Corrosion Products from Metallic Implants Induce ROS and Cell Death in Human Motoneurons In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Cell Culture
2.3. NeuO Staining
2.4. ROS Measurement
2.5. Fluorescence-Assisted Cell Sorting
2.6. Statistics
3. Results
3.1. Cell Death Caused by Metal Ions Is Dose Dependent
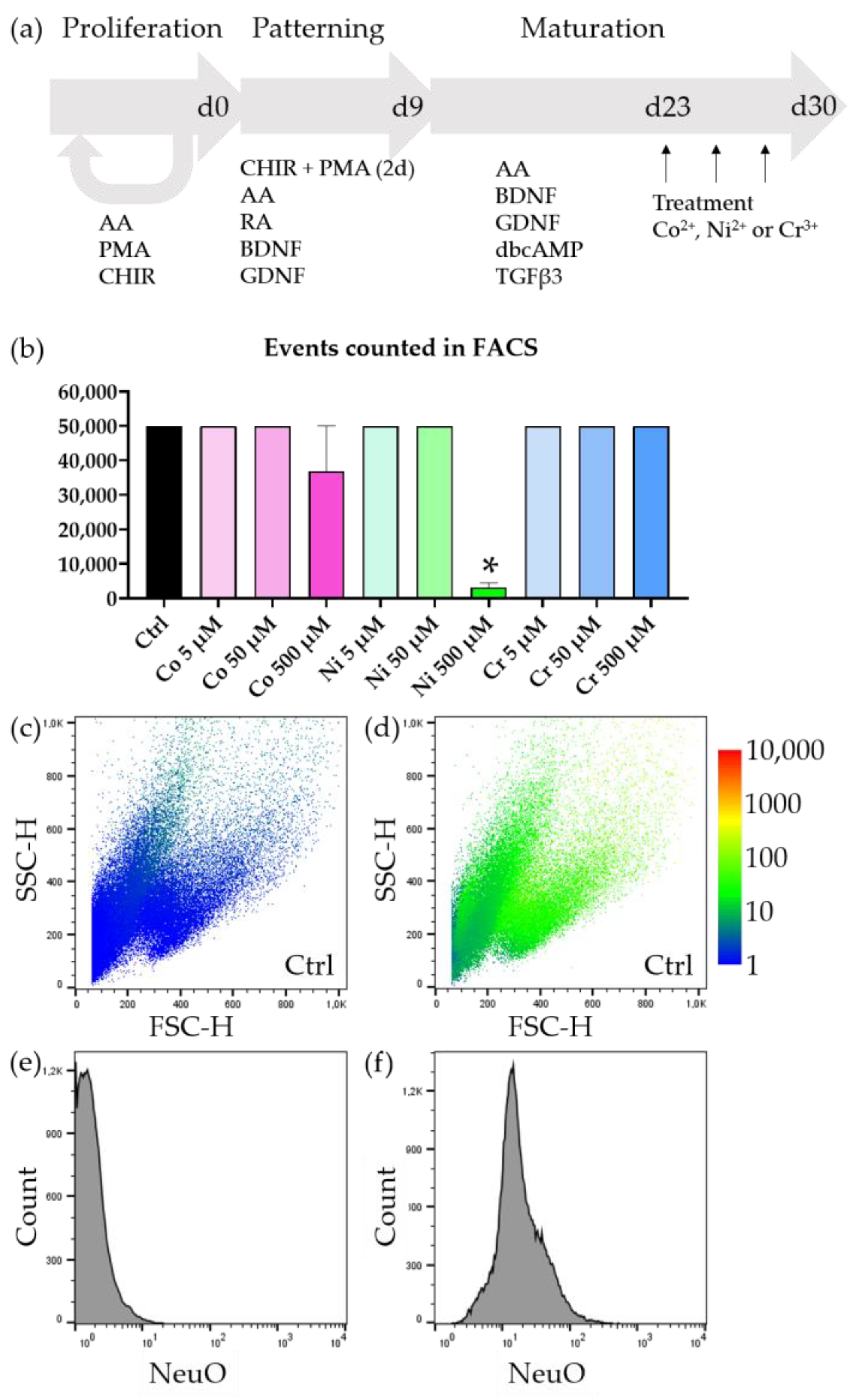
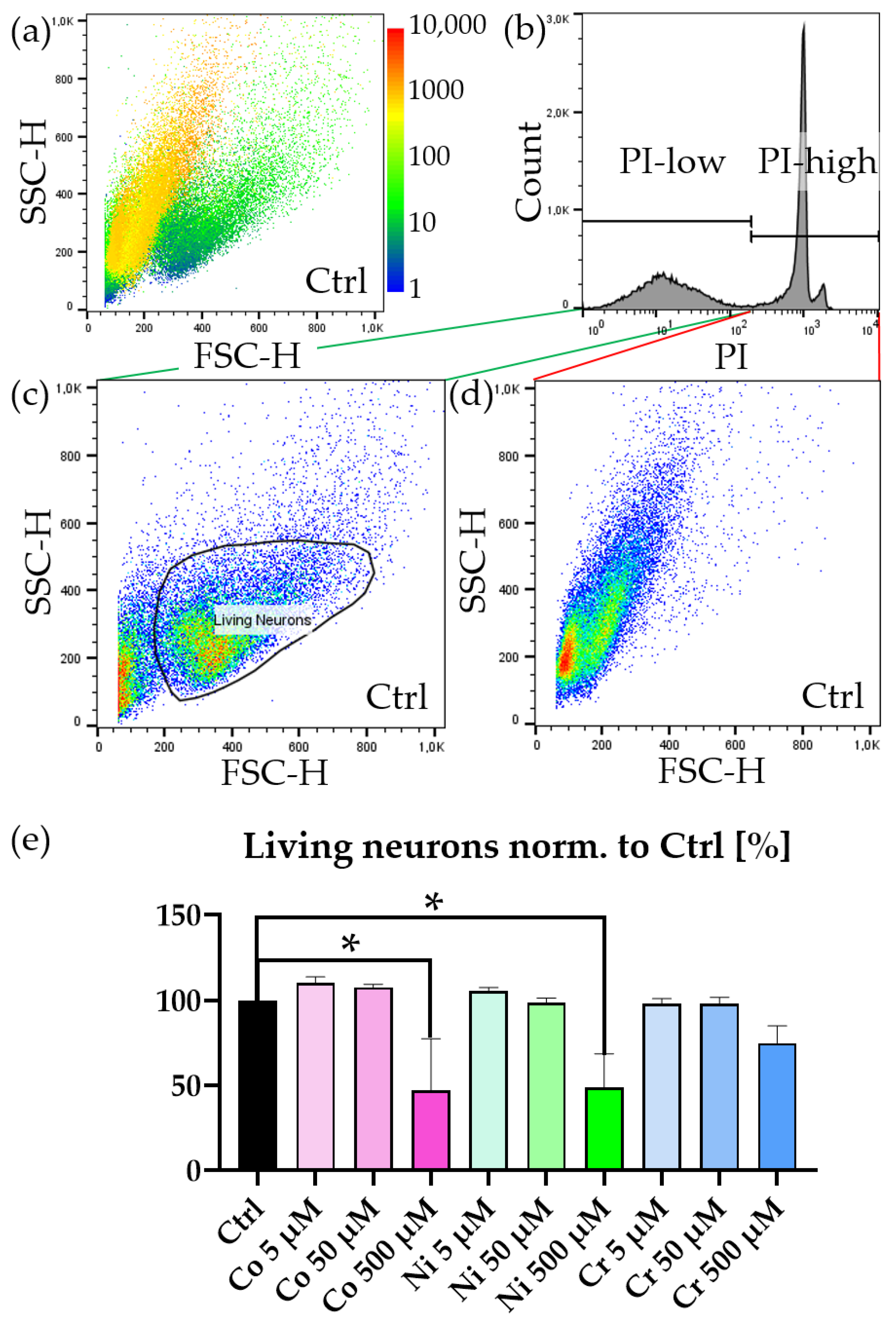
3.2. Motoneurons Are Selectively Vulnerable to Metal Ions
3.3. Neurotoxicity Is Accompanied by Increase in ROS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqi, A.; Levine, B.R.; Springer, B.D. Highlights of the 2021 American Joint Replacement Registry Annual Report. Arthroplast. Today 2022, 13, 205–207. [Google Scholar] [CrossRef]
- Projected Volume of Primary and Revision Total Joint Replacement in the U.S. 2030 to 2060 American Academy of Orthopaedic Surgeons. Available online: http://aaos-annualmeeting-presskit.org/2018/research-news/sloan_tjr/ (accessed on 28 April 2023).
- Losina, E.; Katz, J.N. Total Knee Arthroplasty on the Rise in Younger Patients: Are We Sure That Past Performance Will Guarantee Future Success? Arthritis Rheum. 2012, 64, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, U.; Cotogno, G.; Institute for Health and Consumer Protection. Total Hip Arthroplasty: State of the Art, Prospects and Challenges; Publications Office of the European Union: Luxembourg, 2012; pp. 1–60. [Google Scholar] [CrossRef]
- Matusiewicz, H. Potential Release of in Vivo Trace Metals from Metallic Medical Implants in the Human Body: From Ions to Nanoparticles—A Systematic Analytical Review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Sansone, V. The Effects on Bone Cells of Metal Ions Released from Orthopaedic Implants. A Review. Clin. CASES Miner. BONE Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular Analysis of Chromium and Cobalt-Related Toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef]
- Toh, W.; Tan, X.; Bhowmik, A.; Liu, E.; Tor, S. Tribochemical Characterization and Tribocorrosive Behavior of CoCrMo Alloys: A Review. Materials 2017, 11, 30. [Google Scholar] [CrossRef]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Safavi, M.S.; Bordbar-Khiabani, A.; Walsh, F.C.; Mozafari, M.; Khalil-Allafi, J. Surface Modified NiTi Smart Biomaterials: Surface Engineering and Biological Compatibility. Curr. Opin. Biomed. Eng. 2023, 25, 100429. [Google Scholar] [CrossRef]
- Lohberger, B.; Eck, N.; Glaenzer, D.; Lichtenegger, H.; Ploszczanski, L.; Leithner, A. Cobalt Chromium Molybdenum Surface Modifications Alter the Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells. Materials 2020, 13, 4292. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The Biological Response to Orthopaedic Implants for Joint Replacement: Part I: Metals. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef]
- Hannemann, F.; Hartmann, A.; Schmitt, J.; Lützner, J.; Seidler, A.; Campbell, P.; Delaunay, C.P.; Drexler, H.; Ettema, H.B.; García-Cimbrelo, E.; et al. European Multidisciplinary Consensus Statement on the Use and Monitoring of Metal-on-Metal Bearings for Total Hip Replacement and Hip Resurfacing. Orthop. Traumatol. Surg. Res. 2013, 99, 263–271. [Google Scholar] [CrossRef] [PubMed]
- COS—Overview: Cobalt, Serum. Available online: https://www.mayocliniclabs.com/test-catalog/overview/80084#Clinical-and-Interpretive (accessed on 28 April 2023).
- Kim, C.-H.; Choi, Y.H.; Jeong, M.Y.; Chang, J.S.; Yoon, P.W. Cobalt Intoxication Heart Failure after Revision Total Hip Replacement for Ceramic Head Fracture: A Case Report. Hip Pelvis 2016, 28, 259. [Google Scholar] [CrossRef]
- Jonitz-Heincke, A.; Sellin, M.L.; Seyfarth, A.; Peters, K.; Mueller-Hilke, B.; Fiedler, T.; Bader, R.; Klinder, A. Analysis of Cellular Activity and Induction of Inflammation in Response to Short-Term Exposure to Cobalt and Chromium Ions in Mature Human Osteoblasts. Materials 2019, 12, 2771. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt Metabolism and Toxicology—A Brief Update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Zhao, Y.y.; Cao, C.-l.; Liu, Y.-l.; Wang, J.; Li, S.-y.; Li, J.; Deng, Y. Genetic Analysis of Oxidative and Endoplasmic Reticulum Stress Responses Induced by Cobalt Toxicity in Budding Yeast. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129516. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Grossmann, D.; Malburg, N.; Glaß, H.; Weeren, V.; Sondermann, V.; Pfeiffer, J.F.; Petters, J.; Lukas, J.; Seibler, P.; Klein, C.; et al. Mitochondria–Endoplasmic Reticulum Contact Sites Dynamics and Calcium Homeostasis Are Differentially Disrupted in PINK1-PD or PRKN-PD Neurons. Mov. Disord. 2023. [Google Scholar] [CrossRef] [PubMed]
- Völkner, C.; Liedtke, M.; Petters, J.; Huth, K.; Knuebel, G.; Murua Escobar, H.; Bullerdiek, J.; Lukas, J.; Hermann, A.; Frech, M.J. Generation of an IPSC Line (AKOSi006-A) from Fibroblasts of an NPC1 Patient, Carrying the Homozygous Mutation p.I1061T (c.3182 T > C) and a Control IPSC Line (AKOSi007-A) Using a Non-Integrating Sendai Virus System. Stem Cell Res. 2020, 49, 102056. [Google Scholar] [CrossRef]
- Reinhardt, P.; Glatza, M.; Hemmer, K.; Tsytsyura, Y.; Thiel, C.S.; Hoing, S.; Moritz, S.; Parga, J.A.; Wagner, L.; Bruder, J.M.; et al. Derivation and Expansion Using Only Small Molecules of Human Neural Progenitors for Neurodegenerative Disease Modeling. PLoS ONE 2013, 8, e59252. [Google Scholar] [CrossRef]
- Naumann, M.; Pal, A.; Goswami, A.; Lojewski, X.; Japtok, J.; Vehlow, A.; Naujock, M.; Günther, R.; Jin, M.; Stanslowsky, N.; et al. Impaired DNA Damage Response Signaling by FUS-NLS Mutations Leads to Neurodegeneration and FUS Aggregate Formation. Nat. Commun. 2018, 9, 335. [Google Scholar] [CrossRef]
- Bräuer, S.; Günther, R.; Sterneckert, J.; Glaß, H.; Hermann, A. Human Spinal Motor Neurons Are Particularly Vulnerable to Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients. Int. J. Mol. Sci. 2020, 21, 3564. [Google Scholar] [CrossRef]
- Günther, R.; Pal, A.; Williams, C.; Zimyanin, V.L.; Liehr, M.; von Neubeck, C.; Krause, M.; Parab, M.G.; Petri, S.; Kalmbach, N.; et al. Alteration of Mitochondrial Integrity as Upstream Event in the Pathophysiology of SOD1-ALS. Cells 2022, 11, 1246. [Google Scholar] [CrossRef]
- Niinomi, M. Recent Metallic Materials for Biomedical Applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Green, D.R.; Reed, J.C. Mitochondria and Apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Dixon, S.J. Mechanisms of Ferroptosis. Cell Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Gupta, G.; Gliga, A.; Hedberg, J.; Serra, A.; Greco, D. Cobalt Nanoparticles Trigger Ferroptosis-like Cell Death (Oxytosis) in Neuronal Cells: Potential Implications for Neurodegenerative Disease. FASEB J. 2020, 34, 5262–5281. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Ni, D.; Zhou, Z.; Gu, J.; Zhang, W.; Sun, H.; Liu, F. Alpha Lipoic Acid Antagonizes Cytotoxicity of Cobalt Nanoparticles by Inhibiting Ferroptosis-like Cell Death. J. Nanobiotechnology 2020, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, H.; Jonitz-Heincke, A.; Peters, K.; Mueller-Hilke, B.; Fiedler, T.; Bader, R.; Klinder, A. Comparison of Inflammatory Effects in THP-1 Monocytes and Macrophages after Exposure to Metal Ions. Materials 2020, 13, 1150. [Google Scholar] [CrossRef]
- Filvaroff, E.; Calautti, E.; Reiss, M.; Dotto, G.P. Functional Evidence for an Extracellular Calcium Receptor Mechanism Triggering Tyrosine Kinase Activation Associated with Mouse Keratinocyte Differentiation. J. Biol. Chem. 1994, 269, 21735–21740. [Google Scholar] [CrossRef]
- Cortijo, J.; Milara, J.; Mata, M.; Donet, E.; Gavara, N.; Peel, S.E.; Hall, I.P.; Morcillo, E.J. Nickel Induces Intracellular Calcium Mobilization and Pathophysiological Responses in Human Cultured Airway Epithelial Cells. Chem. Biol. Interact. 2010, 183, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Antholine, W.E.; Myers, C.R. The Intracellular Redox Stress Caused by Hexavalent Chromium Is Selective for Proteins That Have Key Roles in Cell Survival and Thiol Redox Control. Toxicology 2011, 281, 37. [Google Scholar] [CrossRef] [PubMed]
- Pulletikurthi, C.; Munroe, N.; Gill, P.; Pandya, S.; Persaud, D.; Haider, W.; Iyer, K.; McGoron, A. Cytotoxicity of Ni from Surface-Treated Porous Nitinol (PNT) on Osteoblast Cells. J. Mater. Eng. Perform. 2011, 20, 824–829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and Mechanisms of Action of Titanium Dioxide Nanoparticles in Living Organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- Rae, T. The Toxicity of Metals Used in Orthopaedic Prostheses. An Experimental Study Using Cultured Human Synovial Fibroblasts. J. Bone Joint Surg. Br. 1981, 63-B, 435–440. [Google Scholar] [CrossRef]
- Haynes, D.R.; Rogers, S.D.; Hay, S.; Pearcy, M.J.; Howie, D.W. The Differences in Toxicity and Release of Bone-Resorbing Mediators Induced by Titanium and Cobalt-Chromium-Alloy Wear Particles. J. Bone Jt. Surg. 1993, 75, 825–834. [Google Scholar] [CrossRef]
- Mou, Y.H.; Yang, J.Y.; Cui, N.; Wang, J.M.; Hou, Y.; Song, S.; Wu, C.F. Effects of Cobalt Chloride on Nitric Oxide and Cytokines/Chemokines Production in Microglia. Int. Immunopharmacol. 2012, 13, 120–125. [Google Scholar] [CrossRef]
- Radcliffe, P.M.; Olabisi, A.O.; Wagner, D.J.; Leavens, T.; Wong, B.A.; Struve, M.F.; Chapman, G.D.; Wilfong, E.R.; Dorman, D.C. Acute Sodium Tungstate Inhalation Is Associated with Minimal Olfactory Transport of Tungsten (188W) to the Rat Brain. Neurotoxicology 2009, 30, 445–450. [Google Scholar] [CrossRef]
- Tamargo, R.J.; Rossell, L.A.; Kossoff, E.H.; Tyler, B.M.; Ewend, M.G.; Aryanpur, J.J. The Intracerebral Administration of Phenytoin Using Controlled-Release Polymers Reduces Experimental Seizures in Rats. Epilepsy Res. 2002, 48, 145–155. [Google Scholar] [CrossRef]
- Xia, M.; Liang, S.; Li, S.; Ji, M.; Chen, B.; Zhang, M.; Dong, C.; Chen, B.; Gong, W.; Wen, G.; et al. Iatrogenic Iron Promotes Neurodegeneration and Activates Self-Protection of Neural Cells against Exogenous Iron Attacks. Function 2021, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Vowden, R.; Hughes, L.; Qi, D.; Francis, W.; Perino, G.; Pink, R.; Xiao, J.; Li, B.; et al. Combination of Cobalt, Chromium and Titanium Nanoparticles Increases Cytotoxicity in Vitro and pro-Inflammatory Cytokines in Vivo. J. Orthop. Transl. 2023, 38, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA Damage and Reactive Oxygen Species in Neurodegenerative Disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glaß, H.; Jonitz-Heincke, A.; Petters, J.; Lukas, J.; Bader, R.; Hermann, A. Corrosion Products from Metallic Implants Induce ROS and Cell Death in Human Motoneurons In Vitro. J. Funct. Biomater. 2023, 14, 392. https://doi.org/10.3390/jfb14080392
Glaß H, Jonitz-Heincke A, Petters J, Lukas J, Bader R, Hermann A. Corrosion Products from Metallic Implants Induce ROS and Cell Death in Human Motoneurons In Vitro. Journal of Functional Biomaterials. 2023; 14(8):392. https://doi.org/10.3390/jfb14080392
Chicago/Turabian StyleGlaß, Hannes, Anika Jonitz-Heincke, Janine Petters, Jan Lukas, Rainer Bader, and Andreas Hermann. 2023. "Corrosion Products from Metallic Implants Induce ROS and Cell Death in Human Motoneurons In Vitro" Journal of Functional Biomaterials 14, no. 8: 392. https://doi.org/10.3390/jfb14080392
APA StyleGlaß, H., Jonitz-Heincke, A., Petters, J., Lukas, J., Bader, R., & Hermann, A. (2023). Corrosion Products from Metallic Implants Induce ROS and Cell Death in Human Motoneurons In Vitro. Journal of Functional Biomaterials, 14(8), 392. https://doi.org/10.3390/jfb14080392








