Eighty-Four-Month Clinical Outcomes of Autologous Dentin Graft Using Tooth Transformer® and Concentrated Growth Factors in Maxillary Atrophy: A Retrospective Study of 31 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design
- -
- Age between 30 and 75 years (The selected age range of 30 to 75 years represents the population commonly considered for implant rehabilitation procedures with maxillary atrophy because it has a sufficiently stable bone metabolism to support regenerative processes, minimizing the confounding variables associated with very young patients (still in the bone development phase) or very elderly patients (with advanced osteoporosis or multiple comorbidities).
- -
- Residual crestal bone height of 5 mm or more.
- -
- Need for extraction of at least two molars for severe periodontal reasons to ensure a minimum amount of 4 g of available autologous dentin.
- -
- Severe maxillary atrophy candidates for sinus lift and concomitant implant placement.
- -
- Adequate oral hygiene status (Full-Mouth Plaque Score < 20%).
- -
- Non-Smoker or light smoker (<10 cigarettes/day).
- -
- Patients with severe systemic diseases that may interfere with the bone regeneration process including: uncontrolled diabetes mellitus (HbA1c > 7.5%), cardiovascular disease, neoplasms undergoing treatment, advanced chronic liver or kidney disease (stage ≥ 3).
- -
- Infectious or autoimmune diseases (systemic lupus erythematosus, rheumatoid arthritis).
- -
- Amount of autologous dentin less than 2 g or no need for dental avulsions to ensure adequate availability of useful biomaterial for bone regeneration.
- -
- Other factors that could compromise bone regeneration or implant osseointegration (patients undergoing immunosuppressive therapy or intravenous bisphosphonates).
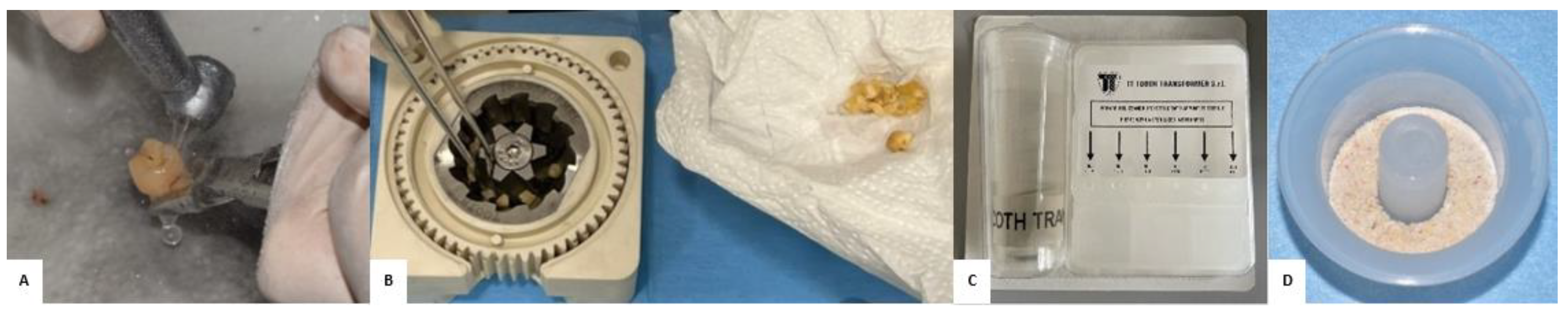
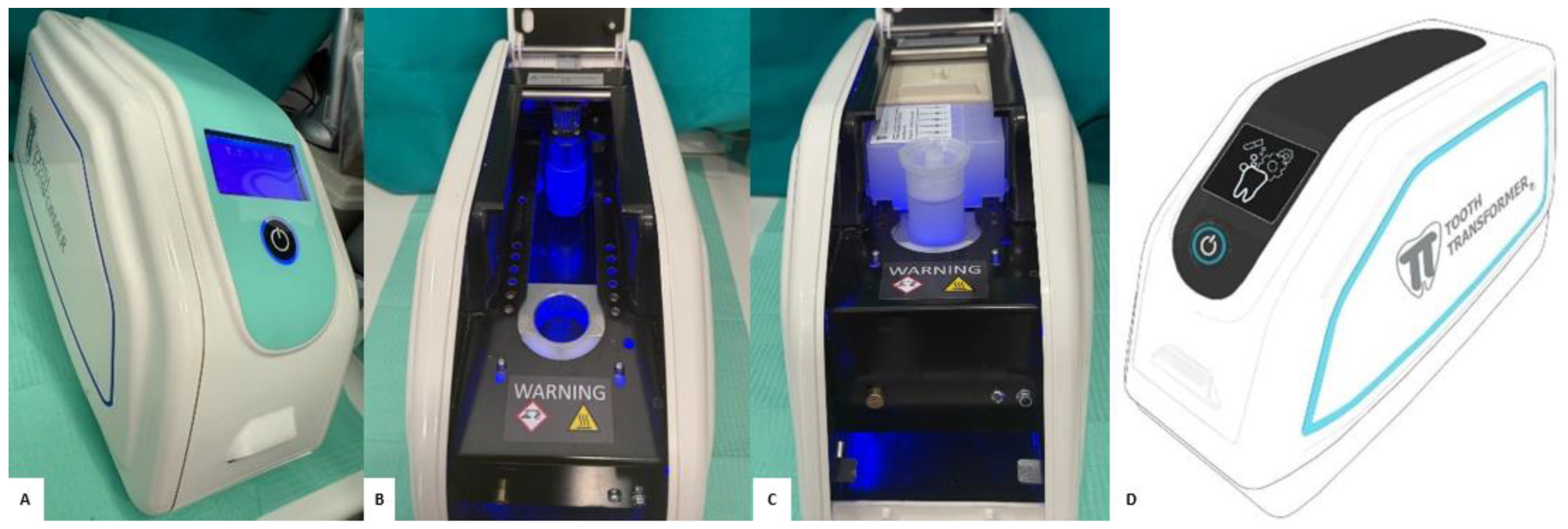
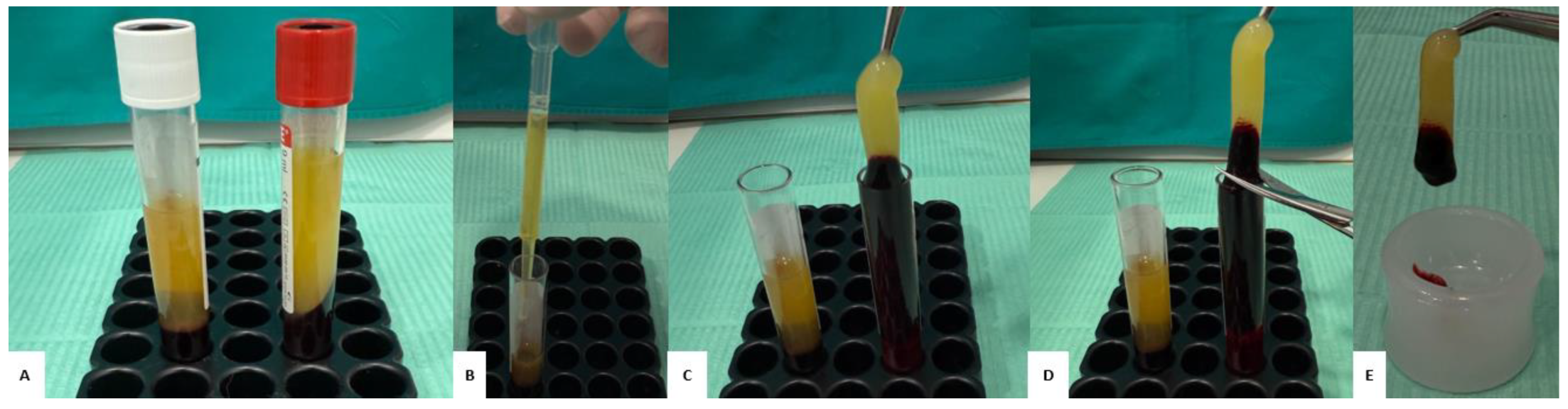
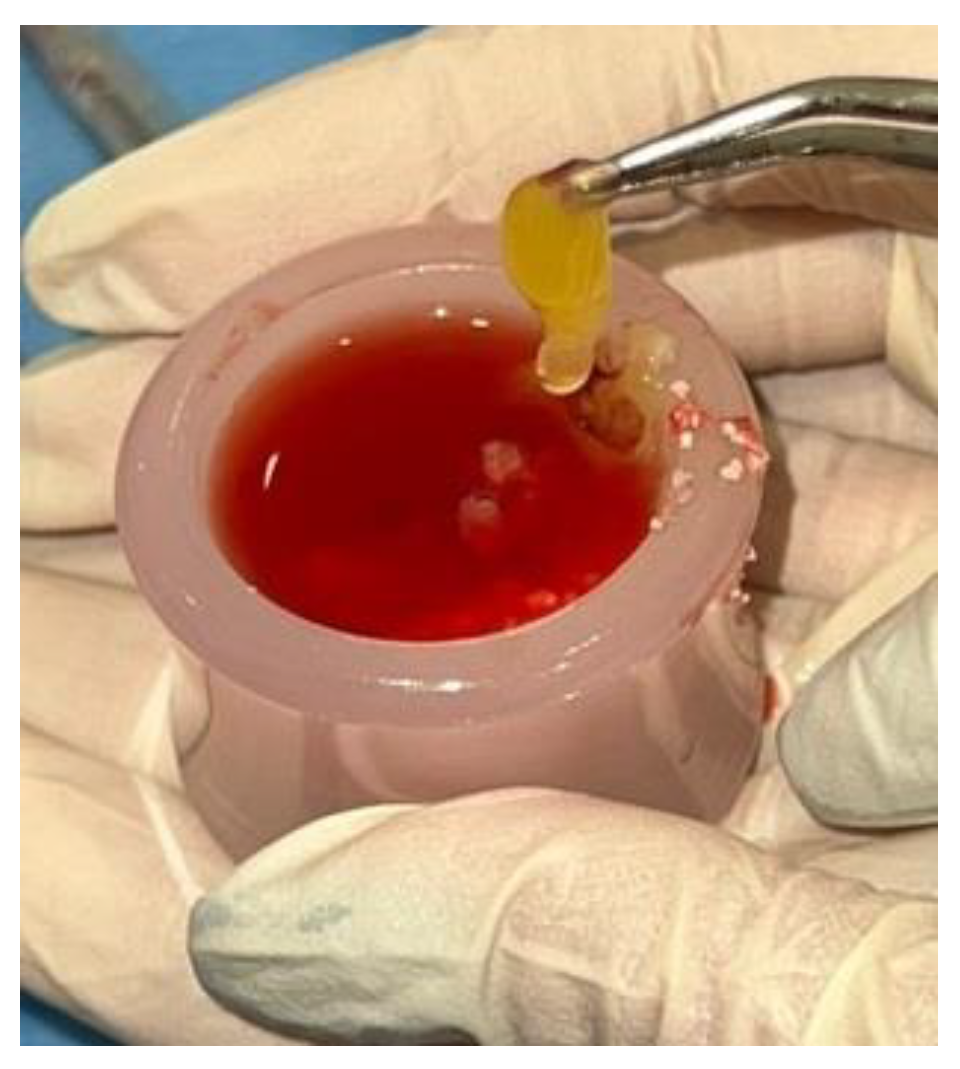
2.3. Preparation of the “Sticky Bone”
2.4. Surgical Procedure
2.5. Clinical and Radiographic Evaluation
3. Results
3.1. CBCT Radiographic Evaluation at T0, T1 and T2
3.2. Implant Survival, Stability and Complications
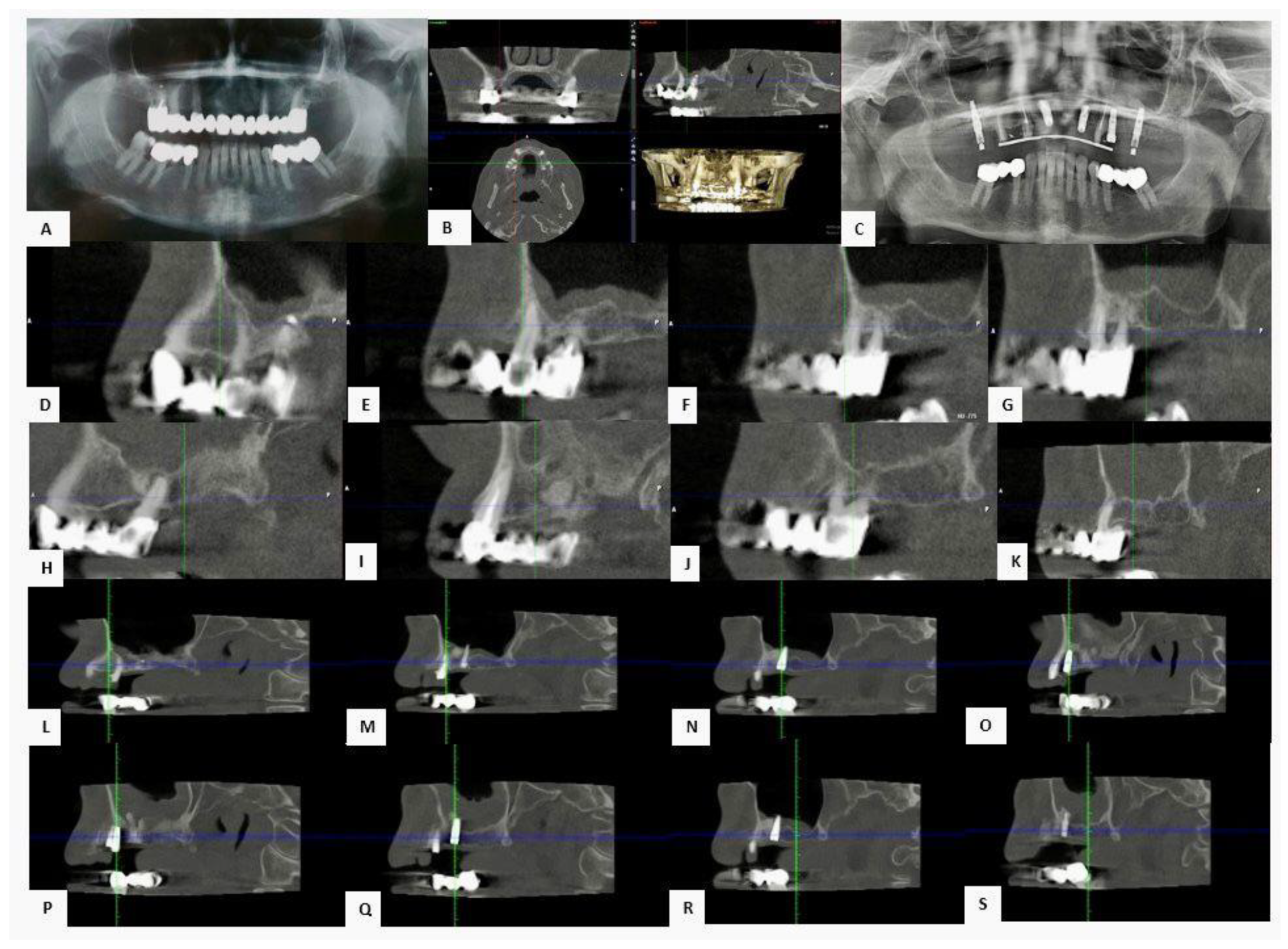
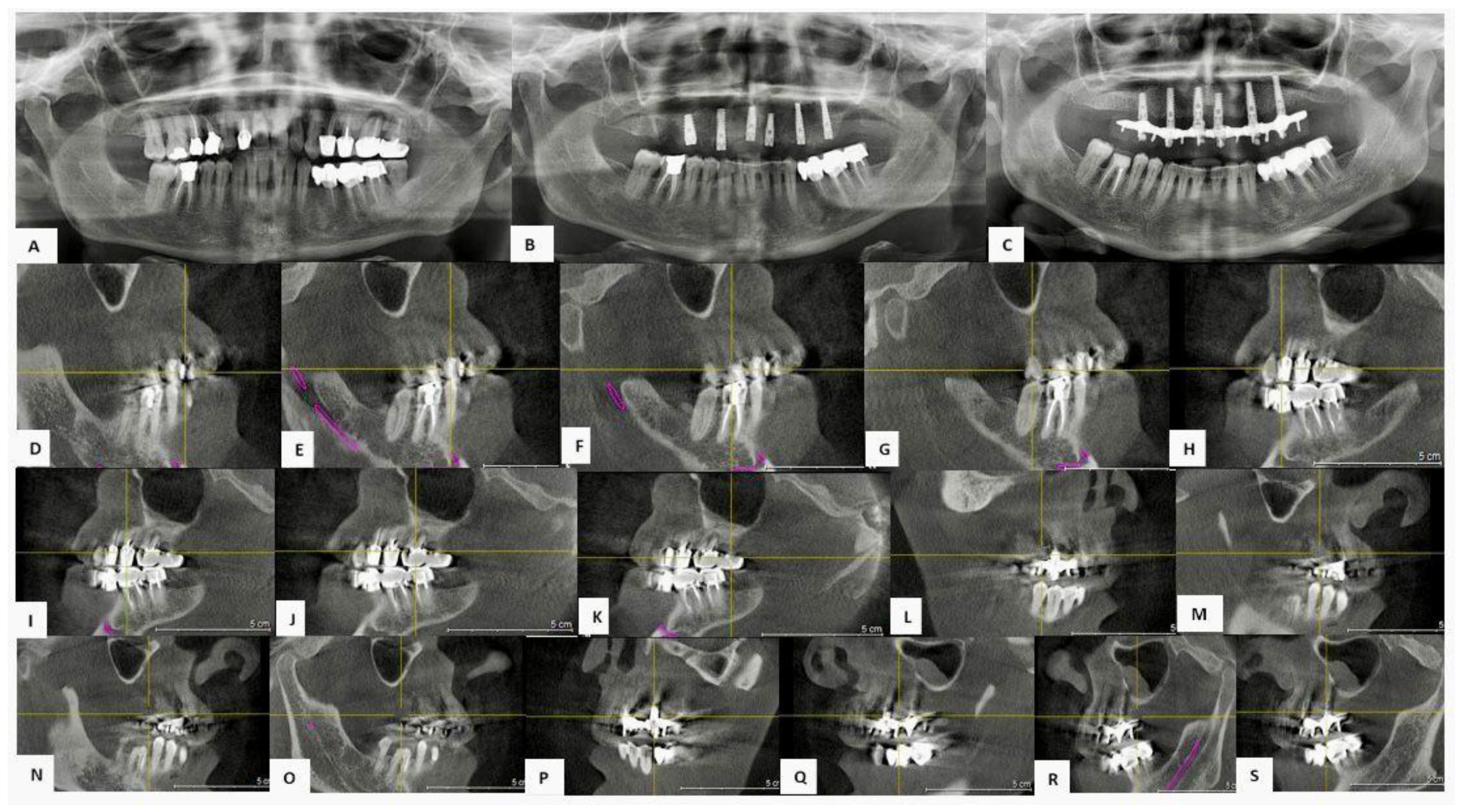
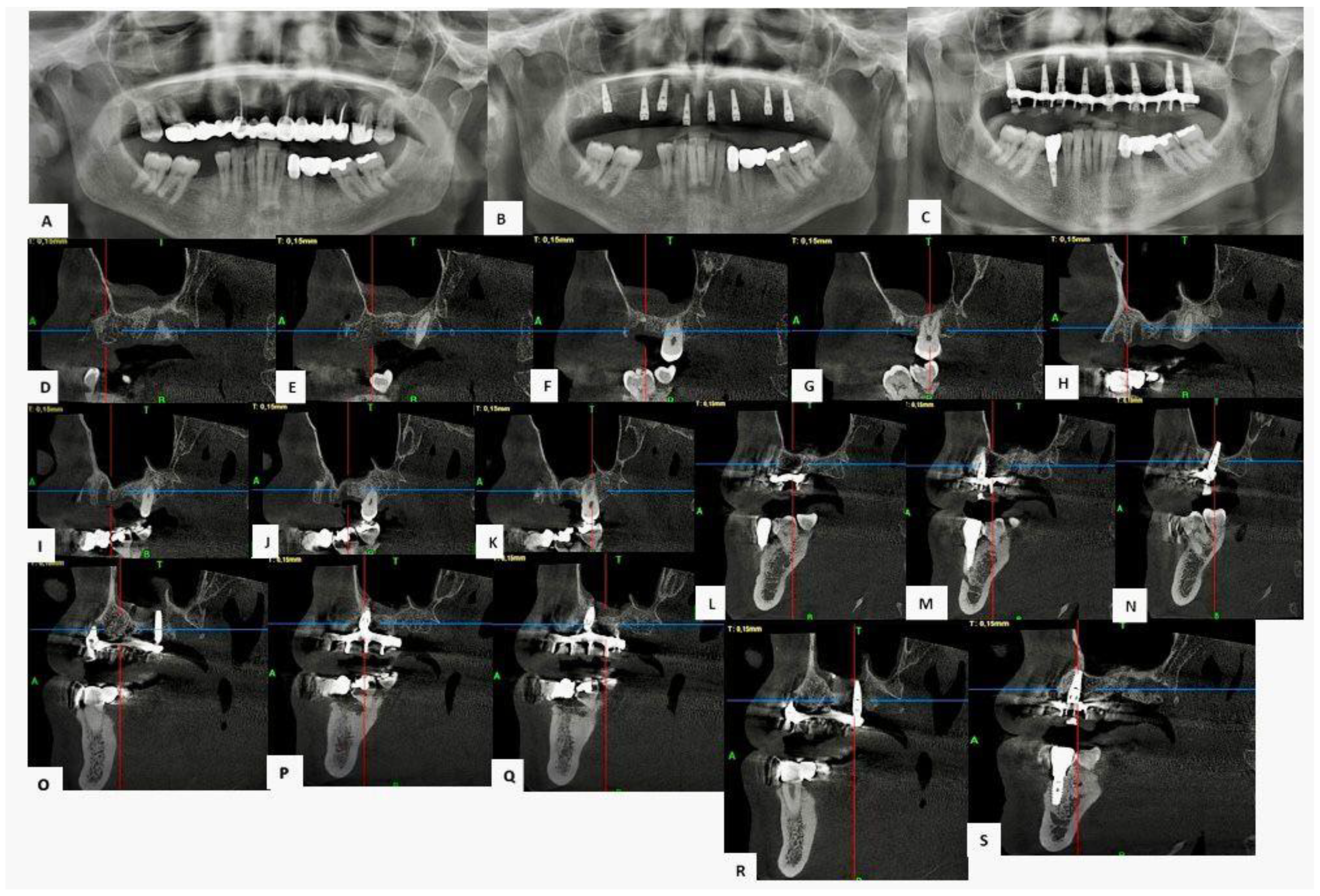
3.3. Clinical Cases
3.3.1. Case 1—Female Patient, 52 Years Old
3.3.2. Case 2—Female Patient, 60 Years Old
3.3.3. Case 3—Male Patient, 58 Years Old
4. Discussion
4.1. Clinical and Radiographic Evaluation of the Regenerative Protocol
4.2. Considerations on Representative Clinical Cases
4.3. Limitations of the Study
4.4. Future Objectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AFG | Autologous Fibrin Glue |
| BMP-2 | Bone morphogenetic protein |
| CBCT | Cone Beam Computed Tomography |
| CGF | Concentrated Growth Factors |
| IGF | Insulin-like growth factor |
| ISQ | Implant Stability Quotient |
| OPT | Orthopantomography |
| PDGF | Platelet-Derived Growth Factor |
| PRF | Platelet rich fibrin |
| PRP | Platelet rich plasma |
| TGF-β1 | Transforming Growth Factor beta 1 |
| VEGF | Vascular Endothelial Growth Factor |
References
- Boyapati, L.; Wang, H.-L. The Role of Platelet-Rich Plasma in Sinus Augmentation: A Critical Review. Implant. Dent. 2006, 15, 160–170. [Google Scholar] [CrossRef]
- Solakoglu, Ö.; Heydecke, G.; Amiri, N.; Anitua, E. The Use of Plasma Rich in Growth Factors (PRGF) in Guided Tissue Regeneration and Guided Bone Regeneration. A Review of Histological, Immunohistochemical, Histomorphometrical, Radiological and Clinical Results in Humans. Ann. Anat. 2020, 231, 151528. [Google Scholar] [CrossRef]
- Bhalla, N.; Dym, H. Update on Maxillary Sinus Augmentation. Dent. Clin. N. Am. 2021, 65, 197–210. [Google Scholar] [CrossRef]
- Juzikis, E.; Gaubys, A.; Rusilas, H. Uses of Maxillary Sinus Lateral Wall Bony Window in an Open Window Sinus Lift Procedure: Literature Review. Stomatologija 2018, 20, 14–21. [Google Scholar] [PubMed]
- Jakse, N.; Tangl, S.; Gilli, R.; Berghold, A.; Lorenzoni, M.; Eskici, A.; Haas, R.; Pertl, C. Influence of PRP on Autogenous Sinus Grafts. An Experimental Study on Sheep. Clin. Oral Implant. Res. 2003, 14, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Roldán, J.C.; Jepsen, S.; Schmidt, C.; Knüppel, H.; Rueger, D.C.; Açil, Y.; Terheyden, H. Sinus Floor Augmentation with Simultaneous Placement of Dental Implants in the Presence of Platelet-Rich Plasma or Recombinant Human Bone Morphogenetic Protein-7. Clin. Oral Implant. Res. 2004, 15, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, H.; Streckbein, P.; Lendeckel, S.; Heidinger, K.S.; Rehmann, P.; Boedeker, R.-H.; Howaldt, H.-P. Sinus Lift Augmentation Using Autogenous Bone Grafts and Platelet-Rich Plasma: Radiographic Results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 106, 673–678. [Google Scholar] [CrossRef]
- Tavelli, L.; Borgonovo, A.E.; Re, D.; Maiorana, C. Sinus Presurgical Evaluation: A Literature Review and a New Classification Proposal. Minerva Dent. Oral Sci. 2017, 66, 115–131. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.-S.; Nally, M.; Lanata, N.; Wang, H.-L.; Quirynen, M. The Impact of the Centrifuge Characteristics and Centrifugation Protocols on the Cells, Growth Factors, and Fibrin Architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) Clot and Membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Mastrangelo, F.; Franceschelli, S.; Annicchiarico, C.; Annicchiarico, A.; Bizzoca, M.E.; De Cecco, F.; Gioia, R.L.; Cervino, G.; Pesce, M. The Influence of Different Preservation Protocols on the Teeth’s Osteoinductive Characteristics: An In Vitro Study. Int. J. Mol. Sci. 2025, 26, 4044. [Google Scholar] [CrossRef]
- Trimmel, B.; Gede, N.; Hegyi, P.; Szakács, Z.; Mezey, G.A.; Varga, E.; Kivovics, M.; Hanák, L.; Rumbus, Z.; Szabó, G. Relative Performance of Various Biomaterials Used for Maxillary Sinus Augmentation: A Bayesian Network Meta-Analysis. Clin. Oral Implant. Res. 2021, 32, 135–153. [Google Scholar] [CrossRef]
- Testori, T.; Weinstein, T.; Taschieri, S.; Wallace, S.S. Risk Factors in Lateral Window Sinus Elevation Surgery. Periodontol. 2000 2019, 81, 91–123. [Google Scholar] [CrossRef]
- Miron, R.J.; Pikos, M.A. Sinus Augmentation Using Platelet-Rich Fibrin With or Without a Bone Graft: What Is the Consensus? Compend. Contin. Educ. Dent. 2018, 39, 355–361, quiz 362. [Google Scholar] [PubMed]
- Nam, J.-H.; Almansoori, A.A.; Kwon, O.-J.; Seo, Y.-K.; Kim, B.; Kim, Y.-K.; Lee, J.-H.; Pang, K. Sinus Augmentation with Poly(ε)Caprolactone-β Tricalcium Phosphate Scaffolds, Mesenchymal Stem Cells and Platelet Rich Plasma for One-Stage Dental Implantation in Minipigs. J. Periodontal Implant. Sci. 2023, 53, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Wiltfang, J.; Schlegel, K.A.; Schultze-Mosgau, S.; Nkenke, E.; Zimmermann, R.; Kessler, P. Sinus Floor Augmentation with Beta-Tricalciumphosphate (Beta-TCP): Does Platelet-Rich Plasma Promote Its Osseous Integration and Degradation? Clin. Oral Implant. Res. 2003, 14, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Mendonça-Caridad, J.; Lopez, P.J.; Fayos, F.V.; Miery, G. A Novel Approach to Human Cranial Tissue Regeneration and Frontal Sinus Obliteration with an Autogenous Platelet-Rich/Fibrin-Rich Composite Matrix: 10 Patients with a 6–10 Year Follow-Up. J. Tissue Eng. Regen. Med. 2013, 7, 491–500. [Google Scholar] [CrossRef]
- Egierska, D.; Perszke, M.; Mazur, M.; Duś-Ilnicka, I. Platelet-Rich Plasma and Platelet-Rich Fibrin in Oral Surgery: A Narrative Review. Dent. Med. Probl. 2023, 60, 177–186. [Google Scholar] [CrossRef]
- Arora, N.S.; Ramanayake, T.; Ren, Y.-F.; Romanos, G.E. Platelet-Rich Plasma in Sinus Augmentation Procedures: A Systematic Literature Review: Part II. Implant. Dent. 2010, 19, 145–157. [Google Scholar] [CrossRef]
- Acerra, A.; Caggiano, M.; Chiacchio, A.; Scognamiglio, B.; D’Ambrosio, F. PRF and PRP in Dentistry: An Umbrella Review. J. Clin. Med. 2025, 14, 3224. [Google Scholar] [CrossRef]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF-Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef]
- Meyer, C.; Chatelain, B.; Benarroch, M.; Garnier, J.-F.; Ricbourg, B.; Camponovo, T. Greffes sinusiennes massives par phosphate tricalcique. Résultats à long terme [Massive sinus-lift procedures with beta-tricalcium phosphate: Long-term results]. Rev. Stomatol. Chir. Maxillofac. 2009, 110, 69–75. [Google Scholar] [CrossRef]
- Tiwana, P.S.; Kushner, G.M.; Haug, R.H. Maxillary Sinus Augmentation. Dent. Clin. N. Am. 2006, 50, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Malcangi, G.; Patano, A.; Palmieri, G.; Di Pede, C.; Latini, G.; Inchingolo, A.D.; Hazballa, D.; de Ruvo, E.; Garofoli, G.; Inchingolo, F.; et al. Maxillary Sinus Augmentation Using Autologous Platelet Concentrates (Platelet-Rich Plasma, Platelet-Rich Fibrin, and Concentrated Growth Factor) Combined with Bone Graft: A Systematic Review. Cells 2023, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Alghamdi, R.; Guallart, I.F.; Bergamini, M.; Yu, P.Y.; Froum, S.J.; Cho, S.-C. Patient-Related Risk Factors for Maxillary Sinus Augmentation Procedures: A Systematic Literature Review. Int. J. Periodontics Restorative Dent. 2021, 41, e121–e128. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, M.J.; Filstein, M.R.; Shanaman, R. Histological Evaluation of Sinus Augmentation Using Platelet Rich Plasma (PRP): A Case Series. J. Int. Acad. Periodontol. 2001, 3, 48–56. [Google Scholar] [PubMed]
- Dłucik, R.; Orzechowska-Wylęgała, B.; Dłucik, D.; Bogus, K. Histological Examination of Tooth-Derived Biomaterials Obtained from Different Devices. Expert Rev. Med. Devices 2023, 20, 979–988. [Google Scholar] [CrossRef]
- Klongnoi, B.; Rupprecht, S.; Kessler, P.; Thorwarth, M.; Wiltfang, J.; Schlegel, K.A. Influence of Platelet-Rich Plasma on a Bioglass and Autogenous Bone in Sinus Augmentation. An Explorative Study. Clin. Oral Implant. Res. 2006, 17, 312–320. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable Platelet Rich Fibrin (i-PRF): Opportunities in Regenerative Dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Rapone, B.; Inchingolo, A.D.; Trasarti, S.; Ferrara, E.; Qorri, E.; Mancini, A.; Montemurro, N.; Scarano, A.; Inchingolo, A.M.; Dipalma, G.; et al. Long-Term Outcomes of Implants Placed in Maxillary Sinus Floor Augmentation with Porous Fluorohydroxyapatite (Algipore® FRIOS®) in Comparison with Anorganic Bovine Bone (Bio-Oss®) and Platelet Rich Plasma (PRP): A Retrospective Study. J. Clin. Med. 2022, 11, 2491. [Google Scholar] [CrossRef]
- Marinelli, G.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Limongelli, L.; Montenegro, V.; Coloccia, G.; Laudadio, C.; Patano, A.; Inchingolo, F.; et al. White Spot Lesions in Orthodontics: Prevention and Treatment. A Descriptive Review. J. Biol. Regul. Homeost. Agents 2021, 35, 227–240. [Google Scholar] [CrossRef]
- Lemos, C.a.A.; Mello, C.C.; dos Santos, D.M.; Verri, F.R.; Goiato, M.C.; Pellizzer, E.P. Effects of Platelet-Rich Plasma in Association with Bone Grafts in Maxillary Sinus Augmentation: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 517–525. [Google Scholar] [CrossRef]
- Riaz, R.; Ravindran, C.; Ramkumar; Nandakumar. Efficacy of Platelet Rich Plasma in Sinus Lift Augmentation. J. Maxillofac. Oral Surg. 2010, 9, 225–230. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth Factor and Pro-Inflammatory Cytokine Contents in Platelet-Rich Plasma (PRP), Plasma Rich in Growth Factors (PRGF), Advanced Platelet-Rich Fibrin (A-PRF), and Concentrated Growth Factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- Gülcan, H.; Gülşen, U.; Billur, D.; Bayram, P.; Dereci, Ö. Histologic and Immunohistochemical Assessment of the Effect of Various Biomaterials on New Bone Formation in the Maxillary Sinus Floor Augmentation Procedure. Int. J. Oral Maxillofac. Implant. 2021, 36, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Sardaro, N.; Topi, S.; Pettini, F.; Bottalico, L.; Cantore, S.; Cascella, G.; Del Prete, R.; Dipalma, G.; Inchingolo, F. The Pivotal Role of Oral Microbiota in Health and Disease. J. Biol. Regul. Homeost. Agents 2020, 34, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Sanz-Sánchez, I.; Sanz-Martín, I.; Ortiz-Vigón, A.; Sanz, M. Complications in Sinus Lifting Procedures: Classification and Management. Periodontol. 2000 2022, 88, 103–115. [Google Scholar] [CrossRef]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 2: Bone Graft, Implant and Reconstructive Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, R.I.B.; Alqutaibi, A.Y.; Kaddah, A. Does the Adjunctive Use of Platelet-Rich Plasma to Bone Graft during Sinus Augmentation Reduce Implant Failure and Complication? Systematic Review and Meta-Analysis. Quintessence Int. 2018, 49, 139–146. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Koga, T.; Nakatani, Y.; Ohba, S.; Hara, M.; Sumita, Y.; Nagai, K.; Asahina, I. Clinical Safety Assessment of Autologous Freeze-Drying Platelet-Rich Plasma for Bone Regeneration in Maxillary Sinus Floor Augmentation: A Pilot Study. J. Clin. Med. 2021, 10, 1678. [Google Scholar] [CrossRef]
- Minetti, E.; Gianfreda, F.; Bollero, P.; Annicchiarico, C.; Daniele, M.; Padula, R.; Mastrangelo, F. Comparative Histological Analysis of Dentine-Derived Tooth Grafts in Maxillary vs Mandibular Socket Preservation: A Retrospective Study of 178 Cases. Dent. J. 2024, 12, 320. [Google Scholar] [CrossRef]
- Dłucik, R.; Orzechowska-Wylęgała, B.; Dłucik, D.; Puzzolo, D.; Santoro, G.; Micali, A.; Testagrossa, B.; Acri, G. Comparison of Clinical Efficacy of Three Different Dentin Matrix Biomaterials Obtained from Different Devices. Expert Rev. Med. Devices 2023, 20, 313–327. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Berardini, M. Comparison of Different Techniques in Post-Extractive Socket Regeneration Using Autologous Tooth Graft: Histological and Clinical Outcomes. Eur. J. Dent. 2024, 18, 477–484. [Google Scholar] [CrossRef]
- Qiu, P.; Zhang, X.; Cao, R.; Xu, H.; Jiang, Z.; Lei, J. Assessment of the Efficacy of Autologous Blood Preparations in Maxillary Sinus Floor Elevation Surgery: A Systematic Review and Meta-Analysis. BMC Oral Health 2024, 24, 1171. [Google Scholar] [CrossRef]
- Minetti, E.; Gianfreda, F.; Palermo, A.; Bollero, P. Autogenous Dentin Particulate Graft for Alveolar Ridge Augmentation with and without Use of Collagen Membrane: Preliminary Histological Analysis on Humans. Materials 2022, 15, 4319. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Inchingolo, A.D.; Patano, A.; Viapiano, F.; Ciocia, A.M.; de Ruvo, E.; Mancini, A.; Inchingolo, F.; Sauro, S.; et al. Autologous Tooth for Bone Regeneration: Dimensional Examination of Tooth Transformer® Granules. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5421–5430. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Lagioia, R.; De Cecco, F.; Minetti, E.; Ballini, A.; Panella, V.; Speranza, L.; Grilli, A.; Mastrangelo, F. Biological Evaluation of the Osteoinductive Potential of Dry Teeth after Chemical Demineralization Treatment Using the Tooth Transformer Device. Biomolecules 2023, 13, 1727. [Google Scholar] [CrossRef] [PubMed]
- Patano, A.; Cirulli, N.; Beretta, M.; Plantamura, P.; Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Marinelli, G.; Scarano, A.; et al. Education Technology in Orthodontics and Paediatric Dentistry during the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6056. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Taschieri, S.; Corbella, S. A Histologic Study on the Use of Tooth as a Graft Material in Oral Surgery: Analysis of 187 Samples. Materials 2025, 18, 2518. [Google Scholar] [CrossRef]
- Minetti, E.; Giacometti, E.; Gambardella, U.; Contessi, M.; Ballini, A.; Marenzi, G.; Celko, M.; Mastrangelo, F. Alveolar Socket Preservation with Different Autologous Graft Materials: Preliminary Results of a Multicenter Pilot Study in Human. Materials 2020, 13, 1153. [Google Scholar] [CrossRef]
- Valentini, P.; Calciolari, E.; Monlezun, S.; Akcalı, A.; Donos, N.; Quirynen, M. APCs in Sinus Floor Augmentation. Periodontol. 2000 2025, 97, 254–270. [Google Scholar] [CrossRef]
- Ohba, S.; Shido, R.; Asahina, I. Application of Hydroxyapatite/Collagen Composite Material for Maxillary Sinus Floor Augmentation. J. Oral Sci. 2021, 63, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Calabria, E.; Adamo, D.; Leuci, S.; Pecoraro, G.; Coppola, N.; Aria, M.; Mignogna, M.D. The Health-Related Quality of Life and Psychological Profile in Patients with Oropharyngeal Pemphigus Vulgaris in Complete Clinical Remission: A Case-Control Study. J. Oral Pathol. Med. 2021, 50, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Canfora, F.; Calabria, E.; Pecoraro, G.; D Aniello, L.; Aria, M.; Marenzi, G.; Sammartino, P.; Mignogna, M.D.; Adamo, D. The Use of Self-Report Questionnaires in an Analysis of the Multidimensional Aspects of Pain and a Correlation with the Psychological Profile and Quality of Life in Patients with Burning Mouth Syndrome: A Case-Control Study. J. Oral Rehabil. 2022, 49, 890–914. [Google Scholar] [CrossRef] [PubMed]
- Adamo, D.; Calabria, E.; Coppola, N.; Pecoraro, G.; Mignogna, M.D. Vortioxetine as a New Frontier in the Treatment of Chronic Neuropathic Pain: A Review and Update. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211034320. [Google Scholar] [CrossRef]
- Adamo, D.; Canfora, F.; Calabria, E.; Coppola, N.; Leuci, S.; Pecoraro, G.; Cuocolo, R.; Ugga, L.; D’Aniello, L.; Aria, M.; et al. White Matter Hyperintensities in Burning Mouth Syndrome Assessed According to the Age-Related White Matter Changes Scale. Front. Aging Neurosci. 2022, 14, 923720. [Google Scholar] [CrossRef]
- Monaco, A.; Sgolastra, F.; Pietropaoli, D.; Giannoni, M.; Cattaneo, R. Comparison between Sensory and Motor Transcutaneous Electrical Nervous Stimulation on Electromyographic and Kinesiographic Activity of Patients with Temporomandibular Disorder: A Controlled Clinical Trial. BMC Musculoskelet. Disord. 2013, 14, 168. [Google Scholar] [CrossRef]
- Monaco, A.; Cattaneo, R.; Mesin, L.; Ciarrocchi, I.; Sgolastra, F.; Pietropaoli, D. Dysregulation of the Autonomous Nervous System in Patients with Temporomandibular Disorder: A Pupillometric Study. PLoS ONE 2012, 7, e45424. [Google Scholar] [CrossRef]
- Monaco, A.; Cattaneo, R.; Mesin, L.; Ortu, E.; Giannoni, M.; Pietropaoli, D. Dysregulation of the Descending Pain System in Temporomandibular Disorders Revealed by Low-Frequency Sensory Transcutaneous Electrical Nerve Stimulation: A Pupillometric Study. PLoS ONE 2015, 10, e0122826. [Google Scholar] [CrossRef]
- Morresi, A.L.; D’Amario, M.; Monaco, A.; Rengo, C.; Grassi, F.R.; Capogreco, M. Effects of Critical Thermal Cycling on the Flexural Strength of Resin Composites. J. Oral Sci. 2015, 57, 137–143. [Google Scholar] [CrossRef]
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J. Funct. Biomater. 2022, 13, 121. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D.; et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering 2023, 10, 472. [Google Scholar] [CrossRef]
- Lo Russo, L.; Ciavarella, D.; Salamini, A.; Guida, L. Alignment of Intraoral Scans and Registration of Maxillo-Mandibular Relationships for the Edentulous Maxillary Arch. J. Prosthet. Dent. 2019, 121, 737–740. [Google Scholar] [CrossRef]
- Troiano, G.; Dioguardi, M.; Cocco, A.; Laino, L.; Cervino, G.; Cicciu, M.; Ciavarella, D.; Muzio, L.L. Conservative vs. Radical Approach for the Treatment of Solid/Multicystic Ameloblastoma: A Systematic Review and Meta-Analysis of the Last Decade. Oral Health Prev. Dent. 2017, 15, 421–426. [Google Scholar] [CrossRef]
- Laino, L.; Troiano, G.; Giannatempo, G.; Graziani, U.; Ciavarella, D.; Dioguardi, M.; Lo Muzio, L.; Lauritano, F.; Cicciù, M. Sinus Lift Augmentation by Using Calcium Sulphate. A Retrospective 12 Months Radiographic Evaluation Over 25 Treated Italian Patients. Open Dent. J. 2015, 9, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Inchingolo, F.; Tari, S.R. Animal Models for Investigating Osseointegration: An Overview of Implant Research over the Last Three Decades. J. Funct. Biomater. 2024, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.M.; Colonna, V.; Marotti, P.; Carone, C.; Ferrante, L.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D. Autologous and Heterologous Minor and Major Bone Regeneration with Platelet-Derived Growth Factors. J. Funct. Biomater. 2025, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Limongelli, L.; Cascardi, E.; Capodiferro, S.; Favia, G.; Corsalini, M.; Tempesta, A.; Maiorano, E. Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case. Diagnostics 2020, 10, 424. [Google Scholar] [CrossRef]
- Pettini, F.; Corsalini, M.; Savino, M.G.; Stefanachi, G.; Di Venere, D.; Pappalettere, C.; Monno, G.; Boccaccio, A. Roughness Analysis on Composite Materials (Microfilled, Nanofilled and Silorane) After Different Finishing and Polishing Procedures. Open Dent. J. 2015, 9, 357–367. [Google Scholar] [CrossRef]
- Solarino, B.; Coppola, F.; Di Vella, G.; Corsalini, M.; Quaranta, N. Vestibular Evoked Myogenic Potentials (VEMPs) in Whiplash Injury: A Prospective Study. Acta Otolaryngol. 2009, 129, 976–981. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef]
- Lucchese, A.; Pilolli, G.P.; Petruzzi, M.; Crincoli, V.; Scivetti, M.; Favia, G. Analysis of Collagen Distribution in Human Crown Dentin by Confocal Laser Scanning Microscopy. Ultrastruct. Pathol. 2008, 32, 107–111. [Google Scholar] [CrossRef]
- Tortarolo, A.; Rotolo, R.; Nucci, L.; Tepedino, M.; Crincoli, V.; Piancino, M.G. Condylar Asymmetry in Children with Unilateral Posterior Crossbite Malocclusion: A Comparative Cross-Sectional Study. Children 2022, 9, 1772. [Google Scholar] [CrossRef]
- Crincoli, V.; Ballini, A.; Fatone, L.; Di Bisceglie, M.B.; Nardi, G.M.; Grassi, F.R. Cytokine Genotype Distribution in Patients with Periodontal Disease and Rheumatoid Arthritis or Diabetes Mellitus. J. Biol. Regul. Homeost. Agents 2016, 30, 863–866. [Google Scholar] [PubMed]
- Celli, D.; Garcovich, D.; Gasperoni, E.; Deli, R. Bimaxillary Protrusion Treated without Extractions. J. Clin. Orthod. 2007, 41, 33–38. [Google Scholar] [PubMed]
- Catalfamo, L.; Gasperoni, E.; Celli, D.; Deli, R. Class II Treatment with the Smart Distalization Technique. J. Clin. Orthod. 2012, 46, 613–624, quiz 631–632. [Google Scholar] [PubMed]
- Quaranta, M.; Scaramella, F.; D’Addona, A.; Caputi, S.; Celli, D. Dentistry for the elderly patient. 2. Endodontic therapy. Dent. Cadmos 1989, 57, 38–50, 53. [Google Scholar] [PubMed]
- Minetti, E.; Palermo, A.; Malcangi, G.; Inchingolo, A.D.; Mancini, A.; Dipalma, G.; Inchingolo, F.; Patano, A.; Inchingolo, A.M. Dentin, Dentin Graft, and Bone Graft: Microscopic and Spectroscopic Analysis. J. Funct. Biomater. 2023, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Inchingolo, A.M.; Ferrante, L.; Marinelli, G.; Inchingolo, F.; Inchingolo, A.D.; Palermo, A.; Dipalma, G. Six-Year Implants Follow-Up After Guided Bone Regeneration Using Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration Tooth Transformer®. J. Funct. Biomater. 2025, 16, 172. [Google Scholar] [CrossRef]
- Matarese, G.; Ramaglia, L.; Fiorillo, L.; Cervino, G.; Lauritano, F.; Isola, G. Implantology and Periodontal Disease: The Panacea to Problem Solving? Open Dent. J. 2017, 11, 460–465. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Inchingolo, A.D.; Nardelli, P.; Latini, G.; Trilli, I.; Ferrante, L.; Malcangi, G.; Palermo, A.; Inchingolo, F.; Dipalma, G. Stem Cells: Present Understanding and Prospects for Regenerative Dentistry. J. Funct. Biomater. 2024, 15, 308. [Google Scholar] [CrossRef]
- Quinzi, V.; Saccomanno, S.; Manenti, R.J.; Giancaspro, S.; Paskay, L.C.; Marzo, G. Efficacy of Rapid Maxillary Expansion with or without Previous Adenotonsillectomy for Pediatric Obstructive Sleep Apnea Syndrome Based on Polysomnographic Data: A Systematic Review and Meta-Analysis. Appl. Sci. 2020, 10, 6485. [Google Scholar] [CrossRef]
- Dinoi, M.T.; Marchetti, E.; Garagiola, U.; Caruso, S.; Mummolo, S.; Marzo, G. Orthodontic Treatment of an Unerupted Mandibular Canine Tooth in a Patient with Mixed Dentition: A Case Report. J. Med. Case Rep. 2016, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Appl. Sci. 2020, 10, 5084. [Google Scholar] [CrossRef]
- Muraglie, S.; Leonardi, R.; Aboulazm, K.; Stumpo, C.; Loreto, C.; Grippaudo, C. Evaluation of Structural Skeletal Asymmetry of the Glenoid Fossa in Adult Patients with Unilateral Posterior Crossbite Using Surface-to-Surface Matching on CBCT Images. Angle Orthod. 2020, 90, 376–382. [Google Scholar] [CrossRef]
- Grippaudo, C.; D’Apolito, I.; Cafiero, C.; Re, A.; Chiurazzi, P.; Frazier-Bowers, S.A. Validating Clinical Characteristic of Primary Failure of Eruption (PFE) Associated with PTH1R Variants. Prog. Orthod. 2021, 22, 43. [Google Scholar] [CrossRef]
- Grippaudo, C.; Paolantonio, E.G.; Deli, R.; La Torre, G. Validation of the Risk Of Malocclusion Assessment (ROMA) Index. Eur. J. Paediatr. Dent. 2007, 8, 136–142. [Google Scholar] [PubMed]
- Bambini, F.; Memè, L.; Procaccini, M.; Rossi, B.; Muzio, L.L. Bone Scintigraphy and SPECT in the Evaluation of the Osseointegrative Response to Immediate Prosthetic Loading of Endosseous Implants: A Pilot Study. Int. J. Oral Maxillofac. Implant. 2004, 19, 80–86. [Google Scholar] [PubMed]
- Muzio, L.L.; Santarelli, A.; Orsini, G.; Memè, L.; Mattioli Belmonte, M.; De Florio, I.; Gatto, R.; Gallusi, G.; Nocini, P.F.; Bertossi, D.; et al. Mg63 and Mc3t3-E1 Osteoblastic Cell Lines Response to Raloxifene. Eur. J. Inflamm. 2013, 11, 797–804. [Google Scholar] [CrossRef]
- Memè, L.; Bambini, F.; Gallusi, G.; Sartini, D.; Pozzi, V.; Emanuelli, M.; Strappa, E.M.; Mummolo, S. The Effect and the Potential Use of Magnetic–Dam Barrier in Guided Bone Regeneration: A Laboratory Study. Appl. Sci. 2023, 13, 1625. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Pacifici, A.; Gargari, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G.; Marrelli, M.; Abenavoli, F.M.; Pacifici, L. Use of Dermal-Fat Grafts in the Post-Oncological Reconstructive Surgery of Atrophies in the Zygomatic Region: Clinical Evaluations in the Patients Undergone to Previous Radiation Therapy. Head Face Med. 2012, 8, 33. [Google Scholar] [CrossRef]
- Di Stasio, D.; Lauritano, D.; Romano, A.; Salerno, C.; Minervini, G.; Minervini, G.; Gentile, E.; Serpico, R.; Lucchese, A. In Vivo Characterization of Oral Pemphigus Vulgaris By Optical Coherence Tomography. J. Biol. Regul. Homeost. Agents 2015, 29, 39–41. [Google Scholar] [PubMed]
- Temelci, A.; Yılmaz, H.G.; Ünsal, G.; Uyanik, L.O.; Yazman, D.; Ayali, A.; Minervini, G. Investigation of the Wetting Properties of Thalassemia Patients’ Blood Samples on Grade 5 Titanium Implant Surfaces: A Pilot Study. Biomimetics 2023, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Alshadidi, A.A.F.; Alshahrani, A.A.; Aldosari, L.I.N.; Chaturvedi, S.; Saini, R.S.; Hassan, S.A.B.; Cicciù, M.; Minervini, G. Investigation on the Application of Artificial Intelligence in Prosthodontics. Appl. Sci. 2023, 13, 5004. [Google Scholar] [CrossRef]
- Ramalingam, K.; Yadalam, P.K.; Ramani, P.; Krishna, M.; Hafedh, S.; Badnjević, A.; Cervino, G.; Minervini, G. Light Gradient Boosting-Based Prediction of Quality of Life among Oral Cancer-Treated Patients. BMC Oral Health 2024, 24, 349. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Corelli, R.; Inchingolo, A.M.; Dipalma, G. Surgical Treatment of Depressed Scar: A Simple Technique. Int. J. Med. Sci. 2011, 8, 377–379. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Flace, P.; Girolamo, F.; Tarullo, A.; Laino, L.; et al. Regenerative Surgery Performed with Platelet-Rich Plasma Used in Sinus Lift Elevation before Dental Implant Surgery: An Useful Aid in Healing and Regeneration of Bone Tissue. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1222–1226. [Google Scholar] [PubMed]
- Manimaran, K.; Sankaranarayanan, S.; Ravi, V.R.; Elangovan, S.; Chandramohan, M.; Perumal, S.M. Treatment of Osteoradionecrosis of Mandible with Bone Marrow Concentrate and with Dental Pulp Stem Cells. Ann. Maxillofac. Surg. 2014, 4, 189–192. [Google Scholar] [CrossRef]
- Alrmali, A.; Saleh, M.H.A.; Mazzocco, J.; Zimmer, J.M.; Testori, T.; Wang, H.-L. Auto-Dentin Platelet-Rich Fibrin Matrix Is an Alternative Biomaterial for Different Augmentation Procedures: A Retrospective Case Series Report. Clin. Exp. Dent. Res. 2023, 9, 993–1004. [Google Scholar] [CrossRef]
- Choukroun, J.; Ghanaati, S. Reduction of Relative Centrifugation Force within Injectable Platelet-Rich-Fibrin (PRF) Concentrates Advances Patients’ Own Inflammatory Cells, Platelets and Growth Factors: The First Introduction to the Low Speed Centrifugation Concept. Eur. J. Trauma Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef]
- Clark, D.; Rajendran, Y.; Paydar, S.; Ho, S.; Cox, D.; Ryder, M.; Dollard, J.; Kao, R.T. Advanced Platelet-Rich Fibrin and Freeze-Dried Bone Allograft for Ridge Preservation: A Randomized Controlled Clinical Trial. J. Periodontol. 2018, 89, 379–387. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Comparison between Traditional Surgery, CO2 and Nd:Yag Laser Treatment for Generalized Gingival Hyperplasia in Sturge-Weber Syndrome: A Retrospective Study. J. Investig. Clin. Dent. 2010, 1, 85–89. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Inchingolo, A.D.; Mancini, A.; Annicchiarico, C.; Inchingolo, F.; Dipalma, G.; et al. Surface Coatings of Dental Implants: A Review. J. Funct. Biomater. 2023, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Viapiano, F.; Netti, A.; Patano, A.; Isacco, C.G.; Inchingolo, A.D.; Inchingolo, F. Sixty-Month Follow Up of Clinical MRONJ Cases Treated with CGF and Piezosurgery. Bioengineering 2023, 10, 863. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Inchingolo, F.; Tartaglia, G.M.; De Francesco, M.; Ferrara, E. Asymmetric Dimethylarginine as a Potential Mediator in the Association between Periodontitis and Cardiovascular Disease: A Systematic Review of Current Evidence. Dent. J. 2024, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Ceci, S.; Limongelli, L.; Corriero, A.; Curatoli, L.; Azzollini, D.; Mezzapesa, P.P.; Marinelli, G.; Malcangi, G.; Coloccia, G.; et al. Cavernous Sinus Involvement and Near Miss Mediastinitis Following Mandibular Tooth Infection Treated during the COVID-19 Pandemic: Clinical Diagnosis and Treatment. Case Rep. Dent. 2022, 2022, 8650099. [Google Scholar] [CrossRef]
- Gelpi, F.; De Santis, D.; Luciano, U.; Bertajola, A.; Bernardello, F.; Zambotti, T.; Causarano, G.; Zarantonello, M.; Iurlaro, A.; Poscolere, A.; et al. Platelet Rich Plasma Grafting Technique Combined with Trans-Sinusal Post-Extractive Implants Placement in the Posterior Maxilla: A Technical Report and Brief Literature Review. J. Biol. Regul. Homeost. Agents 2020, 34, 9–20. [Google Scholar] [PubMed]
- Consolo, U.; Zaffe, D.; Bertoldi, C.; Ceccherelli, G. Platelet-Rich Plasma Activity on Maxillary Sinus Floor Augmentation by Autologous Bone. Clin. Oral Implant. Res. 2007, 18, 252–262. [Google Scholar] [CrossRef]
- Saccomanno, S.; Mummolo, S.; Giancaspro, S.; Manenti, R.J.; Mastrapasqua, R.F.; Marzo, G.; Quinzi, V. Catering Work Profession and Medico-Oral Health: A Study on 603 Subjects. Healthcare 2021, 9, 582. [Google Scholar] [CrossRef]
- Daniele, V.; Macera, L.; Taglieri, G.; Spera, L.; Marzo, G.; Quinzi, V. Color Stability, Chemico-Physical and Optical Features of the Most Common PETG and PU Based Orthodontic Aligners for Clear Aligner Therapy. Polymers 2021, 14, 14. [Google Scholar] [CrossRef]
- Quinzi, V.; Marchetti, E.; Guerriero, L.; Bosco, F.; Marzo, G.; Mummolo, S. Dentoskeletal Class II Malocclusion: Maxillary Molar Distalization with No-Compliance Fixed Orthodontic Equipment. Dent. J. 2020, 8, 26. [Google Scholar] [CrossRef]
- Primozic, J.; Canova, F.F.; Rizzo, F.A.; Marzo, G.; Quinzi, V. Diagnostic Ability of the Primary Second Molar Crown-to-Root Length Ratio and the Corresponding Underlying Premolar Position in Estimating Future Expander Anchoring Teeth Exfoliation. Orthod. Craniofac. Res. 2021, 24, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Gual-Vaqués, P.; Polis-Yanes, C.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Mari-Roig, A.; López-López, J. Autogenous Teeth Used for Bone Grafting: A Systematic Review. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e112–e119. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-B.; Lee, D.-Y.; Ahn, H.-W.; Kim, S.-H.; Kim, E.-C.; Roitman, I. Tooth Movement out of the Bony Wall Using Augmented Corticotomy with Nonautogenous Graft Materials for Bone Regeneration. Biomed. Res. Int. 2014, 2014, 347508. [Google Scholar] [CrossRef] [PubMed]
- Mourão, C.F.d.A.B.; Valiense, H.; Melo, E.R.; Mourão, N.B.M.F.; Maia, M.D.-C. Obtention of Injectable Platelets Rich-Fibrin (i-PRF) and Its Polymerization with Bone Graft: Technical Note. Rev. Col. Bras. Cir. 2015, 42, 421–423. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Agwan, M.A.S.; Ansari, S.A.; Zafar, M.S.; Matinlinna, J.P. Regenerative Potential of Platelet Rich Fibrin (PRF) for Curing Intrabony Periodontal Defects: A Systematic Review of Clinical Studies. Tissue Eng. Regen. Med. 2017, 14, 735–742. [Google Scholar] [CrossRef]
- Gheno, E.; Palermo, A.; Rodella, L.F.; Buffoli, B. The Effectiveness of the Use of Xenogeneic Bone Blocks Mixed with Autologous Concentrated Growth Factors (CGF) in Bone Regeneration Techniques: A Case Series. J. Osseointegration 2014, 6, 37–42. [Google Scholar] [CrossRef]
- Montemurro, N.; Pierozzi, E.; Inchingolo, A.M.; Pahwa, B.; De Carlo, A.; Palermo, A.; Scarola, R.; Dipalma, G.; Corsalini, M.; Inchingolo, A.D.; et al. New Biograft Solution, Growth Factors and Bone Regenerative Approaches in Neurosurgery, Dentistry, and Orthopedics: A Review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7653–7664. [Google Scholar] [CrossRef]
- Del Amo, F.S.L.; Yu, S.-H.; Sammartino, G.; Sculean, A.; Zucchelli, G.; Rasperini, G.; Felice, P.; Pagni, G.; Iorio-Siciliano, V.; Grusovin, M.G.; et al. Peri-Implant Soft Tissue Management: Cairo Opinion Consensus Conference. Int. J. Environ. Res. Public Health 2020, 17, 2281. [Google Scholar] [CrossRef]
- D’Esposito, V.; Lecce, M.; Marenzi, G.; Cabaro, S.; Ambrosio, M.R.; Sammartino, G.; Misso, S.; Migliaccio, T.; Liguoro, P.; Oriente, F.; et al. Platelet-Rich Plasma Counteracts Detrimental Effect of High-Glucose Concentrations on Mesenchymal Stem Cells from Bichat Fat Pad. J. Tissue Eng. Regen. Med. 2020, 14, 701–713. [Google Scholar] [CrossRef]
- Caggiano, M.; Gasparro, R.; D’Ambrosio, F.; Pisano, M.; Di Palo, M.P.; Contaldo, M. Smoking Cessation on Periodontal and Peri-Implant Health Status: A Systematic Review. Dent. J. 2022, 10, 162. [Google Scholar] [CrossRef]
- Saccomanno, S.; Antonini, G.; D’Alatri, L.; D’Angeloantonio, M.; Fiorita, A.; Deli, R. Case Report of Patients Treated with an Orthodontic and Myofunctional Protocol. Eur. J. Paediatr. Dent. 2014, 15, 184–186. [Google Scholar] [PubMed]
- Saccomanno, S.; Passarelli, P.C.; Oliva, B.; Grippaudo, C. Comparison between Two Radiological Methods for Assessment of Tooth Root Resorption: An In Vitro Study. BioMed Res. Int. 2018, 2018, 5152172. [Google Scholar] [CrossRef] [PubMed]
- Saccomanno, S.; Ieria, I.; Manenti, R.J.; Giancaspro, S.; Pirelli, P. Complications of Oral Piercing: A Review of the Literature and Two Case Reports. J. Biol. Regul. Homeost. Agents 2021, 35, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Quinzi, V.; Paskay, L.C.; D’Andrea, N.; Albani, A.; Monaco, A.; Saccomanno, S. Evaluation of the Nasolabial Angle in Orthodontic Diagnosis: A Systematic Review. Appl. Sci. 2021, 11, 2531. [Google Scholar] [CrossRef]
- Botzer, E.; Quinzi, V.; Salvati, S.E.; Paskay, L.C.; Saccomanno, S. Myofunctional Therapy Part 3: Tongue Function and Breastfeeding as Precursor of Oronasal Functions. Eur. J. Paediatr. Dent. 2021, 22, 248–250. [Google Scholar] [CrossRef]
- Conti, P.; Varvara, G.; Murmura, G.; Tete, S.; Sabatino, G.; Saggini, A.; Rosati, M.; Toniato, E.; Caraffa, A.; Antinolfi, P.; et al. Comparison of Beneficial Actions of Non-Steroidal Anti-Inflammatory Drugs to Flavonoids. J. Biol. Regul. Homeost. Agents 2013, 27, 1–7. [Google Scholar] [PubMed]
- Traini, T.; Pettinicchio, M.; Murmura, G.; Varvara, G.; Di Lullo, N.; Sinjari, B.; Caputi, S. Esthetic Outcome of an Immediately Placed Maxillary Anterior Single-Tooth Implant Restored with a Custom-Made Zirconia-Ceramic Abutment and Crown: A Staged Treatment. Quintessence Int. 2011, 42, 103–108. [Google Scholar] [PubMed]
- Shaik, Y.; Sabatino, G.; Maccauro, G.; Varvara, G.; Murmura, G.; Saggini, A.; Rosati, M.; Conti, F.; Cianchetti, E.; Caraffa, A.; et al. IL-36 Receptor Antagonist with Special Emphasis on IL-38. Int. J. Immunopathol. Pharmacol. 2013, 26, 27–36. [Google Scholar] [CrossRef]
- Frydas, S.; Varvara, G.; Murmura, G.; Saggini, A.; Caraffa, A.; Antinolfi, P.; Tete’, S.; Tripodi, D.; Conti, F.; Cianchetti, E.; et al. Impact of Capsaicin on Mast Cell Inflammation. Int. J. Immunopathol. Pharmacol. 2013, 26, 597–600. [Google Scholar] [CrossRef]
- Nicoletti, M.; Neri, G.; Maccauro, G.; Tripodi, D.; Varvara, G.; Saggini, A.; Potalivo, G.; Castellani, M.L.; Fulcheri, M.; Rosati, M.; et al. Impact of Neuropeptide Substance P an Inflammatory Compound on Arachidonic Acid Compound Generation. Int. J. Immunopathol. Pharmacol. 2012, 25, 849–857. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Fish-Hook Injuries: A Risk for Fishermen. Head Face Med. 2010, 6, 28. [Google Scholar] [CrossRef]
- Marrelli, M.; Tatullo, M. Influence of PRF in the Healing of Bone and Gingival Tissues. Clinical and Histological Evaluations. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1958–1962. [Google Scholar] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Scacco, S.; Papa, F.; Inchingolo, A.M.; Dipalma, G. Odontostomatologic Management of Patients Receiving Oral Anticoagulant Therapy: A Retrospective Multicentric Study. Ann. Surg. Innov. Res. 2011, 5, 5. [Google Scholar] [CrossRef]
- Malzoni, C.M.d.A.; Pichotano, E.C.; de Paula, L.G.F.; de Souza, R.V.; Okamoto, R.; Austin, R.S.; Marcantonio, E., Jr.; de Molon, R.S.; Zandim-Barcelos, D.L. Combination of Leukocyte and Platelet-Rich Fibrin and Demineralized Bovine Bone Graft Enhanced Bone Formation and Healing after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Clin. Oral Investig. 2023, 27, 5485–5498. [Google Scholar] [CrossRef] [PubMed]
- Sağlanmak, A.; Arısan, V.; Karabuda, C.; Özyuvacı, H. Dental Implant Rehabilitation of Posterior Maxillary Edentulism via Sinus Augmentation Using the Lateral Window Technique: A Retrospective Analysis of 289 Implants Followed Up for 15 Years. J. Funct. Biomater. 2025, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Grossi-Oliveira, G.A.; Dallazen, E.; Asbi, T.; Fonseca-Santos, J.M.; Ribeiro-Júnior, P.D.; Shibli, J.A.; Grecco, C.M.; Magro-Filho, O.; Mourão, C.F.; Haim, D.; et al. Effects of Platelet-Rich Fibrin on Bone Healing Around Implants Placed in Maxillary Sinuses: A Histomorphometric Assessment in Rabbits. J. Funct. Biomater. 2024, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Prati, C.; Zamparini, F.; Iezzi, G.; Botticelli, D.; Gandolfi, M.G.; Baba, S. ESEM-EDX Mineralization and Morphological Analysis of Human Retrieved Maxillary Sinus Bone Graft Biopsies before Loading. J. Funct. Biomater. 2023, 14, 391. [Google Scholar] [CrossRef]
- Băbțan, A.-M.; Feurdean, C.N.; Ionel, A.; Uriciuc, W.A.; Chifor, R.; Jaques, C.A.B.; Boșca, B.A.; Ilea, A. Insights into Sinus-Lift Bone Grafting Materials: What’s Changed? J. Funct. Biomater. 2025, 16, 133. [Google Scholar] [CrossRef]
- Mangano, C.; Riberti, N.; Orilisi, G.; Tecco, S.; Furlani, M.; Giommi, C.; Mengucci, P.; Giorgini, E.; Giuliani, A. Morphometric, Biomechanical and Macromolecular Performances of β-TCP Macro/Micro-Porous Lattice Scaffolds Fabricated via Lithography-Based Ceramic Manufacturing for Jawbone Engineering. J. Funct. Biomater. 2025, 16, 237. [Google Scholar] [CrossRef]
- Jing, L.; Su, B. Resorption Rates of Bone Graft Materials after Crestal Maxillary Sinus Floor Elevation and Its Influencing Factors. J. Funct. Biomater. 2024, 15, 133. [Google Scholar] [CrossRef]
- Maniwa, N.; Xavier, S.P.; de Souza, S.L.S.; Silva, E.R.; Botticelli, D.; Morinaga, K.; Baba, S. Sequential Bone Repair in Rabbit Sinus Lifts Using Bio-Oss and Hyaluronic Acid-Polynucleotide Gel (Regenfast). J. Funct. Biomater. 2024, 15, 361. [Google Scholar] [CrossRef]
- Idiri, K.; Bandiaky, O.; Soueidan, A.; Verner, C.; Renard, E.; Struillou, X. The Effectiveness of the Addition of Platelet-Rich Fibrin to Bovine Xenografts in Sinus and Bone Ridge Augmentation: A Systematic Review. J. Funct. Biomater. 2023, 14, 389. [Google Scholar] [CrossRef]
- Maspero, C.; Fama, A.; Cavagnetto, D.; Abate, A.; Farronato, M. Treatment of Dental Dilacerations. J. Biol. Regul. Homeost. Agents 2019, 33, 1623–1627. [Google Scholar] [PubMed]
- Tartaglia, G.M.; Mapelli, A.; Maspero, C.; Santaniello, T.; Serafin, M.; Farronato, M.; Caprioglio, A. Direct 3D Printing of Clear Orthodontic Aligners: Current State and Future Possibilities. Materials 2021, 14, 1799. [Google Scholar] [CrossRef]
- Farronato, M.; Boccalari, E.; Del Rosso, E.; Lanteri, V.; Mulder, R.; Maspero, C. A Scoping Review of Respirator Literature and a Survey among Dental Professionals. Int. J. Environ. Res. Public Health 2020, 17, 5968. [Google Scholar] [CrossRef] [PubMed]
- Ergoren, M.C.; Paolacci, S.; Manara, E.; Dautaj, A.; Dhuli, K.; Anpilogov, K.; Camilleri, G.; Suer, H.K.; Sayan, M.; Tuncel, G.; et al. A Pilot Study on the Preventative Potential of Alpha-Cyclodextrin and Hydroxytyrosol against SARS-CoV-2 Transmission. Acta Biomed. 2020, 91, e2020022. [Google Scholar] [CrossRef]
- Inchingolo, F.; Santacroce, L.; Cantore, S.; Ballini, A.; Del Prete, R.; Topi, S.; Saini, R.; Dipalma, G.; Arrigoni, R. Probiotics and EpiCor® in Human Health. J. Biol. Regul. Homeost. Agents 2019, 33, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Oguić, M.; Čandrlić, M.; Tomas, M.; Vidaković, B.; Blašković, M.; Radetić, A.T.J.; Cvek, S.Z.; Kuiš, D.; Peloza, O.C. Osteogenic Potential of Autologous Dentin Graft Compared with Bovine Xenograft Mixed with Autologous Bone in the Esthetic Zone: Radiographic, Histologic and Immunohistochemical Evaluation. Int. J. Mol. Sci. 2023, 24, 6440. [Google Scholar] [CrossRef]
- Olchowy, A.; Olchowy, C.; Zawiślak, I.; Matys, J.; Dobrzyński, M. Revolutionizing Bone Regeneration with Grinder-Based Dentin Biomaterial: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 9583. [Google Scholar] [CrossRef]
- Raghoebar, G.M.; Slater, J.J.H.; Hartog, L.d.; Meijer, H.J.A.; Vissink, A. Comparison of Procedures for Immediate Reconstruction of Large Osseous Defects Resulting from Removal of a Single Tooth to Prepare for Insertion of an Endosseous Implant after Healing. Int. J. Oral Maxillofac. Surg. 2009, 38, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Laudadio, C.; Palmieri, G.; et al. Genetic Pattern, Orthodontic and Surgical Management of Multiple Supplementary Impacted Teeth in a Rare, Cleidocranial Dysplasia Patient: A Case Report. Medicina 2021, 57, 1350. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, L.; Lin, J.; Chen, J.; Huang, W.; Chen, Y. Immediate Implant Placement in Anterior Teeth with Grafting Material of Autogenous Tooth Bone vs Xenogenic Bone. BMC Oral Health 2019, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Alrayyes, Y.; Al-Jasser, R. Regenerative Potential of Platelet Rich Fibrin (PRF) in Socket Preservation in Comparison with Conventional Treatment Modalities: A Systematic Review and Meta-Analysis. Tissue Eng. Regen. Med. 2022, 19, 463–475. [Google Scholar] [CrossRef]
- Andrade, C.; Camino, J.; Nally, M.; Quirynen, M.; Martínez, B.; Pinto, N. Combining Autologous Particulate Dentin, L-PRF, and Fibrinogen to Create a Matrix for Predictable Ridge Preservation: A Pilot Clinical Study. Clin. Oral Investig. 2020, 24, 1151–1160. [Google Scholar] [CrossRef]
- Grawish, M.E.; Grawish, L.M.; Grawish, H.M.; Grawish, M.M.; Holiel, A.A.; Sultan, N.; El-Negoly, S.A. Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review. Tissue Eng. Regen. Med. 2022, 19, 687–701. [Google Scholar] [CrossRef]
- Mendes, V.V.; Martins, F.V.; de Santana, C.M.M.; de Santana, R.B. Do Recombinant, Purified, and Concentrated Growth Factors Enhance the Regenerative Potential of Particulate Bone Graft Substitutes in Maxillary Sinus Floor Augmentation? A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2024, 39, 87–101. [Google Scholar] [CrossRef]
- Damsaz, M.; Castagnoli, C.Z.; Eshghpour, M.; Alamdari, D.H.; Alamdari, A.H.; Noujeim, Z.E.F.; Haidar, Z.S. Evidence-Based Clinical Efficacy of Leukocyte and Platelet-Rich Fibrin in Maxillary Sinus Floor Lift, Graft and Surgical Augmentation Procedures. Front. Surg. 2020, 7, 537138. [Google Scholar] [CrossRef]
- Miron, R.J.; Pikos, M.A.; Estrin, N.E.; Kobayashi-Fujioka, M.; Espinoza, A.R.; Basma, H.; Zhang, Y. Extended Platelet-Rich Fibrin. Periodontol. 2000 2024, 94, 114–130. [Google Scholar] [CrossRef]
- Scaini, R.; Saleh, M.H.A.; Lai, H.-C.; Sangiorgi, M.; Zucchelli, G.; Testori, T. Indications and Regenerative Techniques for Lateral Window Sinus Floor Elevation With Ridge Augmentation. Clin. Implant. Dent. Relat. Res. 2025, 27, e70007. [Google Scholar] [CrossRef]
- Quirynen, M.; Blanco, J.; Wang, H.-L.; Donos, N.; Temmerman, A.; Castro, A.; Pinto, N. Instructions for the Use of L-PRF in Different Clinical Indications. Periodontol. 2000 2025, 97, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura, S.; Ito, K.; Kohgo, T.; Hibi, H.; Nagasaka, T.; Ueda, M. Injectable Tissue-Engineered Bone Using Autogenous Bone Marrow-Derived Stromal Cells for Maxillary Sinus Augmentation: Clinical Application Report from a 2–6-Year Follow-Up. Tissue Eng. Part A 2008, 14, 1699–1707. [Google Scholar] [CrossRef]
- Bernard, G.W. Healing and Repair of Osseous Defects. Dent. Clin. N. Am. 1991, 35, 469–477. [Google Scholar] [CrossRef]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implantol. 2018, 44, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part I: Technological Concepts and Evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef]
- Ghanaati, S.; Al-Maawi, S.; Conrad, T.; Lorenz, J.; Rössler, R.; Sader, R. Biomaterial-Based Bone Regeneration and Soft Tissue Management of the Individualized 3D-Titanium Mesh: An Alternative Concept to Autologous Transplantation and Flap Mobilization. J. Craniomaxillofac Surg. 2019, 47, 1633–1644. [Google Scholar] [CrossRef]
- Gowda, T.M.; Jayashri, M.; Venkatesh, U.G.; Shah, R.; Kumar, A.B.T.; Deepthi, M.; Priya, S. Autologous Tooth Bone Graft Block Compared with Advanced Platelet-Rich Fibrin in Alveolar Ridge Preservation: A Clinico-Radiographic Study. J. Indian Soc. Periodontol. 2023, 27, 619–625. [Google Scholar] [CrossRef]
- Miron, R.J. Optimized Bone Grafting. Periodontol. 2000 2024, 94, 143–160. [Google Scholar] [CrossRef]
- Arshad, S.; Tehreem, F.; Khan, M.R.; Ahmed, F.; Marya, A.; Karobari, M.I. Platelet-Rich Fibrin Used in Regenerative Endodontics and Dentistry: Current Uses, Limitations, and Future Recommendations for Application. Int. J. Dent. 2021, 2021, 4514598. [Google Scholar] [CrossRef]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Stumbras, A.; Krukis, M.M.; Januzis, G.; Juodzbalys, G. Regenerative Bone Potential after Sinus Floor Elevation Using Various Bone Graft Materials: A Systematic Review. Quintessence Int. 2019, 50, 548–558. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative Potential of Leucocyte- and Platelet-Rich Fibrin. Part B: Sinus Floor Elevation, Alveolar Ridge Preservation and Implant Therapy. A Systematic Review. J. Clin. Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.; Pozza, D.H.; Gouveia, S.; Felino, A.; Almeida, R.F.E. The Applications of Regenerative Medicine in Sinus Lift Procedures: A Systematic Review. Clin. Implant. Dent. Relat. Res. 2018, 20, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, W.; Niemczyk, S.; Odrzywolska, O.; Doroz, P.; Hochuł, D.; Zawadzka, K. Application of I-PRF in Dentistry. Wiad. Lek. 2024, 77, 2348–2352. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of Platelet-Rich Fibrin in Regenerative Dentistry: A Systematic Review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]
- Scarano, A.; Cappucci, C.; Rapone, B.; Bugea, C.; Lorusso, F.; Serra, P.; Di Carmine, M.S. Volumetric Evaluations of the Maxillary Sinus before and Post Regenerative Surgery. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 128–134. [Google Scholar] [CrossRef]
- Anitua, E.; Allende, M.; Eguia, A.; Alkhraisat, M.H. Bone-Regenerative Ability of Platelet-Rich Plasma Following Sinus Augmentation with Anorganic Bovine Bone: A Systematic Review with Meta-Analysis. Bioengineering 2022, 9, 597. [Google Scholar] [CrossRef]
- Asahina, I.; Kagami, H.; Agata, H.; Honda, M.J.; Sumita, Y.; Inoue, M.; Nagamura-Inoue, T.; Tojo, A. Clinical Outcome and 8-Year Follow-Up of Alveolar Bone Tissue Engineering for Severely Atrophic Alveolar Bone Using Autologous Bone Marrow Stromal Cells with Platelet-Rich Plasma and β-Tricalcium Phosphate Granules. J. Clin. Med. 2021, 10, 5231. [Google Scholar] [CrossRef]
- Granz, C.L.; Gorji, A. Dental Stem Cells: The Role of Biomaterials and Scaffolds in Developing Novel Therapeutic Strategies. World J. Stem Cells 2020, 12, 897–921. [Google Scholar] [CrossRef]
- Hajibagheri, P.; Basirat, M.; Tabari-Khomeiran, Z.; Asadi-Aria, A. The Efficacy of Platelet-Rich Fibrin (PRF) in Post-Extraction Hard and Soft Tissue Healing and Associated Complications: A Systematic Review and Meta-Analysis of Split-Mouth Randomized Clinical Trials. BMC Oral Health 2025, 25, 869. [Google Scholar] [CrossRef]
- Jensen, O.T.; Cottam, J.; Ringeman, J.; Adams, M. Trans-Sinus Dental Implants, Bone Morphogenetic Protein 2, and Immediate Function for All-on-4 Treatment of Severe Maxillary Atrophy. J. Oral Maxillofac. Surg. 2012, 70, 141–148. [Google Scholar] [CrossRef]
- Rathan, A.C.L.; Satheesan, S.; Divya, V.C.; Narayanan, V.; Ramakrishnan, K. Comparison of Activated Platelet Rich Fibrin and Platelet Rich Fibrin in Osseous Regeneration of Freshly Extracted Socket- A Double Blinded Randomized Clinical Study. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101919. [Google Scholar] [CrossRef]
- Murata, M.; Nezu, T.; Takebe, H.; Hirose, Y.; Okubo, N.; Saito, T.; Akazawa, T. Human Dentin Materials for Minimally Invasive Bone Regeneration: Animal Studies and Clinical Cases. J. Oral Biosci. 2023, 65, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.P.; De Santis, E.; Hochuli-Vieira, E.; Faco, E.F.d.S.; Pantani, F.; Salata, L.A.; Botticelli, D. Deproteinized Bovine Bone Mineral or Autologous Bone at Dehiscence Type Defects at Implants Installed Immediately into Extraction Sockets: An Experimental Study in Dogs. Clin. Implant. Dent. Relat. Res. 2016, 18, 507–516. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Sahin, D.; Sader, R.; Becker, J.; Schwarz, F. Efficacy of Autogenous Teeth for the Reconstruction of Alveolar Ridge Deficiencies: A Systematic Review. Clin. Oral Investig. 2019, 23, 4263–4287. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.M.; Al-Deen, M.S.M.S.; Emam, A.A. Comparative Evaluation of Platelet Rich Plasma (PRP) versus Platelet Rich Fibrin (PRF) Scaffolds in Regenerative Endodontic Treatment of Immature Necrotic Permanent Maxillary Central Incisors: A Double Blinded Randomized Controlled Trial. Saudi Dent. J. 2020, 32, 224–231. [Google Scholar] [CrossRef]
- Inchingolo, F.; Dipalma, G.; Paduaneffi, G.; De Oliveira, L.A.; Inchingolo, A.M.; Georgakopoulos, P.L.; Inchingolo, A.D.; Malcangi, G.; Athanasiou, E.; Fotopoulou, E.; et al. Computer-Based Quantification of an Atraumatic Sinus Augmentation Technique Using CBCT. J. Biol. Regul. Homeost. Agents 2019, 33, 31–39. [Google Scholar] [PubMed]
- DePoi, R.; John, V.; de Mendoza, C.Y.P.; Gossweiler, M.K. Development of an Oro-Antral Fistula Following Sinus Elevation Surgery: A Case Report on Management Using Platelet-Rich Plasma. J. Indiana Dent. Assoc. 2007, 86, 10–16. [Google Scholar] [PubMed]
- Shekarian, M.; Aghajani, F.; Hejazi, Z.S.M.; Iranmanesh, P.; Akbari-Bezenjani, B.M.; Khademi, A. Tooth Graft and Platelet-Rich Fibrin Mixture for Oral Bone Reconstruction and Preservation: A Scoping Review. Clin. Exp. Dent. Res. 2025, 11, e70160. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yin, X.; Yang, C.; Kuang, H.; Luo, W. Advances in Autogenous Dentin Matrix Graft as a Promising Biomaterial for Guided Bone Regeneration in Maxillofacial Region: A Review. Medicine 2024, 103, e39422. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, X.; Lin, J.; Fan, L.; Zheng, Y.; Kuang, W. Dental Stem Cell in Tooth Development and Advances of Adult Dental Stem Cell in Regenerative Therapies. Curr. Stem Cell Res. Ther. 2015, 10, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Tolstunov, L.; Chi, J. Alveolar Ridge Augmentation: Comparison of Two Socket Graft Materials in Implant Cases. Compend. Contin. Educ. Dent. 2011, 32, E146–E155. [Google Scholar] [PubMed]
- Urist, M.R.; Strates, B.S. Bone Morphogenetic Protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef]
- Wang, B.; Feng, C.; Liu, Y.; Mi, F.; Dong, J. Recent Advances in Biofunctional Guided Bone Regeneration Materials for Repairing Defective Alveolar and Maxillofacial Bone: A Review. Jpn. Dent. Sci. Rev. 2022, 58, 233–248. [Google Scholar] [CrossRef]
- Coloccia, G.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Montenegro, V.; Patano, A.; Marinelli, G.; Laudadio, C.; Limongelli, L.; Di Venere, D.; et al. Effectiveness of Dental and Maxillary Transverse Changes in Tooth-Borne, Bone-Borne, and Hybrid Palatal Expansion through Cone-Beam Tomography: A Systematic Review of the Literature. Medicina 2021, 57, 288. [Google Scholar] [CrossRef]
- Tommasato, G.; Piano, S.; Casentini, P.; De Stavola, L.; Chiapasco, M. Digital Planning and Bone Regenerative Technologies: A Narrative Review. Clin. Oral Implant. Res. 2024, 35, 906–921. [Google Scholar] [CrossRef]
- Quirynen, M.; Siawasch, S.; Temmerman, A.; Cortellini, S.; Dhondt, R.; Teughels, W.; Castro, A.B. Do Autologous Platelet Concentrates (APCs) Have a Role in Intra-Oral Bone Regeneration? A Critical Review of Clinical Guidelines on Decision-Making Process. Periodontol. 2000 2023, 93, 254–269. [Google Scholar] [CrossRef]
- Thor, A.; Franke-Stenport, V.; Johansson, C.B.; Rasmusson, L. Early Bone Formation in Human Bone Grafts Treated with Platelet-Rich Plasma: Preliminary Histomorphometric Results. Int. J. Oral Maxillofac. Surg. 2007, 36, 1164–1171. [Google Scholar] [CrossRef]
- Pocaterra, A.; Caruso, S.; Bernardi, S.; Scagnoli, L.; Continenza, M.A.; Gatto, R. Effectiveness of Platelet-Rich Plasma as an Adjunctive Material to Bone Graft: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Int. J. Oral Maxillofac. Surg. 2016, 45, 1027–1034. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an Injectable Platelet-Rich Fibrin on Osteoblast Behavior and Bone Tissue Formation in Comparison to Platelet-Rich Plasma. Platelets 2018, 29, 48–55. [Google Scholar] [CrossRef]
- Wysłouch, E.; Sipika, A.; Grabowska-Jasik, N.; Tyrakowski, M.; Kaczmarzyk, T.; Szuta, M. Use of Autologous Dentin Matrix in Bone Defects Augmentation—A Literature Review. Folia Med. Cracov. 2024, 64, 87–91. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Hao, R.; Yao, J. Comparative Evaluation of Autologous Dental Bone Powder and Deproteinized Bovine Bone Mineral Allografts for Oral Implant Bone Deficits. Clin. Oral Investig. 2024, 28, 637. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Tsai, C.-H.; Chang, Y.-C. Clinical Application of Platelet-Rich Fibrin as the Sole Grafting Material in Maxillary Sinus Augmentation. J. Formos. Med. Assoc. 2015, 114, 779–780. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipalma, G.; Inchingolo, A.D.; Calò, F.; Lagioia, R.; Bassi, P.; de Ruvo, E.; Inchingolo, F.; Palermo, A.; Marinelli, G.; Inchingolo, A.M. Eighty-Four-Month Clinical Outcomes of Autologous Dentin Graft Using Tooth Transformer® and Concentrated Growth Factors in Maxillary Atrophy: A Retrospective Study of 31 Patients. J. Funct. Biomater. 2025, 16, 357. https://doi.org/10.3390/jfb16100357
Dipalma G, Inchingolo AD, Calò F, Lagioia R, Bassi P, de Ruvo E, Inchingolo F, Palermo A, Marinelli G, Inchingolo AM. Eighty-Four-Month Clinical Outcomes of Autologous Dentin Graft Using Tooth Transformer® and Concentrated Growth Factors in Maxillary Atrophy: A Retrospective Study of 31 Patients. Journal of Functional Biomaterials. 2025; 16(10):357. https://doi.org/10.3390/jfb16100357
Chicago/Turabian StyleDipalma, Gianna, Alessio Danilo Inchingolo, Francesca Calò, Rosalba Lagioia, Paola Bassi, Elisabetta de Ruvo, Francesco Inchingolo, Andrea Palermo, Grazia Marinelli, and Angelo Michele Inchingolo. 2025. "Eighty-Four-Month Clinical Outcomes of Autologous Dentin Graft Using Tooth Transformer® and Concentrated Growth Factors in Maxillary Atrophy: A Retrospective Study of 31 Patients" Journal of Functional Biomaterials 16, no. 10: 357. https://doi.org/10.3390/jfb16100357
APA StyleDipalma, G., Inchingolo, A. D., Calò, F., Lagioia, R., Bassi, P., de Ruvo, E., Inchingolo, F., Palermo, A., Marinelli, G., & Inchingolo, A. M. (2025). Eighty-Four-Month Clinical Outcomes of Autologous Dentin Graft Using Tooth Transformer® and Concentrated Growth Factors in Maxillary Atrophy: A Retrospective Study of 31 Patients. Journal of Functional Biomaterials, 16(10), 357. https://doi.org/10.3390/jfb16100357









