Reconstructive Approach in Residual Periodontal Pockets with Biofunctionalized Heterografts—A Retrospective Comparison of 12-Month Data from Three Centers
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P. Focus on intrabony defects–conservative therapy. Periodontology 2000, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Participants, E.W.; Consultants, M.; et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Koidou, V.P.; Nieri, M.; Barbato, L.; Pagliaro, U.; Cairo, F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 320–351. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.; Qahash, M.; Thomson, R.C.; Cook, A.D.; Rohrer, M.D.; Wozney, J.M.; Hardwick, W.R. Space-providing expanded polytetrafluoroethylene devices define alveolar augmentation at dental implants induced by recombinant human bone morphogenetic protein 2 in an absorbable collagen sponge carrier. Clin. Implant Dent. Relat. Res. 2003, 5, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.; Lim, W.H.; Razi, S.S.; Sigurdsson, T.J.; Lee, M.B.; Tatakis, D.N.; Hardwick, W.R. Periodontal repair in dogs: A bioabsorbable calcium carbonate coral implant enhances space provision for alveolar bone regeneration in conjunction with guided tissue regeneration. J. Periodontol. 2003, 74, 957–964. [Google Scholar] [CrossRef]
- Aimetti, M.; Fratini, A.; Manavella, V.; Giraudi, M.; Citterio, F.; Ferrarotti, F.; Mariani, G.M.; Cairo, F.; Baima, G.; Romano, F. Pocket resolution in regenerative treatment of intrabony defects with papilla preservation techniques: A systematic review and meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2021, 48, 843–858. [Google Scholar] [CrossRef]
- Yıldırım, S.; Özener, H.Ö.; Doğan, B.; Kuru, B. Effect of topically applied hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J. Periodontol. 2018, 89, 36–45. [Google Scholar] [CrossRef]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Correction to: Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Investig. 2018, 22, 2961–2962. [Google Scholar] [CrossRef]
- Shirakata, Y.; Nakamura, T.; Kawakami, Y.; Imafuji, T.; Shinohara, Y.; Noguchi, K.; Sculean, A. Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a cross-linked hyaluronic acid gel. An experimental study in dogs. J. Clin. Periodontol. 2021, 48, 570–580. [Google Scholar] [CrossRef]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Shinohara, Y.; Iwata, M.; Setoguchi, F.; Noguchi, K.; Sculean, A.; Dent, M. Cross-linked hyaluronic acid-gel with or without a collagen matrix in the treatment of class III furcation defects: A histologic and histomorphometric study in dogs. J. Clin. Periodontol. 2022, 49, 1079–1089. [Google Scholar] [CrossRef]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Božić, D.; Ćatović, I.; Badovinac, A.; Musić, L.; Par, M.; Sculean, A. Treatment of intrabony defects with a combination of hyaluronic acid and deproteinized porcine bone mineral. Materials 2021, 14, 6795. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Müller, H.-D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In vitro effects of hyaluronic acid on human periodontal ligament cells. BMC Oral Health 2017, 17, 44. [Google Scholar] [CrossRef]
- Nobis, B.; Ostermann, T.; Weiler, J.; Dittmar, T.; Friedmann, A. Impact of cross-linked hyaluronic acid on osteogenic differentiation of SAOS-2 cells in an air-lift model. Materials 2022, 15, 6528. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, S.B.; Sculean, A.; Božić, D. Hyaluronic acid enhances cell migration, viability, and mineralized tissue-specific genes in cementoblasts. J. Periodontal Res. 2023. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Clin. Periodontol 2018. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontology 2015, 68, 282–307. [Google Scholar] [CrossRef]
- Pilloni, A.; Bernard, G. The effect of hyaluronan on mouse intramembranous osteogenesis in vitro. Cell Tissue Res. 1998, 294, 323–333. [Google Scholar] [CrossRef]
- Xing, F.; Zhou, C.; Hui, D.; Du, C.; Wu, L.; Wang, L.; Wang, W.; Pu, X.; Gu, L.; Liu, L.; et al. Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions. Nanotechnol. Rev. 2020, 9, 1059–1079. [Google Scholar] [CrossRef]
- Asparuhova, M.B.; Chappuis, V.; Stähli, A.; Buser, D.; Sculean, A. Role of hyaluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts. Clin. Oral Investig. 2020, 24, 3923–3937. [Google Scholar] [CrossRef] [PubMed]
- Frasheri, I.; Tsakiridou, N.D.; Hickel, R.; Folwaczny, M. The molecular weight of hyaluronic acid influences metabolic activity and osteogenic differentiation of periodontal ligament cells. Clin. Oral Investig. 2023, 27, 5905–5911. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cha, J.; Jang, M.; Kim, P. Hyaluronic acid-based extracellular matrix triggers spontaneous M2-like polarity of monocyte/macrophage. Biomater. Sci. 2019, 7, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Mathew, S.A.; Gupta, P.K.; Bhonde, R.; Totey, S. Glycosaminoglycans enhance osteoblast differentiation of bone marrow derived human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2014, 8, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohara, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: A preclinical study in dogs. Quintessence Int. 2021, 52, 308–316. [Google Scholar] [PubMed]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: A randomized clinical trial. Quintessence Int. 2013, 44, 231. [Google Scholar] [PubMed]
- Amin, H.D.; Olsen, I.; Knowles, J.; Dard, M.; Donos, N. Interaction of enamel matrix proteins with human periodontal ligament cells. Clin. Oral Investig. 2016, 20, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, Y.; Xia, Q.; Meng, M.; Ye, Z.; Tang, Z.; Feng, H.; Chen, X.; Chen, H.; Zeng, X. Enamel matrix derivative (EMD) enhances the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs). Bioengineered 2021, 12, 7033–7045. [Google Scholar] [CrossRef]
- Sordi, M.B.; Cabral da Cruz, A.C.; Panahipour, L.; Gruber, R. Enamel matrix derivative decreases pyroptosis-related genes in macrophages. Int. J. Mol. Sci. 2022, 23, 5078. [Google Scholar] [CrossRef]
- Stout, B.M.; Alent, B.J.; Pedalino, P.; Holbrook, R.; Gluhak-Heinrich, J.; Cui, Y.; Harris, M.A.; Gemperli, A.C.; Cochran, D.L.; Deas, D.E. Enamel matrix derivative: Protein components and osteoinductive properties. J. Periodontol. 2014, 85, e9–e17. [Google Scholar] [CrossRef]
- Sanz, M.; Tonetti, M.S.; Zabalegui, I.; Sicilia, A.; Blanco, J.; Rebelo, H.; Rasperini, G.; Merli, M.; Cortellini, P.; Suvan, J.E. Treatment of intrabony defects with enamel matrix proteins or barrier membranes: Results from a multicenter practice-based clinical trial. J. Periodontol. 2004, 75, 726–733. [Google Scholar] [CrossRef]
- Sculean, A.; Kiss, A.; Miliauskaite, A.; Schwarz, F.; Arweiler, N.B.; Hannig, M. Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J. Clin. Periodontol. 2008, 35, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Windisch, P.; Szendröi-Kiss, D.; Horváth, A.; Rosta, P.; Becker, J.; Gera, I.; Schwarz, F. Clinical and histologic evaluation of an enamel matrix derivative combined with a biphasic calcium phosphate for the treatment of human intrabony periodontal defects. J. Periodontol. 2008, 79, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Meyle, J.; Hoffmann, T.; Topoll, H.; Heinz, B.; Al-Machot, E.; Jervøe-Storm, P.M.; Meiß, C.; Eickholz, P.; Jepsen, S. A multi-centre randomized controlled clinical trial on the treatment of intra-bony defects with enamel matrix derivatives/synthetic bone graft or enamel matrix derivatives alone: Results after 12 months. J. Clin. Periodontol. 2011, 38, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, S.; Tarnow, D.P. Efficacy of enamel matrix derivative with freeze-dried bone allograft or demineralized freeze-dried bone allograft in intrabony defects: A randomized trial. J. Periodontol. 2014, 85, 1351–1360. [Google Scholar] [CrossRef]
- Matarasso, M.; Iorio-Siciliano, V.; Blasi, A.; Ramaglia, L.; Salvi, G.E.; Sculean, A. Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects. A systematic review and meta-analysis. Clin. Oral Investig. 2015, 19, 1581–1593. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials–biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Basha, R.Y.; TS, S.K.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef]
- Kurien, T.; Pearson, R.; Scammell, B. Bone graft substitutes currently available in orthopaedic practice: The evidence for their use. Bone Jt. J. 2013, 95, 583–597. [Google Scholar] [CrossRef]
- Friedmann, A.; Fickl, S.; Fischer, K.R.; Dalloul, M.; Goetz, W.; Kauffmann, F. Horizontal augmentation of chronic mandibular defects by the Guided Bone Regeneration approach: A randomized study in dogs. Materials 2021, 15, 238. [Google Scholar] [CrossRef]
- Falk, H.; Laurell, L.; Ravald, N.; Teiwik, A.; Persson, R. Guided tissue regeneration therapy of 203 consecutively treated intrabony defects using a bioabsorbable matrix barrier. Clinical and radiographic findings. J. Periodontol. 1997, 68, 571–581. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Karring, T. Long-term stability of periodontal conditions achieved following guided tissue regeneration with bioresorbable membranes: Case series results after 6–7 years. J. Clin. Periodontol. 2004, 31, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fok, M.R.; Zhang, Y.; Han, B.; Lin, Y. The role of non-steroidal anti-inflammatory drugs as adjuncts to periodontal treatment and in periodontal regeneration. J. Transl. Med. 2023, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Weaks-Dybvig, M.; Sanavi, F.; Zander, H.; Rifkin, B.R. The effect of indomethacin on alveolar bone loss in experimental periodontitis. J. Periodontal Res. 1982, 17, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Jeffcoat, M.; Kaplan, M.; Goldhaber, P.; Johnson, H.; Wechter, W. Flurbiprofen: A potent inhibitor of alveolar bone resorption in beagles. Science 1985, 227, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Kurtiş, B.; Tüter, G.; Serdar, M.; Pınar, S.; Demirel, İ.; Toyman, U. Gingival crevicular fluid prostaglandin E2 and thiobarbituric acid reactive substance levels in smokers and non-smokers with chronic periodontitis following phase I periodontal therapy and adjunctive use of flurbiprofen. J. Periodontol. 2007, 78, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Buti, J.; Barbato, L.; Cairo, F.; Graziani, F.; Jepsen, S. Adjunctive effect of systemic antibiotics in regenerative/reconstructive periodontal surgery—A systematic review with meta-analysis. Antibiotics 2021, 11, 8. [Google Scholar] [CrossRef]
- Nagano, O.; Saya, H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004, 95, 930–935. [Google Scholar] [CrossRef]
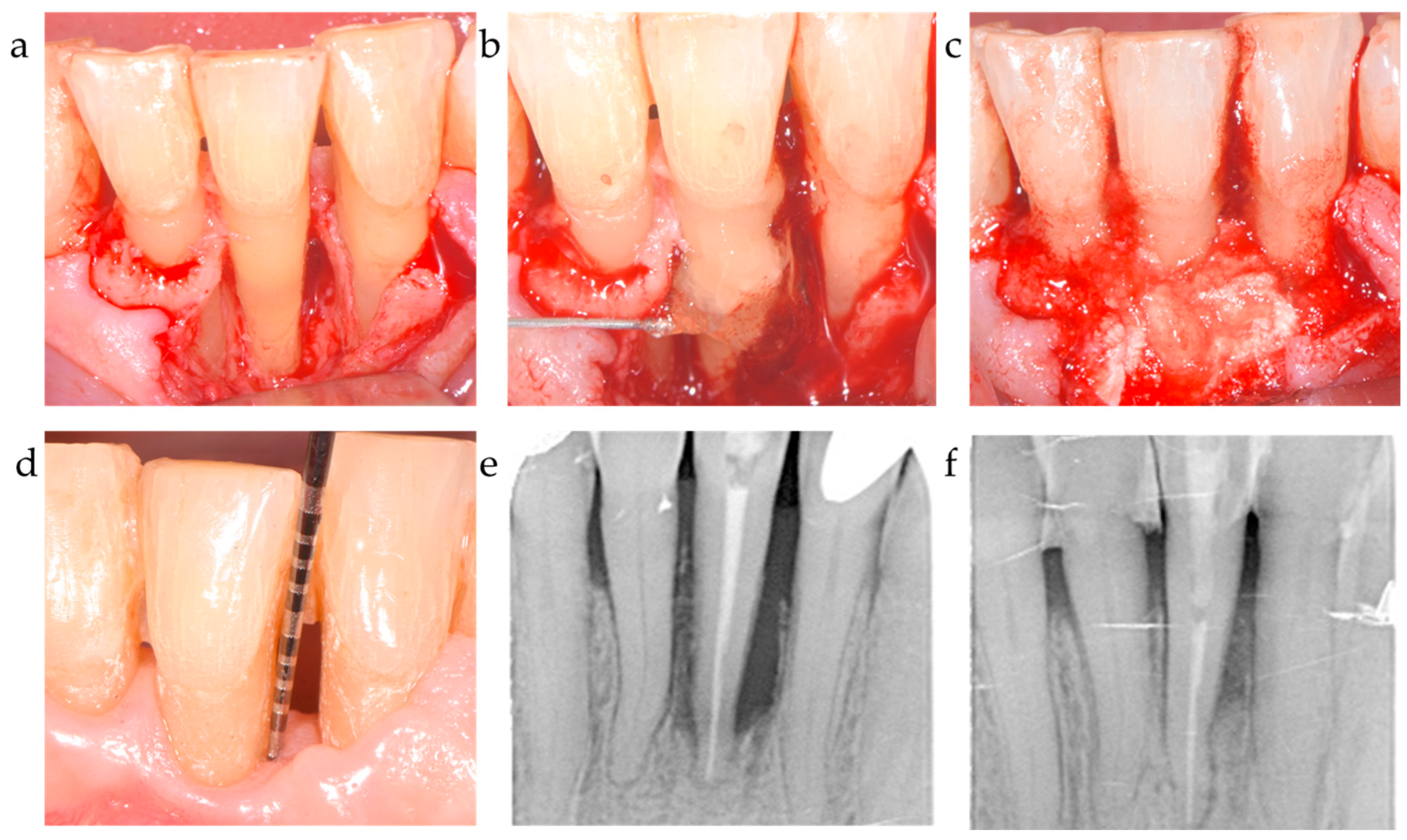

| C1 | xHyA | BDDE-crosslinked hyaluronic acid | HyaDent BG, Regedent, Zürich, Switzerland |
| Collapat II | Bovine collagen + dispersed hydroxyapatite granules | Collapat II, Symatese, France | |
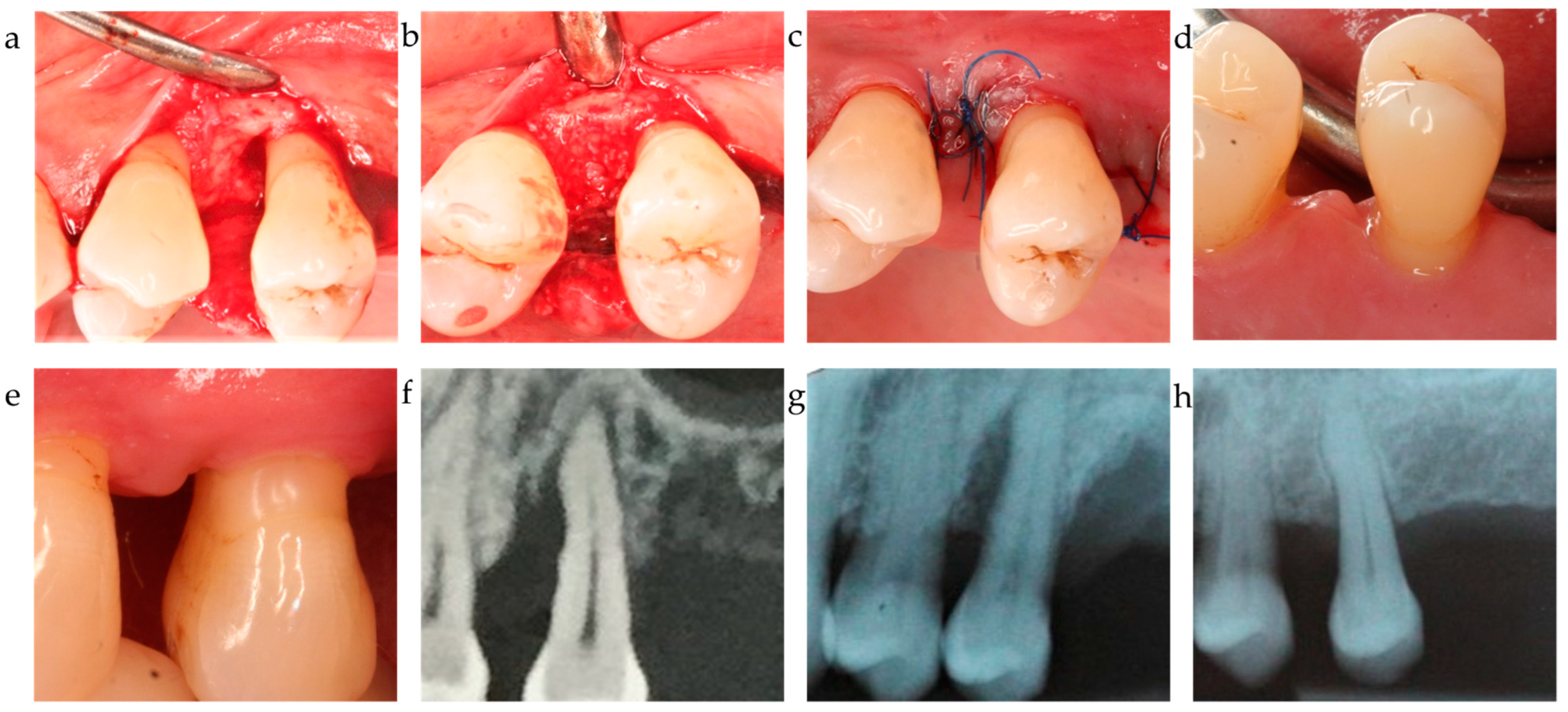
| C2 | EMD | Enamel matrix derivative, Propylenglycolalginate (PGA), water | Emdogain, Straumann Group, USA |
| OraGraft | Cortical/cancellous mineralized particulate 50/50 | LifeNet Health, USA | |
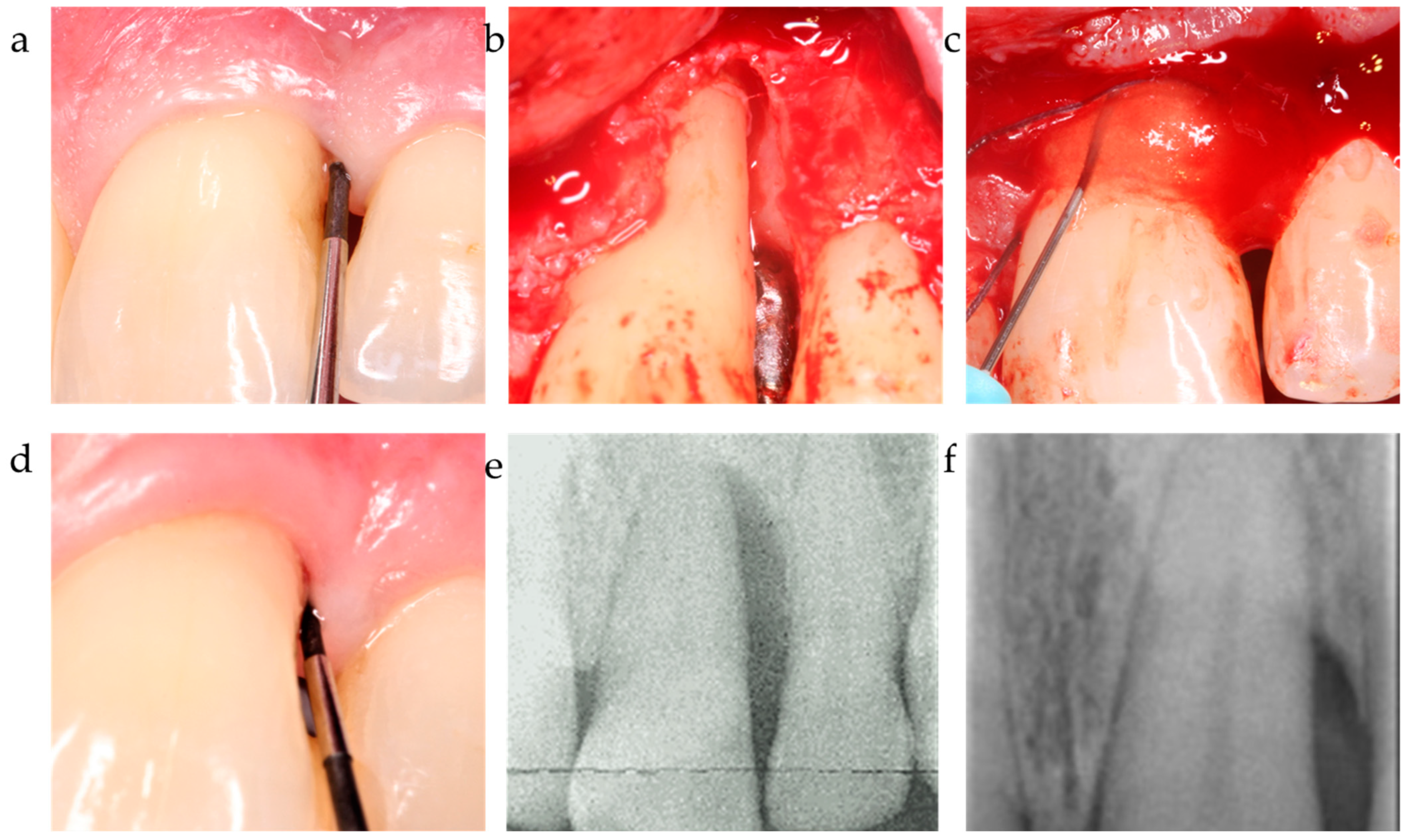
| C3 | xHyA | BDDE-crosslinked hyaluronic acid | HyaDent BG, Regedent, Zürich, Switzerland |
| Guidor matrix barrier | Polylactic polymer | Sunstar, Germany |
| C1 (n = 19) | C2 (n = 21) | C3 (n = 16) | Total (n = 56) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 0.004 | ||||
| Mean ± SD | 58.5 ± 9.2 | 46.6 ± 9.3 | 53.1 ± 13.9 | 52.5 ± 11.7 | |
| Median | 57 | 46 | 54 | 55 | |
| Min | 36 | 32 | 20 | 20 | |
| Max | 75 | 65 | 75 | 75 | |
| Gender | 0.085 | ||||
| Male | 10 (52.6%) | 4 (19.0%) | 6 (18.75%) | 17 (30.4%) | |
| Female | 9 (47.4%) | 17 (81.0%) | 10 (62.5%) | 36 (64.3%) | |
| Smoker | 0.192 | ||||
| Yes | 3 (15.8%) | 4 (19.0%) | 0 (0.0%) | 7 (12.5%) | |
| No | 16 (84.2%) | 17 (81.0%) | 16 (100.0%) | 49 (87.5%) | |
| Localization | 0.480 | ||||
| Mandible | 13 (68.4%) | 11 (52.4%) | 11 (68.8%) | 35 (62.5%) | |
| Maxilla | 6 (9.47%) | 10 (9.02%) | 5 (9.22%) | 21 (37.5%) | |
| Walls (n=) | 0.137 | ||||
| 1 | 4 (21.1%) | 1 (4.8%) | 6 (37.5%) | 11 (19.6%) | |
| 2 | 11 (57.8%) | 17 (81.0%) | 8 (50.0%) | 36 (64.3%) | |
| 3 | 4 (21.1%) | 3 (14.3%) | 2 (12.5%) | 9 (16.1%) | |
| Intraosseous depth (mm) | 0.210 | ||||
| Mean ± SD | 5.96 ± 1.68 | 5.70 ± 3.50 | 7.23 ± 2.44 | 6.22 ± 2.73 | |
| Median | 5.9 | 5.1 | 7.8 | 6.0 | |
| Min | 3.3 | 1.9 | 2.4 | 1.9 | |
| Max | 10.6 | 15.3 | 11.0 | 15.3 | |
| Defect angle (°) | 0.508 | ||||
| Mean ± SD | 28.54 ± 9.80 | 31.32 ± 9.66 | 32.88 ± 14.22 | 30.82 ± 11.12 | |
| Median | 29.6 | 32.4 | 31.35 | 31.7 | |
| Min | 14.0 | 14.6 | 16.5 | 14.0 | |
| Max | 50.5 | 44.2 | 64.5 | 64.5 | |
| Defect width (mm) | 0.375 | ||||
| Mean ± SD | 2.81 ± 099 | 2.55 ± 0.86 | N/A | 2.62 ± 0.92 | |
| Median | 2.7 | 2.4 | 2.55 | ||
| Min | 1.5 | 1.1 | 1.1 | ||
| Max | 4.8 | 4.5 | 4.8 | ||
| Antibiotics | |||||
| Duration (n) | 7 days (19) | -- | 10 days (16) | ||
| Type (mg) | Amoxicillin (2000) | -- | Doxycycline (200) | ||
| Analgesics | |||||
| Duration (n) | If required | If required | If required | ||
| Type (mg) | Prednisone + Paracetamol (80 + 1000) | Ibuprofen (400) | Ibuprofen (600) |
| C1 | C2 | C3 | ||||
|---|---|---|---|---|---|---|
| Baseline | 12-mo | Baseline | 12-mo | Baseline | 12-mo | |
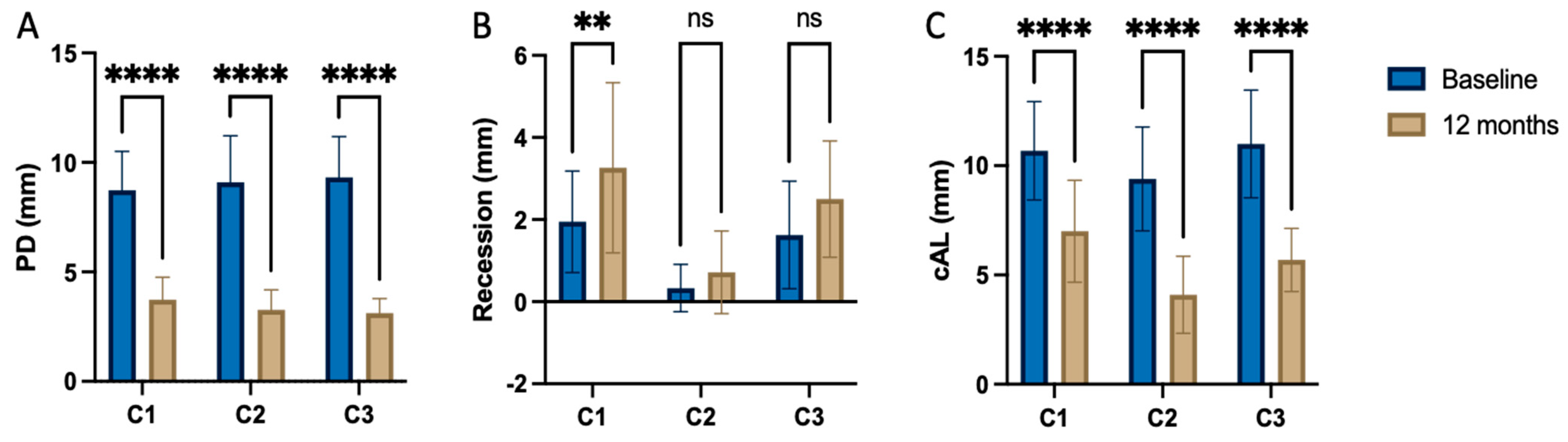
| PPD (mm) | ||||||
| Mean ± SD | 8.74 ± 1.82 | 3.74 ± 1.05 | 9.29 ± 2.13 | 3.38 ± 0.92 | 9.50 ± 1.86 | 3.19 ± 0.66 |
| Median | 8.00 | 4.00 | 9.00 | 3.00 | 9.50 | 3.00 |
| Minimum | 7 | 2 | 7 | 2 | 6 | 2 |
| Maximum | 13 | 6 | 12 | 6 | 12 | 4 |
| p-value | <0.001 | <0.001 | <0.001 | |||
| CAL (mm) | ||||||
| Mean ± SD | 10.68 ± 2.31 | 7.00 ± 2.29 | 9.62 ± 2.38 | 4.10 ± 1.76 | 11.25 ± 2.46 | 5.69 ± 1.45 |
| Median | 10.00 | 7.00 | 9.00 | 4.00 | 11.00 | 6.00 |
| Minimum | 7 | 4 | 7 | 2 | 7 | 3 |
| Maximum | 16 | 15 | 16 | 9 | 15 | 8 |
| p-value | <0.001 | <0.001 | <0.001 | |||
| REC (mm) | ||||||
| Mean ± SD | 1.95 ± 1.27 | 3.26 ± 2.13 | 0.33 ± 0.58 | 0.71 ± 1.10 | 1.62 ± 1.31 | 2.50 ± 1.41 |
| Median | 2.00 | 3.00 | 0.00 | 0.00 | 1.00 | 3.00 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 5 | 10 | 2 | 3 | 4 | 5 |
| p-value | 0.003 | 0.008 | 0.029 | |||
| C1 (n = 19) | C2 (n = 21) | C3 (n = 16) | p-Value | Significant Covariates | ||||
|---|---|---|---|---|---|---|---|---|
| Overall | C1 vs. C2 | C2 vs. C3 | C1 vs. C3 | |||||
| ΔPPD | 4.95 ± 1.71 | 5.81 ± 1.78 | 6.25 ± 1.88 | 0.192 | 0.287 | 1.00 | 0.476 | Intraosseous depth (p < 0.001) |
| ΔCAL | 3.68 ± 1.67 | 5.86 ± 2.37 | 5.53 ± 1.92 | 0.007 | 0.006 | 0.718 | 0.158 | Intraosseous depth (p < 0.001) |
| REC | 1.32 ± 1.67 | 0.04± 0.01 | 1.33 ± 1.11 | 0.015 | 0.031 | 0.038 | 1.00 | - |
| Δdefect fill | 3.33 ± 1.76 | 4.95 ± 2.43 | 5.97 ± 2.49 | 0.002 | 0.003 | 1.00 | 0.014 | Intraosseous depth (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedmann, A.; Liedloff, P.; Eliezer, M.; Brincat, A.; Ostermann, T.; Diehl, D. Reconstructive Approach in Residual Periodontal Pockets with Biofunctionalized Heterografts—A Retrospective Comparison of 12-Month Data from Three Centers. J. Funct. Biomater. 2024, 15, 39. https://doi.org/10.3390/jfb15020039
Friedmann A, Liedloff P, Eliezer M, Brincat A, Ostermann T, Diehl D. Reconstructive Approach in Residual Periodontal Pockets with Biofunctionalized Heterografts—A Retrospective Comparison of 12-Month Data from Three Centers. Journal of Functional Biomaterials. 2024; 15(2):39. https://doi.org/10.3390/jfb15020039
Chicago/Turabian StyleFriedmann, Anton, Pheline Liedloff, Meizi Eliezer, Arthur Brincat, Thomas Ostermann, and Daniel Diehl. 2024. "Reconstructive Approach in Residual Periodontal Pockets with Biofunctionalized Heterografts—A Retrospective Comparison of 12-Month Data from Three Centers" Journal of Functional Biomaterials 15, no. 2: 39. https://doi.org/10.3390/jfb15020039
APA StyleFriedmann, A., Liedloff, P., Eliezer, M., Brincat, A., Ostermann, T., & Diehl, D. (2024). Reconstructive Approach in Residual Periodontal Pockets with Biofunctionalized Heterografts—A Retrospective Comparison of 12-Month Data from Three Centers. Journal of Functional Biomaterials, 15(2), 39. https://doi.org/10.3390/jfb15020039







