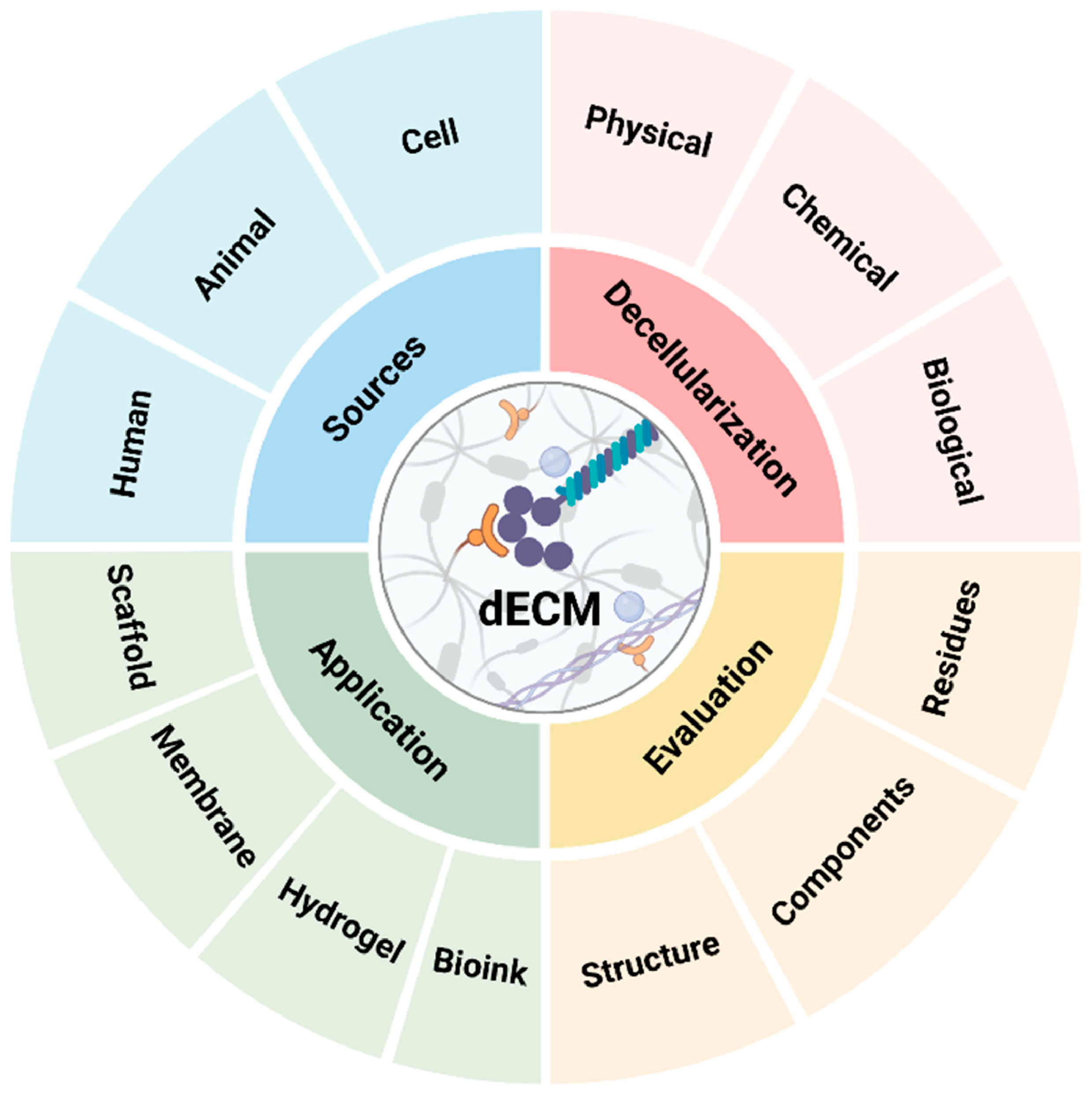
Acellular Extracellular Matrix Scaffolds in Regenerative Medicine: Advances in Decellularization and Clinical Applications
Abstract
1. Introduction
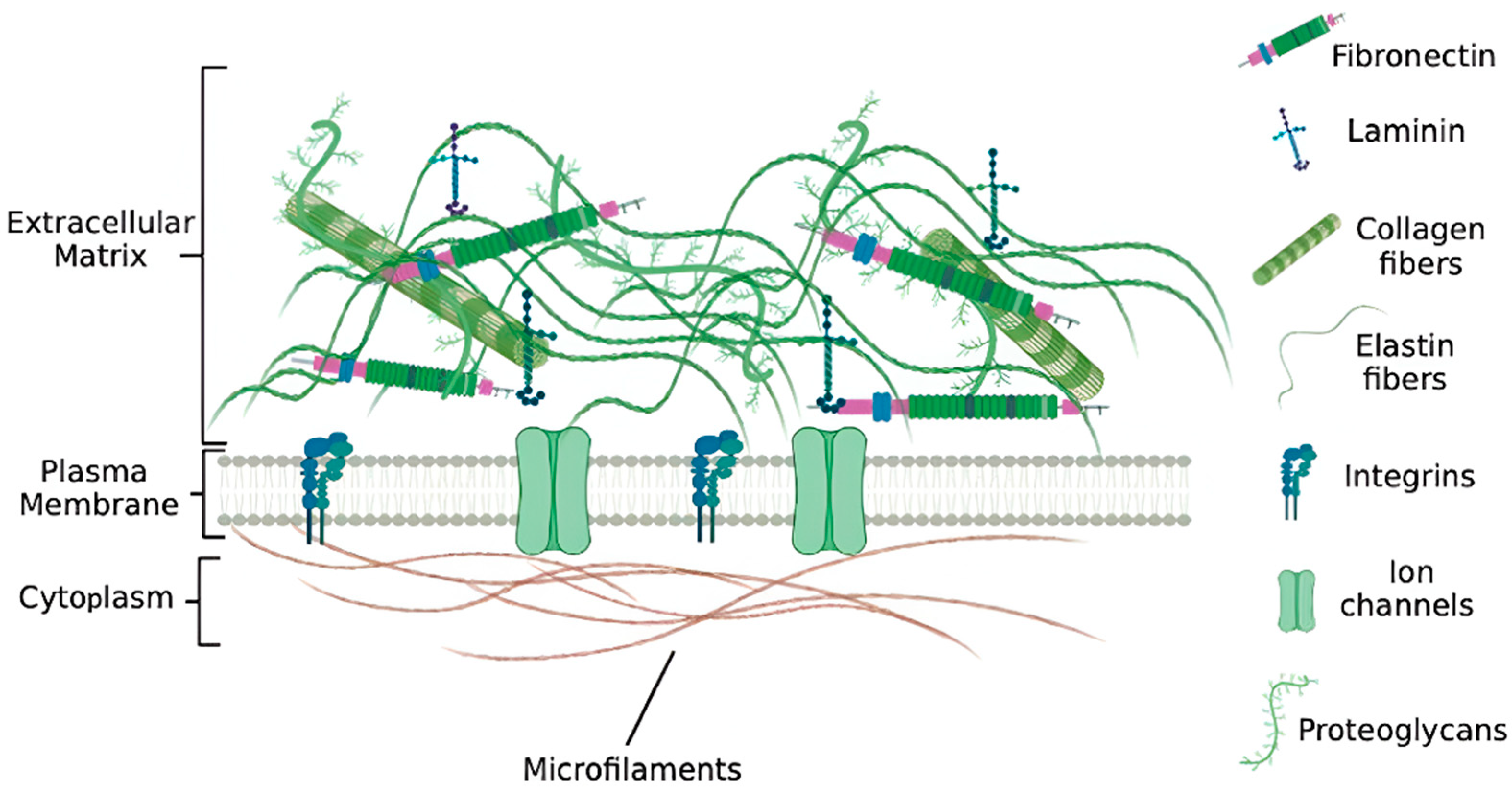
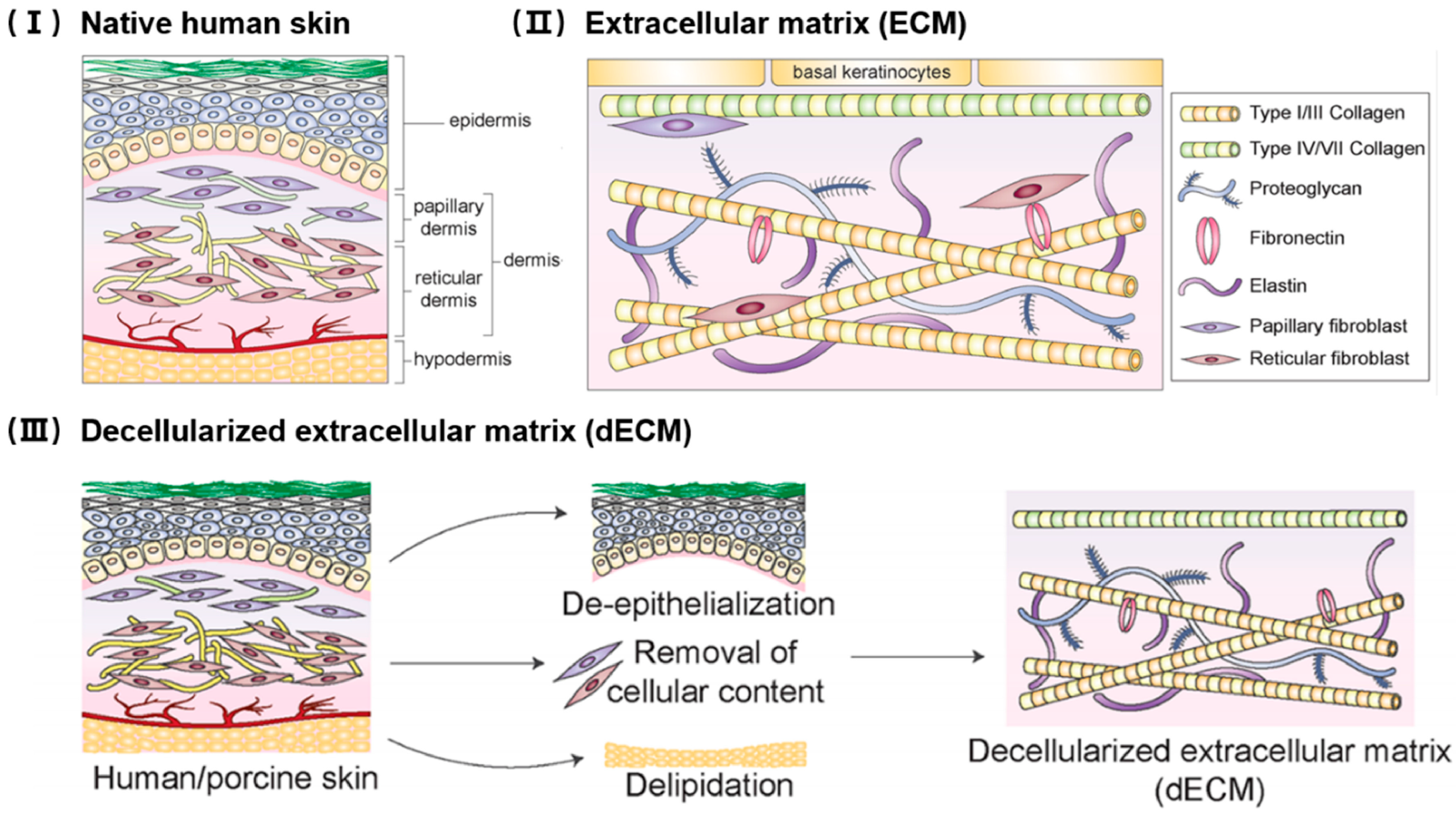
2. Structure and Function of the Extracellular Matrix
3. Decellularization Techniques
3.1. Chemical Methods

3.2. Enzymatic Methods
3.3. Physical Methods
| Category | Sub-Method | Merits | Demerits | Limitations/Notes | References |
|---|---|---|---|---|---|
| Chemical | Ionic detergents (SDS, SDC) | Highly effective at DNA/protein removal | Denatures ECM proteins, cytotoxic residues | Requires extensive washing; risk of growth factor loss | [45,46,47,48,49,50] |
| Non-ionic detergents (Triton X-100, Tween-20) | Mild, preserves collagen alignment | Less efficient DNA removal | Often combined with enzymes for completeness | [45,46,47,48,49,50] | |
| Acids/Bases (Peracetic acid, NaOH, NH4OH) | Good sterilization, DNA solubilization | Collagen/GAG degradation, mechanical weakening | Best for thin tissues; high pH damages ECM | [51,52,53,54] | |
| Hyper/Hypotonic solutions (NaCl, DI water) | Simple, inexpensive, low cytotoxicity | Incomplete decellularization | Usually adjunctive, not sufficient alone | [55,56] | |
| Enzymatic | Nucleases (DNase, RNase) | Specific nucleic acid removal | Expensive, incomplete if penetration limited | Needs detergents/physical methods adjunct | [54] |
| Proteases (Trypsin, Dispase, Collagenase) | Efficient membrane/cell protein removal | Can degrade ECM proteins if overused | Must optimize time/concentration | [58,59,60,61,62] | |
| Lipases | Remove lipids in adipose tissue | ECM damage with prolonged exposure | Narrow use cases | [63,64,65,66,67] | |
| Phospholipase | Degrade membrane phospholipids, enhancing cell lysis | May also attack ECM lipids | Useful for adipose or lipid-rich tissues; often adjunct to detergents | [68,69] | |
| Physical | Freeze–thaw cycles | Simple, disrupts cell membranes | Inefficient DNA removal | Good adjunct to chemical/enzymatic | [71,72] |
| Agitation/Stirring | Enhances reagent diffusion | Possible shear damage | Widely used in lab-scale protocols | [73] | |
| High hydrostatic pressure | Preserves ECM architecture, sterilizing | Requires special equipment | Limited tissue size | [74,75] | |
| Vascular perfusion (whole organs) | Maintains vascular networks, uniform penetration | Technically demanding, costly | Primarily for solid organs (liver, kidney, lung, heart) | [76,77] | |
| Supercritical CO2 extraction | Good for lipid-rich tissues, sterilization | Limited penetration in dense tissues | Still experimental, requires optimization | [82,83] | |
| Sonication | Assists detergent penetration | Local ECM disruption, heat damage | Best used in small samples | [84] | |
| Irreversible electroporation (IRE) | Creates nanopores in cell membranes without harsh chemicals; preserves ECM | Requires precise control; risk of local ECM disruption | Promising for thick tissues, still experimental | [85] | |
| Bioreactor systems (dynamic decellularization) | Provides controlled flow, shear, and nutrient removal; scalable | Requires high cost infrastructure | Essential for whole organ engineering | [86] | |
| Biofabrication (3D printing + dECM bioinks) | Enables patient-specific scaffolds, integration with cells/growth factors | Requires standardization, mechanical strength challenges | Bridges decellularization with regenerative manufacturing | [89,90] |
4. Characterization of Decellularized ECM Scaffolds
4.1. Evaluations for Cellular Residues
4.2. Evaluations for dECM Components
4.3. Evaluations for Cytocompatibility and Immunogenicity
| Assay/Method | Target | Detection Limit/Sensitivity | Advantages | Limitations/Notes | References |
|---|---|---|---|---|---|
| DNA quantification (PicoGreen, qPCR) | Residual nuclear material | ~50 ng/mg tissue | Highly sensitive, quantitative | Cannot distinguish intact vs. fragmented DNA; requires extraction | [99,100,101] |
| Histology (H&E, DAPI, Hoechst) | Cellular remnants, nuclei | Semi-quantitative | Simple, visual localization of residual cells | Limited sensitivity, observer bias | [102,103,104,105,106] |
| Immunohistochemistry/IF | ECM proteins (collagen I/III, laminin, fibronectin) or signaling molecular (TGF-β1, VEGF and bFGF) | N/A | Protein-specific, spatial localization | Antibody-dependent, qualitative | [107] |
| Biochemical assays (FASTIN, SIRCOL, BLYSCAN) | Elastin (FASTIN), collagen (SIRCOL), GAGs (Blyscan) | 1–5 μg/mL | Quantitative, widely used | Destructive, requires standard curves | [108] |
| Residual detergent assays (methylene blue for SDS) | Chemical residues | μg/mL | Safety-relevant, quantitative | Assay-specific, not always standardized | [110] |
| In vitro cytocompatibility (MTT, CCK-8, Live/Dead, cell adhesion assays) | Cell viability and proliferation | N/A | Functional, directly relevant | Cell-type dependent; semi-quantitative | [111,112,113] |
5. Tissue-Specific ECM Scaffolds
5.1. Skin and Dermis
5.2. Peripheral Nerve
5.3. Heart and Vascular Tissue
5.4. Lung
5.5. Adipose Tissue
5.6. Placenta
5.7. Kidney
5.8. Liver
5.9. Alternative Sources
6. Clinical Applications and Current Trials
| Product/Brand | Source Tissue | Company/Manufacturer | Indications/Clinical Use | Regulatory/Market Status | Notes | References |
|---|---|---|---|---|---|---|
| AlloDerm®/AlloDerm RTM/Cymetra® (micronized) | Human dermis | LifeCell/Allergan Aesthetics (AbbVie), Irvine, CA, USA | Breast and abdominal wall reconstruction, chronic wounds, soft-tissue repair | FDA HCT/P; widely used | Gold-standard ADM; micronized Cymetra allows injectable use | [189,190] |
| DermACELL® | Human dermis | LifeNet Health, Virginia Beach, VA, USA | Chronic wounds, breast reconstruction, hernia repair | FDA HCT/P | Matracell® process ensures >97% DNA removal | [191,192] |
| FlexHD® | Human dermis | MTF Biologics, Edison, NJ, USA | Breast reconstruction, hernia repair, soft-tissue reinforcement | FDA HCT/P | Hydrated, pliable ADM | [193,194] |
| GraftJacket® | Human dermis | Wright/Stryker, Kalamazoo, MI, USA | rotator cuff tears, diabetic foot ulcers | FDA cleared | Available in thick or meshed forms | [195,196] |
| DermaMatrix®, AlloPatch® Pliable | Human dermis | MTF Biologics, Edison, NJ, USA | reconstructive surgery | Commercial | Freeze-dried or hydrated formats | [197,198] |
| SureDerm®, CGDerm®, MegaDerm®/MegaDerm® Plus | Human dermis (Korea) | HansBiomed, CGBio, L&C Bio, Seoul, South Korea | Burn care, plastic reconstruction, dental/periodontal | CE/NMPA/Korean MFDS approvals | Widely used in Asia; Mega Derm Plus recently NMPA-approved (China) | [126] |
| Strattice™ RTM/ARTIA™ RTM | Porcine dermis (non-crosslinked) | LifeCell/Allergan Aesthetics, Irvine, CA, USA | Abdominal wall/hernia repair, breast reconstruction | FDA 510(k); CE Mark | Multiple versions (Perforated, Extra-thick, Lap) | [199] |
| XenMatrix™/XenMatrix™ AB | Porcine dermis | BD (C.R. Bard), Franklin Lakes, NJ, USA | Hernia repair, abdominal wall reinforcement | FDA 510(k) | AB version has antibiotic coating (rifampin/minocycline) | [200] |
| Permacol™ | Porcine dermis (crosslinked) | Medtronic, Medtronic Parkway, Minneapolis, MN, USA | Hernia repair, pelvic floor, reconstructive | FDA 510(k) | Long history of clinical use | [201,202] |
| SurgiMend® | Fetal bovine dermis | Integra LifeSciences, Princeton, NJ, USA | Hernia repair, breast reconstruction, soft-tissue reinforcement | FDA 510(k) | Non-crosslinked collagen scaffold | [203,204,205] |
| Edwards Perimount® | Bovine pericardium | Edwards Lifesciences, Irvine, CA, USA | Surgical bioprosthetic heart valves for aortic/mitral valve replacement | FDA and CE approved | One of the most established pericardial valve products with extensive long-term clinical outcome data | [206] |
| CardioCel® | Bovine pericardium | Admedus (now Anteris Technologies), Eagan, MN, USA | Congenital heart defect repair, vascular and pericardial reconstruction | CE Mark; TGA (Australia); FDA clearance for certain indications | Designed to reduce calcification and improve durability; applied in pediatric cardiac surgery | [207] |
| OASIS® Wound Matrix/Flowable | Porcine small intestinal submucosa (SIS) | Cook Biotech/Smith + Nephew, West Lafayette, TN, USA | Acute and chronic wounds (DFU, VLU, pressure ulcers) | FDA 510(k) | Widely adopted in wound care | [208] |
| Biodesign® (formerly Surgisis®) | Porcine SIS | Cook Biotech, West Lafayette, IN, USA | Hernia repair, fistula plugs, esophageal/gastrointestinal applications | FDA 510(k) | Versatile SIS-based product line | [209] |
| CorMatrix® ECM | Porcine SIS | CorMatrix Cardiovascular, Roswell, GA, USA | Vascular/arterial and pericardial repair, cardiac applications | FDA 510(k); CE Mark | Used in congenital heart surgery and vascular repair | [210,211] |
| Cytal®, MicroMatrix®, Gentrix® | Porcine urinary bladder matrix (UBM) | Integra LifeSciences (via ACell), Princeton, NJ, USA | Wound management, hernia repair, pelvic floor | FDA 510(k) | Available in sheets, powders, gels | [212,213] |
| Endoform®/Symphony™/Myriad® | Ovine forestomach matrix (OFM) | Aroa Biosurgery, San Diego, CA, USA | Acute and chronic wounds, reconstructive surgery | FDA 510(k); CE Mark | Multiple formats (natural, antimicrobial, injectable, mesh) | [214] |
| OviTex®/OviTex® PRS | OFM reinforced with synthetic polymer fibers | TELA Bio (with Aroa), Malvern, PA, USA | Hernia and abdominal wall reconstruction; PRS version for plastic/reconstructive surgery | FDA 510(k); CE | Reinforced hybrid scaffold | [215] |
| Avance® Nerve Graft | Human decellularized peripheral nerve | AxoGen, Alachua, FL, USA | Peripheral nerve gap repair | HCT/P in U.S.; transitioning to BLA | Leading nerve ECM product | [216] |
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| dECM | Decellularized extracellular matrix |
| GAG | Glycosaminoglycan |
| ECM | Extracellular matrix |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphoinositide 3-kinase |
| FAK | Focal adhesion kinase |
| TGF-β | Transforming growth factor-β |
| VEGF | Vascular endothelial growth factor |
| FGF | Fibroblast growth factor |
| BMP | Bone morphogenetic protein |
| ADAM | A disintegrin and metalloproteinase |
| MMP | Matrix metalloproteinase |
| TIMP | Tissue inhibitors of metalloproteinase |
| DAMP | Damage-associated molecular pattern |
| PDGF | Platelet-derived growth factor |
| SDS | Sodium dodecyl sulfate |
| SDC | Sodium deoxycholate |
| PAA | Peracetic acid |
| DNase | Deoxyribonuclease |
| RNase | Ribonuclease |
| sCO2 | Supercritical carbon dioxide |
| IRE | Irreversible electroporation |
| dsDNA | Double-stranded DNA |
| HE | Hematoxylin eosin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| MHC | Major histocompatibility complex |
| ACM | Acellular matrix |
| GMP | Good manufacturing practice |
References
- Takahashi, J. Next steps in regenerative medicine. Cell Stem Cell 2023, 30, 509–511. [Google Scholar] [CrossRef]
- McKinley, K.L.; Longaker, M.T.; Naik, S. Emerging frontiers in regenerative medicine. Science 2023, 380, 796–798. [Google Scholar] [CrossRef]
- Xu, P.; Kankala, R.K.; Wang, S.; Chen, A. Decellularized extracellular matrix-based composite scaffolds for tissue engineering and regenerative medicine. Regen. Biomater. 2024, 11, rbad107. [Google Scholar] [CrossRef]
- Edgar, L.; Pu, T.; Porter, B.; Aziz, J.M.; La Pointe, C.; Asthana, A.; Orlando, G. Regenerative medicine, organ bioengineering and transplantation. Br. J. Surg. 2020, 107, 793–800. [Google Scholar] [CrossRef]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, X.; Liu, X.; Wen, G.; Yu, Y. Recent advances in decellularized biomaterials for wound healing. Mater. Today Bio 2023, 19, 100589. [Google Scholar] [CrossRef]
- Niklason, L.E. Understanding the Extracellular Matrix to Enhance Stem Cell-Based Tissue Regeneration. Cell Stem Cell 2018, 22, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, Y.; Yang, Q.; Chen, J.; Liu, L.; Jin, J.; Zhu, S. Adipose-derived stem cells derived decellularized extracellular matrix enabled skin regeneration and remodeling. Front. Bioeng. Biotechnol. 2024, 12, 1347995. [Google Scholar] [CrossRef] [PubMed]
- Solarte David, V.A.; Güiza-Argüello, V.R.; Arango-Rodríguez, M.L.; Sossa, C.L.; Becerra-Bayona, S.M. Decellularized Tissues for Wound Healing: Towards Closing the Gap Between Scaffold Design and Effective Extracellular Matrix Remodeling. Front. Bioeng. Biotechnol. 2022, 10, 821852. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hwang, H.J.; Kim, H.J.; Choi, Y.; Lee, D.; Jung, H.H.; Do, S.H. Effect of Decellularized Extracellular Matrix Bioscaffolds Derived from Fibroblasts on Skin Wound Healing and Remodeling. Front. Bioeng. Biotechnol. 2022, 10, 865545. [Google Scholar] [CrossRef]
- Shaik, R.; Xu, J.; Wang, Y.; Hong, Y.; Zhang, G. Fibrin-Enriched Cardiac Extracellular Matrix Hydrogel Promotes In Vitro Angiogenesis. ACS Biomater. Sci. Eng. 2023, 9, 877–888. [Google Scholar] [CrossRef]
- Luo, P.; Huang, R.; Wu, Y.; Liu, X.; Shan, Z.; Gong, L.; Deng, S.; Liu, H.; Fang, J.; Wu, S.; et al. Tailoring the multiscale mechanics of tunable decellularized extracellular matrix (dECM) for wound healing through immunomodulation. Bioact. Mater. 2023, 28, 95–111. [Google Scholar] [CrossRef]
- Zelus, E.I.; Panduro, A.; Deshmukh, I.; Grime, J.; Alperin, M.; Vahabzadeh-Hagh, A.M.; Christman, K.L. Immunomodulatory extracellular matrix hydrogel induces tissue regeneration in a model of partial glossectomy. Bioact. Mater. 2024, 38, 528–539. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, E.; Mao, Y.; Luan, J.; Fu, S. A Bibliometric Analysis of Research on Decellularized Matrix for Two Decades. Tissue Eng. Part C Methods 2023, 29, 395–409. [Google Scholar] [CrossRef]
- Jiang, S.; Zhuang, Y.; Cai, M.; Wang, X.; Lin, K. Decellularized extracellular matrix: A promising strategy for skin repair and regeneration. Eng. Regen. 2023, 4, 357–374. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.E.; Leeming, D.J.; Henriksen, K.; Mortensen, J.H.; Nielsen, S.H.; Anstee, Q.M.; Sanyal, A.J.; Karsdal, M.A.; Schuppan, D. ECM formation and degradation during fibrosis, repair, and regeneration. NPJ Metab. Health Dis. 2025, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Henninger, H.B.; Ellis, B.J.; Scott, S.A.; Weiss, J.A. Contributions of elastic fibers, collagen, and extracellular matrix to the multiaxial mechanics of ligament. J. Mech. Behav. Biomed. Mater. 2019, 99, 118–126. [Google Scholar] [CrossRef]
- Mattson, J.M.; Turcotte, R.; Zhang, Y. Glycosaminoglycans contribute to extracellular matrix fiber recruitment and arterial wall mechanics. Biomech. Model. Mechanobiol. 2017, 16, 213–225. [Google Scholar] [CrossRef]
- Pan, W.; Roccabianca, S.; Basson, M.D.; Bush, T.R. Influences of sodium and glycosaminoglycans on skin oedema and the potential for ulceration: A finite-element approach. R. Soc. Open Sci. 2019, 6, 182076. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Sharma, D.D.; Jayakumar, G.C.; Madhan, B.; Zameer, F. A review on an imperative by-product: Glycosaminoglycans- A holistic approach. Carbohydr. Polym. Technol. Appl. 2023, 5, 100275. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Vives, R.R.; Schaefer, L.; Gotte, M.; Merline, R.; Passi, A.; Heldin, P.; Magalhaes, A.; Reis, C.A.; Skandalis, S.S.; et al. A biological guide to glycosaminoglycans: Current perspectives and pending questions. FEBS J. 2024, 291, 3331–3366. [Google Scholar] [CrossRef]
- Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Mierke, C.T. Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells. Cells 2024, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Dibus, M.; Joshi, O.; Ivaska, J. Novel tools to study cell-ECM interactions, cell adhesion dynamics and migration. Curr. Opin. Cell Biol. 2024, 88, 102355. [Google Scholar] [CrossRef]
- Morgan, M.R.; Humphries, M.J.; Bass, M.D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007, 8, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; You, J.; Fu, J.; Wang, X.; Zhang, Y. Phosphatidylinositol 3-Kinase/Akt Mediates Integrin Signaling To Control RNA Polymerase I Transcriptional Activity. Mol. Cell Biol. 2016, 36, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Yan, Y.; Song, B.; Zhu, S.; Mei, Q.; Wu, K. Focal adhesion kinase: From biological functions to therapeutic strategies. Exp. Hematol. Oncol. 2023, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Janson, I.A.; Putnam, A.J. Extracellular matrix elasticity and topography: Material-based cues that affect cell function via conserved mechanisms. J. Biomed. Mater. Res. A 2015, 103, 1246–1258. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef]
- Zhu, J.; Clark, R.A.F. Fibronectin at select sites binds multiple growth factors and enhances their activity: Expansion of the collaborative ECM-GF paradigm. J. Investig. Dermatol. 2014, 134, 895–901. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- de Castro Brás, L.E.; Frangogiannis, N.G. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Fu, K.; Zheng, X.; Chen, Y.; Wu, L.; Yang, Z.; Chen, X.; Song, W. Role of matrix metalloproteinases in diabetic foot ulcers: Potential therapeutic targets. Front. Pharmacol. 2022, 13, 1050630. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Vallet, S.D. Fragments generated upon extracellular matrix remodeling: Biological regulators and potential drugs. Matrix Biol. 2019, 75–76, 170–189. [Google Scholar] [CrossRef]
- Chen, W.; Chen, M.; Chen, S.; Wang, S.; Huang, Z.; Zhang, L.; Wu, J.; Peng, W.; Li, H.; Wen, F. Decellularization of fish tissues for tissue engineering and regenerative medicine applications. Regen. Biomater. 2024, 12, rbae138. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Phang, S.J.; Basak, S.; Teh, H.X.; Packirisamy, G.; Fauzi, M.B.; Kuppusamy, U.R.; Neo, Y.P.; Looi, M.L. Advancements in Extracellular Matrix-Based Biomaterials and Biofabrication of 3D Organotypic Skin Models. ACS Biomater. Sci. Eng. 2022, 8, 3220–3241. [Google Scholar] [CrossRef]
- Kumar, S.; Saha, D.; Ray, D.; Aswal, V.K. Surfactant-driven modifications in protein structure. Soft Matter 2025, 21, 4979–4998. [Google Scholar] [CrossRef]
- Soulie, M.; Deletraz, A.; Wehbie, M.; Mahler, F.; Bouchemal, I.; Le Roy, A.; Petit-Hartlein, I.; Keller, S.; Meister, A.; Pebay-Peyroula, E.; et al. Zwitterionic fluorinated detergents: From design to membrane protein applications. Biochimie 2023, 205, 40–52. [Google Scholar] [CrossRef]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the retention of tissue niches. J. Tissue Eng. 2022, 13, 20417314221101151. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.E.; Willacy, O.; Madsen, C.D.; Rafaeva, M.; Elisabeth Heumuller, S.; Bock, F.; Sengle, G.; Koch, M.; Imhof, T.; Zaucke, F.; et al. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat. Protoc. 2019, 14, 3395–3425. [Google Scholar] [CrossRef]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The impact of detergents on the tissue decellularization process: A ToF-SIMS study. Acta Biomater. 2017, 50, 207–219. [Google Scholar] [CrossRef]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef] [PubMed]
- Sueters, J.; de Boer, L.; Groenman, F.; Huirne, J.A.F.; Smit, T.H.; Zaat, S.A.J. A sterilization method for human decellularized vaginal matrices. Sci. Rep. 2024, 14, 31728. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Balestrini, J.L.; Mendez, J.; Calle, E.A.; Zhao, L.; Niklason, L.E. Influence of pH on extracellular matrix preservation during lung decellularization. Tissue Eng. Part C Methods 2014, 20, 1028–1036. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Shevchuk, O.I.; Korcheva, V.V.; Moskalenko, N.S.; Kyryk, V.M.; Kot, K.V.; Krasnienkov, D.S. Application of decellularization methods for scaffold production: Advantages, disadvantages, biosafety and modifications. Front. Bioeng. Biotechnol. 2025, 13, 1621641. [Google Scholar] [CrossRef]
- Cornelison, R.C.; Wellman, S.M.; Park, J.H.; Porvasnik, S.L.; Song, Y.H.; Wachs, R.A.; Schmidt, C.E. Development of an apoptosis-assisted decellularization method for maximal preservation of nerve tissue structure. Acta Biomater. 2018, 77, 116–126. [Google Scholar] [CrossRef]
- Gadre, M.; Kasturi, M.; Agarwal, P.; Vasanthan, K.S. Decellularization and Their Significance for Tissue Regeneration in the Era of 3D Bioprinting. ACS Omega 2024, 9, 7375–7392. [Google Scholar] [CrossRef]
- Allu, I.; Sahi, A.K.; Koppadi, M.; Gundu, S.; Sionkowska, A. Decellularization Techniques for Tissue Engineering: Towards Replicating Native Extracellular Matrix Architecture in Liver Regeneration. J. Funct. Biomater. 2023, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.D.; Nemets, E.A.; Grigoriev, A.M.; Kirsanova, L.A.; Ryzhikova, V.A.; Volkova, E.A.; Basok, Y.B.; Sevastianov, V.I. Effect of trypsin on biochemical and functional properties of decellularized porcine articular cartilage. Russ. J. Transplantology Artif. Organs 2023, 25, 76–86. [Google Scholar] [CrossRef]
- Narciso, M.; Ulldemolins, A.; Junior, C.; Otero, J.; Navajas, D.; Farre, R.; Gavara, N.; Almendros, I. Novel Decellularization Method for Tissue Slices. Front. Bioeng. Biotechnol. 2022, 10, 832178. [Google Scholar] [CrossRef]
- Boone, M.; Draye, J.P.; Verween, G.; Pirnay, J.P.; Verbeken, G.; De Vos, D.; Rose, T.; Jennes, S.; Jemec, G.B.; Del Marmol, V. Real-time three-dimensional imaging of epidermal splitting and removal by high-definition optical coherence tomography. Exp. Dermatol. 2014, 23, 725–730. [Google Scholar] [CrossRef]
- Ribes Martinez, E.; Franko, Y.; Franko, R.; Ferronato, G.A.; Viana, A.E.S.; Windenbach, E.; Stoeckl, J.B.; Fröhlich, T.; Ferraz, M. Developing and characterising bovine decellularized extracellular matrix hydrogels to biofabricate female reproductive tissues. Acta Biomater. 2025, 196, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Kuşoğlu, A.; Yangın, K.; Özkan, S.N.; Sarıca, S.; Örnek, D.; Solcan, N.; Karaoğlu, İ.C.; Kızılel, S.; Bulutay, P.; Fırat, P.; et al. Different Decellularization Methods in Bovine Lung Tissue Reveals Distinct Biochemical Composition, Stiffness, and Viscoelasticity in Reconstituted Hydrogels. ACS Appl. Bio Mater. 2023, 6, 793–805. [Google Scholar] [CrossRef]
- Park, J.-Y.; Park, K.-M.; Haque, A. Lipase and Its Unique Selectivity: A Mini-Review. J. Chem. 2022, 2022, 7609019. [Google Scholar] [CrossRef]
- Young, D.A.; Ibrahim, D.O.; Hu, D.; Christman, K.L. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011, 7, 1040–1049. [Google Scholar] [CrossRef]
- Young, D.A.; Choi, Y.S.; Engler, A.J.; Christman, K.L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 2013, 34, 8581–8588. [Google Scholar] [CrossRef]
- Song, M.; Wang, W.; Ye, Q.; Bu, S.; Shen, Z.; Zhu, Y. The repairing of full-thickness skin deficiency and its biological mechanism using decellularized human amniotic membrane as the wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 739–747. [Google Scholar] [CrossRef]
- Shi, P.; Gao, M.; Shen, Q.; Hou, L.; Zhu, Y.; Wang, J. Biocompatible surgical meshes based on decellularized human amniotic membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Filkin, S.Y.; Lipkin, A.V.; Fedorov, A.N. Phospholipase Superfamily: Structure, Functions, and Biotechnological Applications. Biochemistry 2020, 85 (Suppl. S1), S177–S195. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, Y.; Li, N.; Huang, M.; Duan, H.; Ge, J.; Xiang, P.; Wang, Z. The use of phospholipase A(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials 2009, 30, 3513–3522. [Google Scholar] [CrossRef]
- Rabbani, M.; Zakian, N.; Alimoradi, N. Contribution of Physical Methods in Decellularization of Animal Tissues. J. Med. Signals Sens. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Griffin, M.; Naik, A.; Szarko, M.; Butler, P.E.M. Optimising the decellularization of human elastic cartilage with trypsin for future use in ear reconstruction. Sci. Rep. 2018, 8, 3097. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Hui, L. Preparation and characterization of acellular adipose tissue matrix using a combination of physical and chemical treatments. Mol. Med. Rep. 2018, 17, 138–146. [Google Scholar] [CrossRef]
- He, Y.; Lin, M.; Wang, X.; Guan, J.; Dong, Z.; Lu, F.; Xing, M.; Feng, C.; Li, X. Optimized adipose tissue engineering strategy based on a neo-mechanical processing method. Wound Repair Regen. 2018, 26, 163–171. [Google Scholar] [CrossRef]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhou, Q.; Gao, H.; Li, S.; Dong, M.; Wang, T.; Jia, Y.; Dong, C.; Wang, X.; Guo, Z.; et al. Protectively Decellularized Porcine Cornea versus Human Donor Cornea for Lamellar Transplantation. Adv. Funct. Mater. 2019, 29, 1902491. [Google Scholar] [CrossRef]
- Giatsidis, G.; Guyette, J.P.; Ott, H.C.; Orgill, D.P. Development of a large-volume human-derived adipose acellular allogenic flap by perfusion decellularization. Wound Repair Regen. 2018, 26, 245–250. [Google Scholar] [CrossRef]
- Nicholls, D.L.; Rostami, S.; Karoubi, G.; Haykal, S. Perfusion decellularization for vascularized composite allotransplantation. SAGE Open Med. 2022, 10, 20503121221123893. [Google Scholar] [CrossRef]
- Hülsmann, J.; Aubin, H.; Oberle, F.; Schütterle, N.; Bandesha, S.T.; Iijima, M.; Lichtenberg, A.; Akhyari, P. Mechanistics of biomass discharge during whole-heart decellularization. Biomed. Mater. 2018, 13, 035014. [Google Scholar] [CrossRef]
- Suzuki, T.; Watanabe, T.; Tomiyama, F.; Ito, T.; Okada, Y. Transplantation of Bioengineered Lung Using Decellularized Mouse Lungs and Primary Human Endothelial Cells. J. Vis. Exp. 2025, 28, e67565. [Google Scholar] [CrossRef]
- Daryabari, S.S.; Fendereski, K.; Ghorbani, F.; Dehnavi, M.; Shafikhani, Y.; Omranipour, A.; Zeraatian-Nejad Davani, S.; Majidi Zolbin, M.; Tavangar, S.M.; Kajbafzadeh, A.M. Whole-organ decellularization of the human uterus and in vivo application of the bio-scaffolds in animal models. J. Assist. Reprod. Genet. 2022, 39, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A.M.; Khorramirouz, R.; Nabavizadeh, B.; Ladi Seyedian, S.S.; Akbarzadeh, A.; Heidari, R.; Masoumi, A.; Azizi, B.; Seyed Hossein Beigi, R. Whole organ sheep kidney tissue engineering and in vivo transplantation: Effects of perfusion-based decellularization on vascular integrity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ozudogru, E.; Kurt, T.; Derkus, B.; Cengiz, U.; Arslan, Y.E. Supercritical CO2-Mediated Decellularization of Bovine Spinal Cord Meninges: A Comparative Study for Decellularization Performance. ACS Omega 2024, 9, 48781–48790. [Google Scholar] [CrossRef]
- Sung, S.Y.; Lin, Y.W.; Wu, C.C.; Lin, C.Y.; Hsu, P.S.; Periasamy, S.; Nagarajan, B.; Hsieh, D.J.; Tsai, Y.T.; Tsai, C.S.; et al. Supercritical carbon dioxide-decellularized arteries exhibit physiologic-like vessel regeneration following xenotransplantation in rats. Biomater. Sci. 2023, 11, 2566–2580. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Nascari, D.G.; Saldin, L.T.; Kolich, B.; Lee, Y.C.; Crum, R.J.; El-Mossier, S.O.; D′Angelo, W.; Dziki, J.L.; Badylak, S.F. Ultrasonic cavitation to prepare ECM hydrogels. Acta Biomater. 2020, 108, 77–86. [Google Scholar] [CrossRef]
- Koo, M.A.; Jeong, H.; Hong, S.H.; Seon, G.M.; Lee, M.H.; Park, J.C. Preconditioning process for dermal tissue decellularization using electroporation with sonication. Regen. Biomater. 2022, 9, rbab071. [Google Scholar] [CrossRef]
- Mora-Navarro, C.; Garcia, M.E.; Sarker, P.; Ozpinar, E.W.; Enders, J.R.; Khan, S.; Branski, R.C.; Freytes, D.O. Monitoring decellularization via absorbance spectroscopy during the derivation of extracellular matrix scaffolds. Biomed. Mater. 2021, 17, 015008. [Google Scholar] [CrossRef]
- Barbulescu, G.I.; Buica, T.P.; Goje, I.D.; Bojin, F.M.; Ordodi, V.L.; Olteanu, G.E.; Heredea, R.E.; Paunescu, V. Optimization of Complete Rat Heart Decellularization Using Artificial Neural Networks. Micromachines 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, X.; Wang, Y.; Ouyang, D. Harnessing the power of machine learning into tissue engineering: Current progress and future prospects. Burn. Trauma 2024, 12, tkae053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Pi, Q.; van Genderen, A.M. Microfluidic Bioprinting for Engineering Vascularized Tissues and Organoids. J. Vis. Exp. 2017, 11, e55957. [Google Scholar] [CrossRef]
- Chae, S.; Ha, D.H.; Lee, H. 3D bioprinting strategy for engineering vascularized tissue models. Int. J. Bioprint. 2023, 9, 748. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Li, Y.; Zhou, L.; Dan, N.; Min, J.; Chen, Y.; Wang, Y. Evolution of biomimetic ECM scaffolds from decellularized tissue matrix for tissue engineering: A comprehensive review. Int. J. Biol. Macromol. 2023, 246, 125672. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, G. Comparative Analysis of Decellularization Methods for the Production of Decellularized Umbilical Cord Matrix. Curr. Issues Mol. Biol. 2024, 46, 7686–7701. [Google Scholar] [CrossRef]
- Burk, J.; Erbe, I.; Berner, D.; Kacza, J.; Kasper, C.; Pfeiffer, B.; Winter, K.; Brehm, W. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng. Part C Methods 2014, 20, 276–284. [Google Scholar] [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef]
- Tavares, D.F.; Mano, J.F.; Oliveira, M.B. Advances in abiotic tissue-based biomaterials: A focus on decellularization and devitalization techniques. Mater. Today Bio 2025, 32, 101735. [Google Scholar] [CrossRef]
- Ma, R.; Gao, X.; Jin, Y.; Wang, X.; Li, R.; Qiao, R.; Wang, X.; Liu, D.; Xie, Z.; Wang, L.; et al. Is there a duration-characteristic relationship for trypsin exposure on tendon? A study on anterior cruciate ligament reconstruction in a rabbit model. Front. Med. 2024, 11, 1417930. [Google Scholar] [CrossRef]
- Long, J.; Qin, Z.; Chen, G.; Song, B.; Zhang, Z. Decellularized extracellular matrix (d-ECM): The key role of the inflammatory process in pre-regeneration after implantation. Biomater. Sci. 2023, 11, 1215–1235. [Google Scholar] [CrossRef] [PubMed]
- Naso, F.; Gandaglia, A. Can Heart Valve Decellularization Be Standardized? A Review of the Parameters Used for the Quality Control of Decellularization Processes. Front. Bioeng. Biotechnol. 2022, 10, 830899. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Costa, J.; Rettig Emanuel, J. PicoGreen Quantitation of DNA: Effective Evaluation of Samples Pre-or Psost-PCR. Nucleic Acids Res. 1996, 24, 2623–2625. [Google Scholar] [CrossRef]
- Xiong, G.; Flynn, T.J.; Chen, J.; Trinkle, C.; Xu, R. Development of an ex vivo breast cancer lung colonization model utilizing a decellularized lung matrix. Integr. Biol. 2015, 7, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, K.H.; Ahn, J.S.; Kim, H.B.; Yi, J.H.; Kim, H.S. A simple segmentation and quantification method for numerical quantitative analysis of cells and tissues. Technol. Health Care 2020, 28 (Suppl. S1), 401–410. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Huang, J.; Yang, L.; Xie, Y.; Xiao, G. Automatic extraction of cell nuclei from H&E-stained histopathological images. J. Med. Imaging 2017, 4, 027502. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Hoshiba, T.; Kawazoe, N.; Tateishi, T.; Chen, G. Development of stepwise osteogenesis-mimicking matrices for the regulation of mesenchymal stem cell functions. J. Biol. Chem. 2009, 284, 31164–31173. [Google Scholar] [CrossRef] [PubMed]
- Narciso, M.; Otero, J.; Navajas, D.; Farré, R.; Almendros, I.; Gavara, N. Image-Based Method to Quantify Decellularization of Tissue Sections. Int. J. Mol. Sci. 2021, 22, 8399. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef]
- Isaeva, E.V.; Beketov, E.E.; Arguchinskaya, N.V.; Ivanov, S.; Shegay, P.V.; Kaprin, A.D. Decellularized Extracellular Matrix for Tissue Engineering (Review). Sovrem. Tekhnologii Med. 2022, 14, 57–68. [Google Scholar] [CrossRef]
- Morris, A.H.; Chang, J.; Kyriakides, T.R. Inadequate Processing of Decellularized Dermal Matrix Reduces Cell Viability In Vitro and Increases Apoptosis and Acute Inflammation In Vivo. Biores Open Access 2016, 5, 177–187. [Google Scholar] [CrossRef]
- Zvarova, B.; Uhl, F.E.; Uriarte, J.J.; Borg, Z.D.; Coffey, A.L.; Bonenfant, N.R.; Weiss, D.J.; Wagner, D.E. Residual Detergent Detection Method for Nondestructive Cytocompatibility Evaluation of Decellularized Whole Lung Scaffolds. Tissue Eng. Part C Methods 2016, 22, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, Y.; Lin, J.; Liu, H.H. Direct and Indirect Culture Methods for Studying Biodegradable Implant Materials In Vitro. J. Vis. Exp. 2022, 15, e63065. [Google Scholar] [CrossRef]
- Hu, C.; He, S.; Lee, Y.J.; He, Y.; Kong, E.M.; Li, H.; Anastasio, M.A.; Popescu, G. Live-dead assay on unlabeled cells using phase imaging with computational specificity. Nat. Commun. 2022, 13, 713. [Google Scholar] [CrossRef]
- Busra, F.M.; Lokanathan, Y.; Nadzir, M.M.; Saim, A.; Idrus, R.B.H.; Chowdhury, S.R. Attachment, Proliferation, and Morphological Properties of Human Dermal Fibroblasts on Ovine Tendon Collagen Scaffolds: A Comparative Study. Malays. J. Med. Sci. 2017, 24, 33–43. [Google Scholar] [CrossRef]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.M.; Majidi Zolbin, M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef] [PubMed]
- Mihalečko, J.; Boháč, M.; Danišovič, Ľ.; Koller, J.; Varga, I.; Kuniaková, M. Acellular Dermal Matrix in Plastic and Reconstructive Surgery. Physiol. Res. 2022, 71 (Suppl. S1), S51–S57. [Google Scholar] [CrossRef] [PubMed]
- Mahdian, M.; Tabatabai, T.S.; Abpeikar, Z.; Rezakhani, L.; Khazaei, M. Nerve regeneration using decellularized tissues: Challenges and opportunities. Front. Neurosci. 2023, 17, 1295563. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X.; Jiang, X.; Dai, B.; Zhang, L.; Zhu, Y. Decellularized extracellular matrix materials for treatment of ischemic cardiomyopathy. Bioact. Mater. 2024, 33, 460–482. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Terzini, M.; Bignardi, C.; Castagnoli, C.; Cambieri, I.; Zanetti, E.M.; Audenino, A.L. Ex Vivo Dermis Mechanical Behavior in Relation to Decellularization Treatment Length. Open Biomed. Eng. J. 2016, 10, 34–42. [Google Scholar] [CrossRef]
- Tallapaneni, V.; Kalaivani, C.; Pamu, D.; Mude, L.; Singh, S.K.; Karri, V. Acellular Scaffolds as Innovative Biomaterial Platforms for the Management of Diabetic Wounds. Tissue Eng. Regen. Med. 2021, 18, 713–734. [Google Scholar] [CrossRef]
- Haney, N.M.; Huang, M.M.; Liu, J.L.; Hawksworth, D.J.; Burnett, A.L. Acellular Dermal Matrix Tissues in Genitourinary Reconstructive Surgery: A Review of the Literature and Case Discussions. Sex. Med. Rev. 2021, 9, 488–497. [Google Scholar] [CrossRef]
- Gabriel, A.; Maxwell, G.P. AlloDerm RTU Integration and Clinical Outcomes When Used for Reconstructive Breast Surgery. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1744. [Google Scholar] [CrossRef]
- Roth, J.S.; Zachem, A.; Plymale, M.A.; Davenport, D.L. Complex Ventral Hernia Repair with Acellular Dermal Matrices: Clinical and Quality of Life Outcomes. Am. Surg. 2017, 83, 141–147. [Google Scholar] [CrossRef]
- Zang, J.; Su, L.; Luan, Q.; Liu, G.; Li, S.; Yu, X. Clinical and histological evaluation of the use of acellular dermal matrix (ADM) membrane in peri-implant vertical soft tissue augmentation: A controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Wonski, B.T.; Saliganan, D.M.; Rteil, A.; Kabbani, L.S.; Lam, M.T. Decellularized dermis extracellular matrix alloderm mechanically strengthens biological engineered tunica adventitia-based blood vessels. Sci. Rep. 2021, 11, 11384. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.N.; Zhang, X.R.; Nilsu, D.; Faruq, O.; VAN ANH, L.T.; Nam, S.Y.; Heo, C.Y. In Vivo Comparison of Three Human Acellular Dermal Matrices for Breast Reconstruction. In Vivo 2021, 35, 2719–2728. [Google Scholar] [CrossRef]
- Wilson, R.L.; Kirwan, C.C.; Johnson, R.K.; O’Donoghue, J.M.; Linforth, R.A.; Harvey, J.R. Breast Reconstruction Outcomes with and without Strattice: Long-Term Outcomes of a Multicenter Study Comparing Strattice Immediate Implant Breast Reconstruction with Submuscular Implant Reconstruction. Plast. Reconstr. Surg. 2023, 152, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P.; Gandhi, A.; Saini, S. Integra® Dermal Regeneration Template: From Design to Clinical Use. Cureus 2023, 15, e38608. [Google Scholar] [CrossRef]
- Zilic, L.; Wilshaw, S.P.; Haycock, J.W. Decellularisation and histological characterisation of porcine peripheral nerves. Biotechnol. Bioeng. 2016, 113, 2041–2053. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, G.; Hou, B.; Song, E.; Wen, J.; Ba, Y.; Zhu, D.; Wang, G.; Qin, F. Effects of ECM proteins (laminin, fibronectin, and type IV collagen) on the biological behavior of Schwann cells and their roles in the process of remyelination after peripheral nerve injury. Front. Bioeng. Biotechnol. 2023, 11, 1133718. [Google Scholar] [CrossRef]
- Tusnim, J.; Budharaju, K.; Grasman, J.M. Fabrication of ECM protein coated hollow collagen channels to study peripheral nerve regeneration. Sci. Rep. 2024, 14, 16096. [Google Scholar] [CrossRef]
- Qiu, S.; Deng, P.J.; He, F.L.; Yan, L.W.; Tu, Z.H.; Liu, X.L.; Quan, D.P.; Bai, Y.; Zheng, C.B.; Zhu, Q.T. A decellularized nerve matrix scaffold inhibits neuroma formation in the stumps of transected peripheral nerve after peripheral nerve injury. Neural Regen. Res. 2023, 18, 664–670. [Google Scholar] [CrossRef]
- Ansaripour, A.; Thompson, A.; Styron, J.F.; Javanbakht, M. Cost-effectiveness analysis of Avance(®) allograft for the treatment of peripheral nerve injuries in the USA. J. Comp. Eff. Res. 2024, 13, e230113. [Google Scholar] [CrossRef]
- Mathot, F.; Rbia, N.; Thaler, R.; Bishop, A.T.; van Wijnen, A.J.; Shin, A.Y. Introducing human adipose-derived mesenchymal stem cells to Avance(®) nerve grafts and NeuraGen(®) nerve guides. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1473–1481. [Google Scholar] [CrossRef]
- Whitehead, K.M.; Hendricks, H.K.L.; Cakir, S.N.; de Castro Brás, L.E. ECM roles and biomechanics in cardiac tissue decellularization. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H585–H596. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Montón, C.; Prat-Vidal, C.; Roura, S.; Soler-Botija, C.; Bayes-Genis, A. Cardiac Tissue Engineering and the Bioartificial Heart. Rev. Española Cardiol. (Engl. Ed.) 2013, 66, 391–399. [Google Scholar] [CrossRef]
- McClure, R.S.; Narayanasamy, N.; Wiegerinck, E.; Lipsitz, S.; Maloney, A.; Byrne, J.G.; Aranki, S.F.; Couper, G.S.; Cohn, L.H. Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: Up to 17-year follow-up in 1000 patients. Ann. Thorac. Surg. 2010, 89, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, J.B.; Kim, M.H.; Kim, W.H.; Lee, M.K.; Lee, S.Y. Aortic valve leaflet replacement with bovine pericardium to preserve native dynamic capabilities of the aortic annulus. Tex. Heart Inst. J. 2014, 41, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Ohata, K.; Ott, H.C. Human-scale lung regeneration based on decellularized matrix scaffolds as a biologic platform. Surg. Today 2020, 50, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Muller, M.; Costa, P.F.; Kohl, Y. Advanced In Vitro Lung Models for Drug and Toxicity Screening: The Promising Role of Induced Pluripotent Stem Cells. Adv. Biol. 2022, 6, e2101139. [Google Scholar] [CrossRef]
- Taniguchi, D.; Kamata, S.; Rostami, S.; Tuin, S.; Marin-Araujo, A.; Guthrie, K.; Petersen, T.; Waddell, T.K.; Karoubi, G.; Keshavjee, S.; et al. Evaluation of a decellularized bronchial patch transplant in a porcine model. Sci. Rep. 2023, 13, 21773. [Google Scholar] [CrossRef]
- Gilpin, S.E.; Charest, J.M.; Ren, X.; Tapias, L.F.; Wu, T. Regenerative potential of human airway stem cells in lung epithelial engineering. Biomaterials 2016, 108, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Morissette Martin, P.; Shridhar, A.; Yu, C.; Brown, C.; Flynn, L.E. Decellularized Adipose Tissue Scaffolds for Soft Tissue Regeneration and Adipose-Derived Stem/Stromal Cell Delivery. Methods Mol. Biol. 2018, 1773, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Qiu, L.H.; Xiong, S.H.; Dang, J.L.; Rong, X.K.; Hou, M.M.; Wang, K.; Yu, Z.; Yi, C.G. Decellularized adipose matrix provides an inductive microenvironment for stem cells in tissue regeneration. World, J. Stem Cells 2020, 12, 585–603. [Google Scholar] [CrossRef]
- Chen, C.L.; Wei, S.Y.; Chen, W.L.; Hsu, T.L.; Chen, Y.C. Reconstructing vascular networks promotes the repair of skeletal muscle following volumetric muscle loss by pre-vascularized tissue constructs. J. Tissue Eng. 2023, 14, 20417314231201231. [Google Scholar] [CrossRef]
- Melnick, B.A.; Abu-Romman, A.; Fine, K.S.; Barron-Cervantes, N.M.; Duckworth, E.D.; Chnari, E.; Long, M.; Ramsay, M.D.; O′Connor, M.J.; Ho, K.C.; et al. Decellularized Adipose Matrix for Soft Tissue Regeneration: Enhancing Angiogenesis and Adipogenesis. Tissue Eng. Part B Rev. 2025; ahead of print. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, B.; Shu, J.; Wang, H.; Han, Y.; Zeng, Q.; Chen, Y.; Xi, J.; Tao, R.; Pei, X.; et al. Human decellularized adipose matrix derived hydrogel assists mesenchymal stem cells delivery and accelerates chronic wound healing. J. Biomed. Mater. Res. A 2021, 109, 1418–1428. [Google Scholar] [CrossRef]
- Xiong, C.; Yao, W.; Tao, R.; Yang, S.; Jiang, W.; Xu, Y.; Zhang, J.; Han, Y. Application of Decellularized Adipose Matrix as a Bioscaffold in Different Tissue Engineering. Aesthetic Plast. Surg. 2024, 48, 1045–1053. [Google Scholar] [CrossRef]
- Murchison, A.C.; Odanga, J.J.; Treadwell, M.L.; Breathwaite, E.K.; Weaver, J.R.; Lee, J.B. Human Placenta-Derived ECM Supports Tri-Lineage Differentiation of Human Induced Pluripotent Stem Cells. Int. J. Stem Cells 2020, 13, 432–438. [Google Scholar] [CrossRef]
- Komemi, O.; Shochet, G.E.; Pomeranz, M.; Fishman, A.; Pasmanik-Chor, M.; Drucker, L.; Matalon, S.T.; Lishner, M. Placenta-conditioned extracellular matrix (ECM) activates breast cancer cell survival mechanisms: A key for future distant metastases. Int. J. Cancer 2019, 144, 1633–1644. [Google Scholar] [CrossRef]
- Moghassemi, S.; Nikanfar, S.; Dadashzadeh, A.; Sousa, M.J.; Wan, Y.; Sun, F.; Colson, A.; De Windt, S.; Kwaspen, L.; Kanbar, M.; et al. The revolutionary role of placental derivatives in biomedical research. Bioact. Mater. 2025, 49, 456–485. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, C.; Vishwakarma, S.K.; Raju, N.; Ahmed, S.I.; Bardia, A.; Khan, M.A.; Annamaneni, S.; Khan, A.A. Fabrication of Decellularized Amnion and Chorion Scaffolds to Develop Bioengineered Cell-Laden Constructs. Cell Mol. Bioeng. 2022, 15, 137–150. [Google Scholar] [CrossRef]
- Galvez, P.; Ahmed Omar, N.; Siadous, R.; Durand, M.; Comperat, L.; Lafarge, X.; Gindraux, F.; Sentilhes, L.; Fricain, J.C.; L’Heureux, N.; et al. In vitro and in vivo assessment of a new acellular human amnion/chorion membrane device for guided bone regeneration. Sci. Rep. 2025, 15, 5483. [Google Scholar] [CrossRef]
- He, M.; Callanan, A.; Lagaras, K.; Steele, J.A.M.; Stevens, M.M. Optimization of SDS exposure on preservation of ECM characteristics in whole organ decellularization of rat kidneys. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1352–1360. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, S.; Liu, Y.; Sun, C.; Cheng, G.; Luan, Y.; Li, K.; Wang, J.; Xie, X.; Zhao, S. Porcine kidneys as a source of ECM scaffold for kidney regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Narayanan, K.; Leong, M.F.; Ibrahim, M.S.; Chua, Y.P.; Khoo, V.M.; Wan, A.C. Functional Kidney Bioengineering with Pluripotent Stem-Cell-Derived Renal Progenitor Cells and Decellularized Kidney Scaffolds. Adv. Healthc. Mater. 2016, 5, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Chi, P.L.; Chen, H.Y.; Ou, S.H.; Chou, K.J.; Fang, H.C.; Chen, C.L.; Huang, C.W.; Ho, T.Y.; Lee, P.T. Kidney bioengineering by using decellularized kidney scaffold and renal progenitor cells. Tissue Cell 2022, 74, 101699. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, S.; Bideskan, A.E.; Aslzare, M.; Tavakkoli, M.; Bahrami, A.R.; Hosseinian, S.; Matin, M.M.; Rad, A.K. Decellularization with triton X-100 provides a suitable model for human kidney bioengineering using human mesenchymal stem cells. Life Sci. 2022, 295, 120167. [Google Scholar] [CrossRef]
- Choi, M.; Yang, Y.B.; Park, S.; Rahaman, S.; Tripathi, G.; Lee, B.T. Effect of Co-culture of mesenchymal stem cell and glomerulus endothelial cell to promote endothelialization under optimized perfusion flow rate in whole renal ECM scaffold. Mater. Today Bio 2022, 17, 100464. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, J.; Wu, Q.; Zhou, Y.; Li, Y.; Wu, X.; Shi, Y.; Li, L.; Bu, H. Method for perfusion decellularization of porcine whole liver and kidney for use as a scaffold for clinical-scale bioengineering engrafts. Xenotransplantation 2015, 22, 48–61. [Google Scholar] [CrossRef]
- Naeem, E.M.; Sajad, D.; Talaei-Khozani, T.; Khajeh, S.; Azarpira, N.; Alaei, S.; Tanideh, N.; Reza, T.M.; Razban, V. Decellularized liver transplant could be recellularized in rat partial hepatectomy model. J. Biomed. Mater. Res. A 2019, 107, 2576–2588. [Google Scholar] [CrossRef] [PubMed]
- Willemse, J.; Verstegen, M.M.A.; Vermeulen, A.; Schurink, I.J.; Roest, H.P.; van der Laan, L.J.W.; de Jonge, J. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110200. [Google Scholar] [CrossRef]
- Antarianto, R.D.; Pragiwaksana, A.; Septiana, W.L.; Mazfufah, N.F.; Mahmood, A. Hepatocyte Differentiation from iPSCs or MSCs in Decellularized Liver Scaffold: Cell-ECM Adhesion, Spatial Distribution, and Hepatocyte Maturation Profile. Organogenesis 2022, 18, 2061263. [Google Scholar] [CrossRef]
- Ebrahim, N.; Badr, O.A.M.; Yousef, M.M.; Hassouna, A.; Sabry, D.; Farid, A.S.; Mostafa, O.; Saihati, H.A.A.; Seleem, Y.; Abd El Aziz, E.; et al. Functional Recellularization of Acellular Rat Liver Scaffold by Induced Pluripotent Stem Cells: Molecular Evidence for Wnt/B-Catenin Upregulation. Cells 2021, 10, 2819. [Google Scholar] [CrossRef]
- Campinoti, S.; Almeida, B.; Goudarzi, N.; Bencina, S.; Grundland Freile, F.; McQuitty, C.; Natarajan, D.; Cox, I.J.; Le Guennec, A.; Khati, V.; et al. Rat liver extracellular matrix and perfusion bioreactor culture promote human amnion epithelial cell differentiation towards hepatocyte-like cells. J. Tissue Eng. 2023, 14, 20417314231219813. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Romero, R.; Sorokina, L.V.; Ballinger, K.R.; Place, L.W.; Kipper, M.J.; Khetani, S.R. A polyelectrolyte multilayer platform for investigating growth factor delivery modes in human liver cultures. J. Biomed. Mater. Res. A 2018, 106, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Caires-Júnior, L.C.; Goulart, E.; Telles-Silva, K.A.; Araujo, B.H.S.; Musso, C.M.; Kobayashi, G.; Oliveira, D.; Assoni, A.; Carvalho, V.M.; Ribeiro-Jr, A.F.; et al. Pre-coating decellularized liver with HepG2-conditioned medium improves hepatic recellularization. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111862. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Q.; Wang, S.; Zhang, J.; Fan, S.; Lin, X. Current Advances in the Development of Decellularized Plant Extracellular Matrix. Front. Bioeng. Biotechnol. 2021, 9, 712262. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Zenhausern, F. The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. [Google Scholar] [CrossRef]
- Gorbenko, N.; Rinaldi, G.; Sanchez, A.; Merna, N. Small-Caliber Vascular Grafts Engineered from Decellularized Leaves and Cross-Linked Gelatin. Tissue Eng. Part A 2023, 29, 397–409. [Google Scholar] [CrossRef]
- Kian, M.; Hashemi, S.S.; Derakhshanfar, A.; Darya, G.H.; Shahhossein, Z.; Saharkhiz, M.J. Decellularized Persian walnut leaf (Juglans regia) as a potential wound dressing scaffold: An experimental study. Front. Bioeng. Biotechnol. 2025, 13, 1524956. [Google Scholar] [CrossRef]
- Lombardi, J.; Stec, E.; Edwards, M.; Connell, T.; Sandor, M. Comparison of mechanical properties and host tissue response to OviTex™ and Strattice™ surgical meshes. Hernia 2023, 27, 987–997. [Google Scholar] [CrossRef]
- Shariatzadeh, S.; Shafiee, S.; Zafari, A.; Tayebi, T.; Yazdanpanah, G.; Majd, A.; Haj-Mirzaian, A.; Bahrami, S.; Niknejad, H. Developing a pro-angiogenic placenta derived amniochorionic scaffold with two exposed basement membranes as substrates for cultivating endothelial cells. Sci. Rep. 2021, 11, 22508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Long, D.W.; Huang, Y.; Chen, W.C.W.; Kim, K.; Wang, Y. Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction. Acta Biomater. 2019, 87, 140–151. [Google Scholar] [CrossRef]
- Kong, P.; Dong, J.; Li, W.; Li, Z.; Gao, R.; Liu, X.; Wang, J.; Su, Q.; Wen, B.; Ouyang, W.; et al. Extracellular Matrix/Glycopeptide Hybrid Hydrogel as an Immunomodulatory Niche for Endogenous Cardiac Repair after Myocardial Infarction. Adv. Sci. 2023, 10, e2301244. [Google Scholar] [CrossRef]
- Sutherland, A.J.; Converse, G.L.; Hopkins, R.A.; Detamore, M.S. The bioactivity of cartilage extracellular matrix in articular cartilage regeneration. Adv. Healthc. Mater. 2015, 4, 29–39. [Google Scholar] [CrossRef]
- Zhu, W.; Cao, L.; Song, C.; Pang, Z.; Jiang, H.; Guo, C. Cell-derived decellularized extracellular matrix scaffolds for articular cartilage repair. Int. J. Artif. Organs 2021, 44, 269–281. [Google Scholar] [CrossRef]
- Ma, S.; Hu, H.; Wu, J.; Li, X.; Ma, X.; Zhao, Z.; Liu, Z.; Wu, C.; Zhao, B.; Wang, Y.; et al. Functional extracellular matrix hydrogel modified with MSC-derived small extracellular vesicles for chronic wound healing. Cell Prolif. 2022, 55, e13196. [Google Scholar] [CrossRef]
- Vriend, L.; Sinkunas, V.; Camargo, C.P.; van der Lei, B.; Harmsen, M.C.; van Dongen, J.A. Extracellular Matrix-Derived Hydrogels to Augment Dermal Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2022, 28, 1093–1108. [Google Scholar] [CrossRef]
- Hou, N.; Xu, X.; Lv, D.; Lu, Y.; Li, J.; Cui, P.; Ma, R.; Luo, X.; Tang, Y.; Zheng, Y. Tissue-engineered esophagus: Recellular esophageal extracellular matrix based on perfusion-decellularized technique and mesenchymal stem cells. Biomed. Mater. 2021, 16, 055017. [Google Scholar] [CrossRef]
- Li, H.; Ghorbani, S.; Ling, C.C.; Yong, V.W.; Xue, M. The extracellular matrix as modifier of neuroinflammation and recovery in ischemic stroke and intracerebral hemorrhage. Neurobiol. Dis. 2023, 186, 106282. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, J.; Dang, J.; Rong, X.; Wang, K.; Zhang, Z.; Hou, M.; Yu, Z.; Yi, C. Bioactive decellularized adipose matrix prepared using a rapid, nonchemical/enzymatic method for adipogenesis. Biotechnol. Bioeng. 2024, 121, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Seow, D.; Yasui, Y.; Hurley, E.T.; Ross, A.W.; Murawski, C.D.; Shimozono, Y.; Kennedy, J.G. Extracellular Matrix Cartilage Allograft and Particulate Cartilage Allograft for Osteochondral Lesions of the Knee and Ankle Joints: A Systematic Review. Am. J. Sports Med. 2018, 46, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Tofigh, A.; Tajik, M. Comparing the standard surgical dressing with dehydrated amnion and platelet-derived growth factor dressings in the healing rate of diabetic foot ulcer: A randomized clinical trial. Diabetes Res. Clin. Pract. 2022, 185, 109775. [Google Scholar] [CrossRef]
- Serena, T.E.; Orgill, D.P.; Armstrong, D.G.; Galiano, R.D.; Glat, P.M.; Carter, M.J.; Kaufman, J.P.; Li, W.W.; Zelen, C.M. A Multicenter, Randomized, Controlled, Clinical Trial Evaluating Dehydrated Human Amniotic Membrane in the Treatment of Venous Leg Ulcers. Plast. Reconstr. Surg. 2022, 150, 1128–1136. [Google Scholar] [CrossRef]
- Game, F.; Gray, K.; Davis, D.; Sherman, R.; Chokkalingam, K.; Connan, Z.; Fakis, A.; Jones, M. The effectiveness of a new dried human amnion derived membrane in addition to standard care in treating diabetic foot ulcers: A patient and assessor blind, randomised controlled pilot study. Int. Wound J. 2021, 18, 692–700. [Google Scholar] [CrossRef]
- Hunter, J.D.; Johnson, T.D.; Braden, R.L.; Christman, K.L. Injectable ECM Scaffolds for Cardiac Repair. Methods Mol. Biol. 2022, 2485, 255–268. [Google Scholar] [CrossRef]
- Guagliano, G.; Volpini, C.; Sardelli, L.; Bloise, N.; Briatico-Vangosa, F.; Cornaglia, A.I.; Dotti, S.; Villa, R.; Visai, L.; Petrini, P. Hep3Gel: A Shape-Shifting Extracellular Matrix-Based, Three-Dimensional Liver Model Adaptable to Different Culture Systems. ACS Biomater. Sci. Eng. 2023, 9, 211–229. [Google Scholar] [CrossRef]
- Jansen, L.A.; Macadam, S.A. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: Part, I. A systematic review. Plast. Reconstr. Surg. 2011, 127, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.A.; De Caigny, P.; Guay, N.A.; Lineaweaver, W.C.; Shokrollahi, K. The evidence base for the acellular dermal matrix AlloDerm: A systematic review. Ann. Plast. Surg. 2013, 70, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Tzeng, Y.S.; Wang, C.H. Treatment of severe burn with DermACELL(®), an acellular dermal matrix. Int. J. Burn. Trauma. 2012, 2, 105–109. [Google Scholar]
- Pittman, T.A.; Fan, K.L.; Knapp, A.; Frantz, S.; Spear, S.L. Comparison of Different Acellular Dermal Matrices in Breast Reconstruction: The 50/50 Study. Plast. Reconstr. Surg. 2017, 139, 521–528. [Google Scholar] [CrossRef]
- Eberli, D.; Rodriguez, S.; Atala, A.; Yoo, J.J. In vivo evaluation of acellular human dermis for abdominal wall repair. J. Biomed. Mater. Res. A 2010, 93, 1527–1538. [Google Scholar] [CrossRef]
- Diffley, M.; Tang, A.; Sawar, K.; Al-Saghir, T.; Gonte, M.R.; Hall, J.; Tepper, D.; Darian, V.; Evangelista, M.; Atisha, D. Comparative Postoperative Complications of Acellular Dermal Matrix and Mesh Use in Prepectoral and Subpectoral One-Stage Direct to Implant Reconstruction: A Retrospective Cohort Study. Ann. Plast. Surg. 2025, 94, 521–527. [Google Scholar] [CrossRef]
- Modi, A.; Haque, A.; Deore, V.; Singh, H.P.; Pandey, R. Interposition GraftJacket allografts for irreparable rotator cuff tears. Bone Jt. J. 2022, 104-b, 91–96. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Bohn, G.; Driver, V.R.; Mills, J.L., Sr.; Nanney, L.B.; Williams, M.L.; Wu, S.C. Human acellular dermal wound matrix: Evidence and experience. Int. Wound J. 2015, 12, 646–654. [Google Scholar] [CrossRef]
- Athavale, S.M.; Rangarajan, S.; Dharamsi, L.; Wentz, S.; Phillips, S.; McRackan, T.; Yarbrough, W.G. AlloDerm and DermaMatrix implants for parotidectomy reconstruction: A histologic study in the rat model. Head Neck 2013, 35, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, S.D.; Moss, W.D.; Graham, E.M.; Carter, G.; Agarwal, J.P. The BREASTrial Stage III: Acellular Dermal Matrix Breast Reconstruction Outcomes from 3 Months to 2 Years Postoperatively. Plast. Reconstr. Surg. 2023, 151, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.W.; Chung, J.; Kim, J.M.; Cha, E.S.; Kim, J.H. Imaging Surveillance After Breast-Conserving Surgery for Cancer With Acellular Dermal Matrix Reconstruction. Korean J. Radiol. 2024, 25, 992–1002. [Google Scholar] [CrossRef]
- Ilahi, O.N.; Velmahos, G.; Janis, J.E.; Kovach, S.J., III.; McLean, S.F.; Askari, R.; Sommer, C.A.; Agarwal, S.; Srinivasan, J.; Wong, A.K.; et al. Prospective, multicenter study of antimicrobial-coated, noncrosslinked, acellular porcine dermal matrix (XenMatrix™ AB Surgical Graft) for hernia repair in all centers for disease control and prevention wound classes: 24-month follow-up cohort. Ann. Med. Surg. 2023, 85, 1571–1577. [Google Scholar] [CrossRef]
- Brunner, M.; Schneider, I.; Günther, K.; Grützmann, R.; Matzel, K.E. Permacol™ collagen paste for cryptoglandular and Crohn’s anal fistula. Tech. Coloproctol. 2019, 23, 135–141. [Google Scholar] [CrossRef]
- Dirani, M.; Chahine, E.; D’Alessandro, A.; Chouillard, M.A.; Gumbs, A.A.; Chouillard, E. The use of Permacol® biological mesh for complex abdominal wall repair. Minerva Surg. 2022, 77, 41–49. [Google Scholar] [CrossRef]
- Bassetto, F.; Pandis, L. Clinical experience with Surgimend in breast reconstruction: An overview. Br. J. Hosp. Med. 2020, 81, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Germano, C.; Borriello, G.; Corazzelli, G.; Abbate, V.; Troise, S.; Dell′Aversana Orabona, G.; Piombino, P.; Romano, A.; Collà Ruvolo, C.; Bonavolontà, P. Mitigating complications rate in parotid gland tumor surgery by reconstruction technique with SurgiMend®: A retrospective, single-center, observational study on 300 consecutive patients. J. Craniomaxillofac Surg. 2025, 53, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, M.; Martin, M.; Skinner, R. A preliminary comparison study of two noncrosslinked biologic meshes used in complex ventral hernia repairs. World J. Surg. 2012, 36, 1760–1764. [Google Scholar] [CrossRef]
- Krasniqi, L.; Kronby, M.P.; Riber, L.P.S. Long-term survival after Carpentier-Edwards Perimount aortic valve replacement in Western Denmark: A multi-centre observational study. J. Cardiothorac. Surg. 2021, 16, 130. [Google Scholar] [CrossRef]
- Bell, D.; Betts, K.; Justo, R.; Forde, N.; Venugopal, P.; Corno, A.F.; Smith, P.; Caputo, M.; Marsico, R.; Karl, T.R.; et al. Multicenter Experience With 500 CardioCel Implants Used for the Repair of Congenital Heart Defects. Ann. Thorac. Surg. 2019, 108, 1883–1888. [Google Scholar] [CrossRef]
- Brown-Etris, M.; Milne, C.T.; Hodde, J.P. An extracellular matrix graft (Oasis(®) wound matrix) for treating full-thickness pressure ulcers: A randomized clinical trial. J. Tissue Viability 2019, 28, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.; Adkison, G.; Smith, T.; Jenkins, L.; Cizman, Z. Single Institution Outcome of Minimally Invasive Enterocutaneous Fistula Management Utilizing the Biodesign® Fistula Plug. Cardiovasc. Interv. Radiol. 2022, 45, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Mosala Nezhad, Z.; Poncelet, A.; de Kerchove, L.; Gianello, P.; Fervaille, C.; El Khoury, G. Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 839–850. [Google Scholar] [CrossRef]
- Sood, V.; Heider, A.; Rabah, R.; Si, M.S.; Ohye, R.G. Evaluation of Explanted CorMatrix Tyke Extracardiac Patches in Infants With Congenital Heart Disease. Ann. Thorac. Surg. 2021, 112, 1518–1522. [Google Scholar] [CrossRef]
- Wang, X.; Chung, L.; Hooks, J.; Maestas, D.R.; Lebid, A., Jr.; Andorko, J.I.; Huleihel, L.; Chin, A.F.; Wolf, M.; Remlinger, N.T.; et al. Type 2 immunity induced by bladder extracellular matrix enhances corneal wound healing. Sci. Adv. 2021, 7, eabe2635. [Google Scholar] [CrossRef]
- Paige, J.T.; Lightell, D.J.; Jr Douglas, H.F.; Klingenberg, N.C.; Pham, T.; Woods, T.C. Incubation with porcine urinary bladder matrix yields a late-stage wound transcriptome in endothelial cells and keratinocytes isolated from both diabetic and non-diabetic subjects. Exp. Dermatol. 2023, 32, 1430–1438. [Google Scholar] [CrossRef]
- Liden, B.A.; May, B.C. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv. Skin. Wound Care 2013, 26, 164–167. [Google Scholar] [CrossRef]
- McCranie, A.S.; Blades, C.; Dawson, S.; Foppiani, J.A.; Allenby, T.; Winocour, J.; Cohen, J.; Mathes, D.; Kaoutzanis, C. Abdominal Wall Reinforcement Using OviTex after Deep Inferior Epigastric Perforator Flap. J. Reconstr. Microsurg. 2025; ahead of print. [Google Scholar] [CrossRef]
- Leckenby, J.I.; Furrer, C.; Haug, L.; Juon Personeni, B.; Vögelin, E. A Retrospective Case Series Reporting the Outcomes of Avance Nerve Allografts in the Treatment of Peripheral Nerve Injuries. Plast. Reconstr. Surg. 2020, 145, 368e–381e. [Google Scholar] [CrossRef]
- Biehl, A.; Gracioso Martins, A.M.; Davis, Z.G.; Sze, D.; Collins, L.; Mora-Navarro, C.; Fisher, M.B.; Freytes, D.O. Towards a standardized multi-tissue decellularization protocol for the derivation of extracellular matrix materials. Biomater. Sci. 2023, 11, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2019, 13, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Behre, A.; Tashman, J.W.; Dikyol, C.; Shiwarski, D.J.; Crum, R.J.; Johnson, S.A.; Kommeri, R.; Hussey, G.S.; Badylak, S.F.; Feinberg, A.W. 3D Bioprinted Patient-Specific Extracellular Matrix Scaffolds for Soft Tissue Defects. Adv. Healthc. Mater. 2022, 11, e2200866. [Google Scholar] [CrossRef]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2021, 33, e2005476. [Google Scholar] [CrossRef]
- Baker, H.B.; McQuilling, J.P.; King, N.M. Ethical considerations in tissue engineering research: Case studies in translation. Methods 2016, 99, 135–144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Zhang, X.; Jin, Y.; Chien, P.N.; Heo, C.Y. Acellular Extracellular Matrix Scaffolds in Regenerative Medicine: Advances in Decellularization and Clinical Applications. J. Funct. Biomater. 2025, 16, 383. https://doi.org/10.3390/jfb16100383
Jin C, Zhang X, Jin Y, Chien PN, Heo CY. Acellular Extracellular Matrix Scaffolds in Regenerative Medicine: Advances in Decellularization and Clinical Applications. Journal of Functional Biomaterials. 2025; 16(10):383. https://doi.org/10.3390/jfb16100383
Chicago/Turabian StyleJin, Caijun, Xinrui Zhang, Yongxun Jin, Pham Ngoc Chien, and Chan Yeong Heo. 2025. "Acellular Extracellular Matrix Scaffolds in Regenerative Medicine: Advances in Decellularization and Clinical Applications" Journal of Functional Biomaterials 16, no. 10: 383. https://doi.org/10.3390/jfb16100383
APA StyleJin, C., Zhang, X., Jin, Y., Chien, P. N., & Heo, C. Y. (2025). Acellular Extracellular Matrix Scaffolds in Regenerative Medicine: Advances in Decellularization and Clinical Applications. Journal of Functional Biomaterials, 16(10), 383. https://doi.org/10.3390/jfb16100383






