Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication and Cleaning of Mg and Mg-1.6Li Thin Films for Cell Culture
2.2. Cell Culture
2.3. MTT Assay
2.4. Harvesting Rat Sciatic Nerves
2.5. Preparation of Freeze-Killed Cells and Nerve Extracts
2.6. Treatment of Cells with Injury Stimulants and Thin Films
2.7. Quantification of MCP-1 Release
2.8. Determination of Mg and Li Concentrations
2.9. RNA Extraction and Quantitative RT-PCR
2.10. Statistical Analyses
3. Results
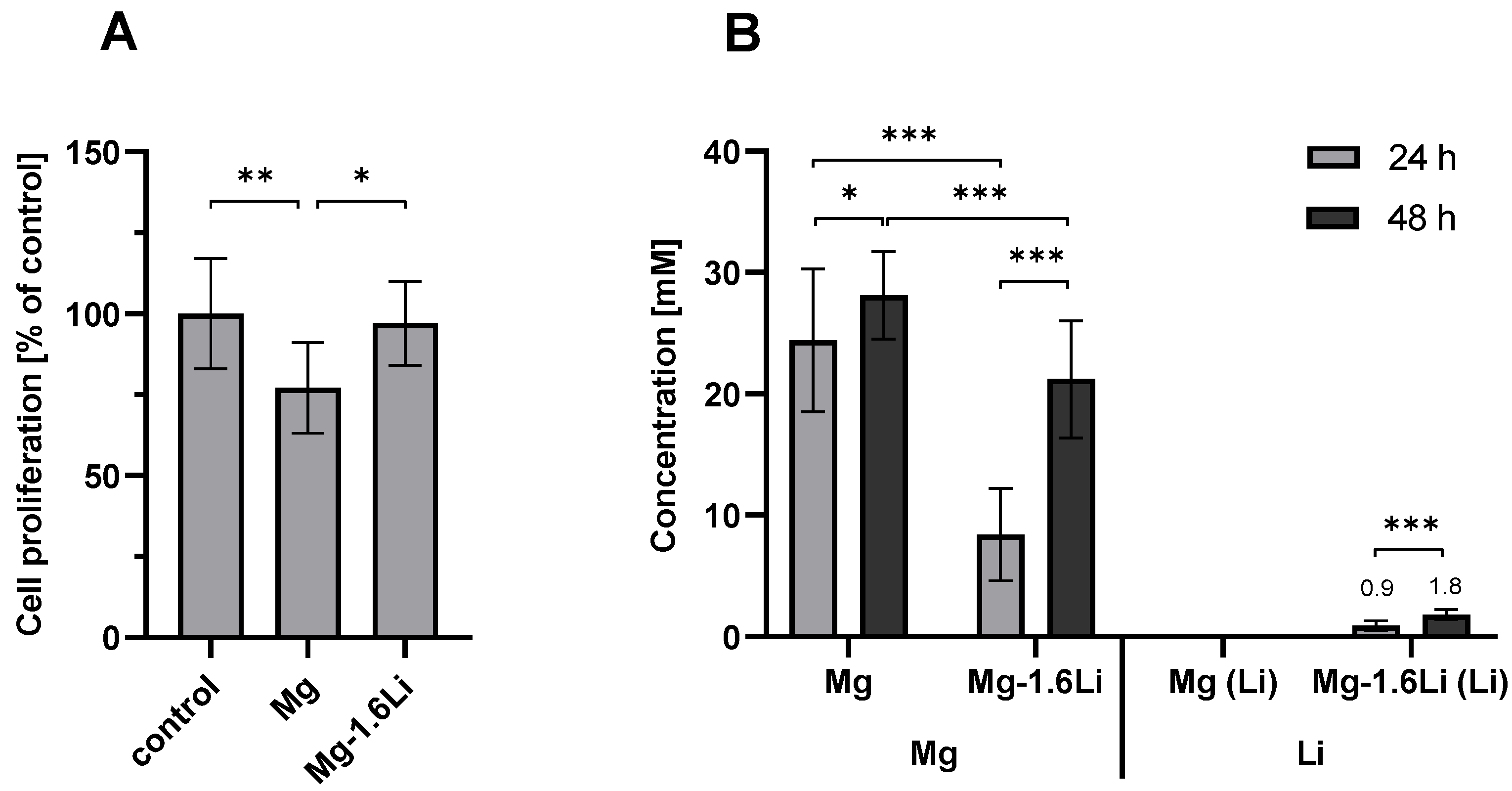
3.1. Mg-Based Extracts Showed Minimal Cytotoxicity with RT4-D6P2T Cells after Short-Term Exposure
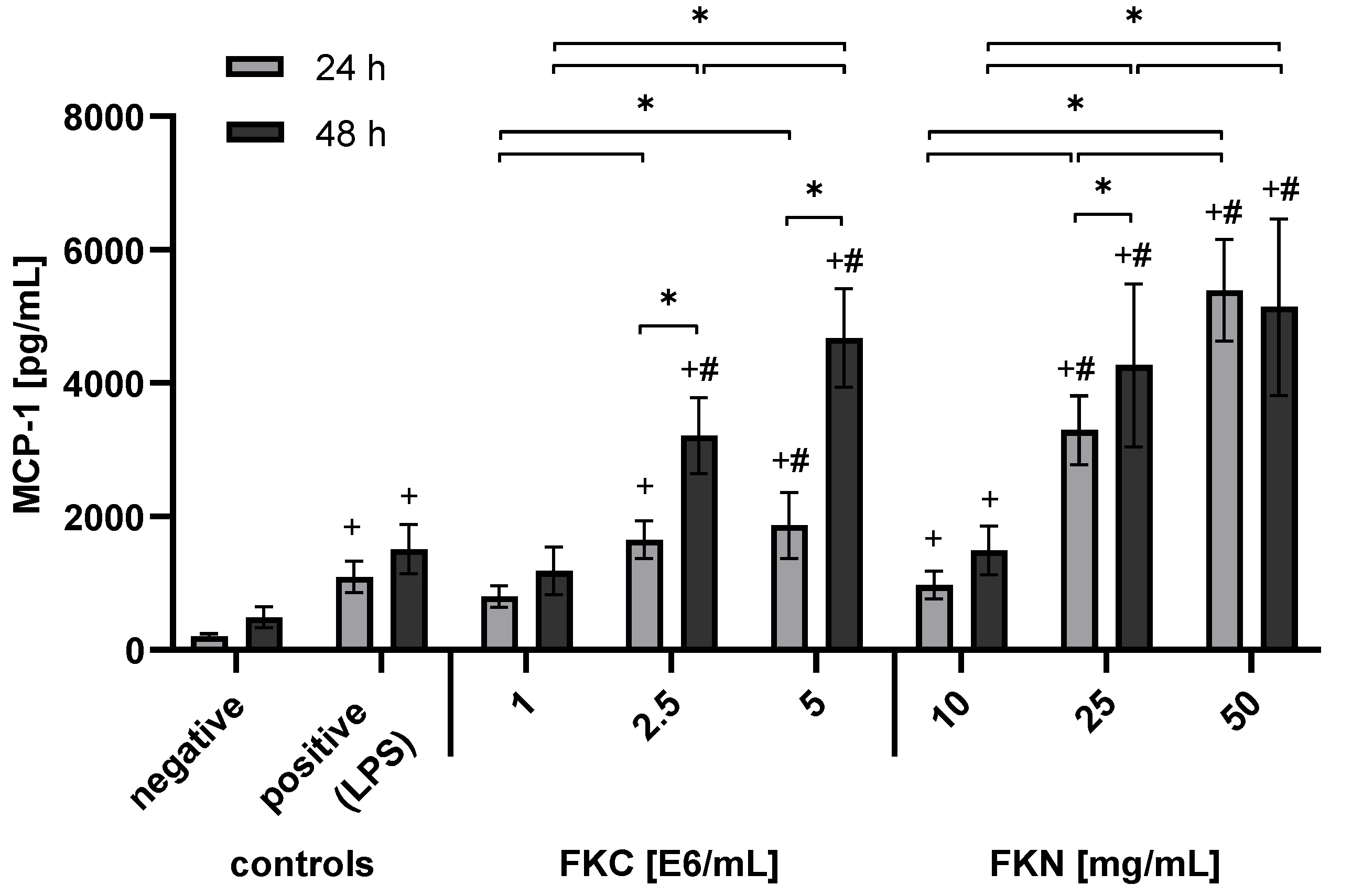
3.2. Extracts from Freeze-Killed Cells and Nerves Triggered MCP-1 Release from RT4-D6P2T Cells
3.3. Influence of Mg-Based Thin Films on RT4-D6P2T Cellular Response to Injury
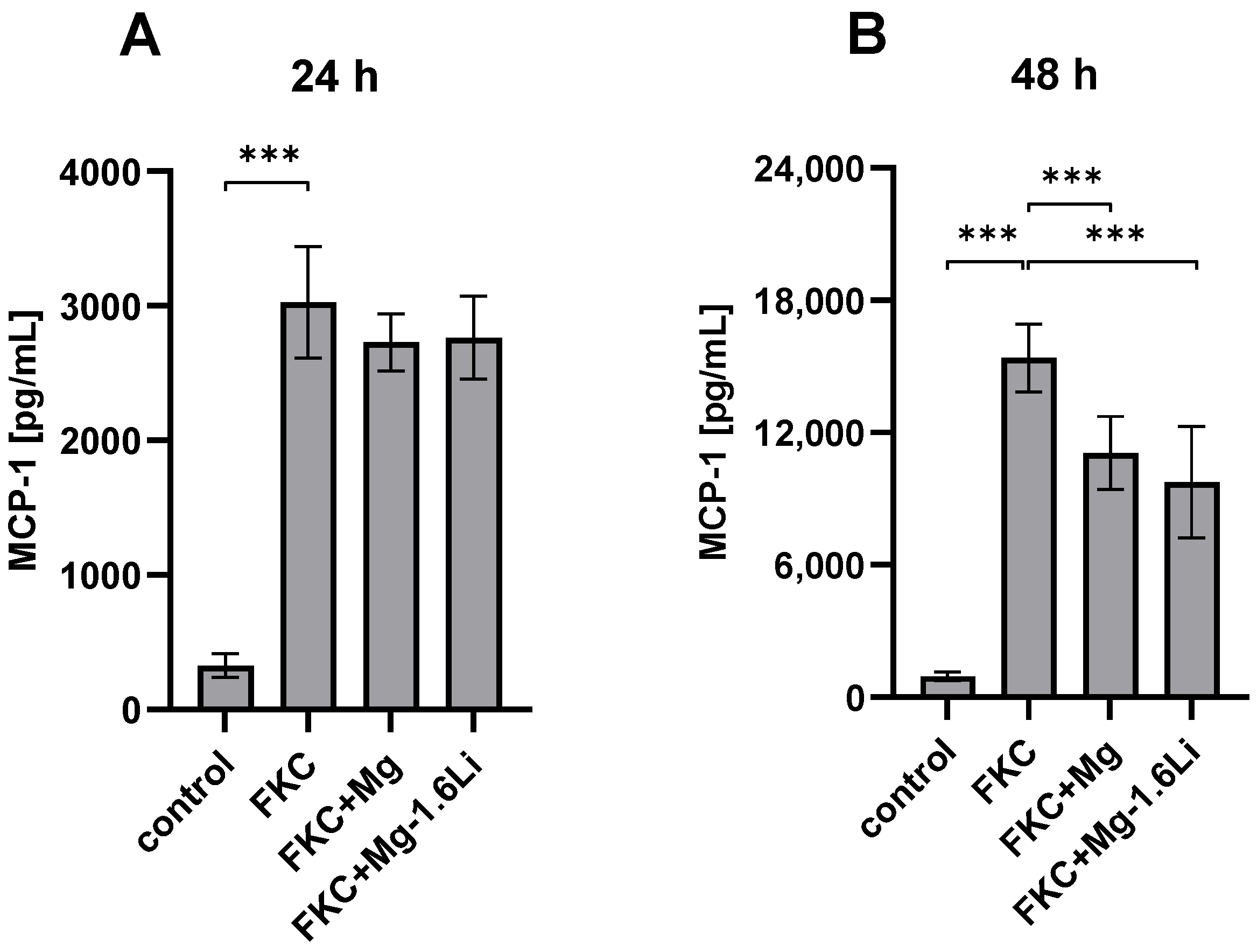
3.3.1. Mg/Mg-1.6Li Thin Films Reduced MCP-1 Release from FKC-Treated RT4-D6P2T Cells
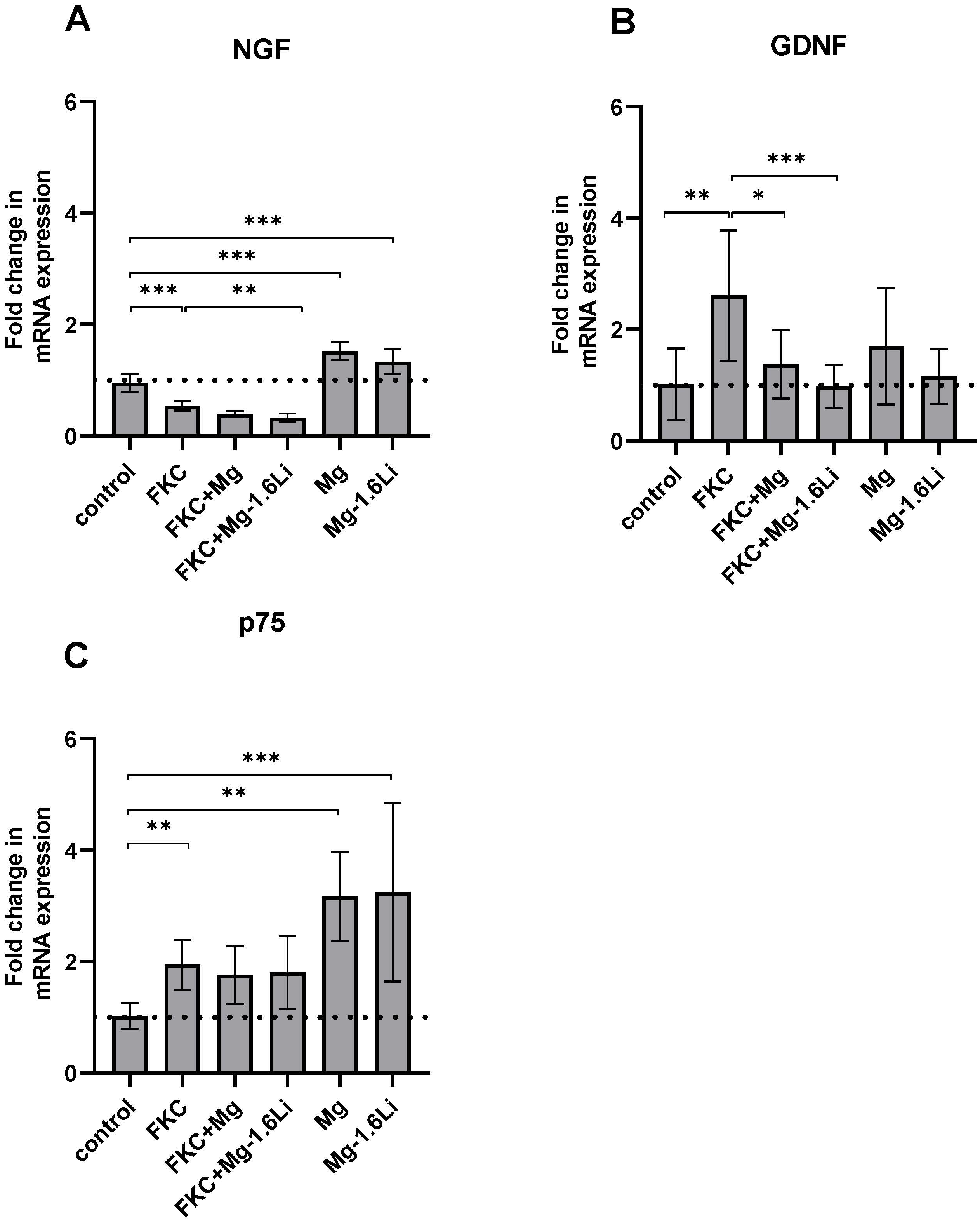
3.3.2. The Gene Expression of Neurotrophins Is Regulated Differently by the Thin Films in the Presence of the Injury Stimulant
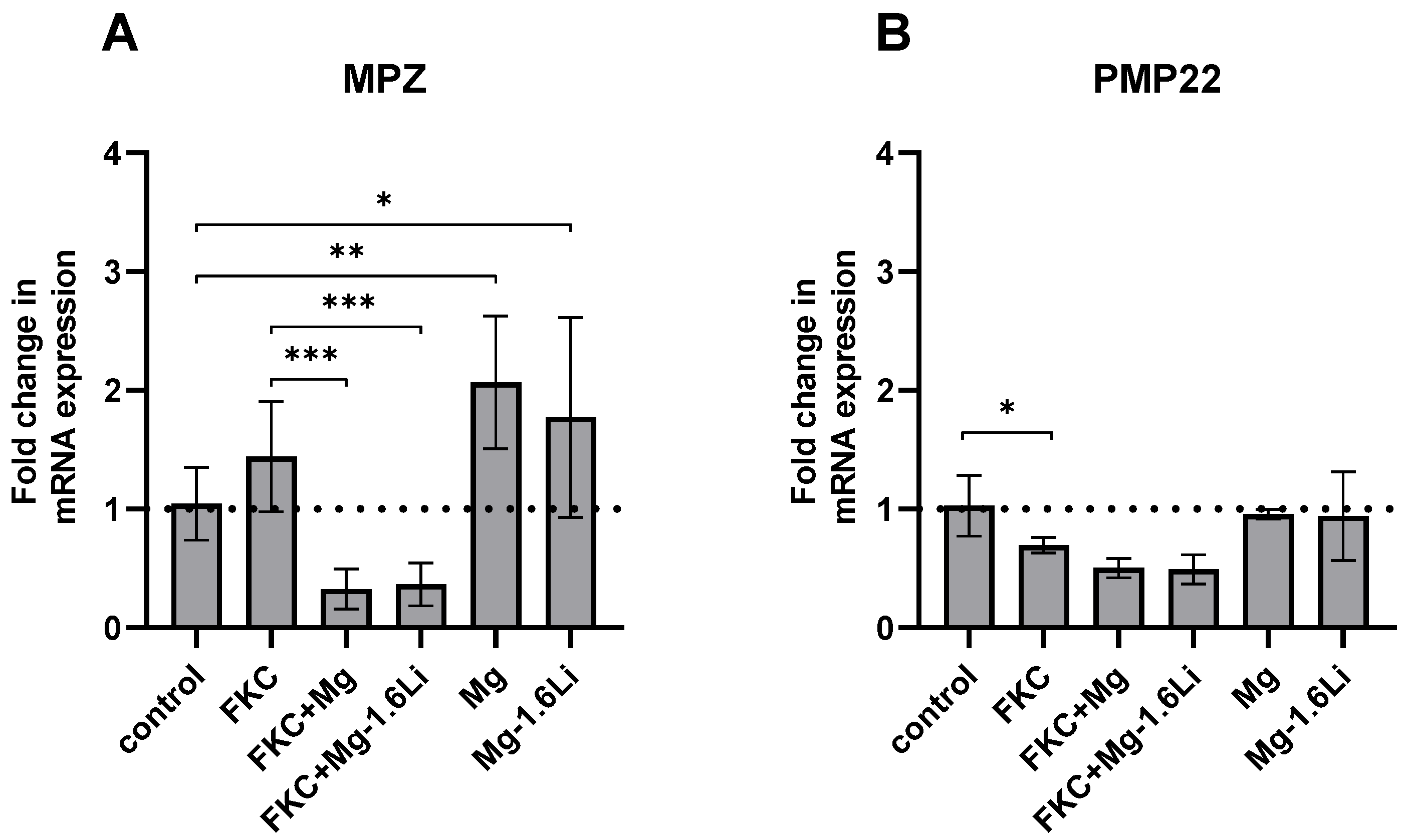
3.3.3. Mg/Mg-1.6Li Thin Films Influenced the Expression of Key Myelin Protein Genes
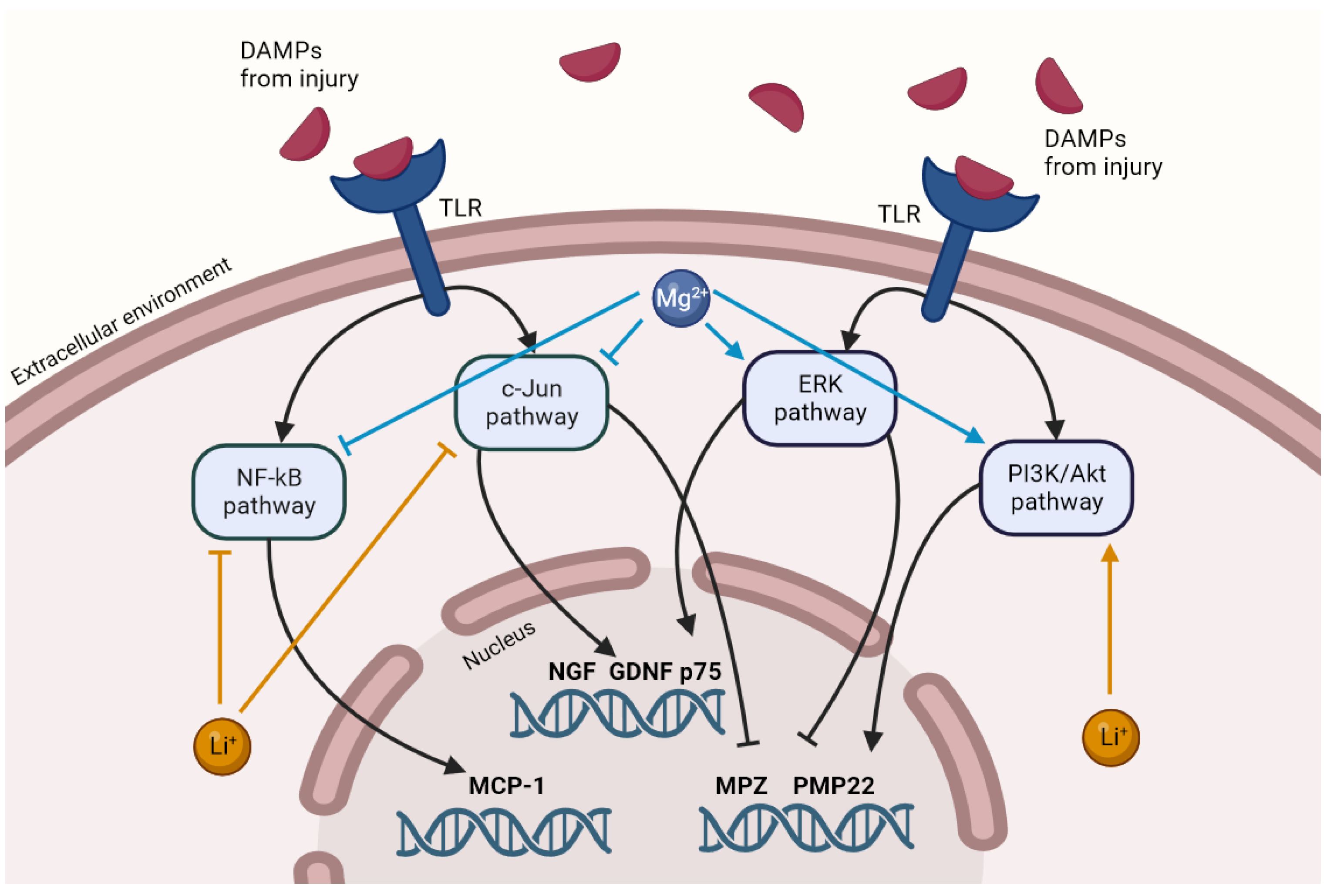
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Lackington, W.A.; Ryan, A.J.; O’Brien, F.J. Advances in Nerve Guidance Conduit-Based Therapeutics for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2017, 3, 1221–1235. [Google Scholar] [CrossRef]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflam. 2011, 8, 109. [Google Scholar] [CrossRef]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular Mechanisms Involved in Schwann Cell Plasticity. Front. Mol. Neurosci. 2017, 10, 38. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Zhang, Q.; Wang, Q.; Wang, X.; Yan, G. Mechanism and application of 3D-printed degradable bioceramic scaffolds for bone repair. Biomater. Sci. 2023, 21, 7304–7050. [Google Scholar] [CrossRef]
- Manescu, P.V.; Antoniac, I.; Antoniac, A.; Laptoiu, D.; Paltanea, G.; Ciocoiu, R.; Nemoianu, I.V.; Gruionu, L.G.; Dura, H. Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives. Biomimetics 2023, 8, 618. [Google Scholar] [CrossRef]
- Li, M.; Jiang, M.; Gao, Y.; Zheng, Y.; Liu, Z.; Zhou, C.; Huang, T.; Gu, X.; Li, A.; Fang, J.; et al. Current status and outlook of biodegradable metals in neuroscience and their potential applications as cerebral vascular stent materials. Bioact. Mater. 2022, 11, 140–153. [Google Scholar] [CrossRef]
- Bhat, K.; Schlotterose, L.; Hanke, L.; Helmholz, H.; Quandt, E.; Hattermann, K.; Willumeit-Römer, R. Magnesium-lithium thin films for neurological applications–An in vitro investigation of glial cytocompatibility and neuroinflammatory response. Acta Biomater. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhang, J.; Lin, W.; Zhang, S. Magnesium Promotes the Regeneration of the Peripheral Nerve. Front. Cell Dev. Biol. 2021, 9, 717854. [Google Scholar] [CrossRef]
- Gordon, T. Brief Electrical Stimulation Promotes Recovery after Surgical Repair of Injured Peripheral Nerves. Int. J. Mol. Sci. 2024, 25, 665. [Google Scholar] [CrossRef]
- Mathew, A.A.; Panonnummal, R.A. Mini Review on the Various Facets Effecting Brain Delivery of Magnesium and Its Role in Neurological Disorders. Biol. Trace Elem. Res. 2023, 201, 4238–4253. [Google Scholar] [CrossRef]
- Pan, H.C.; Sheu, M.L.; Su, H.L.; Chen, Y.J.; Chen, C.J.; Yang, D.Y.; Chiu, W.T.; Cheng, F.C. Magnesium supplement promotes sciatic nerve regeneration and down-regulates inflammatory response. Magnes. Res. 2011, 24, 54–70. [Google Scholar] [CrossRef]
- Li, B.H.; Yang, K.; Wang, X. Biodegradable magnesium wire promotes regeneration of compressed sciatic nerves. Neural Regen. Res. 2016, 11, 2012–2017. [Google Scholar] [CrossRef]
- Yao, Z.; Yuan, W.; Xu, J.; Tong, W.; Mi, J.; Ho, P.C.; Chow, D.H.K.; Li, Y.; Yao, H.; Li, X.; et al. Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration. Adv. Sci. 2022, 9, 2202102. [Google Scholar] [CrossRef]
- Sun, L.; Wang, M.; Chen, S.; Sun, B.; Guo, Y.; He, C.; Mo, X.; Zhu, B.; You, Z. Molecularly engineered metal-based bioactive soft materials—Neuroactive magnesium ion/polymer hybrids. Acta Biomater. 2019, 85, 310–319. [Google Scholar] [CrossRef]
- Vennemeyer, J.J.; Hopkins, T.; Kuhlmann, J.; Heineman, W.R.; Pixley, S.K. Effects of elevated magnesium and substrate on neuronal numbers and neurite outgrowth of neural stem/progenitor cells in vitro. Neurosci. Res. 2014, 84, 72–78. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Zhang, J.; Zhang, M.; Dai, C.; Zhang, Y.; Zhang, L.; Bian, L.; Yang, Y.; Zhang, K.; et al. Advancing neural regeneration via adaptable hydrogels: Enriched with Mg2+ and silk fibroin to facilitate endogenous cell infiltration and macrophage polarization. Bioact. Mater. 2024, 33, 100–113. [Google Scholar] [CrossRef]
- Hopkins, T.M.; Little, K.J.; Vennemeyer, J.J.; Triozzi, J.L.; Turgeon, M.K.; Heilman, A.M.; Minteer, D.; Marra, K.; Hom, D.B.; Pixley, S.K. Short and long gap peripheral nerve repair with magnesium metal filaments. J. Biomed. Mater. Res. A 2017, 105, 3148–3158. [Google Scholar] [CrossRef]
- Vennemeyer, J.J.; Hopkins, T.; Hershcovitch, M.; Little, K.D.; Hagen, M.C.; Minteer, D.; Hom, D.B.; Marra, K.; Pixley, S.K. Initial observations on using magnesium metal in peripheral nerve repair. J. Biomater. Appl. 2014, 29, 1145–1154. [Google Scholar] [CrossRef]
- Tatu, R.; White, L.G.; Yun, Y.; Hopkins, T.; An, X.; Ashraf, A.; Little, K.J.; Hershcovitch, M.; Hom, D.B.; Pixley, S. Effects of Altering Magnesium Metal Surfaces on Degradation In Vitro and In Vivo during Peripheral Nerve Regeneration. Materials 2023, 16, 1195. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhang, Y.; Liu, Z.; He, D.; Xu, W.; Li, S.; Zhang, C.; Zhang, Z. Li–Mg–Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration. Bioact. Mater. 2023, 28, 227–242. [Google Scholar] [CrossRef]
- Monfared, A.; Ghaee, A.; Ebrahimi-Barough, S. Fabrication of tannic acid/poly(N-vinylpyrrolidone) layer-by-layer coating on Mg-based metallic glass for nerve tissue regeneration application. Colloids Surf. B Biointerfaces 2018, 170, 617–626. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Duan, L.; Yao, R.; Yan, Y.; Wang, T.; Wang, J.; Zheng, Z.; Wang, X.; Li, G. Preparation and mechanical optimization of a two-layer silk/magnesium wires braided porous artificial nerve guidance conduit. J. Biomed. Mater. Res. A 2022, 110, 1801–1812. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Manji, H.K.; Zarate, C.A. The role of lithium in the treatment of bipolar disorder: Convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009, 11, 92–109. [Google Scholar] [CrossRef]
- Jakobsson, E.; Argüello-Miranda, O.; Chiu, S.W.; Fazal, Z.; Kruczek, J.; Nunez-Corrales, S.; Pandit, S.; Pritchet, L. Towards a Unified Understanding of Lithium Action in Basic Biology and its Significance for Applied Biology. J. Membr. Biol. 2017, 250, 587–604. [Google Scholar] [CrossRef]
- Wang, F.; Chang, S.; Li, J.; Wang, D.; Li, H.; He, X. Lithium alleviated spinal cord injury (SCI)-induced apoptosis and inflammation in rats via BDNF-AS/miR-9-5p axis. Cell Tissue Res. 2021, 384, 301–312. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Qiao, H.; Liu, D.F.; Li, J.; Li, J.X.; Chang, S.E.; Lu, T.; Li, F.T.; Wang, D.; Li, H.P.; et al. Lithium promotes recovery after spinal cord injury. Neural Regen. Res. 2022, 17, 1324–1333. [Google Scholar] [CrossRef]
- Chen, Y.; Weng, J.; Han, D.; Chen, B.; Ma, M.; Yu, Y.; Li, M.; Liu, Z.; Zhang, P.; Jiang, B. GSK3β inhibition accelerates axon debris clearance and new axon remyelination. Am. J. Transl. Res. 2016, 8, 5410–5420. [Google Scholar]
- Liu, P.; Zhang, Z.; Wang, Q.; Guo, R.; Mei, W. Lithium Chloride Facilitates Autophagy Following Spinal Cord Injury via ERK-dependent Pathway. Neurotox. Res. 2017, 32, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, F.; Zhai, X.; Li, X.H.; He, X.J. Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy. Neural Regen. Res. 2018, 13, 2191–2199. [Google Scholar] [CrossRef]
- Yang, M.; Su, B.; Ma, Z.; Zheng, X.; Liu, Y.; Li, Y.; Ren, J.; Lu, L.; Yang, B.; Yu, X. Renal-friendly Li+-doped carbonized polymer dots activate Schwann cell autophagy for promoting peripheral nerve regeneration. Acta Biomater. 2023, 159, 353–366. [Google Scholar] [CrossRef]
- Ogata, T.; Iijima, S.; Hoshikawa, S.; Miura, T.; Yamamoto, S.; Oda, H.; Nakamura, K.; Tanaka, S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 2004, 24, 6724–6732. [Google Scholar] [CrossRef]
- Makoukji, J.; Belle, M.; Meffre, D.; Stassart, R.; Grenier, J.; Shackleford, G.; Fledrich, R.; Fonte, C.; Branchu, J.; Goulard, M.; et al. Lithium enhances remyelination of peripheral nerves. Proc. Natl. Acad. Sci. USA 2012, 109, 3973–3978. [Google Scholar] [CrossRef]
- Kocman, A.E.; Dag, I.; Sengel, T.; Söztutar, E. The effect of lithium and lithium-loaded hyaluronic acid hydrogel applications on nerve regeneration and recovery of motor functions in peripheral nerve injury. Rend. Lincei. Sci. Fis. Nat. 2020, 31, 889–904. [Google Scholar] [CrossRef]
- Weng, J.; Wang, Y.H.; Li, M.; Zhang, D.Y.; Jiang, B.G. GSK3β inhibitor promotes myelination and mitigates muscle atrophy after peripheral nerve injury. Neural Regen. Res. 2018, 13, 324–330. [Google Scholar] [CrossRef]
- Grandjean, E.M.; Aubry, J.M. Lithium: Updated human knowledge using an evidence-based approach. Part II: Clinical pharmacology and therapeutic monitoring. CNS Drugs 2009, 23, 331–349. [Google Scholar] [CrossRef]
- Hanke, L.; Jessen, L.K.; Weisheit, F.; Bhat, K.; Westernströer, U.; Garbe-Schönberg, D.; Willumeit-Römer, R.; Quandt, E. Structural characterisation and degradation of Mg–Li thin films for biodegradable implants. Sci. Rep. 2023, 13, 12572. [Google Scholar] [CrossRef]
- Lee, H.; Jo, E.K.; Choi, S.Y.; Oh, S.B.; Park, K.; Kim, J.S.; Lee, S.J. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: Implication in Wallerian degeneration. Biochem. Biophys. Res. Commun. 2006, 350, 742–747. [Google Scholar] [CrossRef]
- Karanth, S.; Yang, G.; Yeh, J.; Richardson, P.M. Nature of signals that initiate the immune response during Wallerian degeneration of peripheral nerves. Exp. Neurol. 2006, 202, 161–166. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Endogenous signals initiating inflammation in the injured nervous system. Glia 2009, 57, 351–361. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- International Organization for Standardization. Part 5: Tests for in vitro cytotoxicity. In ISO 10993 Biological Evaluation of Medical Devices, 3rd ed.; International Organization for Standardization (ISO): Geneva, Switzerland, 2009. [Google Scholar]
- Hai, M.; Muja, N.; DeVries, G.H.; Quarles, R.H.; Patel, P.I. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J. Neurosci. Res. 2002, 69, 497–508. [Google Scholar] [CrossRef]
- Geuna, S.; Raimondo, S.; Fregnan, F.; Haastert-Talini, K.; Grothe, C. In vitro models for peripheral nerve regeneration. Eur. J. Neurosci. 2016, 43, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Pereira, C.; Hill, E.E.; Vukcevich, O.; Wang, A. In Vitro, In Vivo and Ex Vivo Models for Peripheral Nerve Injury and Regeneration. Curr. Neuropharmacol. 2022, 20, 344–361. [Google Scholar] [CrossRef]
- Andersen, N.D.; Srinivas, S.; Piñero, G.; Monje, P.V. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci. Rep. 2016, 6, 31781. [Google Scholar] [CrossRef] [PubMed]
- Shojapour, M.; Mosayebi, G.; Hajihossein, R.; Noorbakhsh, F.; Mokarizadeh, A.; Ghahremani, M.H. A Simplified Protocol for the Purification of Schwann Cells and Exosome Isolation from C57BL/6 Mice. Rep. Biochem. Mol. Biol. 2018, 7, 9–15. [Google Scholar]
- Amukarimi, S.; Mozafari, M. Biodegradable magnesium-based biomaterials: An overview of challenges and opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef]
- Thiemicke, A.; Neuert, G. Kinetics of osmotic stress regulate a cell fate switch of cell survival. Sci. Adv. 2021, 7, 1122. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.K.; Li, X.R.; Lu, M.L.; Xu, H. Lithium promotes proliferation and suppresses migration of Schwann cells. Neural Regen. Res. 2020, 15, 1955–1961. [Google Scholar] [CrossRef]
- Piñero, G.; Berg, R.; Andersen, N.D.; Setton-Avruj, P.; Monje, P.V. Lithium Reversibly Inhibits Schwann Cell Proliferation and Differentiation Without Inducing Myelin Loss. Mol. Neurobiol. 2017, 54, 8287–8307. [Google Scholar] [CrossRef]
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent progress on coatings of biomedical magnesium alloy. Smart Mater. Med. 2022, 3, 104–116. [Google Scholar] [CrossRef]
- Albaraghtheh, T.; Willumeit-Römer, R.; Zeller-Plumhoff, B. In silico studies of magnesium-based implants: A review of the current stage and challenges. J. Magnes. Alloy 2022, 11, 2968–2996. [Google Scholar] [CrossRef]
- Mahjoory, M.; Shahgholi, M.; Karimipour, A. Investigation on the size and percentage effects of magnesium nanoparticles on thermophysical properties of reinforced calcium phosphate bone cement by molecular dynamic simulation. Heliyon 2023, 9, e18835. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liang, J.; Cui, M.; Zhang, L.; Ren, S.; Zheng, W.; Dong, X.; Zhang, B. Saturated fatty acids activate the inflammatory signalling pathway in Schwann cells: Implication in sciatic nerve injury. Scand. J. Immunol. 2020, 92, 12896. [Google Scholar] [CrossRef] [PubMed]
- Hammel, G.; Zivkovic, S.; Ayazi, M.; Ren, Y. Consequences and mechanisms of myelin debris uptake and processing by cells in the central nervous system. Cell. Immunol. 2022, 380, 104591. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.B.; Laranjeira, S.G.; Eriksson, T.M.; Jessen, K.R.; Mirsky, R.; Quick, T.J.; Phillips, J.B. Characterising cellular and molecular features of human peripheral nerve degeneration. Acta Neuropathol. Commun. 2020, 8, 51. [Google Scholar] [CrossRef]
- Tomita, K.; Kubo, T.; Matsuda, K.; Fujiwara, T.; Yano, K.; Winograd, J.M.; Tohyama, M.; Hosokawa, K. The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia 2007, 55, 1199–1208. [Google Scholar] [CrossRef]
- Li, R.; Li, D.; Wu, C.; Ye, L.; Wu, Y.; Yuan, Y.; Yang, S.; Xie, L.; Mao, Y.; Jiang, T.; et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 2020, 10, 1649–1677. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Bessa-Gonçalves, M.; Silva, A.M.; Brás, J.P.; Helmholz, H.; Luthringer-Feyerabend, B.J.C.; Willumeit-Römer, R.; Barbosa, M.A.; Santos, S.G. Fibrinogen and magnesium combination biomaterials modulate macrophage phenotype, NF-kB signaling and crosstalk with mesenchymal stem/stromal cells. Acta Biomater. 2020, 114, 471–484. [Google Scholar] [CrossRef]
- Makola, R.T.; Mbazima, V.G.; Mokgotho, M.P.; Gallicchio, V.S.; Matsebatlela, T.M. The Effect of Lithium on Inflammation-Associated Genes in Lipopolysaccharide-Activated Raw 264.7 Macrophages. Int. J. Inflam. 2020, 2020, 8340195. [Google Scholar] [CrossRef] [PubMed]
- Jeub, M.; Siegloch, P.A.; Nitsch, L.; Zimmermann, J.; Mueller, M.M. Reduced inflammatory response and accelerated functional recovery following sciatic nerve crush lesion in CXCR3-deficient mice. NeuroReport 2020, 31, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Talsma, A.D.; Niemi, J.P.; Pachter, J.S.; Zigmond, R.E. The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration. J. Neuroinflam. 2022, 19, 179. [Google Scholar] [CrossRef]
- Niemi, J.P.; DeFrancesco-Lisowitz, A.; Roldán-Hernández, L.; Lindborg, J.A.; Mandell, D.; Zigmond, R.E. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J. Neurosci. 2013, 33, 16236–16248. [Google Scholar] [CrossRef]
- Mutschler, C.; Fazal, S.V.; Schumacher, N.; Loreto, A.; Coleman, M.P.; Arthur-Farraj, P. Schwann cells are axo-protective after injury irrespective of myelination status in mouse Schwann cell–neuron cocultures. J. Cell Sci. 2023, 136, 261557. [Google Scholar] [CrossRef]
- Hongisto, V.; Smeds, N.; Brecht, S.; Herdegen, T.; Courtney, M.J.; Coffey, E.T. Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol. Cell. Biol. 2003, 23, 6027–6036. [Google Scholar] [CrossRef]
- Reddy, C.; Albanito, L.; De Marco, P.; Aiello, D.; Maggiolini, M.; Napoli, A.; Musti, A.M. Multisite phosphorylation of c-Jun at threonine 91/93/95 triggers the onset of c-Jun pro-apoptotic activity in cerebellar granule neurons. Cell Death Dis. 2013, 4, e852. [Google Scholar] [CrossRef]
- Altura, B.M.; Kostellow, A.B.; Zhang, A.; Li, W.; Morrill, G.A.; Gupta, R.K.; Altura, B.T. Expression of the nuclear factor-kappaB and proto-oncogenes c-fos and c-jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: Possible links to hypertension, atherogenesis, and stroke. Am. J. Hypertens. 2003, 16, 701–707. [Google Scholar] [CrossRef]
- Lambuk, L.; Iezhitsa, I.; Agarwal, R.; Agarwal, P.; Peresypkina, A.; Pobeda, A.; Ismail, N.M. Magnesium acetyltaurate prevents retinal damage and visual impairment in rats through suppression of NMDA-induced upregulation of NF-κB, p53 and AP-1 (c-Jun/c-Fos). Neural Regen. Res. 2021, 16, 2330–2344. [Google Scholar] [CrossRef]
- Liao, W.; Jiang, M.; Li, M.; Jin, C.; Xiao, S.; Fan, S.; Fang, W.; Zheng, Y.; Liu, J. Magnesium Elevation Promotes Neuronal Differentiation While Suppressing Glial Differentiation of Primary Cultured Adult Mouse Neural Progenitor Cells through ERK/CREB Activation. Front. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef]
- Kong, Y.; Hu, X.; Zhong, Y.; Xu, K.; Wu, B.; Zheng, J. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res. Ther. 2019, 10, 378. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef]
- Sanchez, A.H.M.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef]
- Jana, A.; Das, M.; Balla, V.K. In vitro and in vivo degradation assessment and preventive measures of biodegradable Mg alloys for biomedical applications. J. Biomed. Mater. Res. A 2022, 110, 462–487. [Google Scholar] [CrossRef]
- Ulum, M.F.; Caesarendra, W.; Alavi, R.; Hermawan, H. In-Vivo Corrosion Characterization and Assessment of Absorbable Metal Implants. Coatings 2019, 9, 282. [Google Scholar] [CrossRef]
| Gene | Primer Sequence |
|---|---|
| GAPDH (reference gene) | Forward GGCAAGTTCAACGGCACAG Reverse CGCCAGTAGACTCCACGAC |
| NGF | Forward AGCTCACCTCAGTGTCTGG Reverse GCTATCTGTGTACGGTTCTGC |
| GDNF | Forward TCGGGCCACTTGGAGTTAAT Reverse CAGCCACGACATCCCATAAC |
| p75 | Forward CAACCAGACCGTGTGTGAACC Reverse GTCTCCTCGTCCTGGTAGTAGC |
| MPZ | Forward CACCACTCAGTTCCTTGTCC Reverse ACTTCCCTGTCCGTGTAAACC |
| PMP22 | Forward TGTACCACATCCGCCTTGG Reverse CTCATCACACACAGACCAGCAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, K.; Hanke, L.; Helmholz, H.; Quandt, E.; Pixley, S.; Willumeit-Römer, R. Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model. J. Funct. Biomater. 2024, 15, 88. https://doi.org/10.3390/jfb15040088
Bhat K, Hanke L, Helmholz H, Quandt E, Pixley S, Willumeit-Römer R. Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model. Journal of Functional Biomaterials. 2024; 15(4):88. https://doi.org/10.3390/jfb15040088
Chicago/Turabian StyleBhat, Krathika, Lisa Hanke, Heike Helmholz, Eckhard Quandt, Sarah Pixley, and Regine Willumeit-Römer. 2024. "Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model" Journal of Functional Biomaterials 15, no. 4: 88. https://doi.org/10.3390/jfb15040088
APA StyleBhat, K., Hanke, L., Helmholz, H., Quandt, E., Pixley, S., & Willumeit-Römer, R. (2024). Influence of Magnesium Degradation on Schwannoma Cell Responses to Nerve Injury Using an In Vitro Injury Model. Journal of Functional Biomaterials, 15(4), 88. https://doi.org/10.3390/jfb15040088







