Luo, Y.; Zhang, A.; Chen, Z.; Yao, Q.; Cai, Z.; et al. The Application of Human Periodontal Ligament Stem Cells and Biomimetic Silk Scaffold for in Situ Tendon Regeneration. Stem Cell Res. Ther. 2021, 12, 596. [Google Scholar] [CrossRef]
- Qian, S.; Wang, Z.; Zheng, Z.; Ran, J.; Zhu, J.; Chen, W. A Collagen and Silk Scaffold for Improved Healing of the Tendon and Bone Interface in a Rabbit Model. Med. Sci. Monit. 2019, 25, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dan, X.; Chen, H.; Li, T.; Liu, B.; Ju, Y.; Li, Y.; Lei, L.; Fan, X. Developing Fibrin-Based Biomaterials/Scaffolds in Tissue Engineering. Bioact. Mater. 2024, 40, 597–623. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Xie, X.; Xu, J.; Ding, D.; Lin, J.; Han, K.; Wang, C.; Wang, F.; Zhao, J.; Wang, L. Janus Membranes Patch Achieves High-Quality Tendon Repair: Inhibiting Exogenous Healing and Promoting Endogenous Healing. Nano Lett 2024, 24, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, S.; Kuss, M.; Kong, Y.; Shi, W.; Streubel, P.N.; Li, T.; Duan, B. 3D Printing of Multilayered Scaffolds for Rotator Cuff Tendon Regeneration. Bioact. Mater. 2020, 5, 636–643. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, H.; Huang, J.; Wang, Z.; Zhang, T.; Hu, X.; He, Q.; Wang, Z.; Fei, Y.; Zhang, Y.; et al. Tissue-Engineered Bicipital Autologous Tendon Patch Enhances Massive Rotator Cuff Defect Repair in a Rabbit Infraspinatus Tendon Defect Model. Clin. Orthop. Relat. Res. 2024, 482, 2239–2255. [Google Scholar] [CrossRef] [PubMed]
- Izadi, H.; Asadi, H.; Bemani, M. Chitin: A Comparison between Its Main Sources. Front. Mater. 2025, 12, 1537067. [Google Scholar] [CrossRef]
- Sekerci, R.; Ogut, E.; Istil, K.A.; Kilicaslan, O.F.; Acar, N.; Keles-Celik, N. A Time-Dependent Evaluation of Chitosan and Therapeutic Ultrasound Treatments on Achilles Tendon Healing: Assessing Their Effects on Tendon Repair, Collagen Synthesis, and Cellular Regeneration. Bratisl. Med. J. 2025, 126, 1716–1731. [Google Scholar] [CrossRef]
- Li, X.; Feng, Q.; Wang, W.; Cui, F. Chemical Characteristics and Cytocompatibility of Collagen-Based Scaffold Reinforced by Chitin Fibers for Bone Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 219–226. [Google Scholar] [CrossRef]
- Fan, J.; Abedi-Dorcheh, K.; Sadat Vaziri, A.; Kazemi-Aghdam, F.; Rafieyan, S.; Sohrabinejad, M.; Ghorbani, M.; Rastegar Adib, F.; Ghasemi, Z.; Klavins, K.; et al. A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers 2022, 14, 2097. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin Scaffolds in Tissue Engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized Extracellular Matrix Scaffolds: Recent Trends and Emerging Strategies in Tissue Engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Jiang, S.; Zhuang, Y.; Cai, M.; Wang, X.; Lin, K. Decellularized Extracellular Matrix: A Promising Strategy for Skin Repair and Regeneration. Eng. Regen. 2023, 4, 357–374. [Google Scholar] [CrossRef]
- Ratcliffe, A.; Butler, D.L.; Dyment, N.A.; Cagle, P.J.; Proctor, C.S.; Ratcliffe, S.S.; Flatow, E.L. Scaffolds for Tendon and Ligament Repair and Regeneration. Ann. Biomed. Eng. 2015, 43, 819–831. [Google Scholar] [CrossRef]
- Cui, J.; Ning, L.-J.; Wu, F.-P.; Hu, R.-N.; Li, X.; He, S.-K.; Zhang, Y.-J.; Luo, J.-J.; Luo, J.-C.; Qin, T.-W. Biomechanically and Biochemically Functional Scaffold for Recruitment of Endogenous Stem Cells to Promote Tendon Regeneration. npj Regen. Med. 2022, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Lun, W.; Wang, H.; Li, M.; Ma, J.; Ding, Y.; Zheng, X.; Cao, X.; Li, Q. Fabrication of MnO2-Modified Decellularized Tendon Membrane for Enhancing Tendon Repair. Adv. Healthc. Mater. 2025, 14, e2402584. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Shiroud Heidari, B.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, Synthetic and Commercially-Available Biopolymers Used to Regenerate Tendons and Ligaments. Bioact. Mater. 2023, 19, 179–197. [Google Scholar] [CrossRef]
- Brebels, J.; Mignon, A. Polymer-Based Constructs for Flexor Tendon Repair: A Review. Polymers 2022, 14, 867. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Theisen, C.; Fuchs-Winkelmann, S.; Knappstein, K.; Efe, T.; Schmitt, J.; Paletta, J.R.J.; Schofer, M.D. Influence of Nanofibers on Growth and Gene Expression of Human Tendon Derived Fibroblast. Biomed. Eng. Online 2010, 9, 9. [Google Scholar] [CrossRef]
- Karp, J.M.; Yeo, Y.; Geng, W.; Cannizarro, C.; Yan, K.; Kohane, D.S.; Vunjak-Novakovic, G.; Langer, R.S.; Radisic, M. A Photolithographic Method to Create Cellular Micropatterns. Biomaterials 2006, 27, 4755–4764. [Google Scholar] [CrossRef] [PubMed]
- Inui, A.; Kokubu, T.; Makino, T.; Nagura, I.; Toyokawa, N.; Sakata, R.; Kotera, M.; Nishino, T.; Fujioka, H.; Kurosaka, M. Potency of Double-Layered Poly L-Lactic Acid Scaffold in Tissue Engineering of Tendon Tissue. Int. Orthop. 2010, 34, 1327–1332. [Google Scholar] [CrossRef]
- Maduka, C.V.; Alhaj, M.; Ural, E.; Habeeb, O.M.; Kuhnert, M.M.; Smith, K.; Makela, A.V.; Pope, H.; Chen, S.; Hix, J.M.; et al. Polylactide Degradation Activates Immune Cells by Metabolic Reprogramming. Adv. Sci. 2023, 10, e2304632. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Nishimoto, H.; Kokubu, T.; Inui, A.; Mifune, Y.; Nishida, K.; Fujioka, H.; Yokota, K.; Hiwa, C.; Kurosaka, M. Ligament Regeneration Using an Absorbable Stent-Shaped Poly-L-Lactic Acid Scaffold in a Rabbit Model. Int. Orthop. 2012, 36, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Stodolak-Zych, E.; Ficek, K.; Wieczorek, J.; Kajor, M.; Gryń, K.; Rapacz-Kmita, A.; Rajca, J.; Kosenyuk, Y.; Stolarz, M.; Błażewicz, S. Assessment of Sheep Knee Joint after ACL Replacement with Achilles Tendon Autograft and PLA-Based Implant. J. Mech. Behav. Biomed. Mater. 2022, 125, 104923. [Google Scholar] [CrossRef]
- Sensini, A.; Gualandi, C.; Focarete, M.L.; Belcari, J.; Zucchelli, A.; Boyle, L.; Reilly, G.C.; Kao, A.P.; Tozzi, G.; Cristofolini, L. Multiscale Hierarchical Bioresorbable Scaffolds for the Regeneration of Tendons and Ligaments. Biofabrication 2019, 11, 035026. [Google Scholar] [CrossRef]
- Heckmann, L.; Fiedler, J.; Mattes, T.; Dauner, M.; Brenner, R.E. Interactive Effects of Growth Factors and Three-Dimensional Scaffolds on Multipotent Mesenchymal Stromal Cells. Biotechnol. Appl. Biochem. 2008, 49, 185–194. [Google Scholar] [CrossRef]
- Maurus, P.B.; Kaeding, C.C. Bioabsorbable Implant Material Review. Oper. Tech. Sports Med. 2004, 12, 158–160. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.; Li, L. A Review on Biodegradable Polymeric Materials for Bone Tissue Engineering Applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Lu, H.H.; Cooper, J.A.; Manuel, S.; Freeman, J.W.; Attawia, M.A.; Ko, F.K.; Laurencin, C.T. Anterior Cruciate Ligament Regeneration Using Braided Biodegradable Scaffolds: In Vitro Optimization Studies. Biomaterials 2005, 26, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly(Lactic-Co-Glycolic Acid): Applications and Future Prospects for Periodontal Tissue Regeneration. Polymers 2017, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Qi, Y.; Xiao, S.; Ran, J.; Wang, J.; Ghamor-Amegavi, E.P.; Zhou, X.; Li, H.; He, T.; Gou, Z.; et al. Integration of Mesenchymal Stem Cell Sheet and BFGF-Loaded Fibrin Gel in Knitted PLGA Scaffolds Favorable for Tendon Repair. J. Mater. Chem. B 2019, 7, 2201–2211. [Google Scholar] [CrossRef]
- Sahoo, S.; Toh, S.L.; Goh, J.C.H. A BFGF-Releasing Silk/PLGA-Based Biohybrid Scaffold for Ligament/Tendon Tissue Engineering Using Mesenchymal Progenitor Cells. Biomaterials 2010, 31, 2990–2998. [Google Scholar] [CrossRef]
- Han, L.; Hu, Y.-G.; Jin, B.; Xu, S.-C.; Zheng, X.; Fang, W.-L. Sustained BMP-2 Release and Platelet Rich Fibrin Synergistically Promote Tendon-Bone Healing after Anterior Cruciate Ligament Reconstruction in Rat. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8705–8712. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Lipner, J.; Manning, C.N.; Schwartz, A.G.; Thomopoulos, S.; Xia, Y. “Aligned-to-Random” Nanofiber Scaffolds for Mimicking the Structure of the Tendon-to-Bone Insertion Site. Nanoscale 2010, 2, 923–926. [Google Scholar] [CrossRef]
- Rothrauff, B.B.; Lauro, B.B.; Yang, G.; Debski, R.E.; Musahl, V.; Tuan, R.S. Braided and Stacked Electrospun Nanofibrous Scaffolds for Tendon and Ligament Tissue Engineering. Tissue Eng. Part A 2017, 23, 378–389. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, F.; Saris, D.B.F.; Creemers, L.B.; Riesle, J.; Willems, W.J.; van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J.A. The Effect of Timing of Mechanical Stimulation on Proliferation and Differentiation of Goat Bone Marrow Stem Cells Cultured on Braided PLGA Scaffolds. Tissue Eng. Part A 2008, 14, 1425–1433. [Google Scholar] [CrossRef]
- Santos, M.; Mariz, M.; Tiago, I.; Alarico, S.; Ferreira, P. Bio-Based Polyurethane Foams: Feedstocks, Synthesis, and Applications. Biomolecules 2025, 15, 680. [Google Scholar] [CrossRef]
- Pedersen, D.D.; Kim, S.; Wagner, W.R. Biodegradable Polyurethane Scaffolds in Regenerative Medicine: Clinical Translation Review. J. Biomed. Mater. Res. A 2022, 110, 1460–1487. [Google Scholar] [CrossRef]
- Encalada-Diaz, I.; Cole, B.J.; Macgillivray, J.D.; Ruiz-Suarez, M.; Kercher, J.S.; Friel, N.A.; Valero-Gonzalez, F. Rotator Cuff Repair Augmentation Using a Novel Polycarbonate Polyurethane Patch: Preliminary Results at 12 Months’ Follow-Up. J. Shoulder Elb. Surg. 2011, 20, 788–794. [Google Scholar] [CrossRef]
- Webb, K.; Hitchcock, R.W.; Smeal, R.M.; Li, W.; Gray, S.D.; Tresco, P.A. Cyclic Strain Increases Fibroblast Proliferation, Matrix Accumulation, and Elastic Modulus of Fibroblast-Seeded Polyurethane Constructs. J. Biomech. 2006, 39, 1136–1144. [Google Scholar] [CrossRef]
- Hong, Y. 19-Electrospun Fibrous Polyurethane Scaffolds in Tissue Engineering. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: London, UK, 2016; pp. 543–559. ISBN 978-0-08-100614-6. [Google Scholar]
- Wu, J.; Zheng, B. Recyclable and Biodegradable Bio-Based Polyurethane Elastomers with Exceptional Mechanically Properties. Appl. Comput. Eng. 2024, 58, 194–207. [Google Scholar] [CrossRef]
- Ntrivala, M.A.; Pitsavas, A.C.; Lazaridou, K.; Baziakou, Z.; Karavasili, D.; Papadimitriou, M.; Ntagkopoulou, C.; Balla, E.; Bikiaris, D.N. Polycaprolactone (PCL): The Biodegradable Polyester Shaping the Future of Materials—A Review on Synthesis, Properties, Biodegradation, Applications and Future Perspectives. Eur. Polym. J. 2025, 234, 114033. [Google Scholar] [CrossRef]
- Tubio, C.R.; Valle, X.; Carvalho, E.; Moreira, J.; Costa, P.; Correia, D.M.; Lanceros-Mendez, S. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Blends with Poly(Caprolactone) and Poly(Lactic Acid): A Comparative Study. Polymers 2023, 15, 4566. [Google Scholar] [CrossRef] [PubMed]
- Simões, C.L.; Viana, J.C.; Cunha, A.M. Mechanical Properties of Poly(ε-Caprolactone) and Poly(Lactic Acid) Blends. J. Appl. Polym. Sci 2009, 112, 345–352. [Google Scholar] [CrossRef]
- Deepthi, S.; Jeevitha, K.; Nivedhitha Sundaram, M.; Chennazhi, K.P.; Jayakumar, R. Chitosan–Hyaluronic Acid Hydrogel Coated Poly(Caprolactone) Multiscale Bilayer Scaffold for Ligament Regeneration. Chem. Eng. J. 2015, 260, 478–485. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Ying Hsi Fuh, J.; San Wong, Y.; Wang, W.; San Thian, E. Direct E-Jet Printing of Three-Dimensional Fibrous Scaffold for Tendon Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 616–627. [Google Scholar] [CrossRef]
- Gwiazda, M.; Kumar, S.; Świeszkowski, W.; Ivanovski, S.; Vaquette, C. The Effect of Melt Electrospun Writing Fiber Orientation onto Cellular Organization and Mechanical Properties for Application in Anterior Cruciate Ligament Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103631. [Google Scholar] [CrossRef]
- Wang, L.; Sun, L.; Qiu, Z.; Zhang, Z.; Shi, Y.; Dang, J.; Tao, W.; He, J.; Fan, H. Gradient Hydroxyapatite Nanoparticles with Spatial Distribution Facilitate the Healing of Tendon-to-Bone Interface. Int. J. Bioprinting 2025, 11, 501–516. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, A.V.; Katakalos, K. The Role of Scaffolds in Tendon Tissue Engineering. J. Funct. Biomater. 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Safinsha, S.; Mubarak Ali, M. Composite Scaffolds in Tissue Engineering. Mater. Today Proc. 2020, 24, 2318–2329. [Google Scholar] [CrossRef]
- Calejo, I.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. A Textile Platform Using Continuous Aligned and Textured Composite Microfibers to Engineer Tendon-to-Bone Interface Gradient Scaffolds. Adv. Healthc. Mater. 2019, 8, e1900200. [Google Scholar] [CrossRef]
- Sensini, A.; Gualandi, C.; Zucchelli, A.; Boyle, L.A.; Kao, A.P.; Reilly, G.C.; Tozzi, G.; Cristofolini, L.; Focarete, M.L. Tendon Fascicle-Inspired Nanofibrous Scaffold of Polylactic Acid/Collagen with Enhanced 3D-Structure and Biomechanical Properties. Sci. Rep. 2018, 8, 17167. [Google Scholar] [CrossRef]
- Kimura, Y.; Hokugo, A.; Takamoto, T.; Tabata, Y.; Kurosawa, H. Regeneration of Anterior Cruciate Ligament by Biodegradable Scaffold Combined with Local Controlled Release of Basic Fibroblast Growth Factor and Collagen Wrapping. Tissue Eng. Part C Methods 2008, 14, 47–57. [Google Scholar] [CrossRef]
- Sensini, A.; Gualandi, C.; Cristofolini, L.; Tozzi, G.; Dicarlo, M.; Teti, G.; Mattioli-Belmonte, M.; Letizia Focarete, M. Biofabrication of Bundles of Poly(Lactic Acid)-Collagen Blends Mimicking the Fascicles of the Human Achille Tendon. Biofabrication 2017, 9, 015025. [Google Scholar] [CrossRef]
- Guo, J.; Su, W.; Jiang, J.; Ning, C.; Zhao, J.; Liu, X. Enhanced Tendon to Bone Healing in Rotator Cuff Tear by PLLA/CPS Composite Films Prepared by a Simple Melt-Pressing Method: An in Vitro and in Vivo Study. Compos. Part B Eng. 2019, 165, 526–536. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Qi, Y.; Cai, J.; Zhao, J.; Duan, B.; Chen, S. Tendon-Bioinspired Wavy Nanofibrous Scaffolds Provide Tunable Anisotropy and Promote Tenogenesis for Tendon Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112181. [Google Scholar] [CrossRef] [PubMed]
- Shiroud Heidari, B.; Muiños Lopez, E.; Harrington, E.; Ruan, R.; Chen, P.; Davachi, S.M.; Allardyce, B.; Rajkhowa, R.; Dilley, R.; Granero-Moltó, F.; et al. Novel Hybrid Biocomposites for Tendon Grafts: The Addition of Silk to Polydioxanone and Poly(Lactide-Co-Caprolactone) Enhances Material Properties, in Vitro and in Vivo Biocompatibility. Bioact. Mater. 2023, 25, 291–306. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Gao, T.; Zhang, R.; Cai, Z.; Liu, J.; Ma, H.; Zhang, W. Controlled Release of BFGF Loaded into Electrospun Core-Shell Fibrous Membranes for Use in Guided Tissue Regeneration. Biomed. Mater. 2020, 15, 035021. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Slimani, S.; Kahn, C.J.F.; Tran, N.; Rahouadj, R.; Wang, X. A Poly(Lactic-Co-Glycolic Acid) Knitted Scaffold for Tendon Tissue Engineering: An in Vitro and in Vivo Study. J. Biomater. Sci. Polym. Ed. 2010, 21, 1737–1760. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.; Tu, T.; Guo, Z.; Song, Z.; Jiang, Y.; Zhou, B.; Lei, D.; Wang, X.; Zhang, W.; et al. Mechanical Loading on Cell-Free Polymer Composite Scaffold Enhances in Situ Regeneration of Fully Functional Achilles Tendon in a Rabbit Model. Biomater. Adv. 2024, 163, 213950. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Dash, M.; Chakraborty, R.; Lukman, H.J.; Kumar, P.; Hassan, S.; Mehboob, H.; Singh, H.; Nanda, H.S. Engineering Considerations in the Design of Tissue Specific Bioink for 3D Bioprinting Applications. Biomater. Sci. 2024, 13, 93–129. [Google Scholar] [CrossRef]
- Silva, M.; Gomes, S.; Correia, C.; Peixoto, D.; Vinhas, A.; Rodrigues, M.T.; Gomes, M.E.; Covas, J.A.; Paiva, M.C.; Alves, N.M. Biocompatible 3D-Printed Tendon/Ligament Scaffolds Based on Polylactic Acid/Graphite Nanoplatelet Composites. Nanomaterials 2023, 13, 2518. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Mock, D.; Fuentes, J.; Michelis, M.Y.; Balciunaite, A.; Paniagua, P.; Hopf, R.; Barteld, A.; Eng, S.; Badolato, A.; et al. Multicellular Muscle-Tendon Bioprinting of Mechanically Optimized Musculoskeletal Bioactuators with Enhanced Force Transmission. Sci. Adv. 2025, 11, eadv2628. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Yang, W.; Yu, K.; Hong, J.; Ji, X.; Yao, M.; Li, S.; Lu, J.; Chen, Y.; et al. 3D-Printed Hydrogel Particles Containing PRP Laden with TDSCs Promote Tendon Repair in a Rat Model of Tendinopathy. J. Nanobiotechnol. 2023, 21, 177. [Google Scholar] [CrossRef]
- Olvera, D.; Schipani, R.; Sathy, B.N.; Kelly, D.J. Electrospinning of Highly Porous yet Mechanically Functional Microfibrillar Scaffolds at the Human Scale for Ligament and Tendon Tissue Engineering. Biomed. Mater. 2019, 14, 035016. [Google Scholar] [CrossRef]
- Guner, M.B.; Dalgic, A.D.; Tezcaner, A.; Yilanci, S.; Keskin, D. A Dual-Phase Scaffold Produced by Rotary Jet Spinning and Electrospinning for Tendon Tissue Engineering. Biomed. Mater. 2020, 15, 065014. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Meng, H.; Wang, Y.; Hu, L.; Su, W.; Xu, J.; Hou, J.; Zhao, R.; Wang, Z.; et al. Coaxial Electrospun Nanofibrous Membranes as Dual-Functional Biomimetic Tendon Sheath for Tendon Repair and Anti-Peritendinous Adhesion. Adv. Healthc. Mater. 2025, 14, e2402074. [Google Scholar] [CrossRef]
- Basile, P.; Dadali, T.; Jacobson, J.; Hasslund, S.; Ulrich-Vinther, M.; Søballe, K.; Nishio, Y.; Drissi, M.H.; Langstein, H.N.; Mitten, D.J.; et al. Freeze-Dried Tendon Allografts as Tissue-Engineering Scaffolds for Gdf5 Gene Delivery. Mol. Ther. 2008, 16, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel Freeze-Drying Methods to Produce a Range of Collagen-Glycosaminoglycan Scaffolds with Tailored Mean Pore Sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chan, V.; Corridon, P.R. Acellular Tissue-Engineered Vascular Grafts from Polymers: Methods, Achievements, Characterization, and Challenges. Polymers 2022, 14, 4825. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Zieliński, P.S.; Gudeti, P.K.R.; Rikmanspoel, T.; Włodarczyk-Biegun, M.K. 3D Printing of Bio-Instructive Materials: Toward Directing the Cell. Bioact. Mater. 2023, 19, 292–327. [Google Scholar] [CrossRef]
- Parihar, A.; Pandita, V.; Kumar, A.; Parihar, D.S.; Puranik, N.; Bajpai, T.; Khan, R. 3D Printing: Advancement in Biogenerative Engineering to Combat Shortage of Organs and Bioapplicable Materials. Regen. Eng. Transl. Med. 2022, 8, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Shinkar, K.; Rhode, K. Could 3D Extrusion Bioprinting Serve to Be a Real Alternative to Organ Transplantation in the Future? Ann. 3D Print. Med. 2022, 7, 100066. [Google Scholar] [CrossRef]
- Sachdev, A.; Acharya, S.; Gadodia, T.; Shukla, S.; J, H.; Akre, C.; Khare, M.; Huse, S. A Review on Techniques and Biomaterials Used in 3D Bioprinting. Cureus 2022, 14, e28463. [Google Scholar] [CrossRef] [PubMed]
- Muskan; Gupta, D.; Negi, N.P. 3D Bioprinting: Printing the Future and Recent Advances. Bioprinting 2022, 27, e00211. [Google Scholar] [CrossRef]
- Jamee, R.; Araf, Y.; Naser, I.B.; Promon, S.K. The Promising Rise of Bioprinting in Revolutionalizing Medical Science: Advances and Possibilities. Regen. Ther. 2021, 18, 133–145. [Google Scholar] [CrossRef]
- Herzog, J.; Franke, L.; Lai, Y.; Gomez Rossi, P.; Sachtleben, J.; Weuster-Botz, D. 3D Bioprinting of Microorganisms: Principles and Applications. Bioprocess Biosyst. Eng. 2024, 47, 443–461. [Google Scholar] [CrossRef]
- Jose, J.; Peter, A.; Thajudeen, K.Y.; Pereira, M.D.L.G.; VP, A.; Michel, H. Recent Advances in the Design and Development of Bioink Formulations for Various Biomedical Applications. Results Eng. 2024, 22, 102060. [Google Scholar] [CrossRef]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef]
- Robinson, A.J.; Pérez-Nava, A.; Ali, S.C.; González-Campos, J.B.; Holloway, J.L.; Cosgriff-Hernandez, E.M. Comparative Analysis of Fiber Alignment Methods in Electrospinning. Matter 2021, 4, 821–844. [Google Scholar] [CrossRef]
- Thangadurai, M.; Ajith, A.; Budharaju, H.; Sethuraman, S.; Sundaramurthi, D. Advances in Electrospinning and 3D Bioprinting Strategies to Enhance Functional Regeneration of Skeletal Muscle Tissue. Biomater. Adv. 2022, 142, 213135. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Maduna, L.; Patnaik, A. Challenges Associated with the Production of Nanofibers. Processes 2024, 12, 2100. [Google Scholar] [CrossRef]
- Zhu, Y.; Dai, B.; Zhang, S.; Liu, J.; Xu, S.; Liu, W.; Chen, X.; Zhang, H.; Li, Q.; Pang, F.O.-S.; et al. Tissue Mimetic Membranes for Healing Augmentation of Tendon-Bone Interface in Rotator Cuff Repair. Adv. Mater. 2025, 37, e2407358. [Google Scholar] [CrossRef]
- Iorio, F.; El Khatib, M.; Wöltinger, N.; Turriani, M.; Di Giacinto, O.; Mauro, A.; Russo, V.; Barboni, B.; Boccaccini, A.R. Electrospun Poly(ε-Caprolactone)/Poly(Glycerol Sebacate) Aligned Fibers Fabricated with Benign Solvents for Tendon Tissue Engineering. J. Biomed. Mater. Res. A 2025, 113, e37794. [Google Scholar] [CrossRef]
- Rivoallan, N.; Baudequin, T.; Mueller, M.; Nicolas, R.; Marin, S.L.; Vigneron, P.; Jellali, R.; Dermigny, Q.; Le Goff, A.; Duprez, D.; et al. Graded Electrospun Scaffold from Aligned Fibers to Honeycomb Micropatterns: Application to Bone-Tendon Tissue Engineering. Biomater. Adv. 2025, 177, 214413. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Li, J.; Chen, S.; Yuan, Z.; Sun, Z.; Lou, T.; Chen, Z.; Liu, H.; Zhou, C.; Fan, C.; et al. Polylactic Acid Electrospun Membranes Coated with Chiral Hierarchical-Structured Hydroxyapatite Nanoplates Promote Tendon Healing Based on a Macrophage-Homeostatic Modulation Strategy. Bioact. Mater. 2025, 47, 460–480. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Huang, Y.; Zhang, F.; Wang, L.; Li, W.; Santos, H.A.; Sun, L. Biological Augmentation Using Electrospun Constructs with Dual Growth Factor Release for Rotator Cuff Repair. ACS Appl. Bio Mater. 2025, 8, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, J.; Su, W.; Yu, L.; Li, T.; Wang, Y.; Zhang, K.; Wu, Y.; Wang, L. Electrospun Aligned Poly(ε-Caprolactone) Nanofiber Yarns Guiding 3D Organization of Tendon Stem/Progenitor Cells in Tenogenic Differentiation and Tendon Repair. Front. Bioeng. Biotechnol. 2022, 10, 960694. [Google Scholar] [CrossRef]
- Dewle, A.; Pathak, N.; Rakshasmare, P.; Srivastava, A. Multifarious Fabrication Approaches of Producing Aligned Collagen Scaffolds for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 779–797. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of Aligned Porous Gelatin Scaffolds by Unidirectional Freeze-Drying Method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Lowe, C.J.; Reucroft, I.M.; Grota, M.C.; Shreiber, D.I. Production of Highly Aligned Collagen Scaffolds by Freeze-Drying of Self-Assembled, Fibrillar Collagen Gels. ACS Biomater. Sci. Eng. 2016, 2, 643–651. [Google Scholar] [CrossRef]
- Katrilaka, C.; Karipidou, N.; Petrou, N.; Manglaris, C.; Katrilakas, G.; Tzavellas, A.N.; Pitou, M.; Tsiridis, E.E.; Choli-Papadopoulou, T.; Aggeli, A. Freeze-Drying Process for the Fabrication of Collagen-Based Sponges as Medical Devices in Biomedical Engineering. Materials 2023, 16, 4425. [Google Scholar] [CrossRef] [PubMed]
- Odziomek, K.; Drabczyk, A.K.; Kościelniak, P.; Konieczny, P.; Barczewski, M.; Bialik-Wąs, K. The Role of Freeze-Drying as a Multifunctional Process in Improving the Properties of Hydrogels for Medical Use. Pharmaceuticals 2024, 17, 1512. [Google Scholar] [CrossRef]
- He, W.; Jiang, C.; Zhou, P.; Hu, X.; Gu, X.; Zhang, S. Role of Tendon-Derived Stem Cells in Tendon and Ligament Repair: Focus on Tissue Engineer. Front. Bioeng. Biotechnol. 2024, 12, 1357696. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- von Stade, D.; Meyers, M.; Johnson, J.; Schlegel, T.; Romeo, A.; Regan, D.; McGilvray, K. Primary Human Macrophage and Tenocyte Tendon Healing Phenotypes Changed by Exosomes Per Cell Origin. Tissue Eng. Part A 2025, 31, 1109–1120. [Google Scholar] [CrossRef]
- Mooij, I.; Apachitei, I.; Zadpoor, A.A.; Fratila-Apachitei, L.E. Biomaterial Multiscale Geometry for Regenerative Immunoengineering of Bone Tissue. Acta Biomater. 2025, 203, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Shelar, S.; Gupta, U.; Kumari, M.; Shabib, M.; Guchhait, B.; Mandal, C.; Kaity, S.; Roy, S. Hybrid Photo-Crosslinked Decellularized Extracellular Matrix Scaffold from Porcine Achilles Tendon: A Biocompatible and Non-Immunogenic Matrix for Tissue Engineering. J. Mater. Chem. B 2025, 37, 11722–11738. [Google Scholar] [CrossRef]
- Chen, S.-H.; Lien, P.-H.; Sun, C.-Y.; Chou, P.-Y.; Chen, Z.-Y.; Chen, S.-H.; Fang, H.-W.; Lin, F.-H. Dextran Sulfate-Containing Thermosensitive Hydrogel Improves Tendon Healing by Modulating Macrophage Polarization. Int. J. Biol. Macromol. 2025, 322, 146864. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Liang, T.; Du, Z.; Deng, L.; Wu, Z.; Li, Y.; Zhong, H.; Ma, J.; Li, R.; et al. Enhanced Rotator Cuff Tendon-Bone Interface Regeneration with Injectable Manganese-Based Mesoporous Silica Nanoparticle-Loaded Dual Crosslinked Hydrogels. Front. Bioeng. Biotechnol. 2025, 13, 1645970. [Google Scholar] [CrossRef]
- Zhou, T.; Ma, T.; Wang, Q.; Wang, Z.; Huang, J.; Zhong, L.; Zhou, D.; Jin, Z.; Niu, Y.; Yin, D.; et al. Hydrogels with Anti-Inflammatory Modulation Microniches for Tendon Repair. Chem. Eng. J. 2025, 519, 164165. [Google Scholar] [CrossRef]
- Shi, W.; Jiang, Y.; Wu, T.; Zhang, Y.; Li, T. Advancements in Drug-Loaded Hydrogel Systems for Bone Defect Repair. Regen. Ther. 2024, 25, 174–185. [Google Scholar] [CrossRef]
- Chen, R.; Chen, F.; Chen, K.; Xu, J. Advances in the Application of Hydrogel-Based Scaffolds for Tendon Repair. Genes Dis. 2024, 11, 101019. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Li, P.; Gao, C.; Fu, L.; Liao, Z.; Tian, G.; Yin, H.; Li, M.; Sui, X.; Yuan, Z.; et al. Recent Advances in Tendon Tissue Engineering Strategy. Front. Bioeng. Biotechnol. 2023, 11, 1115312. [Google Scholar] [CrossRef]
- Fang, L.; Lin, X.; Xu, R.; Liu, L.; Zhang, Y.; Tian, F.; Li, J.J.; Xue, J. Advances in the Development of Gradient Scaffolds Made of Nano-Micromaterials for Musculoskeletal Tissue Regeneration. Nanomicro Lett. 2024, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Y.; Li, Z.; Yang, G.; Cheng, G.; Qin, S.; Wang, H.; Zhu, L. Recent Advances in Gradient Biomimetic Scaffolds for Tendon-Bone Interface Regeneration. Front. Bioeng. Biotechnol. 2025, 13, 1629816. [Google Scholar] [CrossRef]
- Bianchi, E.; Bañobre-Lopez, M.; Ruggeri, M.; Del Favero, E.; Vigani, B.; Ricci, C.; Boselli, C.; Icaro Cornaglia, A.; Albino, M.; Sangregorio, C.; et al. Magnetic Scaffolds for the Mechanotransduction Stimulation in Tendon Tissue Regeneration. Mater. Today Bio 2025, 32, 101699. [Google Scholar] [CrossRef]

| Category | Polymer | Properties | Advantages | Limitations | Applications/Notes |
|---|---|---|---|---|---|
| Natural | Collagen | Biocompatible, biodegradable, porous, low immunogenicity | Excellent cell attachment, bioactivity, ECM mimicry | Low mechanical strength, rapid degradation when denatured | Used in seeded scaffolds, often cross-linked or combined with silk/synthetics |
| Silk | Strong, flexible, biocompatible | Long degradation time, supports cell differentiation | May induce immune response, batch variability | Used in ACL and tendon repair, often aligned for better results | |
| Fibrin | Biodegradable, supports cell adhesion & ECM synthesis | Bioactive, growth factor carrier | Weak mechanical properties | Modified with collagen/silk, used in tendon patches | |
| Chitin/Chitosan | Strong, polysaccharide-based | Promotes tenocyte adhesion, ECM mimicry | High degradation, lower bioactivity | Combined with alginate/synthetics, needs crosslinking | |
| Decellularized Tissues | ECM-based structure | Native architecture, low immunogenicity | Source limitations, processing complexity | FDA-approved (e.g., Arthroflex®), supports endogenous repair | |
| Synthetic | PLLA (PLA) | Biocompatible, slow degradation, good strength | Supports load-bearing, tunable properties | Poor ductility, hydrophobic | Used in electrospun/braided scaffolds, often mixed with collagen or CPS |
| PGA | High strength, hydrophilic | Fast degradation | Risk of tissue damage from glycolic acid | Used in ACL regeneration, needs controlled degradation | |
| PLGA | Copolymer of PLA & PGA, tunable degradation | Versatile, good for growth factor delivery | Poor hydrophilicity, lower bioactivity | Used with growth factors, MSCs, aligned fibers | |
| PCL | Ductile, bioresorbable | Slow degradation, shape memory | Lower mechanical strength | Used in 3D scaffolds, hybrid structures with gelatin/HA | |
| Polyurethanes | Highly tunable, good mechanical properties | Easy to modify, bioactivity when seeded | Potential toxicity of by-products | Used with fibroblasts, enhanced by cyclic strain | |
| Hybrid | Natural + Synthetic | Combines bioactivity & strength | Synergistic properties, better scaffold performance | Complexity of fabrication | PLLA/collagen, PCL/gelatin, PLGA/keratin, etc., used for improved tendon regeneration |
| Technique | Principle | Key Materials | Advantages | Limitations | Relevance to Tendon TE | References |
|---|---|---|---|---|---|---|
| 3D printing/Bioprinting | Layer-by-layer deposition of bioinks or biomaterials using computer-aided design | PLA, PCL, GelMA, PRP hydrogels, bioinks with cells | High precision, customizable geometry, tunable porosity, patient-specific scaffolds | Limited vascularization, potential cell damage from pressure/stress | Enables gradient and aligned structures mimicking tendon; supports cell differentiation and mechanical strength | [115,116,117] |
| Electrospinning | Electric field draws polymer solution into micro/nanofibers; alignment controlled by collector rotation | PCL, gelatin, collagen, GelMA | Produces ECM-like fibrous architecture, tunable alignment, scalable, good cell guidance | Poor pore interconnectivity, limited 3D volume | Aligned fibers guide tenocyte orientation and ECM deposition; coaxial designs combine regeneration + anti-adhesion | [118,119,120] |
| Freeze-Drying | Freezing polymer solution → sublimation under vacuum → porous 3D scaffold | Collagen, gelatin, silk fibroin, PCL blends | Maintains bioactivity, tunable pore size, simple fabrication, good hydrophilicity | Low mechanical strength, slow production | Creates biocompatible scaffolds and allows gene/drug loading; pore control supports cell infiltration and alignment | [121,122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanisova, D.; Bevizova, K.; Vach Agocsova, S.; Danisovic, L.; Culenova, M. Implications of Tissue Engineering for Tendon Repair and Regeneration. J. Funct. Biomater. 2025, 16, 403. https://doi.org/10.3390/jfb16110403
Ivanisova D, Bevizova K, Vach Agocsova S, Danisovic L, Culenova M. Implications of Tissue Engineering for Tendon Repair and Regeneration. Journal of Functional Biomaterials. 2025; 16(11):403. https://doi.org/10.3390/jfb16110403
Chicago/Turabian StyleIvanisova, Dana, Katarina Bevizova, Sara Vach Agocsova, Lubos Danisovic, and Martina Culenova. 2025. "Implications of Tissue Engineering for Tendon Repair and Regeneration" Journal of Functional Biomaterials 16, no. 11: 403. https://doi.org/10.3390/jfb16110403
APA StyleIvanisova, D., Bevizova, K., Vach Agocsova, S., Danisovic, L., & Culenova, M. (2025). Implications of Tissue Engineering for Tendon Repair and Regeneration. Journal of Functional Biomaterials, 16(11), 403. https://doi.org/10.3390/jfb16110403








