Development of Bioceramic Bone-Inspired Scaffolds Through Single-Step Melt-Extrusion 3D Printing for Segmental Defect Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Design and Development of Polymeric-Ceramic Composite Scaffolds
2.2.2. Fabrication Process of 3D-Printed Scaffolds
2.2.3. Morphological Characterisation
2.2.4. Fourier Transform Infrared Spectroscopy
2.2.5. Thermogravimetric Analysis
2.2.6. Mechanical Analysis of Tensile Strength
2.2.7. Water Contact Angle
2.2.8. Cell Studies
2.2.9. Statistical Analysis
3. Results and Discussion
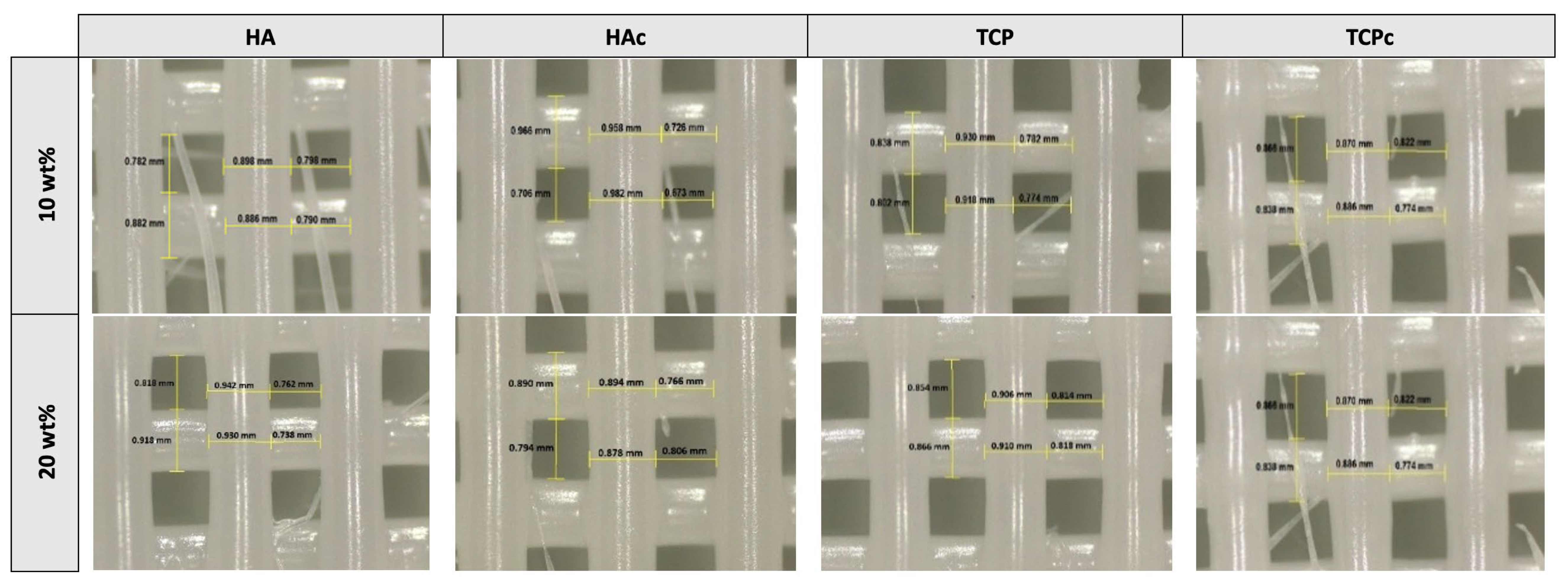
3.1. Printability Studies
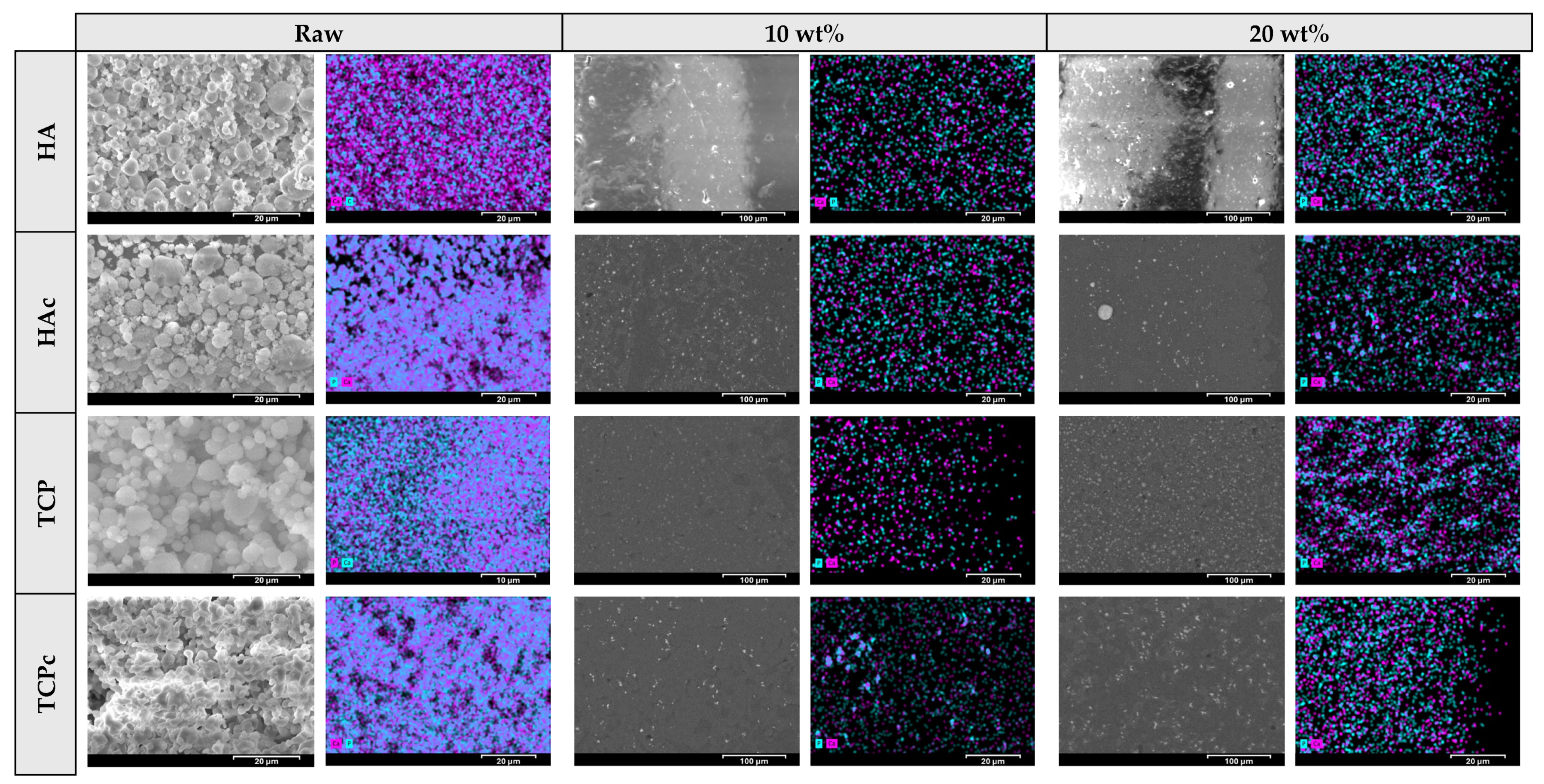
3.2. Microstructural Characteristics of the 3D-Printed Scaffolds
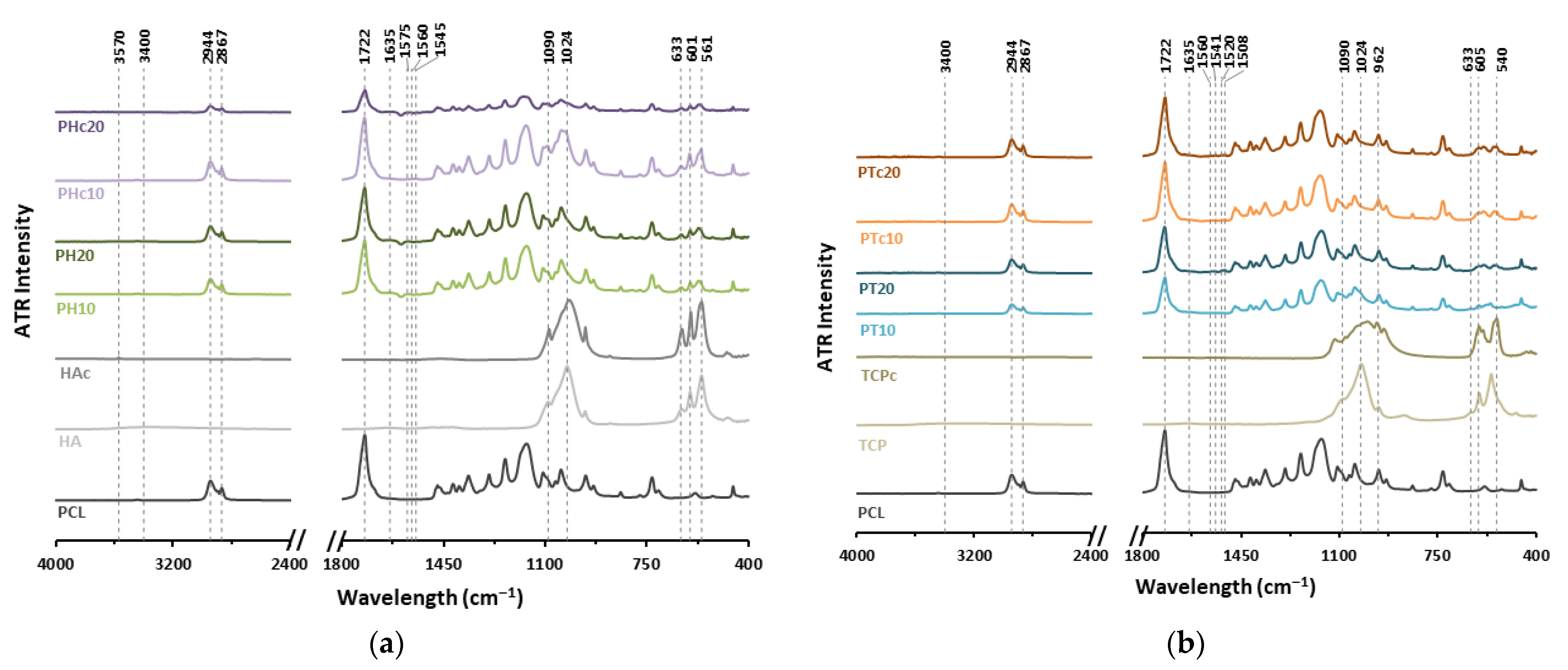
3.3. FTIR Characterisation
3.4. Thermal Degradation Analysis
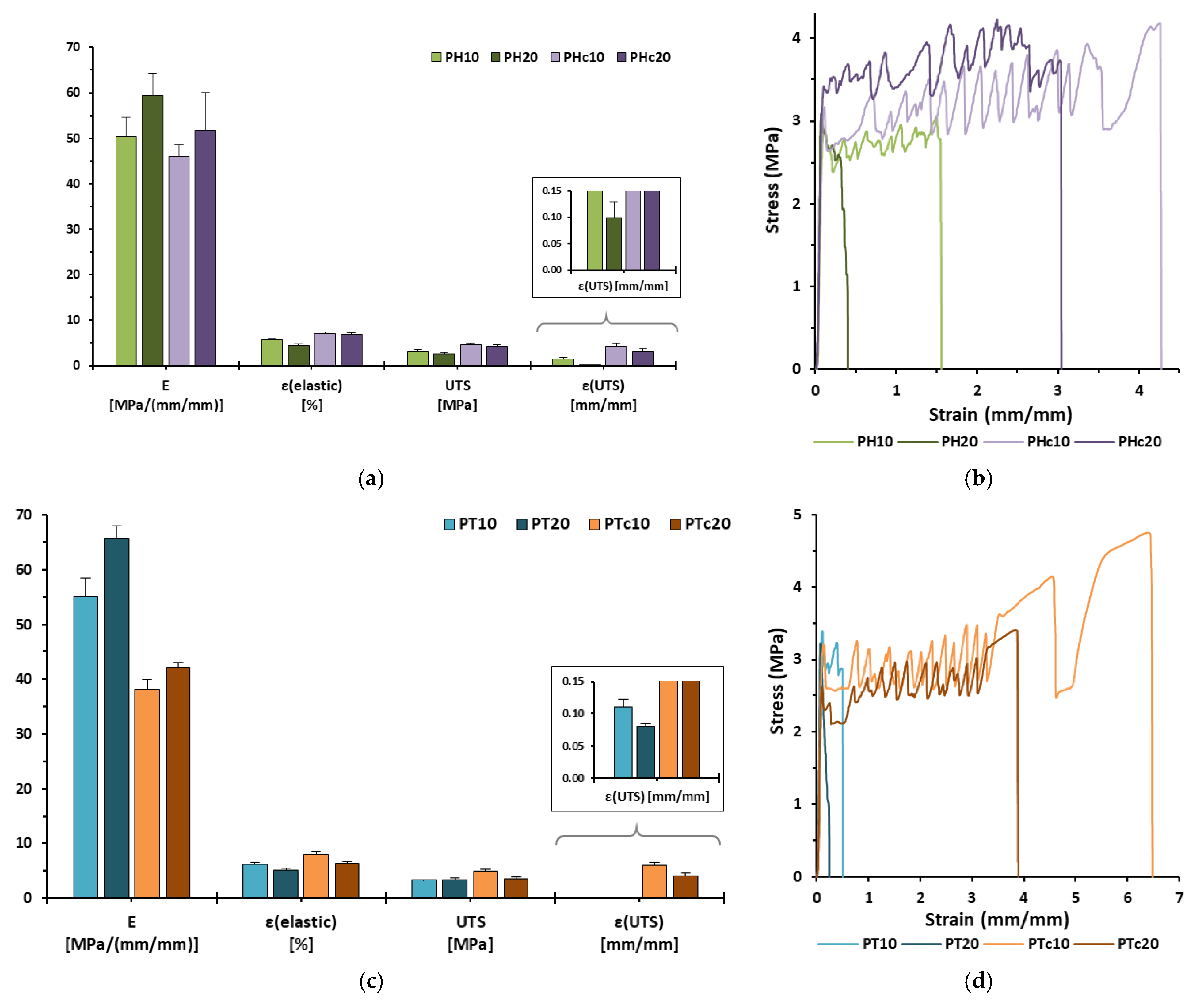
3.5. Mechanical Analysis
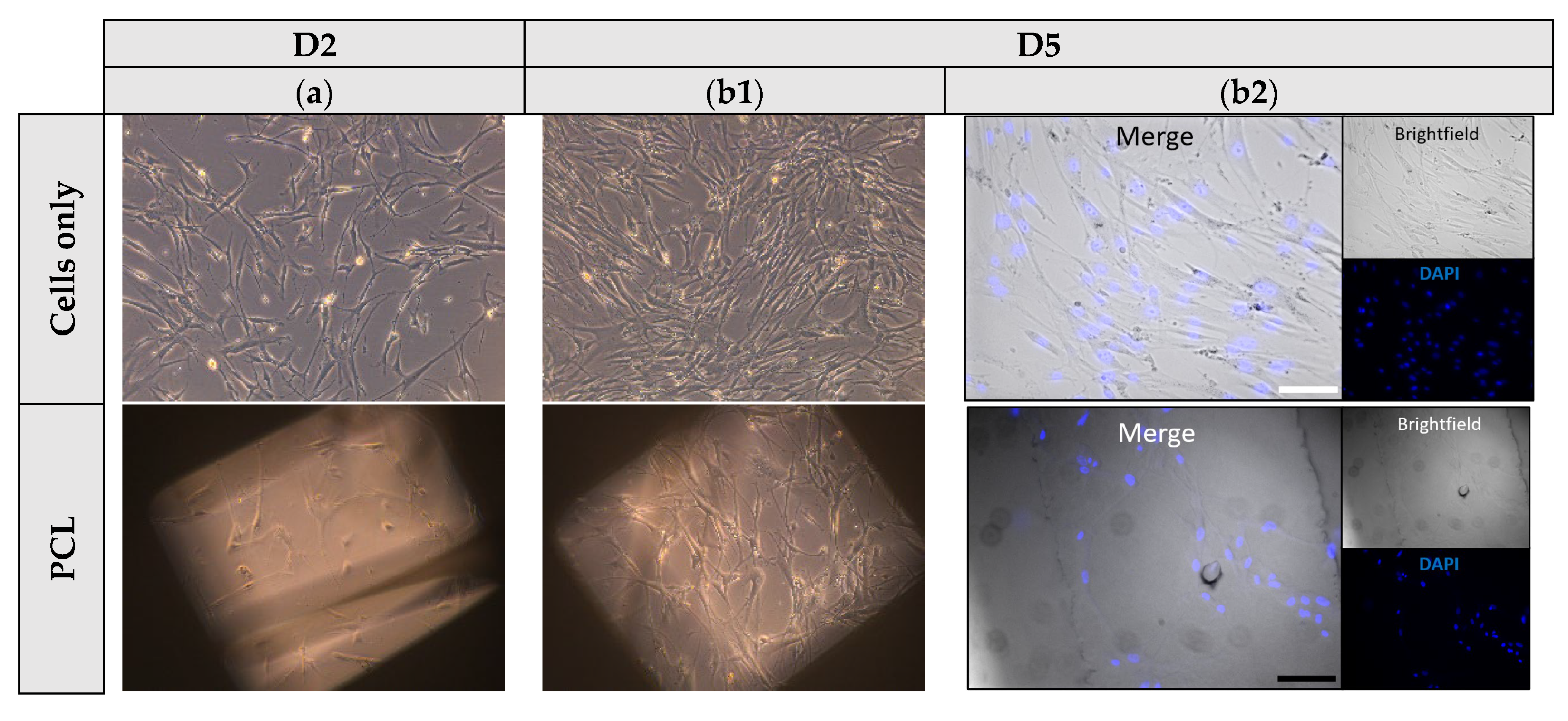
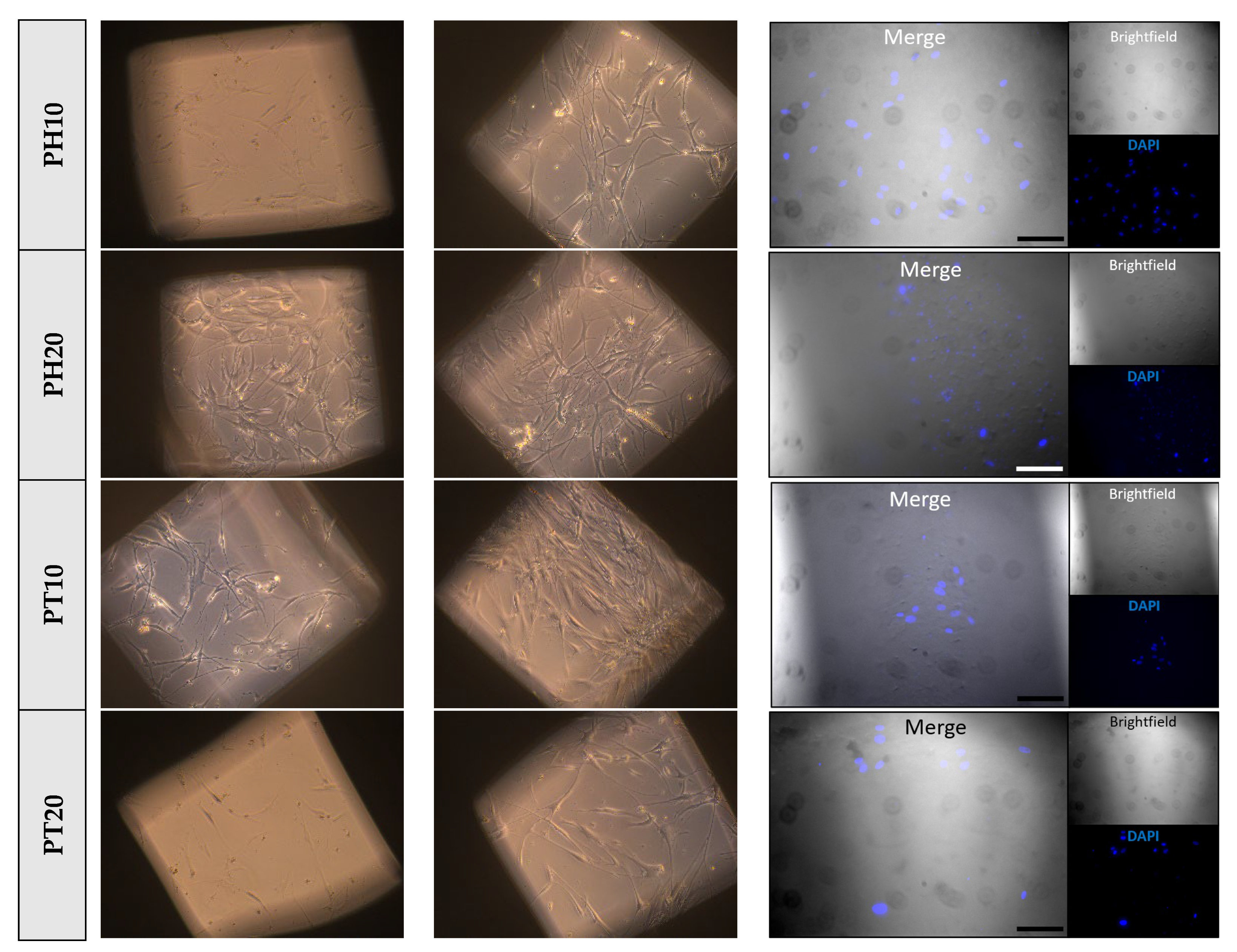
3.6. Cell Attachment and Proliferation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3DP | 3D printing |
| AM | additive manufacturing |
| ANOVA | One-way Analysis of Variance |
| ATR | Attenuated Total Reflectance |
| Avg. | average |
| BTR | bone tissue regeneration |
| CA | contact angle |
| CAD | Computer-Aided Design |
| CAG | Contact Angle Goniometry |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DIW | Direct Ink Writing |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| E | Young’s modulus |
| ECM | Extracellular Matrix |
| EDS | Energy-Dispersive X-ray |
| EM | emerging manufacturing |
| FBS | Fetal Bovine Serum |
| FFF | Fused Filament Fabrication |
| FTIR | Fourier-transform infrared spectroscopy |
| GelMA | gelatin methacryloyl |
| HA | hydroxyapatite |
| HAc | calcined hydroxyapatite |
| HPF | Primary Human Pulmonary Fibroblasts |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| IR | Infra-red |
| MSCs | Mesenchymal Stem Cells |
| MW | Molecular Weight |
| PBS | Phosphate-Buffered Saline |
| PCL | polycaprolactone |
| PLGA | polylactic(L-lactic-co-glycolic acid) |
| PM | Physical Mixture |
| PID | Proportional Integral Derivative |
| PUs | polyurethane |
| S.D. | Standard Deviation |
| SEM | Scanning Electron Microscope |
| SF | Shape Fidelity |
| SSs | Scaffold Samples |
| TCP | tricalcium phosphate |
| TCPc | calcined tricalcium phosphate |
| Tdeg | degradation temperature |
| TE | Tissue Engineering |
| TGA | Thermogravimetric Analysis |
| Tm | melting temperature |
| TP | Thermoplastic Printhead |
| UTS | Ultimate Tensile Strength |
| εelastic | elastic strain limit |
| εf | strain at failure |
| εUTS | strain at UTS |
References
- Lou, Y.; Wang, H.; Ye, G.; Li, Y.; Liu, C.; Yu, M.; Ying, B. Periosteal Tissue Engineering: Current Developments and Perspectives. Adv. Healthc. Mater. 2021, 10, 2100215. [Google Scholar] [CrossRef]
- Buer Boyetey, M.J.; Torgbo, S.; Sukyai, P. Bio-Scaffold for Bone Tissue Engineering with Focus on Bacterial Cellulose, Biological Materials for Hydroxyapatite Synthesis and Growth Factors. Eur. Polym. J. 2023, 194, 112168. [Google Scholar] [CrossRef]
- D’Elía, N.L.; Rial Silva, R.; Sartuqui, J.; Ercoli, D.; Ruso, J.; Messina, P.; Mestres, G. Development and Characterisation of Bilayered Periosteum-Inspired Composite Membranes Based on Sodium Alginate-Hydroxyapatite Nanoparticles. J. Colloid. Interface Sci. 2020, 572, 408–420. [Google Scholar] [CrossRef]
- Kang, J.; Dong, E.; Li, D.; Dong, S.; Zhang, C.; Wang, L. Anisotropy Characteristics of Microstructures for Bone Substitutes and Porous Implants with Application of Additive Manufacturing in Orthopaedic. Mater. Des. 2020, 191, 108608. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Zhang, B.; Shang, S.; Zhao, C.; Zhang, W.; Chen, J.; Zhou, C.; Zhou, H.; Feng, S. 3D Printing of Personalized Magnesium Composite Bone Tissue Engineering Scaffold for Bone and Angiogenesis Regeneration. Chem. Eng. J. 2024, 484, 149444. [Google Scholar] [CrossRef]
- Herath, B.; Suresh, S.; Downing, D.; Cometta, S.; Tino, R.; Castro, N.J.; Leary, M.; Schmutz, B.; Wille, M.L.; Hutmacher, D.W. Mechanical and Geometrical Study of 3D Printed Voronoi Scaffold Design for Large Bone Defects. Mater. Des. 2021, 212, 110224. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, 2000095. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Xin, H.; Yu, H.; Wang, H.; Ma, D.; Cao, X.; Wang, Y. MicroRNA-Activated Hydrogel Scaffold Generated by 3D Printing Accelerates Bone Regeneration. Bioact. Mater. 2022, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D Printed Scaffolds for Promoting Bone Regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef]
- dos Santos, V.I.; Chevalier, J.; Fredel, M.C.; Henriques, B.; Gremillard, L. Ceramics and Ceramic Composites for Biomedical Engineering Applications via Direct Ink Writing: Overall Scenario, Advances in the Improvement of Mechanical and Biological Properties and Innovations. Mater. Sci. Eng. R Rep. 2024, 161, 100841. [Google Scholar] [CrossRef]
- Lopez, C.D.; Diaz-Siso, J.R.; Witek, L.; Bekisz, J.M.; Cronstein, B.N.; Torroni, A.; Flores, R.L.; Rodriguez, E.D.; Coelho, P.G. Three Dimensionally Printed Bioactive Ceramic Scaffold Osseoconduction across Critical-Sized Mandibular Defects. J. Surg. Res. 2018, 223, 115–122. [Google Scholar] [CrossRef]
- Zhao, S.; Xiao, W.; Rahaman, M.N.; O’Brien, D.; Seitz-Sampson, J.W.; Sonny Bal, B. Robocasting of Silicon Nitride with Controllable Shape and Architecture for Biomedical Applications. Int. J. Appl. Ceram. Technol. 2017, 14, 117–127. [Google Scholar] [CrossRef]
- Chan, S.S.L.; Black, J.R.; Franks, G.V.; Heath, D.E. Hierarchically Porous 3D-Printed Ceramic Scaffolds for Bone Tissue Engineering. Biomater. Adv. 2025, 169, 214149. [Google Scholar] [CrossRef] [PubMed]
- Dedeloudi, A.; Farzeen, F.; Lesutan, V.N.; Irwin, R.; Wylie, M.P.; Andersen, S.; Eastwood, M.P.; Lamprou, D.A. Biopolymeric 3D Printed Scaffolds as a Versatile Tissue Engineering Treatment for Congenital Diaphragmatic Hernia. Int. J. Pharm. 2025, 672, 125313. [Google Scholar] [CrossRef] [PubMed]
- Dedeloudi, A.; Martinez-Marcos, L.; Quinten, T.; Andersen, S.K.; Lamprou, D.A. 3D-Printed Biomaterial-Based Scaffolds Loaded with Zoledronic Acid Functionalised Ceramic Microparticles for Sustained Release. Int. J. Pharm. 2025, 683, 126101. [Google Scholar] [CrossRef]
- Wu, L.; Pei, X.; Zhang, B.; Su, Z.; Gui, X.; Gao, C.; Guo, L.; Fan, H.; Jiang, Q.; Zhao, L.; et al. 3D-Printed HAp Bone Regeneration Scaffolds Enable Nano-Scale Manipulation of Cellular Mechanotransduction Signals. Chem. Eng. J. 2023, 455, 140699. [Google Scholar] [CrossRef]
- Gui, X.; Song, P.; Zhang, B.; Lei, H.; Wu, L.; Sun, J.; Tang, R.; Zhang, H.; Qin, Y.; Su, Z.; et al. Natural Loofah Sponge Inspired 3D Printed Bionic Scaffolds Promote Personalized Bone Defect Regeneration. Compos. B Eng. 2025, 288, 111920. [Google Scholar] [CrossRef]
- Shen, H.Y.; Xing, F.; Shang, S.Y.; Jiang, K.; Kuzmanović, M.; Huang, F.W.; Liu, Y.; Luo, E.; Edeleva, M.; Cardon, L.; et al. Biomimetic Mineralized 3D-Printed Polycaprolactone Scaffold Induced by Self-Adaptive Nanotopology to Accelerate Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 18658–18670. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-Printed Silk-Hydroxyapatite Scaffolds for Enhanced Bone Regeneration with Innervation and Vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef]
- Weaver, E.; O’Hagan, C.; Lamprou, D.A. The Sustainability of Emerging Technologies for Use in Pharmaceutical Manufacturing. Expert Opin. Drug Deliv. 2022, 19, 861–872. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Enhanced Osteogenic Differentiation of Stem Cells by 3D Printed PCL Scaffolds Coated with Collagen and Hydroxyapatite. Sci. Rep. 2022, 12, 12359. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Alizadeh Sardroud, H.; Sharma, N.K.; Wilson, L.D.; Chen, X. Fabrication of Chitosan/Alginate/Hydroxyapatite Hybrid Scaffolds Using 3D Printing and Impregnating Techniques for Potential Cartilage Regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef]
- Lee, J.; Song, C.; Noh, I.; Song, S.; Rhee, Y.S. Hot-Melt 3d Extrusion for the Fabrication of Customizable Modified-Release Solid Dosage Forms. Pharmaceutics 2020, 12, 738. [Google Scholar] [CrossRef]
- Huang, H.; Qiang, L.; Fan, M.; Liu, Y.; Yang, A.; Chang, D.; Li, J.; Sun, T.; Wang, Y.; Guo, R.; et al. 3D-Printed Tri-Element-Doped Hydroxyapatite/Polycaprolactone Composite Scaffolds with Antibacterial Potential for Osteosarcoma Therapy and Bone Regeneration. Bioact. Mater. 2024, 31, 18–37. [Google Scholar] [CrossRef]
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D.; et al. Development and Characterization of a PLGA-HA Composite Material to Fabricate 3D-Printed Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2021, 118, 111334. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhang, M.; Zhou, X.; Shao, Y.; Li, J.; Wang, L.; Chu, C.; Xue, F.; Yao, Q.; Bai, J. 3D-Printed Mg-Incorporated PCL-Based Scaffolds: A Promising Approach for Bone Healing. Mater. Sci. Eng. C 2021, 129, 112372. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, N.; Arefian, E.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. 3D-Printed PCL Scaffolds Coated with Nanobioceramics Enhance Osteogenic Differentiation of Stem Cells. ACS Omega 2021, 6, 35284–35296. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cheng, W.; Liu, H.; Luo, R.; Zou, H.; Zhang, L.; Ren, T.; Xu, C. Effect of Mechanical Loading on Bone Regeneration in HA/β-TCP/SF Scaffolds Prepared by Low-Temperature 3D Printing In Vivo. ACS Biomater. Sci. Eng. 2023, 9, 4980–4993. [Google Scholar] [CrossRef]
- Dedeloudi, A.; Martinez-Marcos, L.; Quinten, T.; Andersen, S.; Lamprou, D.A. Biopolymeric 3D Printed Implantable Scaffolds as a Potential Adjuvant Treatment for Acute Post-Operative Pain Management. Expert Opin. Drug Deliv. 2024, 21, 1651–1663. [Google Scholar] [CrossRef]
- Oladeji, S.; Mohylyuk, V.; Jones, D.S.; Andrews, G.P. 3D Printing of Pharmaceutical Oral Solid Dosage Forms by Fused Deposition: The Enhancement of Printability Using Plasticised HPMCAS. Int. J. Pharm. 2022, 616, 121553. [Google Scholar] [CrossRef]
- Corduas, F.; Mathew, E.; McGlynn, R.; Mariotti, D.; Lamprou, D.A.; Mancuso, E. Melt-Extrusion 3D Printing of Resorbable Levofloxacin-Loaded Meshes: Emerging Strategy for Urogynaecological Applications. Mater. Sci. Eng. C 2021, 131, 112523. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.H.; Fernandes, E.M.; Diogo, G.S.; Marques, C.F.; Silva, T.H.; Costa, L.C.; Passador, F.R.; Reis, R.L.; Pessan, L.A. Engineering 3D Printed Bioactive Composite Scaffolds Based on the Combination of Aliphatic Polyester and Calcium Phosphates for Bone Tissue Regeneration. Mater. Sci. Eng. C 2021, 122, 111928. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Gibson, I.R.; Ke, S.; Best, S.M.; Bonfield, W. Calcining Influence on the Powder Properties of Hydroxyapatite. J. Mater. Sci. Mater. Med. 2001, 12, 181–188. [Google Scholar] [CrossRef]
- Pattanayak, D.K.; Dash, R.; Prasad, R.C.; Rao, B.T.; Rama Mohan, T.R. Synthesis and Sintered Properties Evaluation of Calcium Phosphate Ceramics. Mater. Sci. Eng. C 2007, 27, 684–690. [Google Scholar] [CrossRef]
- Muralithran, G.; Ramesh, S. The Effects of Sintering Temperature on the Properties of Hydroxyapatite. Ceram. Int. 2000, 26, 221–230. [Google Scholar] [CrossRef]
- Pitol-Palin, L.; Moura, J.; Frigério, P.B.; de Souza Batista, F.R.; Saska, S.; Oliveira, L.J.M.; Matsubara, E.Y.; Pilatti, L.; Câmara, D.; Lizier, N.; et al. A Preliminary Study of Cell-Based Bone Tissue Engineering into 3D-Printed β-Tricalcium Phosphate Scaffolds and Polydioxanone Membranes. Sci. Rep. 2024, 14, 31184. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D Printing of PCL/Nano-Hydroxyapatite Scaffolds Derived from Biogenic Sources for Bone Tissue Engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- Doyle, H.; Lohfeld, S.; McDonnell, P.; McHugh, P. Evaluation of a Multiscale Modelling Methodology to Predict the Mechanical Properties of PCL/β-TCP Sintered Scaffold Materials. Ann. Biomed. Eng. 2015, 43, 1989–1998. [Google Scholar] [CrossRef]
- Meng, D.; Hou, Y.; Zubairi, H.; Ucan, M.T.; Hall, D.A.; Feteira, A.; Bartolo, P.; Wang, G.; Wang, W. Ceramic-Based Piezoelectric Material Reinforced 3D Printed Polycaprolactone Bone Tissue Engineering Scaffolds. Mater. Des. 2025, 257, 114542. [Google Scholar] [CrossRef]
- Sobhani, A.; Salimi, E. Nanocomposite Films of Polycaprolactone and Amorphous/Sintered Wollastonite Nanoparticles for Potential Biomedical Applications. Ceram. Int. 2024, 50, 26869–26878. [Google Scholar] [CrossRef]
- Popowics, T.E.; Zhu, Z.; Herring, S.W. Mechanical Properties of the Periosteum in the Pig, Sus Scrofa. Arch. Oral. Biol. 2002, 47, 733–741. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Leal, F.; Moreira, A.; Schellhorn, T.; Blahnová, V.H.; Zeiringer, S.; Vocetková, K.; Tetyczka, C.; Simaite, A.; Buzgo, M.; et al. Potential of Electrospun Fibrous Scaffolds for Intestinal, Skin, and Lung Epithelial Tissue Modeling. Adv. Nanobiomed Res. 2023, 3, 2200104. [Google Scholar] [CrossRef]
- Ganesh, M.D.; Manickasamy, M.K.; Joel, P.; Kalyani, D.; Kunnumakkara, A.B.; Dobbidi, P. Tuning the Properties of Hydroxyapatite through Mg/Zn Co-Doping: A Pathway to Advanced Biomedical Devices. ACS Biomater. Sci. Eng. 2025, 11, 4788–4805. [Google Scholar] [CrossRef]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, A.D.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016, 136, 85–97. [Google Scholar] [CrossRef]



| Formulation a,b | Composition (wt%) | ||||
|---|---|---|---|---|---|
| PCL | HA | HAc | β-TCP | β-TCPc | |
| PH10 | 90 | 10 | - | - | - |
| PH20 | 80 | 20 | - | - | - |
| PHc10 | 90 | - | 10 | - | - |
| PHc20 | 80 | - | 20 | - | - |
| PT10 | 90 | - | - | 10 | - |
| PT20 | 80 | - | - | 20 | - |
| PTc10 | 90 | - | - | - | 10 |
| PTc20 | 80 | - | - | - | 20 |
| Formulation | Printing Pressure (kPa) | Raster Width | Pr | |
|---|---|---|---|---|
| (mm) | Relative Error (%) | |||
| PH10 | 75 | 0.89 ± 0.04 | −5.79 | 0.93 |
| PH20 | 90 | 0.88 ± 0.04 | −4.38 | 0.95 |
| PHc10 | 80 | 0.90 ± 0.02 | −7.06 | 0.93 |
| PHc20 | 90 | 0.87 ± 0.03 | −3.02 | 0.97 |
| PT10 | 70 | 0.93 ± 0.04 | −10.60 | 0.89 |
| PT20 | 85 | 0.90 ± 0.04 | −6.61 | 0.91 |
| PTc10 | 75 | 0.88 ± 0.02 | −4.56 | 0.95 |
| PTc20 | 85 | 0.86 ± 0.02 | −2.08 | 0.98 |
| Formulations | Residual wt (%) (T = 500 °C) | |
|---|---|---|
| Raw Materials | HA | 93.73 ± 0.13 |
| HAc | 100.00 | |
| TCP | 94.88 ± 0.17 | |
| TCPc | 100.00 | |
| Scaffold Samples * | PH10 | 8.82 ± 0.59 |
| PH20 | 16.29 ± 0.92 | |
| PHc10 | 9.56 ± 0.01 | |
| PHc20 | 21.33 ± 0.52 | |
| PT10 | 8.64 ± 0.19 | |
| PT20 | 15.89 ± 0.03 | |
| PTc10 | 12.02 ± 0.14 | |
| PTc20 | 17.08 ± 0.11 |
| Formulation | E [MPa/(mm/mm)] | εelastic [%] | UTS [MPa] | εUTS [mm/mm] | εf [%] |
|---|---|---|---|---|---|
| PH10 | 50.32 ± 4.26 | 5.69 ± 0.28 | 3.16 ± 0.29 | 1.45 ± 0.33 | 153.58 ± 40.32 |
| PH20 | 59.46 ± 4.73 | 4.50 ± 0.38 | 2.67 ± 0.25 | 0.10 ± 0.03 | 64.06 ± 21.11 |
| PHc10 | 46.03 ± 2.45 | 7.07 ± 0.34 | 4.58 ± 0.34 | 4.32 ± 0.61 | 489.00 ±60.01 |
| PHc20 | 51.69 ± 8.34 | 6.89 ± 0.37 | 4.21 ± 0.37 | 3.12 ± 0.62 | 350.20 ± 32.57 |
| PT10 | 55.09 ± 3.41 | 6.12 ±0.41 | 3.36 ± 0.03 | 0.11 ± 0.01 | 64.58 ± 11.95 |
| PT20 | 65.66 ± 2.33 | 5.19 ± 0.19 | 3.41 ± 0.24 | 0.08 ± 0.01 | 28.14 ± 2.62 |
| PTc10 | 38.17 ± 1.76 | 8.01 ± 0.56 | 4.92 ± 0.41 | 6.10 ± 0.43 | 664.07 ± 32.82 |
| PTc20 | 42.06 ± 0.90 | 6.43 ± 0.25 | 3.50 ± 0.38 | 4.04 ± 0.63 | 410.91 ± 65.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedeloudi, A.; Bertelli, P.M.; Martinez-Marcos, L.; Quinten, T.; Lengyel, I.; Andersen, S.K.; Lamprou, D.A. Development of Bioceramic Bone-Inspired Scaffolds Through Single-Step Melt-Extrusion 3D Printing for Segmental Defect Treatment. J. Funct. Biomater. 2025, 16, 358. https://doi.org/10.3390/jfb16100358
Dedeloudi A, Bertelli PM, Martinez-Marcos L, Quinten T, Lengyel I, Andersen SK, Lamprou DA. Development of Bioceramic Bone-Inspired Scaffolds Through Single-Step Melt-Extrusion 3D Printing for Segmental Defect Treatment. Journal of Functional Biomaterials. 2025; 16(10):358. https://doi.org/10.3390/jfb16100358
Chicago/Turabian StyleDedeloudi, Aikaterini, Pietro Maria Bertelli, Laura Martinez-Marcos, Thomas Quinten, Imre Lengyel, Sune K. Andersen, and Dimitrios A. Lamprou. 2025. "Development of Bioceramic Bone-Inspired Scaffolds Through Single-Step Melt-Extrusion 3D Printing for Segmental Defect Treatment" Journal of Functional Biomaterials 16, no. 10: 358. https://doi.org/10.3390/jfb16100358
APA StyleDedeloudi, A., Bertelli, P. M., Martinez-Marcos, L., Quinten, T., Lengyel, I., Andersen, S. K., & Lamprou, D. A. (2025). Development of Bioceramic Bone-Inspired Scaffolds Through Single-Step Melt-Extrusion 3D Printing for Segmental Defect Treatment. Journal of Functional Biomaterials, 16(10), 358. https://doi.org/10.3390/jfb16100358









