Wool Keratin Nanofibers for Bioinspired and Sustainable Use in Biomedical Field
Abstract
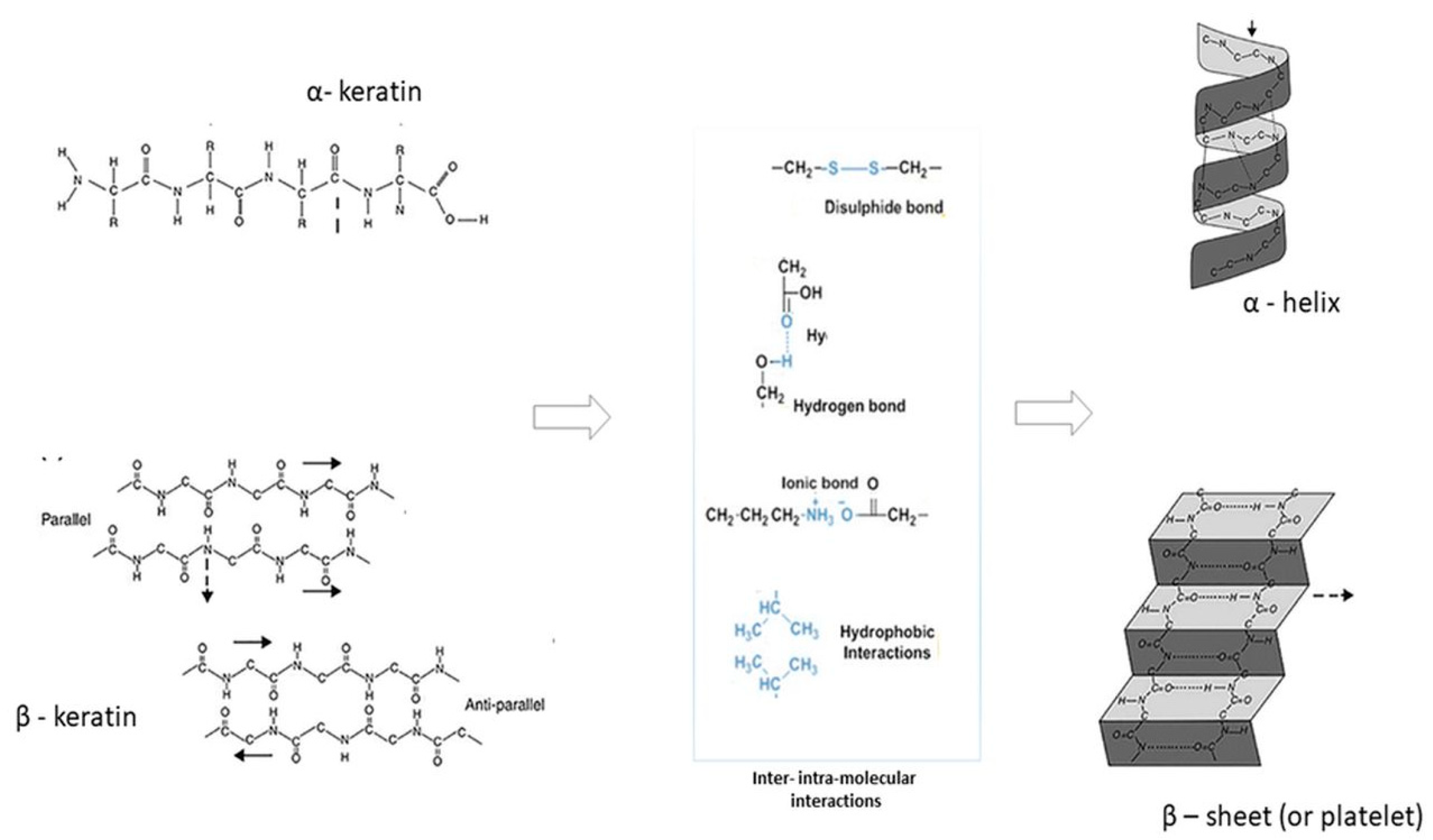
1. Introduction
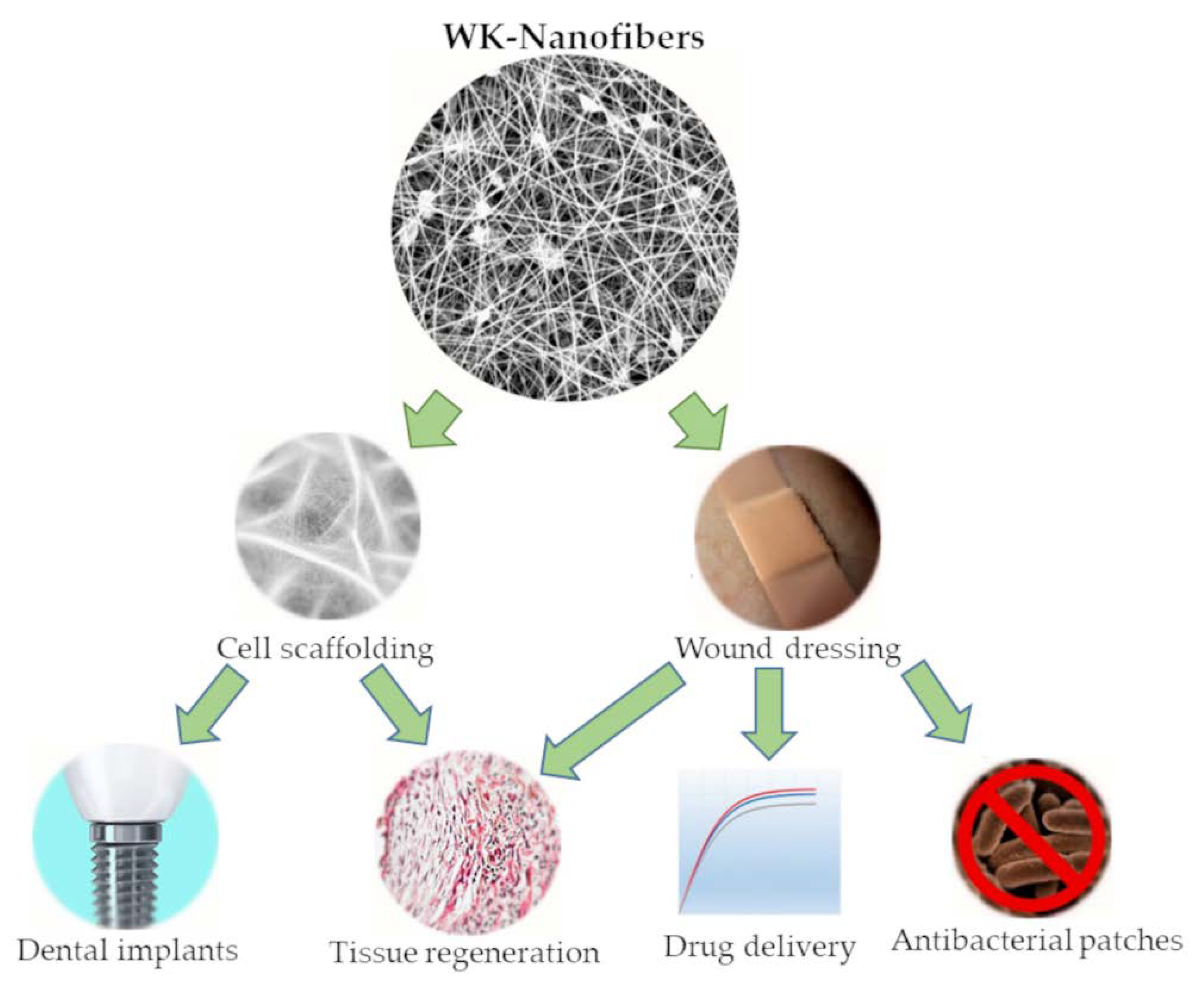
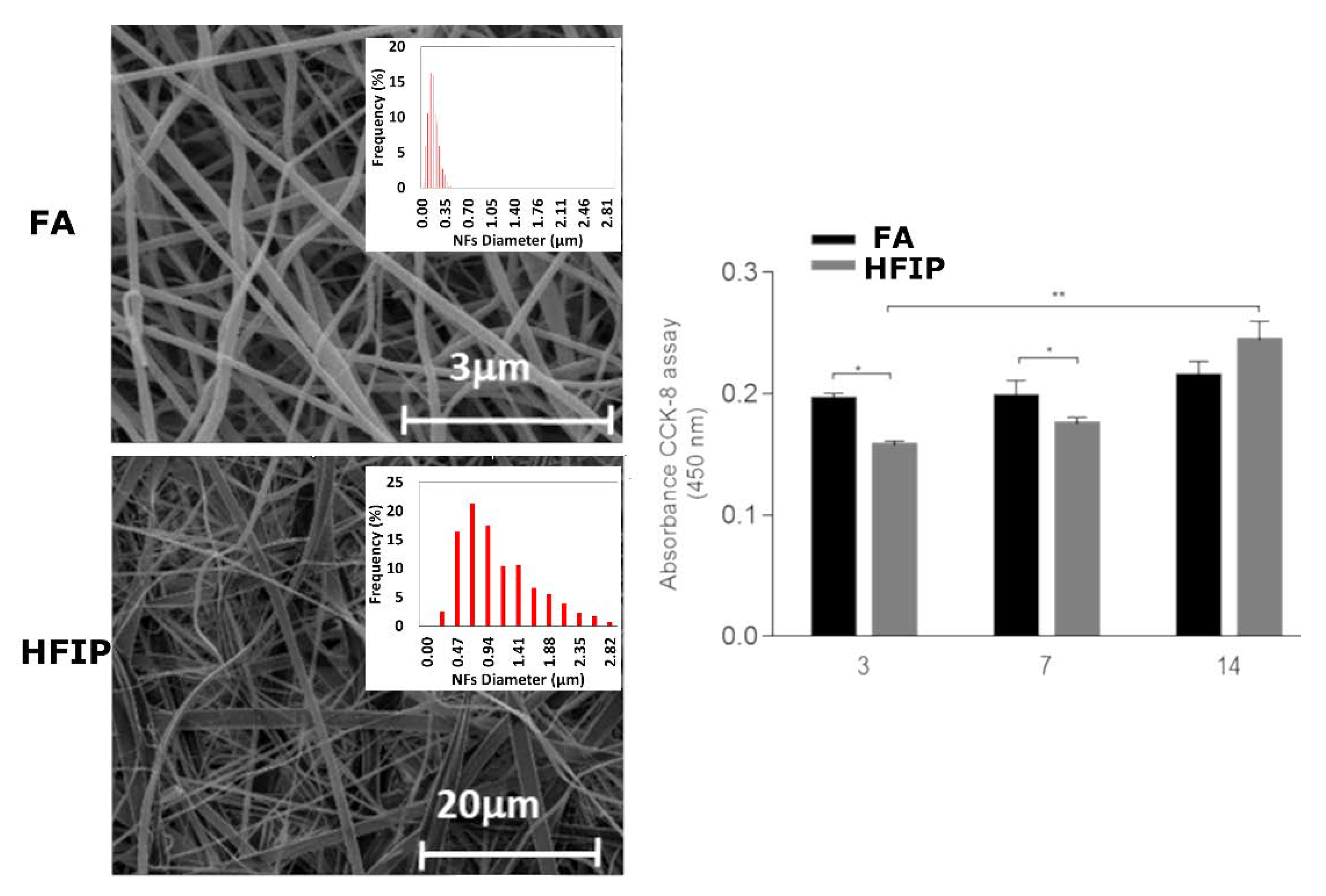
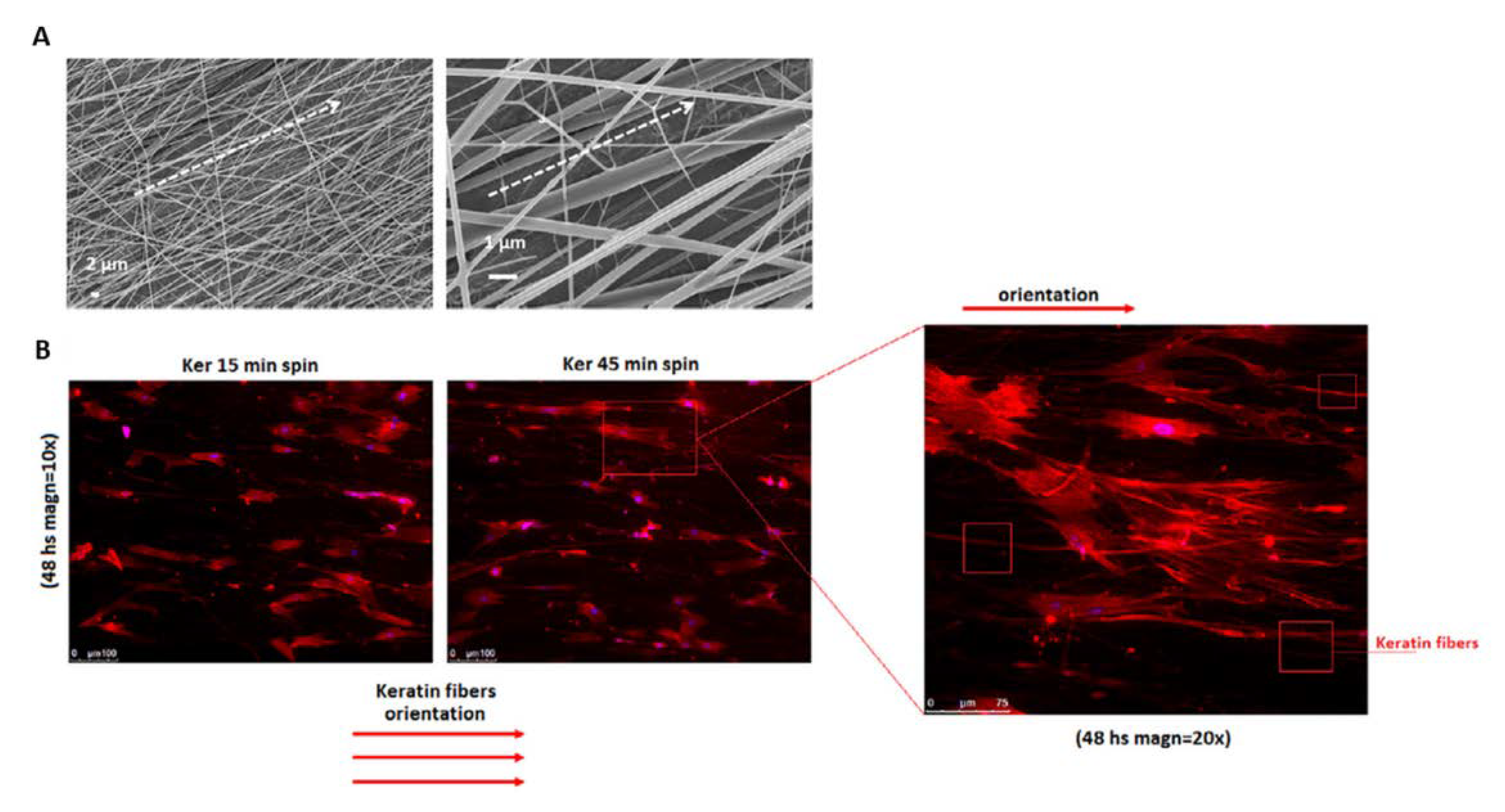
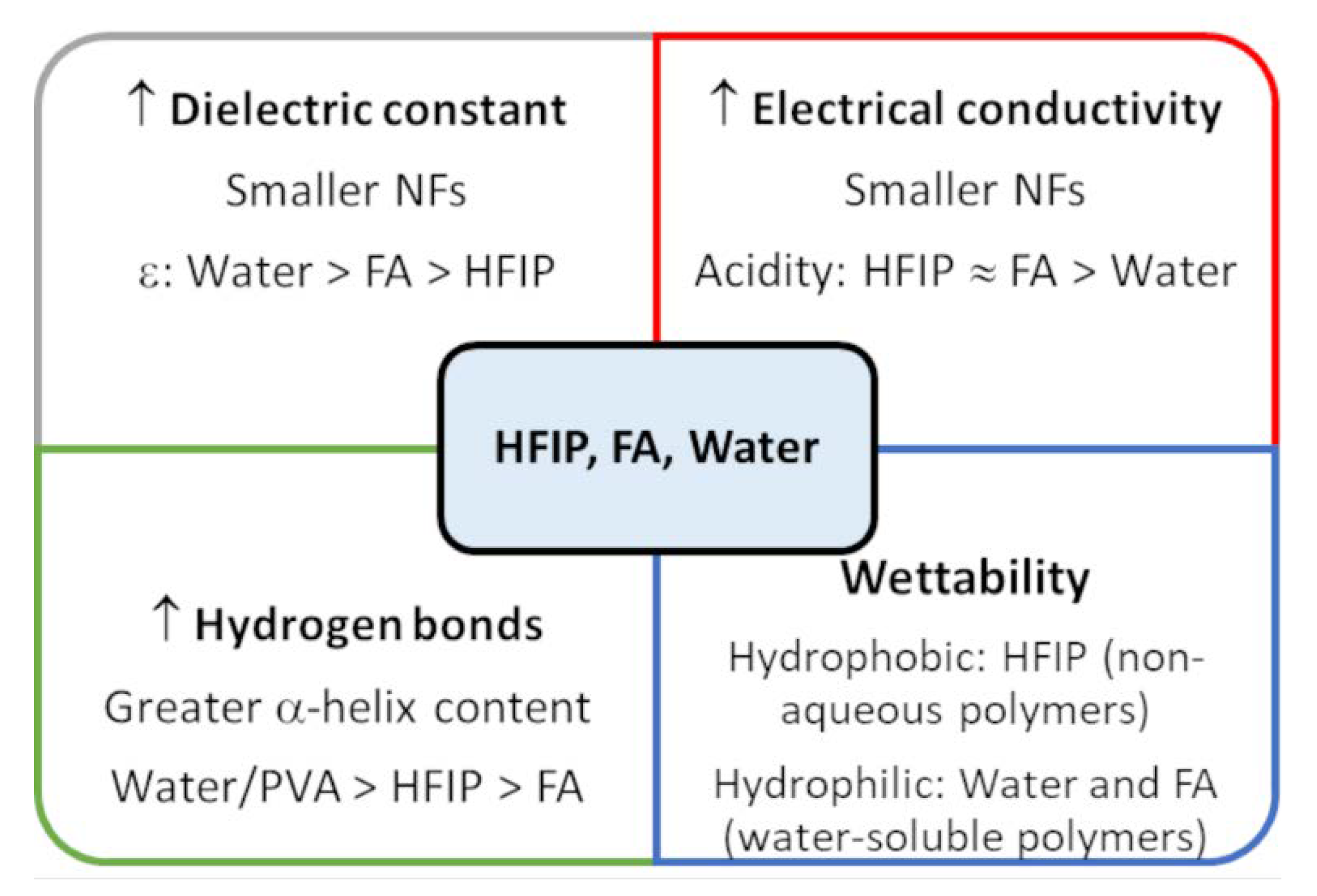
2. Pure WK—NFs
3. WK and Non-Biodegradable Polymers—NFs
4. WK and Biodegradable Polymers—NFs
| Polymer Blend (% wt.) | Polymer Mw * (kDa) | Solvent | Max. Freq. Diameter (nm) | Minimum Diameter (nm) | Maximum Diameter (nm) | Range (μm) | References |
|---|---|---|---|---|---|---|---|
| PVA | 130 | Water | 450 | 110 | 1460 | 1.35 | [33] |
| 17/83 WK/PVA | 130 | Water | 450 | 110 | 1460 | 1.35 | [33] |
| 50/50 WK/PVA | 75 | FA | 75 | 35 | 125 | 0.09 | [74] |
| PBS | 50 | HFIP | 563 | 120 | 1300 | 1.18 | [35] |
| 50/50 WK/PBS | 50 | HFIP | 236 290 | 20 20 | 650 810 | 0.63 0.79 | [34] [35] |
| PCL | 65 | HFIP | 171 | 100 | 250 | 0.15 | [36] |
| 50/50 WK/PCL | 65 | HFIP | 124 144 | 30 50 | 210 260 | 0.18 0.21 | [36] [37] |
| PLA | 138 | HFIP | 600 | 400 | 800 | 0.40 | [75] |
| 50/50 WK/PLA | 119 | HFIP | 120 | 50 | 200 | 0.15 | [15] |
| FIB | n.a. | FA | 800 | 70 | 1620 | 1.55 | [38] |
| 50/50 WK/FIB | n.a. | FA | 220 | 50 | 400 | 0.35 | [38] |
| GEL | n.a. | FA | 114 | 30 | 300 | 0.27 | [39] |
| 23/77 WK/GEL | n.a. | FA | 109 | 30 | 300 | 0.27 | [39] |
| 19/19/62 WK/SER/GEL | n.a. | FA | 200 | 30 | 1500 | 1.47 | [39] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin—Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Silva, R.; Boccaccini, A.R. Fibrous protein-based biomaterials (silk, keratin, elastin, and resilin proteins) for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Woodhead Publishing: Sawston, UK, 2018; pp. 175–204. [Google Scholar] [CrossRef]
- Salama, A.; Guarino, V. Ionic Liquids to Process Silk Fibroin and Wool Keratin for Bio-sustainable and Biomedical Applications. J. Polym. Environ. 2022, 30, 4961–4977. [Google Scholar] [CrossRef]
- Aluigi, A.; Corbellini, A.; Rombaldoni, F.; Zoccola, M.; Canetti, M. Morphological and structural investigation of wool-derived keratin nanofibres crosslinked by thermal treatment. Int. J. Biol. Macromol. 2013, 57, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Truffa Giachet, F.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater. Sci. Eng. C 2017, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Li, L.; Mak, A.F.T.; Ko, F.; Qin, L. Preparation and biodegradation of electrospun PLLA/keratin nonwoven fibrous membrane. Polym. Degrad. Stab. 2009, 94, 1800–1807. [Google Scholar] [CrossRef]
- Ranjit, E.; Hamlet, S.; George, R.; Sharma, A.; Love, R.M. Biofunctional approaches of wool-based keratin for tissue engineering. J. Sci. Adv. Mater. Devices 2022, 7, 100398. [Google Scholar] [CrossRef]
- Su, C.; Gong, J.S.; Ye, J.P.; He, J.M.; Li, R.Y.; Jiang, M.; Geng, Y.; Zhang, Y.; Chen, J.H.; Xu, Z.H.; et al. Enzymatic Extraction of Bioactive and Self-Assembling Wool Keratin for Biomedical Applications. Macromol. Biosci. 2020, 20, 2000073. [Google Scholar] [CrossRef]
- Cochis, A.; Ferraris, S.; Sorrentino, R.; Azzimonti, B.; Novara, C.; Geobaldo, F.; Truffa Giachet, F.; Vineis, C.; Varesano, A.; Sayed Abdelgeliel, A.; et al. Silver-doped keratin nanofibers preserve a titanium surface from biofilm contamination and favor soft-tissue healing. J. Mater. Chem. B 2017, 5, 8366–8377. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Cruz-maya, I.; Vineis, C.; Guarino, V.; Tonetti, C.; Varesano, A. Wool Keratin-Based Nanofibres—In Vitro Validation. Bioengineering 2021, 8, 224. [Google Scholar] [CrossRef]
- Ferraris, S.; Guarino, V.; Cochis, A.; Varesano, A.; Cruz Maya, I.; Vineis, C.; Rimondini, L.; Spriano, S. Aligned keratin submicrometric-fibers for fibroblasts guidance onto nanogrooved titanium surfaces for transmucosal implants. Mater. Lett. 2018, 229, 1–4. [Google Scholar] [CrossRef]
- Varesano, A.; Vineis, C.; Tonetti, C.; Sanchez Ramirez, D.O.; Mazzuchetti, G.; Ortelli, S.; Blosi, M.; Costa, A.L. Multifunctional hybrid nanocomposite nanofibers produced by colloid electrospinning from water solutions. Curr. Nanosci. 2015, 11, 41–48. [Google Scholar] [CrossRef]
- Giuri, D.; Barbalinardo, M.; Sotgiu, G.; Zamboni, R.; Nocchetti, M.; Donnadio, A.; Corticelli, F.; Valle, F.; Gennari, C.G.M.; Selmin, F.; et al. Nano-hybrid electrospun non-woven mats made of wool keratin and hydrotalcites as potential bio-active wound dressings. Nanoscale 2019, 11, 6422–6430. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, S.; Sanchez Ramirez, D.O.; Carletto, R.A.; Varesano, A.; Vineis, C.; Zanzoni, S.; Molinari, H.; Ragona, L. Electrospun Lipid Binding Proteins Composite Nanofibers with Antibacterial Properties. Macromol. Biosci. 2017, 17, 1600300. [Google Scholar] [CrossRef] [PubMed]
- Schifino, G.; Gasparini, C.; Drudi, S.; Giannelli, M.; Sotgiu, G.; Posati, T.; Zamboni, R.; Treossi, E.; Maccaferri, E.; Giorgini, L.; et al. Keratin/Polylactic acid/graphene oxide composite nanofibers for drug delivery. Int. J. Pharm. 2022, 623, 121888. [Google Scholar] [CrossRef] [PubMed]
- Khodir, W.K.W.A.; Razak, A.H.A.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Li, D.; Yin, Y.; Zhou, F.L. Electrospun PHB/Chitosan Composite Fibrous Membrane and Its Degradation Behaviours in Different pH Conditions. J. Funct. Biomater. 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Carter, P.; Bhattarai, N.; Puoci, F. Aloe Vera for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N.; Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef]
- Papa, A.; Guarino, V.; Cirillo, V.; Oliviero, O.; Ambrosio, L. Optimization of Bicomponent Electrospun Fibers for Therapeutic Use: Post-Treatments to Improve Chemical and Biological Stability. J. Funct. Biomater. 2017, 8, 47. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; de la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Kharaziha, M.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; RamaKrishna, S.; Berto, F. Antimicrobial Synthetic and Natural Polymeric Nanofibers as Wound Dressing: A Review. Adv. Eng. Mater. 2022, 24, 2101460. [Google Scholar] [CrossRef]
- IWTO-International Wool Textile Organisation Recycled Wool. Available online: https://iwto.org/sustainability/recycled-wool/ (accessed on 10 November 2022).
- Varesano, A.; Vineis, C.; Tonetti, C.; Sanchez Ramirez, D.O.; Mazzuchetti, G. Chemical and physical modifications of electrospun keratin nanofibers induced by heating treatments. J. Appl. Polym. Sci. 2014, 131, 40532. [Google Scholar] [CrossRef]
- Aluigi, A.; Varesano, A.; Vineis, C.; Del Rio, A. Electrospinning of immiscible systems: The wool keratin/polyamide-6 case study. Mater. Des. 2017, 127, 144–153. [Google Scholar] [CrossRef]
- Zoccola, M.; Montarsolo, A.; Aluigi, A.; Varesano, A.; Vineis, C.; Tonin, C. Electrospinning of polyamide 6/modified-keratin blends. e-Polymers 2007, 7, 1–19. [Google Scholar] [CrossRef]
- Aluigi, A.; Vineis, C.; Varesano, A.; Mazzuchetti, G.; Ferrero, F.; Tonin, C. Structure and properties of keratin/PEO blend nanofibres. Eur. Polym. J. 2008, 44, 2465–2475. [Google Scholar] [CrossRef]
- Varesano, A.; Aluigi, A.; Vineis, C.; Tonin, C. Study on the shear viscosity behavior of keratin/PEO blends for nanofibre electrospinning. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1193–1201. [Google Scholar] [CrossRef]
- Aluigi, A.; Varesano, A.; Montarsolo, A.; Vineis, C.; Ferrero, F.; Mazzuchetti, G.; Tonin, C. Electrospinning of keratin/poly(ethylene oxide)blend nanofibers. J. Appl. Polym. Sci. 2007, 104, 863–870. [Google Scholar] [CrossRef]
- Suarato, G.; Contardi, M.; Perotto, G.; Heredia-Guerrero, J.A.; Fiorentini, F.; Ceseracciu, L.; Pignatelli, C.; Debellis, D.; Bertorelli, R.; Athanassiou, A. From fabric to tissue: Recovered wool keratin/polyvinylpyrrolidone biocomposite fibers as artificial scaffold platform. Mater. Sci. Eng. C 2020, 116, 111151. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Cruz-Maya, I.; Vineis, C.; Tonetti, C.; Varesano, A.; Guarino, V. Design of asymmetric nanofibers-membranes based on polyvinyl alcohol and wool-keratin for wound healing applications. J. Funct. Biomater. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Soccio, M.; Bondi, E.; Posati, T.; Sotgiu, G.; Zamboni, R.; Torreggiani, A.; Corticelli, F.; Lotti, N.; Aluigi, A. Effects of the blending ratio on the design of keratin/poly (Butylene succinate) nanofibers for drug delivery applications. Biomolecules 2021, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Soccio, M.; Posati, T.; Sotgiu, G.; Tiboni, M.; Barbalinardo, M.; Valle, F.; Casettari, L.; Zamboni, R.; Lotti, N.; et al. Regenerated wool keratin-polybutylene succinate nanofibrous mats for drug delivery and cells culture. Polym. Degrad. Stab. 2020, 179, 109272. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Varesano, A.; Vineis, C.; Guarino, V. Comparative study on protein-rich electrospun fibers for in vitro applications. Polymers 2020, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Guarino, V.; Almaguer-Flores, A.; Alvarez-Perez, M.A.; Varesano, A.; Vineis, C. Highly polydisperse keratin rich nanofibers: Scaffold design and in vitro characterization. J. Biomed. Mater. Res. Part A 2019, 107, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Zoccola, M.; Aluigi, A.; Vineis, C.; Tonin, C.; Ferrero, F.; Piacentino, M.G. Study on cast membranes and electrospun nanofibers made from keratin/fibroin blends. Biomacromolecules 2008, 9, 2819–2825. [Google Scholar] [CrossRef]
- Vineis, C.; Cruz Maya, I.; Mowafi, S.; Varesano, A.; Sánchez Ramírez, D.O.; Abou Taleb, M.; Tonetti, C.; Guarino, V.; El-Sayed, H. Synergistic effect of sericin and keratin in gelatin based nanofibers for in vitro applications. Int. J. Biol. Macromol. 2021, 190, 375–381. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Carletto, R.A.; Tonetti, C.; Giachet, F.T.; Varesano, A.; Vineis, C. Wool keratin film plasticized by citric acid for food packaging. Food Packag. Shelf Life 2017, 12, 100–106. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Carletto, R.A.; Truffa Giachet, F. Keratin Processing. In Kerain as a Protein Biopolymer; Sharma, S., Kumar, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 77–121. ISBN 978-3-030-02900-5. [Google Scholar]
- Tachibana, A.; Furuta, Y.; Takeshima, H.; Tanabe, T.; Yamauchi, K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotechnol. 2002, 93, 165–170. [Google Scholar] [CrossRef]
- Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008, 29, 118–128. [Google Scholar] [CrossRef]
- Renkler, N.Z.; Cruz-Maya, I.; Bonadies, I.; Guarino, V. Electro Fluid Dynamics: A Route to Design Polymers and Composites for Biomedical and Bio-Sustainable Applications. Polymers 2022, 14, 4249. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Benfenati, V.; Cruz-Maya, I.; Saracino, E.; Zamboni, R.; Ambrosio, L. Instructive proteins for tissue regeneration. In Functional 3D Tissue Engineering Scaffolds Materials, Technologies and Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 23–49. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valorization 2017, 8, 1043–1048. [Google Scholar] [CrossRef]
- Wang, S.; Taraballi, F.; Tan, L.P.; Ng, K.W. Human keratin hydrogels support fibroblast attachment and proliferation in vitro. Cell Tissue Res. 2012, 347, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, G.M.; Da Ros, F.; Bisconti, S.; De Acutis, A.; Biagini, F.; Lapomarda, A.; Magliaro, C.; De Maria, C.; Montemurro, F.; Bizzotto, D.; et al. Electrospun structures made of a hydrolyzed keratin-based biomaterial for development of in vitro tissue models. Front. Bioeng. Biotechnol. 2019, 7, 174. [Google Scholar] [CrossRef]
- Soroush, E.; Mohammadpour, Z.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Berto, F. Polysaccharides-based nanofibrils: From tissue engineering to biosensor applications. Carbohydr. Polym. 2022, 291, 119670. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Abdelrazek Khalil, K.; Al-Jassir, F.F.; Gamal-Eldeen, A.M. Biocompatibility properties of polyamide 6/PCL blends composite textile scaffold using EA.hy926 human endothelial cells. Biomed. Mater. 2017, 12, 035002. [Google Scholar] [CrossRef]
- Bonura, L.; Bianchi, G.; Sanchez Ramirez, D.O.; Carletto, R.A.; Varesano, A.; Vineis, C.; Tonetti, C.; Mazzuchetti, G.; Lanzarone, E.; Ortelli, S.; et al. Monitoring Systems of an Electrospinning Plant for the Production of Composite Nanofibers. In Factories of the Future; Springer International Publishing: Cham, Switzerland, 2019; pp. 315–337. [Google Scholar]
- Borrotti, M.; Lanzarone, E.; Manganini, F.; Ortelli, S.; Pievatolo, A.; Tonetti, C. Defect minimization and feature control in electrospinning through design of experiments. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible Synthetic Polymers for Tissue Engineering Purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Alonso-López, O.; López-Ibáñez, S.; Beiras, R.; Stadler, F.J.; García-Peñas, A. Assessment of Toxicity and Biodegradability of Poly(vinyl alcohol)-Based Materials in Marine Water. Polymers 2021, 13, 3742. [Google Scholar] [CrossRef]
- Wu, H.F.; Yue, L.Z.; Jiang, S.L.; Lu, Y.Q.; Wu, Y.X.; Wan, Z.Y. Biodegradation of polyvinyl alcohol by different dominant degrading bacterial strains in a baffled anaerobic bioreactor. Water Sci. Technol. 2019, 79, 2005–2012. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Cao, A.; Wang, J. in vitro Evaluation of Biodegradable Poly(butylene succinate) as a Novel Biomaterial. Macromol. Biosci. 2005, 5, 433–440. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Pistner, H.; Bendi, D.R.; Mühling, J.; Reuther, J.F. Poly (l-lactide): A long-term degradation study in vivo: Part III. Analytical characterization. Biomaterials 1993, 14, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.R.M.; Rozema, F.B.; Boering, G.; Nijenhius, A.J.; Pennings, A.J.; Verwey, A.B.; Nieuwenhuis, P.; Jansen, H.W.B. Degradation of and tissue reaction to biodegradable poly(L-lactide) for use as internal fixation of fractures: A study in rats. Biomaterials 1991, 12, 32–36. [Google Scholar] [CrossRef]
- Cerroni, B.; Cicconi, R.; Oddo, L.; Scimeca, M.; Bonfiglio, R.; Bernardini, R.; Palmieri, G.; Domenici, F.; Bonanno, E.; Mattei, M.; et al. In vivo biological fate of poly(vinylalcohol) microbubbles in mice. Heliyon 2018, 4, e00770. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, C.; Nakashima, S.; Hotta, A. Mechanical properties and chemical structures of biodegradable poly(butylene-succinate) for material reprocessing. Polym. Degrad. Stab. 2012, 97, 972–980. [Google Scholar] [CrossRef]
- Mizuno, S.; Maeda, T.; Kanemura, C.; Hotta, A. Biodegradability, reprocessability, and mechanical properties of polybutylene succinate (PBS) photografted by hydrophilic or hydrophobic membranes. Polym. Degrad. Stab. 2015, 117, 58–65. [Google Scholar] [CrossRef]
- Woodward, S.C.; Brewer, P.S.; Moatamed, F.; Schindler, A.; Pitt, C.G. The intracellular degradation of poly(ε-caprolactone). J. Biomed. Mater. Res. 1985, 19, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.; Lv, C.; Zhou, H. Changes in Crystallization Behaviors of Poly(Vinyl Alcohol) Induced by Water Content. J. Vinyl Addit. Technol. 2020, 26, 613–622. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Shen, Y.; Dai, X.; Wang, X.; Deng, K.; Long, X.; Liu, L.; Zhang, X.; Li, Y.; et al. Instant in-situ Tissue Repair by Biodegradable PLA/Gelatin Nanofibrous Membrane Using a 3D Printed Handheld Electrospinning Device. Front. Bioeng. Biotechnol. 2021, 9, 619. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.H. Fabrication and characterization of electrospun wool keratin/poly(vinyl alcohol) blend nanofibers. Adv. Mater. Sci. Eng. 2014, 2014, 163678. [Google Scholar] [CrossRef]
- Xie, J.; Shen, H.; Yuan, G.; Lin, K.; Su, J. The effects of alignment and diameter of electrospun fibers on the cellular behaviors and osteogenesis of BMSCs. Mater. Sci. Eng. C 2021, 120, 111787. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, J.; Ye, Y.; Fang, M.; Lang, J.; Yang, D.; Pan, Z. Biodegradable poly(butylene succinate) nanofibrous membrane treated with oxygen plasma for superhydrophilicity. Surf. Coat. Technol. 2020, 381, 125147. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, H.H.; Xiong, J.; Zhu, Y.T.; Zhang, H.P.; Xiong, X.; Liu, Y.M.; Yu, J.; Guo, Z.X. Improved Mechanical Properties of Poly(butylene succinate) Membrane by Co-electrospinning with Gelatin. Chin. J. Polym. Sci. 2018, 36, 1063–1069. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Taddei, P.; Alvarez-Perez, M.A.; Ambrosio, L. Tuning size scale and crystallinity of PCL electrospun fibres via solvent permittivity to address hMSC response. Macromol. Biosci. 2011, 11, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Colomer, I.; Chamberlain, A.E.R.; Haughey, M.B.; Donohoe, T.J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 2017, 1, 88. [Google Scholar] [CrossRef]

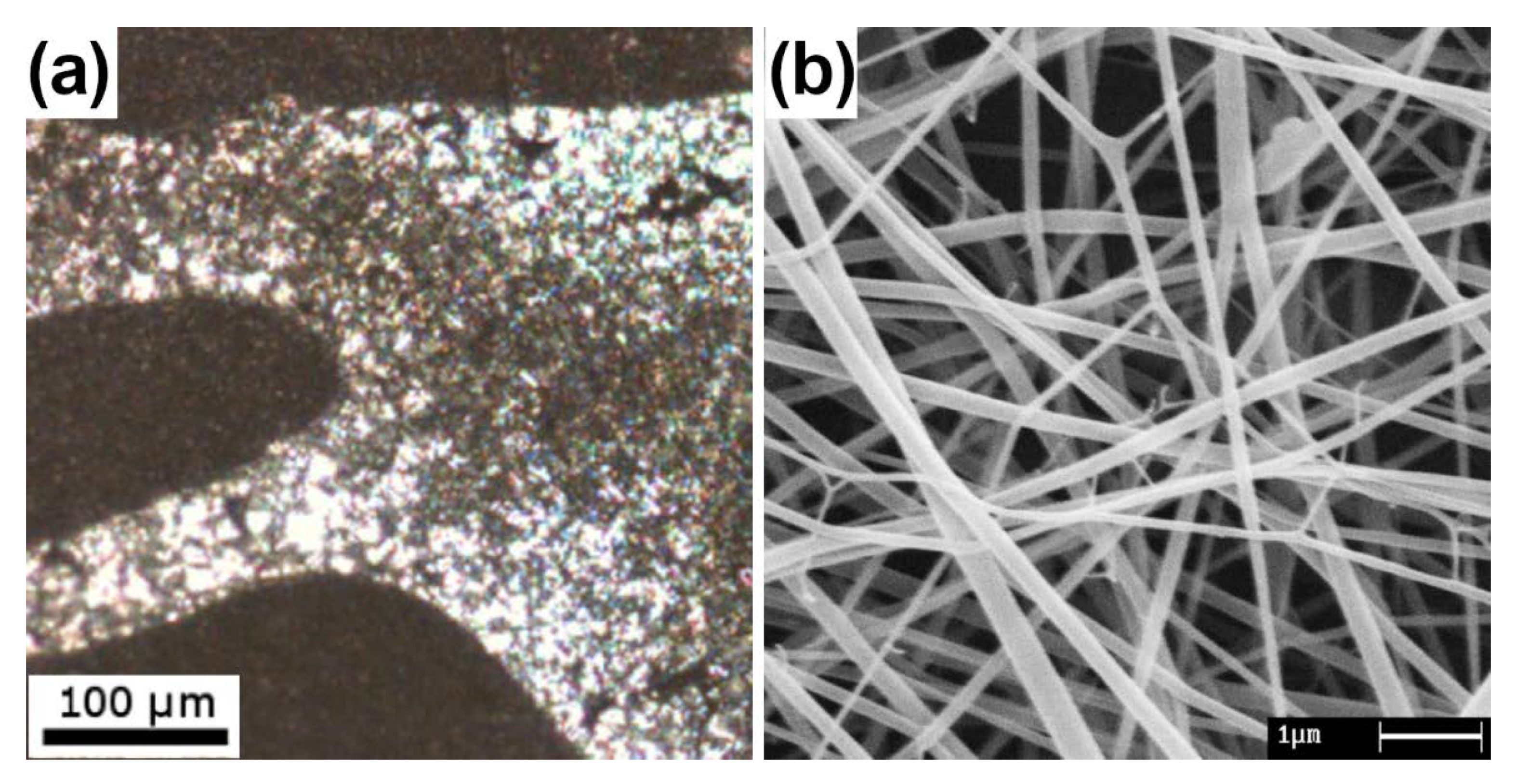



| Polymer Blend (% wt.) | Polymer Mw * (kDa) | Solvent | Biodegradability | Biocompatibility | Reference(s) |
|---|---|---|---|---|---|
| 100 WK | n.a. | FA | + | + | [4,9,26] |
| 100 WK | n.a. | HFIP | + | + | [10,11] |
| 50/50 WK/PA6 | 22 | FA | − | + | [27,28] |
| 70/30 WK/PEO | 400 | Water | − | + | [29,30,31] |
| 25/75 WK/PVP | 1300 | Water | − | + | [32] |
| 17/83 WK/PVA | 130 | Water | + | + | [33] |
| 50/50 WK/PBS | 50 | HFIP | + | + | [34,35] |
| 50/50 WK/PCL | 65 | HFIP | + | + | [36,37] |
| 50/50 WK/PLA | 119 | HFIP | + | + | [15] |
| 50/50 WK/FIB | n.a. | FA | + | + | [38] |
| 23/77 WK/GEL | n.a. | FA | + | + | [39] |
| 19/19/62 WK/SER/GEL | n.a. | FA | + | + | [39] |
| NFs | Additional Polymer(s) | Solvent System | Overall Polymer Conc. | WK Conc. (% on Polymer) | Voltage (kV) | Flow Rate (mL min−1) | Working Distance (cm) | Needle I.D. or Gauge | Collector | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| WK | n.a. | FA | 15% w/w | 100 | 25 | 0.003 | 15 | 0.2 mm | Flat square | [5,9,26] |
| WK | n.a. | HFIP | 10% w/v | 100 | 25 | 0.008 | 15 | 18 Ga | Rotating drum | [11] |
| WK/PA6 | PA6, 22 kDa | FA | 15% w/w | 0–100 | 15, 20, 25, 30 | 0.001, 0.005, 0.01 | 15 | 0.4 mm | Rotating flat disk | [27] |
| WK/PEO | PEO, 400 kDa | Water | 7% w/w | 10–90 | 20 | 0.01 | 20 | 0.2 mm | Rotating flat disk | [29] |
| WK/PEO | PEO, 400 kDa | Water | 5, 7, 10% w/w | 50 | 10–30 | 0.01–0.03 | 20 | - | Rotating flat disk | [31] |
| WK/PVP | PVP, 1300 kDa | Water | 7.6% w/v | 25 | 18 | 0.01 | 20 | 18 Ga | Flat disk | [32] |
| WK/PVA | 130 kDa | Water | 8.8, 10% w/w | 17, 33 | 25 | 0.015 | 25, 30 | 0.4 mm | Flat plate | [33] |
| WK/PBS | PBS, 50 kDa | HFIP | 13% w/v | 30, 50, 70 | 18 | 0.03 | 18 | - | Flat square | [34] |
| WK/PBS | PBS, 50 kDa | HFIP | 15% w/v | 50 | 20 | 0.03 | 15 | 0.8 mm | Flat square | [35] |
| WK/PCL | PCL, 65 kDa | HFIP | 10% w/v | 50, 70 | 15–25 | 0.0017 | 9–12 | - | - | [37] |
| WK/PLA/GO | PLA, 119 kDa | HFIP | 10% w/v | 50 | 12, 15, 18 | 0.03 | 12, 15, 18 | 0.603 mm | Flat plate | [15] |
| WK/FIB | FIB | FA | 15% w/w | 0–100 | 30 | 0.005 | 10 | 0.2 mm | Rotating flat disk | [38] |
| WK/GEL | GEL | FA | 13% w/w | 23 | 30–35 | NFE | 13 | NFE | Flat PP-NW | [39] |
| WK/GEL/SER | GEL, SER | FA | 16% w/w | 19 | 30–35 | NFE | 13 | NFE | Flat PP-NW | [39] |
| Biodegradable Polymer | Advantages | Limitations | Reference(s) |
|---|---|---|---|
| PVA | - Its hydroxyl groups can create physical/chemical interactions with other molecules. - It is a hydrophilic and water-soluble polymer. - It has chemical stability and transport properties that characterize ionotropic polymer. - It has good transparency, good mechanical and thermal properties. - It is resistant to oxygen permeation. - It has a wide range of applications in different industrial–commercial segments. | - Its processing requires relatively large amounts of water and organic plasticizers in extrusion processes. - It can be efficiently degraded by microorganisms whose occurrence in natural environments may be relatively uncommon. - Pure PVA films have disadvantages such as brittleness, low fracture elongation, poor water resistance and processability, which limit its wide application. | [33,58,60,72] |
| PBS | - Its good heat resistance and melting temperature provide a wide processing range. - It has good thermal stability and excellent mechanical properties (comparable to polyethylene and polypropylene). - It contributes to improving the mechanical properties of polymer blend NFs. - It has good clarity, great processability and flexibility. - Its ester bonds can be hydrolyzed by water. | - It is slightly brittle. - Its slow crystallization rate, low melt viscosity and softness limit PBS processing and applications, especially in injection molding. - The strength properties of PBS deteriorate due to a rapid crystallization reaction when combined with other materials. - It has insufficient osteoblast compatibility and bioactivity. | [34,61,70] |
| PCL | - It is easy to manufacture and manipulate into an extensive range of implants and devices thanks to its rheological and viscoelastic properties. - Its crystallinity tends to decrease by increasing its molecular weight. - It has multiple potential applications in the biomedical field thanks to its low melting point (59–64 °C) | - It can be only degraded by outdoor living organisms (bacteria and fungi). - Its bioresorbability process takes much longer, first propagating through hydrolytic degradation. - Pure PCL scaffolds cannot trigger cell adhesion mechanisms due to their intrinsic hydrophobic properties. | [36,63] |
| PLA | - It is widely used for biomedical scaffolds and implants with theranostics and drug delivery systems. - It is simple to synthesize and can be tailored for different therapeutic applications. - It is naturally degraded over time into well-tolerated and safe degradation products, which are secreted from the body. - It has good biocompatibility and mechanical properties. | - It is highly hydrophobic and lacks a cell recognition site, which hinders the rapid adhesion, migration and regeneration of tissue cells. | [65,73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez Ramirez, D.O.; Vineis, C.; Cruz-Maya, I.; Tonetti, C.; Guarino, V.; Varesano, A. Wool Keratin Nanofibers for Bioinspired and Sustainable Use in Biomedical Field. J. Funct. Biomater. 2023, 14, 5. https://doi.org/10.3390/jfb14010005
Sanchez Ramirez DO, Vineis C, Cruz-Maya I, Tonetti C, Guarino V, Varesano A. Wool Keratin Nanofibers for Bioinspired and Sustainable Use in Biomedical Field. Journal of Functional Biomaterials. 2023; 14(1):5. https://doi.org/10.3390/jfb14010005
Chicago/Turabian StyleSanchez Ramirez, Diego Omar, Claudia Vineis, Iriczalli Cruz-Maya, Cinzia Tonetti, Vincenzo Guarino, and Alessio Varesano. 2023. "Wool Keratin Nanofibers for Bioinspired and Sustainable Use in Biomedical Field" Journal of Functional Biomaterials 14, no. 1: 5. https://doi.org/10.3390/jfb14010005
APA StyleSanchez Ramirez, D. O., Vineis, C., Cruz-Maya, I., Tonetti, C., Guarino, V., & Varesano, A. (2023). Wool Keratin Nanofibers for Bioinspired and Sustainable Use in Biomedical Field. Journal of Functional Biomaterials, 14(1), 5. https://doi.org/10.3390/jfb14010005










