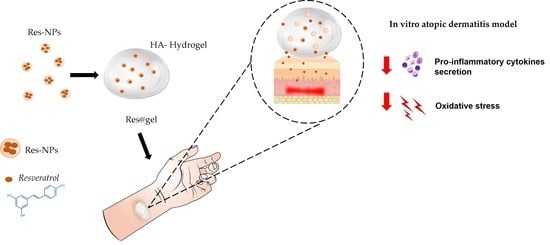
Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Physicochemical Characterization of Res-Loading Nanoparticles (Res-NPs)
2.3. Fabrication and Characterization of Res-Loaded Hydrogel (Res@gel)
2.3.1. HA Hydrogel Preparation
2.3.2. Swelling Test
2.3.3. Rheological Characterization
2.3.4. Short-Term Stability Studies
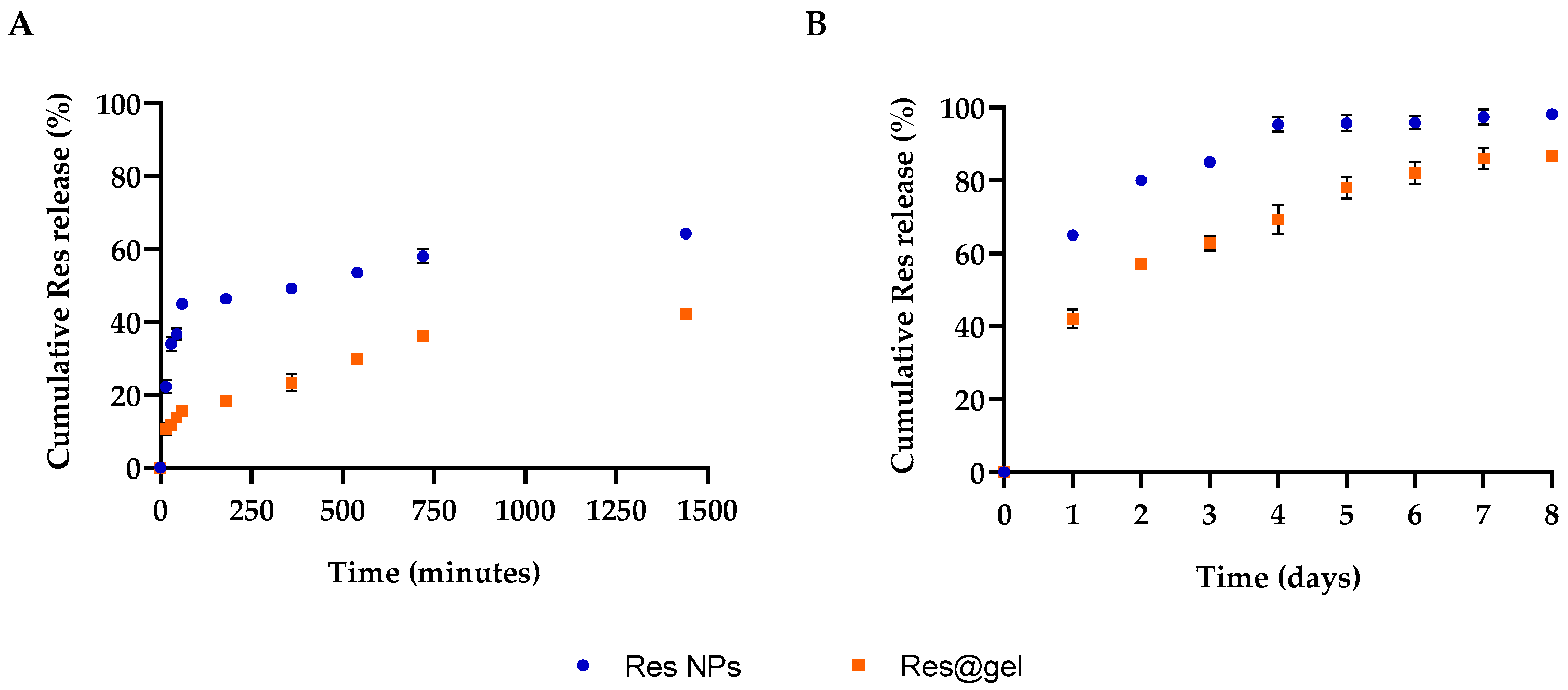
2.3.5. In Vitro Res Release
2.4. In Vitro Cell Studies
2.4.1. Cell Culture and Treatment
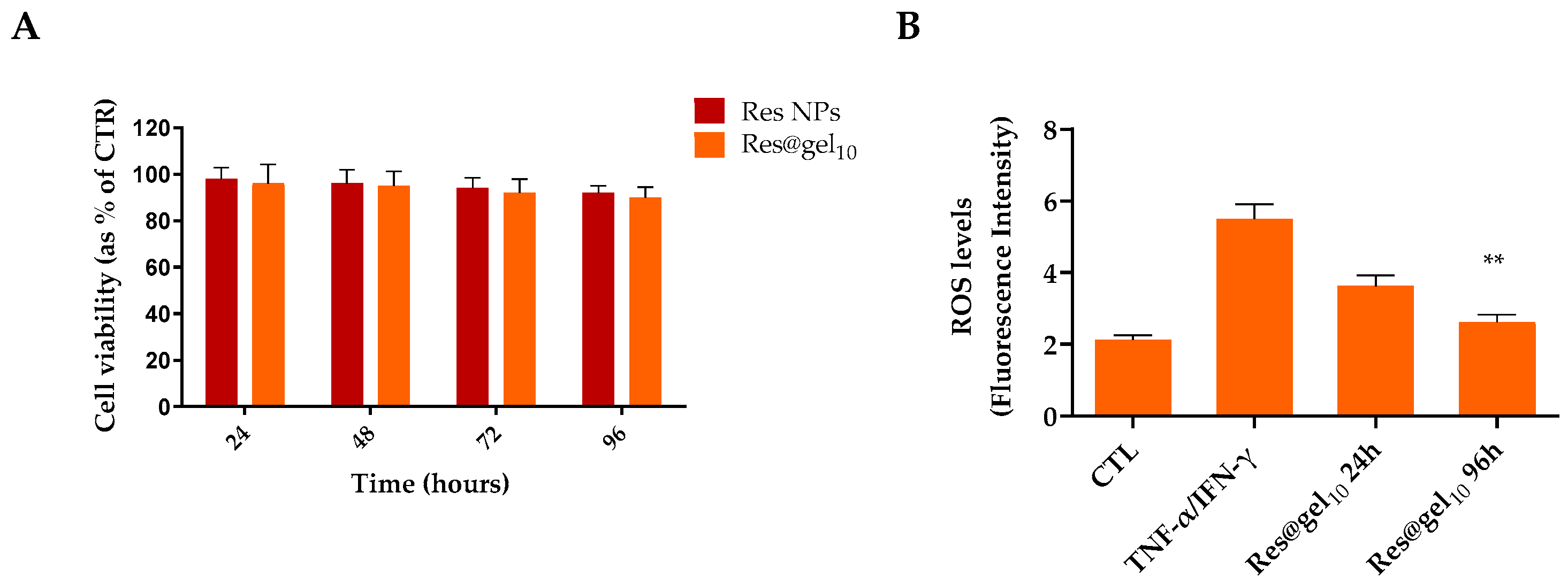
2.4.2. Intracellular Oxidative Stress
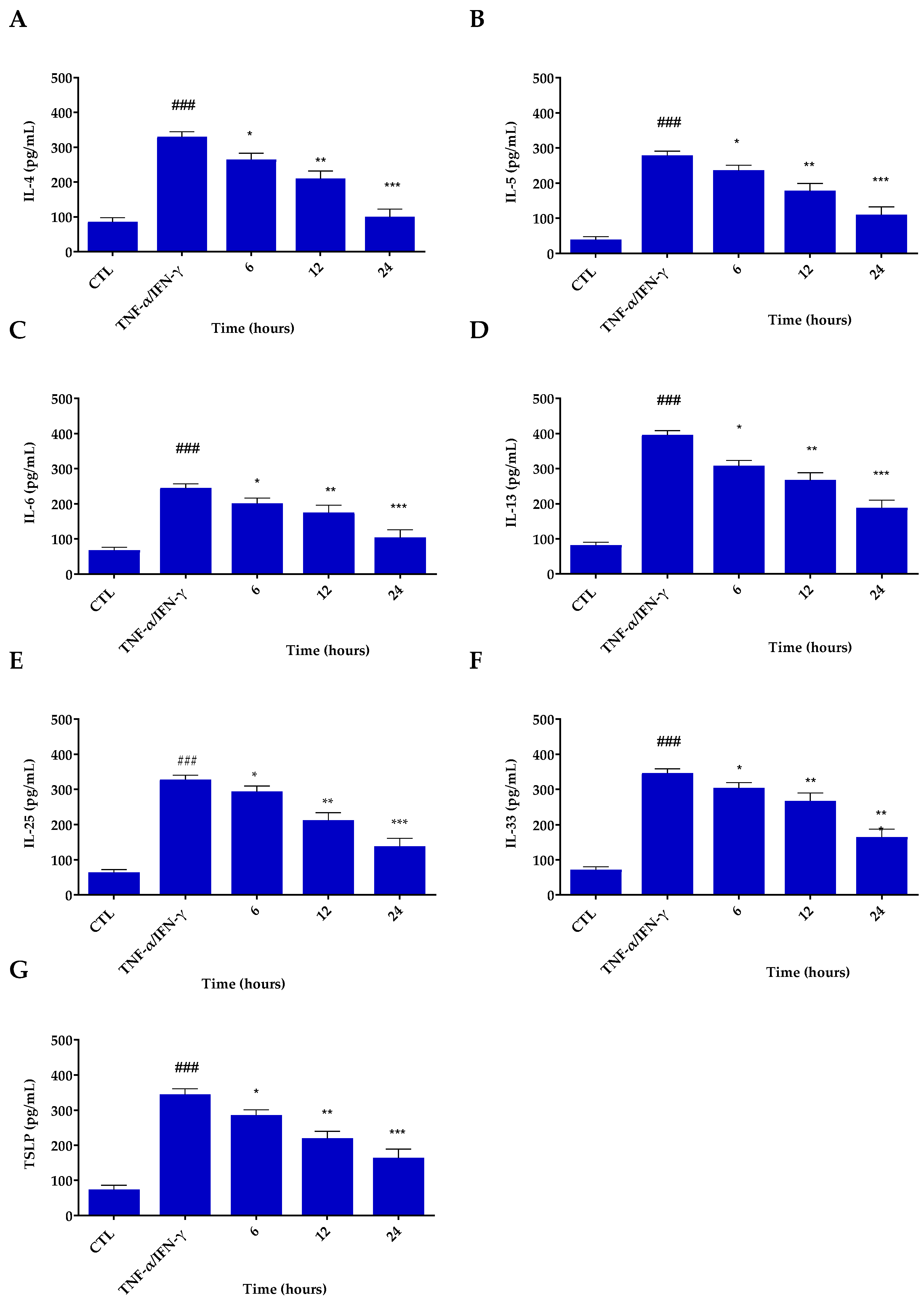
2.4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
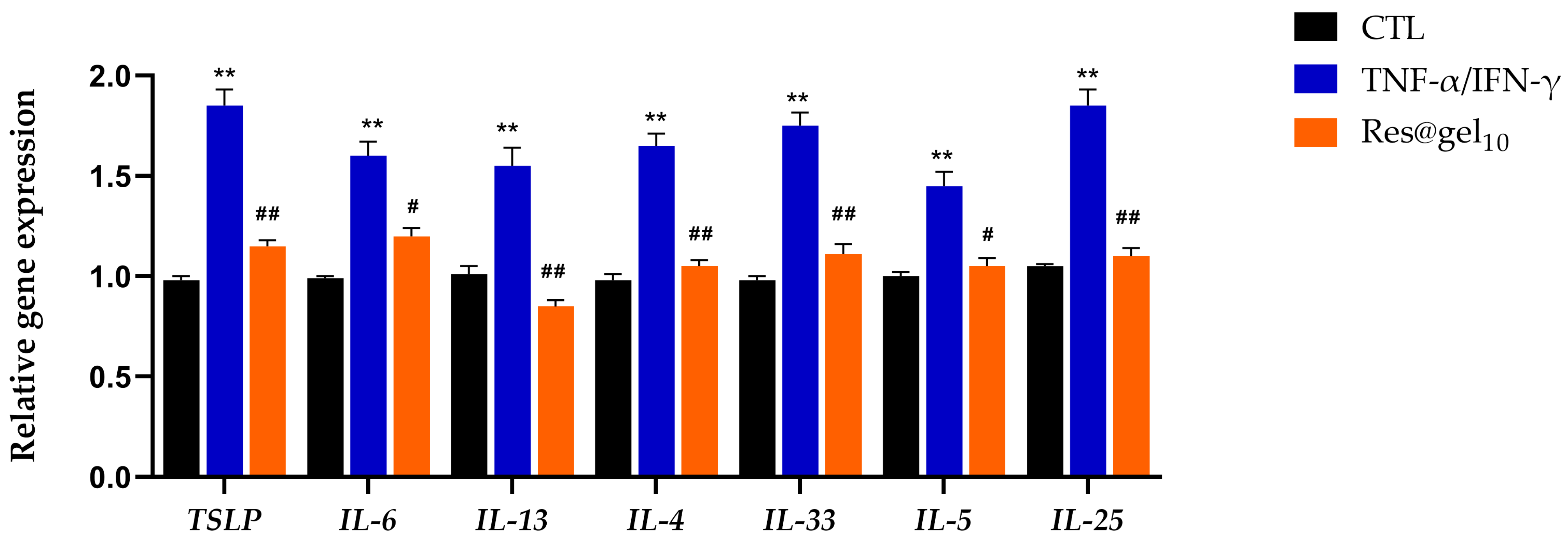
2.4.4. Real-Time Quantitative PCR (qRT-PCR)
2.5. Statistical Analysis
3. Results and Discussion
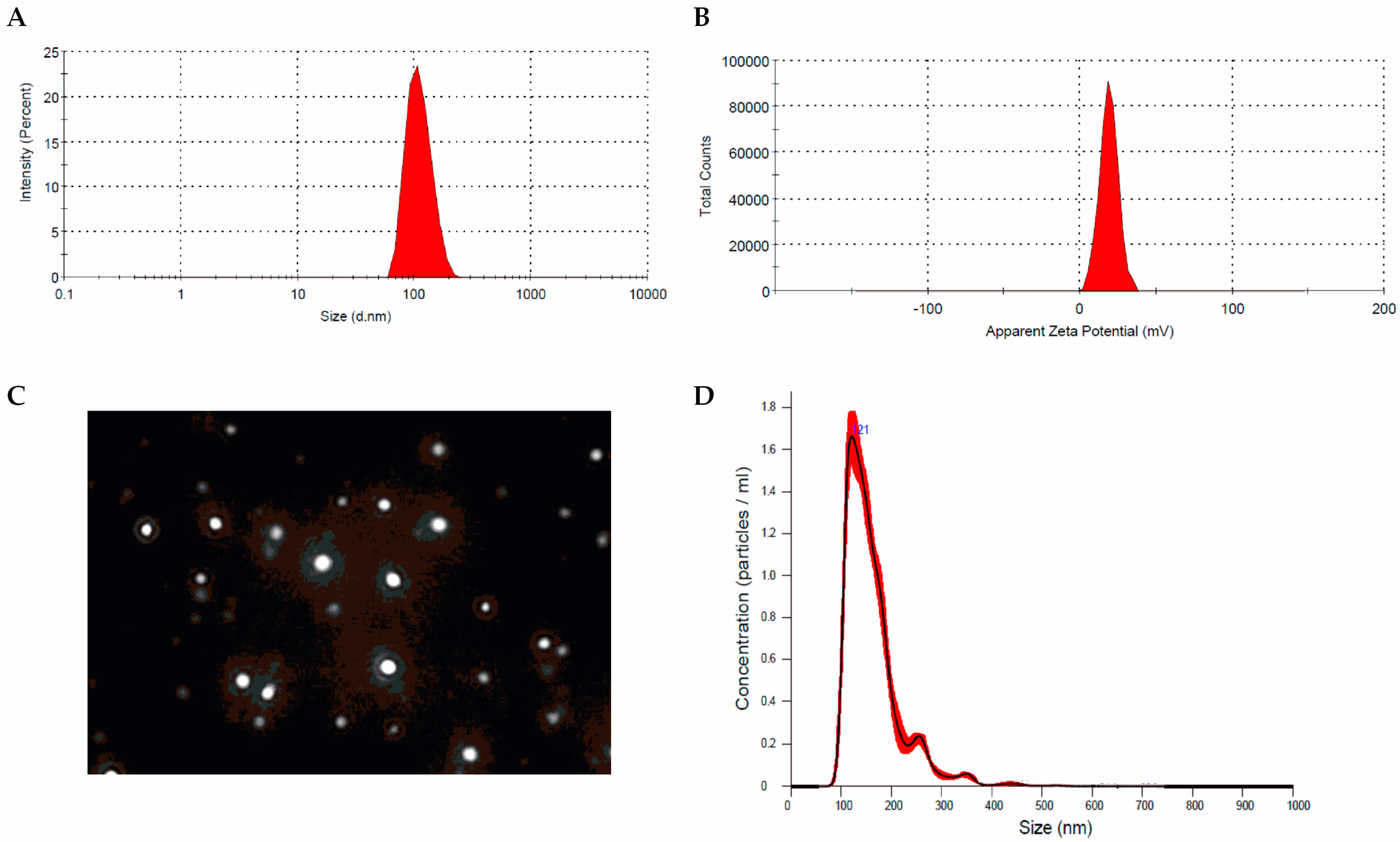
3.1. Preparation and Physicochemical Characterization of Res-Loaded Nanoparticles (Res-NPs)
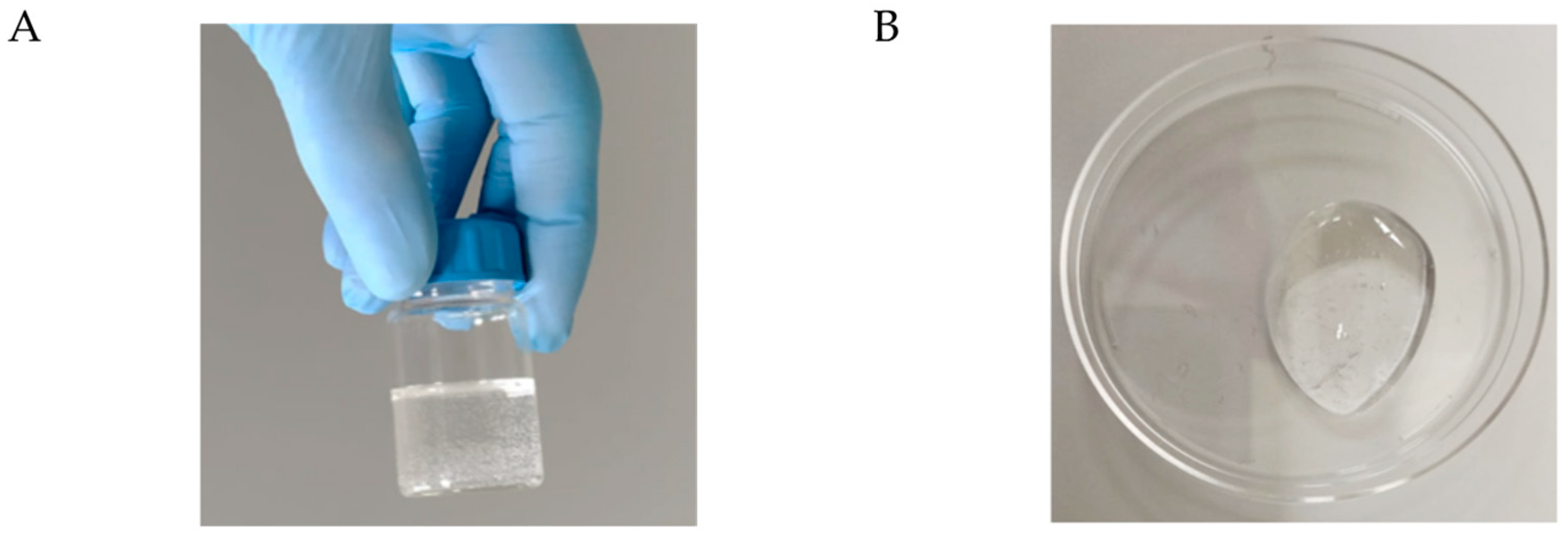
3.2. In Vitro Hydrogel Formulation (Res@gel) and Res Release
3.3. Antioxidant Activity of Res@gel10 in AD-Induced Cellular Model
3.4. Inflammatory Potential of Res@gel10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, S.-H.; Yang, Y.; Jeong, Y.; Kim, Y. Ovalicin attenuates atopic dermatitis symptoms by inhibiting IL-31 signaling and intracellular calcium influx. J. Biomed. Res. 2021, 35, 448–511. [Google Scholar] [CrossRef] [PubMed]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L. Atopic dermatitis: A review of topical treatment options. Curr. Med. Res. Opin. 2010, 26, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Reddy, R.K.K.; Naidu, V.; Madhusudhana, K.; Agwane, S.B.; Ramakrishna, S.; Diwan, P.V. Evaluation of antimicrobial, antioxidant and wound-healing potentials of Holoptelea integrifolia. J. Ethnopharmacol. 2008, 115, 249–256. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; d’Ayala, G.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Johnson, J.L.; Raghavan, V.; Cimmino, A.; Moeini, A.; Petrovic, A.G.; Santoro, E.; Superchi, S.; Berova, N.; Evidente, A.; Polavarapu, P.L. Absolute configurations of chiral molecules with multiple stereogenic centers without prior knowledge of the relative configurations: A case study of inuloxin C. Chirality 2018, 30, 1206–1214. [Google Scholar] [CrossRef]
- Bonadies, I.; Di Cristo, F.; Valentino, A.; Peluso, G.; Calarco, A.; Di Salle, A. pH-Responsive Resveratrol-Loaded Electrospun Membranes for the Prevention of Implant-Associated Infections. Nanomaterials 2020, 10, 1175. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef]
- Shrotriya, S.N.; Ranpise, N.S.; Vidhate, B.V. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Deliv. Transl. Res. 2017, 7, 37–52. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Babaluei, M.; Mojarab, Y.; Kundu, S.C. Advanced Multifunctional Wound Dressing Hydrogels as Drug Carriers. Macromol. Biosci. 2022, 22, 2200111. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2013, 3, 511–529. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chu, C.R.; Payne, K.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef]
- Serrano-Sevilla, I.; Artiga, Á.; Mitchell, S.G.; De Matteis, L.; de la Fuente, J.M. Natural Polysaccharides for siRNA Delivery: Nanocarriers Based on Chitosan, Hyaluronic Acid, and Their Derivatives. Molecules 2019, 24, 2570. [Google Scholar] [CrossRef]
- Dehkordi, N.K.; Minaiyan, M.; Talebi, A.; Akbari, V.; Taheri, A. Nanocrystalline cellulose–hyaluronic acid composite enriched with GM-CSF loaded chitosan nanoparticles for enhanced wound healing. Biomed. Mater. 2019, 14, 035003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhou, H.; Shu, J.; Fu, S.; Yang, Z. Skin wound healing promoted by novel curcumin-loaded micelle hydrogel. Ann. Transl. Med. 2021, 9, 1152. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A. Fungal and Plant Metabolites Formulated into Biopolymers, with Anti-Mold Activity for Food Packaging. Doctoral Dissertation, University of Naples Federico II, Naples, Italy, 2020. [Google Scholar]
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Nesic, A.; Moeini, A.; Santagata, G. 4 Marine biopolymers: Alginate and chitosan. In Sustainability of Polymeric Materials; University of Naples Federico II: Naples, Italy, 2020; pp. 73–92. [Google Scholar] [CrossRef]
- Catanzano, O.; Straccia, M.; Miro, A.; Ungaro, F.; Romano, I.; Mazzarella, G.; Santagata, G.; Quaglia, F.; Laurienzo, P.; Malinconico, M. Spray-by-spray in situ cross-linking alginate hydrogels delivering a tea tree oil microemulsion. Eur. J. Pharm. Sci. 2015, 66, 20–28. [Google Scholar] [CrossRef]
- Straccia, M.C.; Romano, I.; Oliva, A.; Santagata, G.; Laurienzo, P. Crosslinker effects on functional properties of alginate/N-succinylchitosan based hydrogels. Carbohydr. Polym. 2014, 108, 321–330. [Google Scholar] [CrossRef]
- Moeini, A.; Cimmino, A.; Poggetto, G.D.; Di Biase, M.; Evidente, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Leone, A.; Santagata, G.; et al. Effect of pH and TPP concentration on chemico-physical properties, release kinetics and antifungal activity of Chitosan-TPP-Ungeremine microbeads. Carbohydr. Polym. 2018, 195, 631–641. [Google Scholar] [CrossRef]
- Moeini, A.; Mallardo, S.; Cimmino, A.; Poggetto, G.D.; Masi, M.; Di Biase, M.; van Reenen, A.; Lavermicocca, P.; Valerio, F.; Evidente, A.; et al. Thermoplastic starch and bioactive chitosan sub-microparticle biocomposites: Antifungal and chemico-physical properties of the films. Carbohydr. Polym. 2019, 230, 115627. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Conte, R.; Valentino, A.; Di Cristo, F.; Peluso, G.; Cerruti, P.; Di Salle, A.; Calarco, A. Cationic Polymer Nanoparticles-Mediated Delivery of miR-124 Impairs Tumorigenicity of Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 869. [Google Scholar] [CrossRef]
- Valentino, A.; Conte, R.; De Luca, I.; Di Cristo, F.; Peluso, G.; Bosetti, M.; Calarco, A. Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes. Antioxidants 2022, 11, 1210. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef]
- Di Cristo, F.; Valentino, A.; De Luca, I.; Peluso, G.; Bonadies, I.; Calarco, A.; Di Salle, A. PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment. Molecules 2022, 27, 2205. [Google Scholar] [CrossRef]
- Calarco, A.; Di Salle, A.; Tammaro, L.; De Luca, I.; Mucerino, S.; Petillo, O.; Riccitiello, F.; Vittoria, V.; Peluso, G. Long-Term Fluoride Release from Dental Resins Affects STRO-1+ Cell Behavior. J. Dent. Res. 2015, 94, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.L.; Lio, P.A. Alternative, Complementary, and Forgotten Remedies for Atopic Dermatitis. Evid. Based Complement. Altern. Med. 2015, 2015, 676897. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Gunasegaran, T.A.P.; Nathan, S.S.; Shadab; Gorain, B.; Tripathy, M.; Hussain, Z. Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: Fabrication, characterisation, in vitro release kinetics, and dermal targeting. Drug Deliv. Transl. Res. 2019, 9, 520–533. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Wan, T.; Zhai, Y.; Cao, S.; Ruan, W.; Wu, C.; Xu, Y. Tacrolimus nanoparticles based on chitosan combined with nicotinamide: Enhancing percutaneous delivery and treatment efficacy for atopic dermatitis and reducing dose. Int. J. Nanomed. 2018, 13, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.I.; Katas, H.; Jamil, A.; Amin, M.C.I.M.; Ng, S.-F.; Zulfakar, M.H.; Nadeem, S.M. Potential treatment of atopic dermatitis: Tolerability and safety of cream containing nanoparticles loaded with hydrocortisone and hydroxytyrosol in human subjects. Drug Deliv. Transl. Res. 2019, 9, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis. J. Cosmet. Dermatol. 2011, 10, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Evrard, C.; de Rouvroit, C.L.; Poumay, Y. Epidermal Hyaluronan in Barrier Alteration-Related Disease. Cells 2021, 10, 3096. [Google Scholar] [CrossRef]
- Buckley, C.; Murphy, E.J.; Montgomery, T.R.; Major, I. Hyaluronic Acid: A Review of the Drug Delivery Capabilities of This Naturally Occurring Polysaccharide. Polymers 2022, 14, 3442. [Google Scholar] [CrossRef]
- Barbero, C.A.; Martínez, M.V.; Acevedo, D.F.; Molina, M.A.; Rivarola, C.R. Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications. Macromol 2022, 2, 440–475. [Google Scholar] [CrossRef]
- Duarah, S.; Durai, R.D.; Narayanan, V.B. Nanoparticle-in-gel system for delivery of vitamin C for topical application. Drug Deliv. Transl. Res. 2017, 7, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Samy, A.; Abdelaziz, A.E.; Shalaby, K.; Salama, A.; Raslan, M.A.; Abdelgawad, M.A. Polymeric nanoparticles based topical gel of poorly soluble drug: Formulation, ex-vivo and in vivo evaluation. Beni Suef Univ. J. Basic Appl. Sci. 2017, 6, 184–191. [Google Scholar] [CrossRef]
- Hatem, S.; Elkheshen, S.A.; Kamel, A.O.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Elezaby, R.S.; El Hoffy, N.M. Functionalized chitosan nanoparticles for cutaneous delivery of a skin whitening agent: An approach to clinically augment the therapeutic efficacy for melasma treatment. Drug Deliv. 2022, 29, 1212–1231. [Google Scholar] [CrossRef]
- Jana, S.; Manna, S.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Carbopol gel containing chitosan-egg albumin nanoparticles for transdermal aceclofenac delivery. Colloids Surf. B Biointerfaces 2014, 114, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ogston, A.G.; Stanier, J.E. The physiological function of hyaluronic acid in synovial fluid; viscous, elastic and lubricant properties. J. Physiol. 1953, 119, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.-K. Oxidative Stress in Atopic Dermatitis. Oxidative Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Huo, Y.; Yang, D.; Lai, K.; Tu, J.; Zhu, Y.; Ding, W.; Yang, S. Antioxidant Effects of Resveratrol in Intervertebral Disk. J. Investig. Surg. 2022, 35, 1135–1144. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hung, C.-F.; Sung, H.-C.; Yang, S.-C.; Yu, H.-P.; Fang, J.-Y. The Bioactivities of Resveratrol and Its Naturally Occurring Derivatives on Skin. J. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in mice. Int. Immunopharmacol. 2014, 23, 617–623. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Lin, Y.-K.; Yang, S.-C.; Alalaiwe, A.; Lin, C.-J.; Fang, J.-Y.; Lin, C.-F. Percutaneous absorption of resveratrol and its oligomers to relieve psoriasiform lesions: In silico, in vitro and in vivo evaluations. Int. J. Pharm. 2020, 585, 119507. [Google Scholar] [CrossRef]
- Omraninava, M.; Razi, B.; Aslani, S.; Imani, D.; Jamialahmadi, T.; Sahebkar, A. Effect of resveratrol on inflammatory cytokines: A meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2021, 908, 174380. [Google Scholar] [CrossRef]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Ayyildiz, Z.A.; Bağrıyanık, H.A.; Uzuner, N.; Karaman, O. Resveratrol ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis-like lesions through effects on the epithelium. Peerj 2016, 4, e1889. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, J. Resveratrol Exerts Therapeutic Effects on Mice With Atopic Dermatitis. Wounds 2019, 31, 279–284. [Google Scholar] [PubMed]
- Furue, M.; Furue, M. OX40L–OX40 Signaling in Atopic Dermatitis. J. Clin. Med. 2021, 10, 2578. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.; Sung, G.Y. An Interleukin-4 and Interleukin-13 Induced Atopic Dermatitis Human Skin Equivalent Model by a Skin-On-A-Chip. Int. J. Mol. Sci. 2022, 23, 2116. [Google Scholar] [CrossRef]
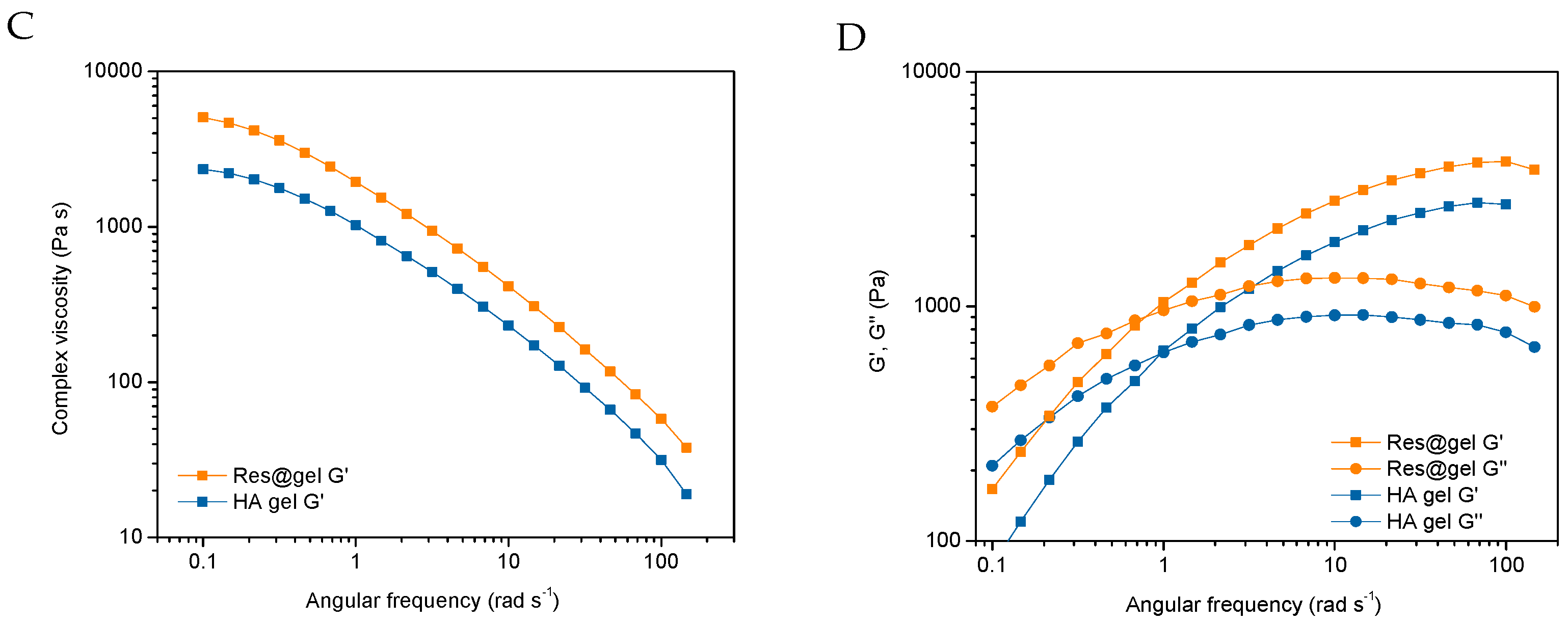
| Gene | Accession Number | Forward | Reverse |
|---|---|---|---|
| IL-4 | NM_000589.4 | ACTGCACAGCAGTTCCACAG | CTCTGGTTGGCTTCCTTCAC |
| IL-5 | NM_000879.3 | TGAGGATGCTTCTGCATTTG | GCAGTGCCAAGGTCTCTTTC |
| IL-6 | NM_000600.5 | CGCCTTCGGTCCAGTTGCC | GCCAGTGCCTCTTTGCTGCTTT |
| IL-13 | NM_002188.3 | CATCGAGAAGACCCAGAGGA | TTTACAAACTGGGCCACCTC |
| IL-25 | NM_022789.4 | GGACTCCTAACCTGCTCCAG | CTCTGCACTGACCTGGTACA |
| IL-33 | NM_033439.4 | CAAAGAAGTTTGCCCCATGT | AAGGCCTTTTGGTGGTTTCT |
| TSLP | NM_033035.5 | ATGAGAGGCAAAACCTGGTG | AATTCCACCCCAGTTTCACA |
| ACTB | NM_001101.5 | ACTCTTCCAGCCTTCCTTCC | CGTACAGGTCTTTGCGGATG |
| Chitosan (mg/mL) | CS:TPP Mass Ratio | Size (nm ± SD) | PDI (nm ± SD) | Z Potential (mV ± SD) | Encapsulation Efficiency (%±SD) |
|---|---|---|---|---|---|
| 0.1 | 1:1 | 563.15 ± 12.24 | 0.37 ± 0.04 | 13.9 ± 0.02 | 16.31 ± 2.23 |
| 0.1 | 5:1 | 352.39 ± 8.19a | 0.28 ± 0.03 | 12.7 ± 0.01 | 39.4 ± 1.31a |
| 0.1 | 10:1 | 247.26 ± 4.21b | 0.19 ± 0.04 | 13.5 ± 0.02 | 41.5 ± 2.23a |
| 0.5 | 1:1 | 514.24 ± 10.13 | 0.31 ± 0.03 | 15.3 ± 0.07 | 23.7 ± 1.45b |
| 0.5 | 5:1 | 338.26 ± 7.49a | 0.33 ± 0.02 | 16.9 ± 0.02 | 46.7 ± 1.12a |
| 0.5 | 10:1 | 177.15 ± 3.12c | 0.22 ± 0.04 | 16.1 ± 0.03 | 55.8 ± 1.64 |
| 1 | 1:1 | 469.31 ± 9.78 | 0.25 ± 0.03 | 17.5 ± 0.07 | 30.3 ± 1.91b |
| 1 | 5:1 | 315.17 ± 7.21b | 0.21 ± 0.02 | 18.6 ± 0.09 | 59.9 ± 2.26 |
| 1 | 10:1 | 121.22 ± 2.43c | 0.24 ± 0.04 | 19.4 ± 0.1 | 76.18 ± 3.16 |
| Res-NPs before Storage | Free Res-NPs | Res-NPs Released from Res@gel at 4 °C (nm ± SD) | Res-NPs Released from Res@gel at 25 °C (nm ± SD) | |
|---|---|---|---|---|
| Average particle size | 123.57 ± 9.11 | 269.18 ± 19.24 | 135.64 ± 9.04 | 141.77 ± 10.21 |
| PDI | 0.15 ± 0.03 | 0.17 ± 0.05 | 0.11 ± 0.03 | 0.19 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, R.; De Luca, I.; Valentino, A.; Cerruti, P.; Pedram, P.; Cabrera-Barjas, G.; Moeini, A.; Calarco, A. Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment. J. Funct. Biomater. 2023, 14, 82. https://doi.org/10.3390/jfb14020082
Conte R, De Luca I, Valentino A, Cerruti P, Pedram P, Cabrera-Barjas G, Moeini A, Calarco A. Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment. Journal of Functional Biomaterials. 2023; 14(2):82. https://doi.org/10.3390/jfb14020082
Chicago/Turabian StyleConte, Raffaele, Ilenia De Luca, Anna Valentino, Pierfrancesco Cerruti, Parisa Pedram, Gustavo Cabrera-Barjas, Arash Moeini, and Anna Calarco. 2023. "Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment" Journal of Functional Biomaterials 14, no. 2: 82. https://doi.org/10.3390/jfb14020082
APA StyleConte, R., De Luca, I., Valentino, A., Cerruti, P., Pedram, P., Cabrera-Barjas, G., Moeini, A., & Calarco, A. (2023). Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment. Journal of Functional Biomaterials, 14(2), 82. https://doi.org/10.3390/jfb14020082