Organ-Specific Strategies in Bioprinting: Addressing Translational Challenges in the Heart, Liver, Kidney, and Pancreas
Abstract
1. Introduction
2. Bioprinting Modalities and Materials: Capabilities, Limitations, and Strategic Alignment
2.1. Overview of Bioprinting Modalities
2.2. Bioink Formulation: Trade-Offs Between Biofunctionality and Printability
2.3. Beyond Printability: Designing for Function and Integration
2.4. Gaps and Future Directions in Bioprinting Modalities
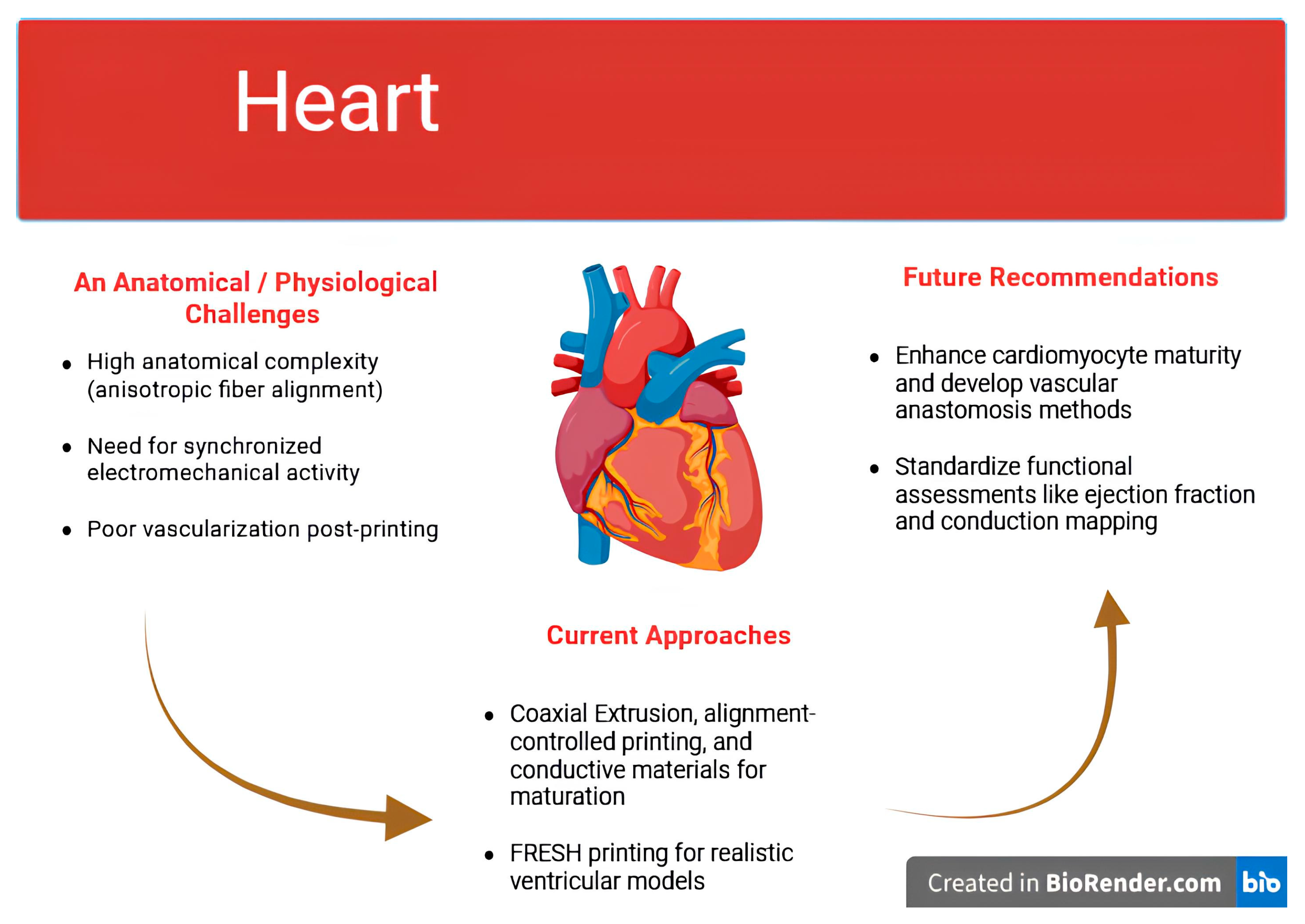
3. Cardiac Bioprinting—From Rhythmic Constructs to Functional Myocardial Repair
3.1. Anatomical and Functional Demands of the Heart
3.2. Advances in Bioprinted Cardiac Patches
3.3. Immaturity and Electromechanical Integration
3.4. Mechanical Demands and Host Integration
3.5. Functional Assessment and Clinical Readiness
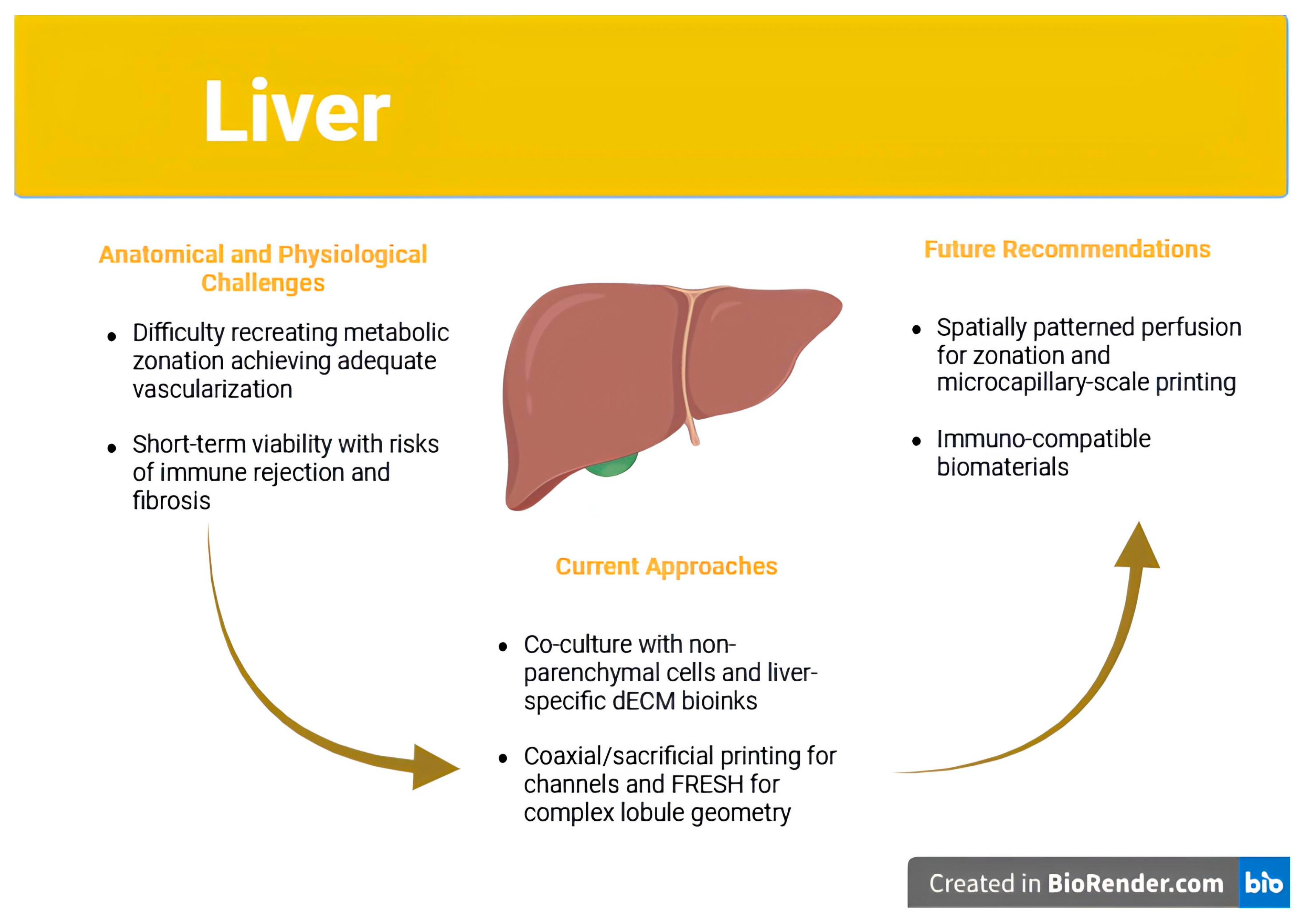
4. Liver Bioprinting—Function, Zonation, and Post-Implantation Challenges
4.1. The Liver as a Bioprinting Target: Unique Advantages and Complexities
4.2. Cell Sources and Bioinks: Beyond Viability
4.3. Vascularization and Zonation: Central Barriers to Functional Equivalence
4.4. Immune Rejection and Fibrotic Encapsulation
4.5. Toward Functional Trials: Realistic Clinical Applications
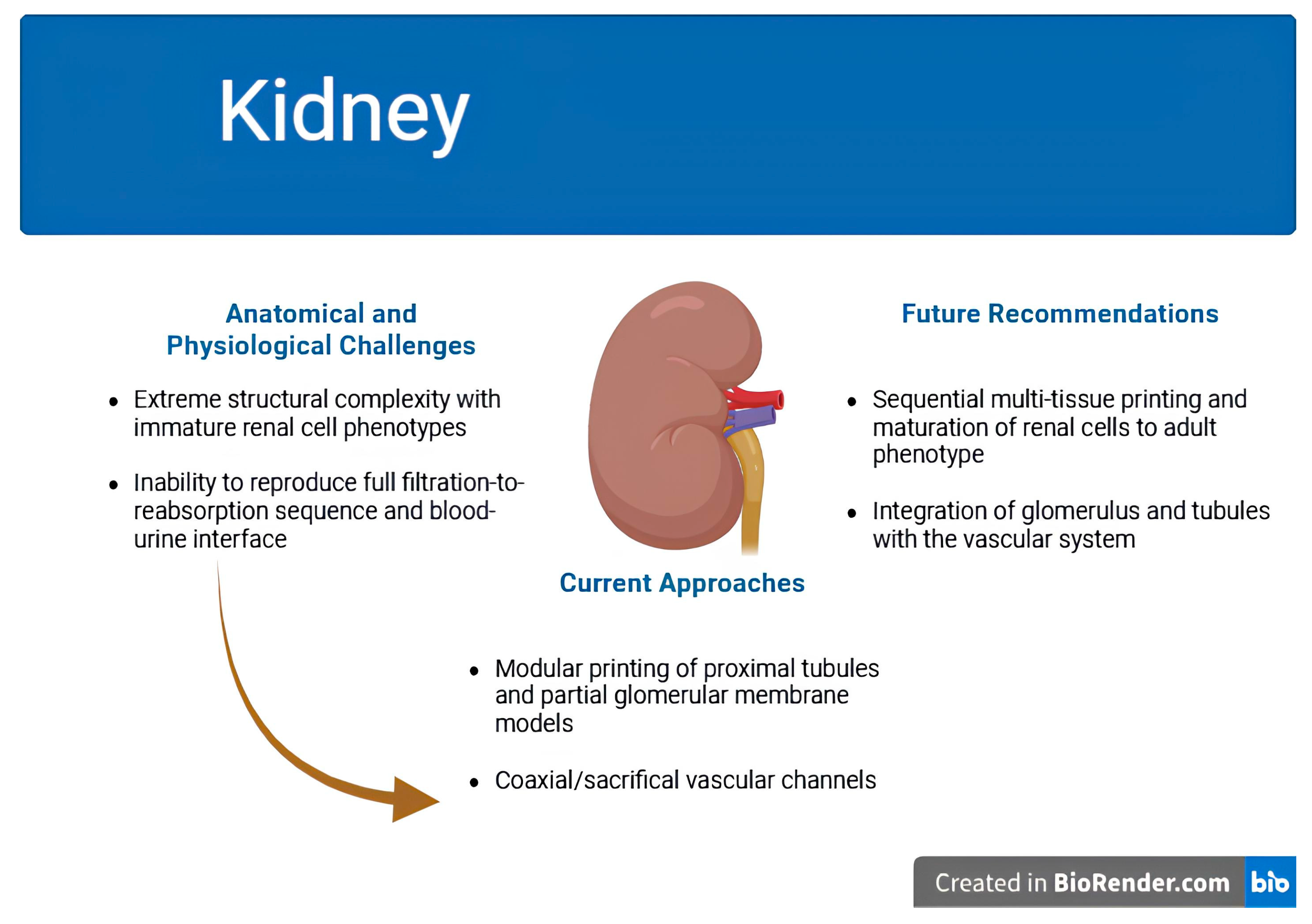
5. Kidney Bioprinting—Navigating Anatomical Complexity and Translational Realities
5.1. The Kidney as the Most Technically Challenging Organ for Bioprinting
5.2. Modular Bioprinting Strategies and Partial Functional Units
5.3. Cellular Immaturity and Patterning Constraints
5.4. Vascularization and Blood–Urine Interface
5.5. Realistic Applications and Clinical Translation Pathways
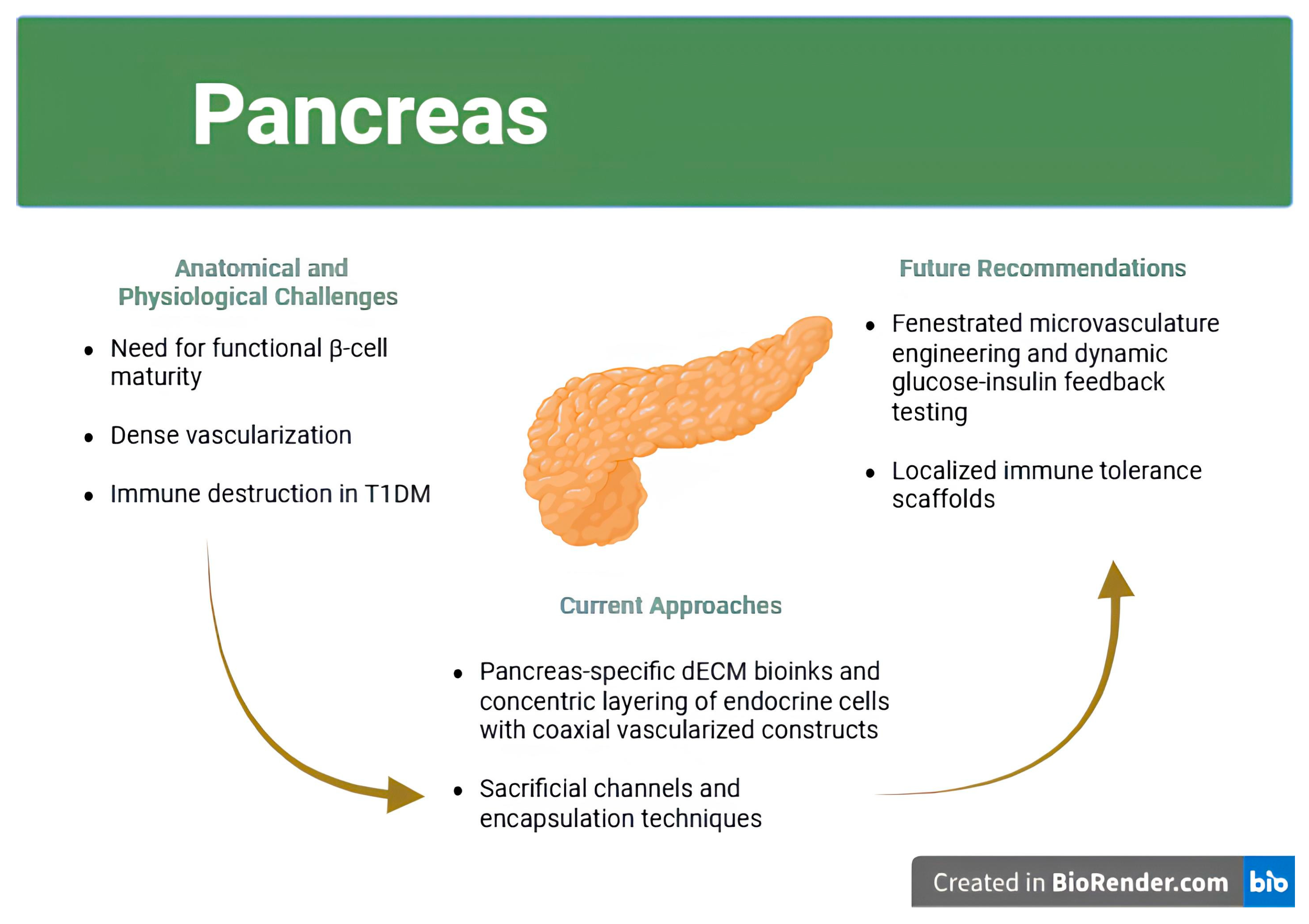
6. Pancreatic Bioprinting—Balancing Function, Vascularization, and Immune Tolerance
6.1. Therapeutic Motivation and Biological Constraints
6.2. Cellular Sources and Islet Microarchitecture
6.3. Vascularization and Dynamic Glucose Responsiveness
6.4. Immune Protection and Scaffold Design
6.5. Translational Considerations and First-in-Human Applications
7. Regulatory Landscape and Clinical Translation—Bridging Innovation and Implementation
7.1. Bioprinted Constructs and the Regulatory Grey Zone
7.2. Functional Uncertainty and Lack of Standardized Potency Metrics
7.3. Designing Ethical and Feasible Clinical Trials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Ozbolat, I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015, 33, 395–400. [Google Scholar] [CrossRef]
- Davoodi, E.; Sarikhani, E.; Montazerian, H.; Ahadian, S.; Costantini, M.; Swieszkowski, W.; Willerth, S.; Walus, K.; Mofidfar, M.; Toyserkani, E.; et al. Extrusion and Microfluidic-based Bioprinting to Fabricate Biomimetic Tissues and Organs. Adv. Mater. Technol. 2020, 5, 1901044. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Chen, F. Preparation and characterization of photocured poly (ε-caprolactone) diacrylate/poly (ethylene glycol) diacrylate/chitosan for photopolymerization-type 3D printing tissue engineering scaffold application. Mater. Sci. Eng. C 2017, 81, 66–73. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D bioprinting and its innovative approach for biomedical applications. MedComm 2023, 4, e194. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Ozbolat, I.; Moncal, K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2016, 13, 179–200. [Google Scholar] [CrossRef]
- Mei, J.; Liu, Y.; Zhou, Y.; Gong, D.; Wu, L.; Zhang, C. Amino-Terminated Poly(propylene oxide) as an Interfacial Dispersant for Low-Conductivity Silica/Carbon Black Hybrid-Filled Natural Rubber Composites. Polymers 2025, 17, 1023. [Google Scholar] [CrossRef] [PubMed]
- Panja, N.; Maji, S.; Choudhuri, S.; Ali, K.A.; Hossain, C.M. 3D Bioprinting of Human Hollow Organs. AAPS PharmSciTech 2022, 23, 139. [Google Scholar] [CrossRef]
- Salg, G.A.; Blaeser, A.; Gerhardus, J.S.; Hackert, T.; Kenngott, H.G. Vascularization in Bioartificial Parenchymal Tissue: Bioink and Bioprinting Strategies. Int. J. Mol. Sci. 2022, 23, 8589. [Google Scholar] [CrossRef]
- Ricci, G.; Gibelli, F.; Sirignano, A. Three-Dimensional Bioprinting of Human Organs and Tissues: Bioethical and Medico-Legal Implications Examined through a Scoping Review. Bioengineering 2023, 10, 1052. [Google Scholar] [CrossRef]
- Jafarkhani, M.; Salehi, Z.; Aidun, A.; Shokrgozar, M.A. Bioprinting in Vascularization Strategies. Iran. Biomed. J. 2019, 23, 9–20. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Chen, T.; Liu, Y.; Wang, Q.; Wang, Z.; Jia, J. Bioprinted vascular tissue: Assessing functions from cellular, tissue to organ levels. Mater. Today Bio 2023, 23, 100846. [Google Scholar] [CrossRef]
- Liang, K. Tissue Bioprinting: Promise and Challenges. Bioengineering 2023, 10, 1400. [Google Scholar] [CrossRef]
- United Network for Organ Sharing. U.S. Surpassed 48,000 Organ Transplants in 2024; Organ Procurement and Transplantation Network: Richmond, VA, USA, 2025.
- Kandaswamy, R.; Stock, P.G.; Miller, J.M.; Handarova, D.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2023 Annual Data Report: Pancreas. Am. J. Transplant. 2025, 25, S138–S192. [Google Scholar] [CrossRef]
- Zhao, X.; Li, N.; Zhang, Z.; Hong, J.; Zhang, X.; Hao, Y.; Wang, J.; Xie, Q.; Zhang, Y.; Li, H.; et al. Beyond hype: Unveiling the Real challenges in clinical translation of 3D printed bone scaffolds and the fresh prospects of bioprinted organoids. J. Nanobiotechnol. 2024, 22, 500. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ren, W.; Xie, L.; Ren, Q.; Zhu, Z.; Jia, Q.; Jiang, W.; Jin, Z.; Yu, Y. Recent advances in 3D bioprinting of tissues and organs for transplantation and drug screening. Virtual Phys. Prototyp. 2024, 19, e2384662. [Google Scholar] [CrossRef]
- Bliley, J.; Tashman, J.; Stang, M.; Coffin, B.; Shiwarski, D.; Lee, A.; Hinton, T.; Feinberg, A. FRESH 3D bioprinting a contractile heart tube using human stem cell-derived cardiomyocytes. Biofabrication 2022, 14, 024106. [Google Scholar] [CrossRef] [PubMed]
- Cross-Najafi, A.A.; Farag, K.; Chen, A.M.; Smith, L.J.; Zhang, W.; Li, P.; Ekser, B. The Long Road to Develop Custom-built Livers: Current Status of 3D Liver Bioprinting. Transplantation 2024, 108, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Loukelis, K.; Koutsomarkos, N.; Mikos, A.G.; Chatzinikolaidou, M. Advances in 3D bioprinting for regenerative medicine applications. Regen. Biomater. 2024, 11, rbae033. [Google Scholar] [CrossRef]
- Ghosh, A.; Sanyal, A.; Mallick, A. Recent Advances in the Development of Bioartificial Pancreas Using 3D Bioprinting for the Treatment of Type 1 Diabetes: A Review. Explor. Med. 2023, 4, 886–922. [Google Scholar] [CrossRef]
- Abraham, N.; Kolipaka, T.; Pandey, G.; Negi, M.; Srinivasarao, D.A.; Srivastava, S. Revolutionizing pancreatic islet organoid transplants: Improving engraftment and exploring future frontiers. Life Sci. 2024, 343, 122545. [Google Scholar] [CrossRef]
- Wu, C.A.; Zhu, Y.; Woo, Y.J. Advances in 3D Bioprinting: Techniques, Applications, and Future Directions for Cardiac Tissue Engineering. Bioengineering 2023, 10, 842. [Google Scholar] [CrossRef]
- Guida, L.; Cavallaro, M.; Levi, M. Advancements in high-resolution 3D bioprinting: Exploring technological trends, bioinks and achieved resolutions. Bioprinting 2024, 44, e00376. [Google Scholar] [CrossRef]
- Askari, M.; Afzali Naniz, M.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2020, 9, 535–573. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Mao, S.; Chen, X.; Na, J.; Sun, W. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication 2015, 7, 044101. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xiong, Z.; Hu, Y.; Wang, S.; Zhang, R.; Zhang, C. Layered manufacturing of tissue engineering scaffolds via multi-nozzle deposition. Mater. Lett. 2003, 57, 2623–2628. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lin, F.; Liu, H.; Yan, Y.; Wang, X.; Zhang, R.; Xiong, Z. Rheological Properties of Cell-Hydrogel Composites Extruding Through Small-Diameter Tips. J. Manuf. Sci. Eng. 2008, 130, 021014. [Google Scholar] [CrossRef]
- Matsusaki, M.; Sakaue, K.; Kadowaki, K.; Akashi, M. Three-dimensional human tissue chips fabricated by rapid and automatic inkjet cell printing. Adv. Healthc Mater. 2013, 2, 534–539. [Google Scholar] [CrossRef]
- Xu, T.; Baicu, C.; Aho, M.; Zile, M.; Boland, T. Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabrication 2009, 1, 035001. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A. 3D bioprinting applications for the printing of skin: A brief study. Sens. Int. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Serra, P.; Fernández-Pradas, J.; Colina, M.; Duocastella, M.; Domínguez, J.; Morenza, J. Laser-induced forward Transfer: A Direct-writing Technique for Biosensors Preparation. J. Laser Micro Nanoeng. 2006, 1, 236–242. [Google Scholar] [CrossRef]
- Burke, M.; Carter, B.M.; Perriman, A.W. Bioprinting: Uncovering the Utility Layer-By-Layer. J. 3D Print Med. 2017, 1, 165–179. [Google Scholar] [CrossRef]
- Wu, P.K.; Ringeisen, B.R. Development of human umbilical vein endothelial cell (HUVEC) and human umbilical vein smooth muscle cell (HUVSMC) branch/stem structures on hydrogel layers via biological laser printing (BioLP). Biofabrication 2010, 2, 014111. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, S.J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 582–590. [Google Scholar] [CrossRef]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Xiao, X.; Huang, Z.; Duan, H.; Yang, L.; Yang, Y.; Li, C.; Feng, L. Photocrosslinkable Biomaterials for 3D Bioprinting: Mechanisms, Recent Advances, and Future Prospects. Int. J. Mol. Sci. 2024, 25, 12567. [Google Scholar] [CrossRef]
- Jing, S.; Lian, L.; Hou, Y.; Li, Z.; Zheng, Z.; Li, G.; Tang, G.; Xie, G.; Xie, M. Advances in volumetric bioprinting. Biofabrication 2023, 16, 012004. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Wu, J.; Chen, Y.; Zhu, Y.; Li, G.; Xie, G.; Tang, G.; Xie, M. A review of 3D bioprinting for organoids. Med. Rev. 2025, 5, 318–338. [Google Scholar] [CrossRef]
- Ribezzi, D.; Zegwaart, J.P.; Van Gansbeke, T.; Tejo-Otero, A.; Florczak, S.; Aerts, J.; Delrot, P.; Hierholzer, A.; Fussenegger, M.; Malda, J.; et al. Multi-material Volumetric Bioprinting and Plug-and-play Suspension Bath Biofabrication via Bioresin Molecular Weight Tuning and via Multiwavelength Alignment Optics. Adv. Mater. 2025, 37, e2409355. [Google Scholar] [CrossRef]
- Duquesne, J.; Parmentier, L.; Vermeersch, E.; Lemaire, F.; Seo, J.W.; Dmitriev, R.I.; Vlierberghe, S.V. Volumetric bioprinting of the osteoid niche. Biofabrication 2025, 17, 025002. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. IJB 2024, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Johari, N.; Adabavazeh, Z.; Baino, F. PVA-based Bioinks for 3D Bioprinting: A Comprehensive Review of Their Applications in Tissue Engineering. Bioprinting 2025, 49, e00419. [Google Scholar] [CrossRef]
- Ali, A.S.M.; Wu, D.; Bannach-Brown, A.; Dhamrait, D.; Berg, J.; Tolksdorf, B.; Lichtenstein, D.; Dressler, C.; Braeuning, A.; Kurreck, J.; et al. 3D bioprinting of liver models: A systematic scoping review of methods, bioinks, and reporting quality. Mater. Today Bio 2024, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Ribezzi, D.; Catala, P.; Pignatelli, C.; Citro, A.; Levato, R. Bioprinting and synthetic biology approaches to engineer functional endocrine pancreatic constructs. Trends Biotechnol. 2025, 43, 2133–2149. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Luo, Z.; Bao, Y. Trends in Photopolymerization 3D Printing for Advanced Drug Delivery Applications. Biomacromolecules 2024, 26, 85–117. [Google Scholar] [CrossRef]
- Kuliavets, V.; Bespalova, O. Consideration of requirements for materials for different bioprinting methods. Technol. Audit. Prod. Reserves 2021, 5, 55–57. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; Van Weeren, P.R.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid and Dextran-Based Semi-IPN Hydrogels as Biomaterials for Bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef]
- Seok, J.M.; Oh, S.H.; Lee, S.J.; Lee, J.H.; Kim, W.D.; Park, S.-H.; Nam, S.Y.; Shin, H.; Park, S.A. Fabrication and characterization of 3D scaffolds made from blends of sodium alginate and poly(vinyl alcohol). Mater. Today Commun. 2019, 19, 56–61. [Google Scholar] [CrossRef]
- Koch, F.; Thaden, O.; Conrad, S.; Tröndle, K.; Finkenzeller, G.; Zengerle, R.; Kartmann, S.; Zimmermann, S.; Koltay, P. Mechanical properties of polycaprolactone (PCL) scaffolds for hybrid 3D-bioprinting with alginate-gelatin hydrogel. J. Mech. Behav. Biomed. Mater. 2022, 130, 105219. [Google Scholar] [CrossRef]
- Khoeini, R.; Nosrati, H.; Akbarzadeh, A.; Eftekhari, A.; Kavetskyy, T.; Khalilov, R.; Ahmadian, E.; Nasibova, A.; Datta, P.; Roshangar, L.; et al. Natural and Synthetic Bioinks for 3D Bioprinting. Adv. NanoBiomed Res. 2021, 1, 2000097. [Google Scholar] [CrossRef]
- Zhang, M.; Wan, T.; Fan, P.; Shi, K.; Chen, X.; Yang, H.; Liu, X.; Xu, W.; Zhou, Y. Photopolymerizable chitosan hydrogels with improved strength and 3D printability. Int. J. Biol. Macromol. 2021, 193, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Blazquez, J.; Yin, G.-Z.; Wang, D.-Y.; Llorca, J.; Echeverry-Rendon, M. A strategy to tailor the mechanical and degradation properties of PCL-PEG-PCL based copolymers for biomedical application. Eur. Polym. J. 2023, 198, 112388. [Google Scholar] [CrossRef]
- Yang, F.; Williams, C.G.; Wang, D.-a.; Lee, H.; Manson, P.N.; Elisseeff, J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 26, 5991–5998. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.; Agarwal, P.; Kasturi, M.; Srinivasan, V.; Seetharam, R.N.; Vasanthan, K.S. Innovative bioinks for 3D bioprinting: Exploring technological potential and regulatory challenges. J. Tissue Eng. 2025, 16, 20417314241308022. [Google Scholar] [CrossRef] [PubMed]
- Ngau, S.; Cheah, K.H.; Wong, V.L.; Khiew, P.; Lim, S.s. 3D-printed poly(ethylene) glycol diacrylate (PEGDA)-chitosan-nanohydroxyapatite scaffolds: Structural characterization and cellular response. Int. J. Biol. Macromol. 2025, 296, 139652. [Google Scholar] [CrossRef]
- Semba, J.A.; Mieloch, A.A.; Tomaszewska, E.; Cywoniuk, P.; Rybka, J.D. Formulation and evaluation of a bioink composed of alginate, gelatin, and nanocellulose for meniscal tissue engineering. IJB 2022, 9, 621. [Google Scholar] [CrossRef]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Dańczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2022, 11, 26–38. [Google Scholar] [CrossRef]
- Albrecht, F.B.; Dolderer, V.; Nellinger, S.; Schmidt, F.F.; Kluger, P.J. Gellan Gum Is a Suitable Biomaterial for Manual and Bioprinted Setup of Long-Term Stable, Functional 3D-Adipose Tissue Models. Gels 2022, 8, 420. [Google Scholar] [CrossRef]
- Jose, J.; Peter, A.; Thajudeen, K.Y.; De Lourdes Gomes Pereira, M.; Athira, V.P.; Bhat, S.G.; Michel, H. Recent advances in the design and development of bioink formulations for various biomedical applications. Results Eng. 2024, 22, 102060. [Google Scholar] [CrossRef]
- Kim, M.; Kang, D.; Han, H.; Jang, J. Light-activated decellularized extracellular matrix-based bioinks for enhanced mechanical integrity. Mater. Today Bio 2025, 32, 101859. [Google Scholar] [CrossRef]
- Zhang, B.; Radisic, M. Organ-on-a-chip devices advance to market. Lab Chip 2017, 17, 2395–2420. [Google Scholar] [CrossRef]
- Finkel, S.; Sweet, S.; Locke, T.; Smith, S.; Wang, Z.; Sandini, C.; Imredy, J.; He, Y.; Durante, M.; Lagrutta, A.; et al. FRESH 3D bioprinted cardiac tissue, a bioengineered platform for in vitro pharmacology. APL Bioeng 2023, 7, 046113. [Google Scholar] [CrossRef]
- Mahdavi, R.; Hashemi-Najafabadi, S.; Ghiass, M.A.; Valaskivi, S.; Valimaki, H.; Kreutzer, J.; Hamngren Blomqvist, C.; Romeo, S.; Kallio, P.; Adiels, C.B. Design, fabrication, and characterization of a user-friendly microfluidic device for studying liver zonation-on-chip (ZoC). Biomed. Microdevices 2025, 27, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lian, X.; Wei, K.; Zhang, J.; Yu, K.; Li, H.; Ma, H.; Cai, Y.; Pang, L. Maturation of pluripotent stem cell-derived cardiomyocytes: Limitations and challenges from metabolic aspects. Stem Cell Res. Ther. 2024, 15, 354. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Kiskin, F.N.; Shen, M.; Zhang, J.Z. Recent advances in regulating the proliferation or maturation of human-induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2023, 14, 228. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, Y.; Li, H.; Yang, Y.; Chen, M.; Wang, X.; Luo, M.; Wang, K. Engineering the maturation of stem cell-derived cardiomyocytes. Front. Bioeng. Biotechnol. 2023, 11, 1155052. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, T.; Lopez, M.E.U.; Hirano, M.; Hasan, A.; Shin, S.R. Recent advancements of human iPSC derived cardiomyocytes in drug screening and tissue regeneration. Microphysiol. Syst. 2020, 4, 2. [Google Scholar] [CrossRef]
- Hwang, D.G.; Choi, Y.M.; Jang, J. 3D Bioprinting-Based Vascularized Tissue Models Mimicking Tissue-Specific Architecture and Pathophysiology for in vitro Studies. Front. Bioeng. Biotechnol. 2021, 9, 685507. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Z.; Wang, X. The Prospect of Hepatic Decellularized Extracellular Matrix as a Bioink for Liver 3D Bioprinting. Biomolecules 2024, 14, 1019. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhou, H.; Aazmi, A.; Yu, M.; Xu, X.; Yang, H.; Huang, Y.Y.S.; Ma, L. Constructing biomimetic liver models through biomaterials and vasculature engineering. Regen. Biomater. 2022, 9, rbac079. [Google Scholar] [CrossRef]
- Barrs, R.W.; Jia, J.; Silver, S.E.; Yost, M.; Mei, Y. Biomaterials for Bioprinting Microvasculature. Chem. Rev. 2020, 120, 10887–10949. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Tang, F.; Jiang, H.; Zhou, Z.; Hao, X.; Zhang, J.M. Application of 3D Bioprinting in Liver Diseases. Micromachines 2023, 14, 1648. [Google Scholar] [CrossRef]
- Freedman, B.S. Physiology assays in human kidney organoids. Am. J. Physiol. Renal. Physiol. 2022, 322, F625–F638. [Google Scholar] [CrossRef]
- Singh, N.K.; Han, W.; Nam, S.A.; Kim, J.W.; Kim, J.Y.; Kim, Y.K.; Cho, D.W. Three-dimensional cell-printing of advanced renal tubular tissue analogue. Biomaterials 2020, 232, 119734. [Google Scholar] [CrossRef]
- Wragg, N.; Burke, L.; Wilson, S. A critical review of current progress in 3D kidney biomanufacturing: Advances, challenges, and recommendations. Ren. Replace. Ther. 2019, 5, 18. [Google Scholar] [CrossRef]
- King, S.M.; Higgins, J.W.; Nino, C.R.; Smith, T.R.; Paffenroth, E.H.; Fairbairn, C.E.; Docuyanan, A.; Shah, V.D.; Chen, A.E.; Presnell, S.C.; et al. 3D Proximal Tubule Tissues Recapitulate Key Aspects of Renal Physiology to Enable Nephrotoxicity Testing. Front. Physiol. 2017, 8, 123. [Google Scholar] [CrossRef]
- Lebedenko, C.G.; Banerjee, I.A. Enhancing Kidney Vasculature in Tissue Engineering-Current Trends and Approaches: A Review. Biomimetics 2021, 6, 40. [Google Scholar] [CrossRef]
- Wan, H.; Xiang, J.; Mao, G.; Pan, S.; Li, B.; Lu, Y. Recent Advances in the Application of 3D-Printing Bioinks Based on Decellularized Extracellular Matrix in Tissue Engineering. ACS Omega 2024, 9, 24219–24235. [Google Scholar] [CrossRef]
- Smandri, A.; Al-Masawa, M.E.; Hwei, N.M.; Fauzi, M.B. ECM-derived biomaterials for regulating tissue multicellularity and maturation. iScience 2024, 27, 109141. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Z.; Zheng, L.-L.; Chen, H.-P.; Chen, W.-H.; Hu, Q.-X. Fabrication of hierarchical polycaprolactone/gel scaffolds via combined 3D bioprinting and electrospinning for tissue engineering. Adv. Manuf. 2014, 2, 231–238. [Google Scholar] [CrossRef]
- Hakim Khalili, M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef]
- Gao, Q.; He, Y.; Fu, J.Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef]
- Mallapaty, S. Mini hearts, lungs and livers made in lab now grow their own blood vessels. Nature 2025, 643, 892. [Google Scholar] [CrossRef]
- Lee, V.K.; Lanzi, A.M.; Haygan, N.; Yoo, S.S.; Vincent, P.A.; Dai, G. Generation of Multi-Scale Vascular Network System within 3D Hydrogel using 3D Bio-Printing Technology. Cell. Mol. Bioeng. 2014, 7, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Harley, W.; Li, C.C.; Toombs, J.; O’Connell, C.; Taylor, H.; Heath, D.; Collins, D. Advances in biofabrication techniques towards functional bioprinted heterogeneous engineered tissues: A comprehensive review. Bioprinting 2021, 23, e00147. [Google Scholar] [CrossRef]
- Pi, Q.; Maharjan, S.; Yan, X.; Liu, X.; Singh, B.; van Genderen, A.M.; Robledo-Padilla, F.; Parra-Saldivar, R.; Hu, N.; Jia, W.; et al. Digitally Tunable Microfluidic Bioprinting of Multilayered Cannular Tissues. Adv. Mater. 2018, 30, e1706913. [Google Scholar] [CrossRef] [PubMed]
- Bliley, J.M.; Stang, M.A.; Behre, A.; Feinberg, A.W. Advances in 3D Bioprinted Cardiac Tissue Using Stem Cell-Derived Cardiomyocytes. Stem Cells Transl. Med. 2024, 13, 425–435. [Google Scholar] [CrossRef]
- Esser, T.U.; Anspach, A.; Muenzebrock, K.A.; Kah, D.; Schrufer, S.; Schenk, J.; Heinze, K.G.; Schubert, D.W.; Fabry, B.; Engel, F.B. Direct 3D-Bioprinting of hiPSC-Derived Cardiomyocytes to Generate Functional Cardiac Tissues. Adv. Mater. 2023, 35, e2305911. [Google Scholar] [CrossRef]
- Thompson, A. 3D bioprinting platform offers realistic cardiac tissue model for drug testing. Scilight 2023, 2023, 481107. [Google Scholar] [CrossRef]
- Marino, S.; Alheijailan, R.; Alonaizan, R.; Gabetti, S.; Massai, D.; Pesce, M. Cardiac Tissue Bioprinting: Integrating Structure and Functions Through Biomimetic Design, Bioinks, and Stimulation. Gels 2025, 11, 593. [Google Scholar] [CrossRef]
- Akter, M.Z.; Tufail, F.; Ahmad, A.; Oh, Y.W.; Kim, J.M.; Kim, S.; Hasan, M.M.; Li, L.; Lee, D.-W.; Kim, Y.S.; et al. Harnessing native blueprints for designing bioinks to bioprint functional cardiac tissue. iScience 2025, 28, 111882. [Google Scholar] [CrossRef]
- Vormann, M.K.; Gijzen, L.; Hutter, S.; Boot, L.; Nicolas, A.; van den Heuvel, A.; Vriend, J.; Ng, C.P.; Nieskens, T.T.G.; van Duinen, V.; et al. Nephrotoxicity and Kidney Transport Assessment on 3D Perfused Proximal Tubules. AAPS J. 2018, 20, 90. [Google Scholar] [CrossRef]
- Sakolish, C.; Weber, E.J.; Kelly, E.J.; Himmelfarb, J.; Mouneimne, R.; Grimm, F.A.; House, J.S.; Wade, T.; Han, A.; Chiu, W.A.; et al. Technology Transfer of the Microphysiological Systems: A Case Study of the Human Proximal Tubule Tissue Chip. Sci. Rep. 2018, 8, 14882. [Google Scholar] [CrossRef]
- Fransen, M.F.J.; Addario, G.; Bouten, C.V.C.; Halary, F.; Moroni, L.; Mota, C. Bioprinting of kidney in vitro models: Cells, biomaterials, and manufacturing techniques. Essays Biochem. 2021, 65, 587–602. [Google Scholar] [CrossRef]
- van den Berg, C.W.; Dumas, S.J.; Little, M.H.; Rabelink, T.J. Challenges in maturation and integration of kidney organoids for stem cell–based renal replacement therapy. Kidney Int. 2025, 107, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.P.; Basit, H.; Knohl, S.J. Physiology, Glomerular Filtration Rate. In StatPearls; National Library of Medicine: Treasure Island, FL, USA, 2025. [Google Scholar]
- Skylar-Scott, M.A.; Mueller, J.; Visser, C.W.; Lewis, J.A. Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 2019, 575, 330–335. [Google Scholar] [CrossRef]
- Liu, X.; Carter, S.D.; Renes, M.J.; Kim, J.; Rojas-Canales, D.M.; Penko, D.; Angus, C.; Beirne, S.; Drogemuller, C.J.; Yue, Z.; et al. Development of a Coaxial 3D Printing Platform for Biofabrication of Implantable Islet-Containing Constructs. Adv. Heal. Mater. 2019, 8, e1801181. [Google Scholar] [CrossRef]
- Rector Iv, J.A.; McBride, L.; Weber, C.M.; Grossman, K.; Sorets, A.; Ventura-Antunes, L.; Holtz, I.; Young, K.; Schrag, M.; Lippmann, E.S.; et al. Fabrication of endothelialized capillary-like microchannel networks using sacrificial thermoresponsive microfibers. Biofabrication 2024, 17, 015023. [Google Scholar] [CrossRef]
- Liu, S.; Wang, T.; Li, S.; Wang, X. Application Status of Sacrificial Biomaterials in 3D Bioprinting. Polymers 2022, 14, 2182. [Google Scholar] [CrossRef] [PubMed]
- Toren, E.; Burnette, K.S.; Banerjee, R.R.; Hunter, C.S.; Tse, H.M. Partners in Crime: Beta-Cells and Autoimmune Responses Complicit in Type 1 Diabetes Pathogenesis. Front. Immunol. 2021, 12, 756548. [Google Scholar] [CrossRef] [PubMed]
- James, E.A.; Joglekar, A.V.; Linnemann, A.K.; Russ, H.A.; Kent, S.C. The beta cell-immune cell interface in type 1 diabetes (T1D). Mol. Metab. 2023, 78, 101809. [Google Scholar] [CrossRef]
- Parvaneh, S.; Kemeny, L.; Ghaffarinia, A.; Yarani, R.; Vereb, Z. Three-dimensional bioprinting of functional beta-islet-like constructs. Int. J. Bioprint. 2023, 9, 665. [Google Scholar] [CrossRef]
- Pignatelli, C.; Campo, F.; Neroni, A.; Piemonti, L.; Citro, A. Bioengineering the Vascularized Endocrine Pancreas: A Fine-Tuned Interplay Between Vascularization, Extracellular-Matrix-Based Scaffold Architecture, and Insulin-Producing Cells. Transpl. Int. 2022, 35, 10555. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Bushev, S.; Abubakirov, A.; Sukikh, G. Bioethical and Legal Issues in 3D Bioprinting. Int. J. Bioprint. 2020, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Hourd, P.; Medcalf, N.; Segal, J.; Williams, D.J. A 3D bioprinting exemplar of the consequences of the regulatory requirements on customized processes. Regen. Med. 2015, 10, 863–883. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Ganetsos, T.; Petrescu, F.I.T.; Alysandratou, E. Bioprinting and Intellectual Property: Challenges, Opportunities, and the Road Ahead. Bioengineering 2025, 12, 76. [Google Scholar] [CrossRef]
- Mladenovska, T.; Choong, P.F.; Wallace, G.G.; O’Connell, C.D. The regulatory challenge of 3D bioprinting. Regen. Med. 2023, 18, 659–674. [Google Scholar] [CrossRef]
- Sekar, M.P.; Budharaju, H.; Zennifer, A.; Sethuraman, S.; Vermeulen, N.; Sundaramurthi, D.; Kalaskar, D.M. Current standards and ethical landscape of engineered tissues-3D bioprinting perspective. J. Tissue Eng. 2021, 12, 20417314211027677. [Google Scholar] [CrossRef]
- Mallya, D.; Gadre, M.A.; Varadharajan, S.; Vasanthan, K.S. 3D bioprinting for the construction of drug testing models-development strategies and regulatory concerns. Front. Bioeng. Biotechnol. 2025, 13, 1457872. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1155–1170. [Google Scholar] [CrossRef]
- Ruland, A.; Gilmore, K.J.; Daikuara, L.Y.; Fay, C.D.; Yue, Z.; Wallace, G.G. Quantitative ultrasound imaging of cell-laden hydrogels and printed constructs. Acta Biomater. 2019, 91, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Arbit, H.M.; Herrick, J.L.; Segovis, S.G.; Maran, A.; Yaszemski, M.J. Tissue engineered constructs: Perspectives on clinical translation. Ann. Biomed. Eng. 2015, 43, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Briones, Y.; Pascua, B.; Tiangco, N.; Crisostomo, I.; Casiguran, S.; Remenyi, R. Assessing the landscape of clinical and observational trials involving bioprinting: A scoping review. 3D Print. Med. 2025, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Tang, N.; Huang, J.; Cao, X.; Wu, S. Global hotspots and emerging trends in 3D bioprinting research. Front. Bioeng. Biotechnol. 2023, 11, 1169893. [Google Scholar] [CrossRef] [PubMed]
| Organ | Anatomical Complexity | Physiological Functions | Key Challenges in Bioprinting |
|---|---|---|---|
| Heart | High | Contraction, conduction | Synchronized electromechanical activity, vascularization, immune response, electrical integration [20] |
| Liver | Moderate–High | Metabolism, detoxification | Lobular zonation, sinusoidal vasculature, long-term viability, fibrosis [11,21] |
| Kidney | Very High | Filtration, excretion | Nephron reconstruction, segment-specific function, vascular–epithelial interface [22] |
| Pancreas | Moderate | Endocrine hormone secretion | Vascularization, immune evasion, β-cell maturity and glucose responsiveness [23,24] |
| Modality | Resolution | Bioink Viscosity Range | Cell Viability | Strengths | Limitations |
|---|---|---|---|---|---|
| Extrusion | 100–300 µm | Medium to high | Moderate (~60–80%) | High cell density, bulk construct formation [3,43] | Low resolution, shear-induced damage |
| Inkjet | 50–100 µm | Low | High (~85–95%) | High spatial precision, suitable for patterning [3,7,8,43] | Limited to low-viscosity inks, nozzle clogging |
| Laser-Assisted (LAB) | 20–100 µm | Low to medium | High (>90%) | Nozzle-free, high precision [3,43] | Expensive, difficult to scale up |
| SLA/DLP | <50 µm | Low | Moderate to high | Excellent for complex microarchitectures [3,7,8,43] | Limited to photocrosslinkable inks, light-induced cytotoxicity |
| Organ | Key Challenge | Current Approaches Mentioned | Remaining Gaps | Future Directions Suggested | References |
|---|---|---|---|---|---|
| Heart |
|
|
|
| [73,74,75,76,77,78] |
| Liver |
|
|
|
| [73,78,79,80,81,82] |
| Kidney |
|
|
|
| [78,83,84,85,86,87] |
| Pancreas |
|
|
|
| [23,88,89] |
| Bioink Composition | Type | Applications | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Alginate | Natural | Cartilage, liver, skin | Biocompatible, easy gelation | Poor cell adhesion, low mechanical strength | [48,57] |
| Gelatin Methacryloyl (GelMA) | Natural/Hydrogel | Cardiac, cartilage, vascular | Cell-friendly, photocrosslinkable | Weak structural stability alone | [4,48] |
| Polycaprolactone (PCL) | Synthetic | Bone, vascular scaffolds | Strong, slow degrading, thermoplastic | Bioinert; requires functionalization | [58,61,90] |
| PEGDA | Synthetic | Liver, vascular tissues | Tunable stiffness, photopolymerizable | Poor bioactivity unless functionalized | [4,62,91] |
| Alginate–Nanocellulose–Gelatin | Hybrid | Meniscus, soft tissues | High print fidelity, cell viability | Requires fine rheological tuning | [66] |
| Pancreas-specific dECM | Natural/dECM | Pancreatic islet maturation | Tissue-specific cues, enhanced insulin expression | Batch variability, regulatory risk | [23,52] |
| FRESH-based Gelatin Support Printing | Process-based | Cardiac, vascularized soft tissues | Enables complex geometry, maintains structure | Labor-intensive, challenging scale-up | [20,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Qassab, M.; Merheb, M.; Sayadi, S.; Salloum, P.; Dabbousi, Z.; Bayeh, A.; Harb, F.; Azar, S.; Ghadieh, H.E. Organ-Specific Strategies in Bioprinting: Addressing Translational Challenges in the Heart, Liver, Kidney, and Pancreas. J. Funct. Biomater. 2025, 16, 356. https://doi.org/10.3390/jfb16100356
Al Qassab M, Merheb M, Sayadi S, Salloum P, Dabbousi Z, Bayeh A, Harb F, Azar S, Ghadieh HE. Organ-Specific Strategies in Bioprinting: Addressing Translational Challenges in the Heart, Liver, Kidney, and Pancreas. Journal of Functional Biomaterials. 2025; 16(10):356. https://doi.org/10.3390/jfb16100356
Chicago/Turabian StyleAl Qassab, Mohamad, Moustafa Merheb, Safaa Sayadi, Pia Salloum, Zeina Dabbousi, Anthony Bayeh, Frederic Harb, Sami Azar, and Hilda E. Ghadieh. 2025. "Organ-Specific Strategies in Bioprinting: Addressing Translational Challenges in the Heart, Liver, Kidney, and Pancreas" Journal of Functional Biomaterials 16, no. 10: 356. https://doi.org/10.3390/jfb16100356
APA StyleAl Qassab, M., Merheb, M., Sayadi, S., Salloum, P., Dabbousi, Z., Bayeh, A., Harb, F., Azar, S., & Ghadieh, H. E. (2025). Organ-Specific Strategies in Bioprinting: Addressing Translational Challenges in the Heart, Liver, Kidney, and Pancreas. Journal of Functional Biomaterials, 16(10), 356. https://doi.org/10.3390/jfb16100356






