Gallium-Doped Hydroxyapatite Shows Antibacterial Activity against Pseudomonas aeruginosa without Affecting Cell Metabolic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Gallium-Doped Hydroxyapatite (GaHAp)
2.2. Characterization Methods
2.2.1. X-ray Diffraction
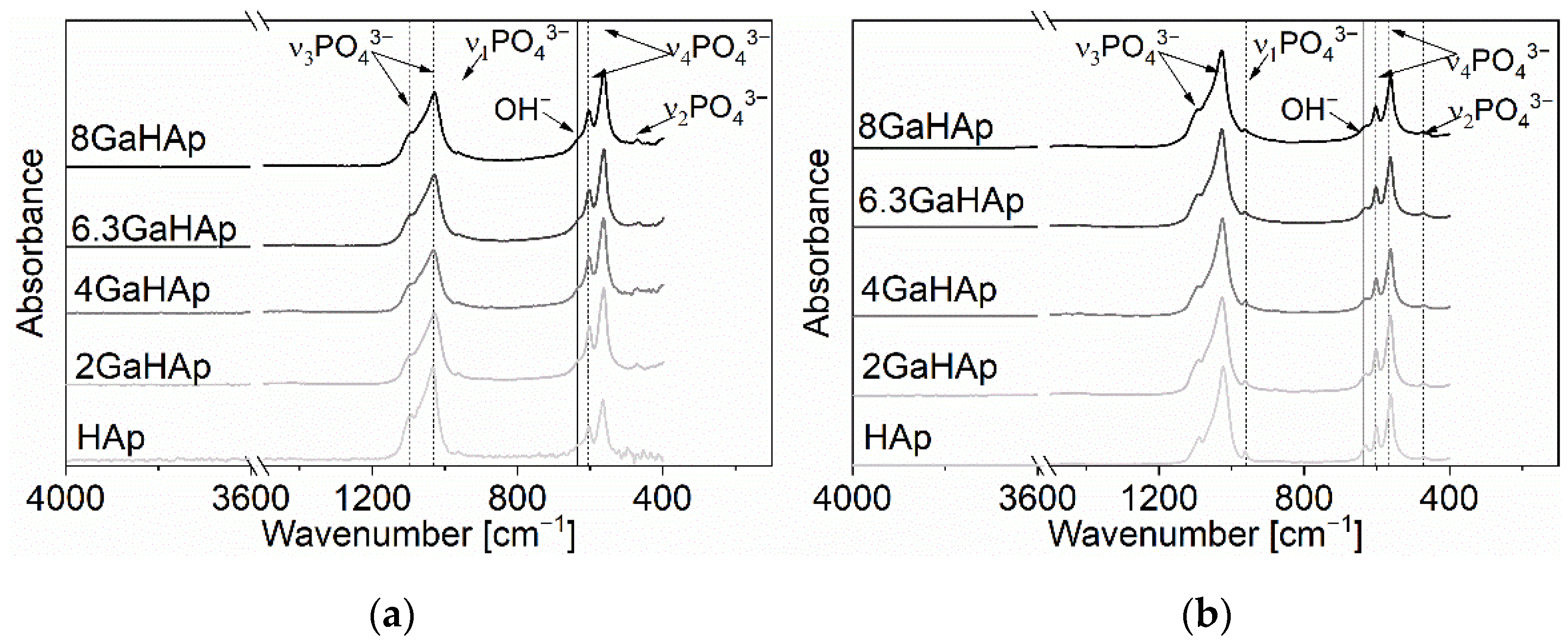
2.2.2. Fourier Transform Infrared Spectrometry
2.2.3. Specific Surface Area and Particle Size
2.2.4. Helium Pycnometry
2.2.5. Transmission Electron Microscope
2.3. In Vitro Release of Gallium Ions
2.4. Antibacterial Tests
2.4.1. Minimal Inhibitory Concentration (MIC) of Ga(NO3)3·4.2H2O
2.4.2. Antibacterial Properties of GaHAp
2.5. Cytocompatibility Test
2.5.1. Cytotoxicity of Ga(NO3)3·4.2H2O
2.5.2. Cytotoxicity of GaHAp
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics
3.2. In Vitro Ga3+ Release
3.3. Antibacterial Activity
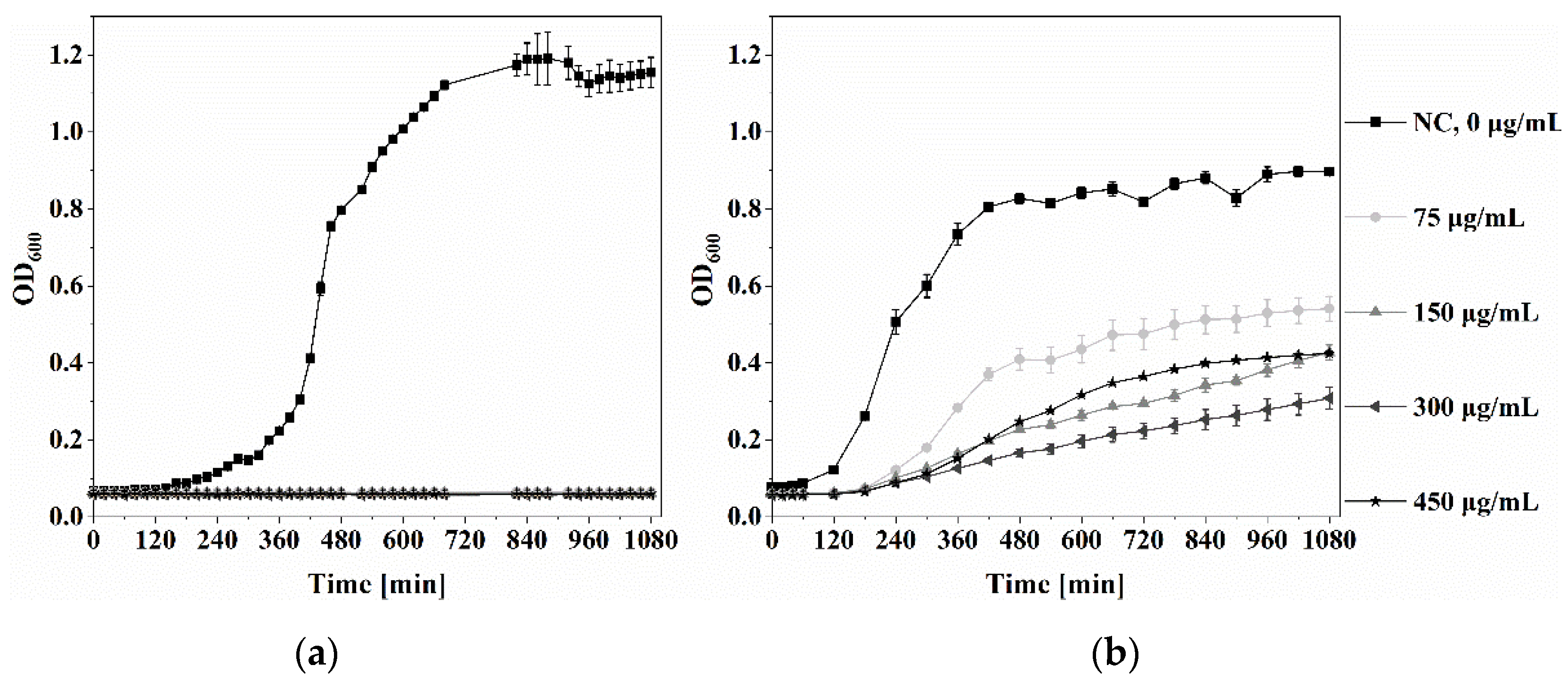
3.3.1. Minimal Inhibitory Concentration of Ga(NO3)3·4.2H2O
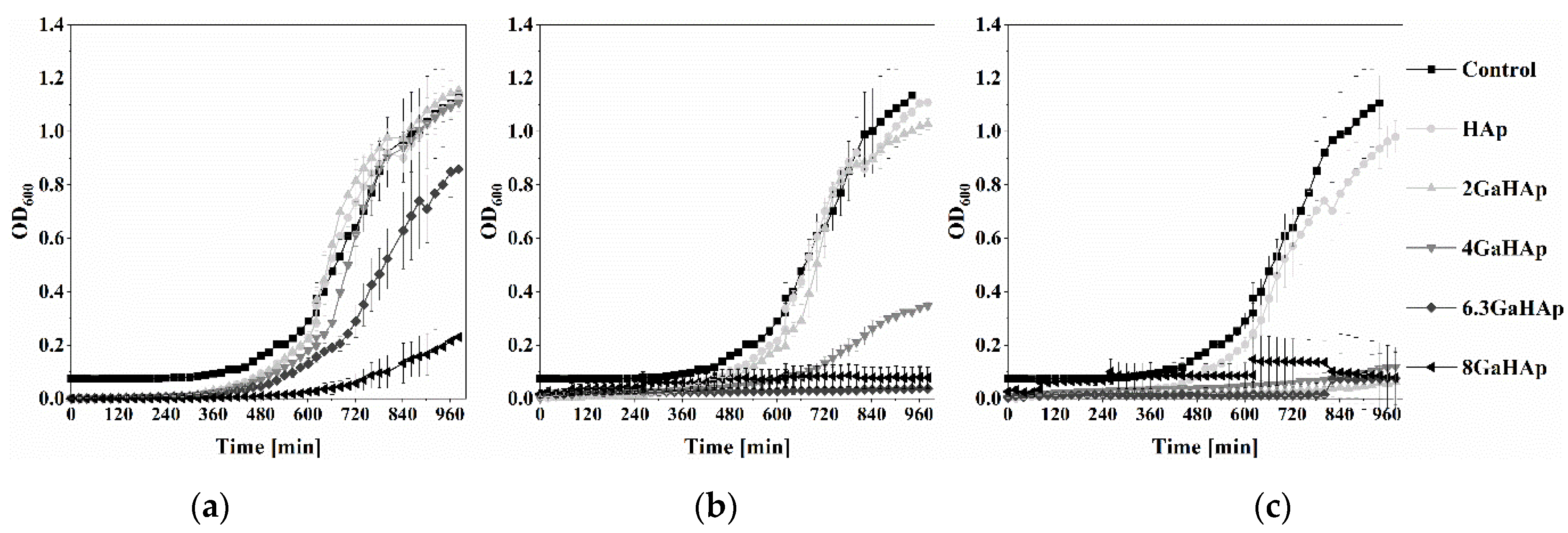
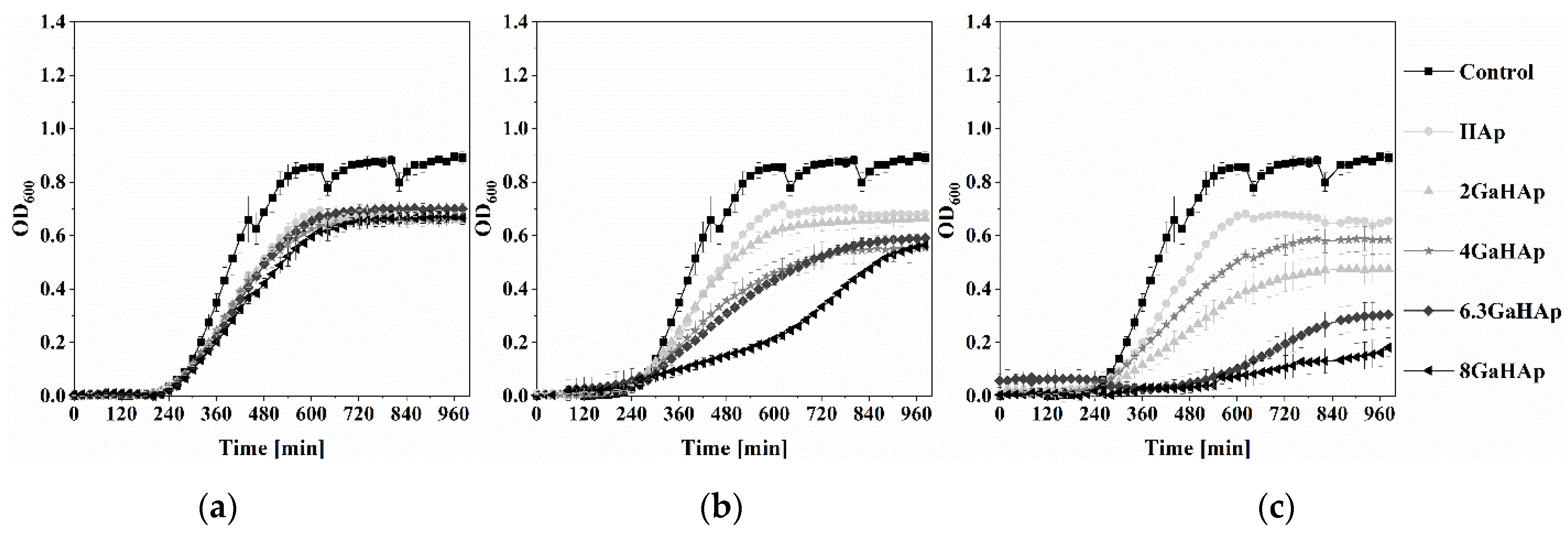
3.3.2. Antibacterial Properties of GaHAp
3.4. Cytocompatibility Test
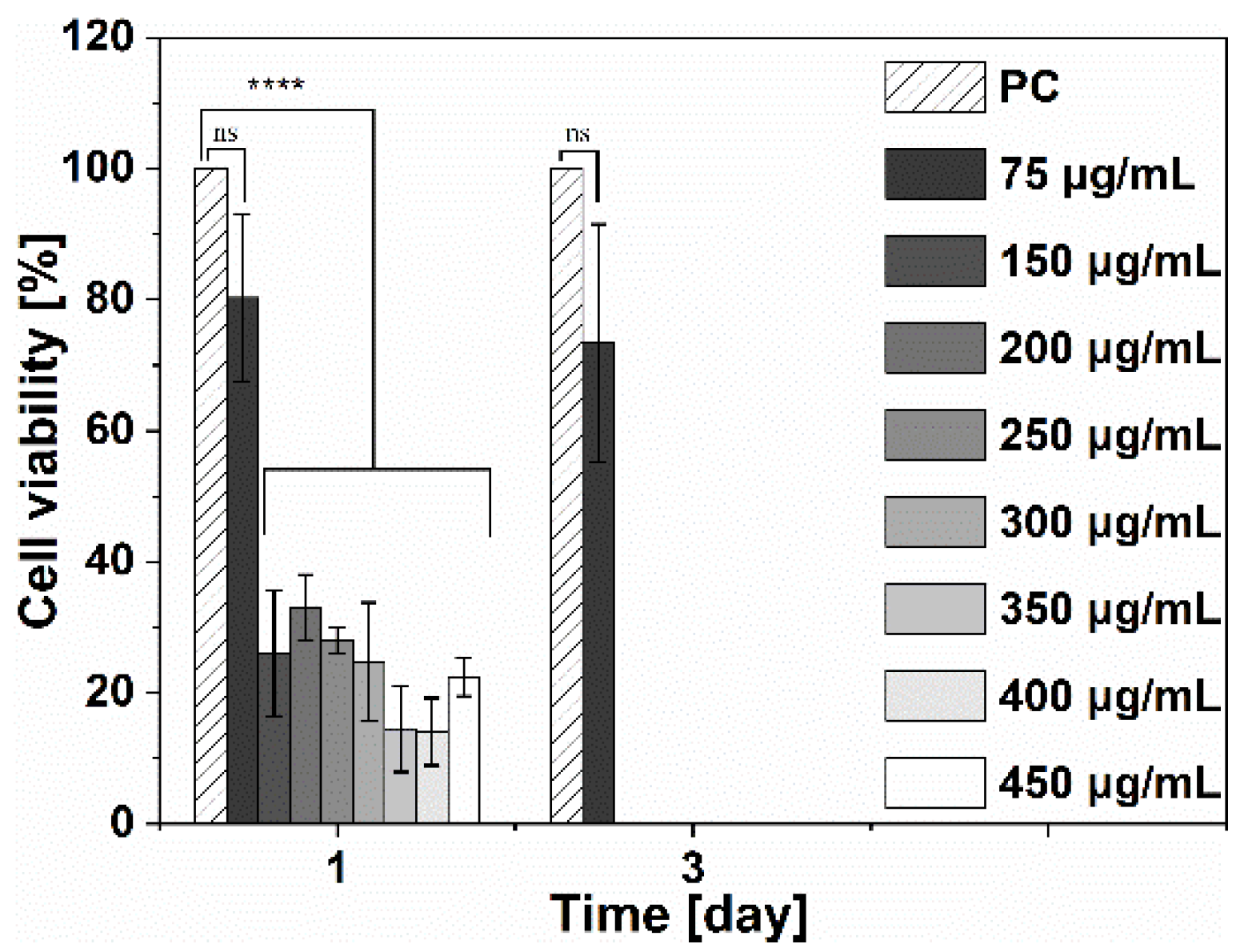
3.4.1. Cytotoxicity of Ga(NO3) 3·4.2H2O
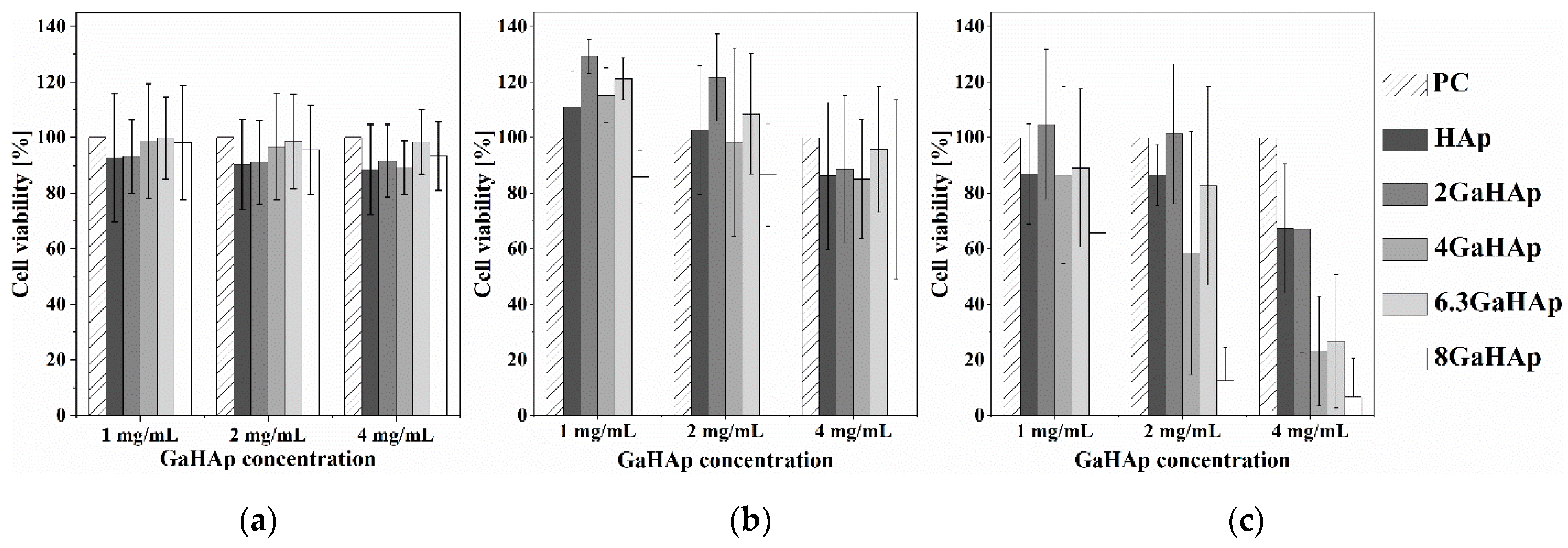
3.4.2. Cytotoxicity of GaHAp
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben-Nissan, B. Advances in Calcium Phosphate Biomaterials; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Samavedi, S.; Poindexter, L.K.; Van Dyke, M.; Goldstein, A.S. Synthetic Biomaterials for Regenerative Medicine Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 81–99. [Google Scholar] [CrossRef]
- Ambard, A.J.; Mueninghoff, L. Calcium Phosphate Cement: Review of Mechanical and Biological Properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, H.L. Calcium Phosphate Scaffolds Combined with Bone Morphogenetic Proteins or Mesenchymal Stem Cells in Bone Tissue Engineering. Chin. Med. J. 2015, 128, 1121–1127. [Google Scholar] [CrossRef]
- Driskell, T.D.; Hassler, C.R.; Tennery, V.J.; McCoy, L. Calcium Phosphate Resorbable Ceramics: A Potential Alternative to Bone Grafting. J. Dent. Res. 1973, 52, 123–131. [Google Scholar]
- Denissen, H.W.; de Groot, K. Immediate Dental Root Implants from Synthetic Dense Calcium Hydroxylapatite. J. Prosthet. Dent. 1979, 42, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Zaner, D.J.; Yukna, R.A. Particle Size of Periodontal Bone Grafting Materials. J. Periodontol. 1984, 55, 406–409. [Google Scholar] [CrossRef]
- Klein, C.P.A.T.; de Groot, K.; Drissen, A.A.; van der Lubbe, H.B.M. Interaction of Biodegradable β-Whitlockite Ceramics with Bone Tissue: An in Vivo Study. Biomaterials 1985, 6, 189–192. [Google Scholar] [CrossRef]
- Brown, W.E.; Chow, L.C.C. A New Calcium Phosphate, Water–Setting Cement. Acc. Chem. Res. 1986, 351–379. [Google Scholar]
- Yuan, H.; Barbieri, D.; Luo, X.; Van Blitterswijk, C.A.; De Bruijn, J.D. Calcium Phosphates and Bone Induction. Compr. Biomater. II 2017, 1, 333–349. [Google Scholar] [CrossRef]
- Lin, L.; Chow, K.L.; Leng, Y. Study of Hydroxyapatite Osteoinductivity with an Osteogenic Differentiation of Mesenchymal Stem Cells. J. Biomed. Mater. Res.-Part A 2009, 89, 326–335. [Google Scholar] [CrossRef]
- Wang, Z.; Han, T.; Zhu, H.; Tang, J.; Guo, Y.; Jin, Y.; Wang, Y.; Chen, G.; Gu, N.; Wang, C. Potential Osteoinductive Effects of Hydroxyapatite Nanoparticles on Mesenchymal Stem Cells by Endothelial Cell Interaction. Nanoscale Res. Lett. 2021, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of Orthopedic Implants with Emphasis on Bacterial Adhesion Process and Techniques Used in Studying Bacterial-Material Interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The Significance of Infection Related to Orthopedic Devices and Issues of Antibiotic Resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef]
- Mosina, M.; Kovrlija, I.; Stipniece, L.; Locs, J. Gallium Containing Calcium Phosphates: Potential Antibacterial Agents or Fictitious Truth. Acta Biomater. 2022, 150, 48–57. [Google Scholar] [CrossRef]
- Coppey, E.; Mommaerts, M.Y. Early Complications from the Use of Calcium Phosphate Paste in Mandibular Lengthening Surgery. A Retrospective Study. J. Oral Maxillofac. Surg. 2017, 75, 1274.e1–1274.e10. [Google Scholar] [CrossRef]
- Mokabber, T. Electrochemically Deposited Antimicrobial Hydroxyapatite Coatings; University of Groningen: Groningen, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Z.; Huang, J. Substituted Hydroxyapatite: A Recent Development. Mater. Technol. 2020, 35, 785–796. [Google Scholar] [CrossRef]
- Stötzel, C.; Müller, F.A.; Reinert, F.; Niederdraenk, F.; Barralet, J.E.; Gbureck, U. Ion Adsorption Behaviour of Hydroxyapatite with Different Crystallinities. Colloids Surf. B Biointerfaces 2009, 74, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem.-Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gopal, B. Copper Substituted Hydroxyapatite and Fluorapatite: Synthesis, Characterization and Antimicrobial Properties. Ceram. Int. 2014, 40, 15655–15662. [Google Scholar] [CrossRef]
- Jacobs, A.; Renaudin, G.; Forestier, C.; Nedelec, J.M.; Descamps, S. Biological Properties of Copper-Doped Biomaterials for Orthopedic Applications: A Review of Antibacterial, Angiogenic and Osteogenic Aspects. Acta Biomater. 2020, 117, 21–39. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic Ions as Therapeutic Agents in Tissue Engineering Scaffolds: An Overview of Their Biological Applications and Strategies for New Developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef]
- Kircheva, N.; Dudev, T. Gallium as an Antibacterial Agent: A DFT/SMD Study of the Ga3+/Fe3+ Competition for Binding Bacterial Siderophores. Inorg. Chem. 2020, 59, 6242–6254. [Google Scholar] [CrossRef] [PubMed]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-Based Anti-Infectives: Targeting Microbial Iron-Uptake Mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Lu, T.; He, F.; Feng, S.; Fang, X.; Zuo, F.; Jiang, Q.; Deng, X.; Ye, J. Influences of Gallium Substitution on the Phase Stability, Mechanical Strength and Cellular Response of β-Tricalcium Phosphate Bioceramics. Ceram. Int. 2020, 46, 16364–16371. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Krajnc, A.; Kramer, L.; Turk, B.; Suvorov, D. Designing Ga(III)-Containing Hydroxyapatite with Antibacterial Activity. RSC Adv. 2016, 6, 112839–112852. [Google Scholar] [CrossRef]
- Wren, A.W.; Jones, M.C.; Misture, S.T.; Coughlan, A.; Keenan, N.L.; Towler, M.R.; Hall, M.M. A Preliminary Investigation into the Structure, Solubility and Biocompatibility of Solgel SiO2-CaO-Ga2O3 Glass-Ceramics. Mater. Chem. Phys. 2014, 148, 416–425. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The Transition Metal Gallium Disrupts Pseudomonas Aeruginosa Iron Metabolism and Has Antimicrobial and Antibiofilm Activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Suvorov, D. Antibacterial Nanocomposite of Functionalized Nanogold and Gallium-Doped Hydroxyapatite. Mater. Lett. 2017, 193, 126–129. [Google Scholar] [CrossRef]
- Rzhepishevska, O.; Ekstrand-Hammarström, B.; Popp, M.; Björn, E.; Bucht, A.; Sjöstedt, A.; Antti, H.; Ramstedt, M. The Antibacterial Activity of Ga3+ Is Influenced by Ligand Complexation as Well as the Bacterial Carbon Source. Antimicrob. Agents Chemother. 2011, 55, 5568–5580. [Google Scholar] [CrossRef] [PubMed]
- Rubenis, K.; Zemjane, S.; Vecstaudza, J.; Bitenieks, J.; Locs, J. Densification of Amorphous Calcium Phosphate Using Principles of the Cold Sintering Process. J. Eur. Ceram. Soc. 2021, 41, 912–919. [Google Scholar] [CrossRef]
- Smits, K.; Grigorjeva, L.; Millers, D.; Kundzins, K.; Ignatans, R.; Grabis, J.; Monty, C. Luminescence Properties of Zirconia Nanocrystals Prepared by Solar Physical Vapor Deposition. Opt. Mater. 2014, 37, 251–256. [Google Scholar] [CrossRef]
- Meredith, D.O.; Eschbach, L.; Wood, M.A.; Riehle, M.O.; Curtis, A.S.G.; Richards, R.G. Human Fibroblast Reactions to Standard and Electropolished Titanium and Ti-6Al-7Nb, and Electropolished Stainless Steel. J. Biomed. Mater. Res.-Part A 2005, 75, 541–555. [Google Scholar] [CrossRef]
- Indrani, D.J.; Soegijono, B.; Adi, W.A.; Trout, N. Phase Composition and Crystallinity of Hydroxyapatite with Various Heat Treatment Temperatures. Int. J. Appl. Pharm. 2017, 9, 87–91. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tamine, K.I.; Kagawa, R.; Hamada, Y.; Okazaki, M.; Takahashi, J. Different Behavior of Implanted Hydroxyapatite Depending on Morphology, Size and Crystallinity. J. Ceram. Soc. Jpn. 2006, 114, 760–762. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD Evaluation of Carbonated Hydroxyapatite Powders Synthesized by Wet Methods. J. Mol. Struct. 2005, 744–747, 657–661. [Google Scholar] [CrossRef]
- Michelot, A.; Sarda, S.; Audin, C.; Deydier, E.; Manoury, E.; Poli, R.; Rey, C. Spectroscopic Characterisation of Hydroxyapatite and Nanocrystalline Apatite with Grafted Aminopropyltriethoxysilane: Nature of Silane–Surface Interaction. J. Mater. Sci. Vol. 2015, 50, 5746–5757. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, Ł.; Zgadzaj, A.; Piotrowska, U.; Kolmas, J. Modifications of Hydroxyapatite by Gallium and Silver Ions—Physicochemical Characterization, Cytotoxicity and Antibacterial Evaluation. Int. J. Mol. Sci. 2020, 21, 5006. [Google Scholar] [CrossRef]
- Melnikov, P.; de Fatima Cepa Matos, M.; Malzac, A.; Rainho Teixeira, A.; de Albuquerque, D.M. Evaluation of In Vitro Toxicity of Hydroxyapatite Doped with Gallium. Mater. Lett. 2019, 253, 343–345. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, X. Hydrothermal Synthesis of Hydroxyapatite Assisted by Gemini Cationic Surfactant. J. Nanomater. 2020, 2020, 5006. [Google Scholar] [CrossRef]
- In, Y.; Amornkitbamrung, U.; Hong, M.H.; Shin, H. On the Crystallization of Hydroxyapatite under Hydrothermal Conditions: Role of Sebacic Acid as an Additive. ACS Omega 2020, 5, 27204–27210. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, N.C.; Cosma, V.; Levine, S. Effect of Gallium on the in Vitro Formation, Growth, and Solubility of Hydroxyapatite. Calcif. Tissue Int. 1989, 45, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their in Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Vrchovecká, K.; Pávková-Goldbergová, M.; Engqvist, H.; Pujari-Palmer, M. Cytocompatibility and Bioactive Ion Release Profiles of Phosphoserine Bone Adhesive: Bridge from In Vitro to In Vivo. Biomedicines 2022, 10, 736. [Google Scholar] [CrossRef]
- Mocanu, A.; Cadar, O.; Frangopol, P.T.; Petean, I.; Tomoaia, G.; Paltinean, G.-A.; Racz, C.P.; Horovitz, O.; Tomoaia-Cotisel, M. Ion Release from Hydroxyapatite and Substituted Hydroxyapatites in Different Immersion Liquids: In Vitro Experiments and Theoretical Modelling Study. R. Soc. Open Sci. 2021, 8, 201785. [Google Scholar] [CrossRef]
- Koppala, S.; Swamiappan, S.; Gangarajula, Y.; Xu, L.; Sadasivuni, K.K.; Ponnamma, D.; Rajagopalan, V. Calcium Deficiency in Hydroxyapatite and Its Drug Delivery Applications. Micro Nano Lett. 2018, 13, 562–564. [Google Scholar] [CrossRef]
- Minandri, F.; Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Promises and Failures of Gallium as an Antibacterial Agent. Future Microbiol. 2014, 9, 379–397. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000182. [Google Scholar] [CrossRef]
- Gasser, V.; Kuhn, L.; Hubert, T.; Aussel, L.; Hammann, P.; Schalk, I.J. The Esterase PfeE, the Achilles’ Heel in the Battle for Iron between Pseudomonas Aeruginosa and Escherichia Coli. Int. J. Mol. Sci. 2021, 22, 2814. [Google Scholar] [CrossRef]
- Ballardini, A.; Montesi, M.; Panseri, S.; Vandini, A.; Balboni, P.G.; Tampieri, A.; Sprio, S. New Hydroxyapatite Nanophases with Enhanced Osteogenic and Anti-Bacterial Activity. J. Biomed. Mater. Res. Part A 2018, 106, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kircheva, N.; Dudev, T. Competition between Abiogenic and Biogenic Metal Cations in Biological Systems: Mechanisms of Gallium‘s Anticancer and Antibacterial Effect. J. Inorg. Biochem. 2021, 214, 111309. [Google Scholar] [CrossRef] [PubMed]
- Lessa, J.A.; Parrilha, G.L.; Beraldo, H. Gallium Complexes as New Promising Metallodrug Candidates. Inorg. Chim. Acta 2012, 393, 53–63. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Verron, E.; Montouillout, V.; Fayon, F.; Lagadec, P.; Bouler, J.M.; Bujoli, B.; Arcos, D.; Vallet-Regí, M. The Response of Pre-Osteoblasts and Osteoclasts to Gallium Containing Mesoporous Bioactive Glasses. Acta Biomater. 2018, 76, 333–343. [Google Scholar] [CrossRef] [PubMed]




| Sample | Theoretical Ga Content (wt%) | Gallium Content (wt%) | Before Sterilization | After Sterilization | ||||
|---|---|---|---|---|---|---|---|---|
| SSA (m2/g) | ρ (g/cm3) | dBET (nm) | SSA (m2/g) | ρ (g/cm3) | dBET (nm) | |||
| HAp | - | - | 71 ± 7 | 2.77 ± 0.04 | 31 ± 3 | 50 ± 2 | 2.94 ± 0.01 | 41 ± 2 |
| 2 GaHAp | 2 | 1.6 ± 0.1 | 95 ± 5 | 2.85 ± 0.06 | 22 ± 1 | 78 ± 3 | 2.86 ± 0.08 | 27 ± 1 |
| 4 GaHAp | 4 | 3.3 ± 0.4 | 117 ± 4 | 2.80 ± 0.06 | 18 ± 1 | 88 ± 5 | 2.92 ± 0.09 | 23 ± 1 |
| 6.3 GaHAp | 6.3 | 5.5 ± 0.1 | 109 ± 2 | 2.83 ± 0.01 | 20 ± 1 | 89 ± 4 | 2.92 ± 0.05 | 23 ± 1 |
| 8 GaHAp | 8 | 6.9 ± 0.5 | 102 ± 5 | 2.79 ± 0.03 | 21 ± 1 | 104 ± 3 | 2.84 ± 0.04 | 20 ± 1 |
| Sample | Plane (hkl) | Before Sterilization | After Sterilization | ||
|---|---|---|---|---|---|
| FWHM | Crystallite Size (nm) | FWHM | Crystallite Size (nm) | ||
| HAp | [002] | 0.274 | 31.1 | 0.219 | 38.9 |
| 2 GaHAp | [002] | 0.347 | 24.5 | 0.284 | 30.0 |
| 4 GaHAp | [002] | 0.384 | 22.2 | 0.279 | 30.5 |
| 6.3 GaHAp | [002] | 0.383 | 22.2 | 0.309 | 27.6 |
| 8 GaHAp | [002] | 0.372 | 22.9 | 0.316 | 27.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosina, M.; Siverino, C.; Stipniece, L.; Sceglovs, A.; Vasiljevs, R.; Moriarty, T.F.; Locs, J. Gallium-Doped Hydroxyapatite Shows Antibacterial Activity against Pseudomonas aeruginosa without Affecting Cell Metabolic Activity. J. Funct. Biomater. 2023, 14, 51. https://doi.org/10.3390/jfb14020051
Mosina M, Siverino C, Stipniece L, Sceglovs A, Vasiljevs R, Moriarty TF, Locs J. Gallium-Doped Hydroxyapatite Shows Antibacterial Activity against Pseudomonas aeruginosa without Affecting Cell Metabolic Activity. Journal of Functional Biomaterials. 2023; 14(2):51. https://doi.org/10.3390/jfb14020051
Chicago/Turabian StyleMosina, Marika, Claudia Siverino, Liga Stipniece, Artemijs Sceglovs, Renats Vasiljevs, T. Fintan Moriarty, and Janis Locs. 2023. "Gallium-Doped Hydroxyapatite Shows Antibacterial Activity against Pseudomonas aeruginosa without Affecting Cell Metabolic Activity" Journal of Functional Biomaterials 14, no. 2: 51. https://doi.org/10.3390/jfb14020051
APA StyleMosina, M., Siverino, C., Stipniece, L., Sceglovs, A., Vasiljevs, R., Moriarty, T. F., & Locs, J. (2023). Gallium-Doped Hydroxyapatite Shows Antibacterial Activity against Pseudomonas aeruginosa without Affecting Cell Metabolic Activity. Journal of Functional Biomaterials, 14(2), 51. https://doi.org/10.3390/jfb14020051