Manganese-Implanted Titanium Modulates the Crosstalk between Bone Marrow Mesenchymal Stem Cells and Macrophages to Improve Osteogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mn-Implanted Titanium
2.2. Characterization
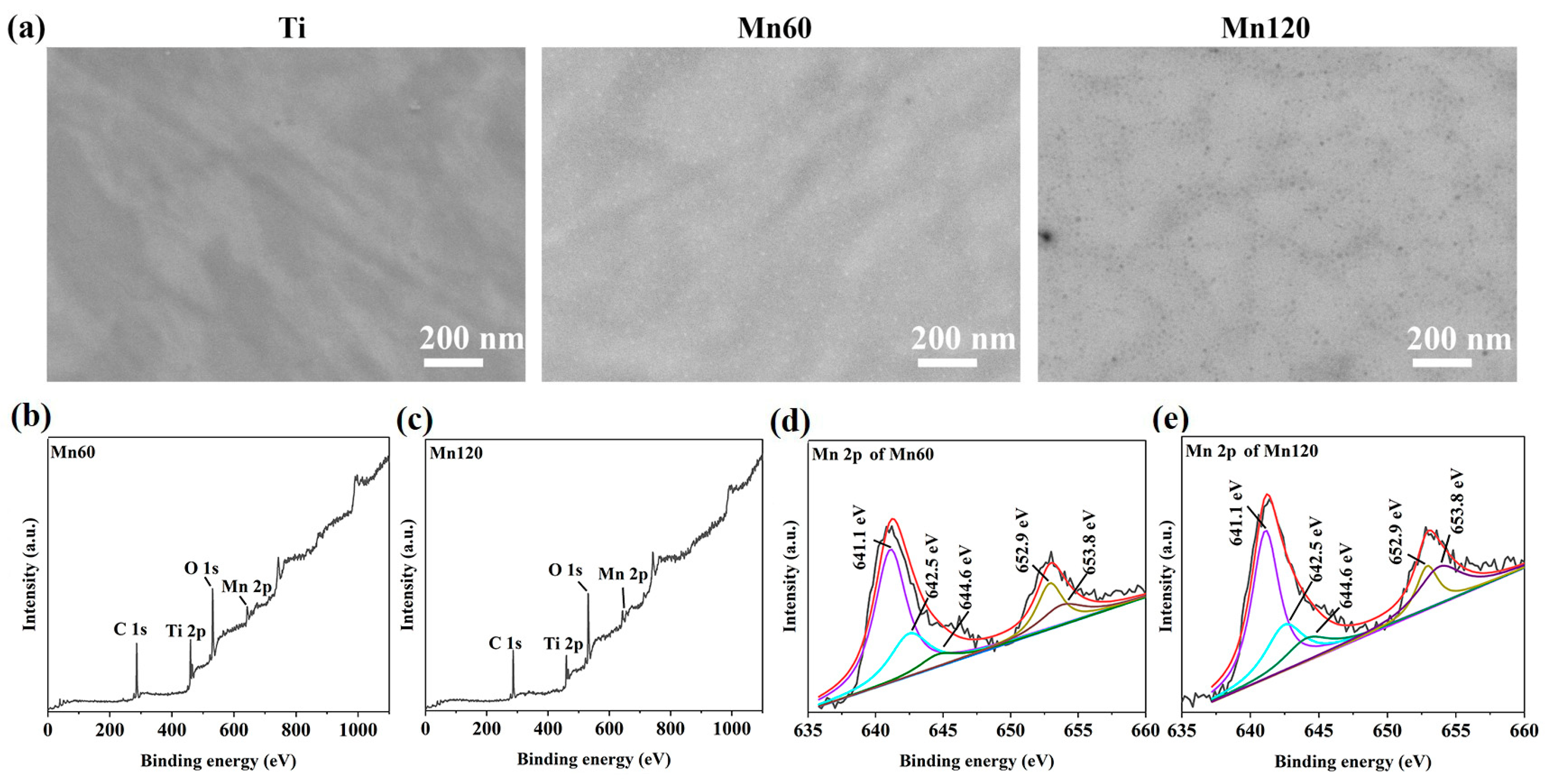
2.2.1. Surface Structure and Physicochemical Characterization
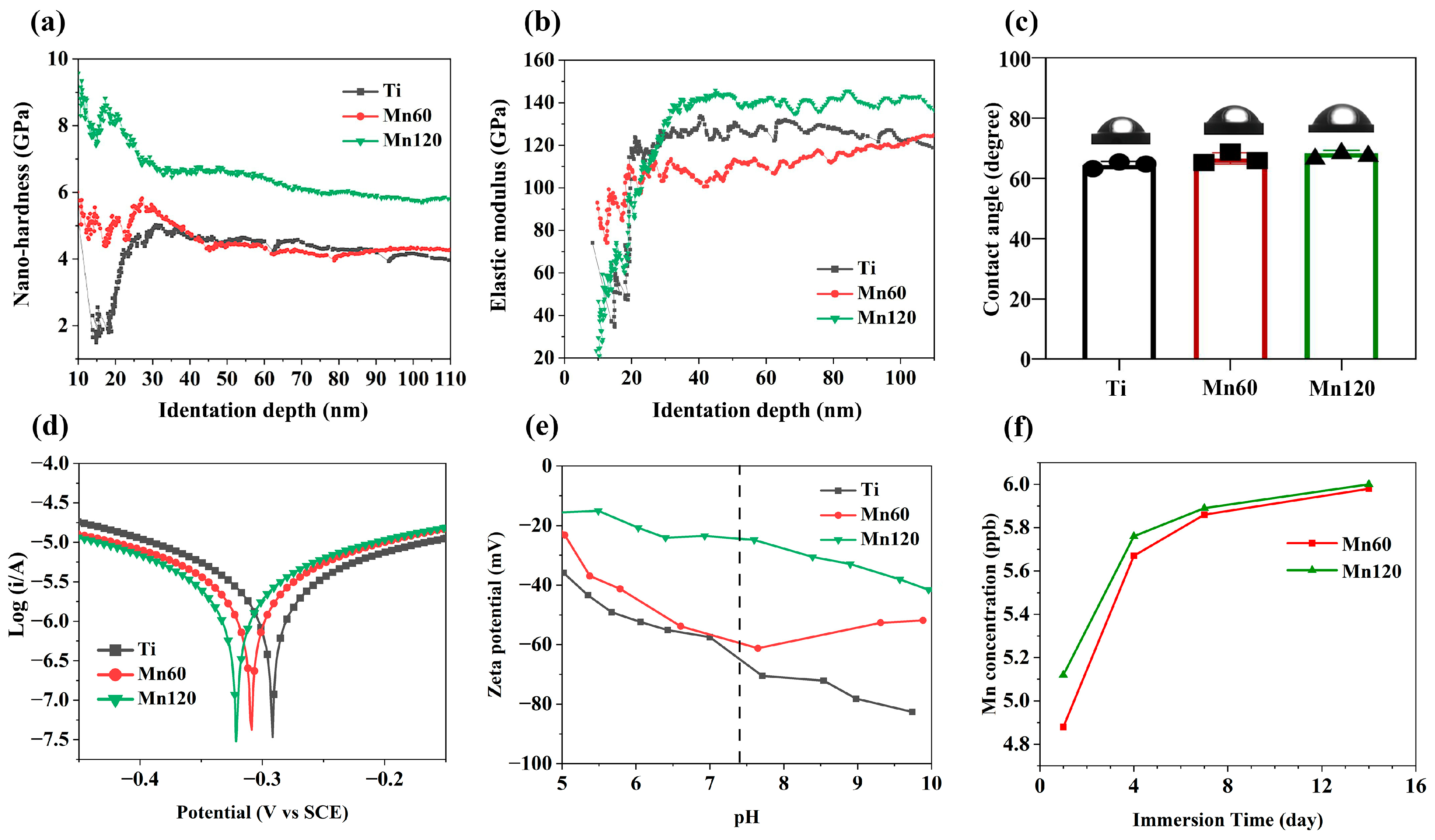
2.2.2. Surface Wettability
2.2.3. Dynamic Potential Polarization Test
2.2.4. Surface Zeta Potential
2.2.5. Concentration of Released Mn ions
2.3. Biological Evaluations
2.3.1. Cell Culture
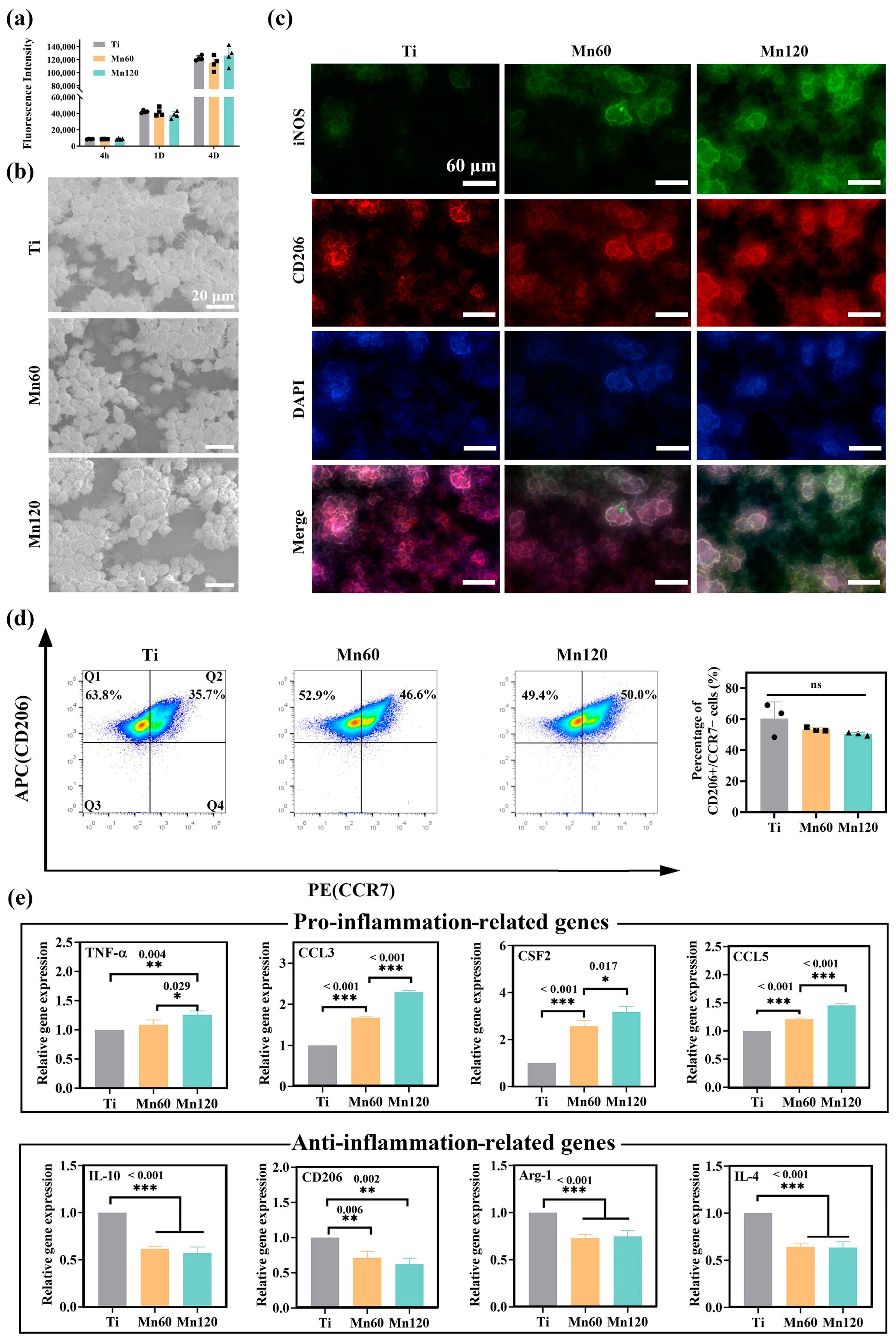
2.3.2. Cell Proliferation and Viability
2.3.3. Cell Morphology
2.3.4. Immunofluorescence Staining of Macrophages
2.3.5. Flow Cytometry Analysis of Macrophages
2.3.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
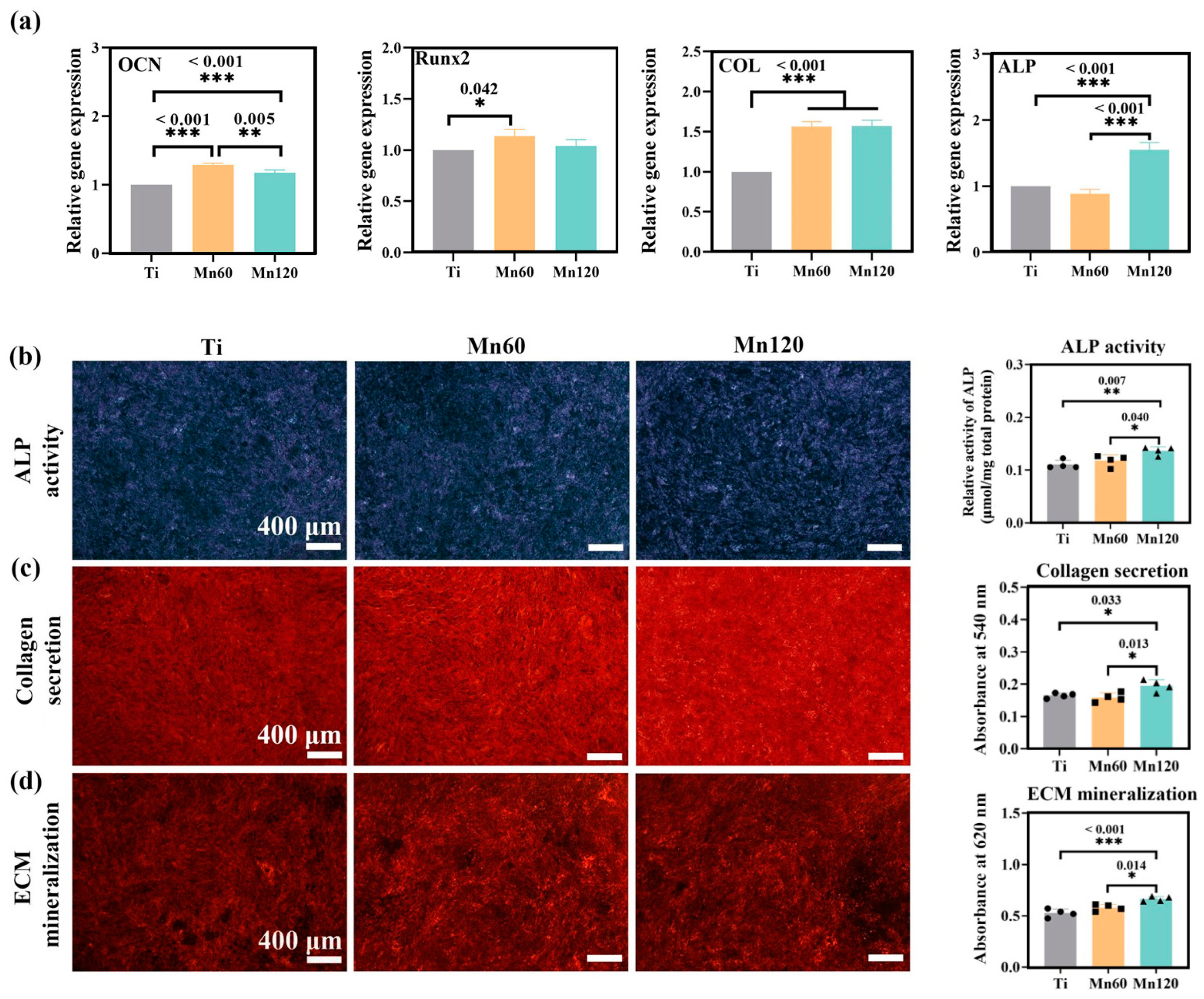
2.3.7. Alkaline Phosphatase (ALP) Activity of mBMSCs
2.3.8. Collagen Secretion of mBMSCs
2.3.9. Extracellular Matrix Mineralization (ECM) of mBMSCs
2.4. Statistical Analysis
3. Results
3.1. Surface Characteristics
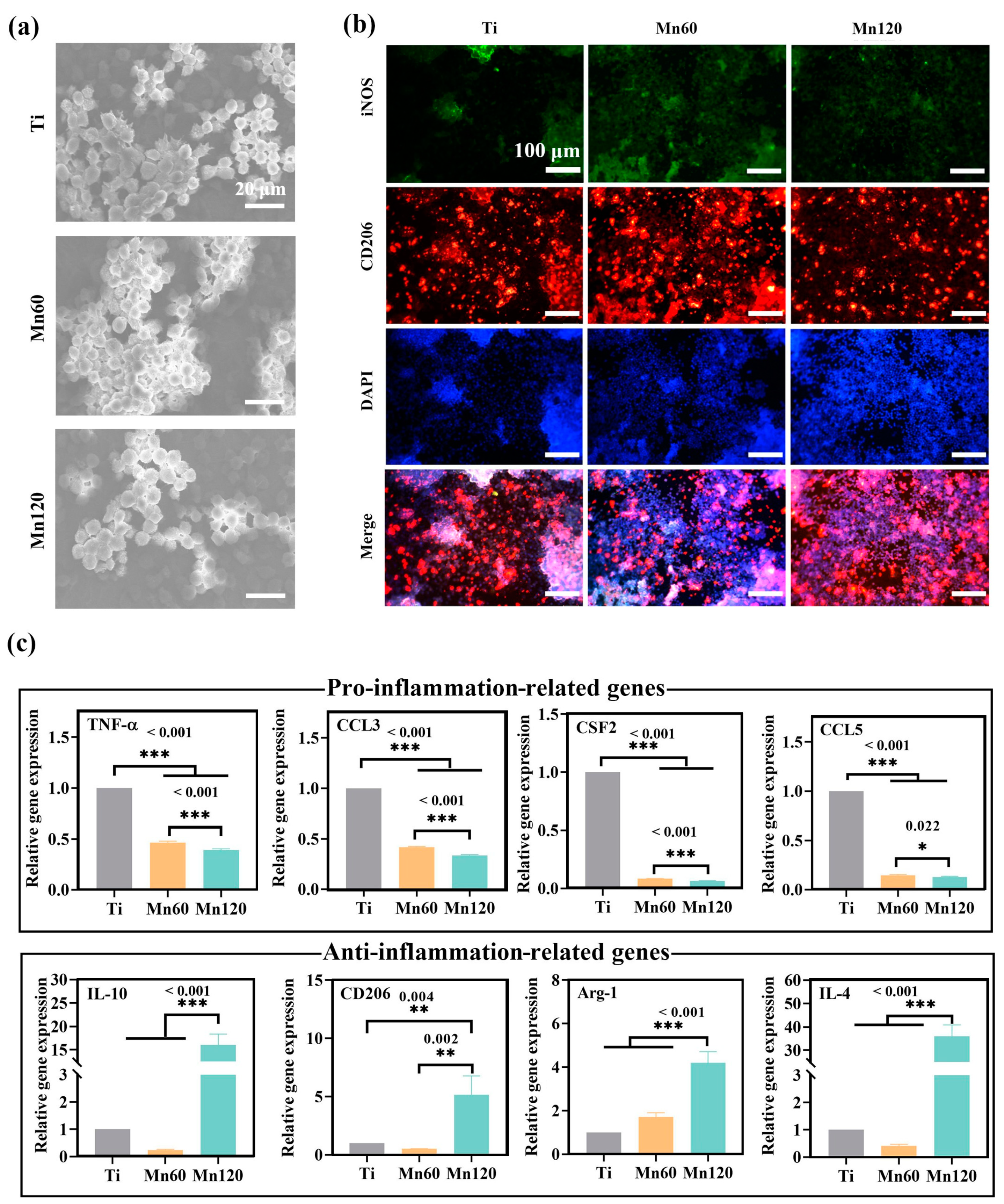
3.2. Effects of Mn Implantation on Cellular Immune Response
3.3. Little Effects of Mn Implantation on Osteogenic Differentiation
3.4. Positive Immunomodulatory Effects of Mn Implantation on Osteogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.-h.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Allard, C. Treating inflammatory bone loss. Nat. Rev. Mater. 2022, 7, 932. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Baht, G.S.; Vi, L.; Alman, B.A. The role of the immune cells in fracture healing. Curr. Osteoporos. Rep. 2018, 16, 138–145. [Google Scholar] [CrossRef]
- Wculek, S.K.; Dunphy, G.; Heras-Murillo, I.; Mastrangelo, A.; Sancho, D. Metabolism of tissue macrophages in homeostasis and pathology. Cell. Mol. Immunol. 2022, 19, 384–408. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; Fischer, H.; Bucher, C.H.; Rendenbach, C.; Duda, G.N.; Schmidt-Bleek, K. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. 2021, 133, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS−) vs. alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Tu, Z.X.; Zhong, Y.L.; Hu, H.Z.; Shao, D.; Haag, R.N.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- Wang, T.; Bai, J.X.; Lu, M.; Huang, C.L.; Geng, D.C.; Chen, G.; Wang, L.; Qi, J.; Cui, W.G.; Deng, L.F. Engineering immunomodulatory and osteoinductive implant surfaces via mussel adhesion-mediated ion coordination and molecular clicking. Nat. Commun. 2022, 13, 160. [Google Scholar] [CrossRef]
- Baseri, M.; Radmand, F.; Hamedi, R.; Yousefi, M.; Kafil, H.S. Immunological aspects of dental implant rejection. Biomed. Res. Int. 2020, 2020, 7279509. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xia, D.; Ni, Z.; Ou, T.; Wang, Y.; Zhang, H.; Mao, L.; Lin, K.; Xu, S.; Liu, J. Calcium silicate bioactive ceramics induce osteogenesis through oncostatin M. Bioact. Mater. 2021, 6, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.-h.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z.; et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. 2017, 35, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Böse, T.; Unger, R.E.; Jansen, J.A.; Kirkpatrick, C.J.; van den Beucken, J.J.J.P. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell Tissue Res. 2017, 369, 273–286. [Google Scholar] [CrossRef]
- Rai, B.; Lin, J.L.; Lim, Z.X.H.; Guldberg, R.E.; Hutmacher, D.W.; Cool, S.M. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials 2010, 31, 7960–7970. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.H.; Cheng, M.Q.; Wang, Q.J.; Qian, Y.B.; Yeung, K.W.K.; Chu, P.K.; Zhang, X.L. A surface-engineered polyetheretherketone biomaterial implant with direct and immunoregulatory antibacterial activity against methicillin-resistant Staphylococcus aureus. Biomaterials 2019, 208, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.C.; Skaar, E.P. Nutritional immunity: The battle for nutrient metals at the host-pathogen interface. Nat. Rev. Microbiol. 2022, 20, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Cui, Y.T.; Liu, H.; Tian, Y.H.; Wang, G.; Fan, Y.; Wang, J.W.; Wu, D.K.; Wang, Y.B. Application of bioactive metal ions in the treatment of bone defects. J. Mater. Chem. B 2022, 10, 9369–9388. [Google Scholar] [CrossRef] [PubMed]
- Knoell, D.; Rink, L. Metal ions in immune function and host defense. Semin. Cell Dev. Biol. 2021, 115, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Renaudin, G.; Forestier, C.; Nedelec, J.M.; Descamps, S. Biological properties of copper-doped biomaterials for orthopedic applications: A review of antibacterial, angiogenic and osteogenic aspects. Acta Biomater. 2020, 117, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, Y.H.; Xu, K.; Zhong, H.S.; Yang, Y.H.; Jin, S.Y.; Yang, K.; Qi, X. Biological applications of copper-containing materials. Bioact. Mater. 2021, 6, 916–927. [Google Scholar] [CrossRef]
- Huang, Q.L.; Ouyang, Z.X.; Tan, Y.N.; Wu, H.; Liu, Y. Activating macrophages for enhanced osteogenic and bactericidal performance by Cu ion release from micro/nano-topographical coating on a titanium substrate. Acta Biomater. 2019, 100, 415–426. [Google Scholar] [CrossRef]
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Manganese superoxide dismutase dysfunction and the pathogenesis of kidney disease. Front. Physiol. 2020, 11, 755. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 2020, 145, 187–241. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y.; et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 2018, 48, 675–687.e7. [Google Scholar] [CrossRef]
- Prasadh, S.; Gupta, M.; Wong, R. In vitro cytotoxicity and osteogenic potential of quaternary Mg-2Zn-1Ca/X-Mn alloys for craniofacial reconstruction. Sci. Rep. 2022, 12, 8259. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shi, H.; Liang, Y.; Lu, T.; Lin, Z.; Ye, J. Improving osteogenesis of calcium phosphate bone cement by incorporating with manganese doped β-tricalcium phosphate. Mater. Sci. Eng. C 2020, 109, 110481. [Google Scholar] [CrossRef] [PubMed]
- Westhauser, F.; Wilkesmann, S.; Nawaz, Q.; Hohenbild, F.; Rehder, F.; Saur, M.; Fellenberg, J.; Moghaddam, A.; Ali, M.S.; Peukert, W.; et al. Effect of manganese, zinc, and copper on the biological and osteogenic properties of mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res. Part A 2021, 109, 1457–1467. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Kong, L.; Smith, W.; Hao, D. Overview of RAW264.7 for osteoclastogensis study: Phenotype and stimuli. J. Cell. Mol. Med. 2019, 23, 3077–3087. [Google Scholar] [CrossRef]
- Berghaus, L.J.; Moore, J.N.; Hurley, D.J.; Vandenplas, M.L.; Fortes, B.P.; Wolfert, M.A.; Boons, G.-J. Innate immune responses of primary murine macrophage-lineage cells and RAW 264.7 cells to ligands of Toll-like receptors 2, 3, and 4. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 443–454. [Google Scholar] [CrossRef]
- Kershner, R.J.; Bullard, J.W.; Cima, M.J. Zeta potential orientation dependence of sapphire substrates. Langmuir 2004, 20, 4101–4108. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Qiu, J.; Zhang, X.; Liu, X.; Qiao, Y.; Liu, X. Synergistic effects of immunoregulation and osteoinduction of ds-block elements on titanium surface. Bioact. Mater. 2021, 6, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xia, C.; Qiao, Y.; Liu, X. Dose-response relationships between copper and its biocompatibility/antibacterial activities. J. Trace Elem. Med. Biol. 2019, 55, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fu, H.; Chen, X.; Gong, P.; Chen, G.; Xia, L.; Wang, H.; You, J.; Wu, Y. Facile and sensitive fluorescence sensing of alkaline phosphatase activity with photoluminescent carbon dots based on inner filter effect. Anal. Chem. 2016, 88, 2720–2726. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Arandiyan, H.; Younis, A.; Scott, J.; Qu, B.; Wan, T.; Lin, X.; Chen, J.; Chu, D. Design and synthesis of CeO2 nanowire/MnO2 nanosheet heterogeneous structure for enhanced catalytic properties. Mater. Today Commun. 2017, 11, 103–111. [Google Scholar] [CrossRef]
- Chang, S.-L.; Anderegg, J.; Thiel, P. Surface oxidation of an Al Pd Mn quasicrystal, characterized by X-ray photoelectron spectroscopy. J. Non-Cryst. Solids 1996, 195, 95–101. [Google Scholar] [CrossRef]
- Pandey, J.; Hua, B.; Ng, W.; Yang, Y.; van der Veen, K.; Chen, J.; Geels, N.J.; Luo, J.-L.; Rothenberg, G.; Yan, N. Developing hierarchically porous MnOx/NC hybrid nanorods for oxygen reduction and evolution catalysis. Green Chem. 2017, 19, 2793–2797. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, E. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Hamilton, J.A. GM-CSF in inflammation. J. Exp. Med. 2020, 217, e20190945. [Google Scholar] [CrossRef] [PubMed]
- Sorgi, C.A.; Rose, S.; Court, N.; Carlos, D.; Paula-Silva, F.W.G.; Assis, P.A.; Frantz, F.G.; Ryffel, B.; Quesniaux, V.; Faccioli, L.H. GM-CSF Priming Drives Bone Marrow-Derived Macrophages to a Pro-Inflammatory Pattern and Downmodulates PGE(2) in Response to TLR2 Ligands. PLoS ONE 2012, 7, e40523. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, Z.Y.; Wang, J.N.; Zhang, W.; Yang, Y.; Zhang, Y.; Qu, X.L.; Zhu, Y.B.; Zou, J.J.; Peng, S.S.; et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 2020, 27, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, Y.S.; Park, K.H.; Ge, X.P.; Xu, R.; Li, N.; Song, M.Y.; Chun, H.; Bok, S.; Charles, J.F.; et al. A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation. Nat. Commun. 2020, 11, 2289. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.Y.; Wu, Y.F.; Mi, R.J.; Liu, W.Z.; Shen, X.; Lu, Y.X.; Jiang, Y.H.; Ma, M.J.; Shen, H.Y. ac4C acetylation of RUNX2 catalyzed by NAT10 spurs osteogenesis of BMSCs and prevents ovariectomy-induced bone loss. Mol. Ther.-Nucl. Acids 2021, 26, 135–147. [Google Scholar] [CrossRef]
- Haase, H. Innate immune cells speak manganese. Immunity 2018, 48, 616–618. [Google Scholar] [CrossRef]
- Gao, M.; Xie, Y.Q.; Lei, K.; Zhao, Y.; Kurum, A.; Van Herck, S.; Guo, Y.; Hu, X.; Tang, L. A manganese phosphate nanocluster activates the cGAS-STING pathway for enhanced cancer immunotherapy. Adv. Ther. 2021, 4, 2100065. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.L. The Mn-Ti (Manganese-Titanium) system. Bull. Alloy Phase Diagr. 1981, 2, 334–343. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Gritsenko, B.P.; Fortuna, S.V.; Perry, A.J. Modification of metallic materials and hard coatings using metal ion implantation. Vacuum 1999, 52, 247–254. [Google Scholar] [CrossRef]
- Jones, E.C.; En, W.; Ogawa, S.; Fraser, D.B.; Cheung, N.W. Anomalous behavior of shallow BF3 plasma immersion ion implantation. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1994, 12, 956–961. [Google Scholar] [CrossRef]
- Thorwarth, G.; Hammerl, C.; Kuhn, M.; Assmann, W.; Schey, B.; Stritzker, B. Investigation of DLC synthesized by plasma immersion ion implantation and deposition. Surf. Coat. Technol. 2005, 193, 206–212. [Google Scholar] [CrossRef]
- Yan, C.; Zeng, Q.F.; He, W.J.; Zhu, J.N. Enhanced surface hardness and tribocorrosion performance of 60NiTi by boron ion implantation and post-annealing. Tribol. Int. 2021, 155, 106816. [Google Scholar] [CrossRef]
- Maremonti, M.I.; Panzetta, V.; Dannhauser, D.; Netti, P.A.; Causa, F. Wide-range viscoelastic compression forces in microfluidics to probe cell-dependent nuclear structural and mechanobiological responses. J. R. Soc. Interface 2022, 19, 20210880. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Kubiak-Ossowska, K.; Jachimska, B.; Al Qaraghuli, M.; Mulheran, P.A. Protein interactions with negatively charged inorganic surfaces. Curr. Opin. Colloid. Interface Sci. 2019, 41, 104–117. [Google Scholar] [CrossRef]
- Wu, Q.; Mu, Q.; Xia, Z.; Min, J.; Wang, F. Manganese homeostasis at the host-pathogen interface and in the host immune system. Semin. Cell Dev. Biol. 2021, 115, 45–53. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Q.; Xu, J.; He, X.; Li, H.A.; Oldridge, D.A.; Li, G.; Qin, L. Overview of methods for enhancing bone regeneration in distraction osteogenesis: Potential roles of biometals. J. Orthop. Transl. 2021, 27, 110–118. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Essentiality of manganese for bone health: An overview and update. Nat. Prod. Commun. 2021, 16, 1934578X211016649. [Google Scholar] [CrossRef]
- Bozec, A.; Soulat, D. Latest perspectives on macrophages in bone homeostasis. Pflug. Arch.-Eur. J. Physiol. 2017, 469, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Wang, J.M. Chemokines: The past, the present and the future. Cell. Mol. Immunol. 2018, 15, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, C.; Guan, Y.; Wei, X.; Sha, M.; Yi, M.; Jing, M.; Lv, M.; Guo, W.; Xu, J.; et al. Manganese salts function as potent adjuvants. Cell Mol. Immunol. 2021, 18, 1222–1234. [Google Scholar] [CrossRef]
- Biswas, L.; Chen, J.; De Angelis, J.; Singh, A.; Owen-Woods, C.; Ding, Z.; Pujol, J.M.; Kumar, N.; Zeng, F.; Ramasamy, S.K.; et al. Lymphatic vessels in bone support regeneration after injury. Cell 2023, 186, 382–397.e24. [Google Scholar] [CrossRef]
- Wang, X.R.; Zhang, M.W.; Chen, D.D.; Zhang, Y.; Chen, A.F. AMP-activated protein kinase rescues the angiogenic functions of endothelial progenitor cells via manganese superoxide dismutase induction in type 1 diabetes. Am. J. Physiol.-Endocrinol. Metab. 2011, 300, E1135–E1145. [Google Scholar] [CrossRef]
- Sielska, M.; Przanowski, P.; Pasierbińska, M.; Wojnicki, K.; Poleszak, K.; Wojtas, B.; Grzeganek, D.; Ellert-Miklaszewska, A.; Ku, M.-C.; Kettenmann, H.; et al. Tumour-derived CSF2/granulocyte macrophage colony stimulating factor controls myeloid cell accumulation and progression of gliomas. Br. J. Cancer 2020, 123, 438–448. [Google Scholar] [CrossRef]

| Parameters | Mn60 | Mn120 |
|---|---|---|
| Target voltage pulse duration (μs) | 500 | 500 |
| Cathodic arc voltage pulse duration (μs) | 800 | 800 |
| Pulsing frequency (Hz) | 5 | 5 |
| Voltage (kV) | −15 | −15 |
| Time (min) | 60 | 120 |
| Pressure (Pa) | 5.0 × 10−3 | 5.0 × 10−3 |
| Sample Name | C 1s (at%) | O 1s (at%) | Ti 2p (at%) | Mn 2p (at%) |
|---|---|---|---|---|
| Mn60 | 56.97 | 32.12 | 7.71 | 3.20 |
| Mn120 | 51.99 | 37.01 | 6.91 | 4.09 |
| Sample Name | Ti | Mn60 | Mn120 |
|---|---|---|---|
| Icorr (A·cm−2) | 3.387 × 10−8 | 4.235 × 10−8 | 2.997 × 10−8 |
| Ecorr (V) vs. SCE | −0.292 | −0.309 | −0.327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, K.; Zhang, X.; Shangguan, L.; Liu, X.; Nie, X.; Qiao, Y. Manganese-Implanted Titanium Modulates the Crosstalk between Bone Marrow Mesenchymal Stem Cells and Macrophages to Improve Osteogenesis. J. Funct. Biomater. 2023, 14, 456. https://doi.org/10.3390/jfb14090456
Ye K, Zhang X, Shangguan L, Liu X, Nie X, Qiao Y. Manganese-Implanted Titanium Modulates the Crosstalk between Bone Marrow Mesenchymal Stem Cells and Macrophages to Improve Osteogenesis. Journal of Functional Biomaterials. 2023; 14(9):456. https://doi.org/10.3390/jfb14090456
Chicago/Turabian StyleYe, Kuicai, Xianming Zhang, Li Shangguan, Xingdan Liu, Xiaoshuang Nie, and Yuqin Qiao. 2023. "Manganese-Implanted Titanium Modulates the Crosstalk between Bone Marrow Mesenchymal Stem Cells and Macrophages to Improve Osteogenesis" Journal of Functional Biomaterials 14, no. 9: 456. https://doi.org/10.3390/jfb14090456
APA StyleYe, K., Zhang, X., Shangguan, L., Liu, X., Nie, X., & Qiao, Y. (2023). Manganese-Implanted Titanium Modulates the Crosstalk between Bone Marrow Mesenchymal Stem Cells and Macrophages to Improve Osteogenesis. Journal of Functional Biomaterials, 14(9), 456. https://doi.org/10.3390/jfb14090456







